Abstract
Background
Colon cancer is the second leading cause of cancer deaths in the United States. The Participatory Research to Advance Colon Cancer Prevention (PROMPT) study is a collaboration between two research institutions and a federally qualified health center (FQHC). The study seeks to raise colon cancer screening rates using a direct-mail fecal immunochemical testing (FIT) and reminder program in an FQHC serving a predominantly Latino population in California.
Methods
PROMPT is a pragmatic trial enrolling 16 clinics. The study will test automated and live prompts (i.e., alerts, reminders) to a direct-mail FIT program in two phases. In Phase I, we tailored and defined intervention components for the pilot using a community-based participatory research approach called boot camp translation. We then plan to conduct a three-arm patient-randomized comparative effectiveness trial in two pilot clinics to compare 1) automated prompts, 2) live prompts, and 3) a combination of automated plus live prompts to alert and remind patients to complete screening. In Phase II, the adapted best practice intervention will be spread to additional clinics within the FQHC (estimated population 27,000) and assessed for effectiveness. Patient and staff interviews will be conducted to explore receptivity to the program and identify barriers to implementation.
Discussion
This pragmatic trial applies innovative approaches to engage diverse stakeholders and will test the effectiveness and spread of a direct-mail plus reminder program. If successful, the program will provide a model for a cost-effective method to raise colon cancer screening rates among Latino patients receiving care in FQHCs.
Trial registration
National Clinical Trial (NCT) Identifier NCT03167125
Keywords: colon cancer screening, fecal immunochemical test, pragmatic study, community-based participatory research, boot camp translation
Introduction
Colon cancer is the second leading cause of cancer-related death in the United States.1 In 2017, an estimated 135,000 persons will be diagnosed with the disease and about 50,000 will die from it.2 Regular screening is effective in reducing the incidence and mortality of colon cancer by detecting precancerous polyps or cancer at early curable stages.3 However, colon cancer screening rates are marked by a pronounced disparity, with Latinos residing in the United States for fewer than ten years and uninsured Latinos having especially low rates.4,5 Since these individuals typically receive care at one of over 1,200 federally qualified health centers (FQHCs) nationwide, FQHCs are the ideal setting for interventions to increase screening rates in this population.6
Studies have shown that mailing fecal immunochemical test (FIT) kits directly to a patient’s home (i.e., “direct-mail”) can increase colon cancer screening among FQHC populations.7–12 Among underserved patients whose screenings were not up-to-date, direct-mail FIT outreach invitations resulted in significantly higher colon cancer screening compared with usual care.11 However, a recent systematic review found that while reminders following direct-mail programs were associated with higher FIT kit returns, studies provided limited comparative detail on optimal timing, content, or format of reminder prompts (i.e., text alerts, automated phone calls).12 Little is known about the effectiveness of these prompts in diverse populations, such as those who receive care at FQHCs. Low screening rates among this population may also be attributed to low awareness about the need for screening and challenges understanding patient health information.13,14 If patients are unable to understand a health condition and related screening options, they will experience difficulties engaging in meaningful conversation with a health care provider, choosing appropriate health action, or adhering to recommended screening measures.15
To address the need for optimally-timed FIT kit reminders and culturally-tailored colon cancer screening messages to improve FIT kit return rates in underserved populations, we implemented boot camp translation15, a community-based participatory research approach in a predominantly Latino-serving FQHC in southern California. As part of the Participatory Research to Advance Colon Cancer Prevention (PROMPT) study, we used boot camp translation to gather input from patients and clinic staff to choose optimal timing and mode of delivery of screening reminders, and refine colon cancer screening messages for an FQHC direct-mail FIT program. We will use key findings to define the intervention components of the PROMPT pilot and follow-up implementation study.
This paper describes the design of this National Institutes of Health (NIH) funded study, which seeks to test the effectiveness of alerts and reminders to a direct-mail colon cancer screening program, and spread the direct-mail and reminder program throughout a large Latino-serving FQHC. PROMPT applies novel strategies to engage stakeholders in adapting the intervention for a Latino population, defining the intervention components, and selecting a best practice for spread. The research aims are threefold: 1) develop personalized messages and define an intervention using boot camp translation to increase colon cancer screening among Latino populations, 2) assess the reach and effectiveness of a three-arm colon cancer screening program among Latino FQHC patients in two pilot clinics, and 3) further refine and test the effectiveness and spread of the program across additional clinics using a two- arm stepped-wedge approach, and develop an implementation guide that includes outreach materials, strategies for incorporating patient input, and resources.
Methods and Design
The PROMPT study was approved by the Institutional Review Board of Kaiser Permanente Northwest (Portland, OR), with ceding agreements from Oregon Health & Science University (OHSU) (Portland, OR) and a large FQHC in southern California. OHSU’s Oregon Rural Practice-based Research Network provided boot camp translation expertise.
Setting
The performance site for PROMPT is a large independent FQHC with 26 medical clinics serving 280,000 patients, the majority of whom are Latino (82%). Colon cancer screening has been an enterprise strategic goal for this clinic system over the last several years. In-clinic distribution of FIT kits and a direct-mail FIT program have improved screening rates from 39 to 64% over the past four years. Due to the minimal risk of the intervention, the requirement for informed consent was waived. The study is registered on ClinicalTrials.gov (NCT03167125).
Research Aims
PROMPT builds on previous research conducted by our research team to pilot-test automated and live reminders to promote colon cancer screening.16,17–19
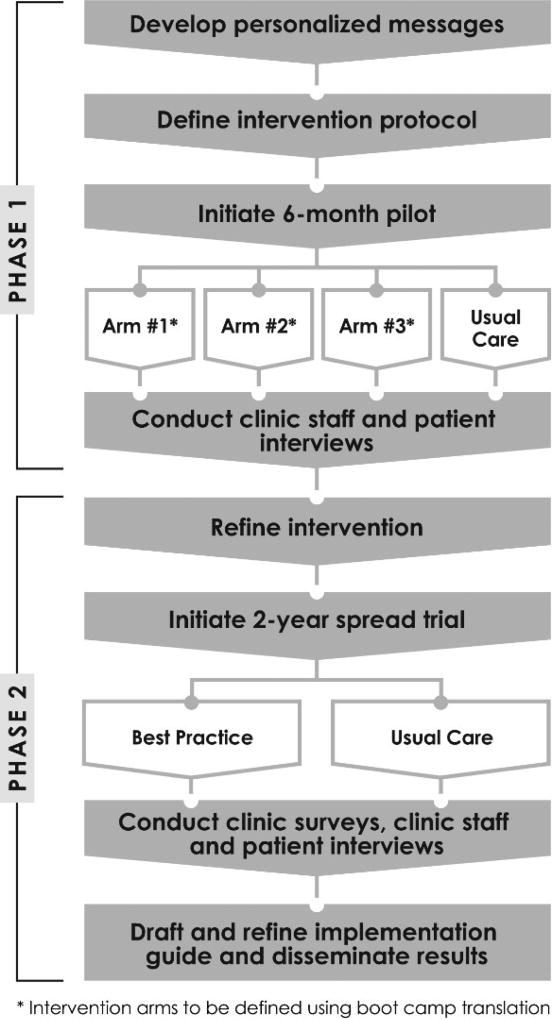
PROMPT has two phases: Phase I (Years 01–02) will design and evaluate a pilot study of a randomized-controlled trial to test systems-based, automated and non-automated prompts to increase colon cancer screening using a direct-mail program. Phase II (Years 03–05) will spread the program to additional clinics (estimated age-eligible patient population 27,000) and assess its effectiveness. Figure 1 provides an overview of the study design.
Figure 1.
The design and evaluation of both phases will be guided by the RE-AIM framework17–19 using Intervention Mapping (IM) focused on Latino patients served by the performance site. We will use IM, developed by Bartholomew and others,20 to identify these factors and to plan each step of the intervention with key stakeholders. The IM model has been implemented in multiple settings and specifies six components that lead to improved program outcomes: needs assessment, matrices, theory and practice, program, implementation, and evaluation.20 IM is increasingly used to systematically plan preventive care interventions and ensure stakeholder input is incorporated in each step.21–23
Boot Camp Translation [Phase I]
During the first six months of the study, we used an adapted version of boot camp translation to develop culturally tailored program materials (e.g., reminder phone scripts for automated calls and text messages) to define the components of the intervention arms in the pilot. Boot camp translation is a method for engaging diverse stakeholders in a consensus-building process.15 It uses an iterative, flexible schedule of face-to-face meetings combined with short, focused teleconferences. The process addressed two questions: ‘What do we need to say in our message to patients?’ and ‘How do we deliver that message to patients?’ The typical boot camp translation process requires about 20 to 25 hours of participant time over a 4- to 12-month time period.15 In our adapted version, participants were asked to commit eight hours of time over the course of three months.24
Our research team aimed to recruit 12 patient participants for each of the English- and Spanish-language versions of boot camp translation. Two clinic staff and two bilingual members of an advisory board were also recruited to participate in this community-based research process. The advisory board, which includes physicians, clinic staff, policy leaders, and patient advocates, advises the PROMPT research team on the design and implementation of the intervention, as well as provides recommendations for the dissemination of results. Eligible patient participants were Latino, ages 50 to 75 years, able to speak English or Spanish, and willing to participate in the in-person meeting and follow- up group phone calls. Participants were compensated up to $200 for their participation in the entire boot camp translation process. Recruitment and materials were developed in both English and Spanish, and separate sessions were held for English- and Spanish-speaking participants.
Pilot [Phase I]
The conditions of the three intervention arms (i.e., number of call attempts, combination of methods) are not prespecified and will be determined by the research team, clinic staff, patients, and the advisory board, using findings from the boot camp translation process. The usual care arm includes a mailed FIT and a potential reminder call delivered by the clinic depending on staff availability. We will test the intervention components, using a patient-level randomized design in two pilot clinics serving Los Angeles and Orange counties. Consistent with the desire of clinic staff to integrate this project into their regular workflows, standardized procedures will be used to manage the interventions so that eligible patients get initial screening on time, are prompted to repeat screening as recommended, and obtain follow-up care or surveillance over time.
The evaluation of Phase I will consist of multiple components. Using the RE-AIM framework, we will track reach and effectiveness of the automated and non-automated prompts, gather business case information, and conduct interviews with staff implementing the program and patients who did and did not complete a FIT to better understand barriers to colon cancer screening and acceptability of the prompts.
Assessment of program reach
We will assess how many patients we reached through the automated, live, and combination interventions. Automated phone call and text message systems report which patients received an automated phone call or text. We will calculate the proportion of patients who received the automated intervention and those who received the live phone calls.
Assessment of program effectiveness
For our primary outcome, we will assess program effectiveness, calculated as the proportion of eligible patients who completed a FIT within 6 months of randomization. We will also assess the percentage of patients who screen positive by FIT. The secondary outcome in this study is the completion of any colon cancer screening including colonoscopy.
Patient and clinic staff interviews
After the pilot study, we will conduct one-on-one patient interviews to elucidate important factors related to both adherence and non-adherence to colon cancer screening among adults who were mailed a FIT and received alerts and reminders. Interviews will be conducted with 45 patients across the three intervention arms: 30 interviews will be conducted with patients who completed the FIT; these interviews will assess acceptability, reaction, and usefulness of the program as well as suggestions for improving content and timing of alerts and reminders. For non-completers of the FIT kit, we will interview 15 patients to understand ongoing barriers to receptivity of the program and completion of the FIT kit. As a comparison, we will conduct an additional 10 interviews with patients in usual care. All interviews will be held by phone and last about an hour.
Eligible participants identified using clinic records, will be invited to participate through an invitation letter and phone call. Interviews will be digitally-recorded, professionally transcribed, and imported into a qualitative analysis program for data management and analysis. Bilingual study team members will independently review transcripts, code key words, and identify common themes that appear throughout the discussion. We will also conduct debrief interviews with clinic staff involved in the implementation of the pilot to understand their experiences, including any unintended consequences (both positive and negative). The same analytic process will be used for analysis of clinic staff interviews.
Statistical analysis and power calculations
For our evaluation of effectiveness, all analyses will be carried out using the intent-to-treat principle. We will use logistic regression to determine whether the automated, live, or combination arms are more effective than the usual care arm in completing a FIT. For PROMPT, the usual care arm consisted of a mailed FIT and potential live reminders delivered at the discretion of each clinic. The dependent variable is completion of a FIT within 6 months, and the independent variables will be dummy vectors representing the arms, with usual care as the reference group. Thus, assuming 1,600 participants and an allocation ratio of 1:1:1 to be randomized to one of three arms (n=533 per arm), a logistic regression model with two dummy vectors correlated at 0.50, and a usual care FIT completion rate of 15%, we will have 80% power to detect an odds ratio as small as 1.58 at a two-tailed alpha level of 0.05. This is equivalent to a 14.9% FIT completion in one or both intervention arms (or 4.9% beyond usual care). We performed power calculations using PASS 13.25
Secondary analysis
We will also explore whether effectiveness differs for patient subgroups, such as language (English vs. Spanish), previous screening history, and poverty status (<100% FPL vs 100%+ FPL). Each factor will be tested separately by adding it as an independent variable to the logistic regression model, as well as the product of each factor with each arm vector. Evidence of differential intervention effectiveness will be supported by a significant joint test of the interaction (product) terms.
Selecting a best practice
Prior to spreading the program to additional sites in the second phase of the trial, clinic staff and advisory board members will review the findings from the pilot study, the data in the business case, and themes from one-on-one interviews with patients and staff to identity a best practice. The best practice could offer flexibility depending on clinic resources. For example, we may determine that the automated phone calls have the highest reach and effectiveness, but adding a live phone call can further boost return rates. Once the best practice is selected, we will test its effectiveness and spread to additional clinics. In Phase II, this “best practice” arm will be evaluated against usual care.
Main Trial [Phase II]
To avoid contamination, the two clinics serving as pilot sites in Phase I will be ineligible for the main trial in Phase II.
Evaluation
We will use the RE-AIM framework to evaluate the main trial. Program adoption, implementation, and maintenance (at the clinic-level) will be assessed using clinic surveys, one-on-one interviews with implementation staff and patients, and data reports. Implementation outcomes will include proportion of patients mailed a FIT kit, proportion unable to be reached, and proportion on the “do not call” list. Reports will be generated monthly during the trial, listing the number of letters, FIT kits, and alerts and reminders sent; number of FIT and other colon cancer screening tests completed; and number of positive FITs and follow-up colonoscopies completed. These reports are to be reviewed regularly by the research team and members of the advisory board. As discussed below, we plan to track program effectiveness and maintenance at both the clinic- and patient-levels.
Assessment of program effectiveness
A stepped-wedge design will be used to assess program effectiveness.26–29 Although randomized controlled trials represent the gold standard for evidence creation, they are not always well-suited for all settings. Clinic leadership endorsed the stepped-wedge design as all practices will receive the intervention and it corresponds with how they would generally roll-out a new initiative. A total of 16 clinics will be randomized to two wedges (or groups) with respect to implementation start date. Clinics will switch from usual care to the program over two 12-month intervals. Both wedges will be in usual care during the first-time period. Wedge 1 will begin the intervention during the second-time period (Year 1, July 2018 – June 2019) while the other wedge remains with usual care. The second wedge will begin the intervention in year 2 (July 2019 – June 2020).
Assessment of adoption, implementation, and maintenance
We will conduct a mixed-methods assessment at three key time points of program implementation: pre-implementation, one-year post- implementation, and two-years post-implementation. The assessments will focus on acceptability (e.g., clinic staff attributes that influence implementation, implementation climate, implementation readiness), adaptability (e.g., changes made to the program, the extent to which the clinic is delivering the intended program), and readiness for maintaining the program over time. Our mixed-methods assessment will involve debrief interviews with clinic outreach workers. Additionally, we will conduct another round of patient interviews (approximately 45) to assess reaction to the program and factors that shaped or hindered adoption, implementation, and maintenance from the patient perspective, using a similar approach as in the pilot phase. We will draft an implementation guide and disseminate findings (in English and Spanish) to local, regional, and national audiences.
Statistical analysis and power calculations
To examine the effectiveness of the program on our primary outcome, FIT completion, we will use the linear mixed model described by Hussey et al.26 If Yijk denotes the individual level response for person k at time j for clinic i, we assume Yijk = µij + αi + βj + Xijθ + εijk, where µij is the average response in clinic i during measurement period j, αi is a random effect for clinic i, αi ~ N(0, τ2), βj is a fixed effect for time interval j, Xij is a binary indicator of treatment status (1=intervention, 0=usual care) for clinic i at time j, and the εijk are normally distributed residual errors. Our primary interest is on the estimated fixed intervention effect, θ, which is assumed to be constant over time. A positive and significant θ would provide evidence for the effectiveness of the intervention. We estimated power assuming 8 clinics per wedge (n=2), using the average clinic size of 2,000 eligible participants per clinic. We assumed an intra-class correlation (ICC) of 0.03.30 (Note that the power for the stepped-wedge design is fairly insensitive to changes in the ICC.)26 Assuming a baseline screening rate of 56.7%, we are estimated to have 80% power to detect an increase as small as 2.8% (to 59.5%) following program implementation, and 90% power to detect an increase of 3.3% (to 60.0%) following program implementation at a two-tailed alpha level of 0.05.
Business Case for intervention
We will perform a cost assessment of the intervention to inform an economic evaluation of the PROMPT program in comparison to usual care. We will collect and report data on the costs to implement and deliver the interventions. We will also report the cost per incremental FIT completed and create a budget impact model.31 Costs and benefits will be inflation- adjusted and discounted at a 2% base rate and adjusted in univariate and multivariate sensitivity analyses.
Discussion
The PROMPT program has great potential to make unique scientific and societal contributions in several ways. First, PROMPT will accelerate the adoption of a direct-mail program proven to address colon cancer screening disparities. Previous studies have shown that direct-mail fecal testing programs successfully raise these screening rates in the general population, and are particularly effective in population subgroups that traditionally forgo screening.11,12,32 PROMPT will accelerate the adoption of this proven approach in an FQHC system with a primary focus on the Latino population. Translating evidence-based programs that are particularly effective in underserved populations is the necessary first step in addressing persistent colon cancer screening disparities.
Second, PROMPT will be step-wise and tailored for language and culture, and will be both practice and patient-centered. Most previous studies have applied a one-size-fits-all approach to prompting colon cancer screening. While such approaches are important, they often cannot answer clinic administrators’ critical questions, such as ‘Will this intervention work in my setting?’ or ‘Can I pare down this intervention and expect the same result?’ Our intervention will be step-wise and will test both low- and higher- intensity follow-up reminder components. All arms of our proposed Phase I intervention – Auto Prompts, Live Prompts, and Auto Plus Live Prompts – will be tailored for cultural factors and language and, as such, represent a real innovation in this field.
Third, PROMPT will sequentially test the effectiveness and spread of our colon cancer screening system. Most previous colon cancer screening trials have either tested the effectiveness of a new intervention33–37 or have tested the implementation of a proven one.38 PROMPT will do both by first adapting and testing the program in two pilot clinics, then, spreading it throughout the performance site’s large and diverse network of clinics in southern California. This approach allows for tailoring based on the best available evidence and local systems, and specifically aims to achieve the goal of long-term sustainability. By testing the program’s scalability across the FQHC’s enterprise, we have the potential to reach over 27,000 patients eligible for colon cancer screening.
Finally, PROMPT applies innovative approaches to engage patients and other stakeholders in all phases of the research process. We apply novel and locally designed approaches, such as boot camp translation, and will incorporate patient and clinic feedback throughout each phase of the research study.
Acknowledgments
Funding
Research reported in this publication was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number U01MD010665. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Trial Registration
National Clinical Trial (NCT) Identifier NCT03167125
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. [Accessed October 11, 2017];Key Statistics for Colorectal Cancer. 2016 https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html.
- 3.Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, Jr, Garcia FAR, Gillman MW, Harper DM, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Owens DK, Phillips WR, Phipps MG, Pignone MP, Siu AL. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315(23):2564–2575. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Facts & Figures for Hispanics/Latinos 2015–2017. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 5.American Cancer Society. Colorectal Cancer Facts & Figures 2017–2019. Atlanta: 2017. [Google Scholar]
- 6.Nath JB, Costigan S, Lin F, Vittinghoff E, Hsia RY. Federally Qualified Health Center Access and Emergency Department Use Among Children. Pediatrics. 2016;138(4) doi: 10.1542/peds.2016-0479. [DOI] [PubMed] [Google Scholar]
- 7.Coronado GD, Golovaty I, Longton G, Levy L, Jimenez R. Effectiveness of a clinic-based colorectal cancer screening promotion program for underserved Hispanics. Cancer. 2011;117(8):1745–1754. doi: 10.1002/cncr.25730. [DOI] [PubMed] [Google Scholar]
- 8.Baker DW, Brown T, Buchanan DR, Weil J, Balsley K, Ranalli L, Lee JY, Cameron KA, Ferreira MR, Stephens Q, Goldman SN, Rademaker A, Wolf MS. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1235–1241. doi: 10.1001/jamainternmed.2014.2352. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg D, Schiff GD, McNutt R, Furumoto-Dawson A, Hammerman M, Hoffman A. Mailings timed to patients' appointments: a controlled trial of fecal occult blood test cards. Am J Prev Med. 2004;26(5):431–435. doi: 10.1016/j.amepre.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Goldman SN, Liss DT, Brown T, Lee JY, Buchanan DR, Balsley K, Cesan A, Weil J, Garrity BH, Baker DW. Comparative Effectiveness of Multifaceted Outreach to Initiate Colorectal Cancer Screening in Community Health Centers: A Randomized Controlled Trial. J Gen Intern Med. 2015;30(8):1178–1184. doi: 10.1007/s11606-015-3234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta S, Halm EA, Rockey DC, Hammons M, Koch M, Carter E, Valdez L, Tong L, Ahn C, Kashner M, Argenbright K, Tiro J, Geng Z, Pruitt S, Skinner CS. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med. 2013;173(18):1725–1732. doi: 10.1001/jamainternmed.2013.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis MM FM, Shannon J, Coronado G, Stange K, Guise JM, Wheeler S, Buckley DI. A Systematic Review of Clinic and Community Intervention to Increase Fecal Testing for Colorectal Cancer in Rural and Low-Income Populations in the United States – How, What and When? 2017 doi: 10.1186/s12885-017-3813-4. [In Press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman MJ, Ogdie A, Kanamori MJ, Canar J, O'Malley AS. Barriers and facilitators of colorectal cancer screening among Mid-Atlantic Latinos: focus group findings. Ethnicity & disease. 2006;16(1):255–261. [PubMed] [Google Scholar]
- 14.Robinson CM, Cassells AN, Greene MA, Beach ML, Tobin JN, Dietrich AJ. Barriers to colorectal cancer screening among publicly insured urban women: no knowledge of tests and no clinician recommendation. Journal of the National Medical Association. 2011;103(8):746–753. doi: 10.1016/s0027-9684(15)30414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norman N, Bennett C, Cowart S, Felzien M, Flores M, Flores R, Haynes C, Hernandez M, Rodriquez MP, Sanchez N, Sanchez S, Winkelman K, Winkelman S, Zittleman L, Westfall JM. Boot camp translation: a method for building a community of solution. J Am Board Fam Med. 2013;26(3):254–263. doi: 10.3122/jabfm.2013.03.120253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coronado GD, Rivelli JS, Fuoco MJ, Vollmer WM, Petrik AF, Keast E, Barker S, Topalanchik E, Jimenez R. Effect of Reminding Patients to Complete Fecal Immunochemical Testing: A Comparative Effectiveness Study of Automated and Live Approaches. J Gen Intern Med. 2017 doi: 10.1007/s11606-017-4184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glasgow RE. RE-AIMing research for application: ways to improve evidence for family medicine. J Am Board Fam Med. 2006;19(1):11–19. doi: 10.3122/jabfm.19.1.11. [DOI] [PubMed] [Google Scholar]
- 18.Glasgow RE, Klesges LM, Dzewaltowski DA, Bull SS, Estabrooks P. The future of health behavior change research: what is needed to improve translation of research into health promotion practice? Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2004;27(1):3–12. doi: 10.1207/s15324796abm2701_2. [DOI] [PubMed] [Google Scholar]
- 19.Glasgow RE, Klesges LM, Dzewaltowski DA, Estabrooks PA, Vogt TM. Evaluating the impact of health promotion programs: using the RE-AIM framework to form summary measures for decision making involving complex issues. Health education research. 2006;21(5):688–694. doi: 10.1093/her/cyl081. [DOI] [PubMed] [Google Scholar]
- 20.Bartholomew LK, Parcel GS, Kok G. Intervention mapping: a process for developing theory- and evidence-based health education programs. Health education & behavior : the official publication of the Society for Public Health Education. 1998;25(5):545–563. doi: 10.1177/109019819802500502. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez ME, Gonzales A, Tortolero-Luna G, Partida S, Bartholomew LK. Using intervention mapping to develop a breast and cervical cancer screening program for Hispanic farmworkers: Cultivando La Salud. Health promotion practice. 2005;6(4):394–404. doi: 10.1177/1524839905278810. [DOI] [PubMed] [Google Scholar]
- 22.Byrd TL, Wilson KM, Smith JL, Heckert A, Orians CE, Vernon SW, Fernandez-Esquer ME, Fernandez ME. Using intervention mapping as a participatory strategy: development of a cervical cancer screening intervention for Hispanic women. Health education & behavior : the official publication of the Society for Public Health Education. 2012;39(5):603–611. doi: 10.1177/1090198111426452. [DOI] [PubMed] [Google Scholar]
- 23.Hou SI, Fernandez ME, Parcel GS. Development of a cervical cancer educational program for Chinese women using intervention mapping. Health promotion practice. 2004;5(1):80–87. doi: 10.1177/1524839903257311. [DOI] [PubMed] [Google Scholar]
- 24.Thompson JH, Davis MM, Michaels L, Rivelli J, Castillo M, Younger B, Castro M, Reich S GC. Using boot camp translation to design a system-based intervention to improve rates of colon cancer screening among Latino patients in community health centers [In Process] 2018 [Google Scholar]
- 25.Hintze J. PASS 13. 2014 www.ncss.com.
- 26.Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemporary clinical trials. 2007;28(2):182–191. doi: 10.1016/j.cct.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Brown CA, Lilford RJ. The stepped wedge trial design: a systematic review. BMC medical research methodology. 2006;6:54. doi: 10.1186/1471-2288-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mdege ND, Man MS, Taylor Nee Brown CA, Torgerson DJ. Systematic review of stepped wedge cluster randomized trials shows that design is particularly used to evaluate interventions during routine implementation. Journal of clinical epidemiology. 2011;64(9):936–948. doi: 10.1016/j.jclinepi.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Hemming K, Lilford R, Girling AJ. Stepped-wedge cluster randomised controlled trials: a generic framework including parallel and multiple-level designs. Statistics in medicine. 2015;34(2):181–196. doi: 10.1002/sim.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green BB, Wang CY, Anderson ML, Chubak J, Meenan RT, Vernon SW, Fuller S. An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: a randomized trial. Annals of internal medicine. 2013;158(5 Pt 1):301–311. doi: 10.7326/0003-4819-158-5-201303050-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauskopf JA, Sullivan SD, Annemans L, Caro J, Mullins CD, Nuijten M, Orlewska E, Watkins J, Trueman P. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices--budget impact analysis. Value Health. 2007;10(5):336–347. doi: 10.1111/j.1524-4733.2007.00187.x. [DOI] [PubMed] [Google Scholar]
- 32.Coronado GD, Vollmer WM, Petrik A, Aguirre J, Kapka T, Devoe J, Puro J, Miers T, Lembach J, Turner A, Sanchez J, Retecki S, Nelson C, Green B. Strategies and opportunities to STOP colon cancer in priority populations: pragmatic pilot study design and outcomes. BMC cancer. 2014;14:55. doi: 10.1186/1471-2407-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen BH, Stewart SL, Nguyen TT, Bui-Tong N, McPhee SJ. Effectiveness of Lay Health Worker Outreach in Reducing Disparities in Colorectal Cancer Screening in Vietnamese Americans. American journal of public health. 2015:e1–e7. doi: 10.2105/AJPH.2015.302713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enard KR, Nevarez L, Hernandez M, Hovick SR, Moguel MR, Hajek RA, Blinka CE, Jones LA, Torres-Vigil I. Patient navigation to increase colorectal cancer screening among Latino Medicare enrollees: a randomized controlled trial. Cancer causes & control : CCC. 2015 doi: 10.1007/s10552-015-0620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steffen LE, Boucher KM, Damron BH, Pappas LM, Walters ST, Flores KG, Boonyasiriwat W, Vernon SW, Stroup AM, Schwartz MD, Edwards SL, Kohlmann WK, Lowery JT, Wiggins CL, Hill DA, Higginbotham JC, Burt R, Simmons RG, Kinney AY. Efficacy of a Telehealth Intervention on Colonoscopy Uptake When Cost Is a Barrier: The Family CARE Cluster Randomized Controlled Trial. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015 doi: 10.1158/1055-9965.EPI-15-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krok-Schoen JL, Young GS, Pennell ML, Reiter PL, Katz ML, Post DM, Tatum CM, Paskett ED. Testing Interventions to Motivate and Educate (TIME): A multi-level intervention to improve colorectal cancer screening. Preventive medicine reports. 2015;2:306–313. doi: 10.1016/j.pmedr.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rawl SM, Christy SM, Monahan PO, Ding Y, Krier C, Champion VL, Rex D. Tailored telephone counseling increases colorectal cancer screening. Health education research. 2015;30(4):622–637. doi: 10.1093/her/cyv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohan EA, Boehm JE, DeGroff A, Glover-Kudon R, Preissle J. Implementing the CDC's Colorectal Cancer Screening Demonstration Program: wisdom from the field. Cancer. 2013;(119 Suppl 15):2870–2883. doi: 10.1002/cncr.28162. [DOI] [PMC free article] [PubMed] [Google Scholar]