Abstract
The early stages of tuber development are characterized by cell division, high metabolic activity, and the predominance of invertase as the sucrose (Suc) cleaving activity. However, during the subsequent phase of starch accumulation the cleavage of Suc occurs primarily by the action of Suc synthase. The mechanism that is responsible for this switch in Suc cleaving activities is currently unknown. One striking difference between the invertase and Suc synthase mediated cleavage of Suc is the direct involvement of inorganic pyrophosphate (PPi) in the latter case. There is presently no convincing explanation of how the PPi required to support this process is generated in potato (Solanum tuberosum) tubers. The major site of PPi production in a maturing potato tubers is likely to be the reaction catalyzed by ADP-glucose pyrophosphorylase, the first committed step of starch biosynthesis in amyloplasts. We present data based on the analysis of the PPi levels in various transgenic plants altered in starch and Suc metabolism that support the hypothesis that PPi produced in the plastid is used to support cytosolic Suc breakdown and that PPi is an important coordinator of cytosolic and plastidial metabolism in potato tubers.
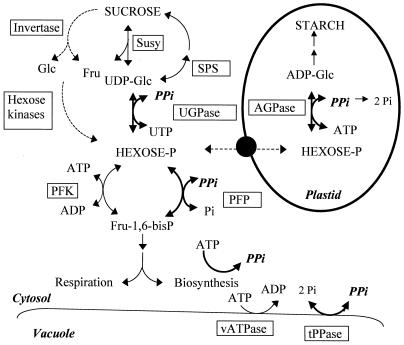
During the development of starch-accumulating storage sinks such as seeds or tubers, there is a remarkable change in the mechanism by which incoming Suc is cleaved to support biosynthesis and growth. Whereas during the early phases of sink development when the rates of cell division and metabolic activity are high, the invertase pathway is the dominate route by which Suc is metabolized, during the later phases of sink development when storage product synthesis predominates, the activity of the invertase pathway declines and is substituted by the Suc synthase (Susy) pathway. Remarkably, the emergence of the Susy pathway is highly correlated with the onset of starch biosynthesis (Quick and Schaeffer, 1996; Appeldoorn et al., 1997, 1999; Weber et al., 1997; Sturm and Tang, 1999). One of the striking differences between the invertase-and Susy-dependent Suc breakdown pathways is that whereas Suc mobilization via invertase is followed by the ATP-dependent phosphorylation of hexoses, UDP-Glc, the product of the Susy reaction, is metabolized to Glc-1-P via the inorganic pyrophosphate (PPi)-dependent enzyme UDP-Glc pyrophosphorylase (UGPase) (ap Rees and Morrell, 1990; Stitt, 1998; Fig. 1). The mechanism that is involved in coordinating this shift in metabolism in developing sink organs is unknown, however, the potential involvement of PPi cannot be ignored. There is also presently no convincing explanation of how PPi is produced to support the Susy-dependent breakdown of Suc. Indeed the question has received virtually no attention, which is somewhat surprising given that the production of PPi could be a crucial regulatory and controlling level in the coordination of this development.
Figure 1.
PPi-dependent (bold line) and PPi-independent (dotted line) Suc breakdown pathways in growing potato tubers. Other PPi-utilizing reactions are shown in bold. Pi, Orthophosphate.
In potato (Solanum tuberosum) tubers it has been estimated that up to 70% of the incoming Suc is metabolized to starch, the remainder being roughly equally divided between respiration, structural polysaccharides, and other storage products (ap Rees and Morrell, 1990). Given the strong predominance of the starch biosynthetic flux in potato tubers in comparison to the flux through other metabolic pathways, there are essentially only two possibilities for the supply of the amount of PPi needed for Suc breakdown via the Susy-dependent pathway: It is either recycled from the starch biosynthesis pathway where PPi is produced in the amyloplast by ADP-Glc pyrophosphorylase (AGPase), or it is provided by a cycling process. In the latter case, two possibilities have been proposed (Taiz, 1986; ap Rees and Morrell, 1990): the PPi-dependent reaction of pyrophosphate:Fru-6-P phosphotransferase (PFP) and the tonoplast pyrophosphatase (vPPase), both of which would produce PPi by coupling to a parallel ATP consuming process (ATP-dependent tonoplast proton pump or phosphofructokinase [PFK], respectively).
It is generally believed that the reaction catalyzed by AGPase is effectively irreversible due to the presence of a highly active alkaline pyrophosphatase in plastids (ap Rees and Morrell, 1990). This view is mainly based upon the presence of a significant pyrophosphatase activity in spinach chloroplasts (Weiner et al., 1987) and soybean plastids (Gross and ap Rees, 1986). However, little is currently known about the nature and regulation of plant inorganic pyrophosphatases. Indeed, there remains no demonstration that the inorganic pyrophosphatase present in plastids catalyzes the complete removal of all the PPi produced by the AGPase reaction in planta. During the development of potato tubers no change in pyrophosphatase activity can be observed at the onset of the starch-accumulating phase (Appeldoorn et al., 1999). The presence of a PPi transporter in chloroplast membranes has been described (Lunn and Douce, 1993) and the existence of a mechanism for exporting PPi from amyloplasts cannot be excluded.
Following the discovery of PFP it was proposed that this enzyme acted in conjunction with PFK to give rise to a tight regulation of cellular PPi levels (ap Rees and Morrell, 1990; Sung et al., 1990). If this were the case in potato tubers, then there would be a requirement for one ATP to be consumed in a PFK/PFP cycle for every Suc that was cleaved. It would therefore be expected that a delicate equilibrium would be set up in potato tubers between the ATP to ADP ratio, PPi content, and the regulation of the Suc to starch conversion. There are now, however, examples of transgenic potato tuber lines that have an elevated ATP to ADP ratio but that accumulate significantly less starch (Trethewey et al., 1998). Furthermore studies on PFP in growing potato tubers indicate that it catalyzes a net flux in the glycolytic direction (Hajirezaei and Stitt, 1991; Hajirezaei et al., 1994) and thus is more likely to consume PPi than generate it. Thus the original ideas about PFP acting as a crucial regulator and facilitator for PPi metabolism have not been supported by more recent transgenic experimentation.
To investigate the question of the source of PPi for potato tuber metabolism and thereby to explore the potential regulatory importance of PPi, we decided to determine the levels of PPi in a range of transgenic potato tubers. The data obtained indicate that changes in the steady-state concentrations of PPi can be related to changes in starch and Suc metabolism and therefore support the hypothesis that PPi acts as a coordinator integrating the pathways of cytosolic Suc breakdown and plastidial starch biosynthesis in potato tubers.
RESULTS
PPi Levels Are Altered Following Changes in Suc and Starch Metabolism in Transgenic Potato Tubers
We used the coupled enzymatic assay of Weiner et al. (1987) to determine the PPi levels in potato tubers. We demonstrated the reliability of our extraction and assay method by performing recovery experiments and obtained a value of 105% ± 1% for PPi based upon six extracts. Because of the significant risk of PPi contamination in the chemicals used in the washing and cleaning procedures, we also prepared pseuodoextracts and confirmed that our solutions and vessels were free of extraneous PPi contamination.
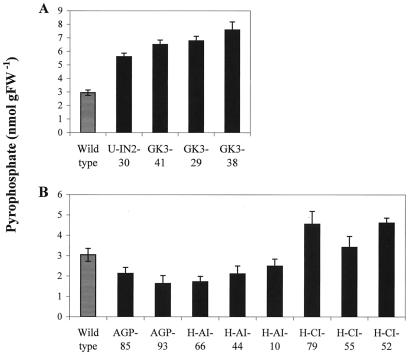
We first determined the levels of PPi in transgenic potato lines that express a yeast invertase in the cytosol either alone or in combination with a bacterial glucokinase (U-IN2-30 and GK3-41/29/38). These lines, which operate a PPi-independent Suc breakdown pathway, were found to contain significantly elevated levels of PPi (Fig. 2). There was a 90% increase in the PPi content in the line U-IN2-30 and a further increase of over 200% with respect to the wild type in the GK3 lines.
Figure 2.
PPi concentration in transgenic tubers. Developing tuber samples were taken from 12-week-old plants grown in 2.5-L pots in the greenhouse. The PPi concentration was measured in TCA extracts by an enzyme-coupled test. A, Wild-type and transgenic potato lines U-IN2-30 and GK3. B, Wild-type and transgenic potato lines AGP, H-AI, and H-CI. The error bars represent the se for determinations on six individual plants per line.
We subsequently analyzed tuber extracts from two lines of transgenic potato plants inhibited in AGPase activity due to the expression of an antisense RNA for the small subunit of this enzyme. The line AGP-93 is characterized by a >95% reduction in AGPase activity and demonstrates a reduction in starch accumulation in excess of 85% (Müller-Röber et al., 1992; Trethewey et al., 1999a). As shown in Figure 2 both the lines tested displayed a lower level of PPi; this reduction was significant in the case of AGP-93.
Transgenic Lines Displaying an Inhibition of the AGPase Activity in Combination with the Expression of an Invertase in the Cytosol Contain Intermediate Levels of PPi
To test whether or not the changes seen in the PPi levels in the AGPase antisense and the invertase expressing lines are due to independent events, we analyzed transgenic plants containing combinations of both genetic modifications. As shown in Figure 2, the PPi level increased significantly in comparison to the AGPase background (AGP-93) when an invertase was additionally expressed in the cytosol (H-CI-lines). However, the PPi levels were below those found in the single transgenic lines expressing the invertase in the cytosol (U-IN2-30).
To ensure that the changes seen as a result of the expression of the invertase are due to its localization in the cytosol, we analyzed transgenic tubers where the invertase was targeted to the apoplast in the AGP-93 background (H-AI-lines). These lines did not differ significantly in PPi content from the AGPase inhibited parental lines (AGP-93; Fig. 2).
Changes in Nucleotide Concentrations Do Not Always Correlate with Changes in PPi Levels
We decided to analyze the levels of nucleotides and nucleotide sugars in the same extracts used for the PPi measurements. Nucleotides are directly or indirectly involved in all PPi-dependent reactions. They are substrates and/or products in both the UGPase and AGPase reactions. Furthermore they are substrates for reactions occurring in parallel to PPi-dependent reactions such as the ATP-dependent phosphorylation of Fru-6-P by PFK (paralleling PFP) or the vacuolar proton-pumping ATPase (paralleling the vPPase). Nucleotide contents in the transgenic lines are presented in Table I. ATP, UTP, and GTP contents in the U-IN2-30 and all GK3 lines were significantly higher than in the wild type. The UTP concentration was elevated by up to 2-fold; the ATP content rose by 40% in U-IN2-30 and by 68% in GK3-38 in comparison to the wild type. These lines do not show strong changes in the ADP, UDP, and GDP contents, only in the GK3 lines was a significant increase in the UDP content observed. There was a tendency for an increase in both the ATP to ADP and UTP to UDP ratios in the U-IN2-30 and GK3-lines. These results are in agreement with the already published data on these transgenic lines (Trethewey et al., 1998), although it was important to confirm in this study that the expected changes and trends actually occurred in the samples used for the PPi analysis.
Table I.
Nucleotide concentrations in transgenic tubers
| Parameter | Wild Type | U-IN2-30 | GK3-41 | GK3-29 | GK3-38 |
|---|---|---|---|---|---|
| nmol g−1 fresh wt | |||||
| ATP | 47.0 ± 2.5 | 66.3 ± 5.3* | 66.3 ± 8.2* | 77.6 ± 7.5* | 78.9 ± 9.0* |
| UTP | 25.2 ± 1.5 | 41.2 ± 2.3* | 45.9 ± 5.0* | 51.7 ± 4.5* | 56.1 ± 5.9* |
| GTP | 11.7 ± 1.1 | 14.5 ± 1.4* | 15.2 ± 1.3* | 16.7 ± 1.7* | 18.2 ± 2.0* |
| ADP | 20.2 ± 1.1 | 19.5 ± 1.9 | 25.8 ± 2.9 | 27.2 ± 3.8 | 23.6 ± 3.1 |
| UDP | 7.5 ± 0.9 | 9.1 ± 0.5 | 13.4 ± 1.6* | 11.8 ± 1.2* | 12.4 ± 0.6* |
| GDP | 6.2 ± 0.3 | 3.5 ± 0.9* | 4.8 ± 0.6 | 5.7 ± 1.5 | 7.1 ± 1.1 |
| ATP/ADP | 2.3 ± 0.1 | 3.5 ± 0.2* | 2.5 ± 0.4 | 2.9 ± 0.3 | 3.5 ± 0.4* |
| UTP/UDP | 3.5 ± 0.3 | 4.6 ± 0.2 | 3.5 ± 0.7 | 4.5 ± 0.6 | 4.6 ± 0.6 |
| ADP-Glc | 1.4 ± 0.3 | 2.3 ± 0.5 | 1.9 ± 0.5 | 2.4 ± 0.8 | 1.3 ± 0.3 |
| Glc-1-P | 16.6 ± 0.8 | 47.0 ± 4.2* | 65.2 ± 4.0* | 54.4 ± 3.2* | 56.3 ± 4.1* |
Developing tuber samples were taken from 12-week-old plants grown in 2.5-L pots in the greenhouse. Nucleotide concentrations were measured in TCA extracts by HPLC. All data represent the means ± se of measurements on six independent plants. Asterisks represent values significantly different (t test P < 0.05) to the wild-type level. Nos. in bold highlight values significantly different (t test P < 0.05) to the parental line.
Changes in the nucleotide contents could also be observed in the AGPase antisense plants and in the H-CI and H-AI lines (Table II). There was a tendency toward higher nucleosidetriphosphate contents in the AGP and H-CI lines, whereas a reduction was observed in the H-AI-lines. We found similar levels of nucleosidediphosphates in the AGP and H-CI lines in comparison to the wild type, but a reduction in the H-AI-lines where there was a decrease in ADP and UDP content of around 35% and 30%, respectively. The ATP to ADP ratio in the AGP-85 and AGP-93 lines was found to be significantly higher than in the wild type.
Table II.
Nucleotide concentrations in transgenic tubers
| Parameter | Wild Type | AGP-85 | AGP-93 | H-AI-66 | H-AI-44 | H-AI-10 | H-CI-79 | H-CI-55 | H-CI-52 |
|---|---|---|---|---|---|---|---|---|---|
| nmol g−1 fresh wt | |||||||||
| ATP | 33.4 ± 2.2 | 44.7 ± 7.2 | 50.3 ± 6.5* | 23.2 ± 3.8 | 30.0 ± 1.7 | 25.5 ± 1.2* | 47.3 ± 5.9 | 52.4 ± 8.2 | 42.0 ± 3.1 |
| UTP | 15.1 ± 1.6 | 18.0 ± 2.8 | 19.3 ± 2.9 | 14.2 ± 2.0 | 12.7 ± 1.9 | 9.3 ± 0.4* | 33.9 ± 4.7* | 22.5 ± 3.1 | 18.6 ± 1.4 |
| GTP | 7.5 ± 0.5 | 8.4 ± 1.3 | 11.4 ± 1.5* | 5.8 ± 0.3* | 7.4 ± 0.7 | 6.2 ± 0.3 | 10.5 ± 1.1* | 12.4 ± 1.6* | 10.7 ± 0.5* |
| ADP | 17.6 ± 0.4 | 17.8 ± 3.0 | 17.6 ± 2.0 | 10.2 ± 0.5* | 12.6 ± 1.1* | 9.9 ± 0.4* | 22.4 ± 1.5* | 17.8 ± 1.1 | 17.4 ± 1.4 |
| UDP | 9.1 ± 1.0 | 7.7 ± 1.2 | 9.3 ± 1.3 | 5.7 ± 0.3* | 6.0 ± 0.7* | 5.8 ± 0.5* | 9.3 ± 0.9 | 7.4 ± 0.8 | 7.5 ± 0.8 |
| GDP | 4.6 ± 0.1 | 3.7 ± 1.0 | 2.9 ± 1.0* | 3.2 ± 1.5 | 3.0 ± 0.6* | 2.8 ± 0.2* | 4.6 ± 0.6 | 5.2 ± 0.2 | 5.3 ± 0.5 |
| ATP/ADP | 1.9 ± 0.1 | 2.5 ± 0.2* | 2.9 ± 0.3* | 2.3 ± 0.4 | 2.5 ± 0.2 | 2.6 ± 0.1* | 2.1 ± 0.3 | 2.9 ± 0.4 | 2.5 ± 0.2* |
| UTP/UDP | 1.8 ± 0.3 | 2.5 ± 0.2 | 2.2 ± 0.3 | 2.6 ± 0.4 | 2.3 ± 0.5 | 1.7 ± 0.2 | 3.6 ± 0.3* | 3.0 ± 0.1* | 2.5 ± 0.2 |
Developing tuber samples were taken from 12-week-old plants grown in 2.5-L pots. Nucleotide concentrations were measured in TCA extracts by HPLC. All data represent the means ± se of measurements on six independent plants. Asterisks represent values significantly different (t test P < 0.05) to the wild-type level. Nos. in bold highlight values significantly different (t test P < 0.05) to the parental line.
No Increase Was Found in the ADP-Glc Content of the Invertase Expressing Lines
No significant change was found in the ADP-Glc content of line U-IN2-30 with respect to the wild type (Table I). However, the Glc-1-P content of tubers of U-IN2-30 was found to be around three times higher than in the wild type. The presence of a bacterial glucokinase in the GK3 lines led to a further increase in the Glc-1-P level in comparison to the parental line U-IN2-30, but no change in the ADP-Glc levels could be found in the GK3 lines (Table I).
DISCUSSION
The aim of this work was to determine whether transgenic potato tubers altered in Suc and starch metabolism could provide evidence of a regulatory role for PPi in the coordination of tuber carbohydrate metabolism. Further, through evaluation of PPi levels, nucleotides, nucleotide sugars, and consideration of what is known about the metabolism in the lines studied (Trethewey et al., 1998, 1999a, 1999b), we aimed to draw some conclusions about the source of PPi for Suc metabolism in potato tubers.
A Reduced AGPase Activity in Transgenic Tubers Leads to a Decrease in the PPi Level
As described in “Results,” antisense inhibition of the AGPase led to a reduction in the steady-state PPi levels in the transgenic tubers. In principle this reduction could be due either to an increased consumption of PPi or to a decreased production of PPi.
An increase in PPi consumption could be achieved by (Fig. 1): (a) an increase in UDP-Glc breakdown via UGPase; (b) an increase in PFP activity in the glycolytic direction; and (c) an increase in the activity of the vPPase.
With respect to the first possibility, the AGPase antisense lines demonstrate no changes in the respiration rate (Geigenberger et al., 1999; Sweetlove et al., 1999). This fact together with the inhibition of starch synthesis makes it unlikely that there is an increase in the net rate of Glc-1-P production and therefore of PPi usage via UGPase in these transgenic tubers. The second possibility, that there is an increase in the net flux through PFP in the glycolytic direction in the transgenic tubers is also unlikely for the same reasons; no increase in the glycolytic flux was found in experimentation by two separate groups (Geigenberger et al., 1999; Sweetlove et al., 1999). The third possibility is more complex to evaluate. There is an increase in the soluble sugar content of tubers from the AGPase antisense lines, and this might represent sugars predominantly stored in the vacuole. If this were the case, then there would be an increased demand for transport of sugar across the tonoplast membrane, a process that would require energy. This energy could be supplied by the vPPase, although the increase in the ATP to ADP ratio in these lines might indicate that the ATP-dependent tonoplast proton pump would be just as likely to participate in the enhanced energization of the tonoplast. Further, the absolute increase in the storage of sugars in the tonoplast is likely to be insignificant in comparison to the reduction in flux through the pathways of Suc mobilization and starch synthesis (ap Rees and Morrell, 1990), and it is therefore questionable whether changes in vPPase activity could have a significant impact on the steady-state level of PPi in a growing potato tuber. Taking these arguments together, we believe that it is unlikely that the decreased content of PPi in the AGPase antisense lines is due to any of the possible explanations centered on an increased consumption of PPi.
We therefore favor the possibility that the observed decrease in PPi in the AGPase antisense lines is due to a decreased production. The most predominant PPi producing reaction in growing potato tubers is the formation of ADP-Glc catalyzed by AGPase (ap Rees and Morrell, 1990; Fig. 1). The reduction in AGPase activity in the antisense lines would directly lead to a reduced production of PPi in the plastid. It is a generally accepted dogma that the AGPase reaction is effectively irreversible due to the action of an alkaline pyrophosphatase in plastids (Gross and ap Rees, 1986; Weiner et al., 1987; ap Rees and Morrell, 1990). However, there is currently no in vivo evidence to support the hypothesis that PPi produced in the amyloplast is immediately cleaved into the component phosphate groups. Therefore, we regard the possibilities that significant levels of PPi are maintained in the amyloplast, or that PPi is rapidly channeled back to the cytosol to be hypotheses worthy of further experimentation. In the case of the latter possibility there is a single report of a PPi transporter located in the spinach chloroplast membrane (Lunn and Douce, 1993). We believe that these two hypotheses represent the most plausible explanations for the reduction in PPi found in the AGPase antisense lines.
The Cytosolic Expression of a Yeast Invertase in Potato Tubers Leads to an Increase in PPi Concentration
It has been shown that in the lines U-IN2-30 and GK3 an intense Suc cycling occurs (Trethewey et al., 1999b; Fig. 3). Suc is cleaved by the yeast invertase to hexoses, which are subsequently phosphorylated by hexose kinases prior to conversion to UDP-Glc and PPi via the action of phosphoglucomutase, phosphoglucoisomerase, and UGPase. UDP-Glc is finally reincorporated into Suc via Suc-P synthase. This cycling, which results in a net production of PPi, is likely to be the explanation for the increase in PPi seen in these lines. Further evidence for this view comes from potato tubers that express a bacterial Suc phosphorylase. These lines also contain a PPi-independent Suc breakdown pathway leading to Glc-1-P and Fru and have been found to contain elevated PPi levels (data not shown). All of these lines have a reduced starch accumulation; however, the reduced flux through the starch biosynthetic pathway is likely to be masked in influence on the PPi levels by the very active nature of the cytosolic Suc cycling (Trethewey et al., 1998, 1999b).
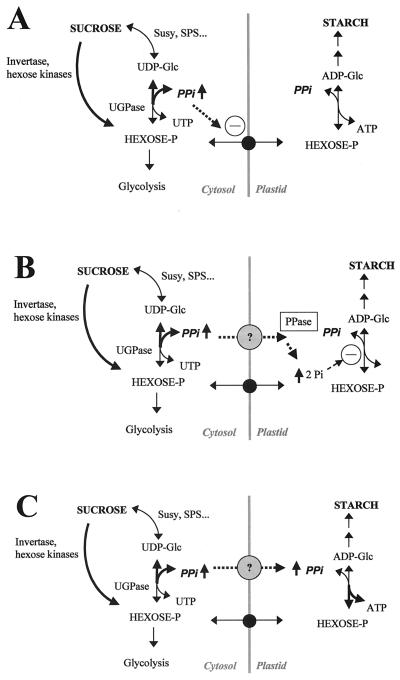
Figure 3.
Possible mechanisms through which an increase in PPi leads to a reduction in starch accumulation in potato tubers that express a yeast invertase in the cytosol. The elevated PPi level may impede plastidial transport process (A); lead to an increase in plastidial Pi, which would inhibit the AGPase reaction (B); and/or an increase in plastidial PPi concentration may influence the rate of the AGPase reaction (C).
Invertase Expressing Lines Exhibit an Increase in Glc-1-P, ATP, and 3-P-Glycerate Levels, However, ADP-Glc-Levels Remain Unchanged
The increase in Glc-1-P, ATP (Table I), and 3-P-glycerate levels (Trethewey et al., 1998) in the invertase expressing line U-IN2-30 and the GK3 lines does not lead to a parallel increase in the ADP-Glc level (Table I). At first sight this is surprising if the reaction catalyzed by AGPase is irreversible due to the removal of PPi by the inorganic pyrophosphatase, and the consumption of ADP-Glc by starch synthases can be assumed to be reduced given the decrease in starch accumulation in these lines. One possible explanation for this observation is that the increase in total Glc-1-P and ATP in the tuber is not reflected in the amyloplast, due to a disturbance of plastidial transport processes by PPi, as has been shown for some photosynthetic systems (Fig. 3A; Heldt and Rapley, 1970; Woldegiorgis et al., 1985; Bölter et al., 1999). An alternative explanation, building on the arguments in the previous section, would be that a higher PPi production in the cytosol leads to an elevated steady-state level of PPi in the amyloplast and this in turn influences either directly or indirectly the activity of the AGPase. An elevated plastidial PPi level may result in an increase in plastidial orthophosphate levels via the action of the inorganic pyrophosphatase, which in turn would lead to a decreased 3-P-glycerate to orthophosphate ratio and a decrease in AGPase activity (Fig. 3B; Sowokinos and Preiss, 1982). Alternatively if the high cytosolic PPi concentration leads to an increase in the plastidial level of PPi, PPi could have a direct effect on the net flux through AGPase by influencing the equilibrium of the reaction (Fig. 3C). All of the hypotheses discussed in this section (Fig. 3) could account for the hitherto unexplained reduction in starch content in the GK3 and U-IN2-30 lines (Sonnewald et al., 1997; Trethewey et al., 1998).
The Decrease in PPi Levels in the AGPase Antisense Lines and the Increase Found in the Cytosolic Invertase Lines Probably Reflect Two Independent Mechanisms
The combined reduction in AGPase activity and expression of a yeast invertase in the cytosol of the H-CI transgenic lines also results in a doubling of the tuber PPi content with respect to the parental line (AGP-93). However, no change in PPi levels was seen when the yeast invertase was expressed in the apoplast in combination with reduced AGPase activity (H-AI-lines). This strongly suggests that the increase in PPi seen in the U-IN2-30 and GK3 lines is indeed due to cytosolic reactions and not to metabolism in the amyloplast, e.g. starch cycling. On the other hand the reduction in PPi content seen in the antisense AGPase lines is probably due to plastidial reactions and not to changes in the cytosolic metabolism.
Hypothesis: PPi Might Integrate the Susy-Dependent Breakdown of Suc, Starch Synthesis, and Glycolysis in Starch-Storing Sinks Like Potato Tubers
Taking all the evidence presented and reviewed here together, we propose the following model to describe the situation in starch-storing sinks. During the early phases of development invertases are active, resulting in an accumulation of PPi. This high PPi level would, by an as-yet-unidentified mechanism (see previous discussion), inhibit starch synthesis and activate glycolysis via the PPi-dependent PFP. This mechanism might be the same one that leads to the reduced starch accumulation found in the U-IN2-30 and GK3 transgenic lines. The switch from an invertase-dependent to a Susy-dependent Suc breakdown during normal tuber development would lead to a decrease in PPi levels following an increased use of PPi by the Susy pathway of Suc mobilization. The subsequent reduction in tuber PPi content would lead to the observed activation in the flux through the starch biosynthetic pathway. The question of where the PPi is generated to support continued Suc mobilization by the Susy pathway has been indirectly addressed in this study and, based upon the results from the analysis of transgenic tubers, we propose that the most likely source of PPi is the AGPase reaction. This proposal runs against the current dogma, although the hypothesis that the PPi level provides a link between catabolic and anabolic reactions has already been proposed in the context of cytosolic metabolism in plant cells (Taiz, 1986). Indeed, a link between the supply of PPi for the cleavage of Suc by the Susy pathway and the production of PPi by the AGPase has been proposed from mainly theoretical considerations for endosperm sink tissue (Doehlert, 1990; Kleczkowski, 1994). Further, the recent discovery that AGPase is localized in the cytosol in endosperms (Denyer et al., 1996; Thorbjørnsen et al., 1996; Shannon et al., 1998) strongly supports the hypothesis that the activities of the AGPase and UGPase enzymes are coupled via the PPi level in these tissues.
The proposed link between PPi and the coordination of cytosolic Suc and plastidial starch metabolism might also explain some discrepancies observed in potato plants expressing an Escherichia coli pyrophosphatase in the cytosol. Initial experiments with these transgenic tubers showed an inhibition of Suc breakdown and a reduction in starch accumulation (Jelitto et al., 1992; Sonnewald, 1992). However, in subsequent experiments an increase in starch content was reported (Geigenberger et al., 1998). The authors argue that differences in the age of the plants or in the growth conditions between the experiments may be the reason for these conflicting results. We propose that an “optimal” PPi level might exist at which Suc breakdown is unrestricted and the AGPase reaction in the plastid is promoted. Such an optimal concentration may have been reached in the circumstances of the later experiments.
In the model presented here we propose that there is a tight integration of cytosolic and plastidial metabolism via PPi. The hypothesis described here is based upon whole tissue measurements of PPi; the achievement of subcellular measurements of PPi is an important task to confirm the model. However, although still speculative in nature, we believe that the model is the only one that can account for all the recent data obtained from transgenic potato tubers while also offering an explanation for the source of PPi to support the continued breakdown of Suc. Finally, the model has the potential to explain the strong correlation seen in all starch-storing sinks between the unloading of Suc, starch biosynthesis, and glycolysis.
MATERIALS AND METHODS
Plant Material
Potato (Solanum tuberosum L. cv Desirée) plants (Saatzucht Lange AG, Bad Schwartau, Germany), along with the transgenic lines U-IN2-30, GK3-29, -38, and -41 (Sonnewald et al., 1997; Trethewey et al., 1998, 1999b), AGP-85 and 93 (Müller-Röber et al., 1992; Trethewey et al., 1999a), H-AI-66, -44, and -10 (Trethewey et al., 1999a), and H-CI-79, -55, and -52 (Trethewey et al., 1999a) were grown in the greenhouse under a 16-h light, 8-h dark regime with supplementary light to ensure a minimum of 250 μmol photons m−2s−1 at 22°C. The term developing tubers is used for tubers over 10 g fresh weight harvested from healthy 2- to 3-month-old plants. U-IN2-30 was the parent line used for transformation with a bacterial glucokinase to generate the GK3 lines (Trethewey et al., 1998). Line AGP-93, expressing an antisense construct targeted against the small subunit of the AGPase under the control of the 35S promoter (Müller-Röber et al., 1992), was transformed with the yeast-derived invertase gene (suc2) under the control of the patatin promoter giving rise to double transgenic lines with an invertase localized either in the cytosol (H-CI-79, -55, -52; Trethewey et al., 1999a) or in the apoplast (H-AI-66, -44, -10; Trethewey et al., 1999a).
Chemicals
All enzymes were purchased from Boehringer Mannheim (Mannheim, Germany), with the exception of the PFP from Propinobacterium freudenreichii shermanii, which was obtained from Sigma-Aldrich (Darmstadt, Germany). Chemicals were obtained from either Sigma or Merck (Darmstadt, Germany).
Biochemical Analysis
Metabolic intermediates were determined in trichloracetic acid (TCA) extracts exactly as described by Trethewey et al. (1998). Nucleotides and ADP-Glc were measured in TCA extracts by HPLC (Trethewey et al., 1998). PPi was determined using a coupled enzymatic assay based upon Weiner et al. (1987). The assay contained 50 mm Tris-acetate (pH 7.5), 2 mm MgCl2, 1 mm EDTA, 20 μm NADH, 0.2 mm Fru-6-P, 0.7 unit mL−1 aldolase, 7 units mL−1 triose-P isomerase, and 2.8 units mL−1 glycerol-3-P-dehydrogenase (all enzymes from rabbit muscle). The reaction was started with the addition of 0.6 unit mL−1 PFP from P. freudenreichii shermanii. Pseudoextracts (without tissue) were also prepared to confirm the absence of significant PPi contamination in all the solutions and vessels used in the procedure. The reliability of the extraction procedure and assay protocol was confirmed using recovery experiments (e.g. Trethewey et al., 1998; Veramendi et al., 1999); in the case of PPi the recovery was found to be 105% ± 1% (se, n = 6).
Statistical Analysis of Data
t Tests were performed using the algorithm integrated into Microsoft Excel 7.0 (Microsoft, Seattle). The word “significant” is used in the text only when the change in question has been confirmed to be statistically significant (P < 0.05) with the t test.
ACKNOWLEDGMENTS
We would like to thank Bruno Marty, Frank Huhn, and Olaf Woiwoide for careful supervision of greenhouse plants. We are indebted to Alisdair Fernie for debating the model presented in this manuscript. R.N.T. would like to acknowledge the excellent tutorship and inspiration of the late Prof. Tom ap Rees, for whom pyrophosphate was a subject of fascination.
Footnotes
This work was supported by grants from the Max-Planck-Gesellschaft (to E.M.F. and R.N.T.).
LITERATURE CITED
- Appeldoorn NJG, de Bruijn SM, Koot-Gronsveld EAM, Visser RGF, Vreugdenhil D, van der Plas LHW. Developmental changes of enzymes involved in conversion of sucrose to hexose-phosphate during early tuberisation of potato. Planta. 1997;202:220–226. [Google Scholar]
- Appeldoorn NJG, de Bruijn SM, Koot-Gronsveld EAM, Visser RGF, Vreugdenhil D, van der Plas LHW. Developmental changes in enzymes involved in the conversion of hexose phosphate and its subsequent metabolites during early tuberization of potato. Plant Cell Environ. 1999;22:1085–1096. [Google Scholar]
- ap Rees T, Morrell S. Carbohydrate metabolism in developing potatoes [Carbohydrate Metabolism Symposium Papers] Am Potato J. 1990;6:835–847. [Google Scholar]
- Bölter B, Soll J, Hill K, Hemmler R, Wagner R. A rectifying ATP-regulated solute channel in the chloroplastic outer envelope from pea. EMBO J. 1999;18:5505–5516. doi: 10.1093/emboj/18.20.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer K, Dunlap F, Thorbjørnsen T, Keeling P, Smith AM. The major form of ADP-glucose pyrophosphorylase in maize endosperm is extraplastidial. Plant Physiol. 1996;112:779–785. doi: 10.1104/pp.112.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlert DC. Distribution of enzyme activities within the developing maize (Zea mays) kernel in relation to starch, oil and protein accumulation. Physiol Plant. 1990;78:560–567. [Google Scholar]
- Geigenberger P, Hajirezaei M, Geiger M, Deiting U, Sonnewald U, Stitt M. Overexpression of pyrophosphatase leads to increased sucrose degradation and starch synthesis, increased activities of enzymes for sucrose-starch interconversions, and increased levels of nucleotides in growing potato tubers. Planta. 1998;205:428–437. doi: 10.1007/s004250050340. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Müller-Röber B, Stitt M. Contribution of adenosine 5′-diphosphoglucose pyrophosphorylase to the control of starch synthesis is decreased by water stress in growing potato tubers. Planta. 1999;209:338–345. doi: 10.1007/s004250050641. [DOI] [PubMed] [Google Scholar]
- Gross P, ap Rees T. Alkaline inorganic pyrophosphatase and starch synthesis in amyloplasts. Planta. 1986;167:140–145. doi: 10.1007/BF00446381. [DOI] [PubMed] [Google Scholar]
- Hajirezaei M, Sonnewald U, Viola R, Carlisle S, Dennis D, Stitt M. Transgenic potato plants with strongly decreased expression of pyrophosphatase show no visible phenotype and only minor changes in metabolic fluxes in their tubers. Planta. 1994;192:16–30. [Google Scholar]
- Hajirezaei M, Stitt M. Contrasting roles for pyrophosphate: fructose-6-phosphate phosphotransferase during aging of tissue slices from potato tubers and carrot storage tissues. Plant Sci. 1991;77:177–183. [Google Scholar]
- Heldt H, Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970;10:143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Jelitto T, Sonnewald U, Willmitzer L, Hajirezeai M, Stitt M. Inorganic pyrophosphate content and metabolites in potato and tobacco plants expressing E. coli pyrophosphatase in their cytosol. Planta. 1992;188:238–244. doi: 10.1007/BF00216819. [DOI] [PubMed] [Google Scholar]
- Kleczkowski L. Glucose activation and metabolism through UDP-glucose pyrophosphorylase in plants. Phytochemistry. 1994;37:1507–1515. [Google Scholar]
- Lunn JE, Douce R. Transport of inorganic pyrophosphatase across the spinach chloroplast envelope. Biochem J. 1993;290:375–379. doi: 10.1042/bj2900375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Röber B, Sonnewald U, Willmitzer L. Inhibition of the ADP-pyrophosphorylase in transgenic potatoes leads to sugar-storing tubers and influences tuber formation and expression of tuber storage protein genes. EMBO J. 1992;11:1229–1238. doi: 10.1002/j.1460-2075.1992.tb05167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick WP, Schaeffer AA. Sucrose metabolism in sources and sinks. In: Zanski E, Schaerffer AA, editors. Photoassimilate Distribution in Plants and Crops: Source-Sink Relationships. New York: Marcel Dekker; 1996. pp. 115–156. [Google Scholar]
- Shannon JC, Pien F-M, Cao H, Liu K-C. Brittle-1, an adenylate translocator, facilitates transfer of extraplastidial synthesized ADP-glucose into amyloplasts of maize endosperms. Plant Physiol. 1998;117:1235–1252. doi: 10.1104/pp.117.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnewald U. Expression of E. coli inorganic pyrophosphatase in transgenic plants alters photoassimilate partitioning. Plant J. 1992;2:571–581. [PubMed] [Google Scholar]
- Sonnewald U, Hajirezaei MR, Kossmann J, Heyer A, Trethewey RN, Willmitzer L. Expression of a yeast invertase in the apoplast of potato tubers increases tuber size. Nat Biotech. 1997;15:794–797. doi: 10.1038/nbt0897-794. [DOI] [PubMed] [Google Scholar]
- Sowokinos JR, Preiss J. Pyrophosphorylases in Solanum tuberosum: III. Purification, physical, and catalytic properties of ADP-glucose pyrophosphorylase in potatoes. Plant Physiol. 1982;69:1459–1466. doi: 10.1104/pp.69.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. Pyrophosphate as an energy donor in the cytosol of plant cells: an enigmatic alternative to ATP. Bot Acta. 1998;111:167–175. [Google Scholar]
- Sturm A, Tang GQ. The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends Plant Sci. 1999;4:401–407. doi: 10.1016/s1360-1385(99)01470-3. [DOI] [PubMed] [Google Scholar]
- Sung S-J, Loboda T, Black C. Sucrose, pyrophosphate, and plastid metabolism in relation to cellular communication. In: Zelitch I, editor. Perspectives in Biochemical and Genetic Regulation of Photosynthesis. New York: Wiley-Liss; 1990. pp. 55–68. [Google Scholar]
- Sweetlove LJ, Müller-Röber B, Willmitzer L, Hill SA. The contribution of adenosine 5′-diphosphoglucose pyrophosphorylase to the control of starch synthesis in potato tubers. Planta. 1999;209:330–337. doi: 10.1007/s004250050640. [DOI] [PubMed] [Google Scholar]
- Taiz T. Are biosynthetic reactions in plant cells thermodynamically coupled to glycolysis and the tonoplast proton motive force? J Theor Biol. 1986;123:231–238. [Google Scholar]
- Thorbjørnsen T, Villand P, Denyer K, Olsen O-A, Smith AM. Distinct isoforms of ADP glucose pyrophosphorylase occur inside and outside the amyloplasts in barley endosperm. Plant J. 1996;10:243–250. [Google Scholar]
- Trethewey RN, Geigenberger P, Riedel K, Hajirezaei M-R, Sonnewald U, Stitt M, Riesmeier JW, Willmitzer L. Combined expression of glucokinase and invertase in potato tubers leads to a dramatic reduction in starch accumulation and a stimulation of glycolysis. Plant J. 1998;15:109–118. doi: 10.1046/j.1365-313x.1998.00190.x. [DOI] [PubMed] [Google Scholar]
- Trethewey RN, Geigenberger P, Sonnewald U, Henning A, Müller-Röber B, Willmitzer L. Induction of the activity of glycolytic enzymes correlates with enhanced hydrolysis of sucrose in the cytosol of transgenic potato tubers. Plant Cell Environ. 1999a;22:71–79. [Google Scholar]
- Trethewey RN, Riesmeier JW, Willmitzer L, Stitt M, Geigenberger P. Tuber-specific expression of a yeast invertase and a bacterial glucokinase in potato leads to an activation of sucrose phosphate synthase and the creation of a sucrose futile cycle. Planta. 1999b;208:227–23. doi: 10.1007/s004250050554. [DOI] [PubMed] [Google Scholar]
- Veramendi J, Roessner U, Renz A, Willmitzer L, Trethewey RN. Antisense repression of hexokinase 1 leads to an overaccumulation of starch in leaves of transgenic potato plants but not to significant changes in tuber carbohydrate metabolism. Plant Physiol. 1999;121:1–11. doi: 10.1104/pp.121.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Wobus U. Sugar import and metabolism during seed development. Trends Plant Sci. 1997;2:169–174. [Google Scholar]
- Weiner H, Stitt M, Heldt HW. Subcellular compartmentation of pyrophosphate and alkaline pyrophospha- tase in leaves. Biochim Biophys Acta. 1987;893:13–21. [Google Scholar]
- Woldegiorgis G, Voss S, Shrago E, Werner-Washburne M, Keesgstra K. Adenine nucleotide translocase-dependent anion transport in pea chloroplasts. Biochim Biophys Acta. 1985;810:340–345. doi: 10.1016/0005-2728(85)90219-1. [DOI] [PubMed] [Google Scholar]