Abstract
Introduction
Aquaporins (AQP) are a family of transmembrane proteins that transport water and small solutes such as glycerol across cell membranes. It is a mediator of transcellular water flow and plays an important role in maintaining intra/extracellular fluid homeostasis by facilitating water transport in response to changing osmotic gradients. In the skin, AQPs permit rapid, regulated, and selective water permeability and have been demonstrated to play a role in skin hydration, cell proliferation, migration, immunity, and wound healing. However, the expression of AQP-3 in the cutaneous burn wound has never been elucidated. We sought to assess the expression of AQP-3 in patients with burn wounds.
Methods
A fresh full thickness biopsy sample was taken from the center of the burn wound, the burn wound edge, and the graft donor site in 7 patients (n = 21), approximately 3–7 days post injury. Fixed, paraffin embedded sections were stained using AQP-3 specific antibody and examined by immunofluorescence. Fresh samples were processed to quantify AQP-3 protein expression with Western blot analysis.
Results
The central portion of the burn wound revealed destruction of the epidermis and dermis with no AQP-3 present. Along the burn wound edge where the epidermal architecture was disrupted, there was robust AQP-3 staining. Western blot analysis demonstrated deeper staining along the burn wound edge compared to unburned skin (control). Quantification of the protein shows a significant amount of AQP-3 expression along the burn wound edge (3.6 ± 0.34) compared to unburned skin (2.1 ± 0.28, N = 7, *p < 0.05). There is no AQP-3 expression in the burn wound center.
Conclusion
AQP-3 expression is increased in the burn wound following injury. While its role in wound healing has been defined, we report for the first time the effect of cutaneous burns on AQP-3 expression. Our data provides the first step in determining its functional role in burn wounds. We hypothesize that development of AQP3 targeted therapies may improve burn wound healing.
Keywords: Aquaporin, Epidermis, Burns
1. Introduction
Aquaporins (AQP) are a family of transmembrane proteins that transport water and small solutes such as glycerol across cell membranes [1]. It is a mediator of transcellular water flow and plays an important role in maintaining intra/extracellular fluid homeostasis by facilitating water transport in response to changing osmotic gradients [2]. AQPs are found in the major systems of the human body such as the nervous, renal, cardiovascular, respiratory, reproductive, digestive, musculoskeletal, and integumentary systems [2].
In the skin, AQPs permit rapid, regulated, and selective water permeability and have been demonstrated to play a role in skin hydration, cell proliferation, migration, immunity, and wound healing [3–5]. Overall, 6 skin AQPs have been identified. At the epidermal level, the most important and abundant of these proteins is AQP-3. It is a member of the aquaglyceroporin subfamily and is found primarily in the plasma membrane of keratinocytes and dermal fibroblasts [6,7]. AQP-3 has function in the transportation of water and glycerol, a natural moisturizing factor that keeps the skin hydrated, in the interstitial space and intracellularly [8].
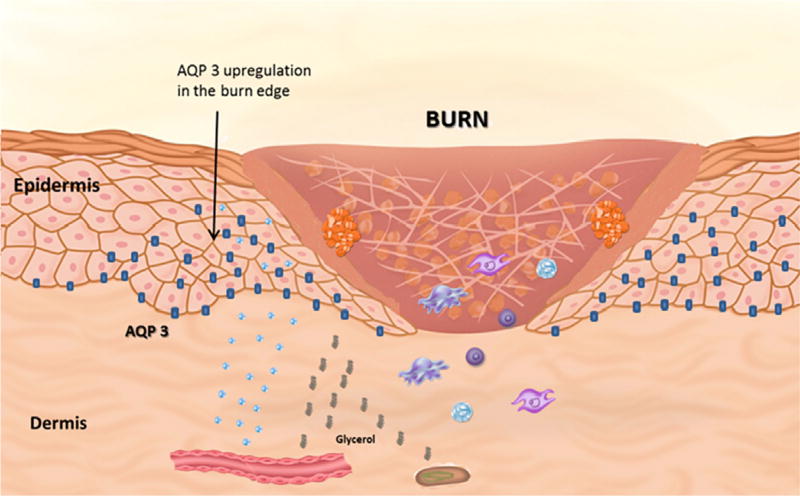
In the epidermis, AQP3 is organized to create a highly selective permeability gradient (Fig. 1). For example, epidermal permeability progressively increases from the stratum corneum to the stratum basale. Not coincidentally, this difference in cell permeability is proportional to the concentration of AQP3 within each epidermal layer, with high concentrations being found in the stratum basale and absent in the stratum corneum and granulosum. In fact, it is this proportionate increase in membrane permeability to AQP3 expression that forms the biologic rationale for an AQP3 mediated water clamp or gate phenomenon, a regulatory mechanism by which water moves across cellular membranes dependent on the conformation of the protein channel in the open or closed position [9].
Fig. 1.
Aquaporin-3 distribution within the epidermis. Aquaporin-3 is bound to keratinocyte membranes in the layers of the epidermis extending from the stratum granulosum to stratum basale. Following a cutaneous burn, there is an increase in AQP-3 expression which will transport water and glycerol. This process plays a key role in the keratinocyte proliferation, hydration, and migration to promote wound healing.
Wound healing in the skin is a multi-step process that involves several cell types, including epidermal keratinocytes, fibroblasts, endothelial cells, and peripheral nerve cells [10]. In addition to cellular involvement, a number of cell signaling molecules and proteins are involved in this process as well. AQP-3 is integral to wound healing by facilitating water and glycerol transport and therefore keratinocyte migration and proliferation respectively [3]. In fact, the absence of AQP3 regulated transport of water and glycerol has been shown to impair wound healing in animal models [11].
While the role of role of AQP-3 in cutaneous wound healing has been defined, its activity in the burn wound has not previously been determined. The aim of our study was to determine the effect of a cutaneous burn on AQP-3 expression within the wound.
2. Materials and methods
Following institutional review board approval (IRB), a total of 7 patients were selected for our study. Full thickness biopsy samples were taken from 3 locations on each patient; the burn wound center, edge, and graft donor sites for a total of 21 specimens (3 per patient) approximately 3–7 days post injury. The areas of burn to be biopsied were determined from physical examination by an experienced burn surgeon. The samples were immediately processed and stored in alloprotect reagent (Qiagen). For western blot analysis, skin samples were minced and processed (Navy Bullet Blender). Bicinchoninic acid protein assay was used to normalize the samples before deglycosylation with PNGase-F (Sigma) for 1 h. Samples were then loaded on to 10% acrylamide gels (Invitrogen) and transferred onto polyvinylidene fluoride membranes (Invitrogen) to detect AQP-3. For immunofluorescence, each skin sample was fixed in formalin and embedded in paraffin. Sections from each block were placed onto glass slides. The slides were then deparaffinized and rehydrated in various gradients of xylene, ethanol, and water. Antigen retrieval was performed with 10 mM citric acid buffer (Sigma). After blocking with 20% donkey serum (Invitrogen), the slides were incubated with anti-AQP-3 antibodies (Sigma) overnight and tagged with 1:400 AlexaFluor488 rabbit secondary antibody (Invitrogen). Standardized measurements of AQP-3 expression were obtained using western blot analysis to determine the AQP-3/actin ratio at each of the various biopsy sites.
3. Results
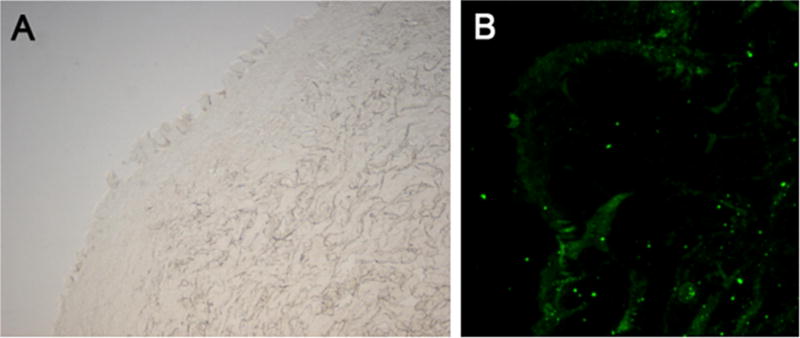
A total of 20 patients were enrolled into our study (N = 7) over a 5-month period. The mean age of the patients selected was 51 years (22–82 years) with 43% male and 57% female. African-Americans accounted for 29% (N = 2) of the patient population, Caucasian 57% (N = 4), Asian 14% (N = 1). The average total body surface area (TBSA) that was burned on these patients was 11.6%. The mechanism of each burn was 57% flame (N = 4), 29% scald (N = 2), and 14% grease (N = 1) (Table 1). Light microscopy of biopsied tissue from the burn wound center demonstrated total loss of an organized epidermis (Fig. 2A). On immunofluorescence specific for AQP-3, there was scattered epidermal staining and an absence of an organized epidermis (Fig. 2B).
Table 1.
Patient demographics.
| Patients | N = 7 (100%) |
|---|---|
| Age | Mean = 51 years (range 22–82 years) |
| Gender | Male N = 3 (43%) |
| Female N = 4 (57%) | |
| Mechanism | Flame N = 4 (57%) |
| Scald N = 2 (29%) | |
| Grease N = 1 (14%) | |
| TBSA | Mean 11.6% (range 1–60%) |
| Ethnicity | Caucasian N = 4 (57%) |
| African-American N = 2 (29%) | |
| Asia N = 1 (14%) |
Fig. 2.
(A) Light microscopy of the burn wound center demonstrating loss of an organized epidermis and (B) AQP-3 staining (green) showing scattered epidermal staining the absence of an organized epidermis. The small dots are background and not an actual cell. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
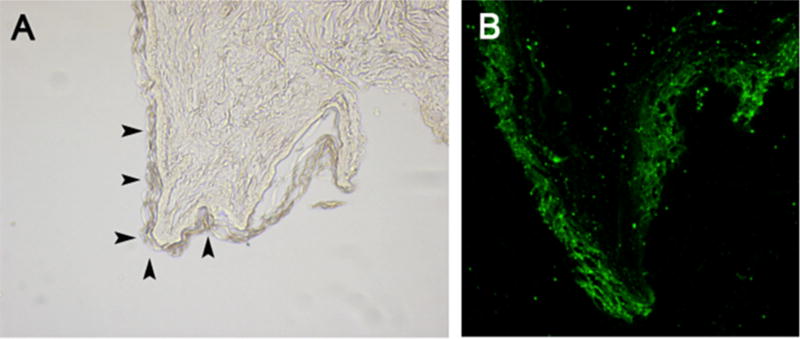
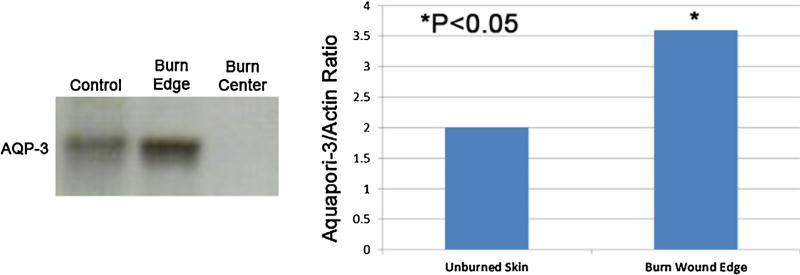
On light microscopy an organized epidermis was seen along the burn wound edge (Fig. 3A, solid arrows). When those same areas were viewed under immunofluorescence, there was robust AQP-3 staining isolated to the epidermis (Fig. 3B). Western blot analysis of AQP-3 recognized at a 36 kDa band demonstrated deeper staining among the burn wound edge compared to unburned skin (control). In the burn wound center there is no AQP-3 expression (Fig. 4, left panel). Quantification of the protein with Anti-AQP3 and B-actin expressed as a ratio shows a significant amount of AQP-3 expression along the burn wound edge (3.6 ± 0.34) compared to unburned skin (2.1 ± 0.28, N = 7, *p < 0.05) (Fig. 4, right panel).
Fig. 3.
(A) Along the burn wound edge there is an organized epidermis (solid arrows) and (B) AQP-3 expression is increased in these areas as well.
Fig. 4.
(A) Western blot analysis of AQP-3 recognized at a 36 kDa band. Along the burn wound edge demonstrates deeper staining compared to unburned skin (control). In the burn wound center there is no AQP-3 expression and (B) quantification of the protein was performed with Anti-AQP3 and B-actin expressed as a ratio shows a significant amount of AQP-3 expression along the burn wound edge compared to unburned skin (N = 7), *p < 0.05.
4. Discussion
We sought to determine the effect of a cutaneous burn on AQP-3 expression in humans using tissue samples from patients admitted to our unit with deep burns to skin. Full thickness biopsy samples were taken from the wound edge (defined clinically as a partial thickness burn), the donor site (normal skin), and wound center (full thickness burn). We evaluated the location and the degree of AQP-3 expression in the skin using immunofluorescence and western blot analysis respectively.
In the center of the burn wound, there was complete loss of the native epidermal architecture and no AQP-3 staining. As a result of this absence of AQP-3 within the burn wound center, we were unable to quantify the level of protein expression. This absence of AQP-3 in the burn wound center likely reflects the high degree of tissue damage and resultant epithelial disorganization within that area.
Light microscopy showed partial loss of epidermal along the burn wound edge. A significant increase in the amount of AQP-3 expression was observed compared to the unburned skin. This finding correlates with previous reporting of AQP-3 as a key facilitator of cell migration and proliferation during wound healing. During cutaneous wound healing AQP-3 expression is increased in hyperproliferating cells [10] and cell proliferation and migration occur from the wound edge [12].
AQP-3 was located throughout the epidermis starting from the burn wound edge and was absent in the dermis. Previous reports have shown epidermal specific AQP-3 expression with increased concentrations within the deeper layers of the epidermis [9]. We believe that this ability to express the protein after a burn is due to the fact that cells along the wound edge remain viable and have been stimulated to execute wound repair. The primary signal for wound repair emanates from these cells which are directly stimulated by the heat source or as a consequence of cell to cell communication originating from viable cells in the burn wound. This signal in turn may act directly or indirectly on the cells involved in wound regeneration. However, detailed molecular analysis would be needed to determine the exact cell signaling process which occurs.
The upstream signals involved in the upregulation of AQP3 at the burn wound edge are currently unknown. A possible activator of this process is Hypoxia Inducible Factor (HIF), a regulatory protein that activates genes known to promote the adaption and survival of cells and has been implicated in the regulation of other aquaporins [13]. It has previously been shown that the margins of the burn wound are hypoxic, which could explain why AQP-3 expression is significantly increased in these sites [14].
Increasing interest in the function of AQP3 in the skin has defined its role in skin hydration, cell proliferation, migration, immunity and wound healing in animal models [10,15,16]. In fact, a recent study looking at the effect of full thickness burns on AQP3 expression in murine and human skin demonstrated an increase within intact keratinocytes surrounding the wound [16]. Although the precise function was not defined, it was postulated that AQP-3 could be stimulated by burn related dehydration and play a vital role to maintain water homeostasis. However, the cellular changes that occur following a burn and the changes that occur during wound healing are very complex. Therefore, further investigation is necessary.
AQP-3 expression is increased in the burn wound following injury. While its role in general wound healing has been defined, we report for the first time the effect of cutaneous burns on AQP-3 expression. Our data provides the first step in determining its functional role in burn wound pathophysiology. This may lead to the development of AQP-3 modulating therapies to improve burn wound healing.
Footnotes
Disclosures
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Author contributions
R. Sebastian, E. Chau, P. Fillmore participated in the development of the study, conducted the experiments, assisted in interpreting the data and writing the manuscript. J. Matthews served in interpreting the data, determining its clinical significance and writing the manuscript. L.A. Price and V. Sidhaye assisted in the design of the experiments and determining its clinical significance. S. Milner oversaw all aspects of the study and obtained the grant that provided funding for the study.
References
- 1.Nakahigashi K, Kabashima K, Ikoma A, Verkman AS, Miyachi Y, Hara-Chikuma M. Upregulation of aquaporin-e is involved in keratinocyte proliferation and epidermal hyperplasia. J Invest Dermatol. 2011;131:865–73. doi: 10.1038/jid.2010.395. [DOI] [PubMed] [Google Scholar]
- 2.Day RE, Kitchen P, Owen DS, Bland C, Marshall L, Conner AC, et al. Human aquaporins: regulators of transcellular water flow. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbagen.2013.09.033. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Hara M, Ma T, Verkman AS. Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J Biol Chem. 2002;277:46616–21. doi: 10.1074/jbc.M209003200. [DOI] [PubMed] [Google Scholar]
- 4.Hara M, Verkman AS. Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc Natl Acad Sci USA. 2003;100:7360–5. doi: 10.1073/pnas.1230416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang GF, Dong CL, Tang GS, Shen Q, Bai CX. Membrane water permeability related to antigen-presenting function of dendritic cells. Clin Exp Immunol. 2008;153:410–9. doi: 10.1111/j.1365-2249.2008.03702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sougrat R, Morand M, Gondran C, Barre P, Gobin R, Bonte F, et al. Functional expression of AQP3 in human skin epidermis and reconstructed epidermis. J Invest Dermatol. 2002;118:678–85. doi: 10.1046/j.1523-1747.2002.01710.x. [DOI] [PubMed] [Google Scholar]
- 7.Bellemère G, Von Stetten O, Oddos T. Retinoic acid increases aquaporin 3 expression in normal human skin. J Invest Dermatol. 2008;128:542–8. doi: 10.1038/sj.jid.5701047. [DOI] [PubMed] [Google Scholar]
- 8.Hara-Chikuma M, Verkman AS. Roles of aquaporin-3 in the epidermis. J Invest Dermatol. 2008;128:2145–51. doi: 10.1038/jid.2008.70. [DOI] [PubMed] [Google Scholar]
- 9.Khandelia H, Jensen MO, Mouritsen OG. To gate or not to gate: using molecular dynamics simulations to morph gated plant aquaporins into constituitively open confirmations. J Phys Chem B. 2009;113:5239–44. doi: 10.1021/jp809152c. [DOI] [PubMed] [Google Scholar]
- 10.Hara-Chikuma M, Verkman AS. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J Mol Med. 2008;86:221–31. doi: 10.1007/s00109-007-0272-4. [DOI] [PubMed] [Google Scholar]
- 11.Ma T, Hara M, Sougrat R, Verbavatz JM, Verkman AS. Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J Biol Chem. 2002;277:17147–53. doi: 10.1074/jbc.M200925200. [DOI] [PubMed] [Google Scholar]
- 12.Shaw T, Martin P. Wound repair at a glance. J Cell Sci. 2009;122:3209–13. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higashida T, Peng C, Li J, Dombos D, Teng K, Li X, et al. Hypoxia-inducible factor-1a contributes to brain edema after stroke by regulating aquaporins and glycerol distribution in brain. Curr Neurovasc Res. 2011;8:44–51. doi: 10.2174/156720211794520251. [DOI] [PubMed] [Google Scholar]
- 14.Xing D, Liu L, Marti GP, Zhang X, Reinblatt M, Milner SM, et al. Hypoxia and hypoxia-inducible factor in the burn wound. Wound Repair and Regen. 2011;19(2):205–13. doi: 10.1111/j.1524-475X.2010.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234:120–41. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubo H, Hayashi T, Ago K, Ago M, Kanekura T, Ogata M. Forensic diagnosis of ante- and postmortem burn based on aquaporin-3 gene expression in the skin. Legal Med. 2014;16:128–34. doi: 10.1016/j.legalmed.2014.01.008. [DOI] [PubMed] [Google Scholar]