Abstract
Background
Laboratory-based evidence of coagulopathy is observed in 25–35% of trauma patients, but clinically-evident coagulopathy (CC) is not well described.
Methods
Prospective observational study of adult trauma patients transported by helicopter from the scene to nine Level 1 trauma centers in 2015. Patients meeting predefined highest-risk criteria who survived ≥30 minutes after hospital arrival were divided into groups based on observation of CC, predefined as surgeon-confirmed bleeding from uninjured sites or injured sites not controllable by sutures. We used A mixed-effects, Poisson regression with robust error variance (controlling for age, injury Severity core(ISS), prehospital transfusions, ED Systolic Blood Presure (SBP), and ED base excess as fixed effects and site as a random effect) to test the hypothesis that abnormalities on rapid thrombelastography (r-TEG) and international normalized ratio (INR) were independently associated with CC+.
Results
Of 1,019 highest-risk patients, CC+ (n=41, 4%) were more severely injured (median ISS 32 vs 17), had increased laboratory-based coagulopathy on r-TEG and INR, received more transfused blood products at 4 hours (37 vs 0 units), and had greater 30-day mortality (59% vs 12%) than CC− (n=978, 96%). The overall incidence of laboratory-based coagulopathy was 39%. 30-day mortality was 22% vs 9% in those with and without laboratory-based coagulopathy. In two separate models, r-TEG K-time >2.5 min (RR 1.3, 95% CI 1.1–1.7), r-TEG mA <55 mm (RR 2.5, 95% CI 2.0–3.2), platelet count <150 × 109/L (RR 1.2, 95% CI 1.1–1.3), and INR >1.5 (RR 5.4, 95% CI 1.8–16.3) were independently associated with CC+. A combined regression model could not be generated, because too few patients underwent both r-TEG and INR.
Conclusion
CC was rare compared to laboratory-based coagulopathy and associated with poor outcomes. Several components of coagulation, both clotting factor and platelet-mediated, were associated with CC, underscoring the complexity of the disease.
TOC Statement
We found that clinically evident coagulopathy (CC) was rare compared to laboratory-evidence of coagulopathy after trauma and identified abnormalities on TEG associated with CC. This increases our understanding of trauma-induced coagulopathy.
Introduction
Injury is the third leading cause of death overall and the leading cause of death in those aged 44 years or younger in the United States.1 In 1969, Simmons et al first reported an association between shock, coagulopathy, and severe injury in combat casualties during the Vietnam War.2 This trauma-induced coagulopathy (TIC) has become the focus of intensive research efforts, although its etiology remains elusive.3 TIC is associated with increased hemorrhage, transfusion requirements, and mortality,4,5 and is independent of traditional iatrogenic causes of post-traumatic coagulopathy such as hemodilution. Between 25% and 35% of civilian and military trauma patients4,5,6 will present with laboratory-based evidence of TIC despite a profound up-regulation in procoagulant mechanisms after injury.7 When unbalanced resuscitation and unrestrained use of crystalloid were the norm, clinical coagulopathy was common. With the change in resuscitation over the last decade, most bleeding after trauma is not coagulopathic and responds readily to surgical techniques, including compression, vessel ligation, and embolization. Clinically-evident coagulopathic bleeding (CC) such as diffuse oozing from injured and uninjured sites is not responsive to such maneuvers and is now uncommon. The relationship between clinical coagulopathic bleeding and laboratory-based evidence of coagulopathy, however, is unclear.
Whereas conventional coagulation tests, such as prothrombin time/international normalized ratio (PT/INR) and partial thromboplastin time (PTT) interrogate, only a small fraction of the coagulation system, viscoelastic whole blood coagulation assays such as thrombelastography (TEG) provide information on multiple facets of coagulation, including clotting factor activity, platelet function, the thrombin burst, and fibrinolysis.8 Abnormalities in the TEG are associated with poor outcome, and enable the targeted use of blood products to correct specific coagulation deficiencies.9,10 Our objectives were therefore two-fold: first, to describe the phenotype of CC, and then to identify specific laboratory coagulation abnormalities which were independently associated with development of CC after trauma.
Methods
Study subjects
This is a secondary analysis of the Prehospital Resuscitation on Helicopters Study (PROHS, clinicaltrials.gov identifier: NCT02272465), which was a prospective, observational study of adult trauma patients transported by helicopter from the scene to nine Level 1 trauma centers in 2015. The study protocol was approved by the institutional review board at all participating sites (University of Texas Health Science Center, Houston, TX; University of Cincinnati, Cincinnati, OH; Mayo Clinic, Rochester, MN; Oregon Health and Science University, Portland, OR; University of Washington, Seattle, WA; University of Maryland, Baltimore, MD; University of Southern California, Los Angeles, CA; University of Alabama, Birmingham, AL; University of Arizona, Tucson, AZ), and patient data were handled in compliance with the Health Insurance Portability and Accountability Act (HIPPA). The highest-risk subset was predefined as those meeting one of the following criteria during prehospital care: heart rate >120 beats per min (bpm), systolic blood pressure (SBP) ≤90 mmHg, penetrating truncal injury, tourniquet application, pelvic binder application, intubation, or pre-hospital transfusion. Prisoners were excluded from enrollment. We also excluded patients who expired within 30 minu from the current study, because these patients expired before operative exploration could be performed (limiting the ability to determine CC+ or CC−) and because of a large proportion of missing data (>20%) in these patients.
Specimens and measurements
Admission blood samples were taken from all highest-risk patients for routine laboratory analysis on arrival to the emergency department (ED) and included complete blood count, venous blood gas, and lactate level. Coagulation tests, such as rapid TEG (r-TEG), kaolin TEG (k-TEG), PT, PTT, and fibrinogen level, were performed according to individual institutional practice. TEGs were performed on citrated whole blood using TEG 5000 Thrombelastograph Hemostasis Analyzer systems (Haemonetics Corporation, Braintree, MA) according to manufacturer specifications. Holcomb et al have demonstrated previously that abnormalities on r-TEG predicted need for transfusions as well as or better than conventional coagulation tests but had a much faster time to obtain the result.9
Outcomes and definitions
Active resuscitation was performed on patients who received blood products during the prehospital phase or within 3 hours of admission. At the end of active resuscitation, research staff asked the attending trauma surgeon if CC had been observed at any previous point. CC was predefined as surgeon-confirmed bleeding from uninjured sites or injured sites not controllable by sutures, which was agreed on by all participating centers and codified in the manual of operations. Active resuscitation was ended when the patient was resuscitated adequately, the patient expired, or further care was deemed futile. Patients who did not require active resuscitation were assumed to be CC−, because these patients were not bleeding. We used cutoffs previously published to define abnormal r-TEG (ACT >128 s, K-time > 2.5min, angle <56°, mA <55mm, LY30 >3%) and abnormal INR (>1.5), PTT (> 35 s), and platelet count (<150 × 109/L).9 To our knowledge, there are no published cutoffs for abnormal k-TEG values in the setting of trauma.
Statistical analysis
Continuous data are expressed as medians with interquartile ranges. Non-parametric comparisons of continuous data were performed by Wilcoxon rank-sum test. Comparisons of categorical data were performed by Chi-squared test or Fisher’s exact test for categories with five or fewer members. We used a mixed-effects, Poisson regression with robust error variance, which has been used previously to estimate relative risks of dichotomous outcomes,11 to identify abnormalities in laboratory coagulation tests (r-TEG parameters and INR) which were associated with CC+. We controlled for age, iInjury Severity core (ISS), prehospital transfusions, admission SBP, and admission base excess as fixed effects. To account for inter-site variation, we included study site as a random effect. These covariates were chosen for two reasons. First, increased injury severity and increased hemorrhage are broadly correlated with increased degree of TIC,3 although the exact mechanistic etiologies are unclear. Second, these variables had an acceptable degree of missingness (all <5%). Stata 14.1 (Stata Corporation, College Station, TX) was used for all statistical calculations.
Results
Demographic and baseline data
A total of 25,118 trauma patients were admitted during the 10-month enrollment period to the nine participating centers, of whom 2,341 arrived by helicopter, and 1,058 met the highest-risk criteria. Thirty-nine (4%) died within 30 minutes of arrival, leaving 1,019 patients for analysis, of whom 340 (33%) underwent active resuscitation. There were 41 (4%) CC+ patients and 978 (96%) CC− patients. The diagnosis of CC+ varied between centers (p<0.05), ranging in incidence from 0% to 22%. Demographic, prehospital, and emergency department (ED) data are summarized in Table 1. CC+ patients were older, more severely injured, hypotensive, with more deranged base excess, and more likely to have received prehospital blood products compared to CC− patients.
Table 1.
Demographics, prehospital, and ED (admission) parameters (n=1,019).
| DEMOGRAPHICS | CC+ (n=41, 4%) | CC− (n=978, 96%) | p |
|---|---|---|---|
|
| |||
| Age (years) | 52 (27, 67) | 37 (25, 55) | 0.03 |
|
| |||
| Male (n, %) | 33 (80%) | 689 (70%) | 0.17 |
|
| |||
| Injury type (n, %) | 0.09 | ||
| Blunt only | 28 (68%) | 792 (81%) | |
| Penetrating only | 12 (30%) | 165 (17%) | |
| Both | 1 (2%) | 21 (2%) | |
|
| |||
| AIS head/neck ≥3 (n, %) | 21 (51%) | 371 (38%) | 0.09 |
|
| |||
| AIS chest ≥3 (n, %) | 20 (49%) | 366 (37%) | 0.14 |
|
| |||
| AIS abd ≥3 (n, %) | 18 (44%) | 168 (17%) | <0.001 |
|
| |||
| AIS extrem ≥3 (n, %) | 13 (32%) | 265 (27%) | 0.52 |
|
| |||
| ISS | 32 (25, 41) | 17 (8, 27) | <0.001 |
|
| |||
| PREHOSPITAL | |||
|
| |||
| Systolic blood pressure (mmHg) | 108 (88, 127) | 127 (106, 147) | <0.001 |
|
| |||
| HR (bpm) | 99 (85, 126) | 100 (83, 122) | 0.44 |
|
| |||
| GCS | 3 (3, 8) | 12 (3, 15) | <0.001 |
|
| |||
| Crystalloid (ml) | 100 (0, 500) | 250 (0, 800) | 0.18 |
|
| |||
| Transfused prehospital RBCs (n, %) | 18 (44%) | 82 (8%) | <0.001 |
|
| |||
| Transfused prehospital plasma (n, %) | 18 (44%) | 104 (11%) | <0.001 |
|
| |||
| ED | |||
|
| |||
| Systolic blood pressure (mmHg) | 100 (80, 125) | 130 (110, 145) | <0.001 |
|
| |||
| Heart rate (bpm) | 104 (89, 128) | 99 (80, 118) | 0.10 |
|
| |||
| Temperature (°C) | 36.2 (35.7, 36.5) | 36.5 (36.1, 36.8) | <0.01 |
|
| |||
| GCS | 3 (3, 8) | 9 (3, 15) | <0.001 |
|
| |||
| pH | 7.22 (7.04, 7.33) | 7.30 (7.23, 7.36) | <0.001 |
|
| |||
| Base excess (mEq/L) | −5 (−12, −3) | −4 (−7, −1) | 0.02 |
|
| |||
| Hemoglobin (g/dL) | 12.0 (10.3, 12.9) | 13.2 (11.7, 14.5) | <0.001 |
|
| |||
| Platelet count (x109/L) | 181 (140, 279) | 230 (184, 278) | 0.07 |
|
| |||
| Emergent (≤90 minutes) OR or IR (n, %) | 25 (81%) | 147 (35%) | <0.001 |
AIS, abbreviated injury scale; ISS, injury severity score; GCS, Glasgow coma score; RBC, red blood cells.
Admission coagulation tests were performed according to each institutional practice and varied considerably between centers. Of the 9 participating centers, INR was performed in 88%–94% of patients at 6 centers, PTT was performed in 79%–94% of patients at 5 centers, fibrinogen was performed in 22%–79% of patients at 3 centers, r-TEG was performed in 85%–95% of patients at 2 centers, and k-TEG was performed in 22%–77% of patients at 2 centers. On k-TEG, CC+ patients had greater R-time (CC+: median 4.2, IQR 3.5–5.6 vs CC−: median 3.3, IQR 2.7–4.0 min, p<0.05) although mA was higher (CC+: median 68.4, IQR 65.2–74.4 vs CC−: median 63.8, IQR 60.2–68.0 mm, p=0.02). There were no differences in K-time, angle, or LY30. Of 774 patients who had r-TEG, PT/INR, and/or PTT performed, 305 patients (39%) had at least one laboratory-based abnormality of coagulation.
Transfusion and mortality data
CC+ patients required increased transfusions of all blood products by a substantial margin and had increased mortality at all time points (all p<0.001) (Table 2). Times to death between CC+ and CC− groups were similar. Although the primary cause of death was most commonly TBI for both groups, exsanguination and TBI death were both more common in the CC+ group. Mortality of CC+ and CC− patients was 59% versus 12%, while mortality of those with and without laboratory-based evidence of coagulopathy was 22% versus 9%.
Table 2.
Outcomes (n=1,019).
| Variable | CC+ (n=41, 4%) | CC− (n=978, 96%) | p |
|---|---|---|---|
| Time to end of resuscitation* | 160 (101, 377) | 107 (39, 210) | <0.01 |
| RBCs at 4 h (units) | 9 (3, 18) | 0 (0, 1) | <0.001 |
| Plasma at 4 h (units) | 7 (3, 15) | 0 (0, 1) | <0.001 |
| Platelets at 4 h (units) | 12 (6, 18) | 0 (0, 0) | <0.001 |
| Cryoprecipitate at 4 h (units) | 0 (0, 10) | 0 (0, 0) | <0.001 |
| Total products at 4 h (units) | 21 (8, 50) | 0 (0, 2) | <0.001 |
| RBCs at 24 h (units) | 10 (4, 20) | 0 (0, 2) | <0.001 |
| Plasma at 24 h (units) | 9 (3, 16) | 0 (0, 1) | <0.001 |
| Platelets at 24 h (units) | 18 (6, 24) | 0 (0, 0) | <0.001 |
| Cryoprecipitate at 24 h (units) | 0 (0, 10) | 0 (0, 0) | <0.001 |
| Total products at 24 h (units) | 37 (9, 54) | 0 (0, 4) | <0.001 |
| Death at 4 h (n, %) | 7 (17%) | 24 (2%) | <0.001 |
| Death at 24 h (n, %) | 16 (39%) | 61 (6%) | <0.001 |
| Death at 30 days (n, %) | 24 (59%) | 113 (12%) | <0.001 |
| Time to death (h) | 8 (3, 31) | 19 (5, 108) | 0.13 |
| Primary cause of death | |||
| Exsanguination (n, %) | 8 (20%) | 13 (1%) | <0.001 |
| TBI (n, %) | 14 (34%) | 81 (8%) | <0.001 |
| Cardiovascular (n, %) | 0 (0%) | 4 (<1%) | 0.85 |
| Sepsis/MOF (n, %) | 1 (2%) | 7 (<1%) | 0.28 |
| Respiratory (n, %) | 0 (0%) | 1 (<1%) | 1.00 |
| Other/Unknown (n, %) | 1 (2%) | 7 (<1%) | 0.28 |
Data for 41 CC+ and 299 CC− patients who underwent active resuscitation.
RBCs, red blood cells; TBI, traumatic brain injury; MOF, multiple organ failure.
Laboratory evidence of coagulopathy: INR
Demographics, baseline, and outcomes data for patients with admission INR data (n=459) are summarized in Table 3. CC+ patients (n=18; 4%) were more severely injured, had an increased INR, and received more blood products at all time points compared to CC− (n=441, 96%) patients, but, there were no differences in mortality or cause of death. A mixed-effects, Poisson regression controlling for age, ISS, prehospital transfusions, admission SBP, and admission base excess as fixed effects and study site as a random effect found that abnormal INR (>1.5) (relative risk [RR] 5.36, 95% confidence interval [CI] 1.77–16.26) and abnormal platelet count (<150 × 109/L) (RR 1.63, 95% CI 1.40–2.36) were significantly associated with CC+. In 373 patients who had admission INR and PTT data, abnormal PTT (>35) was not independently associated with CC+ (RR 2.27, 95% CI 0.83–6.22).
Table 3.
Demographics, baseline, and outcomes data for patients with admission INR (n=459)
| DEMOGRAPHICS | CC+ (n=18, 4%) | CC− (n=441, 96%) | p |
|---|---|---|---|
|
| |||
| Age (years) | 59 (27, 77) | 43 (26, 57) | 0.05 |
|
| |||
| Male (n, %) | 13 (72%) | 318 (72%) | 0.99 |
|
| |||
| Injury type (n, %) | 0.45 | ||
| Blunt only | 13 (72%) | 365 (83%) | |
| Penetrating only | 4 (22%) | 66 (15%) | |
| Both | 1 (6%) | 10 (2%) | |
|
| |||
| ISS | 30 (19, 38) | 20 (10, 29) | <0.01 |
|
| |||
| PREHOSPITAL FLUIDS | |||
|
| |||
| Crystalloid infusion (ml) | 325 (100, 700) | 600 (250, 1100) | 0.07 |
|
| |||
| Transfused prehospital RBCs (n, %) | 7 (39%) | 28 (6%) | <0.001 |
|
| |||
| Transfused prehospital plasma (n, %) | 8 (44%) | 34 (8%) | <0.001 |
|
| |||
| ED | |||
|
| |||
| Systolic blood pressure (mm Hg) | 120 (84, 148) | 130 (109, 148) | 0.30 |
|
| |||
| Heart rate (bpm) | 101 (79, 125) | 99 (81, 115) | 0.84 |
|
| |||
| pH | 7.31 (7.19, 7.35) | 7.31 (7.24, 7.37) | 0.32 |
|
| |||
| Base excess (mEq/L) | −4 (−7.5, −3) | −3.5 (−6.6, −1.3) | 0.36 |
|
| |||
| Hemoglobin (g/dL) | 11.6 (9.9, 12.8) | 13 (11.6, 14.3) | 0.03 |
|
| |||
| Platelet count (x 109/L) | 198 (150, 321) | 223 (181, 269) | 0.83 |
|
| |||
| INR | 1.1 (1.0, 1.3) | 1.4 (1.1, 2.0) | <0.01 |
|
| |||
| INR > 1.5 | 8 (44%) | 51 (12%) | <0.001 |
|
| |||
| Emergent (≤90 minutes) OR or IR (n, %) | 11 (61%) | 75 (17%) | <0.001 |
|
| |||
| OUTCOMES | |||
|
| |||
| Total blood products at 4 hours (units) | 15 (4, 30) | 0 (0, 3) | <0.001 |
|
| |||
| Total blood products at 24 hours (units) | 17 (4, 45) | 0 (0, 5) | <0.001 |
|
| |||
| Death at 4 hours (n, %) | 0 (0%) | 5 (1%) | 0.65 |
|
| |||
| Death at 24 hours (n, %) | 1 (6%) | 25 (6%) | 0.98 |
|
| |||
| Death at 30 days (n, %) | 5 (28%) | 51 (12%) | 0.06 |
|
| |||
| Primary cause of death | |||
|
| |||
| Exsanguination (n, %) | 1 (6%) | 5 (1%) | 0.21 |
|
| |||
| TBI (n, %) | 4 (22%) | 35 (8%) | 0.06 |
|
| |||
| Sepsis/MOF (n, %) | 0 (0%) | 5 (1%) | 1.00 |
|
| |||
| Other/Unknown (n, %) | 0 (0%) | 6 (1%) | 1.00 |
Laboratory evidence of coagulopathy: r-TEG
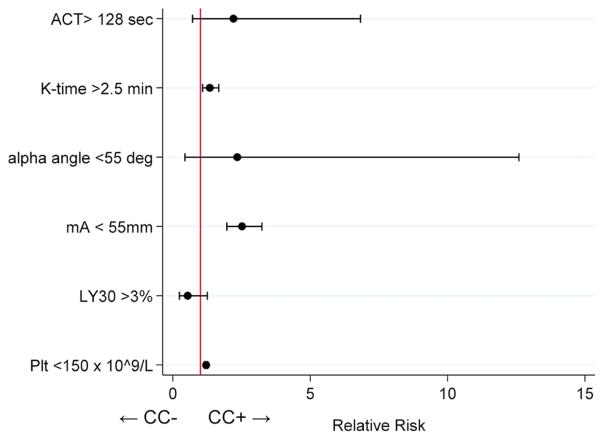
Demographics, baseline, and outcomes data for patients with admission r-TEG data (n=391) are summarized in Table 4. CC+ (n=22, 6%) patients were more severely injured, received more blood products at all time points, and had increased mortality compared to CC− (n=369, 94%) patients. Admission r-TEG parameters are summarized in Table 5. CC+ patients had greater ACT and K-times, as well as well as diminished angle and mA consistent with increased coagulopathy. The LY30 was not different. After dichotomizing all r-TEG parameters based on previously published cutoffs,9 86% of CC+ patients had at least one abnormality on r-TEG compared to 59% of CC− patients (p=0.01). After controlling for the same covariates as above, we found that abnormal K-time (<2.5 min) (RR 1.34, 95% CI 1.08–1.67), abnormal mA (<55 mm) (RR 2.52, 95% CI 1.96–3.24), and abnormal platelet count (<150 × 109/L) (RR 1.21, 95% CI 1.15–1.27) were significantly associated with CC+ (Figure 1). Only 76 patients had both admission r-TEG and INR performed (with only 5 CC+), precluding a combined model with both r-TEG parameters and INR.
Table 4.
Demographics, baseline, and outcomes for patients with admission r-TEG (n=391)
| DEMOGRAPHICS | CC+ (n=22, 6%) | CC− (n=369, 94%) | p |
|---|---|---|---|
|
| |||
| Age (years) | 48 (29, 61) | 34 (25, 51) | 0.09 |
|
| |||
| Male (n, %) | 19 (86%) | 259 (70%) | 0.10 |
|
| |||
| Injury type (n, %) | 0.52 | ||
| Blunt only | 15 (68%) | 286 (77%) | |
| Penetrating only | 7 (32%) | 81 (22%) | |
| Both | 0 (0%) | 3 (1%) | |
|
| |||
| ISS | 30 (25, 38) | 19 (9, 29) | <0.001 |
|
| |||
| PREHOSPITAL FLUIDS | |||
|
| |||
| Crystalloid infusion (ml) | 0 (0, 300) | 0 (0, 270) | 0.78 |
|
| |||
| Transfused prehospital RBCs (n, %) | 0 (0, 1) | 0 (0, 0) | 0.03 |
|
| |||
| Transfused prehospital plasma (n, %) | 1 (0, 1) | 0 (0, 0) | <0.01 |
|
| |||
| ED | |||
|
| |||
| Systolic blood pressure (mm Hg) | 102 (80, 130) | 120 (102, 140) | 0.02 |
|
| |||
| Heart rate (bpm) | 108 (89, 129) | 102 (86, 122) | 0.36 |
|
| |||
| pH | 7.20 (6.99, 7.31) | 7.27 (7.21, 7.35) | <0.01 |
|
| |||
| Base excess (mEq/L) | −6 (−13, −4) | −4 (−7, −1) | 0.01 |
|
| |||
| Hemoglobin (g/dL) | 12.4 (10.5, 13.0) | 13.6 (12.1, 14.8) | <0.01 |
|
| |||
| Platelet count (x 109/L) | 180 (127, 258) | 240 (192, 290) | <0.01 |
|
| |||
| Emergent (≤90 minutes) OR or IR (n, %) | 13 (59%) | 70 (19%) | <0.001 |
|
| |||
| OUTCOMES | |||
|
| |||
| Total blood products at 4 hours (units) | 28 (8, 60) | 0 (0, 4) | <0.001 |
|
| |||
| Total blood products at 24 hours (units) | 37 (9, 70) | 0 (0, 6) | <0.001 |
|
| |||
| Death at 4 hours (n, %) | 5 (23%) | 9 (2%) | <0.001 |
|
| |||
| Death at 24 hours (n, %) | 12 (55%) | 20 (5%) | <0.001 |
|
| |||
| Death at 30 days (n, %) | 18 (82%) | 43 (12%) | <0.001 |
|
| |||
| Primary cause of death | |||
|
| |||
| Exsanguination (n, %) | 5 (23%) | 2 (<1%) | <0.001 |
|
| |||
| TBI (n, %) | 12 (55%) | 35 (9%) | <0.001 |
|
| |||
| Sepsis/MOF (n, %) | 1 (5%) | 2 (<1%) | 0.16 |
|
| |||
| Other/Unknown (n, %) | 0 (0%) | 4 (1%) | 1.00 |
ISS, injury severity score; GCS, Glasgow coma score; TBI, traumatic brain injury; MOF, multiple organ failure.
Table 5.
Rapid TEG parameters (n=391).
| Variable | CC+ (n=22, 6%) | CC− (n=369, 94%) | P |
|---|---|---|---|
| ACT (s) | 128 (113, 191) | 113 (105, 121) | <0.01 |
| K-time (min) | 2.1 (1.5, 2.7) | 1.4 (1.1, 1.8) | <0.001 |
| Angle (degrees) | 67 (52, 73) | 74 (69, 77) | <0.001 |
| mA (mm) | 54 (46, 61) | 64 (58, 68) | <0.001 |
| LY30 (%) | 1.1 (0, 3.2) | 1.3 (0.3, 3.3) | 0.64 |
| Abnormal ACT (>128 s) | 10 (45%) | 50 (14%) | <0.001 |
| Abnormal K-time (>2.5 min) | 8 (38%) | 105 (28%) | 0.34 |
| Abnormal angle (<56°) | 6 (27%) | 12 (3%) | <0.001 |
| Abnormal mA (<55 mm) | 12 (55%) | 59 (16%) | <0.001 |
| Abnormal LY30 (>3%) | 6 (27%) | 98 (26%) | 0.98 |
| Any r-TEG abnormality | 19 (86%) | 217 (59%) | 0.01 |
mA, maximum amplitude; LY30, lysis at 30 minutes.
Figure 1.
Estimated relative risks and 95% confidence intervals for abnormal TEG parameters versus CC+ in 391 patients. K-time >2.5 min, mA <55 mm, and platelet count <150 × 109/L were independently associated with CC+. ACT, activated clotting time. mA, maximum amplitude. LY30, lysis in 30 minutes. Plt, platelet count. CC, clinically evident coagulopathy.
Diagnostics of model performance
Diagnostic studies were performed for both of the above models (see Supplement, online version only). In terms of global goodness-of-fit, Chi-squared tests of the deviance statistic were not significant (p>0.05). Visual inspection of deviance residuals versus predicted values revealed <2% outliers. Due to the dichotomous outcome, the residuals were not expected to be normally distributed. Instead, we used our models to generate simulated data; visual comparison of actual versus simulated residuals did not reveal large deviations.
Discussion
In this study, we performed a secondary analysis of PROHS, which was a prospective observational study of adult trauma patients transported by helicopter from the scene. Overall, we found that the incidence of CC+ was 4% compared to the 39% incidence of laboratory coagulopathy on r-TEG, PT/INR, and/or PTT. CC+ was associated with increased ISS, more deranged physiology on admission, increased transfusion requirements, and increased mortality. Because one of the major objectives of this study was to infer deficiencies in clot formation based on laboratory coagulation abnormalities, we performed subgroup analyses on patients with admission INR and r-TEG data. Differences in demographics and outcomes were mostly preserved in the INR and r-TEG subgroups, except for no difference in mortality between CC+ and CC− patients who had INR performed at admission (Table 3). In patients with admission r-TEG, prolonged K-time (>2.5 min) and diminished mA (<55 mm) were independently associated with development of CC. In a separate model, INR >1.5 was independently associated with CC+. In both models, platelet count <150 × 109/L was associated with CC+.
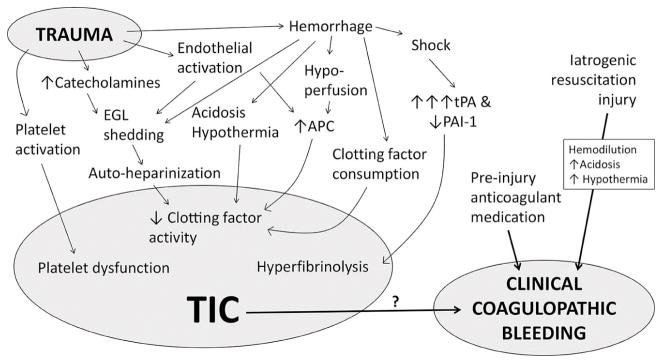
The etiology of TIC is complex, independent from iatrogenic resuscitation injury such as hemodilution, and multifaceted with contributions from decreased clotting factor activity, platelet dysfunction, endothelial injury, hypoperfusion, and hyperfibrinolysis (Figure 2).3 Several proposed pathways include: 1) activated protein C (APC)-mediated deactivation of clotting factors and plasminogen activator inhibitor,12 2) “autoheparinization” by shedding of heparan sulfate and chondroitin sulfate from the endothelial glycocalyx layer (EGL) into the circulation,13 3) dcreased platelet activation to stimuli (adenosine diphosphate [ADP], arachidonic acid [AA], collagen, and thrombin),14 4) hyperfibrinolysis secondary to massive release of tissue plasminogen activator (tPA) by hypoperfusion and/or catecholamines,15 and 5) early consumption of fibrinogen resulting in hypofibrinogenemia.16 Furthermore, TIC is likely modulated by endogenous patient factors, such as genetics and baseline comorbidities, and is exacerbated by any anticoagulant and antiplatelet medication taken prior to injury. Although having an abnormality on coagulation assays is a fairly common finding in trauma patients,4,5,6 its relationship to clinically-evident coagulopathic bleeding, which we defined as bleeding from uninjured sites and/or bleeding from injured sites not controllable by sutures, has not been well-established. Neal et al have proposed a scoring system to better define and study CC,17 but no system is in routine use currently.
Figure 2. Proposed mechanisms of TIC.
Trauma induces a plethora of biochemical and physiologic changes, including activation of both procoagulant and anticoagulant mechanisms. Several, non-mutually exclusive hypotheses have been proposed as the underlying etiology of TIC, but causative mechanisms are currently unclear. Additionally, the relationship between laboratory-based evidence of TIC, upon which most research efforts are based, and clinically-evident coagulopathic bleeding has not been adequately investigated. TIC, trauma-induced coagulopathy; tPA, tissue plasminogen activator; PAI-1, plasminogen activator inhibitor-1; APC, activated protein C; EGL, endothelial glycocalyx layer. Reproduced from Blood 2016;128(8):1043–9. Used with permission.
The cell-based model of hemostasis was a major shift in our understanding of clot formation. Instead of a clotting factor (enzymatic)-driven process, the cell-based model describes three overlapping phases of coagulation driven by the changing properties of cell surfaces: initiation, amplification, and propagation.18 One advantage of TEG over conventional coagulation tests, such as PT/INR and PTT, is that the TEG tracing contains information from whole blood pertaining to all three phases of the cell-based hemostasis model (as well as fibrinolysis). A study by Kawasaki et al analyzed clot formation of blood from healthy volunteers with scanning electron microscopy (SEM) in parallel with kaolin TEG.19 At R-time on the TEG tracing, coarse fibrin formation was observed on SEM. This is consistent with the initiation phase, when exposure of tissue-factor bearing surfaces after blood vessel injury leads to assembly of nascent complexes of clotting factors. At K-time on the TEG tracing, fibrin strands with some entrapped red blood cells were noted on SEM. This is consistent with the amplification phase, when circulating platelets adhere to the site of injury, become activated, and degranulate. Between K-time and the time of mA on TEG, increasing amounts of fibrin with deformed red blood cells were observed on SEM. This is consistent with the propagation phase, when factor Xa/Va complexes are assembled on the activated platelets en masse, resulting in the thrombin burst and large-scale fibrin production. Rivard et al reported in an in vitro study that the alpha angle correlates to the rate of propagation and is reflective of the thrombin burst.20 Abnormalities in the TEG tracing are reflective of specific deficiencies in the coagulation system which can be useful both as a research tool and to guide patient care.
Both platelets and fibrin contribute to the strength of a mature clot. Whereas the fibrin component represents only 20% of the overall clot strength in healthy individuals,21 this increases to 31–44% after trauma.22 A high, local concentration of thrombin is also required to produce a tightly woven, fibrin meshwork.23 Therefore, hypofibrinogenemia, platelet dysfunction, and deficient thrombin generation may all lead to poor clot strength and diminished TEG mA. The fact that the TEG angle was not associated with CC+ suggests that thrombin generation during the propagation phase was not impaired; indeed, the capacity to upregulate thrombin generation after injury is substantial.7
There is evidence to implicate both platelet dysfunction and hypofibrinogenemia as important contributors to TIC. Platelet inhibition to stimuli, such as adenosine diphosphate (ADP), arachidonic acid (AA), collagen, and thrombin receptor activating peptide (TRAP), has been associated with worse outcomes after trauma,14 was detectable within 15 minutes of injury in animal models,24,25 and may occur despite concurrent markers of hypercoagulability, such as increased thrombin generating potential and faster ACT on r-TEG.26 Chambers et al reported that fibrinogen was often the first clotting factor to reach critically low levels during the course of massive transfusion for hemorrhagic shock.27 A recent systematic review and meta-analysis by Cannon et al concluded that early transfusion with high ratios of platelets and plasma decreases mortality after hemorrhagic shock,28 and currently, several randomized trials are investigating the role of early aggressive fibrinogen treatment.29,30,31 Because platelet function assays and fibrinogen measurements were not performed in patients with admission r-TEG, it remains unclear from the present study if platelet dysfunction or hypofibrinogenemia was primarily responsible for the decreased clot strength observed in CC+ patients. The finding that prolonged K-time was associated with CC+ supports the hypothesis that platelet dysfunction contributed to impaired clot amplification, although attenuated clotting factor activity may have also played a role. Our finding that thrombocytopenia was associated with CC+ is consistent with another study which reported an association between thrombocytopenia and mortality after trauma,32 although it is also true that platelet dysfunction occurs at even normal platelet counts.14,32 Of note, blood samples were drawn prior to platelet transfusions. Hyperfibrinolysis (LY30 >3%) has been reported previously as uncommon but associated with increased mortality after trauma.33 In this study, however, the incidence of hyperfibrinolysis was nearly equal between CC+ and CC− (26% versus 27%), and it was not associated with CC+ or mortality. Although INR>1.5 was associated with CC+ in patients with admission INR, there were not enough patients who had both r-TEG and INR data to explore their associations to CC+ in the same model.
Of the 35 CC+ patients with admission r-TEG, PT/INR, or PTT performed, 9 patients (26%) had normal coagulation parameters, and of the 305 patients with laboratory coagulopathy based on these tests, only 26 (9%) were also CC+. This dissociation of hemostatic potential assessed by laboratory values (41%) versus hemostatic function by observed CC+ (4%) suggests that currently used laboratory coagulation assays are suboptimal in assessing the risk of clinical coagulopathic bleeding, however, our reported laboratory coagulation results were obtained at the time of admission, while determination of CC was several hours later. Clinical factors occurring after admission including continued blood loss and variable fluid therapy may modulate the risk of CC+ despite normal admission laboratory coagulation studies.
One final consideration is the relationship between brain injury and CC. Although the incidence of severe TBI by a score of >3 criteria on the the Abbreviated Injury Scale (AIS) was similar between CC+ and CC− patients, CC+ patients did have increased incidence of mortality due to TBI. Previous reports have documented the relationship between TBI, coagulopathy, and mortality.34 Brain-derived microparticles are released into the circulation after TBI, contain high concentrations of procoagulant tissue factor and phosphatidylserine, and have been demonstrated to induce a consumptive coagulopathy in a mouse model of TBI.35 A consumptive process could result in hypofibrinogenemia and explain the poor clot strength and depressed TEG mA observed in CC+ patients’ however, we did not examine subsequent head imaging to analyze progression of intracranial hemorrhage, which can be considered a coagulopathic phenotype. Inclusion of patients with ICH expansion may have increased the incidence of CC+ in the present study.
Our study had several limitations. First, only two of nine participating centers in PROHS performed routine admission r-TEG. Eighty enrolled patients from two other participating centers did have admission k-TEG performed, however, we excluded k-TEG patients from the subgroup analysis for the several reasons. Because of the tissue factor added to r-TEG which is not present in k-TEG, many of the r-TEG and k-TEG parameters are not directly comparable, although some variables are well-preserved.36 Nevertheless, because we are not aware of any method to standardize r-TEG and k-TEG parameters, a pooled analysis with all 5 variables would not be possible. Although we could have used cutoffs to define abnormal k-TEG parameters and performed a pooled analysis using dichotomous variables, we are not aware of any cutoff parameters for k-TEG for the trauma population. Reference ranges based on normal individuals are available from the manufacturer, but because injury induces a plethora of biochemical and physiologic changes including activation of both procoagulant and anticoagulant mechanisms using reference ranges based on normal individuals to define abnormality in the setting of trauma is likely inappropriate.3 Although decreased mA was independently associated with CC+ for 391 patients with r-TEG, mA was actually greater in the CC+ group for 80 patients with k-TEG. The reason for this is unclear but may be related to the inter-site variance in CC+ diagnosis. The two centers which performed admission k-TEG were outliers for CC+ incidence (<1% and 23%, respectively), while the two centers that performed admission r-TEG reported anincidence of CC+ of 6% and 7%, respectively.
Although we analyzed patients with admission r-TEG and PT/INR, we could not combine these patients into a single regression model due to not enough patients with both tests. Furthermore, a limitation inherent in all current coagulation assays is the exclusion of a vital component of in vivo coagulation, the endothelium and the blood vessel itself. We analyzed only admission coagulation tests, while CC developed and was recorded a variable duration of time after admission. During this time, multiple clinical variables, including transfusion of blood products, crystalloid and artificial colloid use, operative procedures, and continued blood loss, may have contributed to the development of CC but were not accounted for in our study. At the end of active resuscitation, research staff asked the attending trauma surgeon if CC had been observed at any point prior. We report the time to end of resuscitation (Table 2), but we do not know the exact time when CC+ was first present. Additionally, patients who developed CC after the end of resuscitation would have been categorized as CC−. In future studies, patients should be assessed for CC at multiple time points and during the different phases of care to account for variations in clinical care. Finally, despite the a priori definition of CC+, there was significant inter-site variability in its incidence.
In conclusion, development of CC was rare (4%) and associated with poor outcomes, whereas an abnormality in laboratory-based coagulation was common (39%). Prolonged K-time and depressed mA on admission r-TEG were independently associated with CC in trauma patients, implicating impaired clot amplification, hypofibrinogenemia, and/or platelet dysfunction as an important contributor to this phenotype. In highlighting the substantial disconnect between laboratory-based evidence of coagulopathy and clinically-evident coagulopathic bleeding, we hope further research will refine our definition of this disease and elucidate its etiology.
Supplementary Material
Acknowledgments
Funding: The Prehospital Resuscitation on Helicopter Study (PROHS) was sponsored by the U.S. National Heart, Lung, and Blood Institute (U01HL077863) and the U.S. Department of Defense. RC is supported by a T32 fellowship (grant no. 5T32GM008792) from the National Institute of General Medical Sciences.
Prehospital Resuscitation on Helicopter Study (PROHS) Study Group
Clinical Coordinating Center: John B. Holcomb, MD; Charles E. Wade, PhD; Erin E. Fox, PhD; Ronald Chang, MD; Jeanette M. Podbielski, RN. Jeffrey S. Tomasek, MD; Deborah J. del Junco, PhD.
Data Coordinating Center: Michael D. Swartz, PhD; Stacia M. DeSantis, PhD; Savitri N. Appana, MS; Thomas J. Greene, MPH; Misung Yi, MS; Michael O. Gonzalez, MS; Sarah Baraniuk, PhD.
Resuscitation Outcomes Consortium at the University of Washington: Gerald van Belle, PhD; Brian G. Leroux, PhD.
PROHS Clinical Sites (listed in order of number of highest risk patients enrolled):
University of Texas Health Science Center at Houston: Carrie L. Howard, MA, MBA; Amanda Haymaker.
Shock, Trauma and Anesthesiology Research - Organized Research Center (STAR-ORC), R Adams Cowley Shock Trauma Center, University of Maryland Medical Center: Deborah M. Stein, MD, MPH; Thomas M. Scalea, MD; Benjamin Ayd; Pratik Das; Anthony V. Herrera, MS.
University of Washington: Eileen M. Bulger, MD; Bryce R. H. Robinson, MD; Patricia Klotz, RN; Aniqa Minhas, BS.
University of Alabama at Birmingham: Jeffrey D. Kerby, MD, PhD; Sherry M. Melton, MD, MSHA; Carolyn R. Williams, RN, MSHI; Shannon W. Stephens, EMTP.
University of Cincinnati: Michael Goodman, MD; Jay A. Johannigman, MD; Jason McMullan, MD; Richard D. Branson, MSc, RRT; Dina Gomaa, BS, RRT; Christopher Barczak, BS, MT(ASCP).
Oregon Health and Science University: Martin A. Schreiber, MD; Samantha J. Underwood, MS; Cheri Watson, BS.
Mayo Clinic: Martin D. Zielinski, MD; James R. Stubbs, MD; Amy Headlee.
University of Arizona: Terence O’Keeffe, MBChB, MSPH; Peter Rhee, MD; Laurel L. Rokowski, RN, BSN, MKT; John Santoro Jr, AA; Andrea Seach, BS; David Bradford, BS; Michelle Fealk, BA; Fortesa Latifi, BS.
University of Southern California: Kenji Inaba, MD; Henry Kim, MD; Carl Chudnofsky, MD; Monica D. Wong, MS.
Footnotes
Presented at the 12th Annual Academic Surgical Congress in Las Vegas, NV February 7–9, 2017
Author contributions: Study inception and design by EEF, DMS, EMB, SMM, MDG, MAS, MDZ, TO, KI, CEW, and JBH. Data acquisition by SMD, DMS, EMB, SMM, MDG, MAS, MDZ, TO, KI, JST, JMP, SA, MY, HH, JS, JS, and PJ. Data analysis and interpretation by RC, EEF, TJG, MDS, HH, JS, PJ, CEW, and JBH. Manuscript drafted by RC with critical appraisal from all authors.
Disclaimer: The opinions or conclusions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of any sponsor.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Vital Statistics System, National Center for Health Statistics, Centers for Disease Control and Prevention. 10 Leading Causes of death by Age Group, United States—2013. Atlanta, GA: Office of Statistics and Programing, National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; [Accessed December 30, 2016]. http://www.cdc.gov/injury/images/lc-charts/leading_causes_of_death_by_age_group_2013-a.gif. [Google Scholar]
- 2.Simmons RL, Collins JA, Heisterkamp CA, Mills DE, Andren R, Phillips LL. Coagulation disorders in combat casualties, I: acute changes after wounding. Ann Surg. 1969;169(4):455–462. doi: 10.1097/00000658-196904000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang R, Cardenas JC, Wade CE, Holcomb JB. Advances in the understanding of trauma-induced coagulopathy. Blood. 2016;128(8):1043–9. doi: 10.1182/blood-2016-01-636423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niles SE, McLaughlin DF, Perkins JG, Wade CE, Li Y, Spinella PC, Holcomb JB. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64(6):1459–63. doi: 10.1097/TA.0b013e318174e8bc. [DOI] [PubMed] [Google Scholar]
- 5.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–30. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 6.Maegele M, Lefering R, Yucel N, Tjardes T, Rixen D, Paffrath T, et al. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38(3):298–304. doi: 10.1016/j.injury.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Cardenas JC, Rahbar E, Pommerening MJ, Baer LA, Matijevic N, Cotton BA, et al. Measuring thrombin generation as a tool for predicting hemostatic potential and transfusion requirements following trauma. J Trauma Acute Care Surg. 2014;77(6):839–845. doi: 10.1097/TA.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 8.Johansson PI. Coagulation monitoring of the bleeding traumatized patient. Curr Opin Anaesthesiol. 2012;25:235–241. doi: 10.1097/ACO.0b013e32834fab76. [DOI] [PubMed] [Google Scholar]
- 9.Holcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg. 2012;256(3):476–86. doi: 10.1097/SLA.0b013e3182658180. [DOI] [PubMed] [Google Scholar]
- 10.Johansson PI, Stensballe J, Oliveri R, Wade CE, Ostrowski SR, Holcomb JB. How I treat patients with massive hemorrhage. Blood. 2014;124(20):3052–8. doi: 10.1182/blood-2014-05-575340. [DOI] [PubMed] [Google Scholar]
- 11.Yelland LN, Salter AB, Ryan P. Performance of the modified Poisson regression approach for estimating relative risks from clustered prospective data. Am J Epidemiol. 2011;174(8):984–92. doi: 10.1093/aje/kwr183. [DOI] [PubMed] [Google Scholar]
- 12.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, Pittet JF. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64(5):1211–17. doi: 10.1097/TA.0b013e318169cd3c. [DOI] [PubMed] [Google Scholar]
- 13.Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg. 2012;73(1):60–66. doi: 10.1097/TA.0b013e31825b5c10. [DOI] [PubMed] [Google Scholar]
- 14.Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, et al. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012;73(1):13–19. doi: 10.1097/TA.0b013e318256deab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman MP, Moore EE, Moore HB, Gonzalez E, Gamboni F, Chandler JG, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J Trauma Acute Care Surg. 2015;80(1):16–23. doi: 10.1097/TA.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davenport R, Brohi K. Fibrinogen depletion in trauma: early, easy to estimate and central to trauma-induced coagulopathy. Crit Care. 2013;17(5):190. doi: 10.1186/cc13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neal MD, Moore HB, Moore EE, Freeman K, Cohen MJ, Sperry JL, Zuckerbraun BS, Park MS TACTIC Investigators. Clinical assessment of trauma-induced coagulopathy and its contribution to postinjury mortality: A TACTIC proposal. J Trauma Acute Care Surg. 2015;79(3):490–2. doi: 10.1097/TA.0000000000000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman M, Monroe DM. A cell-based model of hemostasis. Thromb Haemost. 2001;85(6):958–65. [PubMed] [Google Scholar]
- 19.Kawasaki J, Katori N, Kodaka M, Miyao H, Tanaka KA. Electron microscopic evaluations of clot morphology during thrombelastography. Anesth Analg. 2004;99(5):1440–4. doi: 10.1213/01.ANE.0000134805.30532.59. [DOI] [PubMed] [Google Scholar]
- 20.Rivard GE, Brummel-Ziedins KE, Mann KG, Fan L, Hofer A, Cohen E. Evaluation of the profile of thrombin generation during the process of whole blood clotting as assessed by thrombelastography. J Thromb Haemost. 2005;3(9):2039–43. doi: 10.1111/j.1538-7836.2005.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen L, Lorand L. Contribution of fibrin stabilization to clot strength. Supplementation of factor XIII-deficient plasma with the purified zymogen. J Clin Invest. 1983;71(5):1336–1341. doi: 10.1172/JCI110885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kornblith LZ, Kutcher ME, Redick BJ, Calfee CS, Vilardi RF, Cohen MJ. Fibrinogen and platelet contributions to clot formation: implications for trauma resuscitation and thromboprophylaxis. J Trauma Acute Care Surg. 2014;76(2):255–256. doi: 10.1097/TA.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolberg AS, Campbell RA. Thrombin generation, fibrin clot formation and hemostasis. Transfus Apher Sci. 2008;38(1):15–23. doi: 10.1016/j.transci.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castellino FJ, Chapman MP, Donahue DL, Thomas S, Moore EE, Wohlauer MV, et al. Traumatic brain injury causes platelet adenosine diphosphate and arachidonic acid receptor inhibition independent of hemorrhagic shock in humans and rats. J Trauma Acute Care Surg. 2014;76(5):1169–1176. doi: 10.1097/TA.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sillesen M, Johansson PI, Rasmussen LS, Jin G, Jepsen CH, Imam AM, et al. Platelet activation and dysfunction in a large-animal model of traumatic brain injury and hemorrhage. J Trauma Acute Care Surg. 2013;74(5):1252–1259. doi: 10.1097/TA.0b013e31828c7a6b. [DOI] [PubMed] [Google Scholar]
- 26.Wade CE, Baer LA, Cardenas JC, Folkerson LE, Nutall-Aurora K, Cotton BA, et al. Upon admission coagulation and platelet function in patients with thermal and electrical injuries. Burns. 2016;42(8):1704–1711. doi: 10.1016/j.burns.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Chambers LA, Chow SJ, Shaffer LE. Frequency and characteristics of coagulopathy in trauma patients treated with a low- or high-plasma-content massive transfusion protocol. Am J Clin Pathol. 2011;136(3):364–70. doi: 10.1309/AJCPH16YXJEFSHEO. [DOI] [PubMed] [Google Scholar]
- 28.Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82(3):605–617. doi: 10.1097/TA.0000000000001333. [DOI] [PubMed] [Google Scholar]
- 29.Nascimento B, Callum J, Tien H, Peng H, Rizoli S, Karanicolas P, et al. Fibrinogen in the initial resuscitation of severe trauma (FiiRST): a randomized feasibility trial. Br J Anaesth. 2016;117(6):775–782. doi: 10.1093/bja/aew343. [DOI] [PubMed] [Google Scholar]
- 30.Maegele M, Zinser M, Schlimp C, Schöchl H, Fries D. Injectable hemostatic adjuncts in trauma: Fibrinogen and the FIinTIC study. J Trauma Acute Care Surg. 2015;78(6 Suppl 1):S76–82. doi: 10.1097/TA.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 31.Steinmetz J, Sørensen AM, Henriksen HH, Lange T, Larsen CF, et al. Pilot Randomized trial of Fibrinogen in Trauma Haemorrhage (PRooF-iTH): study protocol for a randomized controlled trial. Trials. 2016;17(1):327. doi: 10.1186/s13063-016-1439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown LM, Call MS, Margaret Knudson M, Cohen MJ Trauma Outcomes Group. A normal platelet count may not be enough: the impact of admission platelet count on mortality and transfusion in severely injured trauma patients. J Trauma. 2011;71(2 Suppl 3):S337–42. doi: 10.1097/TA.0b013e318227f67c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, Schöchl H, et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012 Aug;73(2):365–70. doi: 10.1097/TA.0b013e31825c1234. [DOI] [PubMed] [Google Scholar]
- 34.Galvagno SM, Jr, Fox EE, Appana SN, Baraniuk S, Bosarge PL, Bulger EM, et al. Outcomes Following Concomitant Traumatic Brain Injury and Hemorrhagic Shock: A Secondary Analysis from the PROPPR Trial. J Trauma Acute Care Surg. doi: 10.1097/TA.0000000000001584. Epub June 6, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian Y, Salsbery B, Wang M, Yuan H, Yang J, Zhao Z, et al. Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood. 2015;125(13):2151–9. doi: 10.1182/blood-2014-09-598805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeger V, Willi S, Liu T, Yeh DD, De Moya M, Zimmermann H, Exadaktylos AK. The Rapid TEG α-Angle may be a sensitive predictor of transfusion in moderately injured blunt trauma patients. Scientific World Journal. 2012;2012:821794. doi: 10.1100/2012/821794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.