Abstract
Invasive alien plant species (IAPS) can pose severe threats to biodiversity and stability of native ecosystems, therefore, predicting the distribution of the IAPS plays a crucial role in effective planning and management of ecosystems. In the present study, we use Maximum Entropy (MaxEnt) modelling approach to predict the potential of distribution of eleven IAPS under future climatic conditions under RCP 2.6 and RCP 8.5 in part of Kailash sacred landscape region in Western Himalaya. Based on the model predictions, distribution of most of these invasive plants is expected to expand under future climatic scenarios, which might pose a serious threat to the native ecosystems through competition for resources in the study area. Native scrublands and subtropical needle-leaved forests will be the most affected ecosystems by the expansion of these IAPS. The present study is first of its kind in the Kailash Sacred Landscape in the field of invasive plants and the predictions of potential distribution under future climatic conditions from our study could help decision makers in planning and managing these forest ecosystems effectively.
Introduction
Biological invasion has become one of the major causes of economic and environmental damage in most of the countries across the world [1–3] and its impacts have been predicted to increase even further under future climatic conditions [4–5]. The Convention on Biological Diversity (1992) emphasized biological invasion as one of the major driver of biodiversity decline and the second biggest threat after habitat destruction and ecosystem degradation [6–9]. Climate Change, anthropogenic pressure, and land use change have accelerated bio-invasion [10–11].
The existing unique landscapes, ecosystems, and biota of Hindu Kush Himalayas (HKH) are spawned as a result of diverse climatic, topographic, geological, and altitudinal variations [12]. The HKH region is sensitive and fragile to climate change and is experiencing an annual increase in temperature from 0.03–0.07°C [13–14]. Plant invasions in mountain areas are likely to rise because of increase in trade, tourism activities, climate change, and anthropogenic disturbances and can alter both native floral and faunal species composition inducing prolonged negative impacts [8, 15–16]. Over the last few decades, the study of invasions has received much more attention globally and there are a lot of studies focused on mapping the distribution of invasive species, quantifying economic and ecological impacts, and developing efficient and economical management approaches [17–19]. However, the potential expansion of the invasive plants in the HKH region under predicted climate change remains underexplored, especially in the transboundary context that compels us to undertake this study. Our study provides baseline information for understanding the current distribution and predicting the future distribution of invasive alien plant species (IAPS) which are currently in the early stage of invasion and which could be potential threats in the future.
Limited environmental and historical bioclimatic data and systematic monitoring, coupled with political sensitivities within the region result in the lack of knowledge base, which is crucial for developing scientifically comprehensive transboundary climate change adaptation strategies. Therefore, there is an urgent need for regional cooperation in the HKH region for informed decision-making, risk and vulnerability mapping, effective biodiversity, and conservation management and holistic approach while developing the climate change adaptation strategies [14].
Species distribution models (SDMs) are scientifically proven tools for assessing and predicting the impacts of climate change on flora and fauna [20–23]. SDMs can be used to determine the relationships between species and their environment and predict their distribution from occurrence (presence only or presence/absence) data [24–27]. Understanding the factors influencing species distribution is imperative for ecological research in developing effective adaptation strategies to cope with impacts of climate change [28–29]. Selecting the most suitable modelling algorithm and relevant datasets is a major challenge in species distribution modelling [30]. SDMs dealing with presence-only data might be more advantageous over presence/absence modeling methods, conditional to the suitability for the study [20, 31] eg. MaxEnt.
Maximum entropy (MaxEnt) method is a general purpose machine learning method applied for producing species distribution maps using presence-only data [29, 31–32]. MaxEnt, a bioclimatic model is widely used by conservation practitioners and researchers as a tool for prediction and distribution of species [33]. Wilson et al., [34] used the combination of MaxEnt and Generalized Linear Mixed Models (GLMMs) to identify areas of high conservation value for the endangered species Margaritifera margaritifera (L.). Chitale et al., [20] used MaxEnt to predict the distribution of 637 endemic plants in four global biodiversity hotspots including the Himalaya, by combining climatic and non-climatic variables. Whereas Adhikari et al., [35] modelled hotspots of invasive plant species through Ecological Niche Modeling (ENM) using MaxEnt for guiding the formulation of an effective policy for controlling the IAPS. For instance, West et al., [36] used a MaxEnt model with an invasive species Bromus tectorum (cheatgrass) presence data and evaluated its usefulness in a management context. On the other hand, Qin et al., [37] investigated the spatial patterns of Lantana camara habitat changes from its current distribution to future potential occupied areas using the MaxEnt ecological niche modeling technique. In this study also, MaxEnt modelling was used as it can achieve high predictive accuracy [31]. As the sample size shrinkages the model accuracy decreased however, MaxEnt modelling is less sensitive than other approaches to the number of presence locations [31, 38]. MaxEnt tuned their regularization in relation to sample size to avoid over fitting [38–39]. Presence only data are good enough for species distribution modelling and the AUC scores obtained for predictions from it can be sufficiently accurate [39–40]. SDMs have some limitations such as overestimation of presence of species. Assumption of random sampling of presence of species on the grid cells made by SDMs predicts large probability of presence in each cell, which could be in fact overestimation [29]. MaxEnt uses presence only data and it may give high predicted values for environmental conditions outside the range [32]. In order to avoid the over estimation, we applied a threshold value 0.5 was applied in this study and used only those pixels that have values equal to or higher than 0.5.
In this paper, we use data of distribution of 11 invasive plants (namely Ageratina adenophora L., Ageratum conyzoides L., Ageratum houstonianum Mill., Amaranthus spinosus L., Bidens pilosa L., Erigeron karvinskianus DC., Lantana camara L., Parthenium hysterophorus L., Senna occidentalis (L.) Link., Senna tora L. Roxb. and Xanthium strumarium L.) distributed in four districts of the Kailash Sacred Transboundary Landscape viz., Darchula, Baitadi, and Bajhang in Nepal and Pithoragarh district in India. The species considered in this study have been reported as ‘invasive’ in national level assessments and mapping [41–42]. In Nepal and Himalayas, most of the invasive species have been spreading from southern lowland to mid hills and mountains in the north. Some species (e.g. Ageratina adenophora, Lantana camara) are already widespread in the study region while other species (e.g. Parthenium hysterophorus) are less abundant but they have already established small satellite population at a number of locations indicating their high potential to spread in the landscape. In absence of previous studies on invasive species in the region, it is not possible to estimate the duration for which the species is there. This could be an interesting topic of research for the future. The habitats known to be invaded by these species can be described as below:
Among them, Ageratum conyzoides, A. houstonianum and Erigeron karvinskianus are primarily invading agroecosystem; A. adenophora and L. camara in forest and shrublands; and the remaining six species in grazing lands, roadside vegetation and residential areas [43–44].
The objectives of the study were to i) determine the current distribution pattern and habitat of the selected invasive plant species; ii) use the occurrence data of the selected species to predict their change in distribution under simulated different climate change scenarios in 2050 and 2070; iii) support the formulation of guidelines and management practices on controlling and to provide information to prevent further spread of IAPS in the Kailash Sacred Landscape area based on the information obtained from modelling.
Materials and methods
Study area
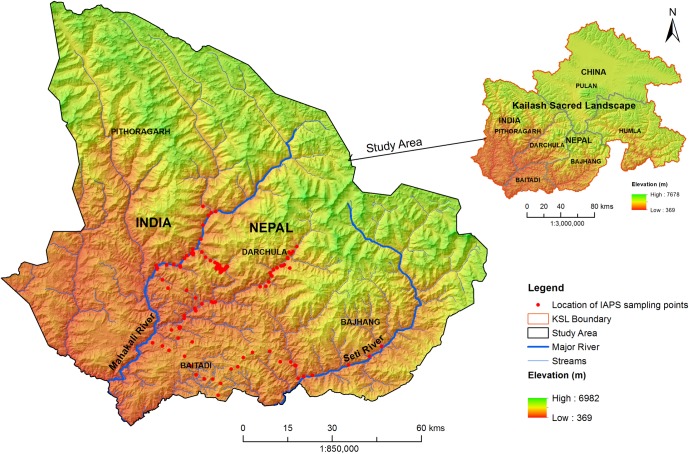
The Kailash Sacred Landscape (KSL) spans across China, India, and Nepal. It represents a unique ‘transboundary cooperation’ and the first cooperation of its kind among China, India and Nepal [45–46], however, this study was focused only in certain regions of Nepal and India. The study area is located between 29.3° to 30.6° N latitudes and 79.86° to 81.56° E longitudes (Fig 1). The landscape covers an area of 13762 km2 and has a wide elevation range from 369 to 6982 m asl (above sea level). This geographic heterogeneity has given rise to a high level of biodiversity including an array of forest types ranging from moist subtropical broadleaf to temperate oak forests, alpine conifers, and pastures [47]. KSL is considered among the most revered sacred landscapes in the world and also the source of four of Asia’s most important rivers, the Brahmaputra, Indus, Karnali, and Sutlej [45–46].
Fig 1. Location of IAPS field sampling points in study area.
Data
Distribution of invasive alien plants
The current distribution of 11 invasive alien plant species (IAPS) in the study area was sampled using geographical positioning system (GPS) during 18–24 June 2015. Since the distribution of the IAPS was most dominant along the road, we recorded the coordinates of the locations of the species using Garmin GPS (GPSmap 62sc) along the road. Road networks often serve as conduit for dispersal of IAPS due to long distance dispersal of propagules by vehicles [48] as well as the road verges being suitable for colonization by the alien plant species [49–50]. Therefore roadside survey is used for rapid assessment of the diversity and distribution of the IAPS at landscape level [51–53]. In landscape level sampling, other methods of mapping is highly expensive and much more time consuming. Instead of single highway, we used several network of roads and trails for mapping. Furthermore, roadside environment provided suitable microhabitat for invasive plants which are ruderal in nature. Dispersal of all the invasive species considered in the study are dispersed directly or indirectly by human activities. However, L. camara seeds is additionally dispersed by birds too.
Interval between successive plots is subjective. Previous study in East Africa Wabuyele et al. used 25 km interval for the survey [51] while another study used 5–30 km interval [53]. We used 5–10 km interval for the survey. When elevation changes sharply, climate as well as the turn-over of species and vegetation also change shortly. The shorter interval in steep landscape was to capture all vegetation types adequately. It is not only the elevation, but in addition aspect and other topographic factors also change drastically in hill and mountains within a short distance. Therefore, shorter interval is required to adequately represent microhabitats.
From the starting point of the survey, at every 10 km distance, field plots of 10 m × 10 m were examined on both sides of the road to record distribution of IAPS. A section of road running south-north parallel to Nepal-India border in Dharcula (India) between Ghatibagar (south) and Tawaghat (north) was examined for the present of invasive alien plant species (IAPS) in every five kilometer road distance. In between these pre-defined locations, some opportunistic observations were also made if we encountered species not recorded in the immediate previous plot. This strategy was used in order to capture maximum possible records and locations of the distribution of IAPS. Altogether 15 IAPS were found in the Nepal region of the KSL; 11 of them with adequate occurrence data have been used in this study (Table 1). Remaining four species (Galinsoga quadriradiata Ruiz & Pav., Ipomoea carnea ssp. fistulosa (Mart. ex Choisy) D.F. Austin, Oxalis latifolia Kunth. and Pistia tratiotes L.) were found only at a few locations and thus excluded in the present analysis. In total 189 plots were examined during the survey.
Table 1. Invasive alien plant species recorded in the Kailash Sacred Landscape, Nepal and included in the present analysis.
| Sl. No. | Scientific Name of IAPS | Common Names | Local Name | Family | Native Range | Sample Number |
|---|---|---|---|---|---|---|
| 1 | Ageratina adenophora L. | Crofton weed | Kalo Banmara | Asteraceae | Mexico | 130 |
| 2 | Ageratum conyzoides L. | Billygoat weed | Raunne/Gandhe | Asteraceae | Central & South America | 69 |
| 3 | Ageratum houstonianum Mill. | Blue billygoat weed | Nilo Gandhe | Asteraceae | Mexico & Central America | 35 |
| 4 | Amaranthus spinosus L. | Spiny pigweed | Kande lude | Amaranthaceae | Tropical America | 19 |
| 5 | Bidens pilosa L. | Blackjack/Hairy Beggar-tick | Kalo kuro | Asteraceae | Tropical America | 101 |
| 6 | Erigeron karvinskianus DC. | Mexican fleabane | Phule Jhar | Asteraceae | Mexico & Central America | 96 |
| 7 | Lantana camara L. | Lantana | Kirne Kanda | Verbenaceae | Central & South America | 20 |
| 8 | Parthenium hysterophorus L. | Parthenium weed | Pati Jhar | Asteraceae | Southern USA to South America | 25 |
| 9 | Senna occidentalis (L.) Link. | Coffee senna | Panwar | Leguminosae | Mexico to South America | 15 |
| 10 | Senna tora (L.) Roxb. | Sickle pod senna | Tapre | Leguminosae | South America | 28 |
| 11 | Xanthium strumarium L. | Rough cockle-Bur | Bhende Kuro | Asteraceae | America | 40 |
For 9 out of 11 species, upper elevation limit of current distribution in the study region is lower than those reported from other parts of Nepal [43–44]. Therefore, it is less likely that the current uppermost distribution of these species has reached to climatic limit. Since dispersal of most of these species occurs through human activities, their further spread also depends on increasing human and livestock movements and opening of roads. However, species specific information on dispersal pattern and adaptation to high elevation region is not available for these study species.
Environmental and bioclimatic data
Nineteen bioclimatic variables of present and future time period (year 2050 and 2070) with a spatial resolution of 1 km2 were downloaded from worldclim datasets (www.worldclim.com). Based on an average annual change in means [54] we used projections of Community Climate System Model (CCSM4) under Representative Concentration Pathways viz., RCP 2.6 and RCP 8.5 for the year 2050 and 2070 as adopted by the IPCC in its Fifth Assessment Report (AR5). RCP 2.6 represents the lowest Greenhouse Gas (GHG) concentration pathway, whereas RCP 8.5 represents the extreme GHG concentration pathway [55] (Table 2). These data are statistically downscaled from a Global Circulation Model (GCM) using WorldClim 1.4 as baseline 'present' climate. Elevation, slope, and aspect were derived from digital elevation data based on the Shuttle Radar Topographic Mission (SRTM) at 90m spatial resolution. Finally, all ancillary layers were resampled to 1 km2 spatial resolution to match with the spatial resolution of climate variables.
Table 2. AR5 global warming increase (°C) projections.
|
Scenario |
Mean and likely range | |
|---|---|---|
| 2046–2065 | 2081–2100 | |
| RCP 2.6 | 1.0 (0.4 to 1.6) | 1.0 (0.3 to 1.7) |
| RCP 4.5 | 1.4 (0.9 to 2.0) | 1.8 (1.1 to 2.6) |
| RCP 6.0 | 1.3 (0.8 to 1.8) | 2.2 (1.4 to 3.1) |
| RCP 8.5 | 2.0 (1.4 to 2.6) | 3.7 (2.6 to 4.8) |
Source: IPCC, AR5 2014
Modeling approach
MaxEnt software (version 3.3.3 k) downloaded from (http://www.cs.princeton.edu/~schapire/maxent/) was used in this study for predicting the distribution of 11 IAPS. MaxEnt generates an estimate of the probability of presence of the species that varies from 0 to 1, i.e. from the lowest to the highest probability of distribution. Species presence data and derived all environmental and physiographic data were converted to ASCII file before running the MaxEnt model. Models were built for individual species to predict the distribution of the IAPS under projected climate change scenarios.
Prediction accuracy and validation of the models were assessed on the basis of Area Under the Receiving Operator Curve (AUC), sensitivity (correctly classified presences) and specificity (correctly classified absences) [20, 56–57]. These measures are estimated from 578 random splits of the field dataset into a calibration subset with 70% of the data and a validation subset with 30% of the data, which is used by the model to assess the statistical significance [20]. AUC values range from 0 to 1. Values between 0.2–0.5 were considered low, 0.5–0.7 moderate and >0.7 as high while validating the model results. The jackknife procedure also called ‘leave one out’ was followed to assess the importance of variables [13, 56]. Jackknife, an alternative approach for assessing variable importance which provides statistics on the significance of each variable in the model [57–59].
Image classification, combination, and analysis
The outputs were imported to ArcGIS 10.4 and converted to a.tiff raster format for further analysis. The output maps of the distribution of IAPS generated by MaxEnt were classified into two classes viz., 0.00 to 0.50 and 0.50 to 1.0. We selected pixels with or more 0.5 value to consider areas that depict at least 50% probability of species occurrence. The value greater than 0.5 depicts areas with a highly suitable habitat, while values lower than 0.5 represents low suitability of habitat for invasive plant species. Change detection maps were generated by using difference function in ArcGIS (subtracting future distribution from present distribution) for all IAPS to understand the expansion/ reduction/ no change in the distribution range of these species. The difference maps were then reclassified into three classes where negative values depicted range expansion, positive values depicted range reduction, and zero values depicted no change, and respectively. We quantified the changes in the altitudinal range in the distribution of these species by using the Digital Elevation Model (DEM).
Results
Prediction accuracy
Overall accuracy was high (~0.90, which refers to 90% accuracy) for predictions under present and future time periods. Prediction accuracy of the model used for analyzing the distribution under present and future time period of 11 IAPS were between 0.942 and 0.997 with training data and between 0.824 and 0.987 with test data, respectively (Table 3, S1 Table). Highest AUC value 0.997 was obtained for L. camara for the year 2070 in RCP 2.6. From the jackknife analysis, three variables out of a total of 22 bioclimatic physiographic variables used as predictor variables showed a major role in predicting the distribution of the IAPS (Table 4, S1 Fig) viz., (i) minimum Temperature of Coldest Month (Bio 6), (ii) mean Temperature of Driest Quarter (Bio 9), and (iii) mean Diurnal Range (Bio 2).
Table 3. Prediction accuracy of invasive species distribution modeling.
| Scenario | Present | Year 2050 | Year 2070 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RCP 2.6 | RCP 8.5 | RCP 2.6 | RCP 8.5 | |||||||
| Training | Test | Training | Test | Training | Test | Training | Test | Training | Test | |
| Minimum AUC Value | 0.942 | 0.829 | 0.949 | 0.827 | 0.942 | 0.83 | 0.942 | 0.825 | 0.944 | 0.824 |
| Maximum AUC Value | 0.995 | 0.978 | 0.995 | 0.987 | 0.995 | 0.981 | 0.997 | 0.984 | 0.993 | 0.984 |
Table 4. Overall relative importance of predictor variables for invasive species from Jackknife test.
| Time Period | Most Significant Variable | Code | Jackknife AUC Value | |
|---|---|---|---|---|
| Present | Mean Temperature of Driest Quarter | Bio 9 | 0.90 | |
| 2050 |
RCP 2.6 | Mean Diurnal Range (Mean of monthly (max temp—min temp) | Bio 2 | 0.95 |
| RCP 8.5 | Minimum Temperature of Coldest Month | Bio 6 | 0.89 | |
| 2070 |
RCP 2.6 | Mean Temperature of Driest Quarter | Bio 9 | 0.90 |
| RCP 8.5 | Minimum Temperature of Coldest Month | Bio 6 | 0.92 | |
Distribution of invasive alien plant species
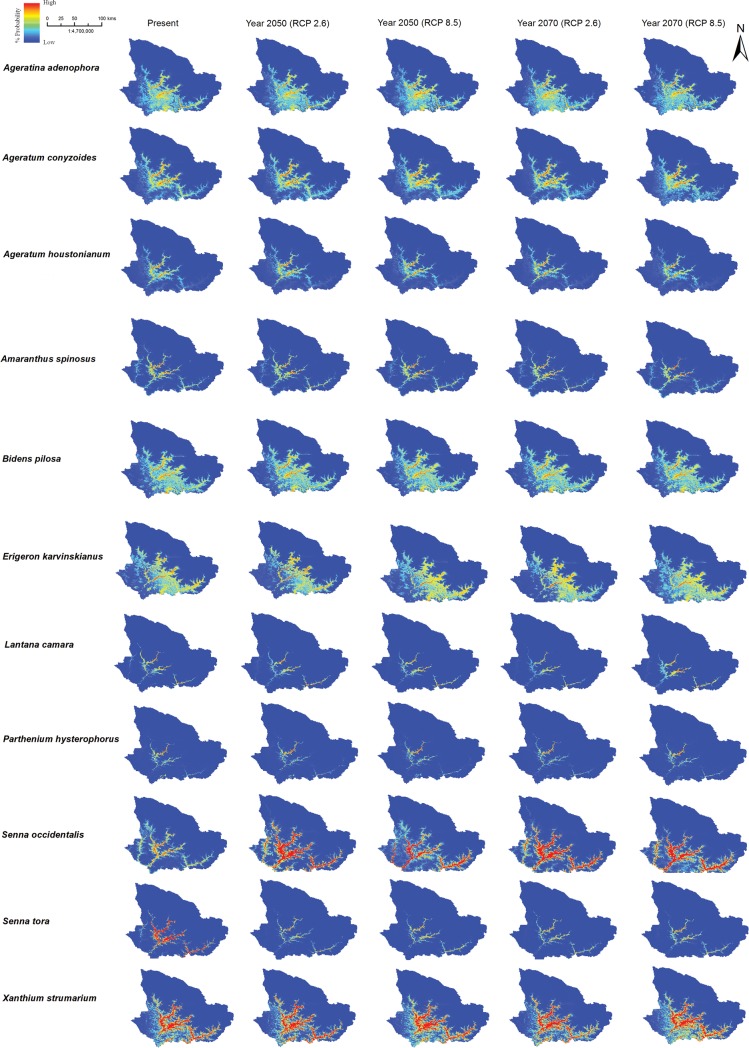
Under current climate conditions, the overall distribution of the IAPS is concentrated towards central South-East (SE) zone of the study area. However, the future distribution is predicted to shift towards North-East (NE) and expand to SE direction compared to the present distribution (Fig 2).
Fig 2. Predictive distribution of IAPS.
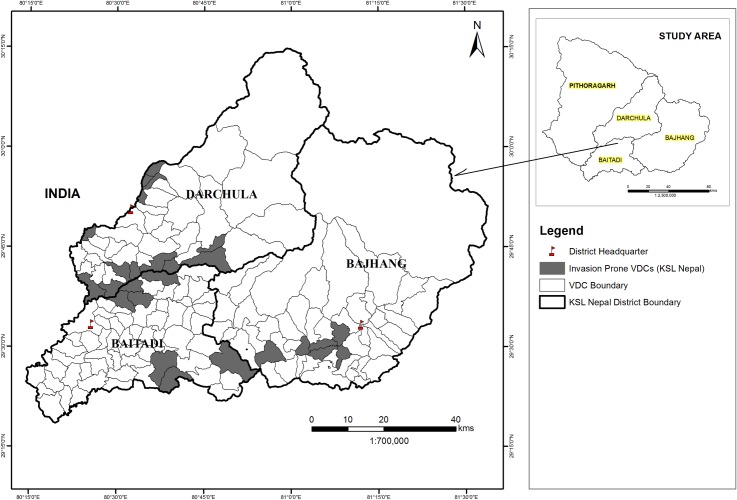
An overall expansion in the modelled distribution of a range of all 11 species is predicted under future climatic conditions under RCP 2.6 and RCP 8.5 by the year 2050 and 2070. Vegetation in the village development committee areas in the following districts of Nepal are highly susceptible to invasion by these IAPS: (i) Darchula district: Boharigaun, Bramhadev, Dattu, Dethala, Dhaulakot, Gokuleshwar, Huti, Lali, Pipalchaur, Sarmauli, Shikhar, Tapoban, (ii) Baitadi district: Gokuleshwor, Rim, Rudreswor, Shivalinga, Siddhapuri, Siddheswor, Sittad, (iii) Bajhang district: Chaudhari, Lamatola, Latinath, Malumela, Matela, Rayal, Subeda (Fig 3).
Fig 3. Highly susceptible areas of KSL Nepal to invasion by IAPS.
Even a moderate climate change scenario will lead to an expansion of the distribution of the 11 IAPS, which might pose threat to the native flora. Out of the 11 species, six are distributed in scrub vegetation and five in the subtropical needle-leaved forest. The current distribution of invasive plant species ranges between an altitude of 622 m to 2865 m; however, under future conditions (RCP 2.6 and RCP 8.5) the distribution is predicted to expand towards both upper and lower elevation ranging from 448 m to 3547 m.
Range expansion in future distribution
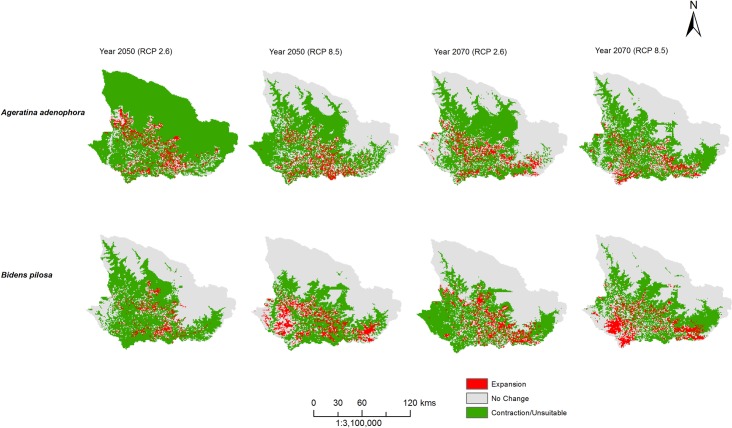
In the beginning of the year 2050, the IAPS will expand towards both upper (1034 m) and lower (246 m) elevation. For instance, Ageratum conyzoides, Erigeron karvinskianus, Xanthium strumarium, Ageratina adenophora, and Bidens pilosa (Fig 4 and S3 Fig) be speculated to expand upward by 1034 m, 981 m, 800 m, 692 m and 682 m respectively from the current elevation range. Similarly, in the beginning of the year 2070, the IAPS range expand upward by 981 m and downward by 359 m. Hence the elevation range expands from “622 m -2865 m” to “448 m-3547 m”. It is predicted that A. adenophora and E. karvinskianus will also expand vertically up to 3547 m and rising 981 m upper elevation than current scenario. While distribution of Lantana camara and Partnenium hysterophorus exhibited a different trend in the future and a significant decrease in their upper elevation range by 598 m and 486 m respectively in the year 2070. Under future climatic conditions, highest range expansion is predicted in the distribution of E. karvinskianus, P. hysterophorus, and L. camara.
Fig 4. Example of predicted range expansion of Ageratina adenophora and Bidens pilosa for year 2050 and 2070 at different climatic scenario.
At present E. karvinskianus is widely distributed and concentrated in central and southeastern parts of the landscape at an elevation range of 677 m to 2566 m with secondary scrub as the dominant vegetation. Under future prediction, it is expected that its distribution will expand in the southern belt, SE of Pithoragarh, SE and SW of Darchula, NE and SE of Baitadi and south of Bajhang. In the future, the distributional range will expand upward by 981m and downward by 200 m. Hence, the distributional elevation range of E. karvinskianus will be from 477 m to 3547 m. Currently, L. camara is distributed between 829 m to 1975 m in the central west and SE region of the landscape (along with the border of West Pithoragarh, and NW of Baitadi and South of Darchula districts) in subtropical needle-leaved forest. In future, L. camara will expand downward by 359 m. The current distribution of P. hysterophorus is dominant in the central eastern of Pithoragarh, SW of Darchula, patches in south of Baitadi and Bajhang in an elevation range from 686 m to 2050 m predominantly in subtropical needle-leaved forest. In the year 2050 its range will expand by 23 m up and 130 m down compare to present distribution. However, in year 2070, the distributional elevation range expand towards lower elevation up to 238 m down but contracts at an upper elevation by 486 m i.e. the distribution of P. hysterophorus in year 2070 will be between 448 m to 1564 m. In future, there will be slight expansion in East of Pithoragarh, NE of Baitadi, SE and SW of Bajhang.
Discussion and conclusions
Research attempt to model the distribution range of 11 IAPS and identify crucial zones for ecosystem management to regulate degradation in part of Western Himalaya. Most of the studies on predicting the distribution of invasive species have focused on one or two species [37, 60–61] and only a few studies have focused on future prediction of more than two species [62–63]. Predicting the future distribution of invasive plants provides insights on their spread under future climate conditions, which in turn can provide the vegetation types prone to degradation because of the invasion. It is essential to identify future invaders and take initial steps in prevention which is the cost-effective means to minimize the spread and impacts of IAPS to new areas.
By year 2050, substantial shifts in bioclimatic conditions is also anticipated throughout the KSL area [14]. Bio-climatic variables like minimum temperature of coldest month, mean temperature of driest quarter and mean diurnal range play major role and contribute more to the invasion by 11 IAPS in the present study. Mostly, the expansion of the invasive species might encroach the natural ecosystems of secondary scrub and subtropical needle-leaved forest. The model predicted that A. adenophora, A. conyzoides, B. pilosa, E. karvinskianus, and X. strumarium will significantly expand both vertically and horizontally under all future climate scenarios and invade secondary scrub and subtropical needle-leaved forest as a potential suitable habitat. Compared to the five IAPS mentioned above, Senna tora slightly increased in lower elevation (by 6 m) in by year 2050, however, it significantly increased in upper elevation in both year 2050 and 2070. L. camara, one of the world’s 100 worst invasive species [64] and a problematic invasive species in forest and shrub lands [43] remarkably expanded downward to 359 m while contracting in upper elevation by 474 to 598 m in year 2050 and 2070 respectively in our study area.
P. hysterophorus, one of the most troublesome weed in the region and one of the worst weed in India [65] has also significantly expanded in Nepal in last 20 years [42, 66] and is rapidly invading in the study area as well [44]. However, in our predicted model for year 2070, P. hysterophorus upper distribution contracts by 486m but expansion takes place at lower elevation by 238 m. Hence in 2070, the elevation range of P. hysterophorus will be from 686–2050 m to 448–1564 m and mostly it is invading Subtropical needle-leaved forest.
Our models have predicted present distribution and range expansion under future climate change, which might pose a threat to the native ecosystems in KSL. Assessment of the threats posed by the combined effects of invasive species and climate change [67] is crucial for conservation and management of the biodiversity of KSL. Knowledge and information about the geographical distribution of these species is vital before applying any control measures [68–69].
Mountain ecosystems are still relatively uninvaded by IAPS than lowland ecosystem but the process may be accelerated due to climate change and anthropogenic disturbance. Thus better investigation and planning is needed for early detection and tracking of these species habitat suitability so that appropriate actions can be taken in time to prevent further invasions [8, 10, 70–71].
Effective management of invasive species requires an integrated approach, which includes mechanical, chemical and biological control techniques [72]. For example, invasive species can be easily controlled in the initial stages of establishment when the small satellite populations can be physically removed to stop its further spread [73]. Uprooting the plant before flowering (to control spread by seeds), flooding for short periods of time (Ageratum conyzoides), shading by intact canopies (Lantana camara) and using herbicides are some of the techniques used to control invasive plant species. Use of biological control agent is one of the tool for management of invasive species. Biological control agents like stem gall fly Procecidochares utilis for Ageratina adenophora [74] and leaf feeding beetle Zygogramma bicolorata for Parthenium hysterophorus [75–76] have been used in the management of invasive species. Moreover, for effective management of invasive species, existing biological control strategies should be complemented with suppressive plants [77] and through re-vegetation of degraded sites with competitive native forage grasses [78]. In addition, there is an urgent need to strengthen capacity of scientific community, local community and other stakeholders to control IAPS through identification, prevention, and early detection. Finding alternative use of invasive species by local communities is one of the strategies in managing the invasive species [79]. For example, L. camara is used to make small scale furniture, bio-briquettes, biochar, farm hedges, fuelwood and as a green manure [80–82]. A. adenophora has been used as animal bedding, composting, extracting essential oils and odors and have been used as contact poisons or repelling agent’s against herbivore pests in parts of China [43,78]. Invasive plant biomass has been also used for the production of biochar [81–82].
Considering the overall modeling scenario, our results demonstrate that the distribution of IAPS in KSL will expand under future climate change and might be a potential threat to the native vegetation. In the future, the distribution range of the species will expand both vertically and horizontally towards South East and North East of KSL. Our model predicted and identified probable areas and types of vegetation invasion. Most of the range expansion of the IAPS will be in the two natural ecosystems viz., secondary scrub and subtropical needle-leaved forest, this will put these natural ecosystems under threat of resource scarcity. Enhancement of our knowledge and understanding about interaction between native species and invasive species considering climate change is imperative for effective conservation and management ecosystems in the Himalaya.
Supporting information
(PDF)
(PDF)
(PDF)
(DOCX)
Acknowledgments
The study was carried out under the Kailash Sacred Landscape Conservation and Development Initiative (KSLCDI) of the International Centre for Integrated Mountain Development (ICIMOD). We would like to thank the Department for International Development (DFID)-UK Aid, German Federal Ministry of Economic Cooperation and Development and German International Cooperation (GIZ) for providing financial support for the KSLCDI. This study was partially supported by core funds from ICIMOD contributed by the Governments of Afghanistan, Australia, Austria, Bangladesh, Bhutan, China, India, Myanmar, Nepal, Norway, Pakistan, Switzerland and the UK. The authors would like to express their gratitude to the three anonymous reviewers whose comments helped us improve the manuscript.
Data Availability
Data are available from the ICIMOD's Institutional Data Access via Regional Database System (RDS) (http://rds.icimod.org/), an online open access system that provides free access to data used in the study for researchers who meet the criteria for access to confidential data. The criteria for access needs the user to log in to the Regional Database System. There is no other criteria that will restrict the researchers from downloading the data.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Pimentel D, Lach L, Zuniga R, Morrison D. Environmental and economic costs of nonindigenous species in the United States. BioScience. 2000;50 (1):53–65. [Google Scholar]
- 2.Simberloff D. How common are invasion-induced ecosystem impacts? Biological invasions. 2011. May 1;13(5):1255–68. [Google Scholar]
- 3.Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F. Impacts of climate change on the future of biodiversity. Ecology letters. 2012. April 1;15(4):365–77. doi: 10.1111/j.1461-0248.2011.01736.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paini DR, Sheppard AW, Cook DC, De Barro PJ, Worner SP, Thomas MB. Global threat to agriculture from invasive species. Proceedings of the National Academy of Sciences. 2016. July 5;113(27):7575–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Early R, Sax DF. Climatic niche shifts between species' native and naturalized ranges raise concern for ecological forecasts during invasions and climate change. Global Ecology and Biogeography. 2014. December 1;23(12):1356–65. [Google Scholar]
- 6.Vitousek PM, Antonio CM, Loope LL, Westbrooks R. Biological invasions as global environmental change. American scientist. 1996. September 1;84(5):468. [Google Scholar]
- 7.Kowarik I. Human agency in biological invasions: secondary releases foster naturalisation and population expansion of alien plant species. Biological Invasions. 2003. December 1;5(4):293–312. [Google Scholar]
- 8.Pauchard A, Kueffer C, Dietz H, Daehler CC, Alexander J, Edwards PJ, et al. Ain't no mountain high enough: plant invasions reaching new elevations. Frontiers in Ecology and the Environment. 2009. November 1;7(9):479–86. [Google Scholar]
- 9.Reddy CS. Assessment of plant invasions across different habitats of India. Bioheral. 2012. December 1:110. [Google Scholar]
- 10.McDougall KL, Khuroo AA, Loope LL, Parks CG, Pauchard A, Reshi ZA, et al. Plant invasions in mountains: global lessons for better management. Mountain Research and Development. 2011. November;31(4):380–7. [Google Scholar]
- 11.Muhlfeld CC, Kovach RP, Jones LA, Al-Chokhachy R, Boyer MC, Leary RF, et al. Invasive hybridization in a threatened species is accelerated by climate change. Nature Climate Change. 2014. July 1;4(7):620–4. [Google Scholar]
- 12.Shrestha UB, Gautam S, Bawa KS. Widespread climate change in the Himalayas and associated changes in local ecosystems. PLoS One. 2012. May 15;7(5):e36741 doi: 10.1371/journal.pone.0036741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.IGES, ICIMOD. 2013. Technical Report: Climate Change Adaptation Needs of People of the Hindu Kush Himalayas Hayama, Japan: IGES [Google Scholar]
- 14.Zomer RJ, Trabucco A, Metzger MJ, Wang M, Oli KP, Xu J. Projected climate change impacts on spatial distribution of bioclimatic zones and ecoregions within the Kailash Sacred Landscape of China, India, Nepal. Climatic change. 2014. August 1;125(3–4):445–60. [Google Scholar]
- 15.Alexander JM, Lembrechts JJ, Cavieres LA, Daehler C, Haider S, Kueffer C, et al. Plant invasions into mountains and alpine ecosystems: current status and future challenges. Alpine Botany. 2016. October 1;126(2):89–103. [Google Scholar]
- 16.Fei S, Phillips J, Shouse M. Biogeomorphic impacts of invasive species. Annual review of ecology, evolution, and systematics. 2014. November 23;45:69–87. [Google Scholar]
- 17.Bhattarai KR, Måren IE, Subedi SC. Biodiversity and invasibility: Distribution patterns of invasive plant species in the Himalayas, Nepal. Journal of Mountain Science. 2014. May 1;11(3):688–96. [Google Scholar]
- 18.Kosaka Y, Saikia B, Mingki T, Tag H, Riba T, Ando K. Roadside distribution patterns of invasive alien plants along an altitudinal gradient in Arunachal Himalaya, India. Mountain Research and Development. 2010. August;30(3):252–8. [Google Scholar]
- 19.Kohli RK, Batish DR, Singh HP, Dogra KS. Status, invasiveness and environmental threats of three tropical American invasive weeds (Parthenium hysterophorus L., Ageratum conyzoides L., Lantana camara L.) in India. Biological Invasions. 2006. October 1;8(7):1501–10. [Google Scholar]
- 20.Chitale VS, Behera MD, Roy PS. Future of endemic flora of biodiversity hotspots in India. PloS one. 2014. December 12;9(12):e115264 doi: 10.1371/journal.pone.0115264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franklin J, Davis FW, Ikegami M, Syphard AD, Flint LE, Flint AL, et al. Modeling plant species distributions under future climates: how fine scale do climate projections need to be?. Global change biology. 2013. February 1;19(2):473–83. doi: 10.1111/gcb.12051 [DOI] [PubMed] [Google Scholar]
- 22.Sobek-Swant S, Kluza DA, Cuddington K, Lyons DB. Potential distribution of emerald ash borer: What can we learn from ecological niche models using Maxent and GARP?. Forest Ecology and Management. 2012. October 1;281:23–31. [Google Scholar]
- 23.Leyequien E, Verrelst J, Slot M, Schaepman-Strub G, Heitkönig IM, Skidmore A. Capturing the fugitive: Applying remote sensing to terrestrial animal distribution and diversity. International Journal of Applied Earth Observation and Geoinformation. 2007. February 28;9(1):1–20. [Google Scholar]
- 24.Pearce JL, Boyce MS. Modelling distribution and abundance with presence‐only data. Journal of applied ecology. 2006. June 1;43(3):405–12. [Google Scholar]
- 25.Wintle BA, Bardos DC. Modeling species–habitat relationships with spatially autocorrelated observation data. Ecological Applications. 2006. October 1;16(5):1945–58. [DOI] [PubMed] [Google Scholar]
- 26.Guisan A, Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecology letters. 2005. September 1;8(9):993–1009. [DOI] [PubMed] [Google Scholar]
- 27.Raxworthy CJ, Martinez-Meyer E, Horning N, Nussbaum RA, Schneider GE, Ortega-Huerta MA, et al. Predicting distributions of known and unknown reptile species in Madagascar. Nature. 2003. December 18;426(6968):837–41. doi: 10.1038/nature02205 [DOI] [PubMed] [Google Scholar]
- 28.Aguirre-Gutiérrez J, Carvalheiro LG, Polce C, van Loon EE, Raes N, Reemer M, et al. Fit-for-purpose: species distribution model performance depends on evaluation criteria–Dutch hoverflies as a case study. PloS one. 2013. May 14;8(5):e63708 doi: 10.1371/journal.pone.0063708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Royle JA, Chandler RB, Yackulic C, Nichols JD. Likelihood analysis of species occurrence probability from presence‐only data for modelling species distributions. Methods in Ecology and Evolution. 2012. June 1;3(3):545–54. [Google Scholar]
- 30.Gallardo B, Aldridge DC. Evaluating the combined threat of climate change and biological invasions on endangered species. Biological Conservation. 2013. April 30;160:225–33. [Google Scholar]
- 31.Phillips SJ, Dudík M. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography. 2008. April 1;31(2):161–75. [Google Scholar]
- 32.Phillips SJ, Dudík M, Schapire RE. Maxent software for species distribution modeling. Á/< w ww. cs. princeton. edu/schapire/maxent. 2005.
- 33.Fourcade Y, Engler JO, Rödder D, Secondi J. Mapping species distributions with MAXENT using a geographically biased sample of presence data: a performance assessment of methods for correcting sampling bias. PloS one. 2014. May 12;9(5):e97122 doi: 10.1371/journal.pone.0097122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson CD, Roberts D, Reid N. Applying species distribution modelling to identify areas of high conservation value for endangered species: A case study using Margaritifera margaritifera (L.). Biological Conservation. 2011. February 28;144(2):821–9. [Google Scholar]
- 35.Adhikari D, Tiwary R, Barik SK. Modelling hotspots for invasive alien plants in India. PloS one. 2015. July 31;10(7):e0134665 doi: 10.1371/journal.pone.0134665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West AM, Kumar S, Brown CS, Stohlgren TJ, Bromberg J. Field validation of an invasive species Maxent model. Ecological Informatics. 2016. November 30;36:126–34. [Google Scholar]
- 37.Qin Z, Zhang JE, DiTommaso A, Wang RL, Liang KM. Predicting the potential distribution of Lantana camara L. under RCP scenarios using ISI-MIP models. Climatic change. 2016. January 1;134(1–2):193–208. [Google Scholar]
- 38.Wisz MS, Hijmans RJ, Li J, Peterson AT, Graham CH, Guisan A. Effects of sample size on the performance of species distribution models. Diversity and distributions. 2008. September 1;14(5):763–73. [Google Scholar]
- 39.Elith J, Graham CH, Anderson RP, Dudík M, Ferrier S, Guisan A, et al. Novel methods improve prediction of species' distributions from occurrence data. Ecography. 2006. April 1:129–51. [Google Scholar]
- 40.Pearce J, Ferrier S. Evaluating the predictive performance of habitat models developed using logistic regression. Ecological modelling. 2000. September 3;133(3):225–45. [Google Scholar]
- 41.Shrestha BB. Invasive Alien Plant Species in Nepal. In Eds.: Jha P.K., Siwakoti M. and Rajbhandary S. Frontiers of Botany.2016269–284 [Google Scholar]
- 42.Tiwari S. An inventory and assessment of invasive alien plant species of Nepal IUCN; Nepal; 2005. [Google Scholar]
- 43.Bisht N, Joshi S, Shrestha BB, Yi S, Chaudhary RP, Kotru R, et al. Manual on invasive alien plant species in Kailash Sacred Landscape-Nepal. Manual on invasive alien plant species in Kailash Sacred Landscape-Nepal. 2016. [Google Scholar]
- 44.Shrestha BB, Joshi S, Bisht N, Yi S, Kotru R, Chaudhary RP, et al. Inventory and impact assessment of invasive plant species in Kailash Sacred Landscape ICIMOD Working Paper 2018 (in press)
- 45.Kotru, R., Chaudhari, S., Lemke, E., Mueller, M., Chettri, R., Basnet, et al. Kailash Sacred Landscape conservation and Development Initiative (2012–2017) Annual Progress Report 2016 (p. 101). Kathmandu: ICIMOD.
- 46.Zomer R, Oli KP, editors. Kailash sacred landscape conservation initiative: feasibility assessment report International Centre for Integrated Mountain Development (ICIMOD); 2011. [Google Scholar]
- 47.Zomer RJ, Trabucco A, Metzger M, Oli KP. Environmental stratification of Kailash Sacred Landscape and projected climate change impacts on ecosystems and productivity. ICIMOD Working Paper. 2013(2013/1).
- 48.von der Lippe M and Kowarik I. 2007. Long-distance dispersal of plants by vehicles as a driver of plant invasions. Conservation Biology 21:986–996. doi: 10.1111/j.1523-1739.2007.00722.x [DOI] [PubMed] [Google Scholar]
- 49.Christen D, Matlack G. The role of roadsides in plant invasions: a demographic approach. Conservation Biology. 2006. April 1;20(2):385–91. [DOI] [PubMed] [Google Scholar]
- 50.Johnston FM and SW Johnston. 2004. Impacts of road disturbance on soil properties and on exotic plant occurrence in subalpine area of the Australian Alps. Arctic, Antarctic and Alpine Research 36:201–207. [Google Scholar]
- 51.Wabuyele E, Lusweti A, Bisikwa J, Kyenune G, Clark K, Lotter WD, et al. 2014. Roadside survey of invasive weed Parthenium hysterophorus (Asteraceae) in East Africa. Journal of East African Natural History 103:49–57. [Google Scholar]
- 52.Shrestha BB. 2014. Distribution of Invasive Alien Weed Parthenium hysterophorus and its Biological Control Agent in Nepal. Unpublished research report submitted to International Foundation for Science, Sweden.
- 53.Kosaka Y, Saikia B, Mingki T, Tag H, Riba Tand Ando K. 2010. Roadside distribution patterns of invasive alien plants along an altitudinal gradient in Arunachal Himalaya, India. Mountain Research and Development 30: 252–258. [Google Scholar]
- 54.Lutz AF, ter Maat HW, Biemans H, Shrestha AB, Wester P, Immerzeel WW. Selecting representative climate models for climate change impact studies: an advanced envelope‐based selection approach. International Journal of Climatology. 2016. October 1;36(12):3988–4005. [Google Scholar]
- 55.Intergovernmental Panel on Climate Change. Climate Change 2014–Impacts, Adaptation and Vulnerability: Regional Aspects Cambridge University Press; 2014. December 29. [Google Scholar]
- 56.Yang XQ, Kushwaha SP, Saran S, Xu J, Roy PS. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecological engineering. 2013. February 28;51:83–7. [Google Scholar]
- 57.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological modelling. 2006. January 25;190(3):231–59. [Google Scholar]
- 58.Baldwin RA. Use of maximum entropy modeling in wildlife research. Entropy. 2009. November 16;11(4):854–66. [Google Scholar]
- 59.Yost AC, Petersen SL, Gregg M, Miller R. Predictive modeling and mapping sage grouse (Centrocercus urophasianus) nesting habitat using Maximum Entropy and a long-term dataset from Southern Oregon. Ecological Informatics. 2008. December 1;3(6):375–86. [Google Scholar]
- 60.Choudhury MR, Deb P, Singha H, Chakdar B, Medhi M. Predicting the probable distribution and threat of invasive Mimosa diplotricha Suavalle and Mikania micrantha Kunth in a protected tropical grassland. Ecological Engineering. 2016. December 31;97:23–31. [Google Scholar]
- 61.Zhu L, Sun OJ, Sang W, Li Z, Ma K. Predicting the spatial distribution of an invasive plant species (Eupatorium adenophorum) in China. Landscape Ecology. 2007. October 1;22(8):1143–54. [Google Scholar]
- 62.Peterson AT, Papes M, Kluza DA. Predicting the potential invasive distributions of four alien plant species in North America. Weed Science. 2003. November;51(6):863–8. [Google Scholar]
- 63.Hoffman JD, Narumalani S, Mishra DR, Merani P, Wilson RG. Predicting potential occurrence and spread of invasive plant species along the North Platte River, Nebraska. Invasive Plant Science and Management. 2008. October;1(4):359–67. [Google Scholar]
- 64.Lowe S, Browne M, Boudjelas S, De Poorter M. 100 of the world's worst invasive alien species: a selection from the global invasive species database Auckland: Invasive Species Specialist Group; 2000. December 12. [Google Scholar]
- 65.Gnanavel I, Natarajan SK. Parthenium hysterophorus L.: a major threat to natural and agro eco-systems in India. International Journal of Agriculture, Environment and Biotechnology. 2013;6(2):261–9. [Google Scholar]
- 66.Shrestha BB, Shabbir A, Adkins SW. Parthenium hysterophorus in Nepal: a review of its weed status and possibilities for management. Weed research. 2015. April 1;55(2):132–44. [Google Scholar]
- 67.Taylor S, Kumar L. Potential distribution of an invasive species under climate change scenarios using CLIMEX and soil drainage: A case study of Lantana camara L. in Queensland, Australia. Journal of environmental management. 2013. January 15;114:414–22. doi: 10.1016/j.jenvman.2012.10.039 [DOI] [PubMed] [Google Scholar]
- 68.Margules CR, Pressey RL. Systematic conservation planning. Nature. 2000. May 11;405(6783):243–53. doi: 10.1038/35012251 [DOI] [PubMed] [Google Scholar]
- 69.Tsoar A, Allouche O, Steinitz O, Rotem D, Kadmon R. A comparative evaluation of presence‐only methods for modelling species distribution. Diversity and distributions. 2007. July 1;13(4):397–405. [Google Scholar]
- 70.Blossey B. Before, during and after: the need for long-term monitoring in invasive plant species management. Biological Invasions. 1999. June 1;1(2):301–11. [Google Scholar]
- 71.Reddy CS. Catalogue of invasive alien flora of India. Life Science Journal. 2008. January 1;5(2):84–9. [Google Scholar]
- 72.DiTomaso JM. Invasive weeds in rangelands: species, impacts, and management. Weed science. 2000. March;48(2):255–65. [Google Scholar]
- 73.Wittenberg R, Cock MJ, editors. Invasive alien species: a toolkit of best prevention and management practices CABI; 2001. [Google Scholar]
- 74.Muniappan R, Shepard BM, Watson GW, Carner GR, Rauf A, Sartiami D, et al. New records of invasive insects (Hemiptera: Sternorrhyncha) in Southeast Asia and West Africa. Journal of Agricultural and Urban Entomology. 2009. October;26(4):167–74. [Google Scholar]
- 75.Dhileepan K, Wilmot Senaratne KA. How widespread is Parthenium hysterophorus and its biological control agent Zygogramma bicolorata in South Asia?. Weed research. 2009. December 1;49(6):557–62. [Google Scholar]
- 76.Dhileepan K, Setter SD, McFadyen RE. Response of the weed Parthenium hysterophorus (Asteraceae) to defoliation by the introduced biocontrol agent Zygogramma bicolorata (Coleoptera: Chrysomelidae). Biological Control. 2000. September 30;19(1):9–16. [Google Scholar]
- 77.Adkins S, Shabbir A. Biology, ecology and management of the invasive parthenium weed (Parthenium hysterophorus L.). Pest Management Science. 2014. July 1;70(7):1023–9. doi: 10.1002/ps.3708 [DOI] [PubMed] [Google Scholar]
- 78.Wan F, Liu W, Guo J, Qiang S, Li B, Wang J, et al. Invasive mechanism and control strategy of Ageratina adenophora (Sprengel). Science China Life Sciences. 2010. November 1;53(11):1291–8. doi: 10.1007/s11427-010-4080-7 [DOI] [PubMed] [Google Scholar]
- 79.Kannan R, Shackleton CM, Krishnan S, Shaanker RU. Can local use assist in controlling invasive alien species in tropical forests? The case of Lantana camara in southern India. Forest Ecology and Management. 2016. September 15;376:166–73. [Google Scholar]
- 80.Love A, Babu S, Babu CR. Management of Lantana, an invasive alien weed, in forest ecosystems of India. Current Science. 2009. November 25;97(10):1421–9. [Google Scholar]
- 81.Duggin JA, Gentle CB. Experimental evidence on the importance of disturbance intensity for invasion of Lantana camara L. in dry rainforest–open forest ecotones in north-eastern NSW, Australia. Forest Ecology and Management. 1998. September 16;109(1):279–92. [Google Scholar]
- 82.Vithanage M, Rajapaksha AU, Tang X, Thiele-Bruhn S, Kim KH, Lee SE, et al. Sorption and transport of sulfamethazine in agricultural soils amended with invasive-plant-derived biochar. Journal of environmental management. 2014. August 1;141:95–103. doi: 10.1016/j.jenvman.2014.02.030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(DOCX)
Data Availability Statement
Data are available from the ICIMOD's Institutional Data Access via Regional Database System (RDS) (http://rds.icimod.org/), an online open access system that provides free access to data used in the study for researchers who meet the criteria for access to confidential data. The criteria for access needs the user to log in to the Regional Database System. There is no other criteria that will restrict the researchers from downloading the data.