Abstract
BACKGROUND
Excessive binge alcohol drinking has acute cardiac arrhythmogenic effects, including promotion of atrial fibrillation (AF), which underlies “Holiday Heart Syndrome.” The mechanism that couples binge alcohol abuse with AF susceptibility remains unclear. We previously reported stress-activated c-Jun N-terminal kinase (JNK) signaling contributes to AF development. This is interesting because JNK is implicated in alcohol-caused organ malfunction beyond the heart.
OBJECTIVES
The purpose of this study was to detail how JNK promotes binge alcohol-evoked susceptibility to AF.
METHODS
The authors found binge alcohol-exposure leads to activated JNK, specifically JNK2. Furthermore, binge alcohol induces AF (24- vs. 1.8-Hz burst pacing-induced episodes per attempt per animal), higher incidence of diastolic intracellular Ca2+activity (Ca2+waves, sarcoplasmic reticulum [SR] Ca2+leakage), and membrane voltage (Vm) and systolic Ca2+ release spatiotemporal heterogeneity (ΔtVm-Ca). These changes were completely eliminated by JNK inhibition both in vivo and in vitro. calmodulin kinase II (CaMKII) is a proarrhythmic molecule known to drive SR Ca2+ mishandling.
RESULTS
The authors report for the first time that binge alcohol activates JNK2, which subsequently phosphorylates the CaMKII protein, enhancing CaMKII-driven SR Ca2+ mishandling. CaMKII inhibition eliminates binge alcohol-evoked arrhythmic activities.
CONCLUSIONS
Our studies demonstrate that binge alcohol exposure activates JNK2 in atria, which then drives CaMKII activation, prompting aberrant Ca2+ waves and, thus, enhanced susceptibility to atrial arrhythmia. Our results reveal a previously unrecognized form of alcohol-driven kinase-on-kinase proarrhythmic crosstalk. Atrial JNK2 function represents a potential novel therapeutic target to treat and/or prevent AF.
Keywords: AF vulnerability, arrhythmic calcium activity, binge alcohol drinking, JNK, kinase inhibition, proarrhythmic calmodulin kinase II, stress-response kinase
Excessive binge drinking affects 38 million adults in the United States (1,2). Despite growing efforts at prevention, there is a significantly rising trend in the prevalence of binge drinking (1–3). The National Institute of Alcohol Abuse and Alcoholism (NIAAA) defines binge drinking as a short-term pattern of consumption (4 to 5 drinks per episode) leading to a blood alcohol level (BAL) >80 mg/dl (2,4).
Excessive binge drinking is a known independent risk factor for cardiac arrhythmias often called Holiday Heart Syndrome (5,6). Atrial fibrillation (AF) is the most frequently diagnosed arrhythmia among Holiday Heart Syndrome patients (5–7). Moreover, AF substantially increases the risk of morbidity and mortality due to embolic stroke and heart failure (8,9). Clinical data suggest that one-third of all new-onset AF cases are related to alcohol intoxication (10,11). Furthermore, clinical studies have revealed that binge alcohol intake creates a higher AF risk among patients with cardiovascular disease (12). Strikingly, this alcohol-associated AF risk exists even in patients without co-existing cardiovascular diseases (7,13,14). Alcohol abstinence can reverse AF, but recurrence is common as repeat binge drinking occurs at an alarmingly high frequency worldwide (5,6). Repeated binge drinking significantly enhances the risk of chronic alcohol abuse that can ultimately lead to alcoholic cardiomyopathy, which can also increase the risk of arrhythmia (15). To date, the molecular mechanisms that couple binge alcohol and enhanced AF propensity remain unclear. Thus, defining those mechanisms will further our understanding of this substantial alcohol-related public health issue.
Stress-activated c-Jun NH(2)-terminal kinase (JNK) is activated by many types of stress stimuli such as cardiovascular diseases, aging, and diabetes (16–19). JNK activation is well known to contribute to alcohol-caused organ damage (20,21). We recently reported that JNK activation promotes AF in aged hearts (18,19). We also showed that direct JNK activation by a JNK activator increased AF risk in young healthy animals (18,19). These findings prompted us to explore whether JNK plays a role in binge alcohol-associated enhancement of atrial arrhythmogenicity.
Emerging evidence suggests alcohol may change intracellular Ca2+dynamics (22) and cardiac contractile function (23), yet the consequences of binge alcohol on atrial sarcoplasmic reticulum (SR) handling of Ca2+and AF propensity remain largely unknown to date. Here, we combined a unique intravital atrial Ca2+ imaging approach in cardiac-specific transgenic mice to detail a causal link between binge alcohol and atrial arrhythmogenicity. The goal of the current studies was to understand how alcohol-evoked JNK regulates calmodulin kinase II (CaMKII), a proarrhythmic molecule (24) that, in turn, drives SR Ca2+ mishandling and consequently enhances AF susceptibility. Our findings indicate that modulating JNK could be a novel therapeutic approach to prevent or treat AF.
METHODS
See the Online Appendix for an expanded Methods section.
HUMAN SAMPLES
Human donor hearts were obtained from Illinois Gift of Hope Organ and Tissue Donor Network. The alcohol-exposed hearts were from donors with a history of repeated binge drinking whereas the control hearts were from donors without a history of alcohol use. All patients had no history of AF or any major cardiovascular diseases. Online Table 1 shows de-identified general data (age, sex, race, and other factors) of the donors obtained from Gift of Hope. The studies were approved by the Human Study Committees of Rush University Medical Center (RUMC) and Illinois Gift of Hope.
ANIMAL MODELS
All animal studies followed Guide for the Care and Use of Laboratory Animals (NIH publication, 8th edition, 2011) and were approved by the Institutional Animal Care and Use Committees of RUMC, Loyola University Chicago, and University of Alabama at Birmingham.
New Zealand White male rabbits were infused with either alcohol or saline (2 g/kg, intravenously every other day for a total of 4 injections) followed by atrial arrhythmia induction procedures and biochemical assays as previously described (18). Rabbit atrial myocytes were isolated as previously described (25). AF induction, confocal imaging, membrane potential (Vm)/Ca2+ dual-channel optical mapping and biochemical assays were conducted to assess the functional role of binge alcohol-evoked JNK in 3 binge alcohol-exposed mouse models including: 1) wild-type (WT) C57/Bj mice; 2) WT mice treated with a JNK2 inhibitor (in vivo); and 3) transgenic (Tg) JNK1/2dn (where “dn” stands for dominant negative) mice with overexpression of inactive cardiac-specific dominant negative JNK1 and JNK2 protein (26). Cardiac function of alcohol exposed mice and sham controls remained unaltered (Online Figure 1). The functional role of CaMKII was assessed in alcohol-exposed WT mice treated with the CaMKII inhibitor KN93 or its inactive analog KN92 prior to the terminal studies (Online Methods).
AF INDUCIBILITY ASSESSMENT, CONFOCAL CA2+ IMAGING, AND DUAL-CHANNEL OPTICAL MAPPING
Atrial arrhythmia inducibility was examined in alcohol-exposed rabbits and mouse models as previously described, with modification (18,19). Ca2+ imaging, and dual-channel optical mapping studies were conducted in intact mouse hearts (24 h after the last treatment of the 4 alcohol injections) as previously described (18,27). The lapse between the activation time of membrane voltage and Ca2+ transient (ΔtVm-Ca) was calculated and the standard deviation of the ΔtVm-Ca was calculated as previously described (28). Results were compared between binge alcohol hearts and sham controls. A well-characterized cultured atrial myocyte line, HL-1, was also used for our studies (18). To assess the roles of JNK and CaMKII, a JNK2-specific inhibitor or the CaMKII inhibitor KN93 was applied 24 h before the alcohol exposure. Diastolic SR Ca2+ leakage was measured using a well-established tetracaine-sensitive SR Ca2+ leakage protocol as previously described (25).
BIOCHEMICAL ASSAYS
Kinase activities were assessed from the production of ADP in the kinase reaction with either JNK-specific substrate c-Jun or CaMKII-specific substrate autocamtide-2 as previously described (19). Protein expression and phosphorylation status were assessed by immunoblotting assay as previously described (18,19,25).
STATISTICAL ANALYSIS
All data are presented as mean ± SEM. Differences between multiple groups or any 2 groups were evaluated using 1-way ANOVA with the post hoc Tukey test or Student’s t-test. When heterogeneity of variance was observed, a nonparametric Mann-Whitney U test or nonparametric 1-way ANOVA was performed. A p value of <0.05 was considered significant.
RESULTS
BINGE ALCOHOL ENHANCES JNK ACTIVATION AND ARRHYTHMIA SUSCEPTIBILITY IN RABBIT AND HUMAN ATRIA
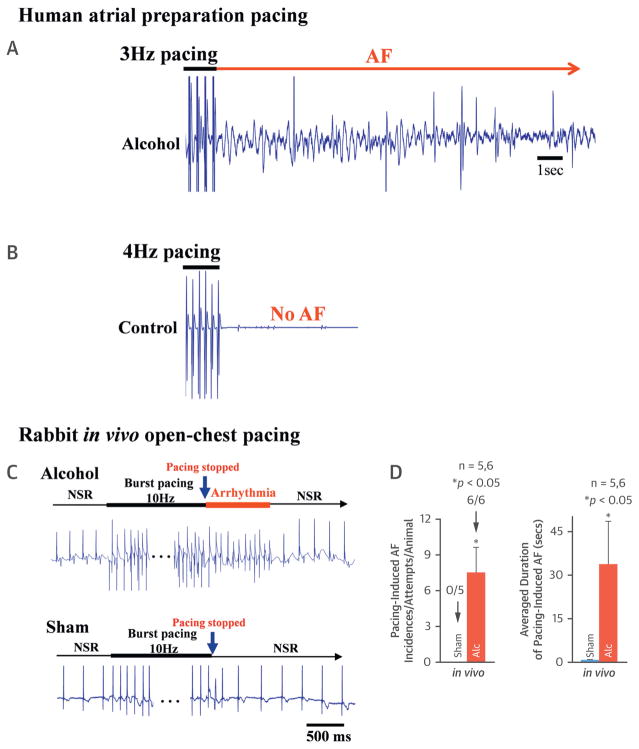
Human hearts from donors with a history of repeat binge alcohol exposure were studied and compared to donor hearts without a history of alcohol exposure. Langendorff-perfused human atrial preparations were challenged with electrical burst stimulations. There was a significantly increased AF incidence immediately after we stopped the electrical stimulation in the alcohol-exposed human atria (3 out of 4 hearts) (Figure 1A, long red arrows). In contrast, no pacing-induced AF events were found in the non–alcohol-exposed age-matched human control atria (0 out of 4 hearts) (Figure 1B). To further confirm these findings in human atria, we assessed proarrhythmic effects of well-controlled alcohol exposure in animals. The binge alcohol regimen consisted of 4 repeated doses (see Methods). In rabbits, there was a significantly increased pacing-induced incidence of AF in binge alcohol-exposed rabbit atria (6 of 6 vs. 0 of 5 in sham controls) (Figures 1C and 1D). The average length of pacing-induced AF was 29.8 ± 11.5 s in alcohol-exposed rabbits compared to 0.7 ± 0.1 s in sham controls (Figure 1D, sham, far right). Immunoblotting revealed the activation of the stress-response kinase JNK (assessed by phosphorylated JNK [JNK-p]), which was markedly enhanced in alcohol-exposed human atria, whereas the total JNK2 and JNK1 protein contents remained unchanged (Figure 2A). Similar to humans, significantly enhanced JNK activation (compared to sham control) was also found in alcohol-exposed rabbit atria (Figure 2B).
FIGURE 1. Binge Alcohol Increases the Inducibility of Atrial Arrhythmia in the Human Donor Heart and Rabbit Heart.
(A) Representative electrogram trace showing burst pacing (3 Hz)-induced AF in a binge alcohol-exposed human donor heart (with normal cardiac function). (B) Representative EG recording from a paced age-matched control human donor heart and there was no AF upon cessation of pacing. (C) Representative electrogram trace showing burst pacing (for 30 s at 6× diastolic threshold; cycle length = 100 ms)-induced AF immediately after cessation of the pacing in open-chest rabbits with binge alcohol pre-exposure, whereas the same burst pacing protocol did not induce any incidence of atrial arrhythmia in sham control rabbit. (D) Summarized data showing enhanced AF inducibility and increased average duration of pacing-induced AF in binge alcohol-exposed rabbits compared to that of sham controls. AF = atrial fibrillation; Alc = alcohol; EG = electrogram; NSR = normal sinus rhythm.
FIGURE 2. JNK Activation in Binge Alcohol-Exposed Atria.
(A) Summarized quantitative immunoblotting data and representative immunoblotting images show increased levels of JNK-P and unchanged expression of total JNK1 and JNK2 proteins in the atrial tissue from binge alcohol-exposed human donor hearts compared to non–alcohol-exposed controls. (B, C) Summarized data and representative immunoblotting images show enhanced level of JNK-P in alcohol-exposed rabbit and mouse atria.
GAPDH = glyceraldehyde 3-phosphate dehydrogenase; JNK = c-Jun N-terminal kinase; JNK-P = phosphorylated JNK; WT = wild type; other abbreviation as in Figure 1.
CAUSAL LINK–BETWEEN ALCOHOL-EVOKED JNK ACTIVATION, ARRHYTHMOGENIC CA2+ ACTIVITIES AND AF
AF inducibility was further measured in binge alcohol-exposed mouse models. In WT mice, binge alcohol exposure significantly increased JNK activation (JNK-p) (Figure 2C), as well as the incidence of burst-pacing–induced atrial arrhythmias. Burst-pacing– induced atrial arrhythmias were absent in sham controls (Figures 3A to 3C). Representative electrogram traces show alcohol-exposed mice developed atrial arrhythmia following 30 s of pacing at 8 Hz (3× diastolic threshold) (Figure 3A1), while sham control animals immediately returned to sinus rhythm (Figure 3A2). Strikingly, alcohol-evoked arrhythmia inducibility was nearly absent in JNK1/2dn mice (JNK1/2dn) (Figures 3A3, 3B, and 3C far right bars). The JNK1/2dn mice have cardiac-specific overexpression of inactive dominant negative JNK1 and JNK2 proteins for competing JNK inhibition. These results imply that JNK activation contributes to the enhanced AF susceptibility of alcohol hearts.
FIGURE 3. JNK Inhibition Precludes Binge Alcohol-Evoked Atrial Arrhythmogenicity in Intact Mouse Atria.
(A) Representative electrogram traces showing burst-pacing induced atrial arrhythmia in a Langendorff-perfused WT mouse heart with binge alcohol pre-exposure (1.), and self-restored NSR after burst pacing in sham control mouse hearts (2.) as well as binge alcohol-exposed JNK1/2dn mouse hearts with overexpression of cardiac-specific inactivated dominant negative JNK (3.). (B, C) Summarized data of enhanced AF inducibility and increased average duration of pacing-induced AF in binge alcohol-exposed WT mouse hearts compared to that of sham controls. JNK1/2dn = inactivated dominant negative JNK1 and JNK2; other abbreviations as in Figures 1 and 2.
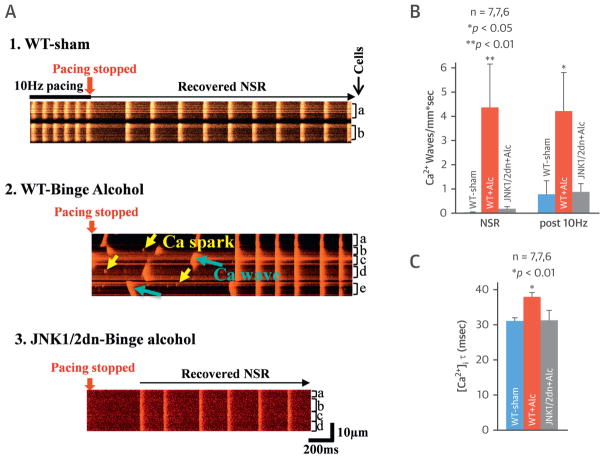
SR Ca2+ mishandling is known to be pivotal for Ca2+-triggered arrhythmic activities, and thus, high-resolution confocal Ca2+ imaging studies were performed using Langendorff-perfused intact mouse hearts. Binge alcohol-exposed WT mouse atria (compared to sham controls) had markedly increased spontaneous diastolic Ca2+ waves during normal sinus rhythm (NSR), as well as more frequent waves following 10-Hz burst pacing (Figures 4A1, 4A2, and 4B). The binge alcohol exposure also prolonged the intracellular Ca2+ decay constant (τ) (37.8 ± 1.26 ms vs. 31.0 ± 0.96 ms in sham) (Figure 4C). In the cardiac-specific JNK1/2dn Tg mouse atria, the expression of inactive JNK1/2dn proteins precluded alcohol-driven atrial Ca2+ waves and Ca2+ transient τ prolongation (31.1 ± 2.9 ms vs. 31.0 ± 0.9 ms in sham) (Figures 4A3, 4B, and 4C, far right bars). This is consistent with the rescue effect of JNK1/2 inhibition on alcohol-evoked AF shown in Figure 3.
FIGURE 4. JNK Inhibition Abolished Binge Alcohol-Evoked Abnormal Atrial Ca2+ Waves in Intact Mouse Atria.
(A) Representative confocal images show increased frequency of Ca2+ sparks (yellow arrows) and Ca2+ waves (blue arrows) in binge alcohol-exposed WT mouse atria after 10-Hz burst pacing (1.) and reversion of NSR in either control WT mice (2.) or JNK1/2dn mice with overexpression of cardiac-specific JNK1/2dn (3.). (B) Summarized data show increased frequency of Ca2+ waves in binge alcohol-exposed mouse atria, while dominant negative overexpression of JNK1/2 precluded binge alcohol-prompted Ca2+waves. (C) Atrial Ca2+decay constant τ was increased in binge alcohol-exposed WT mice, but binge alcohol-exposed JNK1/2dn mice exhibited unchanged τ of Ca2+ decay compared to that of sham controls. Ca2+ = calcium; other abbreviations as in Figures 1 to 3.
JNK2 INHIBITION ATTENUATES ALCOHOL-EVOKED ARRHYTHMOGENIC CA2+ ACTIVATES
Cardiac muscle contains predominantly the JNK1 and JNK2 isoforms, but these 2 isoforms have different functions (26). We immunoprecipitated the JNK2 and JNK1 proteins with JNK isoform-specific antibodies, followed by JNK isoform-specific activity measurement. In alcohol-exposed WT mouse atria, there was a 37% increase in JNK2 activity compared to activity in sham controls (Figure 5A, left bars), whereas JNK1 activity remained unchanged (Figure 5A, right bars). The functional contribution of JNK2 during alcohol-driven atrial arrhythmogenicity was assessed using JNK2-specific inhibition and simultaneous Vm and intracellular Ca2+ dual-channel optical mapping in intact WT mouse atria. Compared to the sham controls, alcohol-exposed atria showed substantially increased spatiotemporal Vm and Ca2+ signal heterogeneity. Summarized data show binge alcohol significantly increases the mean standard deviation of the lapse between the activation time of action potential and Ca2+ transient (ΔtVm-Ca) (Figures 5B and 5C) compared to sham controls. This type of increased spatial heterogeneity of ΔtVm-Ca is arrhythmogenic (28) and indicative of abnormal Ca2+ activities. Binge alcohol-exposed WT mice were then treated with a JNK2 inhibitor in vivo (JNK2I; a total of 3 doses) along with the alcohol exposure. In alcohol-exposed WT mice, the JNK2I effectively suppressed atrial arrhythmogenicity, as shown by reduced ΔtVm-Ca heterogeneity (Figure 5B, far right bar). Specificity of the JNK2I in JNK2 activity was verified by the results of JNK isoform activity measurement. JNK2I significantly reduced JNK2, not JNK1, activity in alcohol-exposed atrial myocytes (Online Figure 2). Recently, we reported (18,19) that JNK was also activated by aging, and this leads to Cx43 gap junction remodeling, which impairs cell–cell communication, slowing atrial action potential conduction velocity. Here, we found that the alcohol-exposed atria had preserved conduction velocity and unchanged Cx43 expression (Online Figure 3). Overall, our results suggest alcohol-evoked JNK2 activation promotes atrial arrhythmias by abnormal atrial Ca2+ activities.
FIGURE 5. JNK2 Inhibition (In Vivo Treatment) Eliminates Binge Alcohol-Evoked Abnormal Ca2+ Handling in Intact Atria and Atrial Myocytes.
(A) Summarized data show enhanced JNK2 kinase activity (measured by ADP production) in JNK2 antibody-specific pulldown JNK2 proteins but unchanged JNK1 activity. (B) Summarized data show enhanced heterogeneity of ΔtVm-Ca in alcohol-exposed mouse left atria, whereas JNK2 inhibitor in vivo treatment abolished this arrhythmogenic abnormality. (C) Representative optical isochronal maps show heterogeneous wavefront propagation of the Vm and Ca2+ signals in an alcohol-exposed atrium compared to a WT sham control. JNK2 inhibition effectively reversed this alcohol-evoked heterogeneity. (D, E) Summarized data show increased tetracaine-sensitive diastolic SR Ca2+ leakage in alcohol-exposed rabbit atrial myocytes (D) and HL-1 myocytes (E), while JNK2 inhibition abolished this alcohol-induced SR Ca2+ leak. ADP = adenosine diphosphate; JNK2I = JNK2 inhibitor; NS = no statistical significance; RLU = relative light units; Vm = membrane potential; other abbreviations as in Figures 1 to 3.
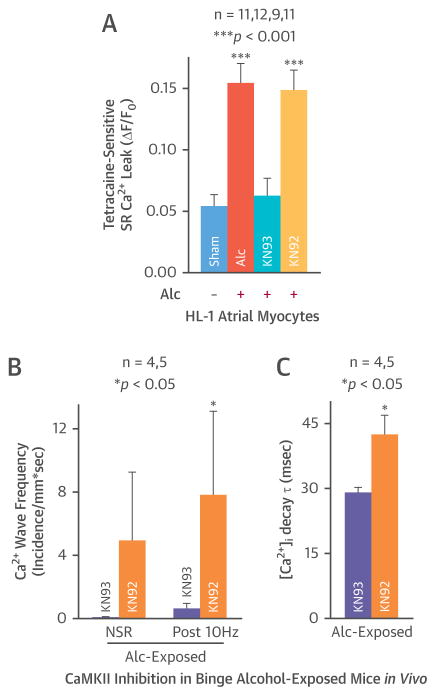
Enhanced diastolic SR Ca2+ leakage is often associated with abnormal diastolic Ca2+ waves (25). Indeed, alcohol-exposed rabbit atrial myocytes and HL-1 atrial myocytes had dramatically increased SR Ca2+ leakage (0.15 ± 0.02 vs. 0.05 ± 0.01 ΔF/F0 in sham controls) (Figures 5D and 5E, middle bars). With the JNK2 inhibition, the alcohol-evoked increase of diastolic SR Ca2+ leakage was abolished by the JNK2I treatment (0.05 ± 0.01 vs. 0.05 ± 0.01 ΔF/F0 in sham controls) (Figures 5D and 5E, far right bars). These data were also consistent with the findings of alcohol-evoked JNK2 action on abnormal Ca2+ activities in intact atria as shown in Figures 4 and 5.
ALCOHOL-EVOKED CaMKII-DEPENDENT SR CA2+ LEAKAGE AND ABERRANT CA2+ ACTIVITIES
CaMKII is well known as an important molecule that promotes SR Ca2+ mishandling and cardiac arrhythmogenicity. Here, atrial myocytes (HL-1 cells) were treated with a CaMKII inhibitor, KN93, or its inactive analog KN92, and then exposed to alcohol (24 h). The alcohol-evoked tetracaine-sensitive SR Ca2+ leakage was abolished by KN93 (0.06 ± 0.02 vs. 0.05 ± 0.01 ΔF/F0 in sham) (Figure 6A). In contrast, KN92 pre-treatment did not alter alcohol-evoked tetracaine-sensitive SR Ca2+ leakage (0.15 ± 0.02 vs. 0.05 ± 0.01 ΔF/F0 in sham) (Figure 6A, far right bar). We measured the functional effects of CaMKII inhibition in alcohol hearts. A single dose of KN93 or KN92 was administered in alcohol-infused mice in vivo prior to the terminal studies. We found that KN93, but not KN92, significantly decreased alcohol-evoked SR Ca2+ waves (Figure 6B) and reversed the alcohol-evoked prolongation of intracellular Ca2+ decay constant (28.9 ± 1.3 in KN93-treated vs. 42.4 ± 4.6 ms in KN92-treated atria) (Figure 6C). Thus, CaMKII inhibition had the same anti-arrhythmic actions as JNK2 inhibition. This suggests that the mechanism that couples alcohol, SR Ca2+ mishandling, and atrial arrhythmogenicity may involve both JNK2 and CaMKII.
FIGURE 6. Like the Effect of JNK2 Inhibition, CaMKII Inhibition Also Abolishes This Binge Alcohol-Evoked Abnormal Ca2+ Handling in Atrial Myocytes and Intact Atria.
(A) Summarized data showing CaMKII inhibition with the inhibitor KN93 (but not its inactive analog KN92) also prevented alcohol-evoked tetracaine-sensitive diastolic SR Ca2+ leakage in HL-1 myocytes. (B) (C) In vivo treatment with a single dose of KN93 eliminated alcohol-evoked Ca2+ waves and shortened [Ca2+]i decay constant τ compared to KN92-treated alcohol hearts. Abbreviations as in Figure 1.
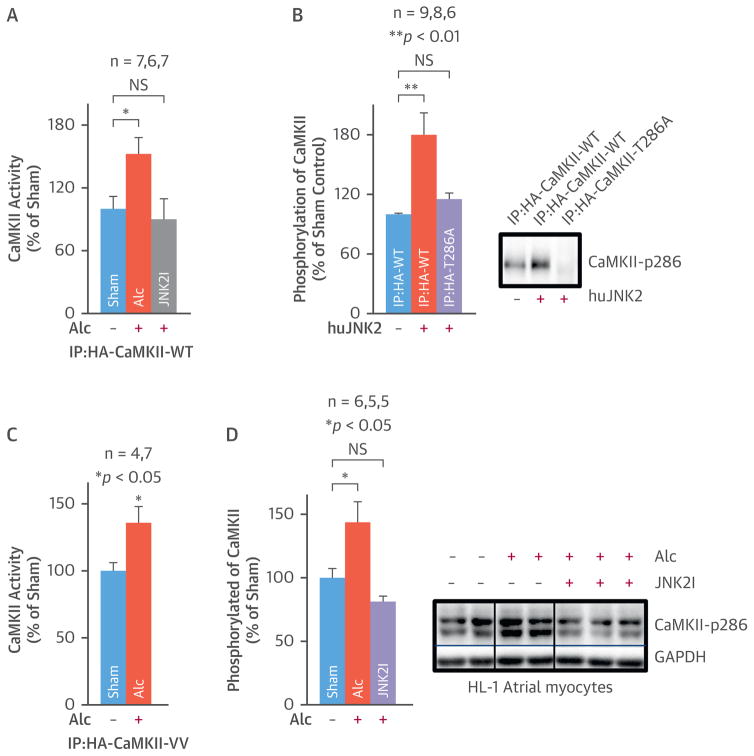
ALCOHOL-EVOKED JNK2 ACTIVATES CaMKII BY PROTEIN PHOSPHORYLATION
To explore the JNK-CaMKII relationship in alcohol-evoked atrial arrhythmogenicity, human influenza hemagglutinin (HA)-tagged CaMKII-WT was overexpressed in alcohol-exposed (24 h) HEK293 cells in the presence or absence of a JNK2 inhibitor. Then, CaMKII activity was assessed by kinase assays in CaMKII-WT proteins immunoprecipitated by an anti-HA antibody. Alcohol alone increased CaMKII activity by 50%. This did not happen with the JNK2I present (Figure 7A). These results indicate that alcohol-evoked JNK2 upregulates CaMKII activity. One possibility is that JNK2 directly phosphorylates the CaMKII protein. To test this possibility, pure active human JNK2 proteins (hJNK2) were incubated with anti-HA antibody-immunoprecipitated CaMKII-WT or mutant CaMKII-T286A proteins. The CaMKII-T286A mutant has a silenced autophosphorylation site. To monitor protein phosphorylation (i.e., ATP consumption), we used the ADP-Glo assay kit (Promega, Madison, Wisconsin) We found that active JNK2 significantly increased phosphorylation of HA-tagged CaMKII-WT proteins but not of the HA-tagged CaMKII-T286A mutated protein (Figure 7B). Immunoblotting data with a phospho-specific anti-CaMKII-P286 antibody confirmed the JNK2 action on CaMKII phosphorylation (Figure 7D). These results suggest JNK2 directly phosphorylates the CaMKII protein, activating CaMKII.
FIGURE 7. Alcohol-Evoked JNK2 Regulates CaMKII Activity.
(A) Summarized data show increased ADP production (reflecting an increased ATP consumption in the CaMKII reaction with its substrate) in HA-pulldown CaMKII-WT proteins from alcohol-exposed cells, while JNK2I suppressed alcohol-evoked CaMKII activity. (B) Pooled ADP production assay data and representative immunoblotting images show that active pure JNK2 proteins increase the phosphorylation of CaMKII-WT, whereas autophosphorylation site mutation prevents this JNK2 action on HA-pulldown CaMKII-T286A proteins. (C) Summarized data show alcohol-enhanced CaMKII activity is not affected by the mutation of CaMKII oxidative sites Met280/281. (D) Summarized quantitative immunoblotting data and representative images show JNK2 inhibition prevented alcohol-enhanced phosphorylation of CaMKII (activated) in cultured HL-1 myocytes. CaMKII = calmodulin kinase; CaMKII-WT = wildtype CaMKII; CaMKII-T286A = CaMKII with Thr287Ala mutation; CaMKII-VV = CaMKII with Met280/281Val mutation; JNK2I = JNK2 inhibitor. Other abbreviations as in Figures 1 and 2.
Note that alcohol may increase production of reactive oxygen species (ROS). Elevated ROS could promote CaMKII activation by oxidizing CaMKII’s Met280 and Met281 sites (29). To assess the potential ROS contribution to alcohol-evoked CaMKII activation, an HA-tagged Met280Val/Met281Val mutant CaMKII vector (CaMKII-VV) was overexpressed in HEK293 cells, and then these cells were exposed to 50 mM alcohol for 24 h. CaMKII-VV was nonresponsive to intracellular ROS challenge but retained the autophosphorylation T286 site function. The activity of HA-tagged CaMKII-VV proteins was significantly higher in alcohol-exposed cells than in sham controls (Figure 7C). The increased CaMKII activity was similar to that of alcohol-exposed CaMKII-WT proteins. This demonstrates that alcohol-evoked JNK2 activation alone is sufficient to explain the observed CaMKII activation.
DISCUSSION
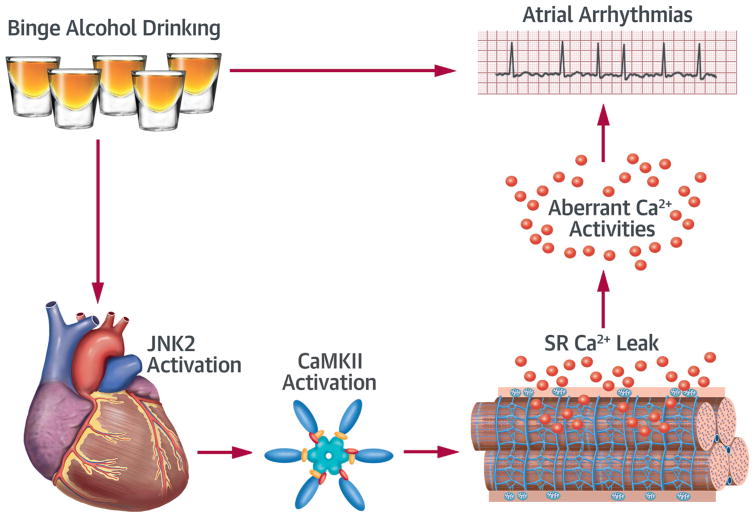
Here we have shown for the first time that alcohol-evoked JNK2 activation enhances atrial susceptibility of arrhythmic Ca2+ activities and AF. We discovered that alcohol-evoked JNK2 targets CaMKII activation. Specifically, the JNK2 phosphorylates the CaMKII protein, activating CaMKII. The resulting enhanced CaMKII activity, in turn, promotes aberrant SR Ca2+ activities and enhanced AF susceptibility (Central Illustration).
CENTRAL ILLUSTRATION. Alcohol-Evoked JNK Activates CaMKII to Promote Atrial Fibrillation.
Proposed mechanism showing that binge alcohol-evoked JNK2 activates CaMKII, which in turn, promotes aberrant SR Ca2+ handling and atrial arrhythmias. Ca2+ = calcium; CaMKII = calmodulin kinase II; JNK2 = c-Jun N-terminal kinase 2; SR = sarcoplasmic reticulum.
In humans, binge drinkers have a significantly increased AF risk compared to nondrinkers (5–7). Accumulating clinical data show binge alcohol exposure promotes cardiac arrhythmias even in the patients who lack a clinical history of cardiovascular diseases. Our AF inducibility studies reproduce the increased AF susceptibility following binge alcohol exposure in both human and animal hearts that had preserved cardiac function and no AF or major cardiovascular disease history. Clinical data also suggest the highest risk time frame for binge alcohol-induced atrial arrhythmia is approximately 24 h following the alcohol exposure (14). Interestingly, BALs have already reversed to the baseline levels by this time (14). Thus, the alcohol is no longer actually present when the atrial arrhythmic ramifications of the binge alcohol exposure occur. Most binge alcohol animal models designed for studying binge alcohol-associated organ damage (i.e., to liver) have extremely high BALs, which indeed lead to severe measurable tissue injury but also likely drive substantial ROS production (30,31). Here, we applied a binge alcohol-exposure model that mimics the human holiday drinking pattern, one with repeated episodes of alcohol intake separated by recovery periods. At the time of terminal studies, alcohol-exposed animals’ BAL had returned to the baseline and was comparable to that in the sham controls. These animals also had preserved cardiac function, unaltered heart weight-to-body weight ratio, as well as no cardiac hypertrophy or pulmonary edema.
This study is the first to report that binge alcohol activates stress response kinase JNK, which leads to SR Ca2+ mishandling and enhances atrial arrhythmogenicity. JNK activation has been observed under various pathological conditions and is involved in the development of cancer, diabetes, and arthritis (16,17). JNK activation has also been shown in various cardiac diseases such as heart failure and ischemic-reperfusion injury (32,33), conditions in which the heart is prone to arrhythmia. Emerging evidence suggests that activated JNK is linked to AF in aged hearts and a tachypacing dog model (18,19,34). Here, we found that alcohol-evoked JNK leads to augmented CaMKII-driven SR Ca2+ mishandling, which ultimately enhances atrial arrhythmogenicity. A causal link among alcohol, JNK activation, and atrial arrhythmogenicity was strongly supported by cardiac-specific JNK inhibition rescue results in alcohol-exposed JNK1/2dn mice, overexpressing inactive JNK1 and JNK2 dominant negative proteins (Figure 3). JNK1 and JNK2 are known to have distinctive functions (17,26). JNK1 has been shown to be critical in preserving cardiac function and promoting apoptosis in hearts with in vitro ischemia-reperfusion. JNK2 has been found to contribute to the development of diabetes, atherosclerosis, and skin tumors. (17,26) Here, we found that elevated JNK2 activity, not JNK1, is implicated in binge alcohol-evoked atrial arrhythmogenicity. The antiarrhythmic action of a JNK2 specific inhibitor in live alcohol-exposed animals further supports the role of JNK2 activation. This new finding suggests that development of drugs specifically targeting the JNK2 isoform may be helpful for future AF prevention and treatment.
Activation of stress-response kinase JNK, a member of the important MAPK family, is a common feature of alcohol-caused tissue injury (20,21). Other MAPK family members (p38 and extracellular ERK) may also contribute in a cellular context and type-dependent fashion (35–37). For example, JNK and p38 have opposite functions (activation or suppression) during cellular senescence (36). In the acutely alcohol-exposed heart, p38 is unchanged, and ERK is suppressed (37). These previous findings are quite consistent with our unpublished results showing unchanged p38 and ERK activation in binge alcohol-exposed atria from both humans and animal models.
Another interesting new finding here is that alcohol-evoked JNK2 activation drives enhancement of CaM-KII activation. This is the first description of this form of kinase–kinase crosstalk, and it may have substantial ramifications in the heart, considering the pivotal role of CaMKII in arrhythmogenesis (24,25,29,38–40). Last, alcohol is known to increase cellular ROS (41), and ROS may activate CaMKII (29). To assess the relative contributions of JNK- and ROS-dependent CaMKII activation here, we used CaMKII mutants missing the ROS-sensing sites (Met280/281). These mutations did not prevent alcohol-induced CaMKII activation. However, JNK2 inhibition effectively suppressed alcohol-evoked CaMKII activation. Thus, this JNK2-CaMKII causal link is critical in binge alcohol-evoked SR Ca2+ mishandling and atrial arrhythmogenicity.
STUDY LIMITATIONS
We note that it is always a great challenge to get accurate donor history of binge drinking habits in human studies. Thus, increasing sample size is required for further human heart studies. However, our current results from well-controlled alcohol exposure in animals provided strong evidence suggesting the pivotal role of JNK2 in enhanced atrial arrhythmogenicity in binge alcohol-exposed hearts.
CONCLUSIONS
Our results indicate that alcohol activates JNK2. The JNK2 then phosphorylates the CaMKII protein, enhancing cellular CaMKII activity and, consequently, atrial arrhythmogenicity. Future therapeutic strategies targeting JNK2-specific inhibition might be helpful for AF prevention and treatment in alcoholic patients. The potential clinical significance of our findings may have implications beyond our alcohol focus as other cellular challenges (such as aging, post-surgical inflammation, heart failure, and so forth) may also cause JNK2 activation.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Excessive “binge” alcohol intake underlies the “holiday heart syndrome” of paroxysmal AF. A causal link between stress-response kinase JNK2 activation and the propensity to AF evoked by binge drinking involves a form of alcohol-driven kinase interaction.
TRANSLATIONAL OUTLOOK
Future studies should explore whether modulating JNK2 activity could prevent the proarrhythmic CaMKII activity associated with AF.
Acknowledgments
Supported by National Institutes of Health grants HL057832 and AA024769 to Dr. Fill; HL113640, AA024769, HL062426 to Dr. Ai; and American Heart Association grant 10GRNT37700 to Dr. Ai.
The authors thank the donor families at Gift of Hope who provided the gifts that made our research possible. They also thank Dr. Dan Bare for assistance with atrial myocyte isolation.
ABBREVIATIONS AND ACRONYMS
- BAL
blood alcohol level
- CaMKII
calmodulin kinase II
- CaMKII-WT
wild-type CaMKII
- CaMKII-VV
mutated CaMKII with Met280/281Val mutation
- CaMKII-T287A
mutated CaMKII with Thr287Ala mutation
- JNK
c-Jun N-terminal kinase
- SR
sarcoplasmic reticulum
- Vm
membrane potential
Footnotes
APPENDIX For a supplemental method section as well as a table and figures, please see the online version of this paper.
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Vital signs: binge drinking prevalence, frequency, and intensity among adults: United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:14–9. [PubMed] [Google Scholar]
- 2.Patrick ME, Azar B. High-intensity drinking. Alcohol Res. 2017;39:e1–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Patrick ME, Terry-McElrath YM, Kloska DD, Schulenberg JE. High-intensity drinking among young adults in the United States: prevalence, frequency, and developmental change. Alcohol Clin Exp Res. 2016;40:1905–12. doi: 10.1111/acer.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbin WR, Zalewski S, Leeman RF, Toll BA, Fucito LM, O’Malley SS. In with the old and out with the new? A comparison of the old and new binge drinking standards. Alcohol Clin Exp Res. 2014;38:2657–63. doi: 10.1111/acer.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tonelo D, Providencia R, Goncalves L. Holiday heart syndrome revisited after 34 years. Arq Bras Cardiol. 2013;101:183–9. doi: 10.5935/abc.20130153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voskoboinik A, Prabhu S, Ling LH, Kalman JM, Kistler PM. Alcohol and atrial fibrillation: a sobering review. J Am Coll Cardiol. 2016;68:2567–76. doi: 10.1016/j.jacc.2016.08.074. [DOI] [PubMed] [Google Scholar]
- 7.Djousse L, Levy D, Benjamin EJ, et al. Long-term alcohol consumption and the risk of atrial fibrillation in the Framingham Study. Am J Cardiol. 2004;93:710–3. doi: 10.1016/j.amjcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 9.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 10.Lowenstein SR, Gabow PA, Cramer J, Oliva PB, Ratner K. The role of alcohol in new-onset atrial fibrillation. Arch Intern Med. 1983;143:1882–5. [PubMed] [Google Scholar]
- 11.Kodama S, Saito K, Tanaka S, et al. Alcohol consumption and risk of atrial fibrillation: a meta-analysis. J Am Coll Cardiol. 2011;57:427–36. doi: 10.1016/j.jacc.2010.08.641. [DOI] [PubMed] [Google Scholar]
- 12.Liang Y, Mente A, Yusuf S, et al. Alcohol consumption and the risk of incident atrial fibrillation among people with cardiovascular disease. CMAJ. 2012;184:E857–66. doi: 10.1503/cmaj.120412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. 2014;64:281–9. doi: 10.1016/j.jacc.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 14.Koskinen P, Kupari M, Leinonen H, Luomanmaki K. Alcohol and new onset atrial fibrillation: a case-control study of a current series. Br Heart J. 1987;57:468–73. doi: 10.1136/hrt.57.5.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnamoorthy S, Lip GY, Lane DA. Alcohol and illicit drug use as precipitants of atrial fibrillation in young adults: a case series and literature review. Am J Med. 2009;122:851–6. doi: 10.1016/j.amjmed.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 17.Bogoyevitch MA. The isoform-specific functions of the c-Jun N-terminal kinases (JNKs): differences revealed by gene targeting. Bioessays. 2006;28:923–34. doi: 10.1002/bies.20458. [DOI] [PubMed] [Google Scholar]
- 18.Yan J, Kong W, Zhang Q, et al. c-Jun N-terminal kinase activation contributes to reduced connexin43 and development of atrial arrhythmias. Cardiovasc Res. 2013;97:589–97. doi: 10.1093/cvr/cvs366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan J, Thomson JK, Zhao W, et al. The stress kinase JNK regulates gap junction Cx43 gene expression and promotes atrial fibrillation in the aged heart. J Mol Cell Cardiol. 2017;114:105–15. doi: 10.1016/j.yjmcc.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Relja B, Weber R, Maraslioglu M, et al. Differential Relevance of NF-kappaB and JNK in the pathophysiology of hemorrhage/resususcitation-induced liver injury after chronic ethanol feeding. PLoS One. 2015;10:e0137875. doi: 10.1371/journal.pone.0137875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker RK, Cousins VM, Umoh NA, et al. The good, the bad, and the ugly with alcohol use and abuse on the heart. Alcohol Clin Exp Res. 2013;37:1253–60. doi: 10.1111/acer.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aistrup GL, Kelly JE, Piano MR, Wasserstrom JA. Biphasic changes in cardiac excitation-contraction coupling early in chronic alcohol exposure. Am J Physiol Heart Circ Physiol. 2006;291:H1047–57. doi: 10.1152/ajpheart.00214.2006. [DOI] [PubMed] [Google Scholar]
- 23.Matyas C, Varga ZV, Mukhopadhyay P, et al. Chronic plus binge ethanol feeding induces myocardial oxidative stress, mitochondrial and cardiovascular dysfunction, and steatosis. Am J Physiol Heart Circ Physiol. 2016;310:H1658–70. doi: 10.1152/ajpheart.00214.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang R, Khoo MS, Wu Y, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–17. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 25.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circulation Res. 2005;97:1314–22. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 26.Liang Q, Bueno OF, Wilkins BJ, Kuan CY, Xia Y, Molkentin JD. c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. EMBO J. 2003;22:5079–89. doi: 10.1093/emboj/cdg474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen B, Guo A, Gao Z, et al. In situ confocal imaging in intact heart reveals stress-induced Ca(2+) release variability in a murine catecholaminergic polymorphic ventricular tachycardia model of type 2 ryanodine receptor(R4496C+/−) mutation. Circ Arrhythm Electrophysiol. 2012;5:841–9. doi: 10.1161/CIRCEP.111.969733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sowell B, Fast VG. Ionic mechanism of shock-induced arrhythmias: role of intracellular calcium. Heart Rhythm. 2012;9:96–104. doi: 10.1016/j.hrthm.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson ME. Oxidant stress promotes disease by activating CaMKII. J Mol Cell Cardiol. 2015;89:160–7. doi: 10.1016/j.yjmcc.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y, Li P, Ma LX, et al. Effects of acute administration of ethanol on experimental arrhythmia. Chin J Physiol. 2012;55:307–13. doi: 10.4077/CJP.2012.BAA053. [DOI] [PubMed] [Google Scholar]
- 31.Anadon MJ, Almendral J, Gonzalez P, Zaballos M, Delcan JL, De Guevara JL. Alcohol concentration determines the type of atrial arrhythmia induced in a porcine model of acute alcoholic intoxication. Pacing Clin Electrophysiol. 1996;19:1962–7. doi: 10.1111/j.1540-8159.1996.tb03262.x. [DOI] [PubMed] [Google Scholar]
- 32.Haq S, Choukroun G, Lim H, et al. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–7. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- 33.Siow YL, Choy PC, Leung WM, OK Effect of Flos carthami on stress-activated protein kinase activity in the isolated reperfused rat heart. Mol Cell Biochem. 2000;207:41–7. doi: 10.1023/a:1017266628572. [DOI] [PubMed] [Google Scholar]
- 34.Li D, Shinagawa K, Pang L, et al. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001;104:2608–14. doi: 10.1161/hc4601.099402. [DOI] [PubMed] [Google Scholar]
- 35.Aroor AR, Shukla SD. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004;74:2339–64. doi: 10.1016/j.lfs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Wada T, Stepniak E, Hui L, et al. Antagonistic control of cell fates by JNK and p38-MAPK signaling. Cell Death Differ. 2008;15:89–93. doi: 10.1038/sj.cdd.4402222. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Dong F, Li Q, Borgerding AJ, Klein AL, Ren J. Cardiac overexpression of catalase antagonizes ADH–associated contractile depression and stress signaling after acute ethanol exposure in murine myocytes. J Appl Physiol (1985) 2005;99:2246–54. doi: 10.1152/japplphysiol.00750.2005. [DOI] [PubMed] [Google Scholar]
- 38.Chelu MG, Sarma S, Sood S, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–51. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan J, Zhao W, Thomson JK, et al. Stress signaling JNK2 crosstalk with CaMKII underlies enhanced atrial arrhythmogenesis. Circ Res. 2018 Jan 19; doi: 10.1161/CIRCRESAHA.117.312536. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao X, Wu X, Yan J, et al. Transcriptional regulation of stress kinase JNK2 in pro-arrhythmic CaMKIIδ expression in the aged atrium. Cardiovasc Res. 2018 Jan 19; doi: 10.1093/cvr/cvy011. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ojeda ML, Barrero MJ, Nogales F, Murillo ML, Carreras O. Oxidative effects of chronic ethanol consumption on the functions of heart and kidney: folic acid supplementation. Alcohol and Alcoholism. 2012;47:404–12. doi: 10.1093/alcalc/ags056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.