Abstract
Background
Duchenne muscular dystrophy (DMD) patients are prone to ventricular arrhythmias, which may be caused by abnormal calcium (Ca2+) homeostasis and elevated reactive oxygen species (ROS). Ca2+/calmodulin-dependent protein kinase II (CaMKII) is vital for normal Ca2+ homeostasis, but excessive CaMKII activity contributes to abnormal Ca2+ homeostasis and arrhythmias in cardiomyocytes. ROS induces CaMKII to become autonomously active. We hypothesized that genetic inhibition of CaMKII oxidation (ox-CaMKII) in a mouse model of DMD can alleviate abnormal Ca2+ homeostasis, thus preventing ventricular arrhythmia. The objective of the study was to test if selective loss of ox-CaMKII affects ventricular arrhythmias in the mdx mouse model of DMD.
Methods and Results
5-(6)-chloromethyl-2,7-dichlorodihydrofluorescein diacetate staining revealed increased ROS production in ventricular myocytes isolated from mdx mice, which coincides with elevated ventricular ox-CaMKII demonstrated by Western blotting. Genetic inhibition of ox-CaMKII by knockin replacement of the regulatory domain methionines with valines (MM-VV) prevented ventricular tachycardia in mdx mice. Confocal calcium imaging of ventricular myocytes isolated from mdx:MM-VV mice revealed normalization of intracellular Ca2+ release events compared to cardiomyocytes from mdx mice. Abnormal action potentials assessed by optical mapping in mdx mice were also alleviated by genetic inhibition of ox-CaMKII. Knockout of the NADPH oxidase regulatory subunit p47phox normalized elevated ox-CaMKII, repaired intracellular Ca2+ homeostasis, and rescued inducible ventricular arrhythmias in mdx mice.
Conclusions
Inhibition of ROS or ox-CaMKII protects against pro-arrhythmic intracellular Ca2+ handling, and prevents ventricular arrhythmia in a mouse model of DMD.
Journal Subject Terms: Arrhythmias, Animal Models of Human Disease, Cell Signaling/Signal Transduction, Oxidant Stress, Cardiomyopathy
Keywords: Duchenne muscular dystrophy, calcium/calmodulin-dependent protein kinase II, oxidation, reactive oxygen species
Introduction
Duchenne muscular dystrophy (DMD) is the most prevalent forms of muscular dystrophy. It is an X-linked recessive disorder, caused by mutations within the dystrophin gene.1 DMD is a fatal disease, affecting 1 in 3,500 male births, characterized by degeneration of muscle tissue with no definitive treatment. Patients with DMD often suffer from cardiomyopathy and may develop cardiac arrhythmias. Cardiomyopathy is found in 25% of DMD patients under the age of 6, 59% of 10 year-old patients, and virtually all adult DMD patients. Moreover, 26% of DMD patients also exhibit ventricular tachycardia (VT) and 51% exhibit an increased heart rate variability.2
Recent studies suggest that increased production of reactive oxygen species (ROS) may contribute to the development of cardiomyopathy in DMD. Studies in mdx mice, a mouse model of DMD, revealed enhanced ROS production in the hearts of these mice.3, 4 Abnormal ROS production is one of the key factors leading to altered excitation-contraction coupling in mdx mice, which may contribute to contractile dysfunction and enhanced arrhythmogenesis.4, 5 ROS can alter cardiac excitation-contraction coupling through oxidation of various phosphatases and kinases, including Ca2+/calmodulin-dependent protein kinase II (CaMKII).6, 7 CaMKII is a serine/threonine-kinase essential for intracellular Ca2+ homeostasis in cardiomyocytes. CaMKII activity is modulated by several post-translational modifications including autophosphorylation and oxidation, and aberrant CaMKII regulation contributes to development of heart failure and arrhythmias.8, 9 Moreover, our studies have revealed that CaMKII-mediated phosphorylation of RyR2 promotes VT in mdx mice.10
Oxidation of two methionine residues (M281 and M282) within the CaMKII regulatory domain locks CaMKII into a constitutively active, Ca2+- and calmodulin-independent conformational state.7 Previous studies suggest that oxidized CaMKII (ox-CaMKII) can cause sinus node dysfunction.11 Ox-CaMKII levels were found to be significantly increased in pacemaker tissues from diabetic patients compared with non-diabetic patients after myocardial infarction. Moreover, activation of a mitochondrial/ox-CaMKII pathway contributes to increased sudden death in diabetic patients after myocardial infarction.12
In this study, we tested the hypothesis that increased ROS production promotes ventricular arrhythmias in a mouse model of DMD as a result of CaMKII oxidation. Our findings revealed increased oxidation of M281/M282 on CaMKII in mdx mice. Genetic inhibition of oxidation of these residues prevented VT induction in mdx mice. We found that ox-CaMKII promoted spontaneous sarcoplasmic reticulum (SR) Ca2+ release events, and Ca2+ waves that can lead to ectopic activity in the hearts of mdx mice. Finally, genetic ablation of p47phox, a regulatory subunit of NADPH oxidase type 2 (NOX2), prevented CaMKII oxidation and VT, suggesting that NOX2 is involved in pathological ROS production in mdx mice.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Experimental Animals
Mdx mice were obtained from Jackson laboratories (C57BL/10ScSn-Dmdmdx/J). CaMKII M281/282V(MM-VV) mice, in which methionine residues 281 and 282 are substituted by valines and thereby cannot undergo oxidation, were previously reported.12 Mdx:MM-VV mice were generated by crossing mdx mice with MM-VV. All studies were performed on male mice according to protocols approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine conforming to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Mouse Ventricular Myocyte Isolation
Mouse ventricular myocytes were isolated as described.4 Briefly, mice were anesthetized and hearts were removed into 0 Ca2+ Tyrode solution (137 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 5 mM HEPES, 10 mM glucose, 3 mM NaOH, pH 7.4). The heart was cannulated through the aorta and connected to a Langendorff system, perfused with 0 Ca2+ Tyrode for 5 minutes, followed by 0 Ca2+ Tyrode containing 20 μg/ml Liberase (Roche, Indianapolis, IN) for 10 to 15 minutes at 37 °C. After digestion, the heart was removed in Krebs-bicarbonate buffer (90 mM KCl, 30 mM K2HPO4, 5 mM MgSO4, 5 mM pyruvic acid, 5 mM β-hydroxybutyric acid, 5 mM creatine, 20 mM taurine, 10 mM glucose, 0.5 mM EGTA, 5 mM HEPES, pH 7.2). The digested heart was minced and agitated in Krebs-bicarbonate buffer, then filtered through a 210 mm polyethylene mesh. The isolated ventricular myocytes were kept in Krebs-bicarbonate buffer before use.
Confocal Imaging
Confocal imaging was performed as described.4, 13 Isolated ventricular myocytes were incubated in 2 mM Fluo-4-acetoxymethyl ester (Fluo-4 AM, Invitrogen, Carlsbad, CA) in normal Tyrode’s solution containing 1.8 mM Ca2+ for 1 hour at room temperature, followed by 15 min for de-esterification with dye-free normal Tyrode’s and loaded on a laser scanning confocal microscope (LSM 510, Carl Zeiss, Thornwood, NY). Fluorescence images were recorded in line-scan mode with 1024 pixels per line at 500 Hz. After myocytes reached steady state with pacing at 1 Hz, pacing was stopping and Ca2+ sparks were counted. 10 mM caffeine was used to induce caffeine-induced Ca2+ transient to calculate SR Ca2+ content.
Western Blotting
Heart lysates were prepared from flash-frozen mouse ventricles as described.14 Ox-CaMKII was detected by using an antibody specific for oxidized M281/M282 on CaMKII, developed by Dr. Anderson (1:1,000).7,10 Refer to supplemental methods for further details.
CaMKII Activity Assay
Total CaMKII and Ca2+/CaM-independent CaMKII activity from mouse ventricular lysate were assessed using the SignaTECT CaMKII activity kit (Promega) in conjunction with γ-32P radio-labeled ATP (Perkin Elmer) according to the manufacturer’s instructions as described.15 See supplemental methods for details.
Programmed Electrical Stimulation
Atrial and ventricular intracardiac electrograms were recorded using a 1.1-F octapolar electrode catheter (EPR-800, Millar Instruments, Houston, TX) inserted into the right ventricle via the right jugular vein, as described.16 VT inducibility was determined using a combination of overdrive pacing and single, double and triple extrastimuli pacing protocols. Sustained VT was defined as ≥10 beats of VT.
Optical mapping
After euthanasia, the mouse heart was quickly removed and rinsed in oxygenated (95% O2, 5% CO2) cold normal Tyrode’s solution containing 2.5 mmol/L Ca2+ and 250 nmol/L isoproterenol. A pacing electrode (Harvard Apparatus, MA, USA) was placed on the surface of the heart, connected to a stimulator (PowerLab 26T, AD Instruments, Sydney, Australia). The anterior epicardial surface was excited using an LED light source centered at 530nm and band pass-filtered from 511 to 551 nm (LEX-2, SciMedia, CA, USA). Blebbistatin (Sigma-Aldrich, B0560-5mg, 50 ul of 2.5 mg/ml in dimethyl sulfoxide) was added to eliminate motion artifacts. The voltage-sensitive dye RH237 was added to the perfusate (Invitrogen, S-1109, 20ul of 2.5mg/ml in dimethyl sulfoxide). The emitted fluorescence Vm signal was collected through a 50mm lens camera (Leica Plan APO 1.0x, SciMedia, CA, USA) and filtered at 700nm. The right atrium was paced at 10-Hz (S1) followed by a single extra stimulus (S2) after 70ms to 40ms, decreasing by 2ms every time.
Statistical Analysis
Results are expressed as mean ± SEM. Continuous variables were evaluated using SPSS or Prism using an unpaired Student t test or ANOVA, after performing the D’Agostino-Pearson normality test for normal distribution of the data. Categorical variables were evaluated with Fisher’s exact test. P<0.05 was considered statistically significant.
Results
Elevated ox-CaMKII increases autonomously activated CaMKII in mdx mice
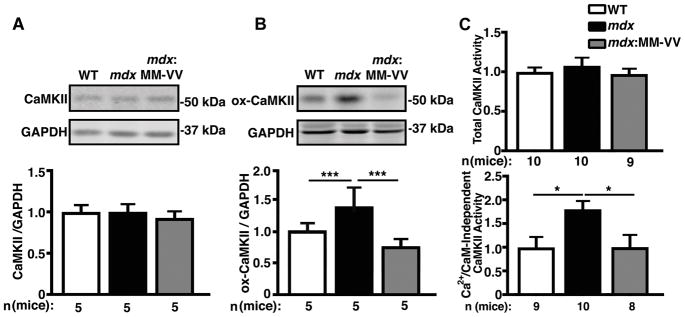
In recent studies, we demonstrated enhanced ROS production in the hearts of 3-month-old mdx mice.4 Consistent with these findings, we found enhanced production of ROS by quantifying 5-(6)-chloromethyl-2,7-dichlorodihydrofluorescein diacetate fluorescence in ventricular myocytes of 5-month-old mdx mice compared to wild-type (WT) littermates (Supplemental Fig. 1). Western blotting revealed unaltered protein levels of CaMKII in the ventricles of mdx mice compared to WT littermates (Fig. 1A). We previously demonstrated unaltered CaMKII autophosphorylation levels of CaMKII in 5-month-old mdx mice.10 In contrast, ox-CaMKII increased by 39±6 % in mdx mice compared to WT controls (P<0.001; Fig. 1B). This enhanced CaMKII oxidation was abrogated by genetic inhibition of CaMKII oxidation in mdx:MM-VV mice, in which M281 and M282 on CaMKII are mutated to valine (MM-VV) to selectively ablate activation by oxidation,12 while maintaining other pathways for activation including autophosphorylation (Fig. 1B). Previously, it has been shown that CaMKII oxidation at M281/M282 can cause constitutive activation of CaMKII independent of Ca2+/CaM activation.15 In order to determine whether this increase in ox-CaMKII indeed enhanced Ca2+/CaM-independent activation of CaMKII, we performed a CaMKII activity assay. Consistent with the finding that total CaMKII protein was unaltered, we found that total Ca2+/CaM-activatable CaMKII activity was not changed between groups (Fig. 1C, upper panel). Conversely, the Ca2+/CaM-independent activity was significantly increased 1.8-fold in mdx hearts compared to WT (P=0.037), and was ameliorated in mdx-MMVV hearts (Fig. 1C, lower panel; P=0.037). These findings suggest that CaMKII activity is increased in the hearts of mdx mice due to enhanced ROS-mediated oxidation of M281/M282.
Figure 1. Elevated ox-CaMKII increases autonomously activated CaMKII in mdx mice.
(A) Representative Western blots and quantification of total CaMKII expression level in mdx mouse ventricular lysates compared to WT and mdx:MM-VV. (B) Representative Western blots and quantification of oxidation at M281/M282 (ox-CaMKII) demonstrating significantly increased expression of oxidized CaMKII in mdx mouse ventricular lysates compared to WT and mdx:MM-VV. (C) Fold change in total CaMKII activity induced by Ca2+/CaM (top), and Ca2+/CaM-independent CaMKII activity (bottom). *P<0.05, ***P<0.001.
Genetic inhibition of ox-CaMKII prevents ventricular arrhythmia in mdx mice
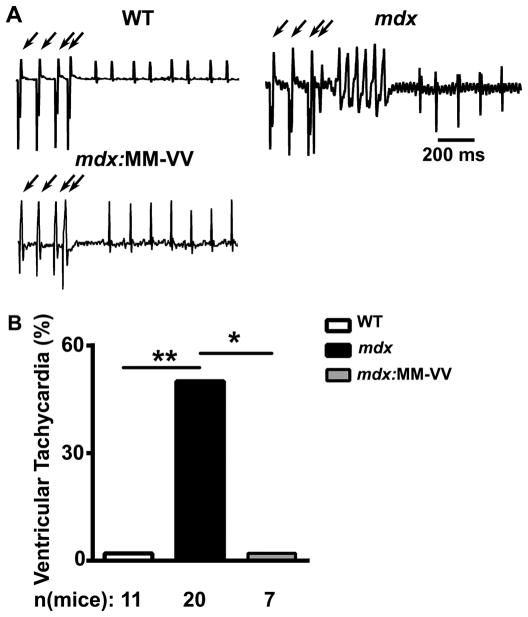
We previously demonstrated that mdx mice develop VT in response to programmed electrical stimulation. Moreover, pharmacological and genetic inhibition of CaMKII prevented VT induction in mdx mice.10 To assess whether oxidation of CaMKII underlies the enhanced susceptibility to arrhythmias, we performed arrhythmia induction studies in mdx:MM-VV mice. While 50% of mdx mice developed reproducible sustained VT following intracardiac stimulation, none of the mdx:MM-VV mice developed VT (P<0.05; Fig. 2). These results suggest that oxidation of CaMKII underlies susceptibility to ventricular arrhythmias in mdx mice.
Figure 2. Genetic inhibition of ox-CaMKII inhibits ventricular tachycardia in mdx mice.
(A) Representative electrograms were recorded from WT, mdx, and mdx:MM-VV mice following programmed electrical stimulation (PES; arrows). (B) Bar graph showing incidence of ventricular tachycardia following PES. *P<0.05, **P<0.01.
Inhibition of ox-CaMKII normalizes intracellular Ca2+ handling in mdx mice
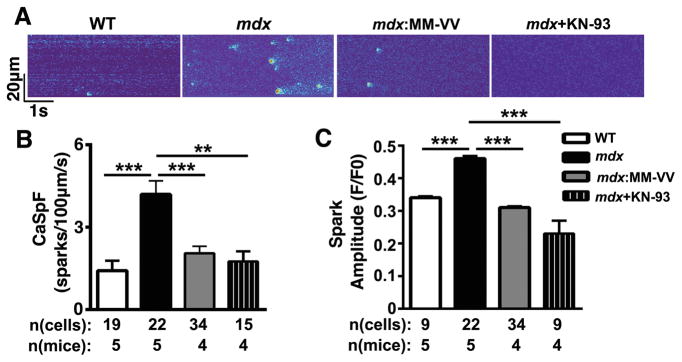
Prior studies suggest that ventricular arrhythmias in mdx mice are in part caused by aberrant Ca2+ release events from the SR.10 Here, we investigated whether ox-CaMKII contributes to abnormal SR Ca2+ handling in mdx mice. First, confocal imaging was performed to quantify spontaneous SR Ca2+ release events known as Ca2+ sparks in ventricular myocytes from WT, mdx, and mdx:MM-VV mice. Compared to WT littermates (1.4 ± 0.4 sparks/100μm/s), mdx mice exhibited a three-fold increase in Ca2+ spark frequency (CaSpF; 4.2 ± 0.5 sparks/100μm/s; P<0.001; Fig. 3A,B). In contrast, the CaSpF was significantly reduced in mdx:MM-VV mice (2.1 ± 0.3 sparks/100μm/s) compared to mdx mice. A similar reduction in CaSpF (1.8 ± 0.4 sparks/100μm/s; P<0.01) was found in mdx cardiomyocytes treated with KN-93, a pharmacological inhibitor of CaMKII. Further analysis of spark properties revealed an increased Ca2+ spark amplitude in mdx mice (0.46 ± 0.008 a.u.) compared to WT mice (0.34 ± 0.005; P<0.001; Fig. 3C). Genetic inhibition of ox-CaMKII significantly reduced the spark amplitude in mdx:MM-VV mice (0.31 ± 0.004; P<0.001 vs. mdx). Again, a similar reduction in spark amplitude was found in mdx cardiomyocytes treated with KN-93 (0.23 ± 0.003; P<0.001).
Figure 3. Genetic inhibition of ox-CaMKII normalizes Ca2+ sparks in mdx mice.
(A) Representative line scan images after 1-Hz pacing recorded from isolated ventricular myocytes from WT, mdx, mdx: MM-VV mice, and mdx myocytes treated with CaMKII inhibitor KN-93. (B) Bar graph of averaged Ca2+ spark frequencies and (C) Ca2+ spark amplitude. **P<0.01,***P<0.001.
To further examine the potential pro-arrhythmic potential of aberrant SR Ca2+ release event, ventricular myocytes were first subjected to a low frequency pacing train (1-Hz) followed by a pause. There was a trend towards an increased frequency of spontaneous Ca2+ waves (SCaWs) in mdx mice (1.5 ± 0.4 events/min) compared to WT mice (0.4 ± 0.3; Supplemental Fig. 2A). The SCaW frequency was significantly lower in mdx:MM-VV mice (0.2 ± 0.2 events/min) compared to mdx mice (P<0.01). In addition, there was a lower Ca2+ transient amplitude in mdx mice compared to WT (P<0.01), although this was not rescued in mdx:MM-VV mice (Supplemental Fig. 2B). There were no significant changes in sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2a) (Supplemental Fig. 2C) or Na+/Ca2+-exchanger (NCX) activity among the three groups of mice (Supplemental Fig. 2D). Finally, pharmacological inhibition of CaMKII using KN-93 had effects on Ca2+ handling similar to genetic inhibition of CaMKII oxidation (which prevents activation).
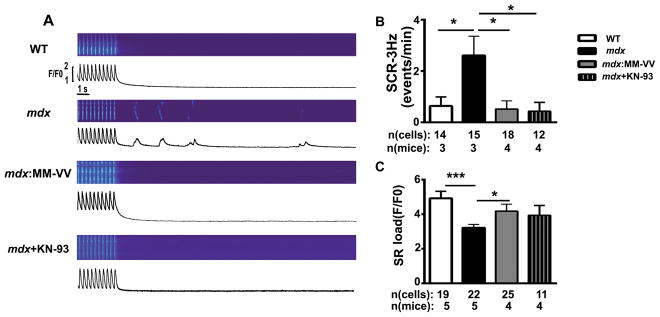
Next, similar experiments were performed in myocytes paced at 3-Hz. Following a 3-Hz pacing train, the incidence of SCaWs was significantly higher in mdx mice (2.6 ± 0.8 events/min) compared to WT littermates (0.6 ± 0.4; P<0.05; Fig. 4A,B). This was reversed by genetic inhibition of ox-CaMKII in mdx:MM-VV mice (0.5 ± 0.3; P<0.05 vs mdx). Pharmacological inhibition of CaMKII using KN-93 also suppressed Ca waves. To determine whether enhanced SR Ca2+ leak altered SR Ca2+ storage, 10 mM caffeine was applied to measure SR Ca2+ content. Myocytes from mdx mice had significantly reduced SR Ca2+ content (3.2 ± 0.2 F/Fo) compared to those from WT mice (4.9 ± 0.4, P<0.001; Fig. 4C). In contrast, inhibition of ox-CaMKII in mdx mice normalized SR Ca2+ content (4.2 ± 0.4; P<0.05 vs mdx). Together, these data demonstrate that CaMKII oxidation is a key determinant of aberrant Ca2+ homeostasis in myocytes from mdx mice.
Figure 4. Genetic inhibition of ox-CaMKII normalizes Ca2+ waves in mdx mice.
(A) Representative line scan images after 3-Hz pacing recorded in ventricular myocytes from WT, mdx, mdx: MM-VV mice, and mdx mice treated with KN-93. (B) Average frequency of spontaneous Ca2+ waves following 3-Hz pacing and (C) quantification of caffeine-induced SR Ca2+ load. *P<0.05, *** P<0.001.
Inhibition of ox-CaMKII prevents triggered activity in isolated mdx mouse hearts
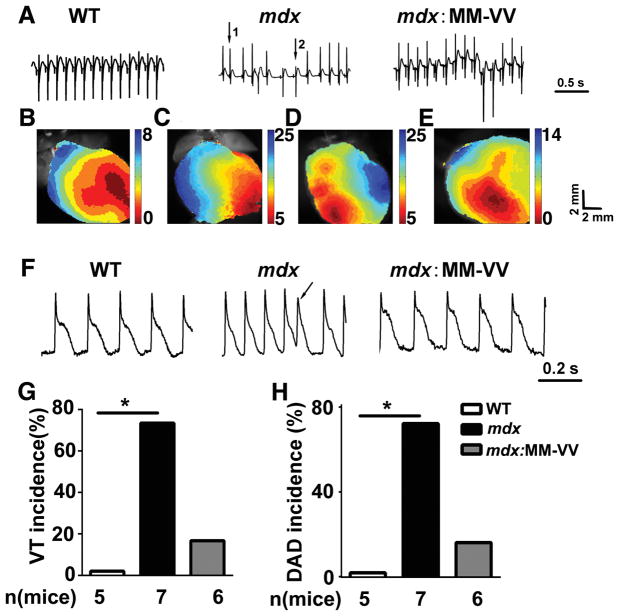
To gain more insight into the mechanisms underlying ventricular arrhythmias in mdx mice, optical mapping with simultaneous ECG monitoring was conducted (Fig. 5). Optical mapping of membrane potential was performed on perfused hearts ex vivo to visualize action potentials and epicardial activation sequences. In hearts from WT mice, sinus rhythm and normal epicardial activation patterns were observed. Following atrial depolarization, ventricular epicardial activation started from the apex of the right ventricle and spread towards the base. These studies revealed that none of the 5 WT mice developed ventricular arrhythmias (Fig. 5,A,B,G). In contrast, 5 of 7 mdx mice (P<0.05 vs WT) exhibited ventricular arrhythmias, initiated by ectopic focal activity (Fig. 5A,C,D,G). In one of these 5 mice, a clear rotor pattern could be seen following arrhythmia initiation. In the other 4 mice, arrhythmias appear to be caused by repeat focal activity. There was a trend towards a reduced ventricular arrhythmia incidence in mdx:MM-VV mice (Fig. 5G; 1 of 6 mice; P=0.10 vs mdx).
Figure 5. Genetic inhibition of ox-CaMKII attenuates ventricular arrhythmias in isolated mdx heart.
(A) Representative electrogram traces in ex vivo hearts from WT, mdx and mdx:MM-VV mice. Following sinus rhythm (arrow 1), ventricular tachycardia (VT) beats emerged spontaneously (arrow 2) in the mdx mouse heart. (B) Normal activation map of WT mouse heart. (C) Activation maps of mdx mouse heart corresponding to normal beat (arrow 1) and (D) the ventricular tachycardia beat (arrow 2). (E) Representative activation map of heart from mdx: MM-VV mice showing normal activation map. (F) Representative action potential (AP) tracings from WT, mdx, and mdx:MM-VV hearts. Arrow denotes delayed after depolarization (DAD). (G–H) Quantification of VT (G) and DAD (H) incidence in ex vivo WT, mdx, and mdx:MM-VV hearts.
Next, we assessed the incidence of delayed afterdepolarizations (DADs) following a pacing train. WT mouse hearts did not exhibit any DADs (0 of 5 WT mice, Fig. 5F,H). In contrast, 5 of 7 mdx mouse hearts developed DAD (P<0.05; arrow in Fig. 5F, and Fig. 5H). There was a trend towards a reduction in DAD incidence in isolated mdx:MM-VV hearts (1 of 6 mdx:MM-VV, P=0.10; Fig. 5F,H). KN-93, which is a small molecule inhibitor of CaMKII, also inhibited ventricular arrhythmia induction in mdx mice (Supplemental Fig. 3), confirming that CaMKII is a key factor responsible for ventricular arrhythmias in mdx mice.
NADPH oxidase mediates CaMKII activation in mdx mice
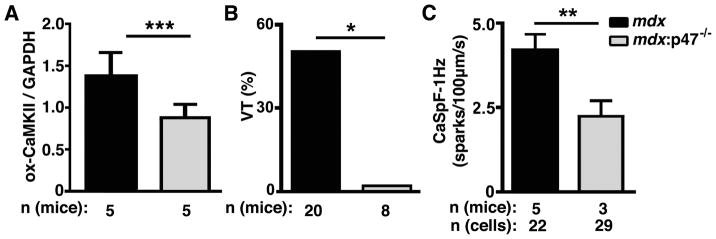
In a final set of experiments, we sought to determine whether NADPH oxidase is the source of ROS in mdx mice.4 p47phox is a key regulatory subunit of the NOX2 complex required for its activity.17 The oxidation level of CaMKII was reduced in mdx:p47−/− mice compared to mdx mice, suggesting that NOX2 mediates increased ox-CaMKII in mdx mice (Fig. 6A). The incidence of inducible VT was also reduced in mdx:p47−/− mice (0 of 8 mice) compared to mdx mice (10 of 20 mice; P<0.05; Fig. 6B). Finally, NOX2 inhibition by p47phox ablation also reduced the frequency of Ca2+ sparks frequency in mdx:p47−/− mice (2.2 ± 0.5 sparks/100μm/s) compared to mdx (4.2 ± 0.5, P<0.01; Fig. 6C). These results further support that NOX2-mediated oxidation of CaMKII is responsible for arrhythmogenic Ca2+ release in mdx mice.
Figure 6. NADPH oxidase activity is required for aberrant ox-CaMKII, heart rhythms, and calcium handling in mdx mice.
(A) Quantification of Western blots demonstrating ox-CaMKII level in mdx mice in which the NADPH oxidase 2 (Nox2) subunit p47phox was absent (mdx:p47−/−). (B) Incidence of ventricular tachycardia (VT) following programmed electrical stimulation in mdx and mdx:p47−/− mice. (C) Reduced Ca2+ spark frequency in myocytes from mdx:p47−/− mice compared to mdx. *P<0.05, **P<0.01, ***P<0.001.
Discussion
Enhanced CaMKII activity levels have been reported in several cardiovascular and pulmonary diseases, as well as cancer.8, 18, 19 Since CaMKII is one of the key protein kinases that regulate intracellular Ca2+ homeostasis in cardiac myocytes, altered CaMKII activity can facilitate the development of arrhythmias. Indeed, CaMKII hyperactivity has been associated with atrial fibrillation and ventricular arrhythmias in heart failure and dystrophic cardiomyopathy.10, 20–22
CaMKII can be activated by several mechanisms including an increased heart rate which leads to autophosphorylation of threonine 287 on neighboring CaMKII subunits within the holoenzyme complex,23, 24 by ROS which leads to oxidation of methionine residues 281 and 282,7 or by O-GlcNAcylation of serine 279.25 In a prior study, we demonstrated that cardiac CaMKII autophosphorylation was not increased in 5-month old mdx mice, whereas ox-CaMKII levels were increased.10 Moreover, in the present study we found that autonomous Ca2+/CaM-independent CaMKII activity was significantly increased in mdx mice (Fig. 1). Together, these findings suggest that ox-CaMKII is responsible for enhanced CaMKII enzymatic activity at least during early stages of dystrophic cardiomyopathy.
CaMKII can phosphorylate several Ca2+ channels and transporters involved in excitation-contraction coupling, including both sarcolemmal and SR channels. In a previous study, we demonstrated that inhibition of CaMKII phosphorylation of RyR2 prevents ventricular arrhythmias and abnormal SR Ca2+ leak in mdx mice.10 KN-93 or the transgenic inhibitory peptide AC3I both suppressed CaMKII activity, and reduced phosphorylation of S2814 on RyR2 in mdx mice.10 RyR2 was proven to be a critical downstream target of CaMKII because mutation of the CaMKII phosphorylation site S2814 on RyR2 to alanine was sufficient to prevent the induction of ventricular arrhythmias in mdx mice. Similar to CaMKII post-translational modifications, enhanced oxidation preceded increased phosphorylation of RyR2 in mdx mice as described before.4 Other post-translational modifications may contribute to enhanced RyR2-mediated SR Ca2+ leak, including enhanced ROS-dependent nitrosylation,26 and increased oxidation of RyR2.5 This may promote a feed-forward mechanism by which enhanced RyR2 channel activity promotes increased ROS production and further protein oxidation. While direct RyR2 oxidation represents one possible mechanism contributing to mdx pathogenesis, our data show that CaMKII inhibition by KN-93 and oxidation site ablation (mdx:MM-VV, Fig. 3) ameliorate aberrant SR Ca2+ release. This demonstrates that targeting CaMKII activity is sufficient to normalize SR Ca2+ in mdx mice.
Another SR channel that can be affected by CaMKII phosphorylation is SERCA2a. CaMKII phosphorylation of phospholamban a negative regulator of SERCA2a de-represses SERCA2a activity. However, there is no evidence of enhanced SERCA2a activity in mdx mice. On the contrary, two prior publications showed reduced SERCA2a activity in mdx mice,27, 28 whereas in our study we did not find significant differences between SERCA2a activity comparing WT and mdx mice (Supplemental Fig. 1). One possible explanation for the discrepancy between our study and the previous studies is that we used mdx mice on a mixed Bl6/Bl10 background, whereas the previous studies were performed on mdx mice on a Bl10 background.
Sarcolemmal ion channels can also be phosphorylated by CaMKII. The L-type Ca2+ channel, Na+ channel, and NCX activity can be enhanced by CaMKII phosphorylation. However, in this study we did not find a significant increase in NCX activity (Supplemental Fig. 1), and did not assess L-type Ca2+ channel or Na+ channel activity. The lack of change in NCX activity may be due to more pronounced ox-CaMKII activity near the SR as compared to the sarcolemma, at least in hearts of 5-month old mdx mice.
In the present study, we found a 3-fold increase in the frequency of Ca2+ sparks and spontaneous Ca2+ waves, consistent with diastolic Ca2+ leak through RyR2.10 Diastolic release of Ca2+ can cause DADs in myocytes from mdx mice, which in turn can lead to triggered ventricular arrhythmias (Fig. 5). In isolated hearts from mdx mice, we observed bidirectional VT and sustained VT, which are also seen in patients with CPVT, a genetic condition caused by gain-of-function RyR2 mutations.29 In addition, in some mdx mice a re-entry pattern with ‘rotor’-like patterns was observed. These findings suggest that triggered arrhythmias may evolve into re-entrant arrhythmias, which may be facilitated by fibrotic structural remodeling,30 or temporary changes in Ca2+ homeostasis as a result of the arrhythmia activity.22
In this study, increased ROS production was detected in cardiomyocytes isolated from mdx mice, consistent with prior studies.4, 31 Jung et al.31 suggested that enhanced fragility of the cell membrane resulting from the lack of dystrophin increases susceptibility to mechanical stress, which in turn causes more ROS production by membrane-associated NADPH. NOX2 and NOX4 are the most abundantly expressed isoforms in adult cardiomyocytes, and increased expression of both has been reported in hearts of mdx mice.28, 32 Prosser et al.33 elegantly demonstrated that NOX2 is activated by disrupted membranes and mechanical stress. Enhanced stretch-dependent ROS production in mdx mice promotes intracellular Ca2+ waves.33 Our data are in agreement and show that ROS produced by NOX2 promotes SR Ca2+ release abnormalities. Previously, we demonstrated increased ROS in mdx cardiomyocytes, which was abrogated by NOX2 inhibitor gp91ds.4 Moreover, this is corroborated by the finding that p47phox knockout reduced ox-CaMKII levels in mdx mice (Fig. 6). Taken together, these data suggest that in mdx mouse cardiomyocytes increased NOX2-mediated ROS production increases ox-CaMKII, thereby promoting aberrant SR Ca2+ release.
Conclusions
In this study, we demonstrated that mdx mice – a small animal model of DMD – are susceptible to pacing-induced ventricular arrhythmias. Ox-CaMKII promotes aberrant SR Ca2+ release through RyR2, which leads to DADs and triggered ventricular arrhythmias. On the other hand, genetic inhibition of ox-CaMKII normalized intracellular Ca2+ handling and prevented ventricular arrhythmias in mdx:MM-VV mice. Knockout of the NADPH oxidase regulatory subunit p47phox normalized elevated ox-CaMKII levels, VT, and Ca2+ homeostasis in mdx mice, suggesting that ox-CaMKII is increased by NADPH oxidase activity in mdx mice. Therefore, targeting this oxidation signaling pathway may represent a novel treatment strategy to prevent ventricular arrhythmias in DMD.
Supplementary Material
What Is Known?
Patients with Duchenne’s muscular dystrophy develop life-threatening ventricular arrhythmias.
Enhanced CaMKII activity is associated with heart disease and arrhythmias, and can be activated by multiple mechanisms including ROS-mediated CaMKII oxidation.
What the Study Adds?
NOX2 mediated ROS-induced CaMKII activation in the mdx mouse model of Duchenne muscular dystrophy promotes aberrant Ca2+ release and triggers ventricular arrhythmias
Genetic inhibition of NOX2 ROS production or CaMKII oxidation, or pharmacological inhibition of CaMKII activity prevents ventricular arrhythmias in the mdx mice.
Targeting CaMKII oxidation may be an effective strategy for treatment of ventricular arrhythmias in Duchenne muscular dystrophy.
Acknowledgments
Sources of Funding: G.G.R. is supported by NIH grants AR053349 and AR-061370, M.E.A. by NIH grants HL070250 and HL096652, N.L. by HL121649, and X.H.T.W. by HL089598, HL091947, HL117641, HL129570, American Heart Association grant 13EIA14560061, and a Muscular Dystrophy Association grant.
Footnotes
Disclosures: M.E.A. is a named inventor on intellectual property claiming to treat arrhythmias by CaMKII inhibition and is a cofounder of Allosteros Therapeutics, a company aiming to develop CaMKII inhibitor therapeutics. X.H.T.W. is a founding partner of Elex Biotech, a start-up company that developed drug molecules that target ryanodine receptors for the treatment of cardiac arrhythmia disorders.
References
- 1.Cox GF, Kunkel LM. Dystrophies and heart disease. Curr Opin Cardiol. 1997;12:329–43. [PubMed] [Google Scholar]
- 2.van Westering TL, Betts CA, Wood MJ. Current understanding of molecular pathology and treatment of cardiomyopathy in duchenne muscular dystrophy. Molecules. 2015;20:8823–55. doi: 10.3390/molecules20058823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science. 2011;333:1440–5. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q, Wang W, Wang G, Rodney GG, Wehrens XH. Crosstalk between RyR2 oxidation and phosphorylation contributes to cardiac dysfunction in mice with Duchenne muscular dystrophy. J Mol Cell Cardiol. 2015;89:177–84. doi: 10.1016/j.yjmcc.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ullrich ND, Fanchaouy M, Gusev K, Shirokova N, Niggli E. Hypersensitivity of excitation-contraction coupling in dystrophic cardiomyocytes. Am J Physiol Heart Circ Physiol. 2009;297:H1992–2003. doi: 10.1152/ajpheart.00602.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–21. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–74. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson ME. Oxidant stress promotes disease by activating CaMKII. J Mol Cell Cardiol. 2015;89:160–7. doi: 10.1016/j.yjmcc.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becerra R, Roman B, Di Carlo MN, Mariangelo JI, Salas M, Sanchez G, Donoso P, Schinella GR, Vittone L, Wehrens XH, Mundina-Weilenmann C, Said M. Reversible redox modifications of ryanodine receptor ameliorate ventricular arrhythmias in the ischemic-reperfused heart. Am J Physiol Heart Circ Physiol. 2016;311:H713–24. doi: 10.1152/ajpheart.00142.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ather S, Wang W, Wang Q, Li N, Anderson ME, Wehrens XH. Inhibition of CaMKII phosphorylation of RyR2 prevents inducible ventricular arrhythmias in mice with Duchenne muscular dystrophy. Heart Rhythm. 2013;10:592–9. doi: 10.1016/j.hrthm.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, Gao Z, He BJ, Luczak ED, Joiner ML, Kutschke W, Yang J, Donahue JK, Weiss RM, Grumbach IM, Ogawa M, Chen PS, Efimov I, Dobrev D, Mohler PJ, Hund TJ, Anderson ME. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest. 2011;121:3277–88. doi: 10.1172/JCI57833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, Yang J, Glynn P, Sossalla S, Swaminathan PD, Weiss RM, Yang B, Rokita AG, Maier LS, Efimov IR, Hund TJ, Anderson ME. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest. 2013;123:1262–74. doi: 10.1172/JCI65268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, Wang Q, De Almeida AC, Skapura DG, Anderson ME, Bers DM, Wehrens XH. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–79. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds JO, Quick AP, Wang Q, Beavers DL, Philippen LE, Showell J, Barreto-Torres G, Thuerauf DJ, Doroudgar S, Glembotski CC, Wehrens XH. Junctophilin-2 gene therapy rescues heart failure by normalizing RyR2-mediated Ca2+ release. Int J Cardiol. 2016;225:371–380. doi: 10.1016/j.ijcard.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guilbert A, Lim HJ, Cheng J, Wang Y. CaMKII-dependent myofilament Ca2+ desensitization contributes to the frequency-dependent acceleration of relaxation. Cell Calcium. 2015;58:489–99. doi: 10.1016/j.ceca.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li N, Wehrens XH. Programmed electrical stimulation in mice. J Vis Exp. 2010:1730. doi: 10.3791/1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 18.Erickson JR, He BJ, Grumbach IM, Anderson ME. CaMKII in the cardiovascular system: sensing redox states. Physiol Rev. 2011;91:889–915. doi: 10.1152/physrev.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulman H, Anderson ME. Ca/Calmodulin-dependent Protein Kinase II in Heart Failure. Drug Discov Today Dis Mech. 2010;7:e117–e122. doi: 10.1016/j.ddmec.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–51. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swaminathan PD, Purohit A, Hund TJ, Anderson ME. Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ Res. 2012;110:1661–77. doi: 10.1161/CIRCRESAHA.111.243956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landstrom AP, Dobrev D, Wehrens XHT. Calcium Signaling and Cardiac Arrhythmias. Circ Res. 2017;120:1969–1993. doi: 10.1161/CIRCRESAHA.117.310083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malinow R, Madison DV, Tsien RW. Persistent protein kinase activity underlying long-term potentiation. Nature. 1988;335:820–4. doi: 10.1038/335820a0. [DOI] [PubMed] [Google Scholar]
- 24.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 25.Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–6. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fauconnier J, Thireau J, Reiken S, Cassan C, Richard S, Matecki S, Marks AR, Lacampagne A. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2010;107:1559–64. doi: 10.1073/pnas.0908540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams IA, Allen DG. Intracellular calcium handling in ventricular myocytes from mdx mice. Am J Physiol Heart Circ Physiol. 2007;292:H846–55. doi: 10.1152/ajpheart.00688.2006. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez DR, Treuer AV, Lamirault G, Mayo V, Cao Y, Dulce RA, Hare JM. NADPH oxidase-2 inhibition restores contractility and intracellular calcium handling and reduces arrhythmogenicity in dystrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2014;307:H710–21. doi: 10.1152/ajpheart.00890.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, DeSimone L, Coltorti F, Bloise R, Keegan R, Cruz Filho FE, Vignati G, Benatar A, DeLogu A. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 30.Silva MC, Magalhaes TA, Meira ZM, Rassi CH, Andrade AC, Gutierrez PS, Azevedo CF, Gurgel-Giannetti J, Vainzof M, Zatz M, Kalil-Filho R, Rochitte CE. Myocardial Fibrosis Progression in Duchenne and Becker Muscular Dystrophy: A Randomized Clinical Trial. JAMA Cardiol. 2017;2:190–199. doi: 10.1001/jamacardio.2016.4801. [DOI] [PubMed] [Google Scholar]
- 31.Jung C, Martins AS, Niggli E, Shirokova N. Dystrophic cardiomyopathy: amplification of cellular damage by Ca2+ signalling and reactive oxygen species-generating pathways. Cardiovasc Res. 2008;77:766–73. doi: 10.1093/cvr/cvm089. [DOI] [PubMed] [Google Scholar]
- 32.Kyrychenko S, Polakova E, Kang C, Pocsai K, Ullrich ND, Niggli E, Shirokova N. Hierarchical accumulation of RyR post-translational modifications drives disease progression in dystrophic cardiomyopathy. Cardiovasc Res. 2013;97:666–75. doi: 10.1093/cvr/cvs425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prosser BL, Khairallah RJ, Ziman AP, Ward CW, Lederer WJ. X-ROS signaling in the heart and skeletal muscle: stretch-dependent local ROS regulates [Ca(2)(+)]i. J Mol Cell Cardiol. 2013;58:172–81. doi: 10.1016/j.yjmcc.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.