Abstract
Rab proteins represent the largest branch of the Ras-like small GTPase superfamily and there are 66 Rab genes in the human genome. They alternate between GTP- and GDP-bound states, which are facilitated by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs), and function as molecular switches in regulation of intracellular membrane trafficking in all eukaryotic cells. Each Rab targets to an organelle and specify a transport step along exocytic, endocytic, and recycling pathways as well as the crosstalk between these pathways. Through interactions with multiple effectors temporally, a Rab can control membrane budding and formation of transport vesicles, vesicle movement along cytoskeleton, and membrane fusion at the target compartment. The large number of Rab proteins reflects the complexity of the intracellular transport system, which is essential for the localization and function of membrane and secretory proteins such as hormones, growth factors, and their membrane receptors. As such, Rab proteins have emerged as important regulators for signal transduction, cell growth, and differentiation. Altered Rab expression and/or activity have been implicated in diseases ranging from neurological disorders, diabetes to cancer.
Keywords: Rab, GTPase, GTP-binding protein, Membrane trafficking, Vesicular transport, GAP, GEF, Effector
1 Introduction
Rab GTPases play an important role in specifying transport pathways in the intracellular membrane trafficking system of all eukaryotes from the last eukaryotic common ancestor (LECA) to mammals. In the LECA, there are at least 20 prototype Rabs forming six groups, e.g., Rab1/Ypt1 and Rab8/Sec4 in group I, Rab5/Ypt51 in group II, Rab7/Ypt7 and Rab9/Ypt9 in group III, Rab11/Ypt31 and Rab4/Ypt4 in group IV, Rab6/Ypt6 in group V, and Rab28 in group VI [1, 2] (Fig. 1). Most of these ancient Rabs are conserved throughout evolution while some are lost in certain species. There is significant expansion of the Rab family in mammalian cells to accommodate increasing complexity of the intracellular trafficking system, with 66 Rab genes in the human genome. Historically Rab GTPases are best characterized in the budding yeast Saccharomyces cerevisiae (S. cerevisiae) and in mammalian cells [3], and evolutionarily older Rabs tend to be more highly and widely expressed and more intensively studied [4]. This volume focuses on the techniques used for biochemical and functional characterizations of these S. cerevisiae and mammalian Rabs.
Fig. 1.
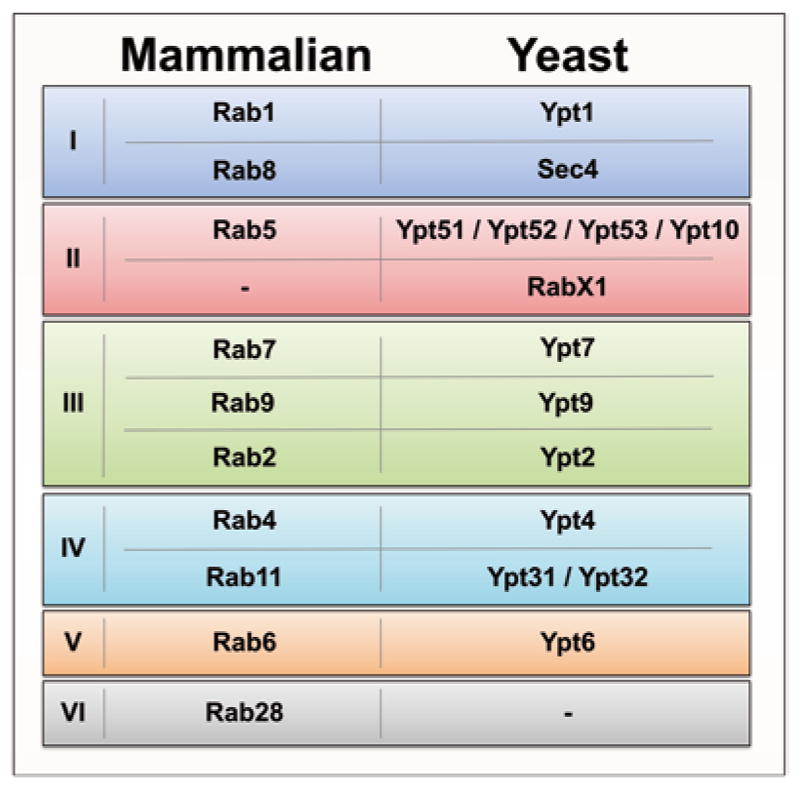
The mammalian and yeast Rab/Ypt homologs. Data from Diekmann et al. and Klöpper et al. [1, 2] reveal six major groups of Rab GTPases from the LECA to mammals. Shown are only the mammalian Rabs with yeast Rab/Ypt homologs. RabX1 is not present in mammalian cells, while there are no members of group VI, including Rab28, found in yeast
The intracellular membrane trafficking system governs protein secretion during exocytosis and uptake of extracellular nutrients during endocytosis in eukaryotic cells. It is also a fundamental transport system for targeting newly synthesized enzymes to correct membrane compartments/organelles, e.g., the lysosomal hydrolases. As such, it is essential for cell physiology. Intracellular membrane trafficking is mediated by vesicular carriers from donor to acceptor compartments and Rab GTPases are involved in every facet of the vesicular transport process via temporal and spatial interactions with a series of effectors [5, 3, 6]. These effectors include cargo proteins to be packaged into the vesicles, motor proteins that facilitate the movement of vesicles along actin and microtubule cytoskeletons, and tethering factors that dock vesicles to target compartments for membrane fusion.
Each Rab specifically targets to a distinct membrane compartment [7], e.g., Rab2 to the transport vesicles between the endoplasmic reticulum (ER) and the Golgi, Rab5 to early endosomes, Rab7 to late endosomes, etc. (Fig. 2). This membrane targeting process requires posttranslational isoprenylation (geranylgeranylation) of the two Cys residues at or near the C-terminus of each Rab as well as a cognate guanine nucleotide exchange factor (GEF) on the target membrane [8] (Fig. 3). In addition, a GDP displacement factor (GDF) is shown to facilitate Rab membrane targeting to endosomes [9] (Fig. 3). Upon membrane association, the GEF catalyzes nucleotide exchange of GDP with GTP on the Rab [10, 11], and the activated GTP-bound Rab then interacts with effectors and is packaged into transport vesicles to mediate the formation and movement of vesicles and their fusion with the target compartment (Fig. 3). The Rab functional cycle is completed by GTP hydrolysis, which is catalyzed by GTPase-activating proteins (GAPs) [12], and recycling back to the donor compartment, which is mediated by the GDP-dissociation inhibitor (GDI) [13–15] (Fig. 3).
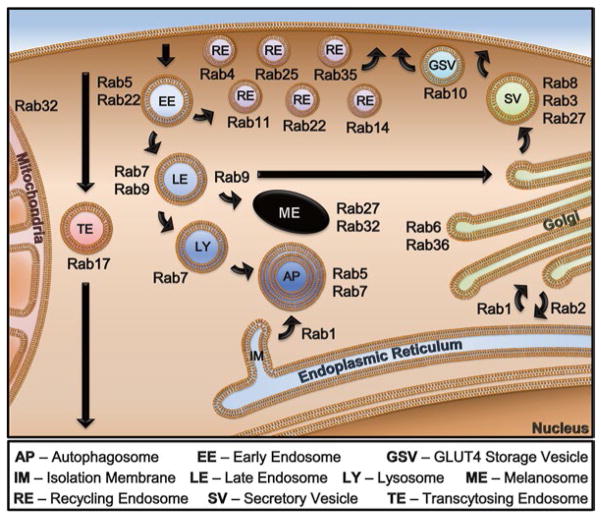
Fig. 2.
Rabs throughout the mammalian cell. Rabs are found in virtually every membranous compartment in eukaryotic cells. Above is a schematic representation of intracellular localization of the Rabs from Fig. 1 and discussed in this volume of MiMB
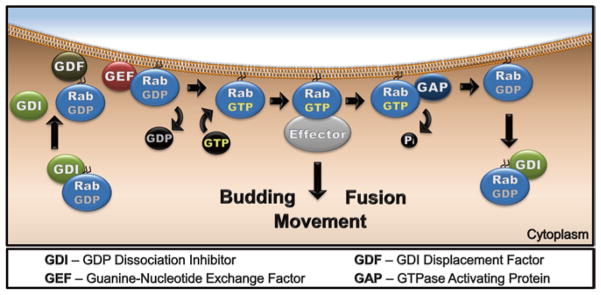
Fig. 3.
The Rab GTPase cycle coupled with membrane targeting. Inactive GDP-bound Rabs are found in the cytosol bound to GDI. Upon approaching the target membrane, GDF may interact with the Rab to facilitate GDI dissociation and Rab insertion into the membrane. On the membrane, GEF catalyzes GDP dissociation, allowing for GTP binding and subsequent activation of the Rab, which in turn interacts with multiple effectors to promote vesicle budding, movement, and fusion. Then GTP hydrolysis by the Rab, accelerated by a cognate GAP, converts it to inactive GDP-bound state. The inactive Rab can be removed from the membrane by GDI and recycled back to the donor compartment
2 Regulation of Rab GTPase Cycle
Like other small GTPases in the Ras superfamily, Rabs show high affinity for guanine nucleotides GTP and GDP (Kd in the nanomolar range) but weak intrinsic GTPase activity in GTP hydrolysis. As a result, both the GDP/GTP exchange reaction and the GTP hydrolysis reaction in a Rab GTPase cycle are accelerated by catalyzing protein factors such as GEFs and GAPs in the cell [12] (Fig. 3).
Rab GEFs show a general mechanism by displacing the switch I region, disrupting Mg2+ coordination, and stabilizing the nucleotide-free form of Rab proteins [12]. As such, the GEFs facilitate GDP dissociation and GTP loading on Rabs in the cell where GTP concentration is two orders of magnitude higher. However, the five families of Rab GEFs identified so far share no sequence and structural homology in the catalytic domain. The Vps9 domain-containing GEFs are specific for the Rab5 subfamily members on early endosomes [16] while the SAND1/Mon1-Ccz1 complex is a specific GEF for Rab7/Ypt7 on late endosomes [17, 18]. These endosomal GEFs promote endocytosis by activation of Rab5 and Rab7. For exocytosis, there are TRAPP complexes [19, 20] and Sec2/Rabin8 proteins [21] that are GEFs for Rab1/Ypt1 and Rab8/Sec4 to promote ER to Golgi transport and post-Golgi transport to the plasma membrane, respectively. In addition, the Ric1/Rgp1 complex is a GEF for Ypt6/Rab6 in the Golgi complex [22]. Finally, the DENN (differentially expressed normal vs. neoplastic) domain-containing GEFs [23] are specific for various Rabs that have no close yeast homologs, such as Rab3, Rab9, Rab10, Rab12, Rab27, Rab28, Rab35, and Rab39.
Rab GAPs, in contrast, contain a TBC (Tre-2/Bud2/Cdc16) domain for catalysis of GTP hydrolysis [12]. The TBC domain contains conserved catalytic motifs IxxDxxR and YxQ from which the Arg and Gln side chains insert into the GTP-binding site on the Rab to stabilize the transition state for GTP hydrolysis in a so-called dual finger mechanism [24].
The GEFs and GAPs are recruited to distinct organelles by proteins and lipids characteristic to each organelle to facilitate the establishment of functional Rab domains on the membrane. In a number of cases, a GEF is recruited to the membrane by an upstream Rab for activation of a downstream Rab, forming a Rab cascade (Fig. 4). For example, Sec2, a GEF for Sec4 in the budding yeast S. cerevisiae, is an effector of the upstream Rab Ypt32 and is recruited by Ypt32-GTP to post-Golgi vesicles for activation of Sec4 in exocytosis [25] (Fig. 4). Their mammalian homologs form a similar Rab cascade where Rab11-GTP recruits Rabin8 to secretory vesicles for activation of Rab8 to facilitate cilia biogenesis in mammalian cells [26] (Fig. 4). In addition, the Ric1/Rgp1 complex is recruited by Rab33B to the Golgi membrane to function as a GEF for Rab6 activation [27] (Fig. 4). Along the endocytic pathway, the Sand1/Mon1–Ccz1 complex is a GEF for Rab7 but an effector of the upstream Rab5 [18, 17]. As such, it is recruited by Rab5-GTP to the endosomal membrane for activation of Rab7 (Fig. 4). With the displacement of Rabex5, a Vps9 domain-containing Rab5 GEF, from the membrane, Rab5-GTP undergoes GTP hydrolysis and converts to Rab5-GDP that is removed from the membrane by GDI, leading to the conversion of early endosomes marked by Rab5 to late endosomes marked by Rab7. Interestingly, Rabex-5 itself can be recruited by another upstream Rab, Rab22, to early endosomes to establish a Rab22–Rabex-5–Rab5 cascade within the early endosomal network [28] (Fig. 4). In addition, within a late endosomal/premelanosomal network, there exists a Rab9–BLOC-3–Rab32/Rab38 cascade where Rab9-GTP recruits BLOC-3 to the membrane to function as a GEF for activation of Rab32/Rab38 [29, 30] (Fig. 4).
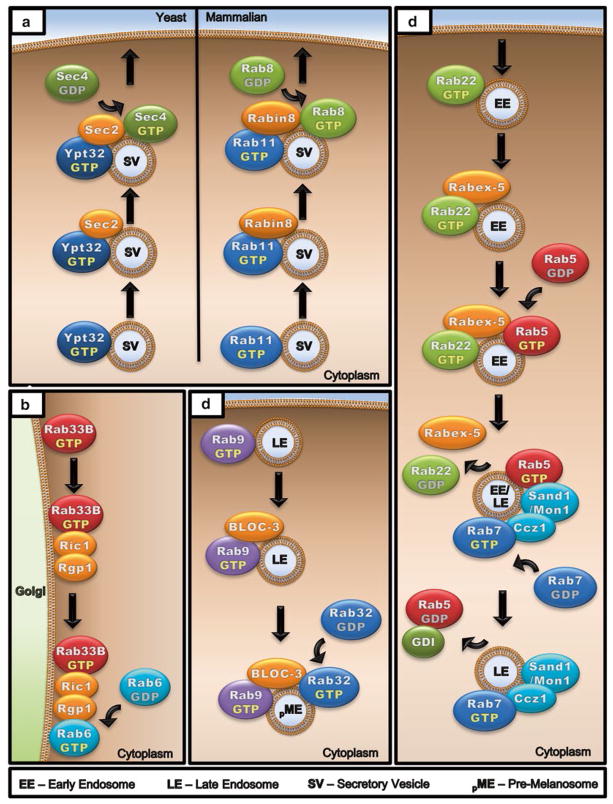
Fig. 4.
Rab activation cascades. The GTP loading and activation of a Rab can be regulated as part of an activation cascade from an upstream Rab. (a) Rab activation cascades are evolutionarily conserved from yeast to mammals. During polarized exocytosis in S. cerevisiae, activated Ypt32 recruits Sec2, a Sec4 GEF, to the membrane of secretory vesicles destined for exocytosis. Sec2 in turn leads to the recruitment and activation of Sec4. The same cascade is seen in mammalian cells with Rab11, Rabin8 (Rab8 GEF), and Rab8. (b) On the Golgi membrane, active Rab33B recruits the Ric1–Rgp1 complex (Rab6 GEF), which activates Rab6. (c) On late endosomes, Rab9 recruits Bloc-3 (Rab32/33 GEF) to the membrane and activates Rab32 as they move toward lysosomes or melanosomes. (d) Active Rab22 binds and recruits Rabex-5 (Rab5 GEF) and activates Rab5 on early endosomes. Active Rab5 in turn binds Sand1 (Mon1 in Yeast) –Ccz1 complex (Rab7 GEF), which recruits and activates Rab7 to facilitate transition to late endosomes. Upon dissociation of Rab22 and Rabex-5, Rab5 is inactivated by GTP hydrolysis and converted to GDP bound state, which is then removed from the membrane by GDI
In contrast to the GEFs recruited by upstream Rabs, a GAP may be recruited by a downstream Rab to the membrane for inactivation of an upstream Rab to establish the boundary between the functional Rab domains. In the budding yeast, it is reported that a Ypt1 GAP, Gyp1, is recruited by a downstream Rab, Ypt32, to the membrane to inactivate and clear Ypt1 from the Ypt32 membrane domain [31]. In mammalian cells, Rab9 is shown to recruit the GAPs (RUTBC1 and RUTBC2) to late endosomes for inactivation of Rab32 and Rab36 on the membrane [32, 33].
The combination of a GEF and a GAP recruited in such a fashion by upstream and downstream Rabs can effectively sharpen the boundary of Rab membrane domains and facilitate the transition from early to late compartments during intracellular transport [34]. It may also generate ultrasensitivity and “all-or-none” switch-like behavior in the Rab activity [35, 36]. In addition to the Rabs, other protein and lipid factors in the membrane are also known to regulate the recruitment and activity of Rab GEFs, which is exemplified by the regulation of Sec2/Rabin8 by phosphatidylinositol-4-phosphate (PI4P) [37] and phosphorylation [38].
3 Rab Functions in Vesicular Transport
Once activated and GTP bound, Rabs can temporally and spatially interact with multiple effectors to facilitate the selection of cargoes into vesicles, vesicle movement on actin and microtubule cables, and tethering of vesicles to target compartment for membrane fusion.
Rabs can interact with the cytoplasmic domains of transmembrane proteins/receptors to facilitate their packaging into transport vesicles. Rab5 and Rab21 on the early endocytic pathway directly bind to the α subunit of β1 integrins and promote their endocytosis and recycling to remodel the cell surface for migration and cytokinesis [39, 40]. In addition, Rab5 also directly interacts with angiotensin II Type 1A receptor (AT1AR) to facilitate its endocytic trafficking [41]. On the exocytic pathway, Rab3b is shown to bind directly to polymeric IgA receptor (pIgR) to modulate its transcytosis in polarized epithelial cells [42]. Furthermore, some Rabs are involved in packaging of cargo proteins into transport vesicles through interactions with adaptor proteins. In this regard, Rab5 is shown to concentrate transferrin receptor into coated pits for endocytosis [43], while Rab9 facilitates the recruitment of the cargo protein (mannose-6-phosphate receptor) into late-endosome derived transport vesicles via its effector TIP47 [44].
Rabs are also known to interact with actin and microtubule motor proteins such as myosins, kinesins, and dyneins to facilitate the movement of transport vesicles on the actin and microtubule cytoskeleton. Class V myosins are actin motors that consist of an N-terminal actin-binding motor domain and a C-terminal cargo-binding globular tail domain (GTD), which can bind to a number of Rabs on post-Golgi secretory vesicles or recycling endosomes and get recruited to these exocytic compartments [45]. These exocytic and recycling Rabs are more closely related in evolution and belong to groups I, IV, and V, including Rab3, Rab6, Rab8/Sec4, Rab10, Rab11, Rab14, Rab25, and Rab39 [1, 46, 2, 45]. In addition, Rab27 indirectly recruits myosin V to melanosomes via a linker protein Slac2/melanophilin [47, 48]. The Rab-myosin V interaction links transport vesicles to actin and facilitate their movement toward the cell surface. These Rabs are also known for recruitment of microtubule-based kinesin and dynein motors, especially Rab6 that directly binds to both kinesin (KIF20A) and dynein (DYNLRB1 and dynactin) [49–51]. Rab14 also directly binds to a kinesin, kinesin-3 (KIF16B) [52]. Some of the Rabs interact with kinesins and dyneins indirectly via linker proteins, e.g., Rab11 proteins can recruit kinesin-1, kinesin-2, dynein LIC1, and dynein LIC2 via Rab11 effectors FIP3 and FIP5 [53–56]. Another interesting example is the endocytic Rab5 that binds and activates one of its effectors hVps34, a PI 3 kinase, and its product PI3P on the membrane in turn recruits the kinesin KIF16B [57]. This plus-end microtubule motor may play a role in the peripheral distribution of Rab5-positive early endosomes, suggesting the necessity of Rab5 removal for transition to late endosomes and movement toward perinuclear region.
Another important Rab function in vesicular transport is to tether transport vesicles to target compartments for membrane fusion. In this regard, Rab5/Vps21 is shown to tether vesicles directly via Rab–Rab interaction in trans [58]. However, the tethering function is more commonly performed by Rab effectors including long coiled-coil homodimers and large multi-subunit complexes. The former may be exemplified by the Rab5 effectors EEA1/Vac1 and Rabenosyn-5 [59–61] and the Rab1/Ypt1 effectors p115/Uso1 [62, 63], while the latter include the Sec4/Rab8 effector exocyst [64, 65], the Rab5/Vps21 effector CORVET (class C core vacuole/endosome tethering) complex [66], the Rab7/Ypt7 effector HOPS (homotypic fusion and vacuole protein sorting) complex [67], and the Rab1/Ypt1 effectors TRAPPI and TRAPPII complexes [68, 69]. These tethering factors are recruited by the Rabs to mediate vesicle docking and often interact with the SM (Sec1–Munc18) proteins to facilitate the assembly of SNARE complexes for membrane fusion. For example, Rabenosyn-5 contains an N-terminal FYVE domain for binding to PI3P on endosomes and a C-terminal Rab5-binding domain for tethering Rab5-positive vesicles. Furthermore, Rabenosyn-5 interacts with hVps45, a SM protein, to facilitate SNARE-mediated membrane fusion [59].
4 Other Rab Functions
The large number of effectors for each Rab, e.g., more than 20 for Rab5 [70], suggests that Rabs may have additional functions beyond intracellular membrane trafficking. Indeed, Rabs play important roles in signal transduction and autophagy. Some of the Rab5 effectors are signaling molecules such as APPL1 and APPL2, which are recruited to early endosomes by Rab5 [71, 72] and in turn recruit Akt and modulate its phosphorylation specificity for GSK-3β rather than TSC2 [73]. This Rab5-mediated APPL signaling on endosomes is essential for cell survival and development in zebrafish [73]. Another Rab5 effector is Vps34 [74], a class III PI 3-kinase that produces PI3P on early endosomes and promotes autophagosome formation during autophagy [75–77]. Vps34 is also an effector for the late endosome-associated Rab7 [78] and may play a similar role in autophagy on late endosomes. In addition, the exocytic Rab1/Ypt1 is also known for its essential role in the formation of preautophagosomal structure (PAS) via TRAPPIII complex during the initiation of autophagy [79–81].
5 Rabs and Disease
The fundamental function of Rabs and membrane trafficking in cell physiology is reflected by various diseases due to mutations or altered expression of Rab genes. Mutations in five of the 66 human Rab genes (Rab7, Rab23, Rab27, Rab38, and Rab39b) are known to cause genetic disorders. Among them, Rab7 is ubiquitously expressed in all tissues while the other four Rabs are expressed only in certain cell types and tissues. Importantly, they are also different in the nature of mutations.
Four gain-of-function mutations in Rab7 are linked to Charcot–Marie–Tooth Type 2B (CMT2B) disease [82–84], which is a form of hereditary motor and sensory neuropathy with symptoms of distal sensory loss and muscle weakness, leading to toe ulcers, infections, and ultimately amputation [85]. These gain-of-function mutations enhance Rab7 activity by increasing the nucleotide exchange reaction independent of GEFs [86, 87]. It is worth noting that enhanced Rab7 activity affects mainly peripheral neurons and CMT2B is a neurological disease, despite the ubiquitous expression of Rab7 in all tissues.
Mutations in the other four Rabs that lead to autosomal recessive disorders are all loss-of-function mutations. Mutations in Rab23 are linked to Carpenter syndrome [88], which is a neurological disorder of craniosynostosis and limb malformation. Rab23 is highly expressed in the brain and neurons [89] and is localized on the plasma membrane and early endosomes [90] involved in sorting and function of signaling molecules in Sonic Hedgehog signal transduction [91]. The open brain (opb) gene that inhibits the Sonic Hedgehog signaling in mice is mapped to Rab23 and the opb mouse model recapitulates some of the neurological defects of Carpenter syndrome [91]. Mutations in Rab27 are linked to Griscelli syndrome, which is an immunological disorder with excessive T lymphocyte and macrophage activation called hemophagocytic syndrome as well as defects in skin pigmentation [92, 93]. Rab27 is expressed in highly secretory cells such as cytotoxic T lymphocytes (CTL) [94] and melanocytes [95, 96] and localized to secretory granules and melanosomes in these cell types. Inactivation of Rab27 by the mutations blocks the transport and function of the secretory granules and melanosomes and contributes to the hemophagocytic syndrome and partial albinism in Griscelli patients [93]. This phenotype can be recapitulated by a Rab27 mutation in ashen mice [97]. Rab38 is one of multiple genes linked to Hermansky–Pudlak syndrome caused by defective melanocytes and platelets [98]. Chocolate mice [99] and Fawn-hooded and Tester-Moriyama rats [100] are animal models for Hermansky–Pudlak syndrome and they contain inactivating mutations in the Rab38 gene. A cell biology study suggests that Rab38 is essential for the biogenesis of melanosomes [101]. Finally, Rab39b is specifically expressed in the brain and neurons and mutations in Rab39b are associated with one form of X-linked mental retardation (XLMR) [102].
In addition to mutations, many Rabs show altered expression level or activity in such diseases as cancer, Alzheimer’s disease, and diabetes. It appears a common theme that a Rab may be up-regulated in certain types of cancers but down-regulated in other types of cancers [4]. For example, Rab25 is known to promote α5β1 integrin recycling in epithelial cells [103] and overexpression of Rab25 is associated with aggressiveness of ovarian and breast cancers [104], suggesting a role for Rab25 in cancer cell invasion and metastasis. However, Rab25 is down-regulated in colon cancer with poor patient prognosis [105], suggesting a tumor suppressor function. Indeed, Rab25 deficiency in mouse models of colon cancer promotes colonic tumor growth [105]. The reconciliation of this apparent conflict over Rab25 function in promoting or blocking tumor growth in different cancers may involve the CLIC3 protein, which is necessary for Rab25-mediated integrin recycling [106]. It is suggested that Rab25 may sort integrins to lysosomes for degradation in cell types that don’t express CLIC3, acting like a tumor suppressor [106]. The opposite may be true in cell types with high levels of CLIC3 where Rab25 can promote integrin recycling and cell migration and invasion [106]. Another example is Rab31, which is overexpressed in breast cancer, brain cancer, skin cancer, and several other types of cancers but is down-regulated in leukemia, lung cancer, and colon cancer [4].
Endocytic Rabs such as Rab5 and Rab7 are overexpressed in hippocampal neurons of Alzheimer’s patients [107] and the enhanced endocytic activity is suggested to promote the proteolytic processing of amyloid precursor protein (APP) in endosomes [108], which may lead to increased production and accumulation of amyloid-β peptide (Aβ) in the brain, a hallmark of Alzheimer’s disease. Recycling Rabs such as Rab10 and Rab14 are activated by insulin signal transduction to promote the translocation of glucose transporter 4 (GLUT4) from intracellular vesicles to the plasma membrane of adipocytes for glucose uptake and metabolism [109]. Rab10 and Rab14 are kept in the inactive GDP-bound state by AS160, a GAP for both Rabs [110]. Upon insulin stimulation, AS160 is inactivated by phosphorylation [111, 112] and consequently Rab10 and Rab14 can be activated by GTP loading to promote docking and fusion of GLUT4-containing vesicles with the plasma membrane [109]. Malfunction of the Rab10- and Rab14-mediated GLUT4 translocation processes is implicated in type II diabetes.
Acknowledgments
The authors’ research program is supported by the NIH/NIGMS grant R01 GM074692 (to G.L.).
References
- 1.Diekmann Y, Seixas E, Gouw M, et al. Thousands of rab GTPases for the cell biologist. PLoS Comput Biol. 2011;7:e1002217. doi: 10.1371/journal.pcbi.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klopper TH, Kienle N, Fasshauer D, et al. Untangling the evolution of Rab G proteins: implications of a comprehensive genomic analysis. BMC Biol. 2012;10:71. doi: 10.1186/1741-7007-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li G, Segev N. Ypt/Rab GTPases and Intracellular Membrane Trafficking: an Overview. In: Li G, Segev N, editors. Rab GTPases and Membrane Trafficking. Bentham Science Publishers; Sharjah: 2012. pp. 3–17. [Google Scholar]
- 4.Rodrigues ML, Pereira-Leal JB. Novel Rab GTPases. In: Li G, Segev N, editors. Rab GTPases and Membrane Trafficking. Bentham Science Publishers; Sharjah: 2012. pp. 155–168. [Google Scholar]
- 5.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfeffer SR. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol. 2013;25:414–419. doi: 10.1016/j.ceb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavrier P, Parton RG, Hauri HP, et al. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- 8.Blumer J, Rey J, Dehmelt L, et al. RabGEFs are a major determinant for specific Rab membrane targeting. J Cell Biol. 2013;200:287–300. doi: 10.1083/jcb.201209113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sivars U, Aivazian D, Pfeffer SR. Yip3 catalyses the dissociation of endosomal Rab-GDI complexes. Nature. 2003;425:856–859. doi: 10.1038/nature02057. [DOI] [PubMed] [Google Scholar]
- 10.Soldati T, Shapiro AD, Svejstrup AB, et al. Membrane targeting of the small GTPase Rab9 is accompanied by nucleotide exchange. Nature. 1994;369:76–78. doi: 10.1038/369076a0. [DOI] [PubMed] [Google Scholar]
- 11.Ullrich O, Horiuchi H, Bucci C, et al. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature. 1994;368:157–160. doi: 10.1038/368157a0. [DOI] [PubMed] [Google Scholar]
- 12.Barr F, Lambright DG. Rab GEFs and GAPs. Curr Opin Cell Biol. 2010;22:461–470. doi: 10.1016/j.ceb.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soldati T, Riederer MA, Pfeffer SR. Rab GDI: a solubilizing and recycling factor for rab9 protein. Mol Biol Cell. 1993;4:425–434. doi: 10.1091/mbc.4.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrett MD, Kabcenell AK, Zahner JE, et al. Interaction of Sec4 with GDI proteins from bovine brain, Drosophila melanogaster and Saccharomyces cerevisiae. Conservation of GDI membrane dissociation activity. FEBS Lett. 1993;331:233–238. doi: 10.1016/0014-5793(93)80343-s. [DOI] [PubMed] [Google Scholar]
- 15.Ullrich O, Stenmark H, Alexandrov K, et al. Rab GDP dissociation inhibitor as a general regulator for the membrane association of rab proteins. J Biol Chem. 1993;268:18143–18150. [PubMed] [Google Scholar]
- 16.Carney DS, Davies BA, Horazdovsky BF. Vps9 domain-containing proteins: activators of Rab5 GTPases from yeast to neurons. Trends Cell Biol. 2006;16:27–35. doi: 10.1016/j.tcb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Nordmann M, Cabrera M, Perz A, et al. The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr Biol. 2010;20:1654–1659. doi: 10.1016/j.cub.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Poteryaev D, Datta S, Ackema K, et al. Identification of the switch in early-to-late endosome transition. Cell. 2010;141:497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Jones S, Newman C, Liu F, et al. The TRAPP complex is a nucleotide exchanger for Ypt1 and Ypt31/32. Mol Biol Cell. 2000;11:4403–4411. doi: 10.1091/mbc.11.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Sacher M, Ferro-Novick S. TRAPP stimulates guanine nucleotide exchange on Ypt1p. J Cell Biol. 2000;151:289–296. doi: 10.1083/jcb.151.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hattula K, Furuhjelm J, Arffman A, et al. A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell. 2002;13:3268–3280. doi: 10.1091/mbc.E02-03-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siniossoglou S, Peak-Chew SY, Pelham HR. Ric1p and Rgp1p form a complex that catalyses nucleotide exchange on Ypt6p. EMBO J. 2000;19:4885–4894. doi: 10.1093/emboj/19.18.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marat AL, Dokainish H, McPherson PS. DENN domain proteins: regulators of Rab GTPases. J Biol Chem. 2011;286:13791–13800. doi: 10.1074/jbc.R110.217067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan X, Eathiraj S, Munson M, et al. TBC-domain GAPs for Rab GTPases accelerate GTP hydrolysis by a dual-finger mechanism. Nature. 2006;442:303–306. doi: 10.1038/nature04847. [DOI] [PubMed] [Google Scholar]
- 25.Ortiz D, Medkova M, Walch-Solimena C, et al. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol. 2002;157:1005–1015. doi: 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knodler A, Feng S, Zhang J, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A. 2010;107:6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pusapati GV, Luchetti G, Pfeffer SR. Ric1-Rgp1 complex is a guanine nucleotide exchange factor for the late Golgi Rab6A GTPase and an effector of the medial Golgi Rab33B GTPase. J Biol Chem. 2012;287:42129–42137. doi: 10.1074/jbc.M112.414565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu H, Liang Z, Li G. Rabex-5 is a Rab22 effector and mediates a Rab22-Rab5 signaling cascade in endocytosis. Mol Biol Cell. 2009;20:4720–4729. doi: 10.1091/mbc.E09-06-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerondopoulos A, Langemeyer L, Liang JR, et al. BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr Biol. 2012;22:2135–2139. doi: 10.1016/j.cub.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kloer DP, Rojas R, Ivan V, et al. Assembly of the biogenesis of lysosome-related organelles complex-3 (BLOC-3) and its interaction with Rab9. J Biol Chem. 2010;285:7794–7804. doi: 10.1074/jbc.M109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivera-Molina FE, Novick PJ. A Rab GAP cascade defines the boundary between two Rab GTPases on the secretory pathway. Proc Natl Acad Sci U S A. 2009;106:14408–14413. doi: 10.1073/pnas.0906536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nottingham RM, Pusapati GV, Ganley IG, et al. RUTBC2 protein, a Rab9A effector and GTPase-activating protein for Rab36. J Biol Chem. 2012;287:22740–22748. doi: 10.1074/jbc.M112.362558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nottingham RM, Ganley IG, Barr FA, et al. RUTBC1 protein, a Rab9A effector that activates GTP hydrolysis by Rab32 and Rab33B proteins. J Biol Chem. 2011;286:33213–33222. doi: 10.1074/jbc.M111.261115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nottingham RM, Pfeffer SR. Defining the boundaries: Rab GEFs and GAPs. Proc Natl Acad Sci U S A. 2009;106:14185–14186. doi: 10.1073/pnas.0907725106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li G, Qian H. Sensitivity and specificity amplification in signal transduction. Cell Biochem Biophys. 2003;39:45–59. doi: 10.1385/CBB:39:1:45. [DOI] [PubMed] [Google Scholar]
- 36.Barr FA. Review series: Rab GTPases and membrane identity: causal or inconsequential? J Cell Biol. 2013;202:191–199. doi: 10.1083/jcb.201306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizuno-Yamasaki E, Medkova M, Coleman J, et al. Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev Cell. 2010;18:828–840. doi: 10.1016/j.devcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stalder D, Mizuno-Yamasaki E, Ghassemian M, et al. Phosphorylation of the Rab exchange factor Sec2p directs a switch in regulatory binding partners. Proc Natl Acad Sci U S A. 2013;110:19995–20002. doi: 10.1073/pnas.1320029110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellinen T, Arjonen A, Vuoriluoto K, et al. Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J Cell Biol. 2006;173:767–780. doi: 10.1083/jcb.200509019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pellinen T, Tuomi S, Arjonen A, et al. Integrin trafficking regulated by Rab21 is necessary for cytokinesis. Dev Cell. 2008;15:371–385. doi: 10.1016/j.devcel.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Seachrist JL, Laporte SA, Dale LB, et al. Rab5 association with the angiotensin II type 1A receptor promotes Rab5 GTP binding and vesicular fusion. J Biol Chem. 2002;277:679–685. doi: 10.1074/jbc.M109022200. [DOI] [PubMed] [Google Scholar]
- 42.Van IJzendoorn SC, Tuvim MJ, Weimbs T, et al. Direct interaction between Rab3b and the polymeric immunoglobulin receptor controls ligand-stimulated transcytosis in epithelial cells. Dev Cell. 2002;2:219–228. doi: 10.1016/s1534-5807(02)00115-6. [DOI] [PubMed] [Google Scholar]
- 43.McLauchlan H, Newell J, Morrice N, et al. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr Biol. 1998;8:34–45. doi: 10.1016/s0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- 44.Carroll KS, Hanna J, Simon I, et al. Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science. 2001;292:1373–1376. doi: 10.1126/science.1056791. [DOI] [PubMed] [Google Scholar]
- 45.Lindsay AJ, Jollivet F, Horgan CP, et al. Identification and characterization of multiple novel Rab-myosin Va interactions. Mol Biol Cell. 2013;24:3420–3434. doi: 10.1091/mbc.E13-05-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Govindan B, Bowser R, Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J Cell Biol. 1995;128:1055–1068. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukuda M, Kuroda TS, Mikoshiba K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J Biol Chem. 2002;277:12432–12436. doi: 10.1074/jbc.C200005200. [DOI] [PubMed] [Google Scholar]
- 48.Wu X, Wang F, Rao K, et al. Rab27a is an essential component of melanosome receptor for myosin Va. Mol Biol Cell. 2002;13:1735–1749. doi: 10.1091/mbc.01-12-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Short B, Preisinger C, Schaletzky J, et al. The Rab6 GTPase regulates recruitment of the dynactin complex to Golgi membranes. Curr Biol. 2002;12:1792–1795. doi: 10.1016/s0960-9822(02)01221-6. [DOI] [PubMed] [Google Scholar]
- 50.Wanschers B, van de Vorstenbosch R, Wijers M, et al. Rab6 family proteins interact with the dynein light chain protein DYNLRB1. Cell Motil Cytoskeleton. 2008;65:183–196. doi: 10.1002/cm.20254. [DOI] [PubMed] [Google Scholar]
- 51.Echard A, Jollivet F, Martinez O, et al. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279:580–585. doi: 10.1126/science.279.5350.580. [DOI] [PubMed] [Google Scholar]
- 52.Ueno H, Huang X, Tanaka Y, et al. KIF16B/Rab14 molecular motor complex is critical for early embryonic development by transporting FGF receptor. Dev Cell. 2011;20:60–71. doi: 10.1016/j.devcel.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Horgan CP, Hanscom SR, Jolly RS, et al. Rab11-FIP3 binds dynein light intermediate chain 2 and its overexpression fragments the Golgi complex. Biochem Biophys Res Commun. 2010;394:387–392. doi: 10.1016/j.bbrc.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 54.Horgan CP, Hanscom SR, Jolly RS, et al. Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J Cell Sci. 2010;123:181–191. doi: 10.1242/jcs.052670. [DOI] [PubMed] [Google Scholar]
- 55.Schonteich E, Wilson GM, Burden J, et al. The Rip11/Rab11-FIP5 and kinesin II complex regulates endocytic protein recycling. J Cell Sci. 2008;121:3824–3833. doi: 10.1242/jcs.032441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simon GC, Prekeris R. Mechanisms regulating targeting of recycling endosomes to the cleavage furrow during cytokinesis. Biochem Soc Trans. 2008;36:391–394. doi: 10.1042/BST0360391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoepfner S, Severin F, Cabezas A, et al. Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B. Cell. 2005;121:437–450. doi: 10.1016/j.cell.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 58.Lo SY, Brett CL, Plemel RL, et al. Intrinsic tethering activity of endosomal Rab proteins. Nat Struct Mol Biol. 2012;19:40–47. doi: 10.1038/nsmb.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohya T, Miaczynska M, Coskun U, et al. Reconstitution of Rab- and SNARE-dependent membrane fusion by synthetic endosomes. Nature. 2009;459:1091–1097. doi: 10.1038/nature08107. [DOI] [PubMed] [Google Scholar]
- 60.Simonsen A, Lippe R, Christoforidis S, et al. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 61.Tall GG, Hama H, DeWald DB, et al. The phosphatidylinositol 3-phosphate binding protein Vac1p interacts with a Rab GTPase and a Sec1p homologue to facilitate vesicle-mediated vacuolar protein sorting. Mol Biol Cell. 1999;10:1873–1889. doi: 10.1091/mbc.10.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sapperstein SK, Walter DM, Grosvenor AR, et al. p115 is a general vesicular transport factor related to the yeast endoplasmic reticulum to Golgi transport factor Uso1p. Proc Natl Acad Sci U S A. 1995;92:522–526. doi: 10.1073/pnas.92.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barroso M, Nelson DS, Sztul E. Transcytosis-associated protein (TAP)/p115 is a general fusion factor required for binding of vesicles to acceptor membranes. Proc Natl Acad Sci U S A. 1995;92:527–531. doi: 10.1073/pnas.92.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.TerBush DR, Maurice T, Roth D, et al. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- 65.Guo W, Roth D, Walch-Solimena C, et al. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balderhaar HJ, Lachmann J, Yavavli E, et al. The CORVET complex promotes tethering and fusion of Rab5/Vps21-positive membranes. Proc Natl Acad Sci U S A. 2013;110:3823–3828. doi: 10.1073/pnas.1221785110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hickey CM, Wickner W. HOPS initiates vacuole docking by tethering membranes before trans-SNARE complex assembly. Mol Biol Cell. 2010;21:2297–2305. doi: 10.1091/mbc.E10-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barrowman J, Bhandari D, Reinisch K, et al. TRAPP complexes in membrane traffic: convergence through a common Rab. Nat Rev Mol Cell Biol. 2010;11:759–763. doi: 10.1038/nrm2999. [DOI] [PubMed] [Google Scholar]
- 69.Sacher M, Kim YG, Lavie A, et al. The TRAPP complex: insights into its architecture and function. Traffic. 2008;9:2032–2042. doi: 10.1111/j.1600-0854.2008.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christoforidis S, McBride HM, Burgoyne RD, et al. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- 71.Miaczynska M, Christoforidis S, Giner A, et al. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 72.Zhu G, Chen J, Liu J, et al. Structure of the APPL1 BAR-PH domain and characterization of its interaction with Rab5. EMBO J. 2007;26:3484–3493. doi: 10.1038/sj.emboj.7601771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schenck A, Goto-Silva L, Collinet C, et al. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 2008;133:486–497. doi: 10.1016/j.cell.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 74.Christoforidis S, Miaczynska M, Ashman K, et al. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat Cell Biol. 1999;1:249–252. doi: 10.1038/12075. [DOI] [PubMed] [Google Scholar]
- 75.Jaber N, Dou Z, Chen JS, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci U S A. 2012;109:2003–2008. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simonsen A, Tooze SA. Coordination of membrane events during autophagy by multiple class III PI3-kinase complexes. J Cell Biol. 2009;186:773–782. doi: 10.1083/jcb.200907014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vergne I, Roberts E, Elmaoued RA, et al. Control of autophagy initiation by phosphoinositide 3-phosphatase Jumpy. EMBO J. 2009;28:2244–2258. doi: 10.1038/emboj.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stein MP, Feng Y, Cooper KL, et al. Human VPS34 and p150 are Rab7 interacting partners. Traffic. 2003;4:754–771. doi: 10.1034/j.1600-0854.2003.00133.x. [DOI] [PubMed] [Google Scholar]
- 79.Lipatova Z, Segev N. A Ypt/Rab GTPase module makes a PAS. Autophagy. 2012;8:1271–1272. doi: 10.4161/auto.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lynch-Day MA, Bhandari D, Menon S, et al. Trs85 directs a Ypt1 GEF, TRAPPIII, to the phagophore to promote autophagy. Proc Natl Acad Sci U S A. 2010;107:7811–7816. doi: 10.1073/pnas.1000063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang J, Menon S, Yamasaki A, et al. Ypt1 recruits the Atg1 kinase to the preautophagosomal structure. Proc Natl Acad Sci U S A. 2013;110:9800–9805. doi: 10.1073/pnas.1302337110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verhoeven K, De Jonghe P, Coen K, et al. Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am J Hum Genet. 2003;72:722–727. doi: 10.1086/367847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Houlden H, King RH, Muddle JR, et al. A novel RAB7 mutation associated with ulcero-mutilating neuropathy. Ann Neurol. 2004;56:586–590. doi: 10.1002/ana.20281. [DOI] [PubMed] [Google Scholar]
- 84.Meggouh F, Bienfait HM, Weterman MA, et al. Charcot-Marie-Tooth disease due to a de novo mutation of the RAB7 gene. Neurology. 2006;67:1476–1478. doi: 10.1212/01.wnl.0000240068.21499.f5. [DOI] [PubMed] [Google Scholar]
- 85.Zuchner S, Vance JM. Molecular genetics of autosomal-dominant axonal Charcot-Marie-Tooth disease. Neuromol Med. 2006;8:63–74. doi: 10.1385/nmm:8:1-2:63. [DOI] [PubMed] [Google Scholar]
- 86.Spinosa MR, Progida C, De Luca A, et al. Functional characterization of Rab7 mutant proteins associated with Charcot-Marie-Tooth type 2B disease. J Neurosci. 2008;28:1640–1648. doi: 10.1523/JNEUROSCI.3677-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCray BA, Skordalakes E, Taylor JP. Disease mutations in Rab7 result in unregulated nucleotide exchange and inappropriate activation. Hum Mol Genet. 2010;19:1033–1047. doi: 10.1093/hmg/ddp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jenkins D, Seelow D, Jehee FS, et al. RAB23 mutations in Carpenter syndrome imply an unexpected role for hedgehog signaling in cranial-suture development and obesity. Am J Hum Genet. 2007;80:1162–1170. doi: 10.1086/518047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Olkkonen VM, Peterson JR, Dupree P, et al. Isolation of a mouse cDNA encoding Rab23, a small novel GTPase expressed predominantly in the brain. Gene. 1994;138:207–211. doi: 10.1016/0378-1119(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 90.Evans TM, Ferguson C, Wainwright BJ, et al. Rab23, a negative regulator of hedgehog signaling, localizes to the plasma membrane and the endocytic pathway. Traffic. 2003;4:869–884. doi: 10.1046/j.1600-0854.2003.00141.x. [DOI] [PubMed] [Google Scholar]
- 91.Eggenschwiler JT, Espinoza E, Anderson KV. Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature. 2001;412:194–198. doi: 10.1038/35084089. [DOI] [PubMed] [Google Scholar]
- 92.Griscelli C, Durandy A, Guy-Grand D, et al. A syndrome associating partial albinism and immunodeficiency. Am J Med. 1978;65:691–702. doi: 10.1016/0002-9343(78)90858-6. [DOI] [PubMed] [Google Scholar]
- 93.Menasche G, Pastural E, Feldmann J, et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 94.Stinchcombe JC, Barral DC, Mules EH, et al. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol. 2001;152:825–834. doi: 10.1083/jcb.152.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hume AN, Collinson LM, Rapak A, et al. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J Cell Biol. 2001;152:795–808. doi: 10.1083/jcb.152.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu X, Rao K, Bowers MB, et al. Rab27a enables myosin Va-dependent melanosome capture by recruiting the myosin to the organelle. J Cell Sci. 2001;114:1091–1100. doi: 10.1242/jcs.114.6.1091. [DOI] [PubMed] [Google Scholar]
- 97.Wilson SM, Yip R, Swing DA, et al. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc Natl Acad Sci U S A. 2000;97:7933–7938. doi: 10.1073/pnas.140212797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Di Pietro SM, Dell’Angelica EC. The cell biology of Hermansky-Pudlak syndrome: recent advances. Traffic. 2005;6:525–533. doi: 10.1111/j.1600-0854.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 99.Loftus SK, Larson DM, Baxter LL, et al. Mutation of melanosome protein RAB38 in chocolate mice. Proc Natl Acad Sci U S A. 2002;99:4471–4476. doi: 10.1073/pnas.072087599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oiso N, Riddle SR, Serikawa T, et al. The rat Ruby (R) locus is Rab38: identical mutations in Fawn-hooded and Tester-Moriyama rats derived from an ancestral Long Evans rat sub-strain. Mamm Genome. 2004;15:307–314. doi: 10.1007/s00335-004-2337-9. [DOI] [PubMed] [Google Scholar]
- 101.Wasmeier C, Romao M, Plowright L, et al. Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. J Cell Biol. 2006;175:271–281. doi: 10.1083/jcb.200606050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Giannandrea M, Bianchi V, Mignogna ML, et al. Mutations in the small GTPase gene RAB39B are responsible for X-linked mental retardation associated with autism, epilepsy, and macrocephaly. Am J Hum Genet. 2010;86:185–195. doi: 10.1016/j.ajhg.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Caswell PT, Spence HJ, Parsons M, et al. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 104.Cheng KW, Lahad JP, Kuo WL, et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med. 2004;10:1251–1256. doi: 10.1038/nm1125. [DOI] [PubMed] [Google Scholar]
- 105.Nam KT, Lee HJ, Smith JJ, et al. Loss of Rab25 promotes the development of intestinal neoplasia in mice and is associated with human colorectal adenocarcinomas. J Clin Invest. 2010;120:840–849. doi: 10.1172/JCI40728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dozynkiewicz MA, Jamieson NB, Macpherson I, et al. Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev Cell. 2012;22:131–145. doi: 10.1016/j.devcel.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ginsberg SD, Alldred MJ, Counts SE, et al. Microarray analysis of hippocampal CA1 neurons implicates early endosomal dysfunction during Alzheimer’s disease progression. Biol Psychiatry. 2010;68:885–893. doi: 10.1016/j.biopsych.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cataldo AM, Peterhoff CM, Troncoso JC, et al. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am J Pathol. 2000;157:277–286. doi: 10.1016/s0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen Y, Wang Y, Zhang J, et al. Rab10 and myosin-Va mediate insulin-stimulated GLUT4 storage vesicle translocation in adipocytes. J Cell Biol. 2012;198:545–560. doi: 10.1083/jcb.201111091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miinea CP, Sano H, Kane S, et al. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J. 2005;391:87–93. doi: 10.1042/BJ20050887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zeigerer A, McBrayer MK, McGraw TE. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol Biol Cell. 2004;15:4406–4415. doi: 10.1091/mbc.E04-04-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sano H, Kane S, Sano E, et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]