Abstract
Introduction
Lung cancer screening represents an opportunity to deliver smoking cessation advice and assistance to current smokers. However, the current tobacco treatment practices of lung cancer screening sites are unknown. The purpose of this study was to describe organizational priority, current practice patterns, and barriers for delivery of evidence-based tobacco use treatment across lung cancer screening sites within the United States.
Methods
Guided by prior work examining readiness of health care providers to deliver tobacco use treatment, we administered a brief online survey to a purposive national sample of site coordinators from 93 lung cancer screening sites.
Results
Organizational priority for promoting smoking cessation among lung cancer screening enrollees was high. Most sites reported that, at the initial visit, patients are routinely asked about their current smoking status (98.9%) and current smokers are advised to quit (91.4%). Fewer (57%) sites provide cessation counseling or refer smokers to a quitline (60.2%) and even fewer (36.6%) routinely recommend cessation medications. During follow-up screening visits, respondents reported less attention to smoking cessation advice and treatment. Lack of patient motivation and resistance to cessation advice and treatment, lack of staff training, and lack of reimbursement were the most frequently cited barriers for delivering smoking cessation treatment.
Conclusions
Although encouraging that lung cancer screening sites endorsed the importance of smoking cessation interventions, greater attention to identifying and addressing barriers for tobacco treatment delivery is needed in order to maximize the potential benefit of integrating smoking cessation into lung cancer screening protocols.
Implications
This study is the first to describe practice patterns, organizational priority, and barriers for delivery of smoking cessation treatment in a national sample of lung cancer screening sites.
Introduction
Lung cancer screening with computed tomography (CT) has demonstrated a significant reduction in lung cancer mortality.1 The United States Preventive Services Task Force now recommends annual low-dose CT screening for lung cancer among adults between 55 and 80 years old who are at high risk for lung cancer because of their age and extensive smoking history.2 In February 2015, the Center for Medicare and Medicaid Services issued a national coverage determination for coverage of annual low-dose CT lung cancer screening for high-risk individuals.3 In addition to the public health benefit of lung cancer screening for early detection of lung cancer, lung cancer screening programs may also provide a “teachable moment” for reaching smokers and delivering evidence-based tobacco cessation treatment.4 Integration of smoking cessation treatment within the context of lung cancer screening is consistent with the Center for Medicare and Medicaid Services and United States Preventive Services Task Force’s acknowledgement of the importance of providing information about tobacco cessation treatment.
To date, several studies have examined the impact of undergoing lung cancer screening on smoking cessation outcomes5–12 and a recent cost–utility analysis13 supports the additional public health benefit of incorporating smoking cessation interventions into lung cancer screening protocols. Poghosyan and colleagues14 summarized nine cross-sectional, longitudinal, and randomized controlled studies examining the impact of CT screening for lung cancer on smoking behaviors and found quit rates ranging from 6.6% to 42%. Although CT screening may provide clinical opportunities to encourage quitting, it is becoming increasingly clear that merely undergoing cancer screening neither adequately promotes smoking abstinence nor utilization of evidence-based cessation strategies.15,16
Despite the potential public health benefits for further reduction in tobacco-related morbidity and mortality, it is unknown whether lung cancer screening sites will value integration of smoking cessation treatment into their protocols and will commit the time and other resources needed to ensure high-quality delivery of evidence-based tobacco treatment. Even with the availability of clinical guidelines for treating tobacco dependence,17 health care providers and clinical settings often vary greatly in their implementation of tobacco use treatment guidelines and patient-, provider-, and systems-level barriers often impede best practices.18 Organizational priority is considered to be a strong prerequisite for practice innovation.19
In order to facilitate the dissemination and implementation of tobacco use treatment within lung cancer screening sites, a greater understanding of current smoking cessation treatment practices is needed. The goals of this study were (1) to describe the current organizational priority and smoking cessation treatment delivery across a national representation of lung cancer screening sites; (2) to identify site characteristics associated with smoking cessation treatment delivery; and (3) to identify perceived barriers for integrating smoking cessation treatment delivery within lung cancer screening sites.
Methods
Participants and Recruitment
To ensure a mix of geography and site characteristics, site coordinators from lung cancer screening sites (n = 152) within the United States that have pledged adherence to best practices for delivery of high-quality lung cancer screening20 were invited to complete an online survey. A cover letter and hyperlink to an online anonymous survey (Survey Monkey) were distributed via E-mail to a list of site coordinators maintained by the Lung Cancer Alliance. The cover letter described the purpose of the voluntary survey as collecting benchmarking data about current site practices, attitudes, and barriers for providing smoking cessation treatment and estimated that 10 minutes would be required for completion. Site coordinators who did not respond after the initial E-mail invitation were recontacted via E-mail up to two additional times within a 4-week period to remind them to complete the online survey. To provide an incentive for survey completion, participants could indicate interest in being entered into a raffle to win an iPad mini tablet. Data were collected from March 14 to April 8, 2014. Ninety-three surveys were completed resulting in a 61% response rate.
Survey Tool
Given our ultimate goal of assessing the readiness of lung cancer screening sites to implement smoking cessation treatment to their screening enrollees, and based on our review of relevant provider surveys, we created a 67-item online survey instrument covering three broad areas of inquiry: (1) demographic characteristics of respondents (ie, age, gender, screening site role) and categorical descriptions of lung cancer screening site (ie, number of patients screened per month, duration of time screening, whether the site is academically affiliated, patient payor mix); (2) current delivery of smoking cessation treatment including smoking cessation advice and assistance practice patterns (at initial enrollment and follow-up) at the screening site; and (3) organizational priority and perceived barriers relevant to implementation of smoking cessation treatment at the respondent’s lung cancer screening site. The survey tool is available from the authors upon request.
Delivery of smoking cessation treatment was assessed using a modified version of a provider survey21 assessing practice patterns related to the 5 A’s brief model of smoking cessation treatment (Ask, Advise, Assess, Assist, Arrange) widely recommended. Respondents were asked how often each of the following smoking cessation treatment practices were offered by their lung cancer screening sites during initial and follow-up (annual repeat) screening visits: Asking patients about current smoking status (ask), advising current smokers to quit (advise), assessing smokers’ readiness to quit (assess), providing brief cessation counseling (assist), referring smokers for additional cessation services such as an onsite cessation program or state quitline and prescribing FDA-approved cessation medications (arrange). Response options were on a 5-point Likert scale (1 = never, 2 = rarely, 3 = sometimes, 4 = most of the time, and 5 = always). Practice behaviors (5 A’s) endorsed as delivered “always” or “most of the time” (defined as ≥4) were considered to be indicative of routine tobacco treatment delivery.
Barriers to Providing Smoking Cessation Treatment
Perceived barriers to providing smoking cessation interventions were assessed using 13 items modified from prior work.22,23 Patient, provider, and systems-level barriers included lack of patient motivation, lack of provider knowledge and tobacco use treatment training, lack of time, lack of relevant cessation resources, lack of direct contact with patients, and lack of reimbursement. Response options were on a 5-point Likert scale (1 = fully disagree, 2 = disagree, 3 = neither agree nor disagree, 4 = agree, and 5 = fully agree). The Cronbach’s alpha coefficient for this scale was 0.81 indicating good internal consistency. Responses were recoded into dichotomized levels of agreement (agree vs. do not agree) and frequencies were reported for each category.
Organizational Priority
The degree to which respondents perceived that their screening site values delivery of smoking cessation treatment was assessed using a nine-item measurement tool.19 Items used to assess perceived organizational priority included “One of this lung cancer screening site’s goals is to integrate best practices for smoking cessation” and “Smoking cessation treatment is a top priority at this lung cancer screening site.” Response options were on a 5-point Likert scale (1 = not true, 2 = slightly true, 3 = somewhat true, 4 = mostly true, and 5 = definitely true). Responses were aggregated into a total scale score (possible scores ranging from 9 to 45) with good internal consistency (Cronbach’s alpha = 0.85). Responses of mostly true and definitely true (≥4) were considered to be indicative of strong endorsement.
Analytic Plan
Data were first explored graphically and by descriptive statistics. Frequencies and other descriptive statistics were calculated for site variables (ie, length of time screening and academic affiliation) and Likert scales (ie, delivery of tobacco use treatment and organizational priority). Proportions and binomial confidence intervals were calculated for dichotomized responses (ie, tobacco treatment practices and barriers). Chi-square was used to test differences between categorical variables and tobacco use treatment delivery (ie, length of time screening and type of site coordinator). Odds ratios were used to test associations between dichotomous variables including site academic affiliation and smoking cessation treatment delivery. Associations between mean scores, such as those describing delivery of smoking cessation services and organizational priority for treatment delivery, were analyzed by Spearman’s rank correlation. Spearman’s rank correlation coefficients were used to test associations between variables with considerable variability, such as the number of patients screened per month (smallest site with one patient per month and largest site with 68 patients per month). Associations between continuous variables were evaluated with the Pearson correlation coefficient (eg, between delivery of tobacco use treatment and summary scores of perceived organizational priority).
To further examine site variation in baseline patterns of tobacco treatment delivery and potential barriers for tobacco treatment, an exploratory Latent Class Analysis (LCA)24 was conducted. LCA is analogous to cluster analysis, in the sense of data reduction, suitable for exploring and identifying patterns of tobacco treatment delivery and barriers. To simplify this exploratory analysis, each of the survey items was dichotomized into a positive response (eg, service offered “most of the time” or “always”) versus a negative (“never,” “rarely,” or “sometimes”). Given that endorsement of “Asking about current smoking” was nearly 100% for all sites, this item was excluded from the LCA because its high homogeneity would cause the LCA computation to become unstable.
Analyses were carried out using the PASW version 22 (SPSS Inc, Chicago, IL) and SAS version 9.2.
Results
Table 1 summarizes survey respondent and lung cancer screening site characteristics. Respondent site coordinators represented 93 screening sites from 34 states across the United States. Most respondents were female (93.5%), aged 40–54 (56.2%), and most identified as being clinicians (45% nurses, 32% physicians). Approximately one-third (29.0%) of screening sites identified as academically affiliated. On average, sites estimated screening 14 patients per month (SD = 13.0) but there was much variation ranging from 1 to 68 patients screened per month. In terms of payor mix, respondents estimated 37.4% Medicare, 7.6% Medicaid, 35% private/commercial, 7.3% no insurance, and 12.7% other insurance. Most screening sites were relatively new with 80.6% having less than 3 years of lung cancer screening experience and 72% had not participated in prior lung cancer screening trials (ie, NLST or I-ELCAP).
Table 1.
Survey Respondent and Lung Cancer Screening Site Characteristics
| Respondent characteristics (n = 93) | n (%) |
|---|---|
| Gender | |
| Female | 87 (94) |
| Ethnicity | |
| Non-Hispanic | 91 (99) |
| Race | |
| White | 84 (90) |
| Black or African American | 6 (7) |
| Asian, Native Hawaiian, or Other Pacific Islander | 1 (1) |
| American Indian or Alaska Native | 2 (2) |
| Primary role | |
| Patient care/clinician | 55 (59) |
| Education | 3 (3) |
| Research | 8 (9) |
| Administration | 17 (18) |
| Other | 10 (11) |
| Primary area of clinical practice | |
| Physician | 30 (32) |
| Nursing | 42 (45) |
| Not applicable (not a clinician) | 18 (19) |
| Other | 3 (3) |
| Mean ± SD | |
|---|---|
| Age (years) | 45.7±9.8 |
| Lung cancer screening site characteristics (n = 93) | n (%) |
|---|---|
| Length of time screening | |
| <1 year | 33 (36) |
| 1–3 years | 42 (45) |
| ≥4 years | 18 (19) |
| Academic affiliation | |
| No | 66 (71) |
| Yes | 27 (29) |
| Mean ± SD, median | |
|---|---|
| Patients screened per month | 14±13, 10 |
| Payor mix | Mean ± SD |
| Medicare | 37.4±25.9 |
| Medicaid | 7.6±12.1 |
| Private commercial | 35±26.4 |
| No insurance | 7.3±17.5 |
| Other insurance | 12.7±30.5 |
Organizational Priority of Smoking Cessation Treatment and Current Practice Patterns
Respondents reported high organizational priority of smoking cessation treatment guideline implementation indicating strong agreement that delivery of smoking cessation treatment is a priority at their screening sites (76.4%). Respondents reported that staff at their lung cancer screening sites think that implementation of smoking cessation treatment is important (86%) and the majority (89.2%) disagreed with the statement “staff don’t care about providing smoking cessation assistance.” Respondents agreed that their sites were encouraged to advise current smokers to quit and provide smoking cessation assistance (74.2% and 81.7%, respectively). In fact, only two sites did not endorse implementing smoking cessation treatment as being one of their quality of care goals.
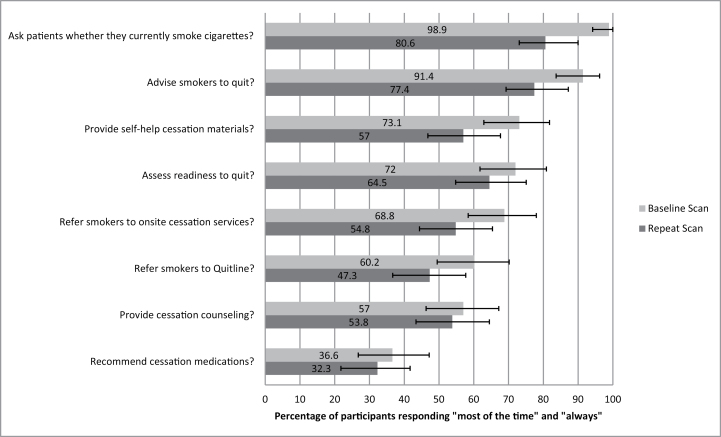
In terms of current delivery of smoking cessation treatment, at the initial visit, most sites reported “always” asking patients about their smoking status (98.9%) and advising current smokers to quit (91.4%). Fewer sites (57%) reported delivery of cessation counseling or referral to the quitline (60.2%) and even fewer (36.6%) reported making recommendations for cessation medications “always” or “most of the time.” As shown in Figure 1, at the follow-up scan visit, sites were less likely to ask about current smoking, advise current smokers to quit, assess smokers’ readiness to quit, document smoking in the medical chart, document smoking cessation treatment plan, provide self-help print materials, refer current smokers to the quitline, and refer current smokers for onsite individual or group counseling.
Figure 1.
Comparison of current tobacco treatment practices at baseline and repeat scans.
Barriers for Providing Smoking Cessation Treatment
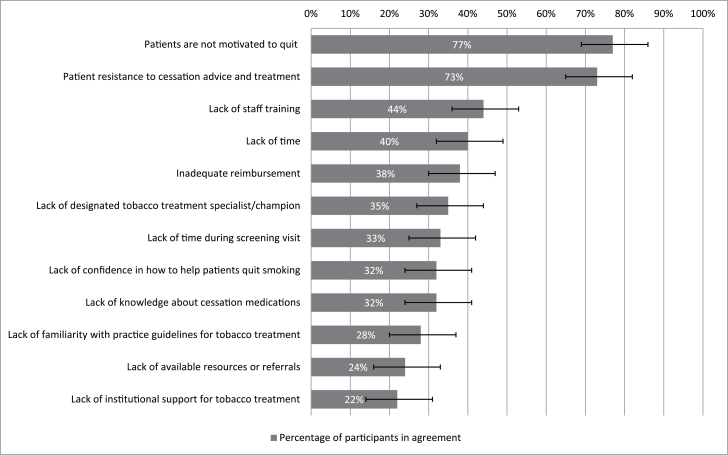
Figure 2 summarizes how respondents identified barriers to providing smoking cessation treatment. The most frequently endorsed barriers (77%, CI = 67% to 85%) were perceptions that patients lacked motivation to quit and were resistant to cessation advice and treatment. Lack of staff time, lack of reimbursement, lack of staff training in tobacco treatment, lack of a designated tobacco treatment specialist/champion, and lack of time/direct contact with patients were also endorsed by at least a third of respondents.
Figure 2.
Barriers to providing smoking cessation interventions to smokers enrolled in lung cancer screening programs.
Site Characteristics Associated With Smoking Cessation Practices
The following variables were examined as correlates of smoking cessation treatment practice patterns (see Table 2): length of time screening, site coordinators’ primary area of clinical practice, site academic affiliation, volume of patients screened per month, and organizational priority summary score. Neither length of time screening, site coordinator primary area of clinical practice nor volume of patients screened per month were significantly associated with reports of tobacco treatment delivery. Sites with an academic affiliation were slightly more likely to assess smokers’ readiness to quit than nonacademically affiliated sites (OR = 3.0; CI = 1.1–8.1).
Table 2.
Site Characteristics and Organizational Priority Associated With Tobacco Use Treatment Practices
| Ask | Advise | Assess | Assist | Arrange | |
|---|---|---|---|---|---|
| Percentage of respondents selecting “most of the time” or “always.” | |||||
| Length of time screening | |||||
| <1 year | 100% | 93.9% | 63.6% | 54.5% | 39.4% |
| 1–3 years | 97.6% | 88.1% | 76.2% | 59.5% | 35.7% |
| ≥4 years | 100% | 94.4% | 77.8% | 55.6% | 33.3% |
| Site coordinator | |||||
| Physician | 96.7% | 93.3% | 80% | 60% | 46.7% |
| Nurse | 100% | 90.5% | 71.4% | 57.1% | 33.3% |
| Not applicable (not a clinician) | 100% | 94.4% | 61% | 50% | 27.8% |
| Odds ratio (confidence interval) | |||||
|---|---|---|---|---|---|
| Academic affiliation | 0.8 (0.1–9.4) | 2.2 (0.6–8.2) | 3.0 (1.1–8.1)* | 1.5 (0.6–3.7) | 2.0 (0.7–5.6) |
| Spearman correlation coefficienta | |||||
|---|---|---|---|---|---|
| Patients screened per month | 0.162 | −0.078 | 0.001 | 0.063 | −0.032 |
| Pearson correlation coefficienta | |||||
|---|---|---|---|---|---|
| Organizational priority | 0.235* | 0.366** | 0.265* | 0.427** | 0.253* |
*Significant at the .05 level (two-tailed).
**Significant at the .01 level (two -tailed).
aRaw continuous scores were used for Ask, Advise, Assess, Assist and Arrange.
Organizational priority mean summary scores were equivalent for academically affiliated and nonacademically affiliated sites (38.9 vs. 38.0, P = .51). Organizational priority was significantly associated with delivery of smoking cessation treatment (all Ps < .05, using Pearson correlation coefficient). Organizational priority was significantly associated with asking patients whether they are current smokers (Pearson correlation coefficient = 0.24, P = .02), advising current smokers to quit (P < .01), assessing smokers readiness to quit (P = .01), providing cessation counseling (P < .01), and prescribing or recommending cessation medications (P = .01).
Barriers and Patterns of Tobacco Treatment Delivery
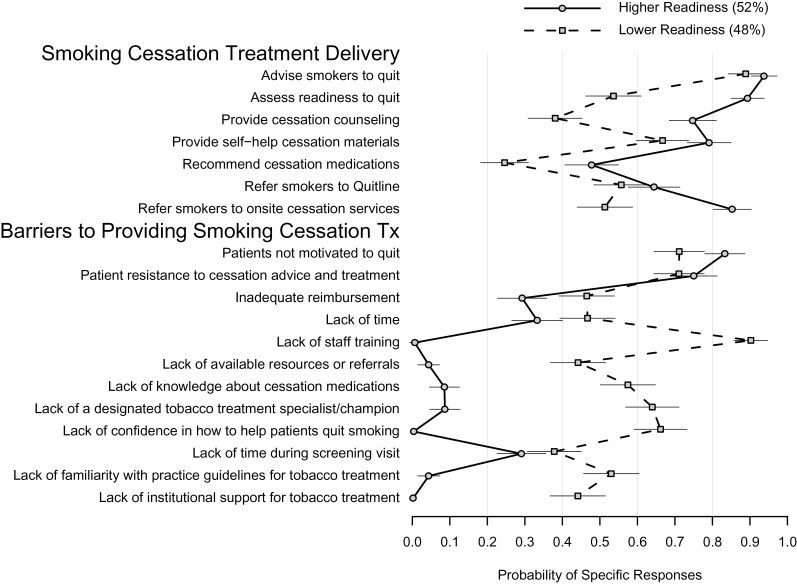
The LCA clustered the sites into two distinct groups suggesting differential readiness to integrate smoking cessation into their lung cancer screening program (Figure 3). One group of screening sites (52% “higher readiness,” with a solid line) offers greater tobacco treatment and identifies fewer barriers and the other group of screening sites (48% “lower readiness,” with a dotted line) offers less tobacco treatment and indentifies more barriers. Figure 3 plots the model-estimated probability and standard error of delivering tobacco use treatment care by readiness group. Sites that belong to the “higher readiness” group are characterized by considerably higher probabilities of providing greater tobacco treatment and fewer barriers than sites in the “lower readiness” group.
Figure 3.
Latent class analysis of smoking cessation treatment delivery and barriers for smoking cessation treatment delivery.
Discussion
Following the recommendations of the United States Preventive Services Task Force, most major medical and professional organizations focusing on cancer prevention and control including the American Society of Clinical Oncology (ASCO), the American College of Chest Physicians (ACCP), the American Association for Thoracic Surgery (AATS), the American Cancer Society (ACS), the National Comprehensive Cancer Network (NCCN), and the International Association for the Study of Lung Cancer (IASLC) have endorsed the public health benefit of lung cancer screening.25–29 Moreover, all of these screening guidelines recommend integration of smoking cessation advice and treatment into lung cancer screening protocols so as to optimize lung cancer prevention and control efforts. To our knowledge, this is the first paper to describe practice patterns, organizational priority, and barriers for delivery of smoking cessation treatment in a community sample of lung cancer screening sites. These findings are especially important in light of observations reported by Park and colleagues30 that smokers who participated in the National Lung Screening Trial reported low rates of cessation assistance and follow-up by their primary care providers.
Fortunately, most screening sites endorsed the organizational priority of integrating smoking cessation into their lung cancer screening protocols; however, there was much variation reported in tobacco treatment practice patterns. Most respondents reported that their screening sites ask patients about their smoking status and advise smokers to quit; however, far fewer sites provide smoking cessation assistance (ie, cessation medication and/or referrals for behavioral counseling). Referral to the quitline and providing self-help print materials were the most frequent cessation treatment efforts endorsed. Tobacco treatment was more commonly delivered at the baseline rather than during follow-up (annual repeat) screening visits. These findings, while promising, indicate that much remains to be done to help screening sites move from organizational priority to actual integration of tobacco use treatment into lung cancer screening protocols, particularly during repeat screening visits. Delivery of evidence-based tobacco treatment should be considered a quality metric for evaluation and quality improvement of lung cancer screening protocols.
In terms of developing a better understanding of observed site variation in tobacco treatment delivery, several site variables were examined. Organizational priority was strongly associated with delivery of tobacco treatment services at initial and follow-up visits. Patient volume, duration of time screening, and payor mix were not associated with organizational priority or tobacco treatment practices. Although there was a nonsignificant trend for sites not affiliated with academic centers to report less delivery of tobacco treatment, academic- and nonacademic-affiliated sites reported similarly high organizational priority.
The exploratory LCA identified two groups of sites. Generally, higher readiness sites reported more routine delivery of tobacco treatment and fewer barriers to providing tobacco treatment. Both groups identified patient resistance, lack of time, and inadequate reimbursement as tobacco treatment delivery barriers indicating a set of common challenges for higher and lower readiness sites. Of note, the LCA identified potential targets for improving implementation of tobacco cessation treatment in lower readiness sites. Since lower readiness sites reported a greater need for staff training in tobacco treatment and a lack of available cessation resources, additional staff training practice facilitation and other effective implementation strategies are likely needed to increase the capacity of screening sites to deliver evidence-based tobacco treatment. Use of LCA to cluster sites into readiness groups that share practice patterns and common barriers represents a methodological tool for targeting subsequent work to develop implementation strategies for addressing sites’ specific training and technical assistance needs.
Although perceived lack of patient motivation to quit was the most frequently reported as a barrier for tobacco treatment delivery, most smokers seeking lung cancer screening do report interest in quitting smoking and receiving tobacco cessation services. In one study,4 approximately two-thirds of smokers seeking lung cancer screening were ready to quit smoking within the next 6 months (25% within the next month) and 60% of smokers expressed strong interest in receiving smoking cessation counseling and medications. When coupled with naturalistic observations of post-screening quitting, prior work suggests that many smokers who undergo lung cancer screening are indeed interested in quitting31,32 and receiving smoking cessation treatment within the context of lung cancer screening. Moreover, recent studies of smokers uninterested in quitting suggest changes to clinical workflows such that tobacco cessation advice and assistance should be offered to all smokers not only those who express quitting interest.33
Given the staff perceptions of patient resistance, staff at lung cancer screening sites will need additional training on how to engage smokers effectively with advice and behavioral counseling tailored to the needs of older, longstanding heavy smokers with variable quitting motivation.34 It may be helpful to train screening site staff in brief, motivational counseling35 and other interventions (ie, prequit nicotine replacement therapy and smoking reduction) targeting smokers not yet ready to quit.36,37 Even sites with high organizational priority for integrating tobacco treatment are likely to identify practical barriers regarding time and staffing resources and therefore facilitating sites’ adoption of an implementation strategy such as Ask, Advise, Connect/Refer38 may be ideal for resource-limited screening sites.
There are several sampling and other methodological limitations that should be noted. First, the findings were derived from self-reports of site coordinators from a newly formed network of lung cancer screening sites that have pledged commitment to implementing a high-quality lung cancer screening program by adopting the National Framework for Excellence in Lung Cancer Screening and Continuum of Care. Therefore, these findings may not be representative of all lung cancer screening sites and may overestimate the perceived organizational priority and actual adoption of tobacco cessation practices in all lung cancer screening sites. Although the 61% survey response rate was as good or higher than prior provider surveys,21,39,40 it is certainly plausible that sites that did not respond to the survey have lower organizational priority for integrating tobacco cessation. Second, health care providers typically report delivery of tobacco use advice and assistance at higher rates than those reported by patients or documented in the medical record.41 As such, these findings reported by site coordinators may overestimate the actual delivery of tobacco use treatment in lung cancer settings. On the other hand, site coordinators may not be fully aware of the full extent of tobacco treatment services delivered. Future studies should corroborate these site coordinator assessments of cessation practices with patient-exit interviews42 and medical chart audits. Finally, we did not collect detailed information on sites’ clinical capacity for tobacco treatment delivery. For instance, we did not assess whether sites had a designated tobacco treatment specialist which is likely needed for sites to provide onsite (in-person) cessation counseling. There is likely to be variation in screening sites’ tobacco treatment capacity depending upon the variability of onsite cessation resources, the screening workflow, and the involvement of the referring physician. One respondent commented, “my state nursing licensure regulations require physical examination as a prerequisite for prescribing medications so the best I can do is refer smokers to the state quitline.” Another respondent indicated that, “Our screening model relies on the referring physician to be responsible for delivering cessation interventions.”
Nonetheless, these descriptive findings provide guidance for the development of strategies to enhance the implementation of smoking cessation advice and treatment in lung cancer screening settings. Effective implementation of smoking cessation treatment within the context of lung cancer screening requires attention to staff training and establishing an effective, scalable, and sustainable clinical work flow that addresses barriers and practical challenges. Although favorable cessation outcomes have been observed, solely enrolling in a screening program is unlikely to be a sufficient catalyst for smoking cessation in this high-risk population of older, heavy smokers. All smokers should be advised to quit smoking and provided with evidence-based smoking cessation treatment. In addition, there are promising opportunities for screening sites and referring physicians to develop and evaluate personalized message framing strategies to communicate the risks of persistent smoking and the benefits of quitting concurrent with notification of CT scan results. Without cessation advice and counseling, those who receive negative screening results may erroneously perceive diminished risks of persistent smoking and those who receive positive scan results may perceive smoking cessation to be of limited clinical benefit. On the other hand, it is well established that smokers who receive advice and evidence-based tobacco treatment from their health care providers have superior cessation outcomes.17 Identifying effective strategies for integrating tobacco use treatment in lung cancer screening sites will be particularly critical as demand for lung cancer screening increases with Medicare and most private insurance plans now covering lung cancer screening and the Affordable Care Act mandating coverage of tobacco cessation treatment. These findings suggest directions for staff training and quality improvement initiatives and guide future research focusing on identifying effective strategies to disseminate and implement evidence-based tobacco treatment into lung cancer screening settings.
Funding
This work was supported in part by philanthropic funds donated by the Society of Memorial Sloan Kettering Cancer Center and by the MSK Cancer Center Support Grant/Core Grant (P30 CA008748).
Declaration of Interests
JSO, DRS, AC, and SPB have no conflicts of interest. CIH receives grants from Flight Attendants Medical Research Institute and American Legacy Foundation. In addition, she has issued and pending patents, some of which are licensed to General Electric Healthcare by Cornell Research Foundation, but has renounced any royalties since April 2009. CIH is the President and serves on the board of the Early Diagnosis and Treatment Research Foundation. She receives no compensation from the Foundation. The Foundation provides grants for projects and conferences involved in early diagnosis and treatment and for developing public databases for the purposes of imaging research. Recipients include the I-ELCAP (projects and conferences). The funding comes from a variety of sources including philanthropic donations, grants and contracts with agencies (federal and nonfederal), and imaging and pharmaceutical companies relating to image processing assessment with a view to the development of public databases.
Acknowledgments
Portions of this paper were presented at the annual meetings of the American Public Health Association in November 2014 and the Society for Nicotine and Tobacco Research in February 2015.
References
- 1. American Cancer Society. Cancer Facts and Figures 2013 2013. www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf. Accessed February 13, 2015.
- 2. U.S. Department of Health and Human Services. The Health Consequences of Smoking - 50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 3. Centers for Medicare & Medicaid Services. Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N) www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274. Accessed July 1, 2015.
- 4. Taylor KL, Cox LS, Zincke N, Mehta L, McGuire C, Gelmann E. Lung cancer screening as a teachable moment for smoking cessation. Lung Cancer. 2007;56(1):125–134. doi:10.1016/j.lungcan.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 5. Anderson CM, Yip R, Henschke CI, Yankelevitz DF, Ostroff JS, Burns DM. Smoking cessation and relapse during a lung cancer screening program. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3476–3483. doi:10.1158/1055-9965.epi-09-0176. [DOI] [PubMed] [Google Scholar]
- 6. Ashraf H, Tonnesen P, Holst Pedersen J, Dirksen A, Thorsen H, Dossing M. Effect of CT screening on smoking habits at 1-year follow-up in the Danish Lung Cancer Screening Trial (DLCST). Thorax. 2009;64(5):388–392. doi:10.1136/thx.2008.102475. [DOI] [PubMed] [Google Scholar]
- 7. Clark MM, Cox LS, Jett JR, et al. Effectiveness of smoking cessation self-help materials in a lung cancer screening population. Lung Cancer. 2004;44(1):13–21. doi:10.1016/j.lungcan.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 8. Cox LS, Clark MM, Jett JR, et al. Change in smoking status after spiral chest computed tomography scan screening. Cancer. 2003;98(11):2495–2501. doi:10.1002/cncr.11813. [DOI] [PubMed] [Google Scholar]
- 9. Ferketich AK, Otterson GA, King M, Hall N, Browning KK, Wewers ME. A pilot test of a combined tobacco dependence treatment and lung cancer screening program. Lung Cancer. 2012;76(2):211–215. doi:10.1016/j.lungcan.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ostroff JS, Buckshee N, Mancuso CA, Yankelevitz DF, Henschke CI. Smoking cessation following CT screening for early detection of lung cancer. Prev Med. 2001;33(6):613–621. doi:10.1006/pmed.2001.0935. [DOI] [PubMed] [Google Scholar]
- 11. Tammemagi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst. 2014;106(6):dju084. doi:10.1093/jnci/dju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Aalst CM, van den Bergh KA, Willemsen MC, de Koning HJ, van Klaveren RJ. Lung cancer screening and smoking abstinence: 2 year follow-up data from the Dutch-Belgian randomised controlled lung cancer screening trial. Thorax. 2010;65(7):600–605. doi:10.1136/thx.2009.133751. [DOI] [PubMed] [Google Scholar]
- 13. Villanti AC, Jiang Y, Abrams DB, Pyenson BS. A cost-utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLoS One. 2013;8(8):e71379. doi:10.1371/journal.pone.0071379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poghosyan H, Kennedy Sheldon L, Cooley ME. The impact of computed tomography screening for lung cancer on smoking behaviors: a teachable moment? Cancer Nurs. 2012;35(6):446–475. doi:10.1097/NCC.0b013e3182406297. [DOI] [PubMed] [Google Scholar]
- 15. Ostroff JS, Henschke C, Yip R, et al. Smoking cessation among current smokers following enrollment in a lung screening program. Under Review.
- 16. Slatore CG, Baumann C, Pappas M, Humphrey LL. Smoking behaviors among patients receiving computed tomography for lung cancer screening. Systematic review in support of the U.S. Preventive Services Task Force. Ann Am Thorac Soc. 2014;11(4):619–627. doi:10.1513/AnnalsATS.201312-460OC. [DOI] [PubMed] [Google Scholar]
- 17. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- 18. Papadakis S, McDonald P, Mullen KA, Reid R, Skulsky K, Pipe A. Strategies to increase the delivery of smoking cessation treatments in primary care settings: a systematic review and meta-analysis. Prev Med. 2010;51(3–4):199–213. doi:10.1016/j.ypmed.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 19. Klein KJ, Conn AB, Sorra JS. Implementing computerized technology: an organizational analysis. J Appl Psychol. 2001;86(5):811–824. doi:10.1037//0021-9010.86.5.811. [DOI] [PubMed] [Google Scholar]
- 20. Lung Cancer Alliance. National Framework for Excellence in Lung Cancer Screening and Continuum of Care www.lungcanceralliance.org/assets/docs/am-i-at-risk/NationalFramework.pdf. Accessed January 17, 2015.
- 21. Association of American Medical Colleges, Center for Health Workforce Studies. Physician Behavior and Practice Patterns Related to Smoking Cessation. Washington, DC: American Legacy Foundation; 2007. [Google Scholar]
- 22. Amemori M, Michie S, Korhonen T, Murtomaa H, Kinnunen TH. Assessing implementation difficulties in tobacco use prevention and cessation counselling among dental providers. Implementat Sci. 2011;6:50. doi:10.1186/1748-5908-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park E, Eaton CA, Goldstein MG, et al. The development of a decisional balance measure of physician smoking cessation interventions. Prev Med. 2001;33(4):261–267. doi:10.1006/pmed.2001.0879. [DOI] [PubMed] [Google Scholar]
- 24. Collins LM, Lanza ST. Latent Class and Latent Transition Analysis. Hoboken, NJ: John Wiley & Sons; 2010. [Google Scholar]
- 25. Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):e78S–e92S. doi:10.1378/chest.12-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Humphrey L, Deffebach M, Pappas M, et al. Screening for Lung Cancer: Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Evidence Synthesis No. 105. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 27. Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144(1):33–38. doi:10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 28. Wender R, Fontham ET, Barrera E, Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63(2):107–117. doi:10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;10(2):240–265. www.jnccn.org/content/13/1/23.full.pdf+html. Accessed January 17, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park ER, Gareen IF, Japuntich S, et al. Primary care provider-delivered smoking cessation interventions and smoking cessation among participants in the National Lung Screening Trial. JAMA Intern Med. 2015. doi:10.1001/jamainternmed.2015.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hahn EJ, Rayens MK, Hopenhayn C, Christian WJ. Perceived risk and interest in screening for lung cancer among current and former smokers. Res Nurs Health. 2006;29(4):359–370. doi:10.1002/nur.20132. [DOI] [PubMed] [Google Scholar]
- 32. Park ER, Ostroff JS, Rakowski W, et al. Risk perceptions among participants undergoing lung cancer screening: baseline results from the National Lung Screening Trial. Ann Behav Med. 2009;37(3):268–279. doi:10.1007/s12160-009-9112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Richter KP, Ellerbeck EF. It’s time to change the default for tobacco treatment. Addiction. 2015;110(3):381–386. doi:10.1111/add.12734. [DOI] [PubMed] [Google Scholar]
- 34. Zbikowski SM, Magnusson B, Pockey JR, Tindle HA, Weaver KE. A review of smoking cessation interventions for smokers aged 50 and older. Maturitas. 2012;71(2):131–141. doi:10.1016/j.maturitas.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 35. Miller WR, Rollnick SR. Motivational Interviewing: Preparing People for Change. 2nd ed. New York, NY: The Guilford Press; 2002. [Google Scholar]
- 36. Stead LF, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst Rev. 2007;(3):CD005231. doi:10.1002/14651858.CD005231.pub2. [DOI] [PubMed] [Google Scholar]
- 37. Lindson-Hawley N, Thompson TP, Begh R. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2015;3:CD006936. doi:10.1002/14651858.CD006936.pub3. [DOI] [PubMed] [Google Scholar]
- 38. Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173(6):458–464. doi:10.1001/jamainternmed.2013.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sarna L, Bialous SA, Wells M, Kotlerman J, Wewers ME, Froelicher ES. Frequency of nurses’ smoking cessation interventions: report from a national survey. J Clin Nurs. 2009;18(14):2066–2077. doi:10.1111/j.1365-2702.2009.02796.x. [DOI] [PubMed] [Google Scholar]
- 40. McPhillips-Tangum C. Results from the first annual survey on addressing tobacco in managed care. Tob Control. 1998;7(suppl):S11–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Conroy MB, Majchrzak NE, Silverman CB, et al. Measuring provider adherence to tobacco treatment guidelines: a comparison of electronic medical record review, patient survey, and provider survey. Nicotine Tob Res. 2005;7(suppl 1):S35–S43. doi:10.1080/14622200500078089. [DOI] [PubMed] [Google Scholar]
- 42. Pbert L, Adams A, Quirk M, Hebert JR, Ockene JK, Luippold RS. The patient exit interview as an assessment of physician-delivered smoking intervention: a validation study. Health Psychol. 1999;18(2):183–188. doi:10.1037/0278-6133.18.2.183. [DOI] [PubMed] [Google Scholar]