Abstract
The mitogen-activated protein kinase (MAPK), especially its extracellular signal-regulated kinase (ERK) subfamily, is a group of kinases enriched in the mammalian brain. While ERK is central to cell signaling and neural activities, the regulation of ERK by transmitters is poorly understood. In this study, the role of acetylcholine in the regulation of ERK was investigated in adult rat striatum in vivo. We focused on muscarinic M1 and M4 receptors, two principal muscarinic acetylcholine (mACh) receptor subtypes in the striatum. A systemic injection of the M1-preferring antagonist telenzepine did not alter ERK phosphorylation in the two subdivisions of the striatum, the caudate putamen and nucleus accumbens. Similarly, telenzepine did not affect ERK phosphorylation in the medial prefrontal cortex (mPFC), hippocampus, and cerebellum. Moreover, telenzepine had no effect on the ERK phosphorylation induced by dopamine stimulation with the psychostimulant amphetamine. In contrast to telenzepine, the M4-preferring antagonist tropicamide consistently increased ERK phosphorylation in the striatum and mPFC. This increase was rapid and transient. Tropicamide and amphetamine when coadministered at subthreshold doses induced a significant increase in ERK phosphorylation. These results demonstrate that mACh receptors exert a subtype-specific modulation of ERK in striatal and mPFC neurons. While the M1 receptor antagonist had no effect on ERK phosphorylation, M4 receptors inhibit constitutive and dopamine-stimulated ERK phosphorylation in these dopamine-innervated brain regions.
Keywords: caudate putamen, nucleus accumbens, prefrontal cortex, acetylcholine, dopamine, tropicamide, telenzepine, amphetamine
1. Introduction
A family of protein kinases, i.e., mitogen-activated protein kinases (MAPK), is central for cell signaling (Nozaki et al., 2001). Among a number of MAPK members that are classified into three subfamilies, the extracellular signal-regulated kinase (ERK) is a prototypic one and has been most extensively studied (Pearson et al., 2001; Volmat and Pouyssegur, 2001). Like all MAPKs, ERK is activated through a phosphorylation-dependent mechanism involving cascade activation of three-tiered protein kinase systems (Volmat and Pouyssegur, 2001). Noticeably, ERK is expressed in brain cells at a high level and is sensitive to changing cellular and synaptic input. Thus, the ERK, as one of key serine/threonine kinase, serves as an important regulator vigorously involved in the regulation of various neuronal and synaptic activities and is linked to the pathogenesis of a number of neurological and neuropsychiatric disorders (reviewed in Sweatt, 2004; Thomas and Huganir, 2004; Wang et al., 2007).
Acetylcholine, an essential transmitter in the central nervous system, interacts with both nicotinic and muscarinic acetylcholine (mACh) receptors to achieve its action. While nicotinic receptors are ion channels, mACh receptors are G protein-coupled receptors (GPCR). Based on the connection to discrete G proteins, mACh receptors are further divided into five subtypes (M1–M5) (Caulfield and Birdsall, 1998). The M1 class (M1, M3, and M5 subtypes) is coupled to Gq proteins (Wess, 1996). Thus, activating them activates the Gq-coupled signaling pathway involving phospholipase Cβ1 and its downstream signaling molecules (diacylglycerol, inositol-1,4,5-triphosphate, and Ca2+). The M2 class (M2 and M4 subtypes) is coupled to Gi/o proteins (Wess, 1996). Activation of these receptors inhibits adenylyl cyclase and thereby suppresses cAMP formation and protein kinase A (PKA) activity, while these receptors also affect other downstream signaling effectors (Lanzafame et al., 2003).
mACh receptors are highly expressed in the mammalian brain. The brain regions showing a high level of mACh receptor expression include the striatum, medial prefrontal cortex (mPFC), and hippocampus (Levey et al., 1991; Hersch et al., 1994; Volpicelli and Levey, 2004). In particular, within the striatum, M1 and M4 receptors are two predominant subtypes that are expressed in this region at a high level (Levey et al., 1991; Yasuda et al., 1993; Hersch et al., 1994; Tice et al., 1996; Chapman et al., 2011). Postsynaptic M1 receptors are present in two subpopulations of medium spiny projection neurons (Hersch et al., 1994; Alcantara et al., 2001; Ztaou et al., 2016), i.e., striatonigral and striatopallidal neurons constituting nearly 95% of the entire striatal neuronal population (Chang et al., 1982; Graveland and DiFiglia, 1985). M4 receptors are coexpressed with dopamine D1 receptors preferentially and postsynaptically in striatonigral neurons (Ince et al., 1997; Santiago and Potter, 2001). Numerous studies have demonstrated various types of interactions between acetylcholine and dopamine, another principal transmitter in the region, for controlling local striatal homeostasis, while molecular mechanisms underlying their interactions are poorly understood.
We here investigated the role of mACh receptors in the regulation of ERK in vivo. We first evaluated the role of striatal M1 and M4 receptors. A systemically active antagonist with moderate subtype selectivity for M1 receptors, telenzepine (Doods et al., 1987; Tanda et al., 2007; Ztaou et al., 2016; Joseph and Thomsen, 2017), or M4 receptors, tropicamide (Lazareno et al., 1990; Ztaou et al., 2016), was injected to adult rats. Their effects on ERK phosphorylation were examined in the two subdivisions of the striatum, the caudate putamen (CPu) and nucleus accumbens (NAc). In addition, other brain regions such as the mPFC, hippocampus and cerebellum were surveyed in comparison with the striatum. Finally, the effect of either antagonist on the ERK phosphorylation induced by the psychostimulant amphetamine (AMPH) was examined in the striatum and mPFC.
2. Results
2.1. Effects of telenzepine on phosphorylation and expression of ERK1/2
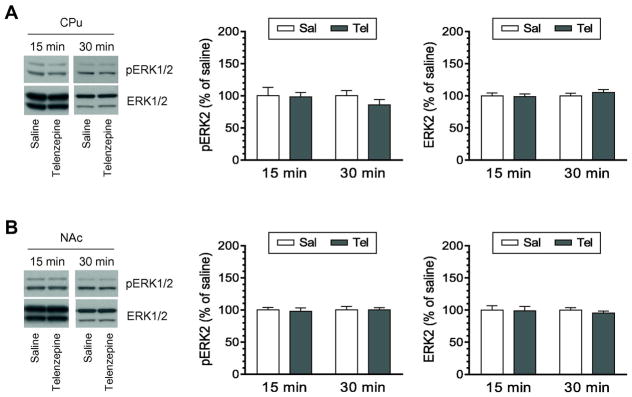
We first investigated the role of M1 receptors in the regulation of ERK1/2 phosphorylation and expression in the rat striatum. To this end, a systemically active M1-preferring antagonist telenzepine was administered at 3 mg/kg (i.p.). Rats were sacrificed 15 or 30 min after drug injection. Changes in levels of phosphorylated ERK1/2 (pERK1/2) and total ERK1/2 proteins were analyzed in the CPu and NAc by Western blot. We chose the dose of 3 mg/kg because this dose of telenzepine following a systemic injection was effective in alleviating behavioral disorders in dopamine-lesioned mice (Ztaou et al., 2016) and in completely blocking cortical dopamine release induced by the M1 agonist N-desmethylclozapine (Li et al., 2005; 2009). A single injection of telenzepine did not alter pERK1/2 levels in the CPu at a 15- min time point (Fig. 1A). Neither did telenzepine at 30 min. At the two time points, telenzepine showed an insignificant effect on expression of total ERK1/2 proteins. Similar to the CPu, pERK1/2 and ERK1/2 in the NAc were insensitive to telenzepine. As shown in Fig. 1B, pERK1/2 and ERK1/2 levels remained unchanged in telenzepine-treated rats compared to saline-treated rats at either time point surveyed. In a separate study, we tested the effect of telenzepine at a higher dose. Telenzepine at 10 mg/kg (15 min) did not alter pERK2 levels in the CPu (108.1 ± 6.9% of saline, p > 0.05) and NAc (102.5 ± 4.4% of saline, p > 0.05). These results demonstrate that blockade of M1 receptors has little impact on phosphorylation and expression of ERK1/2 in the striatum.
Figure 1. Effects of telenzepine on phosphorylation and expression of ERK1/2 in the striatum.
A, Effects of telenzepine on phosphorylation and expression of ERK1/2 in the CPu. B, Effects of telenzepine on phosphorylation and expression of ERK1/2 in the NAc. Representative immunoblots are shown to the left of the quantified data. Note that insignificant changes in pERK2 signals were seen following telenzepine injection in both the CPu (F(1,12) = 0.791, P = 0.391) and NAc (F(1,12) = 0.017, P = 0.899). Rats were given an i.p. injection of saline (Sal) or telenzepine (Tel) at 3 mg/kg and were sacrificed 15 or 30 min after drug injection for Western blot analysis of changes in pERK1/2 and ERK1/2 expression. Data are presented as means ± SEM (n = 4 per group).
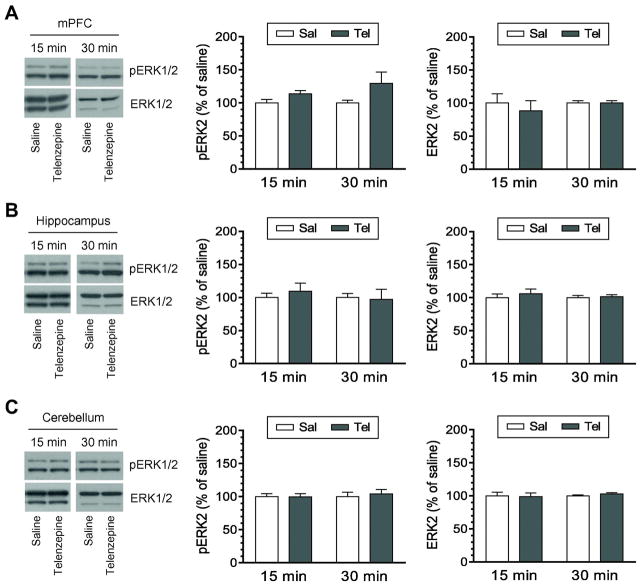
In addition to the striatum, other brain regions were investigated in the ERK1/2 response to telenzepine. In the mPFC, telenzepine (3 mg/kg) did not induce a significant change in pERK1/2 levels at 15 and 30 min after antagonist injection (Fig. 2A). In the hippocampus, similar results were observed. The level of hippocampal pERK1/2 signals in telenzepine (3 mg/kg)-treated rats was not different from that in saline-treated rats at either time point (Fig. 2B). In the cerebellum, phosphorylation of ERK1/2 was not an event sensitive to telenzepine (3 mg/kg) as pERK1/2 levels remained stable in telenzepine-treated rats relative to saline-treated rats (Fig. 2C). In all three regions, expression of total ERK1/2 proteins was not altered at 15 and 30 min following telenzepine administration. In addition, telenzepine at 10 mg/kg (15 min) did not change pERK1/2 levels in three regions (data not shown). These data indicate that the M1 antagonist telenzepine has no ability to alter phosphorylation of ERK1/2 in the three additional brain regions tested.
Figure 2. Effects of telenzepine on phosphorylation and expression of ERK1/2 in different brain regions.
A, Effects of telenzepine on phosphorylation and expression of ERK1/2 in the mPFC. B, Effects of telenzepine on phosphorylation and expression of ERK1/2 in the hippocampus. C, Effects of telenzepine on phosphorylation and expression of ERK1/2 in the cerebellum. Representative immunoblots are shown to the left of the quantified data. Note that insignificant changes in pERK2 signals were seen following telenzepine injection in the mPFC (F(1,12) = 4.397, P = 0.058), hippocampus (F(1,12) = 0.062, P = 0.807), and cerebellum (F(1,12) = 0.25, P = 0.61). Rats were given an i.p. injection of saline (Sal) or telenzepine (Tel) at 3 mg/kg and were sacrificed 15 or 30 min after drug injection for Western blot analysis of changes in pERK1/2 and ERK1/2 expression. Data are presented as means ± SEM (n = 4 per group).
2.2. Effects of tropicamide on phosphorylation and expression of ERK1/2
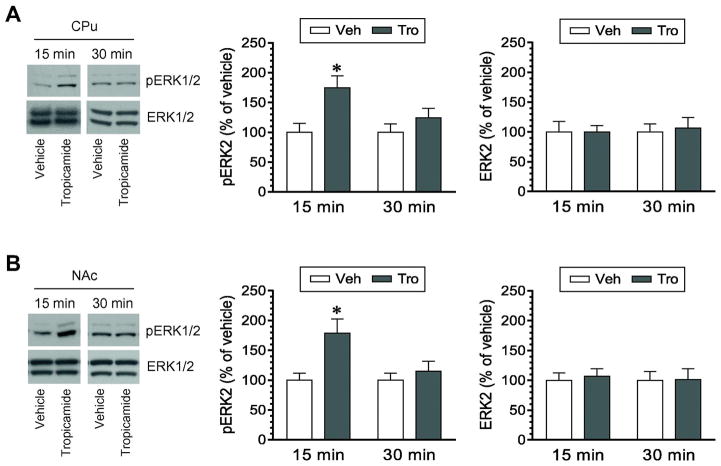
We next attempted to determine the role of M4 receptors in the regulation of ERK1/2 phosphorylation and expression. An M4-preferring antagonist tropicamide was used to block M4 receptors. After administration of this systemically active antagonist at 10 mg/kg (i.p.), rats were sacrificed 15 or 30 min after drug injection. Changes in pERK1/2 and ERK1/2 levels in the striatum and other brain regions were analyzed by Western blot. We found that tropicamide significantly affected phosphorylation of ERK1/2. In the CPu, pERK1/2 levels were markedly increased at 15 min following tropicamide injection (Fig. 3A). The extent of this increase declined to the level insignificant from that seen in vehicle-treated rats at 30 min. Quantification of pERK2 signals confirmed this transient increase in ERK2 phosphorylation at 15 min. In the NAc, we also found that ERK1/2 phosphorylation was altered by tropicamide. A significant increase in pERK2 levels exhibited in the NAc at 15 min although not 30 min after tropicamide administration (Fig. 3B). These results indicate that pharmacological blockade of tropicamide-sensitive M4 receptors leads to a time-dependent upregulation of ERK1/2 phosphorylation in the striatum. Since cellular levels of total ERK1/2 proteins were not changed by tropicamide, the tropicamide-stimulated increase in pERK1/2 signals reflects an accelerated rate of phosphorylation of ERK1/2 rather than an increase in an amount of total ERK1/2 proteins.
Figure 3. Effects of tropicamide on phosphorylation and expression of ERK1/2 in the striatum.
A, Effects of tropicamide on phosphorylation and expression of ERK1/2 in the CPu. B, Effects of tropicamide on phosphorylation and expression of ERK1/2 in the NAc. Representative immunoblots are shown to the left of the quantified data. Note that tropicamide induced a significant increase in pERK2 signals in the CPu (F(1,15) = 7.769, P = 0.014) and NAc (F(1,15) = 6.077, P = 0.026), while the antagonist had no effect on ERK2 expression. Rats were given an i.p. injection of vehicle (Veh) or tropicamide (Tro) at 10 mg/kg and were sacrificed 15 or 30 min after drug injection for Western blot analysis of changes in pERK1/2 and ERK1/2 expression. Data are presented as means ± SEM (n = 4–6 per group). *P < 0.05 versus vehicle at the same time point.
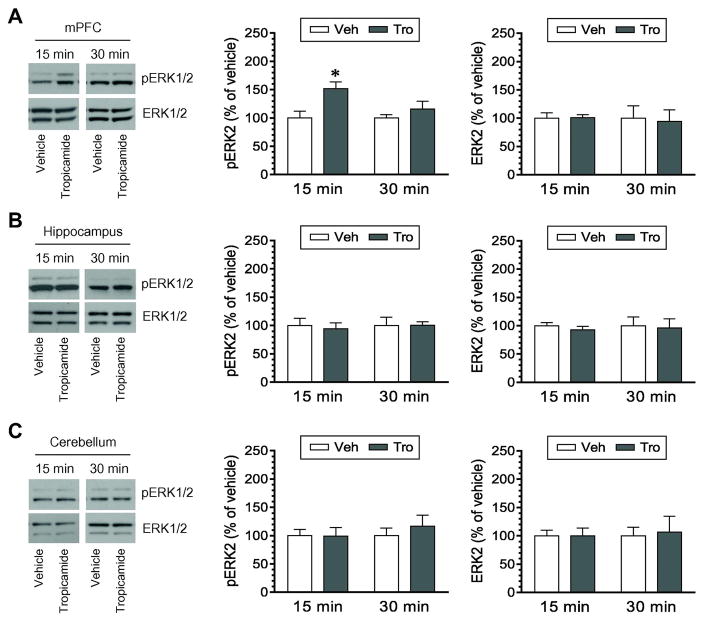
Effects of tropicamide on phosphorylation and expression of ERK1/2 were examined in other three brain regions. Tropicamide increased pERK1/2 levels in the mPFC at 15 min, while it did not at 30 min (Fig. 4A). No significant change in pERK1/2 levels in the hippocampus was observed at the two time points in tropicamide-treated rats compared to vehicle-treated rats (Fig. 4B). Similarly, pERK1/2 levels were not altered in the cerebellum by tropicamide (Fig. 4C). Total ERK1/2 expression remained stable in the three regions after tropicamide injection. Thus, tropicamide is effective in inducing a reliable change in basal ERK1/2 phosphorylation in the mPFC, but not the hippocampus and cerebellum.
Figure 4. Effects of tropicamide on phosphorylation and expression of ERK1/2 in different brain regions.
A, Effects of tropicamide on phosphorylation and expression of ERK1/2 in the mPFC. B, Effects of tropicamide on phosphorylation and expression of ERK1/2 in the hippocampus. C, Effects of tropicamide on phosphorylation and expression of ERK1/2 in the cerebellum. Representative immunoblots are shown to the left of the quantified data. Note that tropicamide induced a significant increase in pERK2 signals in the mPFC (F(1,15) = 8.237, P = 0.012), but not in the hippocampus (F(1,12) = 0.056, P = 0.818) and cerebellum (F(1,12) = 0.433, P = 0.523). Rats were given an i.p. injection of vehicle (Veh) or tropicamide (Tro) at 10 mg/kg and were sacrificed 15 or 30 min after drug injection for Western blot analysis of changes in pERK1/2 and ERK1/2 expression. Data are presented as means ± SEM (n = 4 per group). *P < 0.05 versus vehicle at the same time point.
2.3. Effects of telenzepine on AMPH-stimulated ERK1/2 phosphorylation
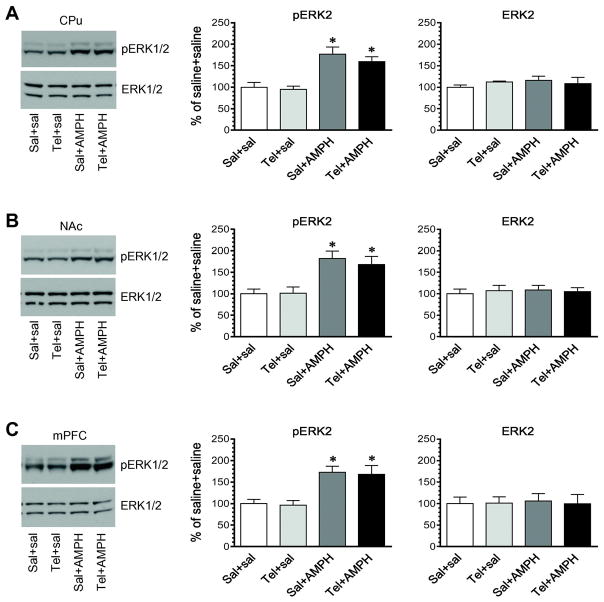
Dopamine stimulation with AMPH is known to stimulate ERK1/2 phosphorylation in the striatum and mPFC (Choe et al., 2002; Choe and Wang, 2002; Valjent et al., 2004; 2005; 2006; Mao et al., 2013). To determine the possible role of M1 receptors in processing ERK1/2 responses to AMPH in striatal and mPFC neurons, we investigated the effect of telenzepine on AMPH-stimulated ERK1/2 phosphorylation in these regions. Telenzepine at 3 mg/kg (i.p.) was administered 5 min prior to a single injection of AMPH at 2.5 mg/kg (i.p.). Rats were then sacrificed 15 min after AMPH injection for Western blot analysis of changes in pERK1/2 levels in the striatum and mPFC. AMPH alone induced a substantial increase in pERK1/2 signals in the CPu (Fig. 5A). In rats pretreated with telenzepine, AMPH elevated pERK1/2 levels to the extent comparable to that induced by AMPH alone. In the NAc, AMPH was effective in producing a moderate increase in ERK1/2 phosphorylation (Fig. 5B). Telenzepine, however, did not affect the effect of AMPH since no change in the amplitude of the AMPH-stimulated ERK1/2 phosphorylation was observed in saline-pretreated rats compared to telenzepine-pretreated rats. In analyzing the mPFC region, we found a significant increase in ERK1/2 phosphorylation in rats treated with saline + AMPH as compared to rats treated with saline + saline (Fig. 5C). This positive response was also observed in rats treated with telenzepine + AMPH. In all three regions, cellular levels of total ERK1/2 proteins showed an insignificant change following administration of telenzepine or AMPH or both. These findings support that activity of telenzepine-sensitive M1 receptors plays an insignificant role in the AMPH-stimulated ERK1/2 phosphorylation in striatal and mPFC neurons.
Figure 5. Effects of telenzepine on AMPH-stimulated phosphorylation of ERK1/2 in the striatum and mPFC.
A, Effects of telenzepine on AMPH-stimulated phosphorylation of ERK1/2 in the CPu. B, Effects of telenzepine on AMPH-stimulated phosphorylation of ERK1/2 in the NAc. C, Effects of telenzepine on AMPH-stimulated phosphorylation of ERK1/2 in the mPFC. Representative immunoblots are shown to the left of the quantified data. Rats were given an i.p. injection of saline (Sal) or telenzepine (Tel, 3 mg/kg) 5 min prior to saline or AMPH (2.5 mg/kg, i.p.) and were sacrificed 15 min after final injection for Western blot analysis of changes in pERK1/2 and ERK1/2 expression. Data are presented as means ± SEM (n = 4 per group) and were analyzed with two-way ANOVA followed by post hoc tests: pERK2 in the CPu (telenzepine: F(1,12) = 0.662, P = 0.432; AMPH: F(1,12) = 33.74, P < 0.0001; interaction: F(1,12) = 0.229, P = 0.641), NAc (telenzepine: F(1,12) = 0.159, P = 0.697; AMPH: F(1,12) = 22.66, P = 0.0005; interaction: F(1,12) = 0.232, P = 0.639), and mPFC (telenzepine: F(1,12) = 0.077, P = 0.787; AMPH: F(1,12) = 24.91, P = 0.0003; interaction: F(1,12) = 0.003, P = 0.954). *P < 0.05 versus saline + saline.
2.4. Effects of tropicamide on AMPH-stimulated ERK1/2 phosphorylation
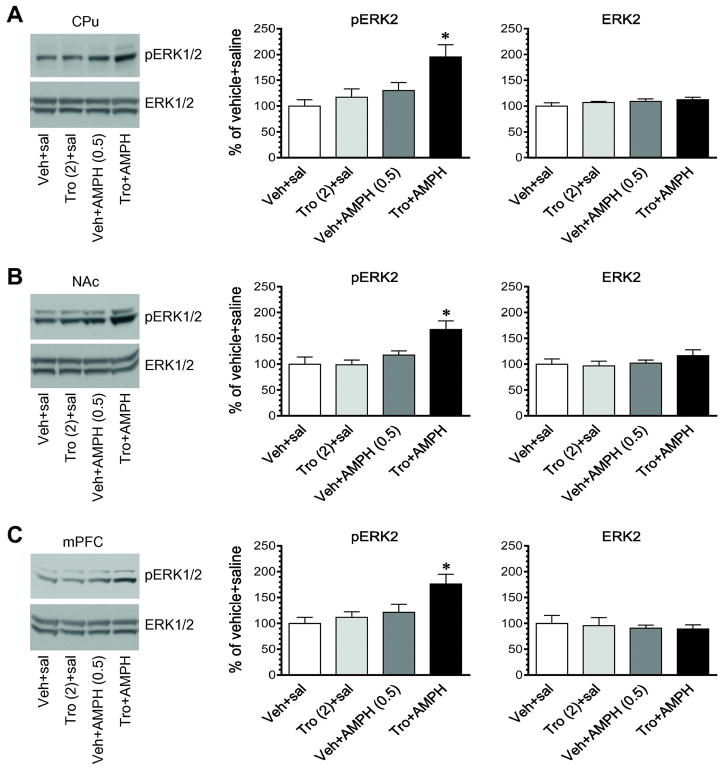
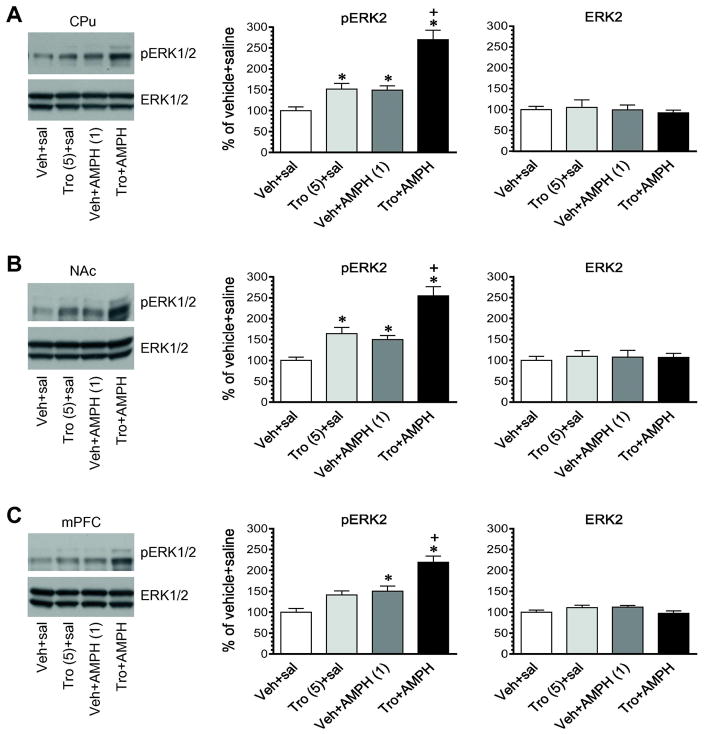
In this study, we determined the effect of coadministration of tropicamide and AMPH on ERK1/2 phosphorylation in the striatum and mPFC. We first administered tropicamide and AMPH at their subthreshold doses (2 mg/kg for tropicamide and 0.5 mg/kg for AMPH). Vehicle or tropicamide was injected alone or 5 min prior to AMPH. Rats were sacrificed 15 min after final injection to assess changes in ERK1/2 phosphorylation. Tropicamide alone did not alter pERK1/2 expression in the CPu (Fig. 6A). Neither did AMPH alone. Remarkably, when the two drugs were coadministered, a reliable increase in pERK1/2 levels was seen in this region. Similar results were seen in the NAc (Fig. 6B) and mPFC (Fig. 6C). There was no significant change in total ERK1/2 expression in all three regions after administration of AMPH in the presence or absence of tropicamide. To substantiate the effect of two drugs in a coadministration regimen, we administered these drugs at higher doses (5 mg/kg for tropicamide and 1 mg/kg for AMPH). In the CPu and NAc, tropicamide alone induced a significant increase in ERK1/2 phosphorylation (Fig. 7A and 7B). AMPH alone also significantly elevated pERK1/2 levels in these regions. Coadministration of tropicamide and AMPH induced a greater increase in ERK1/2 phosphorylation than either drug alone. The pERK2 level in rats treated with tropicamide + AMPH was significantly higher than that in rats treated with tropicamide + saline and vehicle + AMPH (p < 0.05). Similar results were observed in the mPFC (Fig. 7C). Total ERK1/2 protein levels remained stable in three regions in response to all drug treatments. These results demonstrate that coadministration of tropicamide and AMPH can produce a greater increase in ERK1/2 phosphorylation than each drug alone in striatal and mPFC neurons.
Figure 6. Effects of coadministration of tropicamide and AMPH at the subthreshold doses on phosphorylation and expression of ERK1/2 in the striatum and mPFC.
A, Effects of coadministered tropicamide and AMPH on phosphorylation and expression of ERK1/2 in the CPu. B, Effects of coadministered tropicamide and AMPH on phosphorylation and expression of ERK1/2 in the NAc. C, Effects of coadministered tropicamide and AMPH on phosphorylation and expression of ERK1/2 in the mPFC. Representative immunoblots are shown to the left of the quantified data. Note that coadministration of tropicamide and AMPH at subthreshold doses induced a significant increase in pERK2 signals in the CPu (A), NAc (B), and mPFC (C). Rats were given an i.p. injection of vehicle (Veh) or tropicamide (Tro, 2 mg/kg) 5 min prior to saline or AMPH (0.5 mg/kg, i.p.) and were sacrificed 15 min after final injection for Western blot analysis of changes in pERK1/2 and ERK1/2 expression. Data are presented as means ± SEM (n = 4 per group) and were analyzed with two-way ANOVA followed by post hoc tests: pERK2 in the CPu (tropicamide: F(1,12) = 5.628, P = 0.035; AMPH: F(1,12) = 9.835, P = 0.0086; interaction: F(1,12) = 1.93, P = 0.19), NAc (tropicamide: F(1,12) = 4.577, P = 0.054; AMPH: F(1,12) = 13.34, P = 0.003; interaction: F(1,12) = 5.003, P = 0.045), and mPFC (tropicamide: F(1,12) = 5.216, P = 0.041; AMPH: F(1,12) = 8.697, P = 0.012; interaction: F(1,12) = 2.32, P = 0.154). *p < 0.05 versus vehicle + saline.
Figure 7. Effects of coadministered tropicamide and AMPH at the effective doses on phosphorylation and expression of ERK1/2 in the striatum and mPFC.
A, Effects of tropicamide and AMPH on phosphorylation and expression of ERK1/2 in the CPu. B, Effects of tropicamide and AMPH on phosphorylation and expression of ERK1/2 in the NAc. C, Effects of tropicamide and AMPH on phosphorylation and expression of ERK1/2 in the mPFC. Representative immunoblots are shown to the left of the quantified data. Rats were given an i.p. injection of vehicle (Veh) or tropicamide (Tro, 5 mg/kg) 5 min prior to saline or AMPH (1 mg/kg, i.p.) and were sacrificed 15 min after final injection for Western blot analysis of changes in pERK1/2 and ERK1/2 expression. Data are presented as means ± SEM (n = 4 per group) and were analyzed with two-way ANOVA followed by post hoc tests: pERK2 in the CPu (tropicamide: F(1,12) = 58.62, P < 0.0001; AMPH: F(1,12) = 56.00, P < 0.0001; interaction: F(1,12) = 9.56, P = 0.0093), NAc (tropicamide: F(1,12) = 39.31, P < 0.0001; AMPH: F(1,12) = 32.00, P = 0.0001; interaction: F(1,12) = 1.928, P = 0.19), and mPFC (tropicamide: F(1,12) = 23.73, P = 0.0004; AMPH: F(1,12) = 32.57, P < 0.0001; interaction: F(1,12) = 1.532, P = 0.24). *p < 0.05 versus vehicle + saline, and +p < 0.05 versus tropicamide + saline and vehicle + AMPH.
3. Discussion
We investigated the role of mACh receptor subtypes in the regulation of ERK1/2 phosphorylation in the adult rat brain in vivo. Pharmacological blockade of M1 receptors with the antagonist telenzepine did not alter ERK1/2 phosphorylation in the striatum, mPFC, hippocampus and cerebellum. Moreover, telenzepine was ineffective in affecting AMPH-stimulated ERK1/2 phosphorylation. Interestingly, the M4 antagonist tropicamide upregulated ERK1/2 phosphorylation in the striatum (CPu and NAc) and mPFC. This effect was rapid and transient. When coadministered at subthreshold doses, tropicamide and AMPH induced an increase in striatal and cortical ERK1/2 phosphorylation. These results demonstrate that M4 receptors play a significant role in inhibition of basal and dopamine-stimulated ERK1/2 phosphorylation in striatal and mPFC neurons. Blockade of M4 receptors therefore upregulates ERK1/2 phosphorylation under normal and AMPH-stimulated conditions.
Telenzepine exhibited the preferential affinity for M1 receptors relative to other mACh receptor subtypes (Doods et al., 1987; Ztaou et al., 2016). This agent was also 4–10 times more potent than the widely used M1 antagonist pirenzepine (Eltze et al., 1985). Thus, telenzepine is being frequently used to clarify the functional role of M1 receptors in vivo (Tanda et al., 2007; Ztaou et al., 2016; Joseph and Thomsen, 2017). Indeed, an i.p. injection of telenzepine at 3 mg/kg reduced the motor deficits in 6-hydroxydopamine (6-OHDA)-lesioned parkinsonian mice (Ztaou et al., 2016). Subcutaneous administration of telenzepine at 3 mg/kg, which alone did not alter the dopamine concentration in the rat mPFC, completely blocked cortical dopamine release induced by the M1 agonist N-desmethylclozapine (Li et al., 2005; 2009). In the striatum where M1 receptors are a major mACh receptor subtype and are expressed postsynaptically in striatal medium spiny projection neurons (Hersch et al., 1994; Alcantara et al., 2001), we found that telenzepine at 3 mg/kg did not alter ERK1/2 phosphorylation. Thus, M1 receptors may not be involved in the regulation of normal ERK1/2 phosphorylation under basal conditions. Moreover, telenzepine had no effect on the AMPH-stimulated ERK1/2 phosphorylation. Thus, M1 receptors appear to be insignificantly implicated in the AMPH effect on ERK1/2 phosphorylation.
An important finding in this study is that the M4 antagonist tropicamide enhanced basal ERK1/2 phosphorylation in the striatum. Tropicamide has been reported to suppress parkinsonian tremor in a rodent model of Parkinson’s disease (Betz et al., 2007). Similarly, tropicamide after an i.p. injection at 10 mg/kg reduced motor deficits in 6-OHDA-lesioned parkinsonian mice, while tropicamide at this dose lacked the effect on spontaneous locomotion in normal mice (Ztaou et al., 2016). Of note, the tropicamide effect in alleviating motor deficits was lost in mutant mice that express no M4 receptors in D1-bearing neurons (Ztaou et al., 2016). This validates the role of M4 receptors in striatal projection neurons in mediating the effect of tropicamide. In our study, tropicamide was found to be effective in upregulating ERK1/2 phosphorylation in striatal neurons. Thus, M4 receptors are involved in an inhibitory regulation of normal ERK1/2 phosphorylation under basal conditions. Since M4 receptors are predominantly expressed in D1-expressing striatonigral projection neurons (Ince et al., 1997; Santiago and Potter, 2001), tropicamide is believed to exert a primary impact on this phenotype of projection neurons in terms of ERK1/2 phosphorylation.
In addition to the role in determining basal ERK1/2 phosphorylation, M4 receptors noticeably regulate the dopamine-stimulated ERK1/2 phosphorylation. In the presence of tropicamide, AMPH at a subthreshold dose ineffective to stimulate ERK1/2 phosphorylation by itself induced a significant increase in ERK1/2 phosphorylation in the striatum (this study). Thus, the pronounced positive interaction exists between tropicamide- and AMPH-associated systems in an activity-dependent manner. Dopamine stimulation with indirect agonists (cocaine and AMPH) is known to stimulate ERK1/2 phosphorylation in the striatum (Valjent et al., 2000; 2004; 2005; 2006; Choe et al., 2002; Choe and Wang, 2002; Zhang et al., 2004; Jenab et al., 2005). This induction of ERK1/2 phosphorylation was characterized by occurring in a subset of projection neurons, i.e., striatonigral neurons (Valjent et al., 2005; Bertran-Gonzalez et al., 2008), through stimulation of their D1 receptors (Valjent et al., 2000; 2005; Bertran-Gonzalez et al., 2008; Shi and McGinty, 2011). Given the fact that both D1 and M4 receptors are expressed predominantly in striatonigral neurons (Ince et al., 1997; Santiago and Potter, 2001), tropicamide and AMPH are believed to target M4 and D1 receptors in striatonigral neurons, respectively, to achieve the combined effect. At the postreceptor level, Gs-coupled D1 receptors and Gi/o-coupled M4 receptors stimulate and inhibit adenylyl cyclase, respectively, leading to an opposite outcome of the downstream cAMP-PKA activity (Wess, 1996). These different effects on the same signaling transduction pathway by D1 and M4 receptors may account for the different regulation of ERK1/2 phosphorylation by two receptors. In fact, dopamine and acetylcholine are two principal transmitters in the local striatum. They are traditionally viewed to work in concert to form a dynamic balance to control intrinsic homeostats of striatal neurons and outflow of the direct and indirect pathways (Di Chiara et al., 1994; Centonze et al., 2003), although underlying molecular mechanisms are poorly understood. The M4-mediated regulation of dopamine-stimulated ERK1/2 phosphorylation observed in this study provides evidence for an additional layer of molecular mechanism underlying the striatal dopamine-acetylcholine interaction. Consistent with this model, Shen et al. (2015) found that enhancing endogenous M4 signaling with the M4 selective positive allosteric modulator (PAM) VU10010 blocked D1 receptor-dependent long-term potentiation in striatonigral neurons. Similarly, the M4 PAM VU0152100 suppressed the D1 agonist-stimulated phosphorylation of Fyn, NMDA receptor GluN2B subunits, and AMPA receptor GluA1 subunits in the rat striatum (Mao and Wang, 2015; Xue et al., 2017) and reduced cocaine-stimulated hyperlocomotion and cocaine self-administration (Dencker et al., 2012). Selective deletion of M4 receptors in D1 receptor-bearing neurons augmented behavioral responses to dopamine stimulants and reduced the effect of VU0152100 (Jeon et al., 2010; Dencker et al., 2012), indicating that the effect of PAM was in part mediated via M4 receptors that are co-localized with D1 receptors. At the adenylyl cyclase level, the non-selective cholinergic receptor agonist carbachol inhibited the response of adenylyl cyclase to the D1 agonist in the rat nucleus accumbens. This carbachol inhibition was blocked by muscarinic toxin 3, an M4 selective antagonist, but not muscarinic toxin 7, an M1 selective antagonist (Onali and Olianas, 2002).
M4 receptors are present in the mPFC and hippocampus at a middle-to-high level, while the cerebellum contains the smallest number of M4 receptors compared to other brain regions (Levey et al., 1991; Yasuda et al., 1993; Tice et al., 1996; Volpicelli and Levey, 2004; Chapman et al., 2011). In this study, among the three regions surveyed outside the striatum, we found a significant response of ERK1/2 to tropicamide in the mPFC although not in the hippocampus and cerebellum. In details, tropicamide when injected alone increased basal ERK1/2 phosphorylation in the mPFC. When coadministered with AMPH, tropicamide and AMPH induced a greater increase in ERK1/2 phosphorylation than the effect induced by either drug alone. Thus, in addition to the striatum, the mPFC is another dopamine responsive site in the limbic system where basal and dopamine-stimulated ERK1/2 phosphorylation is subject to the regulation by M4 receptors.
4. Experimental procedures
4.1. Animals
In this study, male Wistar rats weighting 250–330 g (Charles River, New York, NY) were used. All animals were individually housed with food and water available ad libitum. Animal rooms were kept at 23°C and humidity of 50 ± 10% on a 12/12 h light/dark cycle with lights on at 0700. All animal use procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
4.2. Drug administration and experimental arrangements
Rats were randomly divided into different groups and were given an i.p. injection of drugs at a volume of ~ 0.5 ml. The dose of all agents was calculated as the salt. Age-matched rats were given an i.p. injection of saline or vehicle (~ 0.5 ml) and served as a control. In the first study, rats were injected with the M1-preferring antagonist telenzepine at a dose of 3 mg/kg and were sacrificed 15 or 30 min after drug injection. The different brain regions, including the CPu, NAc, mPFC, hippocampus and cerebellum, were dissected for Western blot analysis of changes in ERK phosphorylation in responses to telenzepine. In the second study, an M4-preferring antagonist tropicamide was used to test the effect of blockade of M4 receptors on ERK phosphorylation in the CPu, NAc, mPFC, hippocampus and cerebellum. Rats were injected with tropicamide (10 mg/kg, i.p.) and were sacrificed 15 or 30 min after drug injection. In the third study, the effect of telenzepine on the AMPH-stimulated ERK phosphorylation was investigated in the CPu, NAc, and mPFC. Telenzepine was administered at 3 mg/kg (i.p.) 5 min prior to an i.p. injection of AMPH (2.5 mg/kg). Rats were sacrificed 15 min after AMPH injection for Western blot analysis of the effect of telenzepine. In the fourth study, we evaluated the effect of tropicamide on the AMPH-stimulated ERK phosphorylation in the CPu, NAc, and mPFC. Tropicamide at a dose of 2 or 5 mg/kg (i.p.) was administered 5 min prior to AMPH (0.5 or 1 mg/kg). Rats were sacrificed 15 min after AMPH injection for the following Western blot analysis.
4.3. Sample preparation and Western blot analysis
After anesthetized with sodium pentobarbital (65 mg/kg, i.p.), rats were sacrificed by decapitation. Rat brains were removed and cut into coronal slices (~1 mm). Different brain regions of interest on coronal slices were dissected on an ice-cold dissection plate according to the atlas (Paxinos and Watson, 1997). These regions include the CPu, NAc, mPFC, hippocampus and cerebellum. Brain tissue was lysed in RIPA (radioimmunoprecipitation assay) buffer containing 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 1 μg/ml leupeptin (Cell Signaling Technology, Danvers, MA). Lysates were centrifuged at 800 g (10 min, 4°C). Supernatants were collected for immunoblot. Protein concentrations were determined. Samples were stored at −80°C until use.
Western blot analysis was conducted according to our previous work (Mao et al., 2013; 2015). Briefly, brain samples were separated on SDS NuPAGE 4–12% Bis-Tris gels (Invitrogen, Carlsbad, CA). Separated proteins on gel were transferred to polyvinylidene fluoride membranes, which were then incubated in a solution containing a primary antibody overnight at 4°C. After this step, membranes were incubated in a solution containing a secondary antibody (1:2,000). Immunoblots from membranes were developed with the enhanced chemiluminescence reagent (GE Healthcare Life Sciences, Piscataway, NJ). Immunoblots on films were measured using NIH ImageJ gel analysis software. β-Actin was used as a loading control and pERK2 and ERK2 signals were normalized to β-actin in Western blot analysis.
4.4. Antibodies and pharmacological agents
In this study, we used rabbit antibodies against pERK1/2 at Thr202 and Tyr204 (Cell Signaling Technology, Beverly, MA), ERK1/2 (Cell Signaling), or β-actin (1:5000, Sigma-Aldrich, St. Louis, MO). Pharmacological agents include D-amphetamine sulfate, telenzepine dihydrochloride hydrate, and tropicamide which were purchased from Sigma-Aldrich (St. Louis, MO). AMPH and telenzepine were dissolved in physiological saline. Tropicamide was dissolved in a small volume of dimethyl sulfoxide and was then diluted in physiological saline. All agents were freshly prepared at the day of experiments.
4.5. Statistics
In this investigation, data are presented as means ± SEM. These data were evaluated using one- or two-way analysis of variance (ANOVA) followed by a Bonferroni (Dunn) comparison of groups. Probability levels of < 0.05 were considered statistically significant.
Highlights.
The M4R antagonist tropicamide increases ERK phosphorylation in the rat striatum.
Tropicamide also potentiate ERK responses to amphetamine in the striatum and prefrontal cortex.
The M1R antagonist telenzepine did not alter striatal and cortical ERK phosphorylation.
Telenzepine had no effect on amphetamine-stimulated ERK phosphorylation.
Acknowledgments
This work was supported by NIH grants DA10355 (J.Q.W.) and MH61469 (J.Q.W.).
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcantara AA, Mrzljak L, Jakab RL, Levey AI, Hersch SM, Goldman-Rakic PS. Muscarinic m1 and m2 receptor proteins in local circuit and projection neurons of the primate striatum: anatomical evidence for cholinergic modulation of glutamatergic prefronto-striatal pathways. J Comp Neurol. 2001;434:445–460. doi: 10.1002/cne.1186. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz AJ, McLaughlin PJ, Burgos M, Weber SM, Salamone JD. The muscarinic receptor antagonist tropicamide suppresses tremulous jaw movements in a rodent model of parkinsonian tremor: possible role of M4 receptors. Psychopharmacology. 2007;194:347–359. doi: 10.1007/s00213-007-0844-6. [DOI] [PubMed] [Google Scholar]
- Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- Centonze D, Gubellini P, Pisani A, Bernardi G, Calabresi P. Dopamine, acetylcholine and nitric oxide systems interact to induce corticostriatal synaptic plasticity. Rev Neurosci. 2003;14:207–216. doi: 10.1515/revneuro.2003.14.3.207. [DOI] [PubMed] [Google Scholar]
- Chang HT, Wilson CJ, Kitai ST. A Golgi study of rat neostriatal neurons: light microscopic analysis. J Comp Neurol. 1982;208:107–126. doi: 10.1002/cne.902080202. [DOI] [PubMed] [Google Scholar]
- Chapman KL, Vaswani D, Hendry N, Langmead CJ, Kew JN, Watson JM. The muscarinic M(4) receptor is the functionally predominant subtype in rat and mouse striatum as demonstrated using [(35)S] GTPγS binding. Eur J Pharmacol. 2011;652:1–6. doi: 10.1016/j.ejphar.2010.10.079. [DOI] [PubMed] [Google Scholar]
- Choe ES, Chung KT, Mao L, Wang JQ. Amphetamine increases phosphorylation of extracellular signal-regulated kinase and transcription factors in the rat striatum via group I metabotropic glutamate receptors. Neuropsychopharmacology. 2002;27:565–575. doi: 10.1016/S0893-133X(02)00341-X. [DOI] [PubMed] [Google Scholar]
- Choe ES, Wang JQ. CaMKII regulates amphetamine-induced ERK1/2 phosphorylation in striatal neurons. NeuroReport. 2002;13:1013–1016. doi: 10.1097/00001756-200206120-00006. [DOI] [PubMed] [Google Scholar]
- Dencher D, Weikop P, Sorensen G, Woldbye DP, Wortwein G, Wess J, Fink-Jensen A. An allosteric enhancer of M4 muscarinic acetylcholine receptor function inhibits behavioral and neurochemical effects of cocaine. Psychopharmacology. 2012;224:277–287. doi: 10.1007/s00213-012-2751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Doods HN, Mathy MJ, Davidesko D, van Charldorp KJ, de Jonge A, van Zwieten PA. Selectivity of muscarinic antagonists in radioligand and in vivo experiments for the putative M1, M2 and M3 receptors. J Pharmacol Exp Ther. 1987;242:257–262. [PubMed] [Google Scholar]
- Eltze M, Gonne S, Riedel R, Schlotke B, Schudt C, Simon WA. Pharmacological evidence for selective inhibition of gastric acid secretion by telenzepine, a new antimuscarinic drug. Eur J Pharmacol. 1985;112:211–224. doi: 10.1016/0014-2999(85)90498-4. [DOI] [PubMed] [Google Scholar]
- Graveland GA, DiFiglia M. The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res. 1985;327:307–311. doi: 10.1016/0006-8993(85)91524-0. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of m1–m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince E, Ciliax BJ, Levey AI. Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse. 1997;27:357–366. doi: 10.1002/(SICI)1098-2396(199712)27:4<357::AID-SYN9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Jenab S, Festa ED, Nazarian A, Wu HB, Sun WL, Hazim R, Russo SJ, Quinones-Jenab V. Cocaine induction of ERK proteins in dorsal striatum of Fischer rats. Mol Brain Res. 2005;142:134–138. doi: 10.1016/j.molbrainres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Jeon J, Dencker D, Wortwein G, Woldbye DP, Cui Y, Davis AA, Levey AI, Schutz G, Sager TN, Mork A, Li C, Deng CX, Fink-Jensen A, Wess J. A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors. J Neurosci. 2010;30:2396–2405. doi: 10.1523/JNEUROSCI.3843-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph L, Thomsen M. Effects of muscarinic receptor antagonists on cocaine discrimination in wild-type mice and in muscarinic receptor M1, M2, and M4 receptor knockout mice. Beha Brain Res. 2017;329:75–83. doi: 10.1016/j.bbr.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzafame AA, Christopoulos A, Mitchelson F. Cellular signaling mechanisms for muscarinic acetylcholine receptors. Receptors Channels. 2003;9:241–260. [PubMed] [Google Scholar]
- Lazareno S, Buckley NJ, Roberts F. Characterization of muscarinic m4 binding sites in rabbit lung, chicken heart and NG108-15 cells. Mol Pharmacol. 1990;38:805–815. [PubMed] [Google Scholar]
- Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Huang M, Ichikawa J, Dai J, Meltzer HY. N-Desmethylclozapine, a major metabolite of clozapine, increases cortical acetylcholine and dopamine release in vivo via stimulation of M1 muscarinic receptors. Neuropsychopharmacology. 2005;30:1986–1995. doi: 10.1038/sj.npp.1300768. [DOI] [PubMed] [Google Scholar]
- Li Z, Prus AJ, Dai J, Meltzer HY. Differential effects of M1 nad 5-hydroxytryptamine1A receptors on atypical antipsychotic drug-induced dopamine efflux in the medial prefrontal cortex. J Pharmacol Exp Ther. 2009;330:948–955. doi: 10.1124/jpet.109.155663. [DOI] [PubMed] [Google Scholar]
- Mao LM, Reusch JM, Fibuch EE, Liu Z, Wang JQ. Amphetamine increases phosphorylation of MAPK/ERK at synaptic sites in the rat striatum and medial prefrontal cortex. Brain Res. 2013;1494:101–108. doi: 10.1016/j.brainres.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Wang JQ. Dopaminergic and cholinergic regulation of Fyn tyrosine kinase phosphorylation in the rat striatum in vivo. Neuropharmacology. 2015;99:491–499. doi: 10.1016/j.neuropharm.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki K, Nishimura M, Hashimoto N. Mitogen-activated protein kinases and cerebral ischemia. Mol Neurobiol. 2001;23:1–19. doi: 10.1385/MN:23:1:01. [DOI] [PubMed] [Google Scholar]
- Onali P, Olianas MC. Muscarinic M4 receptor inhibition of dopamine D1-like receptor signaling in rat nucleus accumbens. Eur J Pharmacol. 2002;448:105–111. doi: 10.1016/s0014-2999(02)01910-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in sterrotaxic coordinates. Academic; New York: 1997. [Google Scholar]
- Pearson G, Robinson F, Gibson BT, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation of physiological functions. Endo Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Santiago MP, Potter LT. Biotinylated m4-toxin demonstrates more M4 muscarinic receptor protein on direct than indirect striatal projection neurons. Brain Res. 2001;894:12–20. doi: 10.1016/s0006-8993(00)03170-x. [DOI] [PubMed] [Google Scholar]
- Shen W, Plotkin JL, Francardo V, Ko WK, Xie Z, Li Q, Fieblinger T, Wess J, Neubig RR, Lindsley CW, Conn PJ, Greengard P, Bezard E, Cenci MA, Surmeier DJ. Neuron. 2015;88:762–773. doi: 10.1016/j.neuron.2015.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, McGinty JF. D1 and D2 dopamine receptors differentially mediate the activation of phosphoproteins in the striatum of amphetamine-sensitized rats. Psychopharmacology. 2011;214:653–663. doi: 10.1007/s00213-010-2068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Tanda G, Ebbs AL, Kopajtic TA, Elias LM, Campbell BL, Newman AH, Katz JL. Effects of muscarinic M1 receptor blockade on cocaine-induced elevations of brain dopamine levels and locomotor behavior in rats. J Pharmacol Exp Ther. 2007;321:334–344. doi: 10.1124/jpet.106.118067. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signaling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Tice MAB, Hashemi T, Taylor LA, McQuade RD. Distribution of muscarinic receptor subtypes in rat brain from postnatal to old age. Dev Brain Res. 1996;92:70–76. doi: 10.1016/0165-3806(95)01515-9. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006;7:20. doi: 10.1186/1471-2202-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volmat V, Pouyssegur J. Spatiotemporal regulation of the p42/p44 MAPK pathway. Biol Cell. 2001;93:71–79. doi: 10.1016/s0248-4900(01)01129-7. [DOI] [PubMed] [Google Scholar]
- Volpicelli LA, Levey AI. Muscarinic acetylcholine receptor subtypes in cerebral cortex and hippocampus. Prog Brain Res. 2004;145:59–66. doi: 10.1016/S0079-6123(03)45003-6. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Fibuch EE, Mao LM. Regulation of mitogen-activated protein kinases by glutamate receptors. J Neurochem. 2007;100:1–11. doi: 10.1111/j.1471-4159.2006.04208.x. [DOI] [PubMed] [Google Scholar]
- Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- Xue B, Chen EC, He N, Jin DZ, Mao LM, Wang JQ. Integrated regulation of AMPA glutamate receptor phosphorylation in the striatum by dopamine and acetylcholine. Neuropharmacology. 2017;112:57–65. doi: 10.1016/j.neuropharm.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda RP, Ciesla W, Flores LR, Wall SJ, Li M, Satkus SA, Weisstein JS, Spagnola BV, Wolfe BB. Development of antisera selective for m4 and m5 muscarinic cholinergic receptors: distribution of m4 and m5 receptors in rat brain. Mol Pharmacol. 1993;43:149–157. [PubMed] [Google Scholar]
- Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ztaou S, Maurice N, Camon J, Guiraudie-Capraz G, Kerkerian-Le Goff L, Beurrier C, Liberge M, Amalric M. Involvement of striatal cholinergic interneurons and M1 and M4 muscarinic receptors in motor symptoms of Parkinson’s disease. J Neurosci. 2016;36:9161–9172. doi: 10.1523/JNEUROSCI.0873-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]