Abstract
The glucocorticoid receptor gene NR3C1 is an important down-regulator of inflammation and is typically under-expressed in individuals with low socioeconomic status (SES). Negative emotionality has been suggested as a potential mediator of SES disparities in health outcomes. In this study, we expand this literature by naturalistically assessing negative emotionality in a key emotional environment: the family. In a sample of 104 youth with asthma (10–17 years) and their primary caregiver, we assessed SES via caregiver report, emotional expression by youth and parents in the home over four days using the electronically activated recorder (EAR), and NR3C1 expression via blood collected from youth. Although there was not a direct effect of SES on NR3C1 expression, bootstrapping mediation analyses showed a significant indirect path such that lower SES was associated with a more negative family emotional climate, which in turn predicted reduced NR3C1 expression. No mediation effects were found for family positive emotional climate. This research demonstrates the importance of examining the effects of SES on emotion expression in the family context and suggests a critical biopsychosocial pathway underlying SES-based health disparities that may extend beyond youth.
Keywords: Glucocorticoid receptor, socioeconomic status, family emotional climate
1. Introduction
Several common, chronic, and debilitating diseases such as asthma, heart disease, and cancer are closely linked with chronic inflammation (Ershler & Keller, 2000). As these diseases show socioeconomic status (SES) disparities, it is critical to understand the biological and psychosocial mediators of links between SES and inflammatory disease. This paper examines the effects of SES on a key anti-inflammatory regulatory element, the glucocorticoid receptor gene NR3C1, via an important aspect of the social environment, family emotional climate.
NR3C1 is important for regulating neuroendocrine and inflammatory responses. The stress hormone cortisol binds to glucocorticoid receptors produced by NR3C1, which triggers transcriptional processes resulting in reduced inflammation (Hayashi, Wada, Ito, & Adcock, 2004). If NR3C1 is under-expressed, creating fewer receptors for cortisol to bind to and carry out its functions, the inflammatory response can become chronically over-activated and increase susceptibility to inflammatory disease over time(Collaboration, 2010; Howren, Lamkin, & Suls, 2009). Reduced expression of NR3C1 may also lead to glucocorticoid resistance, which occurs when cells are unable to receive signals to terminate the inflammatory response, due to a reduced number of the receptors needed to pick up these signals (Bray & Cotton, 2003; Gross, Lu, & Cidlowski, 2009). Glucocorticoid resistance is especially problematic for individuals with asthma as it limits cortisol’s effectiveness in down-regulating the production of pro-inflammatory cytokines responsible for asthma attacks, thus increasing their frequency (Chen & Miller, 2007). Furthermore, many individuals with asthma rely on corticosteroid medications (e.g., inhalers) to control asthma symptoms (Barnes & Adcock, 2009). Thus, identifying factors affecting NR3C1 expression is important for improving health, particularly for those with asthma.
NR3C1 expression and methylation are affected by adverse social experiences like child abuse (McGowan et al., 2009) and poor maternal care (Stanton et al., 2017; Weaver et al., 2004). Of particular interest is the association between SES and NR3C1 expression, given the large SES disparities in inflammatory disease (Galobardes, Lynch, & Smith, 2008). Low SES is associated with reduced NR3C1 expression and the down-regulation of genes with response elements for the glucocorticoid receptor (Miller & Chen, 2007; Miller et al., 2009). However, psychosocial mediators of the effect of SES on NR3C1 expression are yet to be identified.
More broadly, there is extensive work identifying emotions as key mediators of links between SES and physical health. The reserve capacity model outlines how negative affect serves as a psychosocial mediator of links between SES and health (Gallo, Bogart, Vranceanu, & Matthews, 2005; Gallo & Matthews, 2003). Low SES individuals are prone to experiencing more stressors and negative life events, which leads them to experience negative emotions more often. Further, low SES individuals tend to experience more negative emotions regardless of number of stressors experienced (McLeod & Kessler, 1990) because they tend to interpret ambiguous events as more threatening and have fewer resources (tangible, interpersonal, or intrapersonal) available to help them cope with any stressors that arise (Gallo & Matthews, 2003). Negative emotionality is also associated with poor health outcomes (Kiecolt-Glaser, McGuire, Robles, & Glaser, 2002; Smith, Glazer, Ruiz, & Gallo, 2004) and has been found to mediate links between SES and health for outcomes such as metabolic functioning (Lehman, Taylor, Kiefe, & Seeman, 2005; McCurley et al., 2017), cardiovascular disease risk (Taylor, Lehman, Kiefe, & Seeman, 2006), and mortality (Lazzarino, Hamer, Stamatakis, & Steptoe, 2013; Nabi et al., 2008). It therefore seems likely that emotion should mediate links between SES and inflammation regulatory elements just as with inflammatory disease.
However, the links between SES, negative emotionality, and health are typically studied within the individual: An individual’s SES predicts their own negative emotionality and health. This perspective ignores how an individual’s SES affects the people surrounding them, and how this social environment may explain links between SES and health. In this study, we extend prior work by examining how SES shapes the emotional climate of a key social environment: the family. When, how often, and what types of emotions are expressed by family members in the home is referred to as family emotional climate (Luebbe & Bell, 2014). Families vary greatly in the prevalence and intensity of negative affect expressed in the home (Halberstadt & Eaton, 2003), and one major predictor of family emotional climate is SES (Raver & Spagnola, 2003; Smith & Walden, 1999). Although both positive and negative family emotional climates have been shown to predict children’s anxiety and depressive symptoms (Luebbe & Bell, 2014), only two studies have tested its effects on physical health, and both focused on family negative emotional climate. In these studies, Wood and colleagues (2008; Wood et al., 2007) found that family negative emotional climate predicted asthma severity.
Studies on SES, health, and family emotional climate have mostly used self-report measures of emotion (e.g., Luebbe & Bell, 2014; Wood et al., 2007). This is problematic for two reasons. First, self-reports, particularly at the general, global level (e.g., “How often do you feel sad?”), are subject to bias (Stone et al., 2000). Second, reporting on emotions experienced does not identify how much these emotions are displayed. The damaging effects of family negative emotional climate are at least partially dependent on how much family members express their negative emotions (rather than simply feel negative emotions internally). Indeed, maternal negative emotionality has larger effects on child behavior when mothers are highly expressive (Slatcher & Trentacosta, 2012). In a few cases, emotional expression was rated from lab interaction tasks (Wood et al., 2008), bypassing these issues. However, the extent to which emotional expression in lab situations parallels that in daily life is unclear.
To measure emotional expressivity in daily life, we observed families using the electronically activated recorder (EAR) (Mehl, Pennebaker, Crow, Dabbs, & Price, 2001). Participants wear the EAR and it records short audio clips unobtrusively throughout the day, which are coded for aspects of interaction quality. This is ideal for assessing family emotional climate, as the EAR records what the youth are exposed to. EAR observations of parent-child interactions have been linked to health outcomes including asthma symptoms (Tobin, Kane, Saleh, Naar-King, et al., 2015), diurnal cortisol rhythms (Slatcher & Robles, 2012), and immune responses (Tobin, Kane, Saleh, Wildman, et al., 2015). The EAR addresses the issues of bias, shared method variance, and generalizability that can hinder questionnaire and laboratory measures of emotion.
Thus, in this study, we tested links between SES, family emotional climate, and NR3C1 expression in a sample of youth (ages 10–17) with asthma. We hypothesized that (1) youth in lower SES families will have lower NR3C1 gene expression, and (2) that the link between low SES and NR3C1 expression will be mediated by greater family negative emotional climate.
2. Materials and methods
2.1 Participants
Participants in the current investigation were included from the first wave of an ongoing longitudinal study, Asthma in the Lives of Families Today (ALOFT; recruited from November 2010–June 2014). The ALOFT study explores family dynamics, biological changes, and asthma morbidity among youth from the Metro-Detroit area. Participants were recruited from local area hospitals and schools. To be included in the study, youth were required to be between 10 and 17 years of age and diagnosed with asthma. Youth were screened for medical conditions and medications that might affect asthma and associated biological markers. The full study included 194 youth and their primary caregivers (typically parents). However, only youth with NR3C1 expression data and valid EAR data for measuring family emotional climate (see section 2.3.3) were included in the current investigation1. Thus, the sample was comprised of 104 youth (62 boys and 42 girls), whose average age was 12.86 years old (range = 10.01–16.67 yrs.), and at least one primary caregiver. Fifty-four percent of families had two parents living in the home. The youth in this sample were 69.8% African American/black, 26.4% Caucasian/White, 1.9% Latino, and 1.9% multiracial. Of the parents, 70.2% reported their personal income tax bracket to be below $31,850, but 11.6% of parents earned over $64,251 per year. The modal self-reported parental education (25.5%) was one or more years of college but no degree completed (range = 9th grade completed to Ph.D.).
2.2 Procedures
Families interested in participating in the study called the laboratory and were informed that the purpose of the study was to better understand the links between daily life and asthma. The parent (primary caregiver of the child) completed a telephone screening interview to determine eligibility in the study. Written assent and consent were obtained from the participating youth and their parent, respectively.
The participating youth and parent visited the laboratory for their first visit, where they completed a number of background questionnaires on a computer and individual interviews assessing conflict and stress from the youth and parent's perspective. Also at this visit, the youth and parent were given detailed instructions regarding wearing the EAR. The laboratory visit lasted approximately two hours. Youth then wore the EAR for four days following the lab visit and completed daily diaries at the end of each day (detailed information about the EAR is provided below). Participants returned study materials and the EAR to the lab or during a home-visit. Following the four-day monitoring period, a peripheral blood draw was conducted for each youth participant. Youth and parents were compensated and reimbursed for their time.
2.3 Measures
Descriptive statistics for all measures are in Table 1.
Table 1.
Bivariate correlations and descriptive statistics for study variables
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Female | 1.00 | −0.02 | 0.01 | 0.08 | 0.19† | −0.02 | 0.06 | −0.07 | −0.21* | −0.26** | 0.18 |
| 2. Non-White | 1.00 | −0.34** | −0.27** | −0.18† | −0.49** | 0.20* | 0.16 | −0.02 | 0.13 | −0.10 | |
| 3. Both parents in the house | 1.00 | 0.12 | −0.03 | 0.17† | −0.07 | −0.01 | −0.10 | −0.08 | 0.13 | ||
| 4. Medication | 1.00 | 0.13 | 0.10 | −0.18† | −0.12 | −0.01 | 0.05 | 0.12 | |||
| 5. Child age | 1.00 | 0.03 | −0.19* | −0.31** | −0.21* | −0.29** | 0.00 | ||||
| 6. SES | 1.00 | −0.14 | −0.07 | 0.05 | −0.18† | 0.08 | |||||
| 7. Family stress | 1.00 | 0.19† | −0.05 | −0.05 | −0.01 | ||||||
| 8. Family conflict | 1.00 | 0.07 | 0.25* | −0.03 | |||||||
| 9. Family positive emotional climate | 1.00 | 0.28** | −0.04 | ||||||||
| 10. Family negative emotional climate | 1.00 | −0.25* | |||||||||
| 11. NR3C1 expression | 1.00 | ||||||||||
| Mean (sd) or % | 41.5% | 73.6% | 53.5% | 45.3% | 12.86 (1.78) | .05 (.89) | .002 (.79) | .05 (.05) | −.05 (.91) | −.05 (.65) | 4.00 (2.34) |
Note:
p< .10,
p< .05,
p< .01
2.3.1 Socioeconomic status
To assess socioeconomic status (SES), the parent reported their level of education completed and yearly income. Each was standardized and averaged (r = .56) to create a composite measure of SES.
2.3.2 Medication use
Use of asthma-related medication was obtained from daily logs completed across the four days prior to the blood draw. Each day, youth reported on whether they used (1) inhaled beta-agonist (yes/no), (2) inhaled corticosteroid (yes/no), (3) inhaled combination corticosteroid and beta-agonist (yes/no), (4) oral corticosteroid (yes/no), and/or (5) leukotriene-modifying agent (yes/no). We then created a dichotomous medication use variable (yes/no) by averaging use across four days. If youth had a value above zero for any of the five types of asthma medication, they were given a “yes” score on the dichotomous variable; otherwise, they received a “no” score on the dichotomous variable. Approximately 45.3% of the sample used medication.
2.3.2 Social stress
To ensure that possible associations between negative affect expressivity and NR3C1 gene expression were not simply due to differences in stress experienced by lower versus higher SES participants, each youth and parent completed the Life Stress Interview (LSI) (Adrian & Hammen, 1993; Hammen, 1991). The LSI assesses the extent to which individuals have been exposed to both chronic stress and acutely stressful events over the past six months across a number of domains. To create a measure of overall stress, the number of acute events reported by the parent and the child were standardized and averaged.
2.3.3 EAR measures
In order to assess family emotional climate and family conflict in daily life, each youth wore the Electronically Activated Recorder (EAR; Mehl et al., 2001). Following the laboratory session, the youth participant wore the EAR for four days (two weekdays and two weekend days). Youth were instructed to wear the EAR continuously in their front pocket or in a belt clip provided from the time they woke up until bedtime. Recordings captured 50 seconds of sound every nine minutes.
EAR data were coded using the Everyday Child Home Observation (ECHO) (Slatcher & Tobin, 2012) coding system that specified the youth’s current location, activity, mood, and behaviors related to specific types of parent-child interactions. Inter-coder reliability was determined by a set of training recordings (512 fifty second recordings) independently coded by twenty research assistants. Intraclass correlations (ICCs) based on a two-way random effects model were calculated for each coded behavior. ICCs ranged from .92–.96. EAR data were only used for families who had 30 or more talking files (n=104) (Tobin, Kane, Saleh, Naar-King, et al., 2015; Tobin, Kane, Saleh, Wildman, et al., 2015). These participants had an average of 90.02 (SD = 39.85) talking files. Scores for each EAR-observed behavior reflect a mean of the total recordings in which the behavior was observed during waking hours. EAR coders listened to all participant files prior to coding. During this time, they identified the youth participant and parents based upon the frequency in which speaker was found in sound files and a small snippet of spoken language recorded during the laboratory visit. As only the youth (and not the parents) was wearing the EAR, all codes reflect to behaviors taking place in the presence of the youth.
2.3.3.1 Family emotional climate
The extent to which youth and parent(s) expressed five negative emotions (i.e., sadness, anger, upset, worry, distress; α=.73 for youth, α=.72 for mothers, and α=.66 for fathers) and three positive emotions (i.e., happy, interested, excited; α=.78 for youth, α=.54 for mothers, and α=.53 for fathers) were rated for each EAR file on Likert-type scales from 1 (not at all) to 5 (extremely). Emotion ratings were averaged across all EAR files for each individual and standardized, and then scores were summed to create positive and negative affect ratings for each individual. Finally, positive and negative affect expression scores were averaged across the youth and one or both (if available) parents to create measures of family positive emotional climate and family negative emotional climate.
2.3.3.2 Family conflict
To ensure that links between negative emotionality and NR3C1 expression were not driven only by family conflict, we also measured the level of conflict in the home using the EAR. Coders reported whether each EAR file contained an interpersonal argument, conflict, or fight (regardless of who was involved in the conflict) and whether each EAR file contained the child and/or either parent yelling. Following prior work (Tobin, Kane, Saleh, Naar-King, et al., 2015), these binary ratings were averaged across EAR files and then summed to create a measure of family conflict (α=.60).
2.3.4 NR3C1 gene expression
Each youth provided 8 mL of peripheral blood collected into Vacutainer Cell Preparation Tubes containing sodium heparin (Becton Dickinson and Co., East Rutherford, NJ). In order to assess messenger RNA (mRNA) levels of the glucocorticoid receptor (GR) gene NR3C1, total RNA was extracted from peripheral blood mononuclear cells (PBMCs) following the manufacturer protocol (Becton Dickinson and Co., East Rutherford, NJ). RNA integrity was assessed on an Agilent Bioanalyzer and only samples with RIN>6.0 were included in the study. Total RNA was reverse transcribed to cDNA using SuperScript III kit (Life Tech) and following the manufacturer’s protocol. Gene expression was quantified using TaqMan gene expression assays (Applied Biosystems) on an Applied Biosystems 7500-FAST or StepOnePlus real-time PCR thermocycler, following manufacturer’s protocol. Average CT values were calculated for NR3C1 and the endogenous control (18S rRNA) across three technical replicates for each sample. For each sample, the coefficient of variation across replicates was less than 20%. Relative gene expression values (in CT units) for NR3C1 in each sample were normalized to the endogenous control and expressed as delta CT values.
2.4 Analysis plan
The expectation maximization algorithm, which provides unbiased parameter estimates and improves statistical power of analyses (Enders, 2001; Scheffer, 2002), was used to replace missing values (2.2%) for self-report measures. All self-report variables with missing data were continuous except for medication use and number of parents in the house, which were dichotomous. Because this algorithm does not allow value replacement for dichotomous data, mode replacement was used to replace these missing values (i.e., 1 for medication use and 1 for both parents in the home).
To test hypotheses, all continuous predictors were standardized, while dichotomous variables were coded as 0 and 1 (i.e., 0 = male, 1 = female). For each hypothesis, three multiple regression models of covariates were run: No covariates (Model 1); demographic covariates (i.e., race, sex, age, number of parents in the home, medication use, stress; Model 2); demographic and observational covariates (i.e., family positive emotional climate and family conflict; Model 3) to ensure that results were robust. For Hypothesis 1, regression analyses predicted NR3C1 expression from SES with differing levels of covariates. For Hypothesis 2, indirect effect analyses testing an indirect path from SES to family emotional climate to NR3C1 expression using a bootstrapping approach with 20,000 iterations were run using the PROCESS macro (Model 4) (Hayes, 2013) for all three models.
3. Results
Bivariate correlations were conducted to examine the relations among study variables (see Table 1). There was a marginally significant correlation between SES and family negative emotional climate such that lower SES was associated with greater family negative emotional climate. However, there were no significant correlations between SES and family positive emotional climate, family conflict, or self-reported social stress. Suggesting the potential for an indirect path as specified in Hypothesis 2, NR3C1 gene expression was negatively associated with EAR-measured family negative emotional climate. NR3C1 gene expression was not associated with family positive emotional climate, family conflict, family stress, or SES. EAR-assessed family negative emotional climate and family positive emotional climate were significantly positively associated, suggesting that positive and negative emotional expressivity at least partially tap into an overall emotional climate of the family.
3.2 Hypothesis 1: Lower SES is associated with reduced NR3C1 expression
To test Hypothesis 1, regression analyses predicting NR3C1 expression from SES were conducted for all three models (see Table 2). These models did not support our first hypothesis: SES was not significantly associated with NR3C1 expression in any model (b’s < .08, p’s > .42).
Table 2.
Models predicting NR3C1 expression from SES and covariates
| SES | Female | Non-white | Age | # of parents |
Medication use |
Stress | Conflict | EAR
family positive affect expression |
|
|---|---|---|---|---|---|---|---|---|---|
| b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | |
| Model 1 | .08 (.10) | ||||||||
| Model 2 | .05 (.12) | .18 (.11) | −.02 (.12) | −.07 (.12) | .08 (.11) | .10 (.11) | .01 (.10)) | ||
| Model 3 | .05 (.12) | .18 (.11) | −.02 (.12) | −.09 (.12) | .08 (.11) | .10 (.11) | .01 (.10) | −.04 (.11) | −.01 (.11) |
Note: All statistics standardized.
p< .10,
p< .05,
p< .01
3.3 Hypothesis 2: Family emotional climate as a mediator
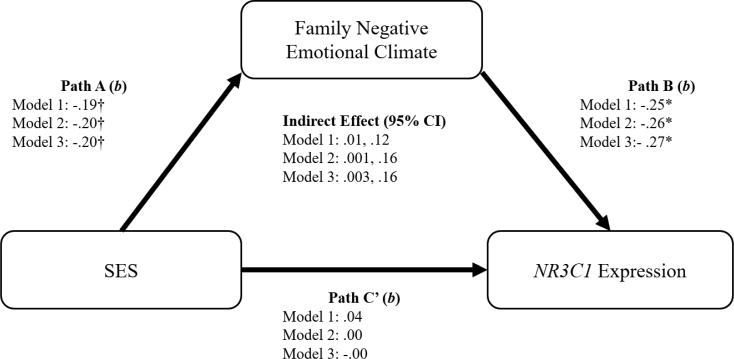
Despite the lack of a direct effect of SES on NR3C1 gene expression, we tested for the hypothesized indirect path from SES to family negative emotional climate to NR3C1 expression, as significant indirect paths can exist in the absence of significant total effects due to Type II errors and can be estimated directly using bootstrapping analyses (see Hayes, 2009, 2013; Shrout & Bolger, 2002; Zhao, Lynch, & Chen, 2010). Bootstrapping analyses revealed a significant indirect effect of SES on NR3C1 via family negative emotional climate in all models (see Figure 1), such that lower SES was associated with greater family negative emotional climate, which in turn was associated with reduced NR3C1 gene expression. Based on model r2 values, Model 1 predicted 6.2% of the variance in NR3C1 expression, and Models 2 and 3 predicted 11% of the variance.
Figure 1.
Mediation models testing an indirect path from SES to NR3C1 expression through negative family emotional climate
† p<.10, *p < .05, **p < .01
Note: Model 1 includes no covariates. Model 2 includes sex, race, age, number of parents in the home, medication usage, and family stress as covariates. Model 3 includes the Model 2 covariates as well as EAR-observed conflict and family positive emotional climate.
4. Discussion
This study tested negative family emotional climate as a mediator of links between SES and expression of NR3C1, an important anti-inflammatory gene, in a sample of youth with asthma. Contrary to Hypothesis 1, we found no direct links between SES and NR3C1 expression; however, as proposed in Hypothesis 2, there was a significant indirect path from lower SES to reduced NR3C1 expression via a more negative family emotional climate (as observed using EAR recordings of everyday family life). This suggests that family emotional climate may be a key mechanism linking SES to health-related biology in youth with asthma.
These effects were specific to family negative emotion climate. Family positive emotional climate was not significantly associated with SES or NR3C1 expression. This aligns with previous work on SES, family emotional climate, and health (Wood et al., 2008; Wood et al., 2007), which has found family negative emotional climate to be more impactful than family positive emotional climate. However, other work has shown that increased levels of positive affect elicited by social experiences can also be linked to beneficial endocrine and inflammatory outcomes (Pressman & Cohen, 2005; Tobin, Kane, Saleh, Wildman, et al., 2015). Future work should establish under what conditions positive versus negative emotions experienced at both the individual and family level modulate inflammatory and neuroendocrine processes.
Furthermore, these findings were robust after controlling for a number of other demographic and psychosocial covariates. In particular, the effects of family negative emotional climate were significant above and beyond the number of stressors assessed with the LSI, showing that the indirect effect of being in a low SES family on NR3C1 expression was not reflective of just experiencing a greater number of stressors, but rather being exposed to greater expressions of emotional negativity beyond that. Furthermore, the reported effects were also not simply driven by the experience greater interpersonal conflict in the home, as evidenced by the robustness of these effects to controlling for observed family conflict. Instead, they appear to show a more global general tendency towards negativity across family members.
This work also suggests that studying emotional dynamics within the broader family unit can better our understanding of the wide range of impacts of SES. As nuclear families share SES levels, everyone in a family are similarly at risk for the adverse consequences of low SES. It may be that the health effects of SES are due as much to the collective family experiences of being low SES as the experience of the individual in question. Interventions at the broader family level may therefore be most effective at improving health (Miller, Brody, Yu, & Chen, 2014).
This study addresses one of the biological pathways underlying SES health disparities in addition to the psychosocial pathways. Glucocorticoid receptor availability and glucocorticoid resistance have important implications for asthma and other inflammatory disorders via the regulation of inflammatory cytokine production and the effectiveness of corticosteroid medications for treating these diseases. An important direction for future research is understanding how mediators like family emotional climate get “under the skin” to affect underlying biology. It is likely that being surrounded by negativity in the home evokes repeated biological stress responses in these children, as chronically high cortisol levels are known to lead to glucocorticoid resistance (Miller, Cohen, & Ritchey, 2002), but this is yet to be shown.
The current research addresses some key methodological issues that have hindered prior work by studying the family emotional climate naturalistically as it unfolds in daily life. Rather than relying on self-reports or lab observations, we rated expressions of negative affect by youth and parents from short audiorecordings of everyday life using the EAR. This allowed us to observe what the youth was hearing and being exposed to in their own home and not under artificial circumstances, maximizing the ecological validity of our measure of family emotional climate.
However, the present study does have some limitations. First, the sample entirely consists of families in which at least one child has asthma, so our ability to generalize these findings is limited. Providing care of a child with a chronic illness may increase stress and lead to higher expression of negative emotions, particularly in low SES families (Wallander & Varni, 1989), and having asthma or use of asthma medications may affect the biological processes assessed here. Our findings should be replicated in more diverse samples to determine their generalizability.
Second, we tested the effects of SES and family emotional climate on only one gene. Although NR3C1 plays an important role in regulating the inflammatory response (Bray & Cotton, 2003; McMahon et al., 2009), many other genes are also involved in regulating inflammation. The extent to which SES and family emotional climate have a widespread effect, resulting in major changes in inflammation and inflammatory disease, is unclear. Studies examining the effects of SES on expression genome-wide find a number of additional genes involved in regulating inflammatory responses with differential expression based on SES levels (Chen et al., 2009), so future work should test the effects of SES and family emotional climate more broadly.
Third, these data are cross-sectional. This makes it difficult to identify causal pathways and also does not inform on whether the effects of family emotional climate are long-lasting. Early adversity, particularly low SES, is known to continue to affect health well into adulthood (Ehrlich, Miller, & Chen, 2016; Miller & Chen, 2013). Although we were unable as of yet to test the extent to which family emotional climate has long-term effects in this study, recent reviews on early life stress and adult inflammation suggest there are many reasons to think these effects may not be transient (Fagundes & Way, 2014; Kim, Evans, Chen, Miller, & Seeman, 2018). Growing up in a negative family emotional climate can result in increased attachment insecurity (Diehl, Elnick, Bourbeau, & Labouvie-Vief, 1998), and attachment insecurity is associated with numerous poor health outcomes (Pietromonaco, Uchino, & Dunkel Schetter, 2013). Alternatively, negative family emotional climates also can result in poor emotion regulation skills, especially for children who are highly reactive (Morris, Silk, Steinberg, Myers, & Robinson, 2007), which can lead them to continue to experience greater levels of negative emotionality (and the health problems that result from it) throughout their lives. Furthermore, there is increasing evidence that pro-inflammatory phenotypes developed while growing up in poverty, particularly during critical periods for plasticity, can lead to the biological embedding of a pro-inflammatory phenotype that persists across the lifetime (Miller & Chen, 2013). Future work should take a longitudinal approach to test the lasting impact of family emotional climate on inflammatory processes.
Fourth, it is difficult to discern the clinical significance of the effects of SES and family negative emotional climate on NR3C1 expression. Our models only explained up to 11% of the variance in NR3C1 expression, and researchers are yet to identify key thresholds for glucocorticoid receptor availability. Thus, it is difficult to determine if changes of this magnitude are associated with clinically significant health outcomes, such as ER visits, pulmonary function, or asthma symptoms. It may also take time for decreases in NR3C1 expression to become reflected in clinical health outcomes, so longitudinal research will be particularly informative in this domain as well.
As inflammatory disease continues to be a major cause of disease, particularly for low SES individuals, it is continually important to uncover the biological and psychosocial pathways underlying these links. This study provides insight into how these processes occur in youth, showing that being in a negatively toned environment is associated with dysregulation in the biological processes designed to keep inflammation in check. By understanding these pathways, we can hopefully find ways to modify them in order to help at-risk individuals live healthier lives.
Highlights.
This study tested links between socioeconomic status and expression of the glucocorticoid receptor gene NR3C1 via naturalistically observed family emotional climate.
There was a significant indirect path from socioeconomic status to NR3C1 expression via family negative emotional climate.
No equivalent path was found for family positive emotional climate.
Acknowledgments
This work was supported by the National Institutes of Health (RO1HL114097) and a Wayne State University Junior Faculty Grant in the Social and Behavioral Sciences. We thank all the research assistants in the Close Relationships Lab for their help with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
These NR3C1 expression values were previously used in the Stanton et al. (2017) paper on the effects of maternal attachment orientations on NR3C1 expression, but findings with the predictors of interest in the current paper have not been published previously.
References
- Adrian C, Hammen C. Stress exposure and stress generation in children of depressed mothers. Journal of Consulting and Clinical Psychology. 1993;61(2):354. doi: 10.1037//0022-006x.61.2.354. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. The Lancet. 2009;373(9678):1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- Bray PJ, Cotton RG. Variations of the human glucocorticoid receptor gene (NR3C1): pathological and in vitro mutations and polymorphisms. Human Mutation. 2003;21(6):557–568. doi: 10.1002/humu.10213. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE. Stress and inflammation in exacerbations of asthma. Brain, Behavior, and Immunity. 2007;21(8):993–999. doi: 10.1016/j.bbi.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Walker HA, Arevalo JM, Sung CY, Cole SW. Genome-wide transcriptional profiling linked to social class in asthma. Thorax. 2009;64(1):38–43. doi: 10.1136/thx.2007.095091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaboration, E. R. F. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. The Lancet. 2010;375(9709):132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl M, Elnick AB, Bourbeau LS, Labouvie-Vief G. Adult attachment styles: Their relations to family context and personality. Journal of Personality and Social Psychology. 1998;74(6):1656. doi: 10.1037//0022-3514.74.6.1656. [DOI] [PubMed] [Google Scholar]
- Ehrlich KB, Miller GE, Chen E. Childhood adversity and adult physical health. Developmental psychopathology 2016 [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annual Review of Medicine. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Way B. Early-life stress and adult inflammation. Current Directions in Psychological Science. 2014;23(4):277–283. [Google Scholar]
- Gallo LC, Bogart LM, Vranceanu A-M, Matthews KA. Socioeconomic status, resources, psychological experiences, and emotional responses: a test of the reserve capacity model. Journal of Personality and Social Psychology. 2005;88(2):386. doi: 10.1037/0022-3514.88.2.386. [DOI] [PubMed] [Google Scholar]
- Gallo LC, Matthews KA. Understanding the Association between Socioeconomic Status and Health: Do Negative Emotions Play a Role? Psychological Bulletin. 2003;129:10–51. doi: 10.1037/0033-2909.129.1.10. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. Journal of Epidemiology and Community Health. 2008;62(5):387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- Gross KL, Lu NZ, Cidlowski JA. Molecular mechanisms regulating glucocorticoid sensitivity and resistance. Molecular and Cellular Endocrinology. 2009;300(1):7–16. doi: 10.1016/j.mce.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. The generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hayashi R, Wada H, Ito K, Adcock IM. Effects of glucocorticoids on gene transcription. European Journal of Pharmacology. 2004;500(1):51–62. doi: 10.1016/j.ejphar.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication monographs. 2009;76(4):408–420. [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press; 2013. [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: New perspectives from psychoneuroimmunology. Annual Review of Psychology. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- Kim P, Evans GW, Chen E, Miller G, Seeman T. Handbook of Life Course Health Development. Springer; 2018. How socioeconomic disadvantages get under the skin and into the brain to influence health development across the lifespan; pp. 463–497. [PubMed] [Google Scholar]
- Lazzarino AI, Hamer M, Stamatakis E, Steptoe A. The combined association of psychological distress and socioeconomic status with all-cause mortality: a national cohort study. JAMA internal medicine. 2013;173(1):22–27. doi: 10.1001/2013.jamainternmed.951. [DOI] [PubMed] [Google Scholar]
- Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relation of childhood socioeconomic status and family environment to adult metabolic functioning in the CARDIA study. Psychosomatic Medicine. 2005;67:846. doi: 10.1097/01.psy.0000188443.48405.eb. [DOI] [PubMed] [Google Scholar]
- Luebbe AM, Bell DJ. Positive and negative family emotional climate differentially predict youth anxiety and depression via distinct affective pathways. Journal of Abnormal Child Psychology. 2014;42(6):897–911. doi: 10.1007/s10802-013-9838-5. [DOI] [PubMed] [Google Scholar]
- McCurley JL, Penedo F, Roesch SC, Isasi CR, Carnethon M, Sotres-Alvarez D, Schneiderman N, Gonzalez P, Chirinos DA, Camacho A. Psychosocial factors in the relationship between socioeconomic status and cardiometabolic risk: the HCHS/SOL Sociocultural Ancillary Study. Annals of Behavioral Medicine. 2017:1–12. doi: 10.1007/s12160-016-9871-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod JD, Kessler RC. Socioeconomic status differences in vulnerability to undesirable life events. Journal of Health and Social Behavior. 1990;31:162–172. [PubMed] [Google Scholar]
- Mehl MR, Pennebaker JW, Crow MD, Dabbs J, Price JH. The Electronically Activated Recorder (EAR): A device for sampling naturalistic daily activities and conversations. Behavior Research Methods, Instruments, and Computers. 2001;33:517–523. doi: 10.3758/BF03195410. [DOI] [PubMed] [Google Scholar]
- Miller GE, Brody GH, Yu T, Chen E. A family-oriented psychosocial intervention reduces inflammation in low-SES African American youth. Proceedings of the National Academy of Sciences. 2014;111(31):11287–11292. doi: 10.1073/pnas.1406578111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosomatic Medicine. 2007;69(5):402–409. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. The biological residue of childhood poverty. Child development perspectives. 2013;7(2):67–73. doi: 10.1111/cdep.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Morris AS, Silk JS, Steinberg L, Myers SS, Robinson LR. The role of the family context in the development of emotion regulation. Social development. 2007;16(2):361–388. doi: 10.1111/j.1467-9507.2007.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi H, Kivimäki M, Marmot MG, Ferrie J, Zins M, Ducimetière P, Consoli SM, Singh-Manoux A. Does personality explain social inequalities in mortality? The French GAZEL cohort study. International Journal of Epidemiology. 2008;37(3):591–602. doi: 10.1093/ije/dyn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietromonaco PR, Uchino B, Dunkel Schetter C. Close relationship processes and health: Implications of attachment theory for health and disease. Health Psychology. 2013;32:499–513. doi: 10.1037/a0029349. Document Type: journal DOI: 10.1037/a0029349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does Positive Affect Influence Health? Psychological Bulletin. 2005;131(6):925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychological Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- Slatcher RB, Robles TF. Preschoolers' everyday conflict at home and diurnal cortisol patterns. Health Psychology. 2012;31(6):834–838. doi: 10.1037/a0026774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatcher RB, Tobin ET. Everyday Child Home Observation Coding System. Wayne State University; Detroit: 2012. [Google Scholar]
- Slatcher RB, Trentacosta CJ. Influences of parent and child negative emotionality on young children's everyday behaviors. Emotion. 2012;12:932–942. doi: 10.1037/a0027148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TW, Glazer K, Ruiz JM, Gallo LC. Hostility, anger, aggressiveness, and coronary heart disease: An interpersonal perspective on personality, emotion, and health. Journal of Personality. Special Issue: Emotions, Personality, and Health. 2004;72(6):1217–1270. doi: 10.1111/j.1467-6494.2004.00296.x. [DOI] [PubMed] [Google Scholar]
- Stanton SC, Zilioli S, Briskin JL, Imami L, Tobin ET, Wildman DE, Mair-Meijers H, Luca F, Kane HS, Slatcher RB. Mothers’ attachment is linked to their children’s anti-inflammatory gene expression via maternal warmth. Social Psychological and Personality Science. 2017 doi: 10.1177/1948550616687125. 1948550616687125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, Turkkan JS, Bachrach CA, Jobe JB, Kurtzman HS, Cain VS. The science of self-report: Implications for research and practice 2000 [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biological Psychiatry. 2006;60(8):819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Tobin ET, Kane HS, Saleh DJ, Naar-King S, Poowuttikul P, Secord E, Pierantoni W, Simon V, Slatcher RB. Naturalistically Observed Conflict and Youth Asthma Symptoms. Health Psychology. 2015;34:622–631. doi: 10.1037/hea0000138. doi: http://dx.doi.org/10.1037/hea0000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin ET, Kane HS, Saleh DJ, Wildman DE, Breen EC, Secord E, Slatcher RB. Asthma-related immune responses in youth with asthma: Associations with maternal responsiveness and expressions of positive and negative affect in daily life. Psychosomatic Medicine. 2015;77:892–902. doi: 10.1097/PSY.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallander JL, Varni JW. Social support and adjustment in chronically ill and handicapped children. American Journal of Community Psychology. 1989;17(2):185–201. doi: 10.1007/BF00931007. [DOI] [PubMed] [Google Scholar]
- Weaver L, Cervoni N, Champagne F, D'Allessio A, Sharma S, Seckl J. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7:847. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wood BL, Lim J, Miller BD, Cheah P, Zwetsch T, Ramesh S, Simmens S. Testing the biobehavioral family model in pediatric asthma: Pathways of effect. Family Process. Special issue on families and asthma. 2008;47(1):21–40. doi: 10.1111/j.1545-5300.2008.00237.x. [DOI] [PubMed] [Google Scholar]
- Wood BL, Lim J, Miller BD, Cheah PA, Simmens S, Stern T, Waxmonsky J, Ballow M. Family emotional climate, depression, emotional triggering of asthma, and disease severity in pediatric asthma: Examination of pathways of effect. Journal of Pediatric Psychology. 2007;32(5):542–551. doi: 10.1093/jpepsy/jsl044. [DOI] [PubMed] [Google Scholar]
- Zhao X, Lynch JG, Chen Q. Reconsidering Baron and Kenny: Myths and truths about mediation analysis. Journal of consumer research. 2010;37(2):197–206. [Google Scholar]