Abstract
Background
The INPP5E gene encodes for the inositol polyphosphate-5-phosphatase (INPP5E) 72 kDa protein that regulates the phosphoinositide signaling pathway and other cellular activities, but the functional role of this gene in embryonic neurodevelopment and neural tube defect (NTD) remains unclear. The aim of this study was to use a mouse model of NTD to investigate the expression levels of the INPP5E gene during neural development and the occurrence of NTD.
Material/Methods
In an established NTD mouse model, stereoscopy was used to look for morphological defects. Transcription and expression levels of the INPP5E gene in neural tissues were detected using real-time fluorescence quantitative polymerase chain reaction (PCR) and Western blotting in the NTD mouse embryos and compared with control mouse embryos.
Results
The expression levels of the INPP5E gene decreased as embryonic development progressed in the neural tissue of control mice embryos, but showed no obvious trend in the neural tissues of the NTD mouse embryos. The expression levels of the INPP5E gene in NTD mouse embryos were significantly lower compared with control embryos, at the time of neural tube closure (gestational day 11.5).
Conclusions
The INPP5E gene regulates the process of embryonic neural development. Abnormal levels of expression of the INPP5E gene may contribute to NTDs. Increased knowledge of the expression pattern of the INPP5E gene may lead to an advanced understanding of the molecular mechanism of embryonic neurodevelopment and identify more specific directions to explore potential treatments for NTDs associated with abnormalities in INPP5E gene expression levels.
MeSH Keywords: Embryonic Development, Gene Expression, Neural Tube Defects
Background
The INPP5E gene is located on q34.3 of chromosome 9 [1], and encodes for the inositol polyphosphate-5-phosphatase E (INPP5E) protein [2,3], a 72 kDa enzyme protein composed of 644 amino acids. The INPP5E protein is found in the neural cytoplasm, cytoskeleton, cilia axial filament, Golgi complex, Golgi membrane, peripheral membrane, and the internal aspect of the plasma membrane [4–7]. The INPP5E protein has a specific affinity for lipid substrates and no affinity for water-soluble inositol phosphates [4]. Previous studies have shown that mutations in the INPP5E gene lead to neural malformations, including Behr syndrome and MORMS syndrome [5,8,9]. Malformations induced by mutations in the INPP5E gene are characterized by mesencephalon-rhombencephalon (deuterencephalon) malformations, cilia abnormalities, retinal dystrophy, cystic renal disease, liver fibrosis, and polydactyly [10–13].
Cilia can be classified as primary or motile, based on their structure and functions [14,15]. Primary cilia play an important role during the development of the nervous system [16,17]. Structural and functional abnormalities of cilia are associated with malformations of the neural tube; other roles for cilia include movement, sensing extracellular stimuli and signal transduction of neural pathways [18–20]. There is evidence that vertebrate embryonic development relies on many signaling pathways that organize and control cellular structure and morphogenesis, with a critical role being the stability of primary cilia [16,21–27].
In mammals, the development of the nervous system has an organized and sequential course, and the occurrence of any abnormality in any part of the development cycle will affect the integrity of the mature nervous system [28–30]. Studies have shown that the phosphoinositide signaling pathway is associated with the stability of primary cilia, which plays a critical role in the closure of neural tube during neural development [22,24,26]. The INPP5E gene is highly expressed in human and mouse brain, with one of the main functions of this gene in eukaryotes being to catalyze the eight members of the phosphoinositide family of phospholipids, PI, PI3P, PI4P, PI5P, PI(3,4)P2, PI(4,5)P2, PI(3,5)P2 and PI(3,4,5)P3, via the PI3K/Akt signaling pathway, regulating cell proliferation [31,32]. Therefore, the INPP5E protein is not only the key enzyme in regulating the synthesis and metabolism of phosphatidylinositol biphosphate (PIP2), it is also a negative regulator of the PI3K/Akt signaling pathway [33–35]. Therefore, mutations in the INPP5E gene would lead to a decreased production of PI (3,4,5) P3 and/or PI (4,5) P2, which through the PI3K signaling pathway, would have a detrimental impact on cell proliferation and the stability of primary cilia, leading to ciliopathy, a genetic disease of primary cilia [36,37]. Ciliopathy is a genetically heterogeneous group of diseases caused by cilia or matrix protein dysfunction, with clinical manifestations that mainly include cystic renal disease, osseous dysplasia, retinosis, mental retardation, and nervous system deformity. Therefore, it is possible to speculate that mutation in the INPP5E gene is related to the occurrence of NTDs.
The aim of this study was to use a mouse model of NTD to investigate the expression levels of the INPP5E gene during neural development and the occurrence of NTDs in mouse embryos. The study was undertaken to provide further information on the role of this gene in embryonic neural development and to provide experimental evidence that may provide information to support future studies on approaches to the treatment and prevention of NTDs.
Material and Methods
Animals and treatment
All the procedures and experiments were approved by Ethics Committee of the Capital Institute of Pediatrics (DWLL2016004). Specific pathogen free (SPF) level C57BL/6J mice, at the age of sexual maturity of 7–8 weeks, weighing 18–20 g (Academy of Military Medical Sciences, Beijing, China) were housed in animal rooms with constant temperature of 22±2°C, and humidity of 50±10%, and were given food and water ad libitum. Upon reaching the weight of 20 g, females were mated overnight (from 6: 00 p.m. to 6: 00 a.m.) with males and examined for the presence of vaginal plugs the following morning, as female mice with vaginal plugs were considered to be at gestational day (GD) 0.5 at 12: 00 a.m.
On GD 7.5, the control group was treated with 0.9% NaCl (0.1ml/10 g) by intraperitoneal injection. The treatment group was treated with an intraperitoneal injection of methotrexate (MTX) (Merck & Co. Inc., Whitehouse Station, NJ, USA) 4.5 mg/kg−1 [38]. At GD 11.5, 13.5, and 15.5, pregnant mice were euthanized by cervical dislocation, and their embryos were examined under a dissecting microscope. Some embryos on GD 11.5 were fixed in cold neutral-buffered formalin for tissue processing, sectioning, and subsequent examination by light microscopy [39]. Neural tissues of control embryos from one litter were isolated and pooled as control samples, and neural tissues of NTD mouse model embryos from another litter were isolated and pooled as embryonic development defect samples. All samples were frozen at −80°C.
Light microscopy and hematoxylin and eosin (H&E) staining
The selected embryos were fixed in neutral-buffered formalin overnight, then dehydrated in graded solutions of alcohol, and embedded in paraffin wax by standard methods. Fixed tissue blocks were then cut into 5 mm-thick sections and stained with hematoxylin and eosin (H&E).
Western blot analysis
The expression levels of the INPP5E gene at different time points were determined by Western blot analysis. Briefly, the extracted neural tissues from control and NTD mouse model embryos at GD 11.5, GD 13.5 and GD 15.5, respectively, were homogenized in CelLytic MT reagents (Sigma-Aldrich, St. Louis, MO, USA). The extracted protein levels were determined using the Bradford spectroscopic protein assay method. Protein extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Thermo Scientific, #88585), which was then sealed by 10% dried skimmed milk powder solution at room temperature for 1.5 h. The PVDF membranes were incubated overnight at 4°C, with the primary antibody to the INPP5E protein (1: 1000) (Santa Cruz Biotechnology), then incubated with the anti-mouse secondary antibody (1: 2000) (Santa Cruz Biotechnology) at room temperature for 2 h. Detection was performed with enhanced chemiluminescent (ECL) substrate (Thermo SuperSignalTM West Pico), and the results were analyzed using a gel image processing system. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control.
Real-time (RT) fluorescence quantitative polymerase chain reaction (PCR)
Real-time (RT) fluorescence quantitative polymerase chain reaction (PCR) was performed to detect the transcription levels of the INPP5E gene in the extracted neural tissues of the NTD mouse embryos and control embryos at GD 11.5, GD 13.5 and GD 15.5, respectively. RT-PCR was performed using the 7500 Fast Real-Time PCR System (Applied Biosystems), and primers were designed by Primer3.0 software. Each test was performed in triplicate using 20 μl of reaction mixture containing 10 μl of SYBR Premix DimerEraser™ (2×) (Perfect Real Time) (Applied Biosystems), 1.2 μl of 10 μM forward (0.6 μl) and reverse (0.6 μl) primers, 0.4 μl ROX Reference Dye (50×) (Thermo Fisher Scientific) and 2 μl of template. Sequence analysis and melting curve analysis were used to examine the specificity of the PCR product. Amplification was carried out according to the following three stages: (1) pre-degeneration at 95°C for 30 seconds, performed in duplicate; (2) PCR reaction at 95°C for 3 seconds; 55°C for 30 seconds; 72°C for 32 seconds; repeated 40 times; and (3) extension for 34 seconds.
Statistical analysis
All data were expressed as the mean ± standard deviation (SD) and analyzed using one-factor ANOVA. P<0.05 was set as the statistically significant level. The results of PCR were the means test performed in triplicate. Transcription levels of the control and NTD mouse model embryos were compared using two-tailed Student’s t-test. All data were analyzed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA).
Results
Neural tube defect (NTD) mouse model
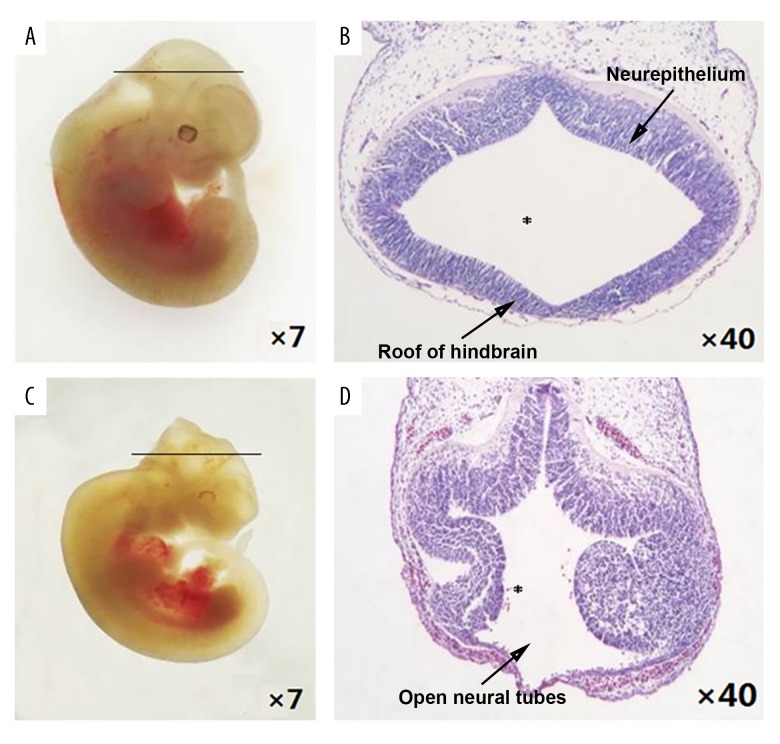
The gestational day (GD) 11.5 embryos were observed using stereoscopy (Figure 1). For the control embryos, the appearance was plump and smooth, and the forebrain, midbrain, and metencephalon primary brain vesicles were already developed. The appearance of the spine of the control embryos was complete with no cleft, otic vesicles and ocular vesicles were visible, and the tail was flexural and slender (Figure 1A). The hematoxylin and eosin (H&E) staining of the tissue sections on light microscopy, under ×40 magnification, showed that the neural tube was closed, the hindbrain was well developed, and the surrounding mesenchymal cells of the 4th ventricle were homogeneously and densely distributed, with regular ventricular wall and smooth internal and external membranes of the neuroepithelium (Figure 1B).
Figure 1.
Morphological changes in the neural tube defect (NTD) mouse model, gestational day (GD) 11.5. (A) Control embryos viewed under the dissecting microscope. (B) Photomicrograph of the light microscopy of the hematoxylin and eosin (H&E)-stained section of control mouse embryonic hindbrain. (C) NTD mouse model embryos viewed under the dissecting microscope. (D) Photomicrograph of the light microscopy of the hematoxylin and eosin (H&E)-stained section of the NTD mouse model embryos. Slice site: * fourth ventricle.
The NTD mouse model embryos (Figure 1C) had open neural tubes in the hindbrain, and their neural tissue was exposed to the external environment with partial degeneration. Although the metencephalon vesicle in mice with exencephalia had developed, the thickness of ventricular walls varied. The cephalic plate of the hindbrain was not fused but was encased with one layer of amnia and mesenchyma (Figure 1D). Also, there were significant differences in the crown-rump length and body weight between the NTD mouse model embryos and the control embryos (4.35±0.37 vs. 6.39±0.53, P<0.01; 9.59±3.79 vs. 22.39±3.33, P<0.01) (Table 1).
Table 1.
Effects of MTX on mouse embryos.
| Group | Litters n | Embryonic number n | Crown-rump length mm | Body weight mg |
|---|---|---|---|---|
| Control | 4 | 33 | 6.39±0.53 | 22.39±3.33 |
| MTX (4.5 mg/kg) | 4 | 31 | 4.35±0.37** | 9.59±3.79** |
P<0.01 compared to the control group.
Expression levels of the INPP5E gene in neural tissues from control embryos
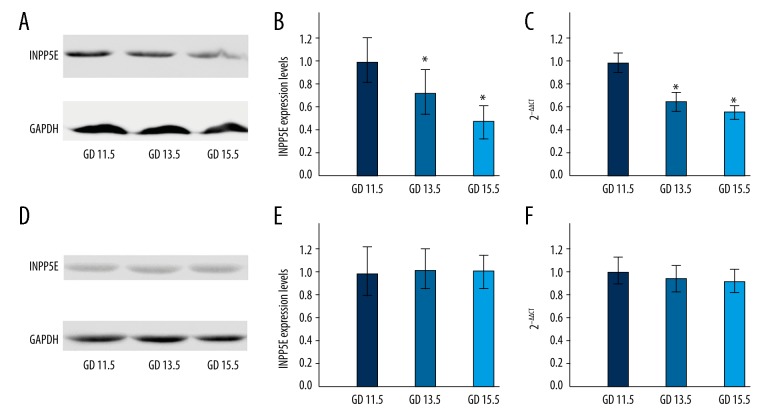
Western blot and real-time (RT) fluorescence quantitative polymerase chain reaction (PCR) were used to investigate the expression levels of the INPP5E gene in embryonic neural tissues at GD 11.5, GD 13.5 and GD 15.5 (Figure 2A, 2B), and quantitative analysis of the expression levels of genes was performed (Figure 2C). The results showed that there was a difference in the expression levels of the INPP5E gene at different time points during embryonic neurodevelopment. On GD 11.5 of neurodevelopment, the transcription of the INPP5E gene and expression levels were greater than that on GD 13.5, which was greater than that on GD 15.5. As the embryonic neural tissues developed and matured, the INPP5E gene transcription and translation levels showed a decreasing trend.
Figure 2.
INPP5E gene expression levels at different time points in the control mouse embryos and the neural tube defect (NTD) mouse model embryonic neural tissues at gestational days (GD) 11.5, 13.5, and15.5 days. (A, B) The expression levels of INPP5E protein in control embryos. (C) The transcription levels of the INPP5E gene in control embryos. (D, E) The expression levels of INPP5E protein in neural tube defect (NTD) mouse model embryos. (F) The transcription levels of the INPP5E gene in NTD mouse model embryos.
Expression levels of the INPP5E protein and INPP5E gene in neural tissues from the NTD mouse model embryos
Western blot was used to detect the expression level of INPP5E protein in neural tissues from GD 11.5, GD 13.5 and GD 15.5 (Figure 2D, 2E). RT-PCR was used for the quantitative analysis of the expression level of the INPP5E gene in neural tissues from GD 11.5, GD 13.5 and GD 15.5 (Figure 2F). The results showed no signifcant difference between the expression levels and transcription levels of the INPP5E gene in NTD mouse model embryo neural tissues from different time points, compared with control tissues. As development progressed, there was no significant trend by which the transcription and translation levels of the INPP5E gene changed.
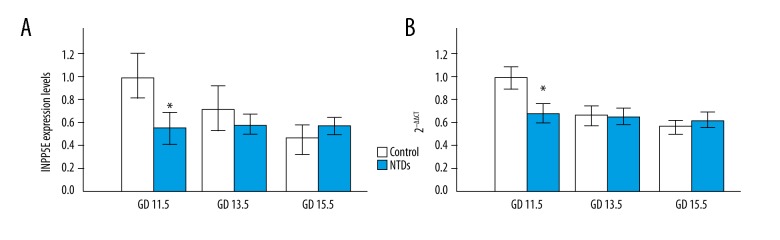
Comparison of expression levels of the INPP5E gene between neural tissues of the NTD mouse model embryos and the control mice
SPSS software was used for the systematic analysis of the expression levels of the INPP5E gene in the NTD mouse model embryos and the control mice. The mechanism of the INPP5E gene in embryonic neurodevelopment was explored preliminarily. The results showed that the expression levels of the INPP5E gene were significantly lower in the NTD mouse model embryos on GD 11.5, when the neural tube would have just closed; on GD 13.5 and GD 15.5, there was no significant difference in the expression levels of the INPP5E gene between the control and the NTD mouse model embryo (Figure 3).
Figure 3.
Comparison of the expression levels of the INPP5E gene in the neural tissues of the control mouse embryos and the neural tube defect (NTD) mouse model embryos. (A) Comparison of INPP5E protein expression in the neural tissues of the control mouse embryos and the neural tube defect (NTD) mouse model embryos. (B) Comparison of the INPP5E gene transcription in the neural tissues of the control mouse embryos and the neural tube defect (NTD) mouse model embryos.
Discussion
The INPP5E gene regulates the activity of the phosphoinositide signaling pathway and is involved in embryonic neurodevelopment. In recent years, studies have also shown that the control of the expression of the INPP5E gene is critical to the stability of primary cilia, which affects the controlled closure of the neural tube [33–35].
Based on the control mouse model with normal neurodevelopment, the expression levels of the INPP5E gene would have been expected to be different at different time points. However, the expression levels of the INPP5E gene decreased as the embryo developed so that on (gestational day) GD 11.5 expression levels of the INPP5E gene were greater than on GD 13.5, which in turn was greater than that on GD 15.5. These results suggest that the INPP5E gene is involved in the development of the nervous system, particularly during the earlier stages. Embryos that were developed as early as GD 15.5, at the critical point when the neural tube closes, were obtained and studied. However, the role of the INPP5E gene during embryonic neurodevelopment prior to GD 11.5 was more difficult to examine but should be further studied. The results of this study also indicate that a higher level of expression of the INPP5E gene was required to regulate the closure of the neural tube at the early stage of neurodevelopment. However, to explain the gradual decrease of expression levels of the INPP5E gene later in development, further investigations still need to be done.
Expression levels of the INPP5E gene in the NTD mouse model neural tissue were also measured at different developmental time points, but no significant difference was found between them. In the NTD mouse model, the inherent dynamic change in the expression levels of the INPP5E gene disappeared, which is suggested to be associated with NTD embryonic neurodevelopment. Specifically, on GD 11.5, the expression levels of the INPP5E gene were not significantly greater than that at other time points, indicating this was a potential factor causing the failure of neural tube closure. But whether or not this finding was due to the effect of the INPP5E gene on the formation of primary cilia still needs to be explored.
The present study demonstrated a correlation between the INPP5E gene mutation and neural tube defects, which were the main manifestations of ciliopathies and are known to be associated with serious birth defects characterized by partial or complete deficiency of neural tube functions, thereby further affecting the course of neurodevelopment. With regard to the mechanism of the INPP5E protein, the currently accepted theory is that mutation of the INPP5E gene leads to a reduced production of PI (3, 4, 5) P3 and/or PI (4, 5) P2, which then, by altering the PI3K signaling pathway, could affect cell proliferation, survival, and apoptosis, as well as the stability of the primary cilia, thereby leading to hindbrain malformation and ciliopathies [36,40,41].
This study found that the expression levels of the INPP5E gene in the NTD mouse model embryos were significantly lower than that of control embryos on GD 11.5, the precise time when the neural tube closes. But the levels were not significantly different between control embryos and the NTD mouse model embryos on GD 13.5 and GD 15.5. This result indicated that the decreased transcription and expression levels of the INPP5E gene might lead to a failure of neural tube closure in mice, causing NTDs.
The effect of the INPP5E gene on embryonic neurodevelopment was further investigated by interfering with the intracellular expression level of the INPP5E gene, then detecting the cellular amount of PI (3,4,5) P3 and/or PI (4,5) P2, as well as looking at the formation of the primary cilia. However, at the molecular level, how the levels of INPP5E gene expression affect the phosphoinositide signaling pathway, affects the formation of the primary cilia, and leads to failure of neural tube closure still needs to be studied further. Studies on the expression pattern of the INPP5E gene might offer a more detailed understanding of the molecular mechanism of embryonic neurodevelopment, and provide insight into potential future approaches leading to treatment for INPP5E gene-associated neurological developmental defects.
Conclusions
Normal expression of the INPP5E gene regulates the closure of the neural tube during embryonic development in mice. The expression levels of the INPP5E gene in mice with abnormal neurodevelopment, the NTD mouse model embryos, were significantly lower compared with normal control mice, during the period of neural tube closure, suggesting that the decreased expression of this gene was closely associated with neural tube deformities. Future studies and an increased understanding the mechanisms by which the INPP5E gene regulates embryonic neurodevelopment would be beneficial for the advancement of therapeutic approaches to treat diseases associated with neurodevelopmental defects.
Acknowledgements
The authors gratefully acknowledge all the participants in the study, and sincerely thank Anqi Chen for writing assistance.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (No.81571443, 81600984), the Ministry of Science and Technology of China, National “973” Project on Population and Health (Project 2013CB945404), Beijing Natural Science Foundation (No.7172038, 7174285)
Conflict of interest
None.
References
- 1.Humphray SJ, Oliver K, Hunt AR, et al. DNA sequence and analysis of human chromosome 9. Nature. 2004;6990:369–74. doi: 10.1038/nature02465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horan KA, Watanabe K, Kong AM, et al. Regulation of FcgammaR-stimulated phagocytosis by the 72-kDa inositol polyphosphate 5-phosphatase: SHIP1, but not the 72-kDa 5-phosphatase, regulates complement receptor 3 mediated phagocytosis by differential recruitment of these 5-phosphatases to the phagocytic cup. Blood. 2007;13:4480–91. doi: 10.1182/blood-2007-02-073874. [DOI] [PubMed] [Google Scholar]
- 3.Kong AM, Speed CJ, O’Malley CJ, et al. Cloning and characterization of a 72-kDa inositol-polyphosphate 5-phosphatase localized to the Golgi network. J Biol Chem. 2000;31:24052–64. doi: 10.1074/jbc.M000874200. [DOI] [PubMed] [Google Scholar]
- 4.Kisseleva MV, Wilson MP, Majerus PW. The isolation and characterization of a cDNA encoding phospholipid-specific inositol polyphosphate 5-phosphatase. J Biol Chem. 2000;26:20110–16. doi: 10.1074/jbc.M910119199. [DOI] [PubMed] [Google Scholar]
- 5.Thomas S, Wright KJ, Le Corre S, et al. A homozygous PDE6D mutation in Joubert syndrome impairs targeting of farnesylated INPP5E protein to the primary cilium. Hum Mutat. 2014;1:137–46. doi: 10.1002/humu.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bielas SL, Silhavy JL, Brancati F, et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet. 2009;9:1032–36. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguilar A. Ciliopathies: Inpp5e links lipids, cysts and cilia. Nat Rev Nephrol. 2016;9:508. doi: 10.1038/nrneph.2016.120. [DOI] [PubMed] [Google Scholar]
- 8.Travaglini L, Brancati F, Silhavy J, et al. Phenotypic spectrum and prevalence of INPP5E mutations in Joubert syndrome and related disorders. Eur J Hum Genet. 2013;10:1074–78. doi: 10.1038/ejhg.2012.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slaats GG, Isabella CR, Kroes HY, et al. MKS1 regulates ciliary INPP5E levels in Joubert syndrome. J Med Genet. 2016;1:62–72. doi: 10.1136/jmedgenet-2015-103250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phua SC, Chiba S, Suzuki M, et al. Dynamic remodeling of membrane composition drives cell cycle through primary cilia excision. Cell. 2017;1–2:264–279e215. doi: 10.1016/j.cell.2016.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plotnikova OV, Seo S, Cottle DL, et al. INPP5E interacts with AURKA, linking phosphoinositide signaling to primary cilium stability. J Cell Sci. 2015;2:364–72. doi: 10.1242/jcs.161323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacoby M, Cox JJ, Gayral S, et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet. 2009;9:1027–31. doi: 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- 13.Dyson JM, Conduit SE, Feeney SJ, et al. INPP5E regulates phosphoinositide-dependent cilia transition zone function. J Cell Biol. 2017;1:247–63. doi: 10.1083/jcb.201511055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ooms LM, Horan KA, Rahman P, et al. The role of the inositol polyphosphate 5-phosphatases in cellular function and human disease. Biochem J. 2009;1:29–49. doi: 10.1042/BJ20081673. [DOI] [PubMed] [Google Scholar]
- 15.Kleene SJ, Van Houten JL. Electrical signaling in motile and primary cilia. Bioscience. 2014;12:1092–102. doi: 10.1093/biosci/biu181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gazea M, Tasouri E, Tolve M, et al. Primary cilia are critical for Sonic hedgehog-mediated dopaminergic neurogenesis in the embryonic midbrain. Dev Biol. 2016;1:55–71. doi: 10.1016/j.ydbio.2015.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trulioff A, Ermakov A, Malashichev Y. Primary cilia as a possible link between left-right asymmetry and neurodevelopmental diseases. Genes (Basel) 2017;8(2) doi: 10.3390/genes8020048. pii: E48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fry AM, Leaper MJ, Bayliss R. The primary cilium: Guardian of organ development and homeostasis. Organogenesis. 2014;1:62–68. doi: 10.4161/org.28910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paridaen JT, Huttner WB, Wilsch-Brauninger M. Analysis of primary cilia in the developing mouse brain. Methods Cell Biol. 2015;127:93–129. doi: 10.1016/bs.mcb.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Shi WG, Ma XN, Chen KM. [The role of primary cilium in signal transduction and its mechanism]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2014;3:359–65. doi: 10.3785/j.issn.1008-9292.2014.04.006. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 21.Manli Z, Yanping L, Yali L. [Correlation between primary cilium and Wnt signaling pathway]. Yi Chuan. 2015;3:233–39. doi: 10.16288/j.yczz.14-252. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharyya S, Rainey MA, Arya P, et al. Endocytic recycling protein EHD1 regulates primary cilia morphogenesis and SHH signaling during neural tube development. Sci Rep. 2016;6:20727. doi: 10.1038/srep20727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiprilov EN, Awan A, Desprat R, et al. Human embryonic stem cells in culture possess primary cilia with hedgehog signaling machinery. J Cell Biol. 2008;5:897–904. doi: 10.1083/jcb.200706028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cortes CR, Metzis V, Wicking C. Unmasking the ciliopathies: Craniofacial defects and the primary cilium. Wiley interdisciplinary reviews. Dev Biol. 2015;6:637–53. doi: 10.1002/wdev.199. [DOI] [PubMed] [Google Scholar]
- 25.Veland IR, Awan A, Pedersen LB, et al. Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol. 2009;3:39–53. doi: 10.1159/000208212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willaredt MA, Tasouri E, Tucker KL. Primary cilia and forebrain development. Mech Dev. 2013;6–8:373–80. doi: 10.1016/j.mod.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Vogel TW, Carter CS, Abode-Iyamah K, et al. The role of primary cilia in the pathophysiology of neural tube defects. Neurosurg Focus. 2012;4:E2. doi: 10.3171/2012.6.FOCUS12222. [DOI] [PubMed] [Google Scholar]
- 28.Greene ND, Copp AJ. Development of the vertebrate central nervous system: formation of the neural tube. Prenat Diagn. 2009;4:303–11. doi: 10.1002/pd.2206. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Bao Y, Lu X, et al. Polymorphisms in MTHFD1 gene and susceptibility to neural tube defects: A case-control study in a Chinese Han population with relatively low folate levels. Med Sci Monit. 2015;21:2630–37. doi: 10.12659/MSM.895155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu J, Li X, Yang J, et al. Effects of simazine exposure on neuronal development-related factors in MN9D cells. Med Sci Monit. 2016;22:2831–38. doi: 10.12659/MSM.896460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao B, Zheng Z. Insulin growth factor 1 protects neural stem cells against apoptosis induced by hypoxia through Akt/Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase (Akt/MAPK/ERK) pathway in hypoxia-ishchemic encephalopathy. Med Sci Monit. 2018;24:1872–79. doi: 10.12659/MSM.901055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Liang W, Lai Y, Zhu M, et al. Combretastatin A4 regulates proliferation, migration, invasion, and apoptosis of thyroid cancer cells via PI3K/Akt signaling pathway. Med Sci Monit. 2016;22:4911–17. doi: 10.12659/MSM.898545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eramo MJ, Mitchell CA. Regulation of PtdIns(3,4,5)P3/Akt signalling by inositol polyphosphate 5-phosphatases. Biochem Soc Trans. 2016;1:240–52. doi: 10.1042/BST20150214. [DOI] [PubMed] [Google Scholar]
- 34.Hakim S, Dyson JM, Feeney SJ, et al. Inpp5e suppresses polycystic kidney disease via inhibition of PI3K/Akt-dependent mTORC1 signaling. Hum Mol Genet. 2016;11:2295–313. doi: 10.1093/hmg/ddw097. [DOI] [PubMed] [Google Scholar]
- 35.Hakim S, Bertucci MC, Conduit SE, et al. Inositol polyphosphate phosphatases in human disease. Curr Top Microbiol Immunol. 2012;362:247–314. doi: 10.1007/978-94-007-5025-8_12. [DOI] [PubMed] [Google Scholar]
- 36.Luo N, Lu J, Sun Y. Evidence of a role of inositol polyphosphate 5-phosphatase INPP5E in cilia formation in zebrafish. Vision Res. 2012;75:98–107. doi: 10.1016/j.visres.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franco I, Gulluni F, Campa CC, et al. PI3K class II alpha controls spatially restricted endosomal PtdIns3P and Rab11 activation to promote primary cilium function. Dev Cell. 2014;6:647–58. doi: 10.1016/j.devcel.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Wang J, Guan T, et al. Role of methotrexate exposure in apoptosis and proliferation during early neurulation. J Appl Toxicol. 2014;8:862–69. doi: 10.1002/jat.2901. [DOI] [PubMed] [Google Scholar]
- 39.Dasiman R, Rahman NS, Othman S, et al. Cytoskeletal alterations in different developmental stages of in vivo cryopreserved preimplantation murine embryos. Med Sci Monit Basic Res. 2013;19:258–66. doi: 10.12659/MSMBR.884019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greene ND, Leung KY, Copp AJ. Inositol, neural tube closure and the prevention of neural tube defects. Birth Defects Res. 2017;2:68–80. doi: 10.1002/bdra.23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Travaglini L, Brancati F, Silhavy, et al. Phenotypic spectrum and prevalence of INPP5E mutations in Joubert syndrome and related disorders. Eur J Hum Genet. 2013;10:1074–78. doi: 10.1038/ejhg.2012.305. [DOI] [PMC free article] [PubMed] [Google Scholar]