Abstract
Background
Pulmonary nodules (PN) are frequently detected incidentally during coronary computed tomography angiography (CTA). We evaluated whether the 2017 Fleischner Society guidelines may result in a decrease of follow-up testing of incidental PN as compared to prior guidelines in patients undergoing coronary CTA.
Methods
We conducted a retrospective study of a registry of emergency department patients who underwent coronary CTA for acute coronary syndrome assessment between 2012 and 2017. Based on guidelines, patients <35 years, history of cancer, or prior exams showing stability of PN were excluded. Patients >60 years, history of smoking, irregular/spiculated PN morphology, or PN size >20mm were classified as high-risk for lung cancer. Radiological findings pertaining to PN were identified (PN size, morphology, quantity) through review of radiology reports. PN follow-up recommendations were established using 2017 Fleischner Society Guidelines and compared with prior guidelines for solid (2005) and subsolid (2013) PN. Data were analyzed with Student’s t-test.
Results
The registry included 2,066 patients (female 45.1%, 52.9±11.0 years), of which 578 (28.0%) reported PN. 438 of those (21.2%) were eligible for guideline-based follow-up evaluation. 205 (4 6.8%) were classified as high-risk for lung cancer. 2017 guidelines reduced the number of individuals requiring follow-up by 64.5%, from 264 (12.8%) to 94 patients (4.5%) when compared to prior guidelines (p<0.001). The minimum number of follow-up chest CTs decreased by 55.8% from 430 to 190 (p<0.001).
Conclusion
Application of the 2017 Fleischner Society Guidelines resulted in a significant decrease of follow-up testing for incidental PN in patients undergoing coronary CTA for suspected acute coronary syndrome.
Keywords: Pulmonary nodule, lung nodule, coronary computed tomography angiography, Fleischner Society Guidelines, Management of Incidental lung nodules, incidental findings, follow-up CT, acute coronary syndrome
Introduction
Coronary computed tomography angiography (CTA) has become an alternative to functional testing for patients presenting to the emergency department with acute chest pain and low to intermediate risk for acute coronary syndrome (ACS)1–3. Coronary CTA datasets not only provide visualization of cardiac anatomy, but also non-cardiac anatomy including the lower parts of the lungs below the level of carina, mediastinum, bones, and the upper parts of the abdomen4. Thus, extra-cardiac incidental findings are common5–11, the majority being pulmonary nodules (PN) with a prevalence of 16-23%12, 13. Those extra-cardiac findings result in a substantial number of additional downstream tests, especially follow-up chest CTs.
Recommendations for follow-up of solid PN were widely standardized by the 2005 Fleischner Society Guidelines for the management of incidental lung nodules and later for subsolid PN by additional 2013 guidelines14, 15. Guideline-based PN follow-up substantially increases costs in patients with CTA-based, anatomic testing of acute and chronic chest pain, but has shown to result in only a minimal reduction in mortality from lung cancer and is only cost-effective in smokers, but not in non-smokers5, 8, 12, 16.
In 2017, the Fleischner Society updated their guidelines recommending follow-up testing in patients with an estimated risk for lung cancer of 1% or greater17. Calculation of lung cancer risk was determined by PN characteristics such as size, location, and morphology, but also by clinical risk factors such as age, smoking history, and exposure to toxic substances. The highest impact on the number of patients with follow-up recommendations might be the increased threshold for solid PN follow-ups from 5 mm (2005 guidelines) to 6mm in the 2017 guidelines, below which a follow-up is not recommended. Also, while all patients with high risk for lung cancer received recommendations for follow-up chest CT per 2005 guidelines, patients with high risk for lung cancer, but solid PN <6 mm would not get follow-up per 2017 guidelines.
The aim of our study was to compare the number of patients with need for follow-up testing per revised 2017 Fleischner Society Guidelines for management of incidental PN versus the relevant prior applicable guidelines (2005 guidelines for solid PN, and 2013 guidelines for subsolid PN) in patients with acute chest pain who underwent coronary CTA for ACS assessment.
Materials and Methods
Subjects
This retrospective, IRB-approved and HIPPA-compliant study was conducted using a prospectively acquired registry which included 2,066 emergency department patients (52.9 ± 11.0 years; 45.1% female), who underwent coronary CTA for ACS assessment in a tertiary, academic hospital from 2012 to 2017. The registry’s inclusion criteria for ACS assessment by coronary CTA were summarized previously18, in a manuscript that dealt with the efficiency and safety of implementation of coronary CTA in the emergency department, but not related to incidental findings (and reporting only 1,022 of 2,066 patients included in the present study)18.
Coronary CT angiography
All coronary CTA scans were performed on second- and third-generation dual-source CT scanners (Siemens Somatom Flash and Siemens Somatom Force, Siemens Healthineers, Forchheim, Germany) and described in above mentioned manuscript18. Based on the scout, the scan length was set from the carina to the diaphragm covering the heart, but not the entire lungs or was tailored based on the Calcium scan images. To visualize the lungs, an image series with a maximum field of view covering the lungs over the entire scan length were reconstructed with 1.5 mm slice thickness in addition to image series with a field of view tailored to the heart. Image quality (but not incidental findings) between second- and third-generation dual-source CT scanners was reported in a subset of 246 patients19.
Pulmonary nodule assessment
PN evaluation was mandatory as part of the structured coronary CTA report and confirmed by two board-certified radiologists specialized on cardiac imaging. PN were measured in long and short axis and an averaged diameter rounded to the nearest millimeter was noted in the report (Figure 1). All radiology reports were extracted from our Research Electronic Data Capture (RedCap, Harvard Catalyst, Boston, Massachusetts) and further evaluated using Microsoft Excel (Microsoft Corporation, Redmond, Washington). Reports were screened for PN evaluation by a cardiac imaging fellow (J.S.). In cases where the radiology report did not clearly describe PN characteristics (type, morphology, size, quantity), images were reviewed by a board-certified radiologist using dedicated picture archiving and communication system (PACS) software (AGFA Impax 6.6.1.3004, AGFA, Mortsel, Belgium).
Fig 1.
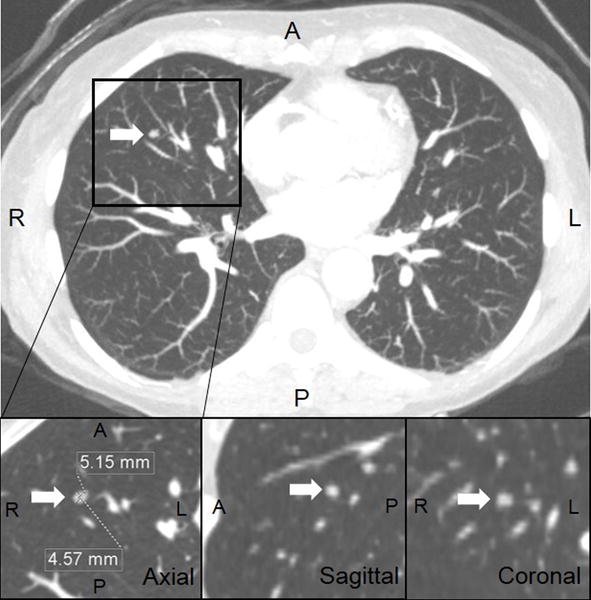
62-year-old female presented with acute chest pain to the emergency department. Coronary CT angiography ruled out coronary artery disease. Reconstructed maximum field-of-view axial 3-mm-thick maximum intensity projection (MIP) images revealed a single 5-mm solid pulmonary nodule in the middle lobe (top image). Average of long and short axes were measured in 1.5 mm multiplanar reformations (MPR) and rounded to the nearest millimeter (bottom images). She was considered low-risk for lung cancer and therefore needed no follow-up chest CT per 2017 guidelines. Prior guidelines from 2005 would have recommended follow-up chest CT at 12 months.
Management of incidental PN followed the 2017 published guidelines of the Fleischner Society17. Patients under 35 years or with a history of cancer were excluded, as these patients would benefit from a case-by-case based follow-up recommendation17. Patients with existing prior chest CT confirming stability (and thus no need for additional follow-up) or findings of granuloma or hamartoma were not included for further evaluation. Patients were divided into subgroups based on the amount of PN (single vs. multiple), type (solid vs. subsolid (subdivided into pure groundglass or partly PN), size, and risk for lung cancer. As the registry only included classification of smokers into former and current smokers, further risk stratification of former smokers depending on the time since quitting could not be evaluated. Thus, all patients with a history of smoking were classified as high risk for lung cancer. Never-smokers were classified as low risk for lung cancer. Further, patients over 60 years, irregular/spiculated nodule, or nodule size >20mm were classified as high risk for lung cancer17. To allow a comparison of prior guidelines from 2005 and 2013 with the current guidelines, all solid PN were subdivided based on their size into groups of ≤ 4 mm, 5 mm, 6-8 mm, and > 8 mm, single subsolid PN into < 5 mm, 5 mm, and ≥6 mm, and multiple subsolid PN into < 6 mm and ≥ 6 mm. Follow-up recommendations were compared between 2017 Fleischner Society Guidelines and previous versions which were from 2005 for solid PN and from 2013 for subsolid PN (Table 2)14, 15, 17 which were bases for our previous or current hospital’s internal guidelines. Cases that did not require PN follow-up, but with a comment for optional follow-up (in 12 month) were counted as no need for follow-up. Minimal number of recommended follow-up chest CT per current (2017) and prior (2005, 2013) guidelines were calculated.
Table 2.
Fleischner Society Guidelines-applicable pulmonary nodule management classified per pulmonary nodule characteristics (type, number, and size) and lung cancer risk.
| Characteristics | No. of patients with PN n (%) |
Recommendation for PN follow-up testing per guidelines (minimal no. of chest CTs) | No. of patients with recommended follow-up CT | |||
|---|---|---|---|---|---|---|
| 2005/2013 | 2017 | 2005/2013 n |
2017 n |
Difference % |
||
| All | 438 (100) | 264 | 94 | −64.5* | ||
| Low risk | 233(53.2) | 63 | 39 | −38.1* | ||
| High risk | 205 (46.8) | 201 | 55 | −72.8* | ||
|
| ||||||
| Solid PN (Single and multiple) | 398 (90.9) | 231 | 64 | −72.3* | ||
| Low risk | 219 (50.0) | 52 | 28 | −46.2* | ||
| High risk | 179 (40.9) | 179 | 36 | −80.0* | ||
| ≤4 mm | 288(65.8) | 121 | – | |||
| Low risk | 167 (38.1) | No | No | – | – | |
| High risk | 121 (27.6) | Yes (1) | No | 121 | – | |
| 5 mm | 46 (10.5) | 46 | – | |||
| Low risk | 24 (5.5) | Yes (1) | No | 24 | – | |
| High risk | 22 (5.0) | Yes (1) | No | 22 | – | |
| 6-8 mm | 54 (12.3) | 54 | 54 | |||
| Low risk | 24 (5.5) | Yes (1-2) | Yes (1) | 24 | 24 | |
| High risk | 30 (6.8) | Yes (2-3) | Yes (2) | 30 | 30 | |
| >8 mm | 10 (2.3) | 10 | 10 | |||
| Low risk | 4 (0.7) | Yes (3**) | Yes (1-2**) | 4 | 4 | |
| High risk | 6 (1.4) | Yes (3**) | Yes (1-2**) | 6 | 6 | |
| Subsolid PN | 40 (9.1) | 34 | 30 | −11.8* | ||
| Low risk | 14 (3.2) | 11 | 11 | 0.0 | ||
| High risk | 26 (5.9) | 23 | 19 | −17.4* | ||
| Single subsolid PN | 24 (5.5) | 18 | 14 | |||
| Pure ground glass PN | 21 (4.8) | 15 | 12 | |||
| <5 mm | 6 (1.4) | No | No | – | – | |
| 5 mm | 3 (0.7) | Yes (4) | No | 3 | – | |
| ≥6 mm | 12 (2.7) | Yes (4) | Yes (6) | 12 | 12 | |
| Partly solid PN | 3 (0.7) | 3 | 2 | |||
| <5 mm | 0 | Yes (4) | No | 0 | – | |
| 5 mm | 1 (0.2) | Yes (4**) | No | 1 | – | |
| ≥6 mm | 2 (0.5) | Yes (4**) | Yes (6) | 2 | 2 | |
| Multiple subsolid PN | 16 (3.7) | 16 | 16 | |||
| <6 mm | 10 (2.3) | Yes (2**) | Yes (3) | 10 | 10 | |
| ≥6 mm | 6 (1.4) | Yes (1-4**) | Yes (1**) | 6 | 6 | |
Values are n (%),
CT, computed tomography; PN, pulmonary nodule
p<0.05
Further examinations (PET, biopsy) might be necessary in addition to chest CT based on the 2005, 2013, and 2017 Fleischner Society Guidelines
Additionally, number of recommended PN follow-ups were categorized into coronary artery disease (CAD) severity (no CAD, non-obstructive CAD, obstructive CAD) recorded within the registry using Coronary Artery Disease - Reporting and Data System (CAD-RADS,)20. No CAD included patients with a CAD-RADS 0 (no plaque nor stenosis) classification. Non-obstructive CAD included CAD-RADS 1 (1-24%/minimal stenosis), 2 (25-49%/mild stenosis), and 3 (50-69%/moderate stenosis). Obstructive CAD included CAD-RADS 4A (70-99%/severe stenosis), 4B (>50% in left main coronary artery or obstructive (70-99%) 3-vessel disease), and 5 (100%/occlusion). Scans with at least one non-evaluable coronary segment, and no other obstructive CAD were classified as CAD-RADS N (non-diagnostic).
Additional extra-cardiac findings
Further incidental extra-cardiac findings (in addition to PN) were noted. In case of reported, suspected malignancy or pulmonary inflammation, patient’s electronic health record was screened to confirm diagnoses.
Statistical analysis
Continuous variables were shown as mean ± standard deviation. Groups were compared with Student’s t-test for independent samples. In the case of non-normally distributed variables, median (P25-P75) were reported, and Mann-Whitney U test was used for comparison. Categorical variables were expressed as frequencies and percentages; differences were assed using the chi-square test. Recommended follow-ups were patient-based and displayed as total numbers and percentage. P-values were two-sided, and a P-value of less than 0.05 was considered statistically significant. Data were analyzed with dedicated software (IBM SPSS Statistics v.19, IBM, Armonk, New York).
Results
PN were reported in 578 (28.0%) of the 2,066 patients included in the registry. Per guidelines, individual follow-up strategies were recommended in 22 patients with a history of cancer and in 18 patients younger than 35 years and, thus, excluded from guideline-based PN follow-up evaluation. Further, patients with previous CT examinations confirming stability (n=70) and patients with a reported calcified granuloma or hamartoma (n=29) were excluded from guideline-based follow-up evaluation (Figure 2). In total, 438 (21.2%) patients (54.5 ± 11.0 years; 43.4% female) had incidental PN with need for guideline-based PN follow-up evaluation (Table 1).
Fig 2.
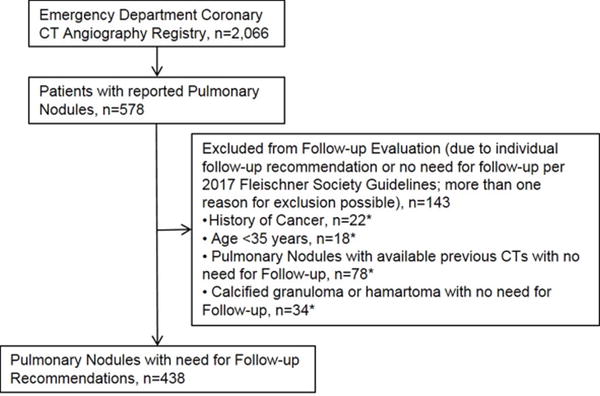
Flow chart of patients with incidentally detected pulmonary nodules within the registry of acute chest pain patients who underwent coronary CT angiography.
Table 1.
Baseline characteristics
| Characteristics | Patients with need for pulmonary nodule follow-up evaluation n=438 |
|---|---|
| Age (years) | 54.5 ± 11.0 |
| Female | 190 (43.4) |
| Male | 248 (56.6) |
| Race | |
| Caucasian | 298 (68.0) |
| African-American | 46 (10.5) |
| Asian | 9 (2.1) |
| Other/Unknown | 85 (19.4) |
| Hispanic Ethnicity | 44 (10.0) |
| BMI (kg/m2) | 29.7 ± 6.3 |
| Cardiovascular risk factors | |
| Past or current smoker | 123 (28.1) |
| Current smoker | 37 (8.4) |
| Diabetes Mellitus | 41 (9.4) |
| Dyslipidemia | 126 (28.8) |
| Hypertension | 168 (38.4) |
| Family history of CAD | 81 (18.5) |
| History of cancer | – |
Values are mean ± standard deviation or n (%)
BMI, body mass index; CAD, coronary artery disease
Pulmonary nodule assessment
Of the 438 patients with need for guidelines-applicable PN evaluation, 94 (21.5%) would receive recommendations for follow-up of incidental PN per 2017 guidelines compared to 264 (60.3%) per prior (2005 and 2013) guidelines, which resulted in a significant reduction of 64.5% (p<0.001) (Figure 3, Table 2). Thus, only 4.5% of all patients undergoing emergent coronary CTA would receive PN follow-up testing per 2017 guidelines versus 12.8% per prior guidelines. The minimal number of follow-up chest CTs would decrease from 430 per 2005/2013 guidelines to 190 per 2017 guidelines; a reduction of 55.8% (p<0.001).
Fig 3.
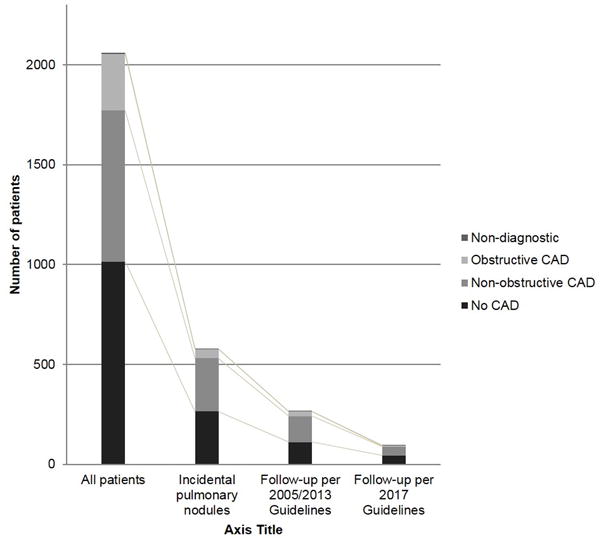
Patients with recommended follow-ups of incidental pulmonary nodules based on prior (2005 and 2013) and currently revised (2017) Fleischner Society Guidelines compared to all patients, subdivided into coronary artery disease severity.
High risk criteria for lung cancer were noted in 205 (46.8%) of the 438 patients: age over 60 years (n=108), smoking history (n=123), PN in the upper lobe (n=100), PN size > 20mm (n=1), and irregular/spiculated PN morphology (n=1). 233 (53.2%) were classified low risk for lung cancer. Per prior guidelines (2005 and 2013), almost all patients classified as high risk for lung cancer (201/205; 98.0%) would have received recommendation for follow-up testing compared to only 55 (26.8%) patients per 2017 guidelines; a reduction of 72.8% (Figure 4, Table 2).
Fig 4.
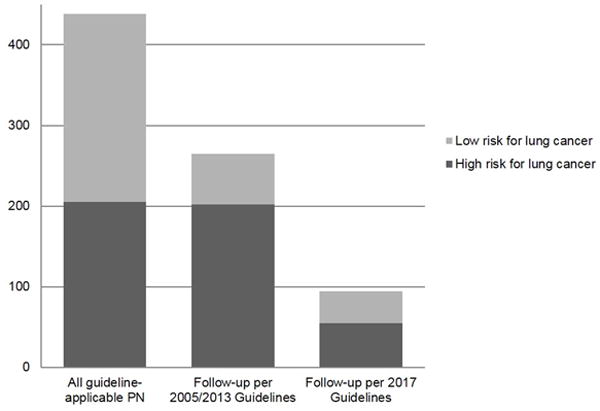
Patients with incidental pulmonary nodules and need for follow-up testing per prior (2005 and 2013) and revised (2017) Fleischner Society Guidelines subdivided into low and high risk for lung cancer. High risk for lung cancer included history of smoking, age >60 years, upper lobe location of PN, irregular morphology PN, and PN size >20 mm.
Evaluation of PN characteristics revealed 398 (90.9%) patients with solid PN, of who the majority (n=334) had solid PN smaller 6 mm which did not require follow-up testing per 2017 guidelines. Number of patients with PN, guideline-based recommendation for PN follow-up, and changes between the old and new guidelines are listed in Table 2 with breakdown into PN characteristics.
Coronary CT angiography
Coronary CTA ruled out obstructive CAD in most patients (n=1771; 85.7%) in that registry; the majority with no need for additional testing. 241 of those (13.6%) patients would receive recommendation for PN follow-up testing per 2005/2013 guidelines. Application of the 2017 guidelines, only 88 (5.0%) patients without obstructive CAD would receive recommendation for PN follow-ups, a reduction of 63.5% (p<0.001) (Figure 3, Table 3). Similar, the minimum number of follow-up chest CTs in patients without obstructive CAD would decrease by 55.9% from 392 to 173 follow-up chest CT scans (p<0.001). In all CAD categories (no CAD, non-obstructive CAD, obstructive CAD), the number of patients with need for PN follow-up testing and the minimal number of follow-up chest CTs would be significantly lower applying the revised 2017 guidelines instead prior (2005, 2013) guidelines (Table 3).
Table 3.
Detection of pulmonary nodules which are applicable to the Fleischner Society Guidelines, subdivided into coronary artery disease severity by coronary CTA and lung cancer risk.
| Findings | Registry | No. of patients with need for PN follow-up (%**) |
No. of patients with need for PN follow-up | Minimal total no. of follow-up chest CT*** | ||||
|---|---|---|---|---|---|---|---|---|
| 2005/2013 n (%**) |
2017 n (%**) |
Difference % |
2005/2013 n |
2017 n |
Difference % |
|||
| All | 2,066 | 438 (21.2) | 264 (12.8) | 94 (4.5) | −64.4* | 430 | 190 | −55.8* |
| Low risk | 233 | 63 | 39 | −38.1* | 108 | 64 | −40.1* | |
| High risk | 205 | 201 | 55 | −72.8* | 322 | 126 | −60.9* | |
| No CAD | 1014 | 202 (19.9) | 111 (10.9) | 44 (4.3) | −60.3* | 177 | 74 | −58.2* |
| Low risk | 130 | 39 | 24 | −38.5* | 57 | 32 | −43.9* | |
| High risk | 72 | 72 | 20 | −72.2* | 120 | 42 | −65.0* | |
| Non-obstructive CAD | 757 | 203 (26.8) | 130 (17.2) | 44 (5.8) | −66.2* | 215 | 99 | −54.0* |
| Low risk | 92 | 22 | 13 | −40.1* | 44 | 25 | −43.2* | |
| High risk | 111 | 108 | 31 | −71.3* | 171 | 74 | −56.7* | |
| Obstructive CAD | 281 | 32 (11.4) | 23 (8.2) | 5 (1.8) | −78.3* | 36 | 16 | −55.6* |
| Low risk | 10 | 2 | 1 | −50.0 | 5 | 6 | 20.0 | |
| High risk | 22 | 21 | 4 | −81.0* | 31 | 10 | −67.7* | |
| Non-diagnostic | 9 | 1 (11.1) | 1 (11.1) | 1 (11.1) | 0.0 | 2 | 1 | −50.0 |
| Low risk | 1 | 1 | 1 | 0.0 | 2 | 1 | −50.0 | |
| High risk | 0 | 0 | 0 | – | 0 | 0 | – | |
Values are n (%), differences are in %
CAD, coronary artery disease; PN, pulmonary nodule
No CAD, no coronary artery stenosis nor plaque; Non-obstructive CAD, 1-69% stenosis or left main coronary artery 1-49% stenosis; Obstructive CAD, ≥ 70% stenosis or left main coronary artery ≥ 50% stenosis;
Low risk for lung cancer: age ≤ 60 years, no current smoker, PN not within upper lobe, no irregular or spiculated morphology of PN
High risk for lung cancer: age > 60 years, current smoker, PN in upper lobe location; irregular or spiculated morphology of PN
p<0.05
In percentage to total number of patients per CAD category
Further examinations (PET, biopsy) might be necessary in addition to chest CT based on the 2005, 2013, and 2017 Fleischner Society Guidelines
Additional extra-cardiac findings
Incidentally detected malignancies were reported and clinically confirmed in 2 patients (0.1%) (Esophageal cancer, n=1; Cholangiocarcinoma, n=1)Further extra-cardiac findings are listed in Table 4.
Table 4.
Extra-cardiac findings in coronary CT angiography.
| Characteristics | Emergency Department Coronary CT Angiography Registry n=2,066 |
|---|---|
| Pulmonary findings | |
| Pulmonary nodules | 578 (28.0) |
| Pulmonary embolism | 6 (2.9) |
| Pneumonia | 13 (6.3) |
| Pneumonitis | 2 (0.1) |
| Pleural effusion | 30 (1.5) |
| Aortic dissection | 4 (0.2) |
| GERD | 29 (1.4) |
| Tumor | 3 (0.1) |
| Newly detected malignancies | 2 (0.1) |
| Esophageal cancer | 1 (0.0) |
| Cholangiocarcinoma | 1 (0.0) |
| Non-specific paraoesophageal mass | 1 (0.0) |
Values are n (%)
GERD, gastroesophageal reflux disease
Discussion
A rate of 28% incidental PN in coronary CTA is in accordance with prior studies6, 7, 9. Our results showed that the 2017 Fleischner Society Guidelines for the management of incidental PN could lower the number of follow-up recommendations by 64.5% compared to prior, 2005 and 2013, guidelines in coronary CTA patients. Thus, the revised guidelines would decrease recommendation for follow-up testing of incidental PN from 12.8 to 4.5% of all patients presenting to the emergency department with acute chest pain and low to intermediate risk for CAD whom underwent coronary CTA for ACS assessment.
Revised 2017 Fleischner Society Guidelines did not recommend follow-up testing of single or multiple solid PN <6mm in both nonsmokers and smokers, which represented the majority (76%) of our cohort17, 21–24. In comparison, 2005 guidelines for solid PN did not recommend follow-up only in low-lung-cancer-risk patients with solid PN ≤4 mm. Thus, 72.3% less follow-up testing of solid PN would be recommended per 2017 guidelines to 2005. Due to the relatively few patients with single subsolid PN <6 mm, the impact of a higher diameter threshold value of <6 mm (2017 guidelines) instead <5 mm (2013) for follow-up testing did only result in a reduction of 11.8% follow-up testing recommendations. There is no change in the follow-up recommendations in patients with multiple subsolid PN, independent of the PN size. Suspicious morphology, upper lobe location, or both, increase cancer risk to 1-5% and, thus, follow-up chest CT “may be considered”17. As coronary CTA only covers the lower lung zones, potential lung cancers in the apices might be missed. However, imaging of the entire lungs within emergent coronary CTA without clinical necessity is ethically problematic and not encouraged by the 2017 Fleischner Society Guidelines themselves17.
The number of patients actually completing the recommended follow-up care is substantially lower than the number of recommendations25, 26. Addition of the Fleischner Society Guidelines within the radiology report has shown to significantly increase the likelihood of undergoing recommended follow-up care25. In combination with the significantly lower number of follow-up recommendations per 2017 guidelines, we speculate that the likelihood of completing recommended follow-up care may increase. The substantial reduction of recommended PN follow-up testing in patients with ruled out CAD per CTA might also increase the acceptability of coronary CTA, which has been proven to be an excellent non-invasive alternative to rule out CAD/ACS compared to standard care1, 3, but has not been widely accepted27. Although extra-cardiac findings are common, clinically significant incidental findings are less prevalent. Nevertheless, 2 of our patients had incidentally detected extra-cardiac malignancies which would have been missed by functional testing for CAD assessment. To keep the number of missed extra-cardiac findings low, we propose that readings should be performed by imagers familiar with cardiac CT; this becomes more important as the technology diffuses, appropriate indications widen, and CTA volume increases28.
There are several limitations that require acknowledgment. First, our experience is based on a single-center registry of a tertiary urban academic hospital. Second, the retrospective design of our study did not allow evaluation of the actual follow-through and performance of recommended follow-up chest CTs29, 30. Our study focused on the expected amount of recommended follow-up testing comparing prior and revised Fleischner Society Guidelines. Further, not all risk factors for lung cancer could be determined based on the registry data (for example, date of smoking cessation, which might downgrade cancer risk). Third, coronary CTA does not cover the entire lungs which might underestimate nodule burden, and thus lung cancer risk in this cohort (this point is the basis of a work focusing on coronary CTA applicability of nodule follow-up guidelines, as opposed to whole-chest CTA).
In conclusion, management of incidental PN as per 2017 Fleischner Society Guidelines would reduce the number of recommended follow-up testing by 64.5% from 12.8 to 4.5% in patients undergoing coronary CTA compared to prior guidelines. This will lower cost for follow-up testing of incidental findings in coronary CTA, and may increase the acceptability of coronary CTA.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
- ACS
acute coronary syndrome
- CAD
coronary artery disease
- CAD-RADS
Coronary Artery Disease - Reporting and Data System
- CTA
computed tomography angiography
- PN
pulmonary nodule
Footnotes
Conflict of interest:
Following authors have conflicts of interest (none related to this work): Dr. Brian B. Ghoshhajra (minor–Medtronic, Inc., and Siemens Healthcare, Inc.—both for unrelated educational consulting for valve replacement imaging). All other authors have no conflict of interest.
References
- 1.Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of Anatomical versus Functional Testing for Coronary Artery Disease. New England Journal of Medicine. 2015;372:1291–1300. doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litt HI, Gatsonis C, Snyder B, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. The New England journal of medicine. 2012;366:1393–1403. doi: 10.1056/NEJMoa1201163. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT Angiography versus Standard Evaluation in Acute Chest Pain. New England Journal of Medicine. 2012;367:299–308. doi: 10.1056/NEJMoa1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Earls JP. The Pros and Cons of Searching for Extracardiac Findings at Cardiac CT: Studies Should Be Reconstructed in the Maximum Field of View and Adequately Reviewed to Detect Pathologic Findings. Radiology. 2011;261:342–346. doi: 10.1148/radiol.11111099. [DOI] [PubMed] [Google Scholar]
- 5.Machaalany J, Yam Y, Ruddy TD, et al. Potential clinical and economic consequences of noncardiac incidental findings on cardiac computed tomography. J Am Coll Cardiol. 2009;54:1533–1541. doi: 10.1016/j.jacc.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Mueller J, Jeudy J, Poston R, White CS. Cardiac CT angiography after coronary bypass surgery: prevalence of incidental findings. AJR. American journal of roentgenology. 2007;189:414–419. doi: 10.2214/AJR.06.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirsch J, Araoz PA, Steinberg FB, Fletcher JG, McCollough CH, Williamson EE. Prevalence and significance of incidental extracardiac findings at 64-multidetector coronary CTA. Journal of thoracic imaging. 2007;22:330–334. doi: 10.1097/RTI.0b013e31813434a9. [DOI] [PubMed] [Google Scholar]
- 8.Budoff MJ, Fischer H, Gopal A. Incidental findings with cardiac CT evaluation—Should we read beyond the heart? Catheterization and cardiovascular interventions. 2006;68:965–973. doi: 10.1002/ccd.20924. [DOI] [PubMed] [Google Scholar]
- 9.Koonce J, Schoepf JU, Nguyen SA, Northam MC, Ravenel JG. Extra-cardiac findings at cardiac CT: experience with 1,764 patients. European radiology. 2009;19:570–576. doi: 10.1007/s00330-008-1195-3. [DOI] [PubMed] [Google Scholar]
- 10.Johnson KM, Dennis JM, Dowe DA. Extracardiac Findings on Coronary CT Angiograms: Limited Versus Complete Image Review. American Journal of Roentgenology. 2010;195:143–148. doi: 10.2214/AJR.08.1050. [DOI] [PubMed] [Google Scholar]
- 11.Kim JW, Kang E-Y, Yong HS, et al. Incidental extracardiac findings at cardiac CT angiography: comparison of prevalence and clinical significance between precontrast low-dose whole thoracic scan and postcontrast retrospective ECG-gated cardiac scan. The international journal of cardiovascular imaging. 2009;25:75. doi: 10.1007/s10554-008-9417-y. [DOI] [PubMed] [Google Scholar]
- 12.Goehler A, McMahon PM, Lumish HS, et al. Cost-effectiveness of follow-up of pulmonary nodules incidentally detected on cardiac computed tomographic angiography in patients with suspected coronary artery disease. Circulation. 2014;130:668–675. doi: 10.1161/CIRCULATIONAHA.113.007306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehman SJ, Abbara S, Cury RC, et al. Significance of cardiac computed tomography incidental findings in acute chest pain. The American journal of medicine. 2009;122:543–549. doi: 10.1016/j.amjmed.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 14.MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society 1. Radiology. 2005;237:395–400. doi: 10.1148/radiol.2372041887. [DOI] [PubMed] [Google Scholar]
- 15.Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266:304–317. doi: 10.1148/radiol.12120628. [DOI] [PubMed] [Google Scholar]
- 16.Lee CI, Tsai EB, Sigal BM, Plevritis SK, Garber AM, Rubin GD. Incidental extracardiac findings at coronary CT: clinical and economic impact. AJR. American journal of roentgenology. 2010;194:1531–1538. doi: 10.2214/AJR.09.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;161659 doi: 10.1148/radiol.2017161659. [DOI] [PubMed] [Google Scholar]
- 18.Ghoshhajra BB, Takx RAP, Staziaki PV, et al. Clinical implementation of an emergency department coronary computed tomographic angiography protocol for triage of patients with suspected acute coronary syndrome. European radiology. 2017;27:2784–2793. doi: 10.1007/s00330-016-4562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyersohn NM, Szilveszter B, Staziaki PV, et al. Coronary CT angiography in the emergency department utilizing second and third generation dual source CT. Journal of cardiovascular computed tomography. 2017;11:249–257. doi: 10.1016/j.jcct.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cury RC, Abbara S, Achenbach S, et al. CAD-RADS(TM) Coronary Artery Disease - Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. Journal of cardiovascular computed tomography. 2016;10:269–281. doi: 10.1016/j.jcct.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Horeweg N, van der Aalst CM, Vliegenthart R, et al. Volumetric computed tomography screening for lung cancer: three rounds of the NELSON trial. European Respiratory Journal. 2013;42:1659–1667. doi: 10.1183/09031936.00197712. [DOI] [PubMed] [Google Scholar]
- 22.Samet JM, Avila-Tang E, Boffetta P, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clinical Cancer Research. 2009;15:5626–5645. doi: 10.1158/1078-0432.CCR-09-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. New England Journal of Medicine. 2013;369:910–919. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. The Lancet Oncology. 2014;15:1332–1341. doi: 10.1016/S1470-2045(14)70389-4. [DOI] [PubMed] [Google Scholar]
- 25.McDonald JS, Koo CW, White D, Hartman TE, Bender CE, Sykes A-MG. Addition of the Fleischner Society Guidelines to Chest CT Examination Interpretive Reports Improves Adherence to Recommended Follow-up Care for Incidental Pulmonary Nodules. Academic radiology. 2017;24:337–344. doi: 10.1016/j.acra.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey HB, Wu CC, Gilman MD, et al. Correlation of the Strength of Recommendations for Additional Imaging to Adherence Rate and Diagnostic Yield. Journal of the American College of Radiology : JACR. 2015;12:1016–1022. doi: 10.1016/j.jacr.2015.03.038. [DOI] [PubMed] [Google Scholar]
- 27.Levin DC, Parker L, Halpern EJ, Julsrud PR, Rao VM. The lack of growth in use of coronary CT angiography: is it being appropriately used. AJR. American journal of roentgenology. 2011;196:862–867. doi: 10.2214/AJR.10.5236. [DOI] [PubMed] [Google Scholar]
- 28.Kmietowicz Z. NICE guideline focuses on diagnosis of acute chest pain to improve outcomes. BMJ. 2010;340 doi: 10.1136/bmj.c1670. [DOI] [PubMed] [Google Scholar]
- 29.Little BP, Gilman MD, Humphrey KL, et al. Outcome of recommendations for radiographic follow-up of pneumonia on outpatient chest radiography. AJR. American journal of roentgenology. 2014;202:54–59. doi: 10.2214/AJR.13.10888. [DOI] [PubMed] [Google Scholar]
- 30.Harvey HB, Gilman MD, Wu CC, et al. Diagnostic yield of recommendations for chest CT examination prompted by outpatient chest radiographic findings. Radiology. 2015;275:262–271. doi: 10.1148/radiol.14140583. [DOI] [PMC free article] [PubMed] [Google Scholar]


