Abstract
Decapitation-induced axillary bud outgrowth is a vital mechanism whereby shoots are able to continue normal growth and development. In many plants, including wild-type garden pea (Pisum sativum L.), this process can be inhibited by exogenous auxin. Using the ramosus (rms) increased branching mutants of pea, we present evidence that this response to auxin is dependent on graft-transmissible substance(s) regulated by the genes Rms1 and Rms2. The response to exogenous auxin is massively diminished in decapitated rms1 and rms2 mutant plants. However, basipetal auxin transport is not reduced in intact or decapitated mutants. Grafting rms1 or rms2 shoots onto wild-type rootstocks restored the auxin response, indicating that Rms1 and Rms2 gene action in the rootstock is sufficient to enable an auxin response in mutant shoots. We conclude that Rms1 and Rms2 act in the rootstock and shoot to control levels of mobile substance(s) that interact with exogenous auxin in the inhibition of bud outgrowth after decapitation. At least for rms1, the reduced auxin response is unlikely to be due to an inability of auxin to decrease xylem sap cytokinin content, as this is already low in intact rms1 plants. Consequently, we have genetic evidence that auxin action in decapitated plants depends on at least one novel long-distance signal.
Apical dominance is generally defined as the control exerted by a shoot apex over outgrowth of lateral buds. However, widespread use of the term apical dominance has led to an emphasis on the apical bud as the prime source of control and underestimates the contribution that roots, stem, or leaves can make to the regulation of shoot branching (Hosokawa et al., 1990). For example, grafting studies with increased branching mutants from pea (Pisum sativum; ramosus [rms]) and petunia (decreased apical dominance; [dad]) demonstrate the regulatory power of roots and stem. Wild-type (WT) roots can inhibit branching in rms1 and rms2 pea shoots (Beveridge et al., 1994, 1997b). In petunia, a small section of WT interstock interrupting the stem at the base of dad1 plants can completely revert the mutant shoot to WT (Napoli, 1996). Therefore, we restrict apical dominance to situations where the apical bud is the major source of regulatory control and prefer to use terms such as branching and bud outgrowth (Napoli et al., 1999).
Early experiments on the control of branching employed decapitation as a means to test the importance of the apical bud in controlling growth of lateral buds. Results of studies modifying exogenous auxin supply to decapitated plants (Thimann and Skoog, 1933, 1934; Snow, 1937) led to the hypothesis that auxin synthesized in the apical bud plays a role in the inhibition of axillary buds.
There is some evidence that auxin inhibits lateral bud outgrowth via an indirect mechanism. For example, Snow (1937) and Morris (1977) showed that auxin supplied to one decapitated shoot of a plant with two or more decapitated shoots can inhibit branching at most nodes of the other shoot, even though radiolabeled auxin applied to one shoot does not move acropetally into other shoot(s) (Morris, 1977). These results indicate that auxin might act through another signal.
The promotion of lateral bud outgrowth by application of exogenous cytokinin to intact plants led to the theory that the ratio of cytokinin to auxin may control branching (Sachs and Thimann, 1964, 1967; Bangerth, 1994; Li et al., 1995). Decapitation experiments indicate that reduced auxin levels after decapitation may induce bud outgrowth by causing a transient increase in cytokinin content (Bangerth, 1994; Li et al., 1995). Intact transgenic plants with modified hormone concentrations have also provided some support for the auxin to cytokinin ratio hypothesis (Klee and Estelle, 1991). However, changes in hormone concentrations in these transgenics often do not correlate well with changes in bud outgrowth (for review, see Napoli et al., 1999). For example, expression of 35S-ipt or 35S-iaaL genes in tobacco caused increased cytokinin or decreased auxin, respectively, in juvenile plants, but were not accompanied by increased bud release or subsequent lateral shoot growth until the plants approached maturity (Medford et al., 1989; Romano et al., 1991; C.P. Roma-no, personal communication).
Another approach, rather than manipulating hormone level through bacterial transgenes, is to use auxin- or cytokinin-deficient or overproducing mutants. To date, such mutants are very rare. One example is amp1 from Arabidopsis which does have elevated cytokinin levels (6-fold) and increased branching (Chaudhury et al., 1993). However, the pleiotropic phenotype of the mutant precludes analysis of branching in isolation.
The possibility that signals other than auxin and cytokinin may contribute to the regulation of branching in plants has not yet received major attention (for review, see Napoli et al., 1999). Mutants screened for altered branching provide not only an opportunity to investigate the role of cytokinin and auxin in the control of branching, but also the potential to uncover novel or unpredicted genetic regulation of branching.
Axillary bud outgrowth occurs at numerous vegetative nodes of rms mutants (Arumingtyas et al., 1992) but the mutations do not cause a highly pleiotropic phenotype. In contrast with predicted increases in cytokinin levels and/or decreased auxin content, rms1 is one of three nonallelic mutants that have reduced root xylem sap cytokinin levels and normal or increased shoot auxin levels (Beveridge et al., 1994, 1996, 1997a, 1997b; for review, see Beveridge, 2000). Combined with results of grafting experiments, these data indicate that Rms1 controls the level or transport of graft-transmissible substance(s), other than cytokinin or auxin, that influence branching (Beveridge et al., 1997b). Branching in rms2 plants is also caused by altered graft-transmissible signaling, but, in comparison with WT plants, rms2 plants have a slightly elevated xylem sap cytokinin concentration and up to a 5-fold increase in shoot auxin level compared with WT plants (Beveridge et al., 1994, 1997b). Using these two mutants, we have investigated auxin responses in decapitated pea to determine possible effects of the graft-transmissible signals controlled by Rms1 and Rms2 on auxin response. This report provides the first clear evidence for genetic control of a mobile substance, other than cytokinin, that interacts with auxin in the control of axillary bud outgrowth.
RESULTS
Auxin Transport
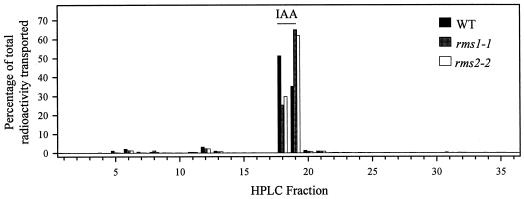
Basipetal transport of [3H]indole-3-acetic acid (IAA) was measured in plants with six to nine fully expanded leaves. In both intact and decapitated plants, radioactivity appeared to move down the stem in a single basipetal wave (Figs. 1 and 2). There were no significant differences among intact plants of different genotypes in the mean distances that the radioactivity was transported during the 18 h after application (Table I). The radioactivity was transported 14% to 22% further in decapitated rms1 and rms2 mutant plants than in decapitated WT plants (significant at P < 0.05 and P < 0.01, respectively; Table II). Eighteen to 19 h after application of [3H]IAA, the mean wave maxima of radioactivity transported in mutant and WT plants were between 9.7 and 11.1 cm in intact plants, and between 14.1 and 17.7 cm in decapitated plants (Tables I and II). The percentage of radioactivity transported from the apical bud of intact plants and from the application site of decapitated plants ranged from 6% to 12% and 13% to 24%, respectively, for the three genotypes. In absolute terms, the export of [3H]IAA in rms1-1 and rms2-2 plants was greater than in WT plants, up to 2-fold in the case of rms1-1 (P < 0.01; Tables I and II). HPLC analysis of the metabolism of radioactive compounds exported from the apical bud into the stem over an 18-h period after application of [3H]IAA, showed that greater than 92% of radioactivity co-eluted with [3H]IAA (Fig. 3). The metabolic profiles were similar among the three genotypes.
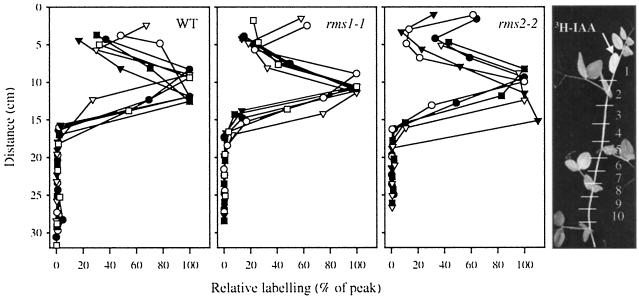
Figure 1.
Distribution of radioactivity in intact WT (cv Parvus), rms1-1, and rms2-2 shoots 18 h after supplying [3H]IAA (37 kBq per plant) to the apical bud. The diagram on the far right shows the numbering of stem segments. Each point is the value for a single segment and each line is a single plant. Data shown are from segments 2 to 12. Data are expressed as a percentage of the radioactivity recovered in the segment with the greatest 3H content within the basipetal wave. Absolute values are given in Table I; n = 5 or 6.
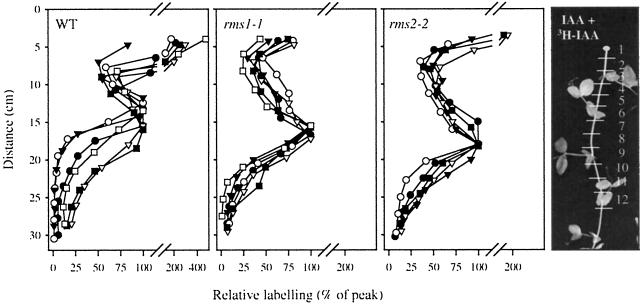
Figure 2.
Distribution of radioactivity in decapitated auxin-treated WT (cv Parvus), rms1-1, and rms2-2 plants 19 h after supplying [3H]IAA (8 kBq per plant) to the cut stem surface. The diagram on the far right shows the numbering of stem segments. Unlabeled auxin (5,000 mg/L in lanolin) was applied to the stem stump before and after supplying the [3H]IAA. Each point is the value for a single segment and each line is a single plant. Data shown are from segments 3 to 12. Data are expressed as a percentage of the radioactivity recovered in the segment with the greatest 3H content within the basipetal wave. Absolute values are given in Table II; n = 5 or 6.
Table I.
Auxin transport in intact WT (cv Parvus), rms1-1, and rms2-2 plants
| Measure of [3H]IAA Movement | Genotype
|
||
|---|---|---|---|
| WT | rms1-1 | rms2-2 | |
| Quantity of radioactivity recovered in segments 2 to 10 (pmol equivalents) | 2.22 ± 0.16 | 4.38 ± 0.57 | 2.91 ± 0.36 |
| Distance of wave maxima (cm) | 9.7 ± 0.9 | 11.1 ± 0.2 | 10.4 ± 0.9 |
| Mean velocity of wave maxima (mm h−1) | 5.4 ± 0.5 | 6.2 ± 0.1 | 5.8 ± 0.5 |
| Stem segment with highest radioactivity | 3.7 ± 0.2 | 4.5 ± 0.2 | 4.3 ± 0.6 |
[3H]IAA (37 kBq; 37 pmol) was supplied to the apical bud of each plant. Plants were harvested after 18 h. Stem segments were numbered basipetally from the apical bud (Fig. 1). The distances of wave maxima are relative to the apical bud. Data are means ± se for the same plants as those represented in Figure 1. n = 5 or 6.
Table II.
Auxin transport in decapitated WT (cv Parvus), rms1-1, and rms2-2 plants
| Measure of [3H]IAA Movement | Genotype
|
||
|---|---|---|---|
| WT | rms1-1 | rms2-2 | |
| Quantity of radioactivity recovered in segments 3 to 12 (pmol plant−1) | 1.06 ± 0.19 | 1.96 ± 0.13 | 1.78 ± 0.11 |
| Distance of wave maxima (cm) | 14.1 ± 0.6 | 16.2 ± 0.3 | 17.7 ± 0.3 |
| Mean velocity of wave maxima (mm h−1) | 7.4 ± 0.3 | 8.4 ± 0.2 | 9.0 ± 0.2 |
| Stem segment with highest radioactivity | 7.2 ± 0.3 | 8.0 ± 0.0 | 9.1 ± 0.2 |
[3H]IAA (8 kBq; 8 pmol) was supplied to the cut stem stump of plants 2 d after decapitation. Plants were harvested 19 h later. Unlabeled auxin (5,000 mg/L in lanolin) was applied to the stem stump before and after supplying the [3H]IAA. Stem segments were numbered basipetally from the stem stump (Fig. 2). The distances of wave maxima are relative to the site of label application. Data are means ± se for the same plants as those represented in Figure 2. n = 5 or 6.
Figure 3.
HPLC radio-histograms of pooled stem segments 2 to 8 from shoots of individual WT (cv Parvus), rms1-1, and rms2-2 plants fed with [3H]IAA, as described in Figure 1. The retention time of IAA is shown by the bar. Two additional replicates for each genotype yielded very similar results.
Auxin Response in Decapitated Plants
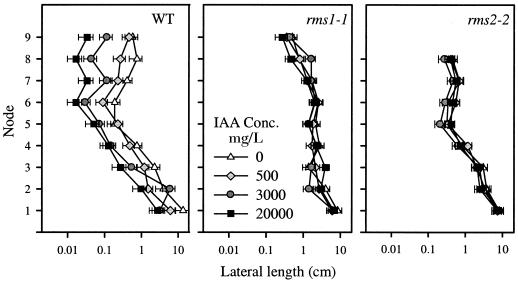
WT (cv Parvus), rms1-1, and rms2-2 plants with eight or nine leaves expanded were decapitated and were treated daily with 0 (control), 500, 3,000, or 20,000 mg/L IAA in lanolin applied to the decapitated stump. All plants had primary axillary buds removed at the time of decapitation. Release and subsequent growth of secondary lateral buds of control plants occurred at all nodes of all genotypes following decapitation (Fig. 4). At some nodes, especially further up the stem, mean lateral shoot lengths were significantly greater in rms1-1 and rms2-2 decapitated control plants than in comparable WT plants. In WT plants, 500 mg/L IAA was sufficient to inhibit bud outgrowth at nodes 2 and 8, and 3,000 mg/L IAA inhibited bud growth at most nodes. However, neither of these auxin treatments had a significant effect on bud growth in mutant plants. Even at 20,000 mg/L, IAA had only a small inhibitory effect on bud growth at any node of rms1-1 and rms2-2 plants (Fig. 4). This highest concentration of IAA caused about a 20-fold or greater inhibition at upper nodes of WT plants, but less than a 2-fold inhibition at similar nodes of rms1-1 and rms2-2 plants. Similar results were obtained from experiments using different mutant alleles (rms1-2 and rms2-1) in other genetic backgrounds (data not shown; Figs. 5 and 6).
Figure 4.
Effect of auxin application on decapitation-induced branching in WT (cv Parvus), rms1-1, and rms2-2 plants. Auxin was supplied to the cut stem stump as IAA in lanolin (approximately 20 μL per plant), and was replaced daily. n = 6 to 12. Data were collected 6 d after decapitation, and are plotted for each node as means ± se on a logarithmic scale.
Figure 5.
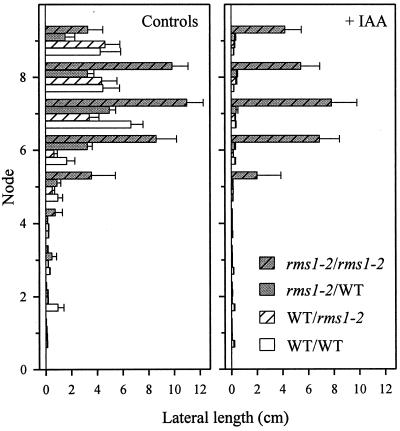
Effect of auxin application on decapitation-induced branching in reciprocally grafted WT (cv Weitor) and rms1-2 plants. Immediately after decapitation, auxin was applied to the cut stem stump as IAA (2,000 mg/L) in lanolin (approximately 20 μL per plant), and was replaced daily. n = 10 to 13. Data were collected 7 d after decapitation, and are plotted for each node as means ± se. Notation is scion/rootstock.
Figure 6.
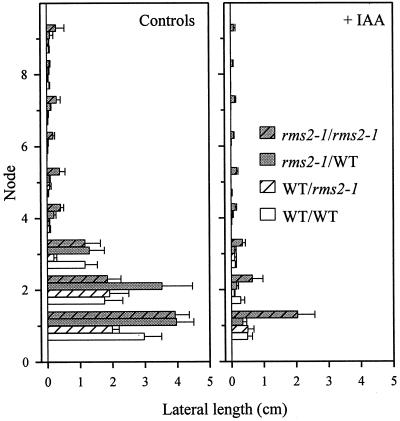
Effect of auxin application on decapitation-induced branching in reciprocally grafted WT (cv Torsdag) and rms2-1 plants. Immediately after decapitation, auxin was applied to the cut stem stump as IAA (2,000 mg/L) in lanolin (approximately 20 μL per plant), and was replaced daily. n = 10. Data were collected 7 d after decapitation, and are plotted for each node as means ± se. Notation is scion/rootstock.
All doses of exogenous IAA above 500 mg/L caused stem swelling in the stump of all genotypes, perhaps due to auxin-induced ethylene synthesis. Particularly at basal nodes, even the highest IAA treatment (20,000 mg/L) did not reduce bud lengths of decapitated WT plants down to that of non-decapitated controls (data not shown; lengths of secondary buds of non-decapitated control plants were always less than 5 mm). Furthermore, the inhibition by exogenous auxin was often associated with bud swelling that did not occur in inhibited buds of intact WT plants.
Auxin Response in Decapitated Grafted Plants
WT (cv Weitor) and rms1-2 reciprocally grafted plants were decapitated (and had primary buds removed) when they had about nine leaves expanded. Following this treatment, bud release and subsequent lateral shoot growth occurred at nodes above node 4 or 5 for all graft combinations without exogenous auxin (Fig. 5). Except at the uppermost node, where lateral lengths were similar for all graft-combinations, lateral lengths above node 4 of rms1-2 self-grafted plants were significantly greater, typically 2-fold, than those of other graft combinations. IAA applied at 2,000 mg/L to the decapitated stump almost completely inhibited bud outgrowth in plants of all graft combinations except for rms1-2 self-grafts (Fig. 5). Lateral lengths were significantly reduced only at node 8 of rms1-2 self-grafted auxin-treated plants. The difference in lateral bud lengths between auxin-treated rms1-2/WT (scion/rootstock) and rms1-2/rms1-2 plants was 12- to 28-fold at the five uppermost nodes. Similar trends were observed for rms1-1 and cv Parvus reciprocally grafted plants (data not shown).
Decapitation (and primary bud removal) above node 9 or 10 of WT (cv Torsdag) and rms2-1 reciprocally grafted plants caused lateral bud outgrowth predominantly at nodes 1 to 3 in all graft combinations (Fig. 6). With the exception of decapitated WT/rms2-1 plants, where lateral lengths tended to be shorter, the extent of lateral bud growth at nodes 1 to 3 was relatively similar among plants of different graft combinations. Auxin application (2,000 mg/L) reduced lateral bud growth at all nodes of all genotypes, but was substantially less effective at inhibiting bud release in rms2-1 self-grafted plants (Fig. 6). The difference in lateral bud lengths between auxin-treated rms2-1 shoots grafted to either WT or rms2-1 rootstocks was 3- to 6-fold at the three basal nodes and was significant at all other nodes. With a different mutant allele, rms2-2, and another background (cv Parvus), auxin again was less effective at inhibiting bud outgrowth in mutant self-grafted plants than in plants of any other graft combination (data not shown).
DISCUSSION
Decapitated rms1 and rms2 mutant plants clearly have a reduced response to exogenous auxin in comparison with decapitated WT plants (Fig. 4). However, the reduced auxin response is probably not a consequence of a direct lesion in auxin reception or signal transduction because mutant shoots have a completely restored auxin response when grafted to WT rootstocks. We have determined that exogenous auxin applied to the decapitated stump interacts with a signal or signals controlled by genes Rms1 and Rms2. These signal(s) may have been supplied by the shoot or rootstock because branching was inhibited by auxin in decapitated shoots of both graft combinations, mutant/WT and WT/mutant (Figs. 5 and 6).
The lateral shoot growth shown for plants of all genotypes (Figs. 4–6) was from secondary axillary buds (Stafstrom and Sarup, 2000) that would have remained inhibited throughout the life of equivalent intact plants. Because intact rms mutants display extensive lateral branching, whereas WTs do not (Beveridge et al., 1997b), removal of the primary axillary buds or shoots from every node at the time of decapitation neatly provided an equalized opportunity for bud outgrowth in mutant and WT shoots. This is demonstrated by the relatively similar decapitation-induced bud outgrowth among control plants of all genotypes and graft combinations. Auxin application enabled a test of the ability of auxin to replace the shoot tip in maintaining bud inhibition in WT and mutant shoots.
There are several reasons why the failure to respond to exogenous auxin in decapitated rms1-1 and rms2-2 plants is unlikely to be due to impaired polar auxin transport. Intact plants of both mutant genotypes exported at least as much exogenous [3H]IAA in the polar transport stream as WT plants, and this auxin moved at least as far over an 18-h period (Tables I and II). The quantity of auxin applied to intact plants (37 pmol) was within normal physiological ranges (typically 60–1,700 pmol/g; Beveridge et al., 1994, 1996, 1997a). The movement of radiolabeled auxin (Figs. 1 and 2) was consistent with the expected characteristics of polar auxin transport, namely a single basipetal wave of radioactivity. The distance traveled by the wave maxima was equivalent to a mean linear velocity of about 5 to 9 mm h−1, similar to that reported previously for pea (Johnson and Morris, 1989; Cambridge and Morris, 1996). Furthermore, a polar auxin transport inhibitor, triiodobenzoic acid, applied in a ring around the stem below the apical bud, inhibited the downward movement of [3H]IAA (data not shown; Johnson and Morris, 1989). As demonstrated by Morris and Johnson (1990), decapitation can result in loss of polar auxin transport, but this loss can be prevented by supplying auxin to the cut stump. It is important to note that the polar transport system was still functional in decapitated auxin-treated plants of mutant and WT genotypes (Fig. 2). [3H]IAA moved slightly further down the stem in decapitated than in intact plants over a similar time interval. Perhaps transfer into the polar transport stream from the cut stump of decapitated plants was more rapid than following application to the intact apical bud. In all genotypes, most of the radioactivity in the polar transport wave was recovered as unmetabolized IAA (Fig. 3), as previously reported by Morris and Johnson (1990). In contrast, a large proportion of the radioactivity remaining near the fed portion (Fig. 1) corresponded to metabolized [3H]IAA (data not shown).
It is interesting to note that, particularly for decapitated plants, slightly more auxin may have moved slightly further in rms1 and rms2 plants than in WT plants (Fig. 2; Tables I and II). Mutant rms1 and rms2 shoot tips also have slightly higher endogenous auxin levels than comparable WT shoot tips (Beveridge et al., 1994, 1997b). Consequently, in comparison with WT plants, it is possible that a greater quantity of auxin is transported from rms1 and rms2 shoot tips through the basipetal transport stream over a given period.
Decapitation in bean and pea can lead to transient increases in cytokinin levels in the xylem sap or shoot, and exogenous auxin application can partially suppress these increases (Bangerth, 1994; Li et al., 1995). However, our studies have shown that Rms1 regulates a rootstock-derived signal other than cytokinin (Beveridge et al., 1997b) that interacts with auxin in decapitated plants. Branching occurs in intact rms1 shoots, even though the root xylem sap already has considerably less zeatin riboside (the major cytokinin in pea xylem sap) than in WT sap (Beveridge et al., 1997b). Other isoprenoid cytokinins are also depleted in root xylem sap of rms1 plants (S.E. Morris, C.A. Beveridge, and C.G.N. Turnbull, unpublished data). Consequently, it is unlikely that Rms1 in the rootstock acts to inhibit bud release by enabling exogenous auxin to reduce xylem sap cytokinin levels even further. Interestingly, rms3 and rms4 shoots also have a reduced response to exogenous auxin (Beveridge, 2000). Like rms1, these mutants already have depleted xylem sap cytokinin levels and normal or elevated shoot auxin levels and transport (Beveridge et al., 1996, 1997a; Beveridge, 2000). It is not yet possible to ascertain whether Rms3 and Rms4 directly affect auxin perception or signal transduction, or whether they control responses to the signals regulated by Rms1 and Rms2.
It is not yet clear whether regulation of branching involves the same signals and processes in decapitated plants as in intact plants (Rubinstein and Nagao, 1976; Napoli et al., 1999). Decapitation-induced branching is a vital recovery mechanism, whereas branching in intact plants is under variable environmental and ontogenetic control. Furthermore, as suggested by Trewavas (1986), a treatment as disruptive as shoot decapitation is likely to influence subsequent development in more ways than simply through loss of an auxin supply (Husain and Linck, 1966; McIntyre and Cessna, 1990; Stahlberg and Cosgrove, 1992; Sherriff et al., 1994). Unlike intact WT plants, where complete bud inhibition is common (i.e. buds remaining 1 mm or less in length), basal buds of decapitated WT plants treated with 20,000 mg/L IAA grew to around 10 mm in length (Fig. 4). This discrepancy may reflect differences in patterns of auxin supply between exogenous and endogenous sources. In decapitated plants, an intermittent exogenous supply was generated by daily replacement of the auxin-lanolin mixture, whereas intact plants presumably have a steady basipetal auxin stream. In some tissues, however, the 20,000 mg/L dose of auxin is likely to have caused auxin levels in WT plants to exceed those normally present in the tissue because the treatment caused phenotypic effects, such as stem swelling, that are associated with high auxin levels but that are not normally associated with bud inhibition in intact WT plants. In addition, attempts to correlate auxin delivery with bud outgrowth have indicated that a higher concentration of auxin is required to inhibit bud outgrowth in decapitated plants than in intact or largely intact plants (Kotov, 1996). However, it is worth considering the question of whether either of the signals regulated by Rms1 and Rms2 may be produced at the shoot tip and hence, like auxin, are affected by decapitation.
In conclusion, we have demonstrated that the effectiveness of exogenous auxin in inhibiting branching is massively diminished in decapitated rms1 and rms2 mutant plants (Fig. 5), and this finding is consistent across four alleles at two loci in three different genetic backgrounds. However, the response to auxin in rms1 and rms2 shoots is fully restored by grafting to WT rootstocks (Figs. 5 and 6). Thus, by separating the location of the gene from its final site of action, we have evidence that Rms1 and Rms2 control one or more mobile signals that interact with auxin in the inhibition of bud outgrowth. The nature of these signals is not yet known, but at least for Rms1, cytokinin is an unlikely candidate.
MATERIALS AND METHODS
Plant Materials
The branching lines used have been described in detail (Arumingtyas et al., 1992; Beveridge et al., 1994, 1997b, and refs. therein). Lines WL5237 (rms1-1) and WL5951 (rms2-2) are derived from cv Parvus, line WL5147 (rms1-2) from cv Weitor, and line K524 (rms2-1) from cv Torsdag, respectively. All lines are tall and photoperiod responsive. The mutations are all recessive and were induced by S. Blixt (Weibullsholm Plant Breeding Institute, Landskrona, Sweden) and K. Sidorova (Institute of Cytology and Genetics, Novosibirsk, Russia) using irradiation or ethyl methane sulfonate.
Growth Conditions and Bud Growth Measurements
Plants were grown under greenhouse conditions with the natural daylength (9–12 h) extended to 16 or 18 h with weak incandescent lighting. The growth medium was as described in Beveridge et al. (1996) or was a mixture of peat:sand:perlite (1:1:1, v/v). Nodes were numbered acropetally with the first scale leaf as node 1. A leaf was considered expanded when the leaflets and stipules were completely unfolded even though further expansion growth followed. Lateral shoot lengths were measured from the leaf axil at the main stem to the estimated position of the axillary shoot apex, to an accuracy of 1 mm. Epicotyl wedge grafts were performed at d 6 or 7 as described by Beveridge et al. (1994). Genotypes of graft combinations are given as scion/rootstock.
Auxin Transport
Auxin transport was measured in plants supplied with [5(n)-3H]IAA; specific activity 1015 Bq mol−1 (Amersham International, Buckinghamshire, UK). For intact plants with six to seven leaves expanded, 37 kBq (37 pmol) of [3H]IAA in 5 μL of ethanol was applied to the apical bud inside the stipules of the leaf two nodes above the highest expanded leaf.
Eighteen hours after treatment with [3H]IAA, internode lengths were measured and the stem was divided into sections (Fig. 1) that were then placed into liquid nitrogen. Sections included the apical bud and the oldest unexpanded leaf, the node at the highest expanded leaf, the two lower nodes, and the internodes adjacent to these nodes (Fig. 1). Internodes were divided into two sections of equal length and leaves and stipules were removed from all but the fed portions. Each section was ground individually under liquid nitrogen, suspended in 7 mL of methanol:water (4:1, v/v), then shaken gently at 4°C for at least 18 h to facilitate extraction. Aliquots were mixed with liquid scintillation cocktail and analyzed in a liquid scintillation counter. As the region of stem containing the basipetal [3H]IAA wave maximum may have fallen equally or disproportionately within two adjacent stem sections, the exact distance of the wave maximum from the apical bud was interpolated for each plant from adjacent data points (Table I).
Similar experiments were conducted with decapitated plants. The primary bud at each node was removed prior to decapitation. Plants were decapitated below the highest expanded leaf (nodes 7–9) and pretreated daily with IAA (5,000 mg/L in lanolin) applied to the cut shoot stump for 2 d prior to supplying [3H]IAA (8.8 kBq, 8.8 pmol per plant). Label was applied to the stem stump in 3 μL of ethanol after cutting off an additional 1 cm of stem to remove the unlabeled auxin and lanolin. Ten minutes after adding the labeled auxin, unlabeled auxin in lanolin was again placed on the stump. Plants were harvested after 19 h and extracted essentially as described above for intact plants.
To determine the extent of metabolism of the [3H]IAA transported from the apical bud, [3H]IAA was applied to intact plants as above. After 18 h, the fed portion (apical bud) was discarded, and the stem sections without stipules or leaves were harvested, quickly frozen in liquid nitrogen, and stored at −20°C. Extracts were prepared as above, except that the extraction volume was 10 mL and contained 0.25 mg mL−1 butylated hydroxytoluene. Extracts were fractionated by reversed phase HPLC as described by Hasan et al. (1994). Solvents were methanol and distilled water containing 0.4% (v/v) acetic acid; the solvent program ran from 20% to 55% (v/v) methanol over 40 min and then from 55% to 100% (v/v) methanol over 5 min at a flow rate of 2 mL min−1. The retention time of [3H]IAA was between 17 and 18 min. The 3H content of individual 1-min HPLC fractions was analyzed by scintillation counting.
Auxin Response in Decapitated Ungrafted and Grafted Plants
The response of axillary buds to exogenous auxin was tested following removal of the apical bud. To avoid complications due to differing degrees of lateral bud outgrowth in WT and mutant plants prior to auxin application, the primary lateral bud was removed from all nodes of all plants prior to decapitation. Decapitation of these plants resulted in significant secondary lateral bud release in all genotypes, including WT. These secondary lateral buds are adjacent to the primary bud in the leaf axil of the main stem. Before decapitation, secondary buds at upper nodes are usually microscopic. At nodes 1 and 2, secondary buds may be up to 1 mm long. All such buds, including those of mutant plants, would have remained inhibited if the plants were left completely intact. After dissolving IAA in ethanol, various concentrations were prepared as mixtures with lanolin:ethanol (92:8). Approximately 20 μL of the auxin-lanolin mixture was applied daily to the decapitated internode (stump) above the highest expanded leaf.
ACKNOWLEDGEMENTS
We are grateful for helpful comments from Drs. John Ross and Ian Dodd and we thank Kathy Crew and John Finlay for technical assistance. We also thank J. Hendrikz for advice on statistical analysis and calculations for the auxin transport experiments.
Footnotes
This work was supported by grants from the Australian Research Council and by a University of Queensland Enabling Grant. G.M.S. was supported by an Australian Postgraduate Award.
LITERATURE CITED
- Arumingtyas EL, Floyd RS, Gregory MJ, Murfet IC. Branching in Pisum: inheritance and allelism tests with 17 ramosus mutants. Pisum Genet. 1992;24:17–31. [Google Scholar]
- Bangerth F. Response of cytokinin concentration in the xylem exudate of bean (Phaseolus vulgaris L.) plants to decapitation and auxin treatment, and relationship to apical dominance. Planta. 1994;194:439–442. [Google Scholar]
- Beveridge CA (2000) Long-distance signalling and a mutational analysis of branching in pea. Plant Growth Regul (in press)
- Beveridge CA, Murfet IC, Kerhoas L, Sotta B, Miginiac E, Rameau C. The shoot controls zeatin riboside export from pea roots: evidence from the branching mutant rms4. Plant J. 1997a;11:339–345. [Google Scholar]
- Beveridge CA, Ross JJ, Murfet IC. Branching mutant rms-2 in Pisum sativum: grafting studies and endogenous indole-3-acetic acid levels. Plant Physiol. 1994;104:953–959. doi: 10.1104/pp.104.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge CA, Ross JJ, Murfet IC. Branching in pea: action of genes Rms3 and Rms4. Plant Physiol. 1996;110:859–865. doi: 10.1104/pp.110.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge CA, Symons GM, Murfet IC, Ross JJ, Rameau C. The rms1 mutant of pea has elevated indole-3-acetic acid levels and reduced root sap zeatin riboside content but increased branching controlled by graft transmissible signal(s) Plant Physiol. 1997b;15:1251–1258. [Google Scholar]
- Cambridge AP, Morris DA. Transfer of exogenous auxin from the phloem to the polar auxin transport pathway in pea (Pisum sativum L.) Planta. 1996;199:583–588. [Google Scholar]
- Chaudhury AM, Letham S, Craig S, Dennis ES. amp1: a mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. Plant J. 1993;4:907–916. [Google Scholar]
- Hasan O, Ridoutt BG, Ross JJ, Davies NW, Reid JB. Identification and quantification of endogenous gibberellins in apical buds and the cambial region of Eucalyptus. Physiol Plant. 1994;90:475–480. [Google Scholar]
- Hosokawa Z, Shi L, Prasad TK, Cline MG. Apical dominance control in Ipomoea nil: the influence of the shoot apex, leaves and stem. Ann Bot. 1990;65:547–556. [Google Scholar]
- Husain SM, Linck AJ. Relationship of apical dominance to the nutrient accumulation pattern in Pisum sativum var. Alaska Physiol Plant. 1966;19:992–1010. [Google Scholar]
- Johnson CF, Morris DA. Applicability of the chemiosmotic polar diffusion theory to the transport of indol-3yl-acetic acid in the intact pea (Pisum sativum L.) Planta. 1989;178:242–248. doi: 10.1007/BF00393200. [DOI] [PubMed] [Google Scholar]
- Klee H, Estelle M. Molecular approaches to plant hormone biology. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:529–551. [Google Scholar]
- Kotov AA. Indole-3-acetic acid transport in apical dominance: a quantitative approach: influence of endogenous and exogenous IAA apical source on inhibitory power of IAA transport. Plant Growth Regul. 1996;19:1–5. [Google Scholar]
- Li CJ, Guevara E, Gerrera J, Bangerth F. Effect of apex excision and replacement by 1-naphthylacetic acid on cytokinin concentration and apical dominance in pea plants. Physiol Plant. 1995;94:465–469. [Google Scholar]
- McIntyre GI, Cessna AJ. Apical dominance in Phaseolus vulgaris: effect of nitrogen supply. Can J Bot. 1990;69:1337–1343. [Google Scholar]
- Medford JI, Horgan R, El-Sawi Z, Klee HJ. Alterations of endogenous cytokinins in transgenic plants using a chimeric isopentenyl transferase gene. Plant Cell. 1989;1:403–413. doi: 10.1105/tpc.1.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DA. Transport of exogenous auxin in two-branched dwarf pea seedlings (Pisum sativum L.): some implications for polarity and apical dominance. Planta. 1977;136:91–96. doi: 10.1007/BF00387930. [DOI] [PubMed] [Google Scholar]
- Morris DA, Johnson CF. The role of auxin efflux carriers in the reversible loss of polar auxin transport in the pea (Pisum sativum L.) stem. Planta. 1990;181:117–124. doi: 10.1007/BF00202333. [DOI] [PubMed] [Google Scholar]
- Napoli CA. Highly branched phenotype of the petunia dad1-1 mutant is reversed by grafting. Plant Physiol. 1996;111:27–37. doi: 10.1104/pp.111.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli CA, Beveridge CA, Snowden KC. Re-evaluating concepts of apical dominance and the control of axillary bud outgrowth. Curr Topics Dev Biol. 1999;44:127–169. doi: 10.1016/s0070-2153(08)60469-x. [DOI] [PubMed] [Google Scholar]
- Romano CP, Hein MB, Klee HJ. Inactivation of auxin in tobacco transformed with the indoleacetic acid-lysine synthetase gene of Pseudomonas savastanoi. Genes Dev. 1991;5:438–446. doi: 10.1101/gad.5.3.438. [DOI] [PubMed] [Google Scholar]
- Rubinstein B, Nagao MA. Lateral bud outgrowth and its control by the apex. Bot Rev. 1976;42:83–113. [Google Scholar]
- Sachs T, Thimann KV. Release of lateral buds from apical dominance. Nature. 1964;201:939–940. [Google Scholar]
- Sachs T, Thimann KV. The role of auxins and cytokinins in the release of buds from apical dominance. Amer J Bot. 1967;45:136–144. [Google Scholar]
- Sherriff LJ, McKay M, Ross JJ, Reid JB, Willis CL. Decapitation reduces the metabolism of gibberellin A20 to A1 in Pisum sativum L., decreasing the Le/le difference. Plant Physiol. 1994;104:227–280. doi: 10.1104/pp.104.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow R. On the nature of correlative inhibition. New Phytol. 1937;36:283–300. [Google Scholar]
- Stafstrom JP, Sarup VB. Development of supernumerary buds from the axillary meristem of pea Pisum sativum (Fabaceae) Aust J Bot. 2000;48:271–278. [Google Scholar]
- Stahlberg R, Cosgrove DJ. Rapid alterations in growth rate and electrical potentials upon stem excision in pea seedlings. Planta. 1992;187:523–531. doi: 10.1007/BF00199972. [DOI] [PubMed] [Google Scholar]
- Thimann KV, Skoog F. Studies on the growth hormone in plants: III. The inhibiting action of growth substances on bud development. Proc Natl Acad Sci USA. 1933;19:714–716. doi: 10.1073/pnas.19.7.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann KV, Skoog F. On the inhibition of bud development and other functions of growth substances in Vicia faba. Proc Roy Soc London Ser B. 1934;114:317–339. [Google Scholar]
- Trewavas A. Understanding the control of plant development and the role of growth substances. Aust J Plant Physiol. 1986;13:447–457. [Google Scholar]