Abstract
The relations between early deprivation and the development of the neuroendocrine and central components of the mammalian stress response have been examined frequently. However, little is known about the impact of early deprivation on the developmental trajectories of autonomic function. Children adopted between 15–36 months from institutional care were examined during their first 16 months post-adoption (N=60). Comparison groups included same-aged peers reared in their birth families (N=50) and children adopted internationally from overseas foster care (N=46). The present study examined trajectories of baseline autonomic nervous system function longitudinally following entry into adopted families. Post-institutionalized children had higher sympathetic tone, measured by pre-ejection period (PEP). Individual differences in PEP soon after adoption served as a mediator between early deprivation and parent-reported behavioral problems two years post-adoption. There were no group differences in parasympathetic function, indexed by respiratory sinus arrhythmia. All three groups showed similar trajectories of ANS function across the 16 month period.
Keywords: Early Deprivation, Sympathetic Nervous System, Parasympathetic Nervous System, Adoption, Child Problem Behavior
For mammals, maternal care is necessary for survival. Decades of research using animal models has consistently demonstrated that social deprivation, in which an animal’s basic survival needs are met but social contact with the mother is disrupted or missing entirely, leads to the altered development of neural systems that underlie stress responses and the regulation of emotion and behavior. In rodents and primates, regular maternal separation not only acutely activates the hypothalamic-adrenal-pituitary (HPA) axis, but it also leads to changes in the functioning of this system that persist into adulthood. Behavioral profiles consistent with early social deprivation include increased fearfulness and freezing (Cirulli & Alleva, 2003; Gunnar & Quevedo, 2007; Sánchez, Ladd, & Plotsky, 2001). This early chronic stress also has a direct, negative impact on prefrontal cortex development (Arnsten, 2009).
Children who have experienced institutional or orphanage care provide a human analogue of these early social deprivation models. While conditions vary across institutions, this type of care is characterized by a lack of supportive social interactions with caregivers (Smyke et al., 2007). Although social deprivation may be confounded with physical deprivation, it is important to note that the lack of a consistent, responsive caregiver in high-quality physical environments is associated with poorer behavioral regulation later in childhood (Roy, Rutter, & Pickles, 2004). This suggests that supportive, reciprocal relationships with caregivers are of unique importance during early development. In fact, children who have been adopted from institutionalized care (post-institutionalized; PI) experience marked initial delays in physical (e.g., Johnson et al., 2010) and cognitive (Kreppner et al., 2001; Nelson et al., 2007) development. While a great deal of catch-up is evident across these domains (MacLean, 2003), difficulties with attention, emotion, and behavior regulation persist across childhood and adolescence (Stevens et al., 2008), and these early difficulties appear to place PI children at heightened risk for the development of emotional disorders during adolescence (Kreppner et al., 2010). Studies that have investigated the impact of early deprivation on neurobiological development have noted decreased gray matter volume in the prefrontal cortex (Hodel et al., 2015; McLaughlin et al., 2013; Sheridan, Fox, Zeanah, McLaughlin, & Nelson, 2012) and disrupted organization in prefrontal white matter tracts (e.g., Govindan, Behen, Helder, Makki, & Chugani, 2010; Hanson et al., 2013). Functional studies show decreased glucose metabolism in areas of the prefrontal cortex related to attention and behavior regulation (Chugani et al., 2001), increased amygdala activity to threat (Maheu et al., 2010; Tottenham et al., 2011), and immature patterns of EEG power while children are still in deprived care or shortly thereafter (Tarullo, Garvin, & Gunnar, 2011; McLaughlin et al., 2010; Vanderwert, Marshall, Nelson, Zeanah, & Fox, 2010). Studies of the HPA axis have noted altered basal activity for both children who remained in the orphanage setting (e.g., Carlson & Earls, 1997) and for those who had been adopted into supportive families (Gunnar, Morison, Chisholm, & Shuder, 2001; Gunnar & Vazquez, 2001; Johnson, Bruce, Tarullo, & Gunnar, 2011; Koss, Hostinar, Donzella, & Gunnar, 2014). Taken together, these studies provide overwhelming evidence that early social deprivation negatively impacts the neural systems that underlie stress responses and behavior and emotion regulation, and these effects persist for years after removal from the institutional setting.
While evidence is rapidly accumulating in the domains listed above, relatively less is known about the functioning of the autonomic nervous system (ANS) following a discrete period early social deprivation. The autonomic nervous system consists of two limbs with opposing functions: the parasympathetic nervous system (PNS), which mediates functions related to growth and rest while the sympathetic nervous system is involved in fight/flight responses. The PNS exerts tonic inhibitory control over heart rate via projections of the myelinated vagus nerve from the nucleus ambiguus to the sinoatrial node. This vagal “brake” slows heart rate in contexts where no threat or stressor is present, but under challenging conditions, suppression of vagal influence results in a removal of the “brake” and a subsequent increase in heart rate (Porges, 1995; 2007). Respiratory sinus arrhythmia (RSA) is a measure of heart rate variability associated with breathing and reflects this vagal influence on the heart. Higher levels of basal RSA are associated with the regulation of attention, behavior, and emotion (Beauchaine et al., 2001). In contrast, lower basal RSA is associated with both internalizing (Beauchaine et al., 2013; Porges, 2007) and externalizing (Beauchaine, Gatzke-Kopp, & Mead, 2007) problems in children and youth, although relations between basal RSA and externalizing behaviors have been less consistent (e.g., Calkins et al. 2007). Thus, basal RSA is thought to represent a stable, trait-like capacity for emotion regulation (Hinnant et al., 2011).
The sympatho-adrenomedullary (SAM) branch of the ANS consists of sympathetic pre-ganglionic neurons originating in the intermediolateral gray matter of the spinal cord that project axons through the ventral root to the chromaffin cells of the adrenal medulla, which release epinephrine (Epi) and norepinephrine (NE) into circulation. These peripheral catecholamines act on multiple target organs, including the heart, to increase cardiac output via increased stroke volume and heart rate (Gunnar & Quevedo, 2007). In humans, the activity of this system can be measured noninvasively through the pre-ejection period (PEP), which is a temporal measure reflecting the amount of time elapsed between the onset of the heartbeat and the ejection of blood into the aorta. Greater sympathetic tone is indexed by shorter PEP (Berntson et al., 1994). Greater sympathetic tone is associated with fearfulness and poorer emotion regulation (Buss et al., 2004). The PNS and SAM system represent complementary systems that consistently work together to allow individuals to appropriately meet situational demands. The coordination of these systems is accomplished by prefrontally-mediated neural networks that inhibit drive on the SAM system from the amygdala. Nodes in these cortical networks have been independently associated with self-regulatory capacities like attention regulation, inhibitory control, and emotion regulation (Thayer & Lane, 2009).
Longitudinal studies that have tracked the development of these systems note significant change across the early years of life. During typical development, RSA increases across infancy and early childhood (Alkon et al., 2006; Alkon et al. 2011; Bar-Haim, Marshall, & Fox, 2000) with stability in this measure increasing after the end of the first year (Alkon et al., 2011). RSA continues to increase across the early school years (Alkon et al., 2003; Marshall & Stevenson-Hinde, 1998), and appears to level off and stabilize during middle childhood or adolescence (El-Sheikh, 2005; Hinnant, Elmore-Staton, & El-Sheikh, 2011; Salomon, 2005). Although longitudinal investigations of PEP across infancy and childhood are more rare, several studies show decreases in basal sympathetic tone from infancy through the early school years (Alkon et al., 2011; De Rogalski Landrot et al., 2007). The changes in this system during the first year of life do not appear to be substantial (Alkon et al., 2006), but repeated measures of PEP are moderately correlated during the preschool years (Alkon et al., 2011). PEP continues to increase significantly across middle childhood and shows stability during this time period (Hinnant et al., 2011).
Early adversity has been shown to impact the development of the autonomic system in diverse samples of children, including those who have experienced maltreatment. A recent study found that preschool-aged children exposed to maltreatment demonstrated lower RSA during a teaching task and greater RSA suppression compared to baseline collection (Skowron et al., 2011). Similar findings show that early adversity is associated with decreased RSA reactivity at age three, but no effects of adversity on longitudinal changes in baseline RSA were noted (Conradt et al., 2014). The literature examining autonomic function in high-risk populations often focuses on its role as a moderator of environmental risk, with greater RSA reactivity indicating greater sensitivity to context. Evidence from maltreated (e.g., Skowron et al., 2014) and community (e.g., Obradovic et al., 2010) samples supports the notion that among young children, greater RSA reactivity in supportive contexts predicts improved outcomes. Higher baseline RSA may also moderate environmental risk, with greater basal RSA serving a protective role for children exposed to maltreatment, though this effect appears to be further moderated by the sympathetic system (Gordis et al., 2009). While a thorough review of the literature on autonomic function among diverse risk populations is beyond the scope of the current work, it appears that, at least when there is no marked change in care settings, early adversity shapes ANS development as a mediator of behavioral functioning, and ANS function may further moderate exposure to subsequent environments.
The relations between early deprivation and the development of the central and peripheral systems that underlie emotion regulation are documented. Despite this research, little work has examined the effect of early deprivation on the developmental trajectories of autonomic function. To our knowledge, very few studies have investigated autonomic functioning following early social deprivation. Gunnar and colleagues (2009) collected RSA and PEP from 10–12-year-olds using a standardized stressor task, and found that in the context of a laboratory visit in which the child would give a speech, PI children had significantly greater sympathetic tone compared to age-matched peers who had been raised in their families of origin who were of similar socioeconomic class to the families who adopt internationally. Most recently, using the same speech stressor context as was used by Gunnar and colleagues (2009), McLaughlin and colleagues (2015) found that youth reared in institutional care settings, in comparison to youth randomized to a high-quality foster care setting early in life, had blunted ANS reactivity to a social stressor task during adolescence. This previous work assessed children at one point in time nearly a after decade removal from institutional settings, and while these studies demonstrated that early social deprivation may lead to enduring shifts in the functioning of this system, they failed to address the severity of this impact on autonomic function immediately following adoption or the extent to which recovery may be possible.
The present study sought to examine differences in RSA and PEP following adoption in a group of internationally-adopted children. In addition to a group of post-institutionalized children adopted from overseas orphanage or institutional settings, the study included two comparison groups. One comparison group included a group of non-adopted same-aged peers who were born into their families of origin in the United States. Notably these families were chosen to be of a similar socioeconomic class to families who adopt internationally. A second comparison group included internationally-adopted children who were adopted from foster care settings; this comparison group allowed an examination of whether differences in ANS function were specific to the conditions experienced in institutional care or may reflect other factors, including prenatal conditions, which tend to be shared by orphaned and abandoned children regardless of their placement in different types of care settings (foster family or institution). To examine differences in RSA and PEP soon after adoption and changes in these systems over the course of the first 16 months post-adoption we fit linear growth models to data collected over three time points. We hypothesized that post-institutionalized children would have higher sympathetic tone and lower RSA consistent with the argument that these systems were shaped by chronic stress (Porges, 1995). Group differences in trajectories of PEP and RSA were conducted to examine evidence of recovery in ANS function following removal from settings of deprivation. Lastly, we examined whether individual differences in PEP and RSA at the start of the study (soon after adoption for PI youth) and change across the study serve as mechanisms for differences in behavioral problems two years post-adoption.
Method
Participants
Participants in the present analysis included 156 children ranging in age from 18 to 36 months at the start of the study. Children were drawn from a larger longitudinal examination of recovery from early life adversity. Participants included 60 children (37 girls, 23 boys; S1 M age 25.97 months, SD = 5.00) who were adopted from overseas institutional care settings (post-institutionalized, PI). Two comparison groups were included. First, 50 same-aged non-adopted children (NA; 25 girls, 25 boys; S1 M age 27.72 months, SD = 5.74) were included. Additionally, 46 children (19 girls, 27 boys; S1 M age 32.64 months, SD = 5.00) who were internationally adopted from international foster care (post foster care, PFC) were included as a second comparison group.
PI children were recruited from families identified as having recently adopted internationally through an adoption medical clinical and adoption agencies. Inclusion criteria for the PI group required children attend the first laboratory session within 3 months of entry into the family, were between the ages of 15 and 36 months at entry into the family (18 to 36 months at recruitment), and were adopted out of an international institution setting. On average, PI children spent the majority of their pre-adoptive lives in institutional care (M = 74%). Regions of origin for the PI children included Africa (n=22), Southeast Asia (n=19), Russia or Eastern Europe (n=14), and Latin America or the Caribbean (n=5).
PFC children were recruited from the International Adoption Project registry. Enrollment on the registry occurred through mailed invitations to families identified as adopting internationally through area adoption agencies. Inclusion criteria for the PFC children required children were adopted internationally, were between the ages of 18 and 36 months at recruitment, and spent less than 50% of pre-adoptive lives in institutional settings. PFC children spent the majority of their pre-adoptive life in foster care (M = 87% of pre-adoptive life in foster care) and as is typical for children adopted internationally from foster care, entry into the family occurred within the first year of life (M = 8.93 months, SD = 1.57). Region of origin for the PFC children included Southeast Asia (n=35) and Latin America or the Caribbean (n=11). PFC children were adopted earlier to reflect the typical age at adoption for children coming from foster care overseas.
NA children were recruited through the department’s participant pool. Families are enrolled through letters sent to all families of live births. Inclusion criteria for the NA children required that children were between the ages of 18 and 36 months at recruitment and were raised in their family of origin. Families were recruited to match the demographics (e.g., income, parental education) as parents who adopt internationally.
For the PI children, 38% were Asian, 38% were Black/African, 13% were White/Caucasian, 3% were American Indian/Alaska Native, 3% were biracial or multiracial, and 3% reported other or unknown racial backgrounds. For the PFC children, 76% were Asian, 17% were American Indian/Alaska Native, and 7% reported other or unknown racial backgrounds. For the NA children, 90% were White/Caucasian, 8% were biracial or multiracial, and 2% were Asian. Additionally, 24% of PFC children, 5% of PI children, and 4% of NA children were Hispanic/Latino. For a complete description of the child and family demographics for each group see Koss et al. 2014.
Children were screened for fetal alcohol exposure using the FAS Facial Photographic Analysis Software (Astley & Clarren, 2000); 8 children suspected of prenatal alcohol exposure were excluded (6 PI, 2 PFC). Children were also excluded for congenital and cognitive disorders (2 PI, 1 PFC). NA children were excluded for atypical developmental experiences (3 NA; 1 maltreatment, 1 autism, 1 childhood cancer). In the present study 9 children were excluded for parent-reported heart defects (5 PI, 1 NA, 3 PFC). Prior to data imputation, children with no ANS data at any of the three time points were excluded (2 PI, 1 NA). This resulted in a sample size of 156 for the present study (60 PI, 50 NA, 46 PFC).
Procedures
Participants completed four laboratory sessions spaced roughly eight months apart. To capture the transition to family care for the PI group, the initial laboratory session occurred within three months of the child’s arrival into the family for PI youth (S1 M = 1.66 months, SD = .74; S2 M = 8.30, SD = .59; S3 M = 16.23, SD = .49; S4 M = 24.12, SD=.53). Parents provided informed consent at the beginning of the longitudinal study. Families received a gift card and small prizes as compensation for their time at each session.
Autonomic Data Acquisition
Cardiac electrophysiological data were collected approximately one hour into the testing session. All cardiac measures were obtained from a single electrode montage (one set of electrodes for all these measures), acquired with BioPac M100 amplifiers and AcKnowledge v3.8.1 software, and analyzed using James Long software. Four spot electrodes were placed on the thorax: attached to the nape of the child’s neck, below the collarbone, on the backside of the ribcage, and on the lower abdomen. A 500 µA signal at 50 kHz was applied to the outer two electrodes, and both transthorasic impedance and ECG were measured by the inner two electrodes. Baseline data were collected while children sat within reach of a primary caregiver during two 3-minute segments of a non-arousing animated film set to classical music.
Measures
Respiratory Sinus Arrhythmia (RSA)
Raw ECG was digitized with a sample rate of 1000Hz and band pass filtered at .05 – 35 Hz, then processed using James Long IBI Analysis software. R-waves were automatically detected by the software, followed by a visual inspection by trained researchers to identify and replace outliers, artifact, and spurious data with interpolated r-waves. If needed, sections of artifact data were removed from analyses. Revisions typically ranged from 0 to 5 per trial (∼1%), although data were retained for analysis if up to 10% of beats needed revision. Thirty percent of the sample was double-scored for reliability and artifact replacement was reliable with Pearson correlations of .95 or greater.
The R-wave times were converted to inter-beat-intervals (IBIs) and then pro-rated into 125 ms windows. The pro-rated IBIs were de-trended using a moving polynomial. The residuals were discrete Fourier transformed yielding spectral power attributable to respiratory sinus arrhythmia. Using 64 second overlapping epochs of data, inter-beat interval variance that fell in the expected respiration band for children (0.24–1.04 Hz, Bar-Haim, Marshall & Fox, 2000) was extracted to reflect vagal tone or the parasympathetic input to the heart (Porges, 1995).
Pre-Ejection Period (PEP)
Raw impedance and ECG were digitized at 1000 Hz with a low pass filter at 10Hz, and then processed using James Long’s ERPview. Ensemble averages of 64 second non-overlapping epochs were created and displayed for time-locked ECG and dZ/dt (change in impedance over change in time). Software identified two points of interest: one point to serve as the onset of R and a second point at the peak of the dZ/dt wave within an analysis window of 50–200 msec. The Q-point or the onset of R is difficult to reliable spot (Berntson et al, 2004). As a proxy for the R-wave onset, using the ensemble averaged ECG wave, the software automatically detects the steep point of the slope of the rising R-wave (James Long, personal communication 6/13/2012). This is defined as the zero point, and thus the latency to dZ/dt maxima becomes PEP. The ensemble average for each epoch was displayed and trained researchers determined if the placement of each fiducial point was reasonable. Epochs where the average appeared to be influenced by artifact were dropped and participants had to retain 2 artifact-free epochs to be retained for analysis. Thirty percent of the sample was double-scored for reliability and resultant PEP scores were reliable (Pearson rs of .95 or greater).
Child Problem Behavior
The primary caregiver completed the problem behavior subscale of the Brief Infant Toddler Social and Emotional Assessment (BITSEA; Briggs-Gowan et al. 2002) at the fourth assessment (approximately 2 years post-adoption). The BITSEA problem behavior scale is comprised of 31 items tapping into emotional dysregulation, internalizing and externalizing problems, and additional atypical problems in toddlers. Participants rated each item on a three-point Likert scale and higher scores indicate more problem behaviors. The BITSEA had adequate internal reliability in the present sample (Cronbach’s α=.73).
Child Height
Children’s height was measured at each of the laboratory sessions. Height-for-age was calculated using World Health Organization standards (W.H.O., 2011). Height-for-age measures were standardized (z-scores reported).
Missing ANS Data and Data Imputation
A total of 156 children had ANS data at one or more time points. Reasons for missing ANS data included child/parent refused ANS task (T1 n = 16, T2 n = 10, T3 n = 16), data lost to artifact (S1 PEP n = 16, S1 RSA n = 6, S2 PEP n = 11, S2 RSA n = 2, S3 PEP n = 11, S3 RSA n = 3), and mechanical failures (S1 PEP n = 6, S1 RSA n = 3, S2 PEP n = 2, S3 PEP n = 9, S3 RSA n = 4). Additionally, six children did not participate in the study at T2 and twelve children did not participate at T3. For PEP, this resulted 88 children (30 PI, 30 NA, 28 PFC) with complete data, 40 children (15 PI, 13 NA, 12 PFC) with two time points of useable data, 22 children (10 PI, 6 NA, 6 PFC) with one time point of useable data, and 6 children (5 PI, 1 NA) with no useable data. For RSA, this resulted in 108 children (37 PI, 34 NA, 37 PFC) with complete data, 32 children (14 PI, 13 NA, 5 PFC) with two time points of useable data, and 16 children (9 PI, 3 NA, 4 PFC) with one time point of useable data. To account for missing data in the present study, multiple imputation was conducted in MPLUS (1998–2012) using Bayesian analysis. Multiple imputation estimates predicted scores for missing data based on the available data. All subsequent analyses and descriptive statistics utilized the imputed data aggregated across five imputed datasets.
Results
Descriptive Statistics
Means, standard deviations, and correlations among study variables are presented in Table 1. Both ANS measures were moderately stable across the three time points and there were weak associations among PEP and RSA. Child age was positively correlated with ANS measures and included as a covariate in subsequent analyses.
Table 1.
Correlations and Descriptive Statistics
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. S1 RSA | 1.00 | |||||||||||||
| 2. S2 RSA | .66*** | 1.00 | ||||||||||||
| 3. S3 RSA | .50*** | .62*** | 1.00 | |||||||||||
| 4. S1 PEP | .18* | .07 | .01 | 1.00 | ||||||||||
| 5. S2 PEP | .07 | .11 | .05 | .28*** | 1.00 | |||||||||
| 6. S3 PEP | .27*** | .16* | .12 | .34*** | .45*** | 1.00 | ||||||||
| 7. S1 Age | .36*** | .19* | .04 | .32*** | .17* | .19* | 1.00 | |||||||
| 8. S2 Age | .34*** | .19* | .04 | .31*** | .17* | .18* | .99*** | 1.00 | ||||||
| 9. S3 Age | .34*** | .18* | .04 | .32*** | .18* | .19* | .99*** | .99*** | 1.00 | |||||
| 10. S1. Height for Age | .07 | −.04 | −.09 | −.02 | .03 | .29*** | .01 | .01 | .02 | 1.00 | ||||
| 11. S2. Height for Age | .05 | −.02 | −.07 | .02 | −.14 | .22** | −.07 | −.07 | −.05 | .88*** | 1.00 | |||
| 12. S3. Height for Age | .03 | −.02 | −.05 | .02 | −.11 | .23** | −.10 | −.11 | −.10 | .81*** | .93*** | 1.00 | ||
| 13. S4 BITSEA | −.12 | −.08 | −.08 | −.30*** | −.21** | −.23** | −.02 | .00 | −.01 | −.22** | −.18* | −.19* | 1.00 | |
| 14. Child Sex | .03 | .04 | .03 | .10 | .20* | .11 | .13 | .13 | .14 | .02 | .02 | .02 | .18* | 1.00 |
|
| ||||||||||||||
| M | 5.22 | 5.79 | 6.21 | 104.64 | 110.32 | 114.04 | 28.50 | 35.48 | 43.42 | −.81 | −.55 | −.49 | 5.70 | -- |
| SD | 1.31 | 1.32 | 1.22 | 13.78 | 11.07 | 14.17 | 5.91 | 6.18 | 6.09 | 1.18 | 1.07 | .97 | 4.35 | -- |
Note. Data averaged across multiple imputation data sets. Child sex 0=girls, 1=boys.
p<.05,
p<.01,
p<.001.
One-way between-subjects analysis of variance (ANOVA) was conducted to examine group differences in children’s age and height-for-age (z-scores) at each of the time points. There was a significant group difference in children’s age at each of the time points (T1 F(2,153) = 21.86, p< .001; T2 F(2,153) = 24.82, p< .001; T3 F(2,153) = 26.06, p< .001). Post-hoc comparisons indicate that PFC children were older (T1 M = 32.64 months, SD = 5.00, range 20.81–37.38; T2 M = 39.98, SD = 5.06, range 28.47–44.52; T3 M = 47.93, SD = 4.99, range 36.26–52.50) than both PI (T1 M = 25.97 months, SD = 5.00, range 18.97–36.66; T2 M = 32.62, SD = 5.23, range 24.92–44.25; T3 M = 40.52, SD = 5.07, range 33.27–61.58) and NA (T1 M = 27.72 months, SD = 5.74, range 18.54–36.92; T2 M = 34.76, SD = 5.88, range 25.58–44.05; T3 M = 42.74, SD = 5.81, range 36.26–52.50) children (all ps<.001 for mean difference comparisons). Additionally, NA children were older than PI children at T2 and T3 (ps< .05).
There were also significant group differences in height-for-age (T1 F(2,153) = 30.42, p< .001; T2 F(2,153) = 12.59, p< .001; T3 F(2,153) = 4.39, p< .05). Post-hoc comparisons indicate that PI children were shorter relative to same aged children (T1 M = −1.54, SD = 1.12; T2 M = −.98, SD = 1.10; T3 M = −.66, SD = 1.10) compared to NA children at all three time points (T1 M = −.06, SD = .90, p< .001; T2 M= −.03, SD = .84, p <.001; T3 M = −.16, SD = .75, p< .01) and PFC children at the first two time points (T1 M = −.68, SD = .94, p< .001; T2 M = −.56, SD = 1.00, p< .05; T3 M = −.62, SD = .95, ns). Additionally, PFC children were shorter than NA children at all three time points (T1 p< .01, T2 p< .01, T3 p< .05). Thus child age and height-for-age were included as time-varying covariates of RSA and PEP.
There was a significant child sex difference in T4 BITSEA scores such that boys had higher rates of problem behavior (M = 5.25, SD = 1.34) compared to girls (M = 5.18, SD = 1.28; F(1,154) = 5.32, p<.05). Child sex was included as a covariate of BITSEA scores in subsequent analyses.
Trajectories of PEP and RSA
Data Analytic Plan
Linear growth models were fit to simultaneously model change in RSA and PEP across the three time points using MPLUS. Multiple imputation was conducted prior to estimating trajectories of autonomic functioning. To examine the effects of early life adversity on ANS functioning, group was dummy-coded (PI and PFC codes with NA children used as the reference category). Group was included as a predictor of the intercept and slope variables for both PEP and RSA linear growth models. To examine ANS functioning as a potential mediator between early life adversity and child behavior problems, T4 BITSEA scores were included as an outcome variable. PEP and RSA intercept and slope variables and the group dummy-codes were included as predictors of T4 BITSEA scores. To account for variation in children’s body mass, age and height-for-age were included as time-varying covariates of both PEP and RSA. Child sex was examined as a potential covariate of RSA and PEP intercepts and slopes; child sex was not related and the covariate was not included to reduce the number of estimated parameters. Child sex was included as a covariate of T4 BITSEA scores.
The model provided good fit to the data (χ2(55) = 72.58, p = .06; χ2/df = 1.32; CFI = .92, RMSEA = .05). Unstandardized parameter estimates for the model are provided in Table 2. Child age was a significant time-varying covariate of RSA at T1 and T2; greater age was associated with greater RSA. Height-for-age was a significant time-varying covariate of PEP at T1 and T2; greater height was associated with shorter PEP.
Table 2.
Linear Growth Model Results
| Unstandardized (SE) | β | R2 | |
|---|---|---|---|
| Predictors of PEP and RSA | |||
| PI Group→PEP Intercept | − 8.79 (3.43)** | −.45 | |
| PFC Group→PEP Intercept | − 4.20 (2.85) | −.20 | |
| PI Group→PEP Slope | 3.98 (2.37)† | .45 | |
| PFC Group→PEP Slope | 3.25 (1.84)† | .34 | |
| PI Group→RSA Intercept | .05 (.28) | .03 | |
| PFC Group→ RSA Intercept | − .11 (.27) | −.05 | |
| PI Group→ RSA Slope | .15 (.14) | .28 | |
| PFC Group→ RSA Slope | .16 (.14) | .28 | |
| Time-varying Covariates | |||
| T1 Age→T1 PEP | .39 (.22)† | .17 | |
| T2 Age→T2 PEP | .27 (.16)† | .13 | |
| T3 Age→T3 PEP | .22 (.23) | .10 | |
| T1 Height for Age→T1 PEP | − 2.82 (1.02)** | −.24 | |
| T2 Height for Age→T2 PEP | − 3.80 (1.26)** | −.31 | |
| T3 Height for Age→T3 PEP | 1.84 (1.27) | .13 | |
| T1 Age→T1 RSA | .08 (.02)*** | .35 | |
| T2 Age→T2 RSA | .04 (.02)* | .20 | |
| T3 Age→T3 RSA | .01 (.02) | .06 | |
| T1 Height for Age→T1 RSA | .07 (.11) | .06 | |
| T2 Height for Age→T2 RSA | .03 (.10) | .03 | |
| T3 Height for Age→T3 RSA | − .01 (.10) | −.01 | |
| Predictors of T4 BITSEA | |||
| PI Group→S4 BITSEA | − .07 (1.85) | −.01 | |
| PFC Group→S4 BITSEA | .92 (1.43) | .10 | |
| PEP Intercept→S4 BITSEA | − .20 (.08)* | −.44 | |
| PEP Slope→S4 BITSEA | − .07 (.28) | −.06 | |
| RSA Intercept→S4 BITSEA | − .51 (.43) | −.12 | |
| RSA Slope→S4 BITSEA | .85 (4.75) | .05 | |
| Child Sex→S4 BITSEA | 1.82 (.67)** | .21 | |
| PEP Intercept Mean | 95.93 (6.16)*** | ||
| PEP Intercept Variance | 76.25 (24.11)** | ||
| PEP Slope Mean | 4.50 (6.08) | ||
| PEP Slope Variance | 17.27 (13.13) | ||
| RSA Intercept Mean | 3.08 (.58)*** | ||
| RSA Intercept Variance | 1.02 (.18)*** | ||
| RSA Slope Mean | 1.20 (.35)*** | ||
| RSA Slope Variance | .07 (.05) | ||
| PEP Intercept-Slope Covariance | −13.28 (15.47) | ||
| RSA Intercept-Slope Covariance | − .07 (.07) | ||
| PEP Intercept-RSA Intercept Covariance | 1.36 (1.03) | ||
| PEP Slope-RSA Slope Covariance | .10 (.31) | ||
| R2 PEP Intercept | .16 | ||
| R2 PEP Slope | .20 | ||
| R2 RSA Intercept | .01 | ||
| R2 RSA Slope | .08 | ||
| R2 S4 BITSEA | .30 |
Note. Group dummy-coded (NA children as comparison group).
p< .10,
p< .05,
p< .01,
p< .001.
Group Effects on PEP and RSA Trajectories
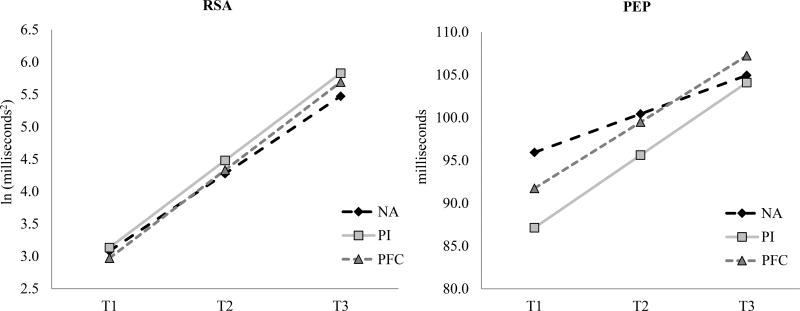
Group was modeled as a predictor of the intercept and slope variables for PEP and RSA. The predicted trajectories for each group are displayed in Figure 1. Early life adversity was associated with greater SNS activity. PI children had shorter PEP at T1 (e.g., latent intercept). Additionally, there was a statistical trend for both the PI and PFC groups on the PEP slope variable with both adopted groups showing steeper gains in PEP across the three time points. Early life adversity (e.g., PI and PFC groups) was not associated with PNS activity; neither group variable predicted the RSA intercept or slope latent variables. The model accounted for a modest amount of the variance in PEP (PEP Intercept R2=.16; PEP Slope R2=.20) and very little variance in RSA (RSA Intercept R2=.01, RSA Slope R2=.09).
Figure 1.
Trajectories of Parasympathetic and Sympathetic Nervous System Activity among Post-Institutionalized (PI), Post-Foster Care (PFC), and Non-Adopted (NA) Children
ANS activity as a Mediator between Group and Child Behavior
PNS and SNS intercepts and slopes were included as predictors of the T4 BITSEA scores. The intercept of the PEP latent variable was negatively associated with BITSEA scores. Greater SNS activity, indexed by shorter PEP at T1, was predictive of greater problem behavior at T4. To examine whether greater SNS activity was a mediator between early life adversity and problem behavior, RMediation (Tofighi & MacKinnon, 2011) was used to test the indirect effect using bootstrapped confidence intervals. The 95% confidence interval did not contain zero indicating a significant indirect effect of the PI group on T4 BITSEA scores through the intercept of PEP (indirect effect estimate =−1.77, SE=1.04; 95% CI: −4.15, −.15). PI children had greater SNS activity soon after adoption (e.g., T1 latent intercept) and greater SNS activity was in turn related to higher levels of problem behavior at T4. An effect size for the indirect effect was calculated using the proportion of the maximum indirect effect (K2= .20; Preacher & Kelley, 2011). Lastly, the PEP slope and RSA intercept and slope were not associated with problem behavior. The model as a whole accounted for a moderate amount of the variance in children’s BITSEA scores (R2=.30).
Discussion
The present study sought to examine the impact of early adversity on children’s autonomic nervous system function following removal from institutional care settings. Findings suggest that PI children had greater baseline sympathetic tone soon after adoption compared to non-adopted youth. In turn, greater sympathetic tone soon after adoption was associated with greater behavioral difficulties for children two years post-adoption. In contrast, there was no evidence for differences in baseline PNS activity soon after adoption or in the 16 months following adoption.
Previous work with children who experienced early adverse care supports the current finding that the SAM system is more strongly impacted by early experience. In a design using similar comparison groups, PI but not children adopted internationally from foster care demonstrated higher sympathetic tone even after spending years in supportive families (Gunnar, Frenn, Wewerka, & Van Ryzin, 2009). A recent study of children in foster families also notes higher sympathetic tone during a laboratory assessment among similarly aged children with a history of neglect (Oosterman et al., 2010). Furthermore, in the present study this higher sympathetic tone served as a mediator between early deprivation for PI youth and parent-reported behavioral problems two years post-adoption. These findings provide support for the notion that early life experiences may shape and become embedded in children’s stress-sensitive biological systems. This in turn may relate to individual differences in later health and well-being serving as a mechanism for these differences across the lifespan.
In contrast, results for the PFC children suggest no statistical differences in PEP compared to the NA children. Examination of their initial PEP responses at the start of the study suggests that the PFC children’s SNS function fall somewhere in between our PI and NA groups. At recruitment into the study, PFC children have spent a longer period of time in their adopted families and a shorter duration in overseas foster care. This combination of factors likely resulted in higher quality parenting for a longer period of time prior to the first measurement and shorter duration of early life adversity both which may contribute to the present findings. Similarly, McLaughlin and colleagues (2015) found that youth raised in institutional care settings had higher baseline sympathetic tone compared to both youth randomized into foster care settings and same-aged never-institutionalized youth during adolescence. Youth receiving the foster care intervention, indicative of higher quality early care, had baseline PEP measures similar to typically developing adolescents. Together with the present findings, these results point to the quality of early care as one mechanism that may shape the development of SNS function.
The results also yielded evidence that over time SNS tone in the PI and in the PFC children decreased (PEP was higher). There is a challenge, of course, in interpreting this finding. With each assessment the experience of being in the laboratory, having electrodes placed for ANS measurement, and other aspects of the experience were becoming less novel. Thus, it is possible that the first assessment reflected SNS tone under highly novel conditions, while the later assessments reflected something more akin to baseline tone. This might be, of course, why the earlier assessment was predictive of children’s behavior problems, even though those problems were assessed after there were no longer SNS differences among the groups. This hypothesis contrast with the argument that with time in the family, SNS differences reflecting early adversity diminished. If this hypothesis is the accurate one, it is challenging to explain why the SNS measure predicted later behavior problems, unless there was a third variable, such as the degree of early adversity, that predicted both initial SNS tone and later behavior problems. If so, then SNS activity near adoption was merely a marker for the degree of pre-adoption adversity. The design of the present study does not allow us to distinguish between these two hypotheses.
Contrary to our hypotheses, the present study did not find support for differences in PNS function suggesting that by 2 months post-adoption any impact of early life adversity on the PNS system, if it was ever there, is not present in baseline RSA. Alternatively, it may be that PNS function is more robust to early environmental adversity. Replication of these findings is needed; however, if RSA is robust to the impact of early life adversity, this provides encouraging news that this PNS function may be preserved in children experiencing early social deprivation. RSA has been implicated in emotion and behavioral regulation (Porges, 2007; Thayer & Lane, 2009) and may help to promote resilience in children experiencing environmental risk. An additional interpretation of these results argues that lower baseline RSA may be the result of initially high RSA reactivity in a context of chronic stress (Hinnant, Erath, & El-Sheikh, 2015); thus, the lack of significant baseline RSA differences between groups simply may not yet be present. However, continued exposure to stress would likely be a necessary component of this developmental trajectory, and the children in this study currently live in supportive and highly-resourced family environments.
There was not strong support for change, indicative of recovery, in the sympathetic system following adoption. Results for group-specific effects on the change in PEP activity were at trend-level and should be interpreted with caution. However, the pattern of results does seem to suggest that both PI and PFC youth exhibit a sharper rise in SNS function across the 16 months post-adoption. The growth trajectories of PEP in the internationally adopted youth appears to converge with levels of PEP function in NA children by the last collection assessment suggesting recovery following early heightened sympathetic tone. This change may correspond with and be indicative of the vast gains in physical growth PI children experience after removal from settings of deprivation. Following placement in enriching environments, PI youth do exhibit rapid catch-up growth in height and weight (Van IJzendoorn, Bakermans-Kranenburg, & Juffer, 2007). On the other hand, there was no evidence of group differences in the growth trajectories of RSA suggesting similar developmental change in PNS function during the toddler years independent of different early life environmental experiences.
The present study is not without a number of limitations. In particular, our sample sizes are limited by the number of families available for recruitment and willing to participate during this transitional time. As such our analyses may be underpowered to detect differences in trajectories of responses. Although we screened and excluded for concerns of fetal alcoholism, we are unable to account for prenatal experiences that may impact differences in ANS function, including alcohol exposure that did not co-occur with the development of facial features. Although we covaried age in our analyses, the differences in age between the groups may have contributed to this pattern of results. Lastly, the present study utilized a baseline measure of ANS function. Because the stress response system responds in context-dependent ways, findings in the present study may be difficult to interpret in the absence of measures of reactivity. Studies that incorporate measures of RSA reactivity in addition to basal RSA note differences in reactivity following exposure to adversity, even when no differences in baseline RSA were detected (e.g., Crowell et al., 2006; Fortunato et al., 2013). Therefore, it is possible that differences in RSA reactivity may be present despite a lack of noted differences in baseline RSA. Measures of baseline function were collected as we were cautious to further provoke children soon after the transition into their new families given the many changes and challenges these children and families faced. Further, we wanted to avoid collecting ANS data within the context of social interaction with the parent, which is a commonly used challenge in reactivity studies (e.g., Skowron et al., 2014), given the possibility that patterns of parent-child interactions may not have stabilized by the first laboratory assessment shortly after adoption. Future research with measures of reactivity is needed to understand how early life deprivation may alter the responses of both the SNS and PNS under conditions of stress during the transition to family care.
Despite these limitations, the present study is one of the first to examine the longitudinal trajectories of ANS function following placement into enriching environments for children who spent their early years in conditions of deprivation. This study also contributes to the little developmental work examining trajectories of both the SNS and PNS. Individual differences in PEP soon after adoption contribute to prediction of later problem behavior providing evidence for one of the mechanisms that may account for how environmental adversity shapes later problem behavior. On the other hand, children’s RSA in the present study seems to be preserved despite conditions of early life adversity. Future work is necessary to understand which biological systems are impacted by early life adversity that may serve as mechanisms for later difficulties throughout the lifespan.
Acknowledgments
The authors would like to thank the other members of the Minnesota International Adoption Team for their efforts in collecting these data: Shanna Mliner, Kristin Frenn, Bao Moua, Alyssa Miller, and Meg Bale, as well as Nathan A. Fox and James Long for their consultation regarding ANS data processing. We would also like to thank the parents and children without whom this study would not have been possible. This work was supported by grants R01 MH080905 and P50 MH078105 from the National Institute of Mental Health awarded to Megan Gunnar. Support was provided to Elisa Esposito by an Interdisciplinary Training Program in Cognitive Science (T32 HD007151) and to Kalsea Koss by a National Institute of Mental Health training grant (T32 MH015755) during the preparation of this article.
References
- Alkon A, Boyce WT, Davis NV, Eskenazi B. Developmental changes in autonomic nervous system resting and reactivity measures in Latino children from 6 to 60 months of age. Journal of Developmental & Behavioral Pediatrics. 2011;32:668–677. doi: 10.1097/dbp.0b013e3182331fa6. [DOI] [PubMed] [Google Scholar]
- Alkon A, Goldstein LH, Smider N, Essex MJ, Kupfer DJ, Boyce WT. Developmental and contextual influences on autonomic reactivity in young children. Developmental Psychobiology. 2003;42:64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- Alkon A, Lippert S, Vujan N, Rodriquez ME, Boyce WT, Eskenazi B. The ontogeny of autonomic measures in 6- and 12-month old infants. Developmental Psychobiology. 2006;48:197–208. doi: 10.1002/dev.20129. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Stress signaling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley S, Clarren S. Diagnosing the full spectrum of fetal alcohol exposed individuals: introducing the 4-digit diagnostic code. Alcohol and Alcoholism. 2000;35:400–410. doi: 10.1093/alcalc/35.4.400. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Marshall PJ, Fox NA. Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology. 2000;37:44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Neuhaus E, Chipman J, Reid MJ, Webster-Stratton C. Sympathetic- and parasympathetic-linked cardiac function and prediction of externalizing behavior, emotion regulation, and prosocial behavior among preschoolers treated for ADHD. Journal of Consulting and Clinical Psychology. 2013;81:481–493. doi: 10.1037/a0032302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Katkin ES, Strassberg Z, Snarr J. Disinhibitory psychopathology in male adolescents: Discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. Journal of Abnormal Psychology. 2001;110:610–624. doi: 10.1037/0021-843X.110.4.610. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Binkley PF, Uchino BN, Quigley KS, Fieldstone A. Autonomic cardiac control: III. Psychological stress and cardiac response in autonomic space as revealed by pharmacological blockades. Psychophysiology. 1994;31:599–608. doi: 10.1111/j.1469-8986.1994.tb02352.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Lozano DL, Chen YJ, Cacioppo JT. Where to Q in PEP. Psychophysiology. 2004;41:333–337. doi: 10.1111/j.1469-8986.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Carter AS, Irwin JR, Wachtel K, Cicchetti DV. The brief infant-toddler social and emotional assessment: screening for social-emotional problems and delays in competence. Journal of Pediatric Psychology. 2002;29:143–155. doi: 10.1093/jpepsy/jsh017. [DOI] [PubMed] [Google Scholar]
- Buss KA, Davidson RJ, Kalin NH, Goldsmith HH. Context-specific freezing and associated physiological reactivity as a dysregulated fear response. Developmental Psychology. 2004;40:583–594. doi: 10.1037/0012-1649.40.4.583. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Keane SP. Cardiac vagal regulation differentiates among children at risk for behavioral problems. Biological Psychology. 2007;74:144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences. 1997;15:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhász C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: A study of postinstitutionalized Romanian orphans. Neuroimage. 2001;14:1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Alleva BE. Early disruption of the mother-infant relationship: Effects on brain plasticity and implications for psychopathology. Neuroscience and Biobehavioral Reviews. 2003;27:73–82. doi: 10.1016/s0149-7634(03)00010-1. [DOI] [PubMed] [Google Scholar]
- Conradt E, Degarmo D, Fisher D, Abar B, Lester BM, Lagasse LL, … Hammond JA. The contributions of early adverse experiences and trajectories of respiratory sinus arrhythmia on the development of neurobehavioral disinhibition among children with prenatal substance exposure. Development and Psychopathology. 2014;26:901–916. doi: 10.1017/S095457941400056X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rogalski Landrot I, Roche F, Pichot V, Teyssier G, Gaspoz JM, Barthelemy JC, Patural H. Autonomic nervous system activity in premature and full-term infants from theoretical term to 7 years. Autonomic Neuroscience: Basic and Clinical. 2007;136:105–109. doi: 10.1016/j.autneu.2007.04.008. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M. Stability of respiratory sinus arrhythmia in children and young adolescents: A longitudinal examination. Developmental Psychobiology. 2005;46:66–74. doi: 10.1002/dev.20036. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Feres N, Olezeski CL, Rabkin AN, Trickett PK. Skin conductance reactivity and respiratory sinus arrhythmia among maltreated and comparison youth: Relations with aggressive behavior. Journal of Pediatric Psychology. 2009;35:547–558. doi: 10.1093/jpepsy/jsp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT. Altered water diffusivity in cortical association tracts in children with early deprivation identified with tract-based spatial statistics (TBSS) Cerebral Cortex. 2010;20:561–569. doi: 10.1093/cercor/bhp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10-12-year-old children. Psychoneuroendocrinology. 2009;34:62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Shuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Development and Psychopathology. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Adluru N, Chung MK, Alexander AL, Davidson RJ, Pollak SD. Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Development. 2013;84:1566–1578. doi: 10.1111/cdev.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnant JB, Elmore-Staton L, El-Sheikh M. Developmental trajectories of respiratory sinus arrhythmia and preejection period in middle childhood. Developmental Psychobiology. 2011;53:59–68. doi: 10.1002/dev.20487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnant JB, Erath SA, El-Sheikh M. Harsh parenting, sympathetic activity, and development of delinquency and substance use. Journal of Abnormal Psychology. 2015;124:137–151. doi: 10.1037/abn0000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel AS, Hunt RH, Cowell RA, Van Den Hoevel SE, Gunnar MR, Thomas KM. Duration of early adversity and structural brain development in post-institutionalized adolescents. Neuroimage. 2015;105:112–119. doi: 10.1016/j.neuroimage.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AE, Bruce J, Tarullo AR, Gunnar MR. Growth delay as an index of allostatic load in young children: Predictions to disinhibited social approach and diurnal cortisol activity. Development and Psychopathology. 2011;23:859–871. doi: 10.1017/s0954579411000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Guthrie D, Smyke AT, Koga SF, Fox NA, Zeanah CH, Nelson CA. Growth and associations between auxology, caregiving environment, and cognition in socially deprived Romanian children randomized to foster vs ongoing institutional care. Archives of Pediatrics & Adolescent Medicine. 2010;164:507–516. doi: 10.1001/archpediatrics.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss KJ, Hostinar CE, Donzella B, Gunnar MR. Social deprivation and the HPA axis in early development. Psychoneuroendocrinology. 2014;50:1–13. doi: 10.1016/j.psyneuen.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreppner JM, O’Connor TG, Rutter M, the English and Romanian Adoptees Study Team Can inattention/overactivity be an institutional deprivation syndrome? Journal of Abnormal Child Psychology. 2001;29:513–528. doi: 10.1023/A:1012229209190. [DOI] [PubMed] [Google Scholar]
- Kreppner J, Kumsta R, Rutter M, Beckett C, Castle J, Stevens S, Sonuga-Barke EJ. IV. Developmental course of deprivation-specific psychological patterns: Early manifestation, persistence to age 15, and clinical features. Monographs of the Society for Research in Child Development. 2010;75:79–101. doi: 10.1111/j.1540-5834.2010.00551.x. [DOI] [PubMed] [Google Scholar]
- MacLean K. The impact of institutionalization on child development. Development and Psychopathology. 2003;15:853–884. doi: 10.1017/s0954579403000415. doi: 10.1017.S0954579403000415. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, Jenness J, Lau JY, Ackerman JP, Pine DS, Ernst M. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Stevenson-Hinde J. Behavioral inhibition, heart period, and respiratory sinus arrhythmia in young children. Developmental Psychobiology. 1998;33:283–292. doi: 10.1002/(sici)1098-2302(199811)33:3<283::aid-dev8>3.3.co;2-f. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Fox NA, Zeanah CH, Sheridan MA, Marshall P, Nelson CA. Delayed maturation in brain electrical activity partially explains the association between early environmental deprivation and symptoms of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2010;68:329–336. doi: 10.1016/j.biopsych.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, Nelson CA. Causal effects of the early caregiving environment on development of stress response systems in children. Proceedings of the National Academy of Sciences. 2015 doi: 10.1073/pnas.1423363112. Advanced online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, Nelson CA. Widespread reductions in cortical thickness following severe early-life deprivation: A neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biological Psychiatry. 2013;76:629–638. doi: 10.1016/j.biopsych.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Seventh. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Obradovic J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Psychology. 2010;81:270–189. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterman M, De Schipper JC, Fisher P, Dozier M, Schuengel C. Autonomic reactivity in relation to attachment and early adversity among foster children. Development and Psychopathology. 2010;22:109–118. doi: 10.1017/S0954579409990290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Kelley K. Effect sizes for mediation models: Quantitative strategies for communicating indirect effects. Psychological Methods. 2011;16:93–115. doi: 10.1037/a0022658. [DOI] [PubMed] [Google Scholar]
- Roy P, Rutter M, Pickles A. Institutional care: Associations between overactivity and lack of selectivity in social relationships. Journal of Child Psychology and Psychiatry. 2004;45:866–873. doi: 10.1111/j.1469-7610.2004.00278.x. [DOI] [PubMed] [Google Scholar]
- Salomon K. Respiratory sinus arrhythmia during stress predicts resting respiratory sinus arrhythmia 3 years later in a pediatric sample. Health Psychology. 2005;24:66–76. doi: 10.1037/0278-6133.24.1.68. [DOI] [PubMed] [Google Scholar]
- Sánchez MM, Ladd CO, Plotsky P. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Development and Psychopathology. 2001;13:419–50. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA. Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences. 2012;109:12927–12932. doi: 10.1073/pnas.1200041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowron EA, Loke E, Gatzke-Kopp LM, Cipriano-Essel EA, Woehrle PL, Van Epps JJ, … Ammerman RT. Mapping cardiac physiology and parenting processes in maltreating mother-child dyads. Journal of Family Psychology. 2011;25:663–674. doi: 10.1037/a0024528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowron EA, Cipriano-Essel E, Gatzke-Kopp LM, Teti DM, Ammerman RT. Early adversity, RSA, and inhibitory control: Evidence of children’s neurobiological sensitivity to social context. Developmental Psychobiology. 2013;56:964–978. doi: 10.1002/dev.21175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyke AT, Koga SF, Johnson DE, Fox NA, Marjall PJ, Nelson CA, Zeanah CH, the BEIP Core Group The caregiving context in institution-reared and family-reared infants and toddlers in Romania. Journal of Child Psychology and Psychiatry. 2007;48:210–218. doi: 10.1111/j.1469-7610.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- Stevens SE, Sonuga-Barke E, Kreppner JM, Beckett C, Castle J, Colvert E, Groothues C, Hawkins A, Rutter M. Inattention/overactivity following early severe institutional deprivation: Presentation and associations in early adolescence. Journal of Abnormal Child Psychology. 2008;36:385–398. doi: 10.1007/s10802-007-9185-5. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Garvin MC, Gunnar MR. Atypical EEG power correlates with indiscriminately friendly behavior in internationally adopted children. Developmental Psychology. 2011;47:417–431. doi: 10.1037/a0021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Tofighi D, MacKinnon DP. RMediation: An R package for mediation analysis confidence intervals. Behavior Research Methods. 2011;43:692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gihlooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental Science. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwert RE, Marshall PJ, Nelson CA, Zeanah CH, Fox NA. Timing of intervention affects brain electrical activity in children exposed to severe psychosocial neglect. PLoS One. 2010;5:e11415. doi: 10.1371/journal.pone.0011415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Bakermans-Kranenburg MJ, Juffer F. Plasticity of growth in height, weight, and head circumference: Meta-analytic evidence of massive catch-up after international adoption. Journal of Developmental & Behavioral Pediatrics. 2007;28:334–343. doi: 10.1097/DBP.0b013e31811320aa. [DOI] [PubMed] [Google Scholar]
- W.H.O. Antro (Version 3.2.2) [Software] 2011 Available from http://www.who.int/childgrowth/software/en/