Positive relationships between carbon storage and taxonomic diversity are not predominant at the local scale.
Abstract
Protecting aboveground carbon stocks in tropical forests is essential for mitigating global climate change and is assumed to simultaneously conserve biodiversity. Although the relationship between tree diversity and carbon stocks is generally positive, the relationship remains unclear for consumers or decomposers. We assessed this relationship for multiple trophic levels across the tree of life (10 organismal groups, 3 kingdoms) in lowland rainforests of the Congo Basin. Comparisons across regrowth and old-growth forests evinced the expected positive relationship for trees, but not for other organismal groups. Moreover, differences in species composition between forests increased with difference in carbon stock. These variable associations across the tree of life contradict the implicit assumption that maximum co-benefits to biodiversity are associated with conservation of forests with the highest carbon storage. Initiatives targeting climate change mitigation and biodiversity conservation should include both old-growth and regenerating forests to optimally benefit biodiversity and carbon storage.
INTRODUCTION
Biodiversity loss and climate change are among the most important threats that humanity faces in the 21st century (1). Consequently, the international community has engaged in a series of initiatives that aim at protecting either biodiversity or carbon stocks. The United Nations Framework Convention on Climate Change (UNFCCC) and the Convention on Biological Diversity are binding multilateral commitments that include specific targets [the Paris Agreement (2) and Aichi Biodiversity Targets (3)]. However, actions that simultaneously minimize carbon loss and maximize biodiversity conservation represent the best use of limited resources and available land. In particular, the UNFCCC REDD+ (Reducing Emissions from Deforestation and Forest Degradation) mechanism is generally recognized for its potential to simultaneously address declines in forest-based carbon stocks and biodiversity (4). Still, empirical research underpinning these policies has been limited to the relationship between tree diversity and carbon storage. Although trees are the structural components and the energetic foundation of forests, biodiversity at multiple trophic levels is required to maintain forest ecosystem functioning (5), including the carbon cycle (6). Current state of knowledge is therefore insufficient to inform the design and implementation of policies such as REDD+ or to ensure that biodiversity and carbon conservation are optimized effectively.
Efforts to mitigate biodiversity and carbon loss often focus on the conservation of tropical forests because they store vast amounts of carbon and are among the most biodiverse terrestrial habitats in the world (7). However, the relationship between biodiversity and carbon storage in tropical forests is complex. Theory predicts that tree species richness has a positive effect on productivity through niche complementarity and selection effects (8, 9). Nonetheless, empirical relationships between tree species richness, taxonomic diversity or functional diversity, and aboveground carbon (AGC) storage differ, depending on the spatial extent of analysis (8–11). In contrast, limited research on heterotrophic groups (mammals and birds) suggests that their species richness, taxonomic diversity, and trait diversity are not associated with AGC storage (12). Likewise, studies on relationships between taxonomic biodiversity and production (or its surrogates) are dependent on spatial scale (13), taxonomic identity of the focal group (14), or metric of biodiversity (15).
We highlight three reasons why many studies on relationships between biodiversity and AGC storage in tropical forests represent incomplete assessments for conservation planning. First, the relationship between tree diversity and carbon storage is unlikely to be representative for analogous relationships for decomposers and consumers. Although plant diversity is directly affected by the distribution of mass or energy production among plant species, the biodiversity of consumer and decomposer taxa is a result of their consumption of plants and the distribution of resources between consumer and decomposer species (6, 16). Consequently, the shapes of relationships between the biodiversity of consumer or decomposer taxa and AGC stocks are likely influenced by a host of environmental factors, each of which depends on the taxonomic group under consideration (13). Second, most studies evaluate biodiversity only at the local or community level (α-diversity), whereas understanding differences in species composition between communities (β-diversity) is vital for the effective conservation of regional biodiversity (17). Moreover, species can exhibit different degrees of habitat specificity, making those specialized to a particular habitat more susceptible to habitat loss (18). Third, most empirical studies examine relationships between biodiversity and carbon storage only in old-growth forests (8–12). However, contemporary tropical forest landscapes also include forests that are subject to complex anthropogenic disturbance regimes (19, 20). Because disturbed forests are becoming increasingly abundant, they must be considered in conservation and restoration planning (19, 20). Therefore, relationships between biodiversity and carbon storage should consider both disturbed and undisturbed forests to be broadly applicable to forest landscapes.
We evaluate the relationships between AGC storage and different aspects of taxonomic biodiversity for primary producers (trees and lichens), decomposers (fungi), and consumers (slime molds, vertebrates, and invertebrates) at the landscape scale. We characterize taxonomic biodiversity using metrics that describe richness and diversity of local communities (α-diversity) and metrics that capture variation in species identity between communities (β-diversity). In addition, we determine which species could be of particular conservation interest (specialization). This allows us to address three critical questions: (i) How are different aspects of taxonomic biodiversity related to AGC? (ii) To what extent are those relationships taxon-dependent? and (iii) What are the implications of these empirical relationships to conservation programs designed to safeguard biodiversity and carbon storage?
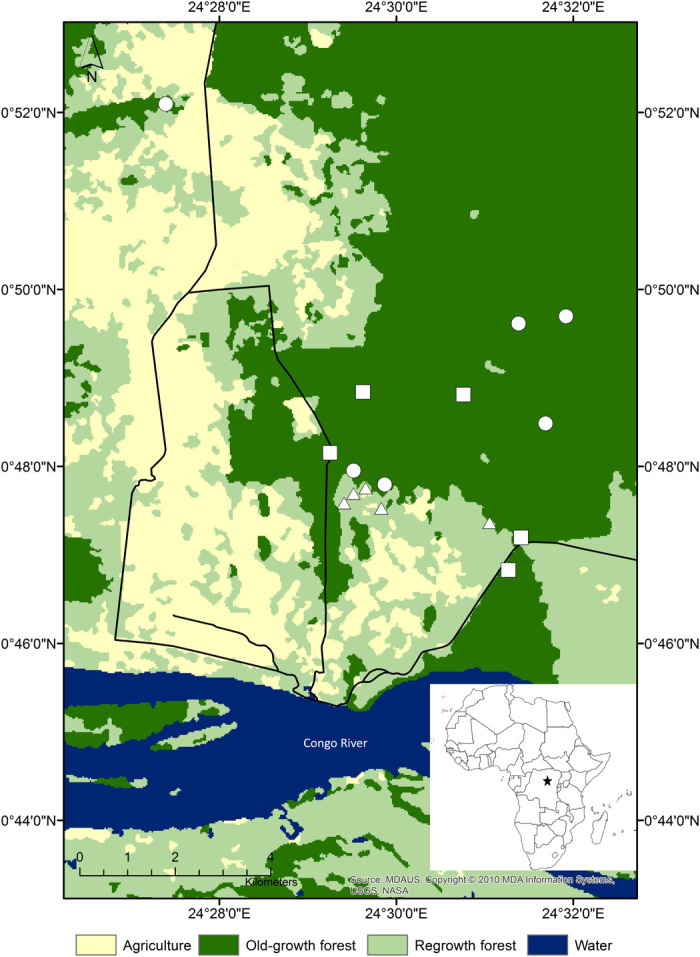
Our study, executed in a data-poor tropical region (21), represents the first assessment of relationships between tropical rainforest biodiversity and AGC storage that includes a wide phylogenetic range of life forms. We collected data in lowland rainforest of the Yangambi Biosphere Reserve, situated in the center of the Congo Basin in the Democratic Republic of the Congo (DRC) (Fig. 1). We determined AGC stock and biodiversity for a diverse array of 10 organismal groups (trees, plasmodial slime molds, fungi, leaf lichens, bark lichens, flies, arboreal-dwelling ants, understory birds, and ground-dwelling rodents and shrews; table S1) from up to 16 1-ha plots in regrowth (n = 5) and old-growth forests (n = 11). AGC values ranged from 2 Mg ha−1 in young regrowth forests to 183 Mg ha−1 in old-growth forests (fig. S1).
Fig. 1. Location of the plots within regrowth (Δ), mixed old-growth (□), and monodominant old-growth (○) forest in the Yangambi Biosphere Reserve (DRC), situated in the center of the African continent (lower right inset).
RESULTS
We first explore the relationships between α-diversity and AGC stock. Each measure of α-diversity (that is, species richness and Shannon or Simpson diversity) is expressed in effective numbers of species and is standardized for sample completeness. Relationships between α-diversity and AGC are quantified via orthogonal polynomial regression, which decomposes the general relationship from ordinary polynomial regression into additive polynomials (linear and quadratic), with coefficients that represent independent contributions that can be statistically evaluated in an unbiased fashion. Effect sizes and forms of relationships between α-diversity and AGC differ considerably among organismal groups (Fig. 2, fig. S2, and table S2). Species richness of trees and leaf lichens increases with AGC, whereas species richness of slime molds decreases with increasing AGC. Species richness of fungi, bark lichens, flies, ants, rodents, or shrews is not related to AGC. Relationships between Shannon or Simpson diversity and AGC differ from those of species richness for leaf lichens and birds. Species richness and Shannon diversity of leaf lichens increase nonlinearly, whereas Simpson diversity increases linearly with AGC. For birds, Shannon diversity decreases nonlinearly with increasing AGC, whereas species richness and Simpson diversity show no significant relationships.
Fig. 2. Relationships between metrics of α biodiversity and carbon storage differ between organismal groups.
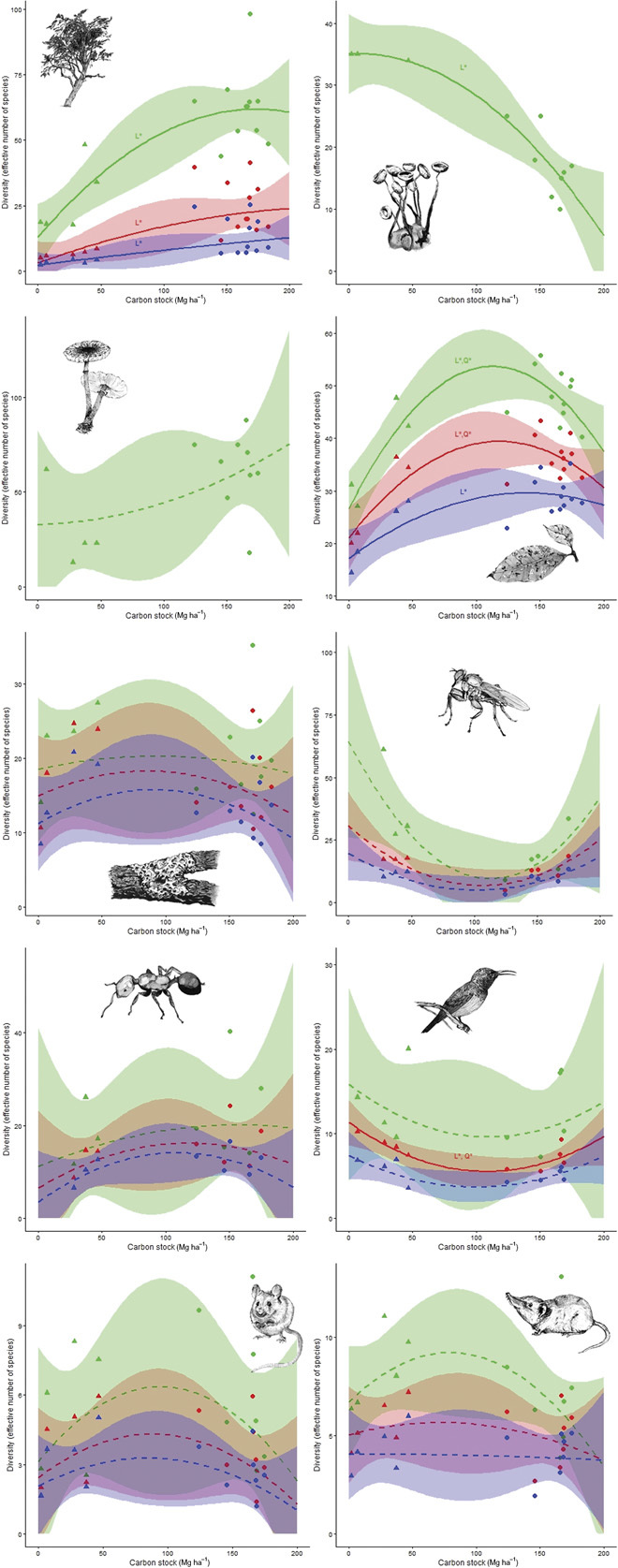
Graphical representations of polynomial regressions for species richness (green), Shannon diversity (red), and Simpson diversity (blue), with associated shaded areas representing 95% confidence regions. Regrowth forests are depicted with ▲ and old-growth forests with ●. Significant linear and quadratic components are indicated with “L*” and “Q*,” respectively, whereas nonsignificant regressions are shown with dashed lines (table S2). Across rows from top to bottom: trees, slime molds, fungi, leaf lichens, bark lichens, flies, ants, birds, rodents, and shrews.
Except for slime molds and shrews, differences in species composition increase with differences in AGC (Fig. 3, table S3, and fig. S3). We use Mantel tests to quantify associations between differences in species composition (β-diversity, represented by the Sørensen and Morisita-Horn indices) and differences in AGC among all possible pairs of plots. As the largest differences in AGC are observed between regrowth and old-growth forest plots, these positive associations indicate that communities from the same forest type tend to have more similar species compositions than do communities from different forest types (which is not due to spatial autocorrelation; table S4). Sørensen and Morisita-Horn indices reveal similar associations for each group, except for ants and birds. For ants, the association for Sørensen dissimilarity is close to significance (P = 0.07), whereas the high relative abundance of Yellow-whiskered greenbul (Andropadus latirostris; 34% of all captures in regrowth and 42% in old-growth) makes the association for Morisita-Horn dissimilarity nonsignificant in birds. This indicates that, although for the majority of groups, species richness and diversity showed no significant correlations with AGC, their species composition does differ with carbon storage.
Fig. 3. For most groups, community composition differs more between forests with larger differences in carbon stock.
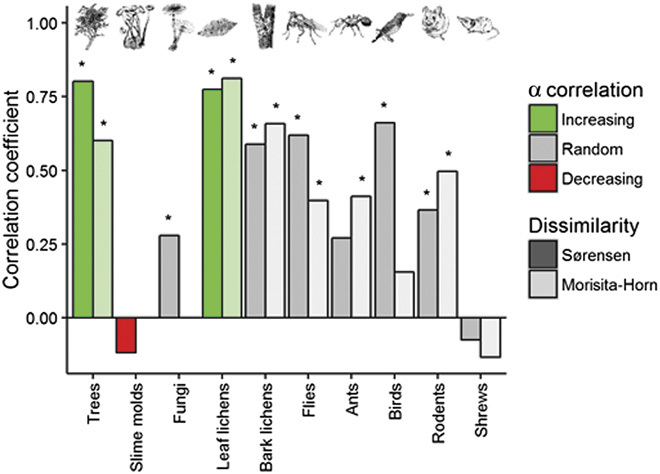
The Pearson correlation coefficient illustrates the strength of association between species dissimilarity (Sørensen and Morisita-Horn) and difference in carbon stock (no abundances were available for fungi and slime molds). For each index, significance (α = 0.05) is indicated by an asterisk. Colors indicate whether the correlation of α diversity with carbon storage was found to be increasing, decreasing, or random for the associated α diversity measures (species richness and Simpson diversity).
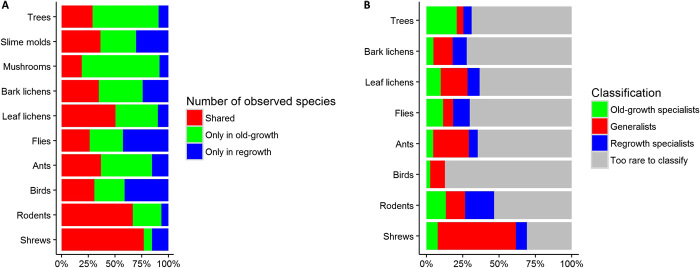
As a last step, we assess the specific conservation value of regrowth and old-growth forests by evaluating (for each organismal group) how many species are specialized to each forest type. Generally, the level of endemism or threat status is used to assess the conservation value of individual species (22). However, because our data contain a broad phylogenetic range of species from a poorly studied area, the ecological characteristics of most species remain unknown. Consequently, we use two alternative methods to assess the level of specialization of species on regrowth or old-growth forests: one based on species presence and one based on the abundance distribution of species. First, we count the number of species that are encountered solely in regrowth or old-growth forests versus the number of species that occur in both forest types. Except for flies, birds, and shrews, more species were found exclusively in old-growth compared to regrowth forests (Fig. 4A and table S5). Second, we use a statistical model (18) that assigns each species into one of four classes based on its relative abundance: (i) generalists, (ii) regrowth specialists, (iii) old-growth specialists, or (iv) too rare to classify with confidence. For the purposes of this analysis, we assume that old-growth specialists are species that only occur within old-growth forests and are therefore irreplaceable (21), whereas regrowth specialists could be regenerating forest specialists as well as opportunistic species that are abundant even outside forest habitats (23). For each group, most species occur at low abundances and could not be reliably classified as generalists or specialists. As such, the actual number of specialists is probably much greater than that indicated in the analysis. Nevertheless, all organismal groups contain species that are classified as either old-growth forest specialists or (except for birds) regrowth forest specialists (Fig. 4B and table S5), indicating that both forest types are important for biodiversity conservation.
Fig. 4. Specialization of species in regrowth or old-growth forests for each organismal group.
(A) Percentage of observed species occurring only in old-growth, only in regrowth, or in both forest types. (B) Percentage of species classified as old-growth specialists, regrowth specialists, generalists, or too rare to classify. Assessments executed for each species group separately.
DISCUSSION
Only for trees is the relationship between α-diversity and AGC both positive and linear. All other groups show random (bark lichens, fungi, flies, ants, birds, rodents, and shrews), nonlinear (leaf lichens), or negative (slime molds) relationships with AGC (Fig. 2). For trees, a positive relationship was expected because both tree α-diversity and AGC are known to be lower in regrowth forests compared to old-growth forests (21, 24). When confining the analysis to plots within old-growth forests, none of the organismal groups show significant relationships between each of the aspects of taxonomic biodiversity and AGC storage, supporting findings in the literature (11, 12). Much of the variability among the relationships between taxonomic diversity and AGC for different organismal groups or different regions may be attributed to differences among studies in spatial scale (in particular, spatial grain and extent). We analyze relationships at a spatial grain relevant for AGC (1-ha sampling units), but the ecological ramifications of this choice of scale likely differ among groups and depend on organism size or mobility (for example, birds versus ants versus lichens), and thus the grain at which organisms respond to their environment. The scale at which biodiversity relates to AGC depends on the life history attributes of the group under consideration (13). For instance, many of the organismal groups whose biodiversity varies randomly with AGC in our study are more mobile (birds, rodents, and shrews), and their sensitivity to environmental variation differs markedly from that of sessile life forms such as plants or fungi. Coarse-grained studies (25, 26) found positive relationships with AGC storage for species richness or diversity of birds, amphibians, or mammals, whereas a fine-grained study (12) revealed random relationships for ground-dwelling mammals and birds. Furthermore, the likelihood of finding no significant relationship between animal diversity and net primary production declines with increasing spatial extent (14). Patterns evaluated at large spatial extents (for example, global or regional) may obscure patterns at small spatial scales, resulting in global conservation strategies that are not effective for local biodiversity. This problem is likely to be exacerbated in studies that do not explicitly include β-diversity in their assessments.
When combining the associations between different aspects of taxonomic biodiversity and AGC for each group, we demonstrate that trees show different patterns compared to most of the other organismal groups. The different organismal groups in the Yangambi Biosphere Reserve can be broadly divided into four categories. First, for trees and leaf lichens, differences in species composition are positively associated with differences in AGC. As α-diversity of these groups also increases with AGC, tree and leaf lichen species composition in low-carbon forests are nested subsets of the species found in high-carbon forests. Most tree species (75.8%) and leaf lichen species (83.5%) occurring in regrowth forests are also recorded in old-growth forests (table S4). Second, for groups with a random relationship to AGC, the increase in β-diversity with difference in AGC indicates turnover in species composition (fungi, bark lichens, flies, ants, rodents, and birds). Third, the taxonomic diversity of shrews does not show relationships with AGC at α- or β-levels. This likely results from the high proportion of shrews classified as generalists (>20% of all shrew species) within the study area. Fourth, as slime molds thrive better under less humid conditions found in regrowth forests, their species richness decreases with increasing carbon storage (27). Our analyses, considering different aspects of taxonomic biodiversity, show that relationships between tree diversity and carbon storage are not representative of those for groups of consumers or decomposers. Nevertheless, because other dimensions of biodiversity (for example, functional diversity) are known to respond differently to carbon storage (11, 28), future research should include functional and phylogenetic biodiversity to better inform conservation policy (29).
Our findings suggest that countries that are developing national guidelines based on international initiatives on climate change mitigation and biodiversity conservation should explicitly integrate forest conservation and regeneration to reach the committed targets. In concordance with literature about other tropical forests, our study indicates that both old-growth and regrowth forests are vital to the persistence of forest species in tropical, human-modified landscapes (21, 30). The observed turnover in species composition between regrowth and old-growth forests and the specialization of species to both forest types illustrate the significance of old-growth forests and the added value of regrowth forests for biodiversity conservation. Old-growth forests are disappearing rapidly (31) and should be priority protection targets because they are largely irreplaceable (Aichi Target 11), whereas regrowth forests could be restored to buffer against the loss of biodiversity and ecosystem services generally provided by old-growth forests (Aichi Target 14) (32). However, the inferred importance of regrowth forests for biodiversity probably depends on their proximity to old-growth forests, as is the case in Yangambi (33). Therefore, we suggest that landscape restoration actions, such as forest regeneration, should occur close to forest remnants (32). Forest regeneration will benefit forest biodiversity both directly, by providing habitat for species adapted to early successional stages, and indirectly, by increasing connectivity and reducing fragmentation of forest fragments, two important threats for forest biodiversity (Aichi Target 15) (34). This integrated approach will also benefit carbon conservation because regrowth and old-growth forests are important sinks and reservoirs of AGC (Paris Agreement, Article 5) (7, 24). Forest regeneration, or the expansion of total forest cover in general, will be necessary to meet the ambitious goal of keeping global warming within 2°C (Paris Agreement, Article 2). In conclusion, approaches that affect the complete forest succession (conservation of old-growth forests with protection and regeneration of regrowth forests) are the ones likely to optimize benefits for biodiversity and carbon storage.
METHODS
Study area
Fieldwork was conducted in the UNESCO Biosphere Reserve in Yangambi, 100 km west of Kisangani, in the DRC. The reserve comprises 6297 km2, and all study plots were within the southwestern portion (00°47′N, 24°30′E) of the reserve (Fig. 1). The study area contains old-growth and human-modified forests, a landscape increasingly characterizing the Paleotropics and Neotropics (19). Vegetation in the reserve is a semideciduous tropical rainforest with fragments of evergreen rainforest, transition forest, agricultural land, fallow land, and swamp forest (35). One-hectare sampling plots (100 m × 100 m) were established in forest types with different carbon stocks (36). Five plots were placed in regrowth forests that represent different stages of regrowth after abandonment of slash-and-burn agriculture, including young Musanga regrowth forest (n = 2; age since disturbance ± 7 years) and older Musanga regrowth forest (n = 3; age since disturbance ± 20 years). These plots are dominated by Musanga cecropioides, the most common pioneer species in these regeneration stages in the area (35). Disturbance history was estimated on the basis of communication with local farmers. Eleven plots were situated in old-growth (or primary) forests that represent a range of climax vegetation, including mixed semideciduous forest (n = 5), monodominant forest of Gilbertiodendron dewevrei (De Wild.) J. Leonard (n = 5), and monodominant forest of Brachystegia laurentii (De Wild.) Hoyle (n = 1).
Because monodominant stands are relatively common in Central Africa compared with the rarity of monodominance in Amazonia or Southeast Asia, we assessed their influence on the relationships between taxonomic diversity and carbon storage. Although structural differences exist between mixed and monodominant forests (36), the omission of monodominant plots from analyses did not alter overall conclusions for α- or β-diversity (β-level associations were significant for most of the groups; table S3). We will therefore refer to old-growth forests as the collection of mixed and monodominant plots in our study area.
Plot inventory and carbon stock estimation
A standardized international inventory protocol for tropical forest was used (37) to assure comparability with other studies. All live stems with a diameter larger than 10 cm were tagged, measured for diameter at breast height (DBH) at 1.3 m, and identified to species. Buttressed trees (although rare in the region) and stilt-rooted trees were measured at 50 cm above the highest root, where the trunk shape is cylindrical. When a deformity was present at breast height, the diameter was measured 2 cm lower. On the basis of this inventory, a subset of trees was selected for height measurement by stratified random sampling. Two levels of strata were formed: species identity and DBH classes of 10 to 20, 20 to 30, 30 to 50, and ≥50 cm. Next, two individuals were randomly selected within each stratum when possible [excluding damaged or leaning (>10%) trees]. These individuals were measured for tree height using a Nikon Laser Rangefinder Forestry Pro hypsometer. The top of the tree was determined from different view angles, and multiple measurements were made to account for over- or underestimation. When the top of an individual was not visible, a different individual using the same selection criteria was selected. The same individuals selected for tree height measurements were selected for wood sampling to determine species wood density, defined as the ratio of oven dry weight to fresh volume. Wood samples with an average size of 5 cm × 5 cm × 5 cm were taken under the bark. For those species with no estimates of wood density, a genus-level average was taken, or when genus-level data were unavailable, a family-level average was used. For the few remaining species for which the family did not occur elsewhere in the plot and for the remaining unidentified individuals, a site average was used. Mean wood density for each plot was weighted by basal area. Stand-specific height-diameter regression models were developed for each forest type. All trees known to be broken, damaged, or leaning more than 10% were excluded from the analysis. Weibull, Chapman-Richards, logistic, power, and two- and three-parameter exponential models were compared. The optimal model was selected on the basis of the Akaike Information Criterion and the residual standard error and was used to determine tree heights for AGC stock estimation. The relation of Chave et al. (38) for moist tropical forest, including height and wood density, was selected for AGC stock estimation, with biomass assumed to be 50% carbon.
The Yangambi Biosphere Reserve is dominated by old-growth forests and recently disturbed regrowth forests. Older regrowth forests that store intermediate quantities of AGC are rare in the area (36) because these sites are often selected by the local community to be cleared for agricultural activities (local communication). The absence of these forests results in greater uncertainty in regression analyses because data for intermediate portions of AGC cannot contribute to the form or parameterization of relationships. However, because our analysis did include data over the full extent of the carbon gradient in our study area (2 to 183 Mg ha−1), the data gap will likely have limited influence on the linear component of regressions (that is, the direction of the biodiversity-carbon relationship: positive or negative). Nonetheless, the diversity of older regrowth forests could influence the quadratic component of the regression (saturating, hump-, or U-shaped). However, older regrowth forests generally converge with old-growth forests in terms of species richness and species composition of trees and animals (39, 40), indicating that inclusion of these forests would not appreciably alter conclusions.
Data collection
To obtain a representative sample of the ecological structure and biodiversity of the Yangambi Biosphere Reserve (41), we included a broad range of life forms from multiple trophic levels: trees, plasmodial slime molds, fungi, lichens, flies, ants, birds, rodents, and shrews. All trees with a DBH ≥10 cm were identified to species level. For individuals that could not be identified to species level in the field, botanical specimens were collected and identified on the basis of a comparison with herbarium material and DNA sequencing. For plasmodial slime molds, the total sampling time per plot was 6 hours (except for plots GIL5 and BRA1, which were sampled for 4 hours). Sampling was done by walking through a plot and searching through substrates. We collected field specimens and various aerial and ground substrates for moist chamber cultures to document as many species as possible. Plots were searched for fungi for 3 days, recording the presence of species. We collected lichens from bark and leaves and corticolous and foliicolous lichens, respectively (for clarity, we refer to these groups as bark lichens and leaf lichens). For the sampling of bark lichens, 12 trees were selected in each plot in a standardized manner (42). Depending on the DBH of trees, lichen species were collected in four frequency ladders of 10 × 50 cm (trees with DBH >36 cm), or between 100 and 150 cm above the ground (DBH ≤36 cm). In each plot, 18 leaves were examined for leaf, lichens: 6 leaves of Scaphopetalum thonneri, 6 of Marantaceae sp., and 6 of other trees and shrubs. An ellipsoid grid of 16 × 6.4 cm, covering an area of ca. 100.5 cm2, was placed on the upper and under sides of the leaf, with one edge of the grid touching one of the margins of the leaf. Empidoid flies were collected via standardized net sweeping. At least two 20-min periods of net sweeping were performed per plot. Arboreal-dwelling ants were collected according to a standardized protocol using bait spread every 5 m along a rope (43). One end of the rope was tied around the trunk, and the other was positioned over a branch in the canopy, forming a loop. Baits comprised a mixture of proteins and carbohydrates and were left for about 4 hours before collection. Birds were caught in the understory using 20 ground-level mist nets, simultaneously established in up to three adjacent plots. Opened nets were checked regularly during daytime. Nets were deployed for 2 to 5 days in each plot. Mist nets were set for a total of 22,717 meter-net-hours (mnh). Sampling effort in plots ranged from 1272 to 3828 mnh. Rodents and shrews were collected using the Paceline method, which consists of placing traps at 5-m intervals on transects (44). On each trapline, three types of traps were used: Sherman LFA traps, Victor snap traps, and Pitfall traps. Traplines were monitored for 21 nights in each plot. Species were identified using DNA barcoding.
For all taxa, specimens that could not be accurately associated with recognized taxa or to morphospecies were excluded from analyses. Because of logistical constraints, only trees and lichens were sampled in all plots. Details on sample storage locations are listed in table S1.
Species richness, diversity, and composition
We characterized the taxonomic dimension of biodiversity using metrics at α- and β-levels. We defined α-level of biodiversity as measurements of local biodiversity using three frequently used measures: species richness, Shannon entropy (45), and the Gini-Simpson index (46). These metrics differ in the extent to which they weight interspecific differences in abundances (species richness, to the zeroth power; Shannon entropy, to the first power; Gini-Simpson index, to the second power). Each metric was transformed to its effective number of species (or Hill number) to represent “true” biodiversity (47), which is the effective number of equally abundant species that would be needed to produce the same value as that of an empirical metric (48). Species richness is an empirical count of the number of species and is already expressed as a Hill number. We used the exponential of Shannon entropy (hereafter referred to as Shannon diversity) and the inverse of the Gini-Simpson index (hereafter referred to as Simpson diversity) as appropriate Hill transformations of abundance-weighted metrics. Because empirical estimates of biodiversity are a function of sample size, α-level measures were standardized for completeness. Sample completeness is the proportion of the total number of individuals in an assemblage that are estimated to belong to the species represented in the sample and can be derived from sampling curves (49). As recommended, extrapolation for each metric at each plot was executed to twice the empirical sample size before standardization (49).
β-Diversity was estimated using pairwise dissimilarities in species composition between plots as represented by the Sørensen (50) and Morisita-Horn (51) indices. The Sørensen index quantifies similarity based on the ratio of the number of shared species (S12) in two plots (1 and 2) to the mean number of species in those same plots [(S1 + S2)/2]. Because the Morisita-Horn index is based on squared differences of the relative abundances of species, its magnitude is primarily determined by the most abundant species, with rare species contributing relatively little (52). When two species assemblages are equally diverse and consist entirely of equally abundant species, the Morisita-Horn index is equal to the Sørensen index. On the basis of additive inverse relationships, we transformed each similarity measure to its corresponding dissimilarity measure (β-diversity).
To determine whether the detected species are habitat specialists, we assessed the occurrence of species in regrowth forests and old-growth forests, respectively, representing forests with lowest and highest carbon stocks in our study area. We used two different methods: one based on species presence and one based on the abundance distribution of species. First, we counted the number of species that were encountered only in regrowth or only in old-growth forest versus the number of species that occurred in both forest types. Second, we used a statistical classification (18) to identify habitat specialists versus generalists. Using a multinomial model based on relative abundances of species in each of two forest types, the method minimizes bias due to differences in sampling intensities between forest types, as well as bias due to insufficient sampling within each forest type. The method permits a robust classification of generalists and specialists without a priori exclusion of rare species. This analysis resulted in the classification of each species into one of four categories: (i) generalist, (ii) regrowth specialist, (iii) old-growth specialist, or (iv) too rare to classify with confidence.
To ensure the use of reliable estimates of biodiversity, we omitted plots with an extrapolated coverage lower than 0.85 from all analyses, except for the identification of specialist species. When abundances were unavailable, as in the case of slime molds and fungi, only species richness, Sørensen’s index, and specialist species based on occurrences were analyzed. All calculations were performed in R 3.1 (R Core Team, 2014) using the packages iNEXT (sample completeness) (53) and SpadeR (similarity indices) (54).
Statistical analyses
To quantify relationships (that is, random, linear, and nonlinear) between aspects of taxonomic biodiversity at the α-level and AGC, we used orthogonal polynomial regression (55). Because we had no a priori empirical evidence or theoretical argument on which to base the exploration of higher-order polynomials, we used second-order polynomials to capture linear and nonlinear relationships. Orthogonal polynomial regression decomposes the general relationship from ordinary polynomial regression into a suite of additive independent polynomials (for example, zeroth-, first-, and second-order relationships), whose coefficients (b*0, b*1, and b*2, respectively) represent their independent contributions and whose statistical significance can then be evaluated in an unbiased fashion. Metrics of biodiversity were weighted by inverses of their SD, thereby giving greater weights to values with a higher certainty. We considered relationships to be significant if both the model and the linear or nonlinear components were significant at an α-level of 0.05. Relationships with significant quadratic terms were subsequently subjected to the Mitchell-Olds and Shaw (MOS) test (56) to distinguish monotonic relationships from those with peaks or troughs. The MOS test, executed in R using vegan (57), is based on a determination of whether the predicted maximum or minimum of a quadratic relationship occurred within the bounds of the empirical data.
We used Mantel tests (58) to quantify associations between differences in species composition (β-diversity) and AGC among all possible pairs of plots. We hypothesized that dissimilarity in species composition would increase with increasing differences in AGC (that is, a one-sided test). A Pearson correlation coefficient was used to determine the direction of association in cases for which the Mantel test was significant. We also used a Mantel test to evaluate the extent to which differences in species composition were related to differences in geographic distance between pairs of plots (spatial autocorrelation); we did so for regrowth and old-growth forests separately, as well as for the entire data set (59). Mantel tests were performed with the R package ecodist (60), and significance levels were assessed with a Monte Carlo procedure with 10,000 permutations.
Supplementary Material
Acknowledgments
We thank I. Janssens and three anonymous reviewers for their comments on an earlier version of this manuscript (artwork by S. Verhulst). Funding: This study is a deliverable of the COBIMFO project (Congo Basin integrated monitoring for forest carbon mitigation and biodiversity; contract no. SD/AR/01A) and was funded by the Belgian Science Policy Office. F.V.d.P. was supported by a Ph.D. fellowship from the Research Foundation–Flanders. M.R.W. and S.J.P. were supported by the Center for Environmental Sciences and Engineering at the University of Connecticut, as well as by NSF grants DEB-1239764 and DEB-1546686. Author contributions: H.V., P.B., D.H., H.B., S.D., H.L., and E.V. developed the project. F.B.A., S.C., M.d.H., A.D.K., D.V.d.B., P.G., S.B.J., M.L., P.M.K., F.V.d.P., and B.W. provided biodiversity data, whereas H.V., P.B., H.B., and E.K. provided AGC data. F.V.d.P. analyzed the data. F.V.d.P., M.R.W., S.J.P., H.L., and E.V. interpreted the results. H.L., S.D., and E.V. supervised the research. F.V.d.P. with assistance from M.R.W., S.J.P., H.L., and E.V. wrote the paper. All authors approved the final version of the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: The carbon inventory data are available in the forestplot.net database (www.forestplots.net/) and in the sPlot database (www.idiv.de/sdiv/working_groups/wg_pool/splot/splot_database.html). All biodiversity data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/3/eaar6603/DC1
fig. S1. AGC increases from regrowth to old-growth forest (mixed or monodominant).
fig. S2. Orthogonal polynomial regression between each aspect of taxonomic biodiversity at the α level and carbon stock for each group.
fig. S3. Relationships between compositional dissimilarity (Sørensen and Morisita-Horn indices) and difference in carbon stocks (in Mg ha−1) between plots.
table S1. Overview of the sampled groups.
table S2. Parameter estimates for orthogonal polynomial regression between each of three measures of taxonomic biodiversity at the α-level and carbon storage, separately for each organismal group.
table S3. For most groups, community composition differs more between forests with larger differences in carbon stock.
table S4. Compositional dissimilarity (Sørensen and Morisita-Horn) is unrelated to geographic distance between pairs of plots, except for trees based on Sørensen dissimilarity in old-growth forests.
table S5. Number of observed individuals and species in regrowth and old-growth forests for each organismal group.
Biodiversity data
REFERENCES AND NOTES
- 1.Millennium Ecosystem Assessment, “Ecosystems and human well-being” (2005); www.millenniumassessment.org/documents/document.356.aspx.pdf.
- 2.UNFCCC, “Adoption of the Paris Agreement FCCC/CP/2015/10/Add.1” (United Nations Office, 2015). [Google Scholar]
- 3.Convention on Biological Diversity, “Strategic plan for biodiversity 2011–2020 and the Aichi Targets” (2011); www.cbd.int/sp/targets/default.shtml.
- 4.Phelps J., Webb E. L., Adams W. M., Biodiversity co-benefits of policies to reduce forest-carbon emissions. Nat. Clim. Change 2, 497–503 (2012). [Google Scholar]
- 5.Soliveres S., van der Plas F., Manning P., Prati D., Gossner M. M., Renner S. C., Alt F., Arndt H., Baumgartner V., Binkenstein J., Birkhofer K., Blaser S., Blüthgen N., Boch S., Böhm S., Börschig C., Buscot F., Diekötter T., Heinze J., Hölzel N., Jung K., Klaus V. H., Kleinebecker T., Klemmer S., Krauss J., Lange M., Morris E. K., Müller J., Oelmann Y., Overmann J., Pašalić E., Rillig M. C., Schaefer H. M., Schloter M., Schmitt B., Schöning I., Schrumpf M., Sikorski J., Socher S. A., Solly E. F., Sonnemann I., Sorkau E., Steckel J., Steffan-Dewenter I., Stempfhuber B., Tschapka M., Türke M., Venter P. C., Weiner C. N., Weisser W. W., Werner M., Westphal C., Wilcke W., Wolters V., Wubet T., Wurst S., Fischer M., Allan E., Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature 536, 456–459 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Sobral M., Silvius K. M., Overman H., Oliveira L. F. B., Raab T. K., Fragoso J. M. V., Mammal diversity influences the carbon cycle through trophic interactions in the Amazon. Nat. Ecol. Evol. 1, 1670–1676 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Lewis S. L., Tropical forests and the changing earth system. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 195–210 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poorter L., van der Sande M. T., Thompson J., Arets E. J. M. M., Alarcón A., Álvarez-Sánchez J., Ascarrunz N., Balvanera P., Barajas-Guzmán G., Boit A., Bongers F., Carvalho F. A., Casanoves F., Cornejo-Tenorio G., Costa F. R. C., de Castilho C. V., Duivenvoorden J. F., Dutrieux L. P., Enquist B. J., Fernández-Méndez F., Finegan B., Gormley L. H. L., Healey J. R., Hoosbeek M. R., Ibarra-Manríquez G., Junqueira A. B., Levis C., Licona J. C., Lisboa L. S., Magnusson W. E., Martínez-Ramos M., Martínez-Yrizar A., Martorano L. G., Maskell L. C., Mazzei L., Meave J. A., Mora F., Muñoz R., Nytch C., Pansonato M. P., Parr T. W., Paz H., Pérez-García E. A., Rentería L. Y., Rodríguez-Velazquez J., Rozendaal D. M. A., Ruschel A. R., Sakschewski B., Salgado-Negret B., Schietti J., Simões M., Sinclair F. L., Souza P. F., Souza F. C., Stropp J., ter Steege H., Swenson N. G., Thonicke K., Toledo M., Uriarte M., van der Hout P., Walker P., Zamora N., Peña-Claros M., Diversity enhances carbon storage in tropical forests. Glob. Ecol. Biogeogr. 24, 1314–1328 (2015). [Google Scholar]
- 9.Cavanaugh K. C., Stephen Gosnell J., Davis S. L., Ahumada J., Boundja P., Clark D. B., Mugerwa B., Jansen P. A., O’Brien T. G., Rovero F., Sheil D., Vasquez R., Andelman S., Carbon storage in tropical forests correlates with taxonomic diversity and functional dominance on a global scale. Glob. Ecol. Biogeogr. 23, 563–573 (2014). [Google Scholar]
- 10.Day M., Baldauf C., Rutishauser E., Sunderland T. C. H., Relationships between tree species diversity and above-ground biomass in Central African rainforests: Implications for REDD. Environ. Conserv. 41, 64–72 (2013). [Google Scholar]
- 11.Sullivan M. J. P., Talbot J., Lewis S. L., Phillips O. L., Qie L., Begne S. K., Chave J., Cuni-Sanchez A., Hubau W., Lopez-Gonzalez G., Miles L., Monteagudo-Mendoza A., Sonké B., Sunderland T., Ter Steege H., White L. J., Affum-Baffoe K., Aiba S.-i., de Almeida E. C., de Oliveira E. A., Alvarez-Loayza P., Dávila E., Andrade A., Aragão L. E., Ashton P., Aymard G. A. C., Baker T. R., Balinga M., Banin L. F., Baraloto C., Bastin J.-F., Berry N., Bogaert J., Bonal D., Bongers F., Brienen R., Camargo J. L., Cerón C., Moscoso V. C., Chezeaux E., Clark C. J., Pacheco Á. C., Comiskey J. A., Valverde F. C., Coronado E. N., Dargie G., Davies S. J., De Canniere C., Djuikouo K. M. N., Doucet J.-L., Erwin T. L., Espejo J. S., Ewango C. E., Fauset S., Feldpausch T. R., Herrera R., Gilpin M., Gloor E., Hall J. S., Harris D. J., Hart T. B., Kartawinata K., Kho L. K., Kitayama K., Laurance S. G., Laurance W. F., Leal M. E., Lovejoy T., Lovett J. C., Lukasu F. M., Makana J.-R., Malhi Y., Maracahipes L., Marimon B. S., Junior B. H., Marshall A. R., Morandi P. S., Mukendi J. T., Mukinzi J., Nilus R., Vargas P. N., Camacho N. C., Pardo G., Peña-Claros M., Pétronelli P., Pickavance G. C., Poulsen A. D., Poulsen J. R., Primack R. B., Priyadi H., Quesada C. A., Reitsma J., Réjou-Méchain M., Restrepo Z., Rutishauser E., Salim K. A., Salomão R. P., Samsoedin I., Sheil D., Sierra R., Silveira M., Slik J. W., Steel L., Taedoumg H., Tan S., Terborgh J. W., Thomas S. C., Toledo M., Umunay P. M., Gamarra L. V., Vieira I. C., Vos V. A., Wang O., Willcock S., Zemagho L., Diversity and carbon storage across the tropical forest biome. Sci. Rep. 7, 39102 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaudrot L., Kroetz K., Alvarez-Loayza P., Amaral E., Breuer T., Fletcher C., Jansen P. A., Kenfack D., Lima M. G. M., Marshall A. R., Martin E. H., Ndoundou-Hockemba M., O’Brien T., Razafimahaimodison J. C., Romero-Saltos H., Rovero F., Roy C. H., Sheil D., Silva C. E. F., Spironello W. R., Valencia R., Zvoleff A., Ahumada J., Andelman S., Limited carbon and biodiversity co-benefits for tropical forest mammals and birds. Ecol. Appl. 26, 1098–1111 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Scheiner S. M., Chiarucci A., Fox G. A., Helmus M. R., McGlinn D. J., Willig M. R., The underpinnings of the relationship of species richness with space and time. Ecol. Monogr. 81, 195–213 (2011). [Google Scholar]
- 14.Mittelbach G. G., Steiner C. F., Scheiner S. M., Gross K. L., Reynolds H. L., Waide R. B., Willig M. R., Dodson S. I., Gough L., What is the observed relationship between species richness and productivity? Ecology 82, 2381–2396 (2001). [Google Scholar]
- 15.Vance-Chalcraft H. D., Willig M. R., Cox S. B., Lugo A. E., Scatena F. N., Relationship between aboveground biomass and multiple measures of biodiversity in subtropical forest of Puerto Rico. Biotropica 42, 290–299 (2010). [Google Scholar]
- 16.Groner E., Novoplansky A., Reconsidering diversity-productivity relationships: Directness of productivity estimates matters. Ecol. Lett. 6, 695–699 (2003). [Google Scholar]
- 17.Socolar J. B., Gilroy J. J., Kunin W. E., Edwards D. P., How should beta-diversity inform biodiversity conservation? Trends Ecol. Evol. 31, 67–80 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Chazdon R. L., Chao A., Colwell R. K., Lin S.-Y., Norden N., Letcher S. G., Clark D. B., Finegan B., Arroyo J. P., A novel statistical method for classifying habitat generalists and specialists. Ecology 92, 1332–1343 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Gardner T. A., Barlow J., Chazdon R., Ewers R. M., Harvey C. A., Peres C. A., Sodhi N. S., Prospects for tropical forest biodiversity in a human-modified world. Ecol. Lett. 12, 561–582 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Lewis S. L., Edwards D. P., Galbraith D., Increasing human dominance of tropical forests. Science 349, 827–832 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Gibson L., Lee T. M., Koh L. P., Brook B. W., Gardner T. A., Barlow J., Peres C. A., Bradshaw C. J. A., Laurance W. F., Lovejoy T. E., Sodhi N. S., Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478, 378–381 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues A. S. L., Pilgrim J., Lamoreux J., Hoffmann M., Brooks T., The value of the IUCN Red List for conservation. Trends Ecol. Evol. 21, 71–76 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Pfeifer M., Lefebvre V., Peres C. A., Banks-Leite C., Wearn O. R., Marsh C. J., Butchart S. H. M., Arroyo-Rodríguez V., Barlow J., Cerezo A., Cisneros L., D’Cruze N., Faria D., Hadley A., Harris S. M., Klingbeil B. T., Kormann U., Lens L., Medina-Rangel G. F., Morante-Filho J. C., Olivier P., Peters S. L., Pidgeon A., Ribeiro D. B., Scherber C., Schneider-Maunoury L., Struebig M., Urbina-Cardona N., Watling J. I., Willig M. R., Wood E. M., Ewers R. M., Creation of forest edges has a global impact on forest vertebrates. Nature 551, 187–191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chazdon R. L., Broadbent E. N., Rozendaal D. M., Bongers F., Zambrano A. M., Aide T. M., Balvanera P., Becknell J. M., Boukili V., Brancalion P. H., Craven D., Almeida-Cortez J. S., Cabral G. A., de Jong B., Denslow J. S., Dent D. H., DeWalt S. J., Dupuy J. M., Durán S. M., Espírito-Santo M. M., Fandino M. C., César R. G., Hall J. S., Hernández-Stefanoni J. L., Jakovac C. C., Junqueira A. B., Kennard D., Letcher S. G., Lohbeck M., Martínez-Ramos M., Massoca P., Meave J. A., Mesquita R., Mora F., Muñoz R., Muscarella R., Nunes Y. R., Ochoa-Gaona S., Orihuela-Belmonte E., Peña-Claros M., Pérez-García E. A., Piotto D., Powers J. S., Rodríguez-Velazquez J., Romero-Pérez I. E., Ruíz J., Saldarriaga J. G., Sanchez-Azofeifa A., Schwartz N. B., Steininger M. K., Swenson N. G., Uriarte M., van Breugel M., van der Wal H., Veloso M. D., Vester H., Vieira I. C., Bentos T. V., Williamson G. B., Poorter L., Carbon sequestration potential of second-growth forest regeneration in the Latin American tropics. Sci. Adv. 2, e1501639 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strassburg B. B. N., Kelly A., Balmford A., Davies R. G., Gibbs H. K., Lovett A., Miles L., Orme C. D. L., Price J., Turner R. K., Rodrigues A. S. L., Global congruence of carbon storage and biodiversity in terrestrial ecosystems. Conserv. Lett. 3, 98–105 (2010). [Google Scholar]
- 26.Anderson B. J., Armsworth P. R., Eigenbrod F., Thomas C. D., Gillings S., Heinemeyer A., Roy D. B., Gaston K. J., Spatial covariance between biodiversity and other ecosystem service priorities. J. Appl. Ecol. 46, 888–896 (2009). [Google Scholar]
- 27.Stephenson S. L., Schnittler M., Lado C., Ecological characterization of a tropical myxomycete assemblage—Maquipucuna Cloud Forest Reserve, Ecuador. Mycologia 96, 488–497 (2004). [PubMed] [Google Scholar]
- 28.van der Sande M. T., Poorter L., Kooistra L., Balvanera P., Thonicke K., Thompson J., Arets E. J. M. M., Garcia Alaniz N., Jones L., Mora F., Mwampamba T. H., Parr T., Peña-Claros M., Biodiversity in species, traits, and structure determines carbon stocks and uptake in tropical forests. Biotropica 49, 593–603 (2017). [Google Scholar]
- 29.Cadotte M. W., Carscadden K., Mirotchnick N., Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 48, 1079–1087 (2011). [Google Scholar]
- 30.Chazdon R. L., Peres C. A., Dent D., Sheil D., Lugo A. E., Lamb D., Stork N. E., Miller S. E., The potential for species conservation in tropical secondary forests. Conserv. Biol. 23, 1406–1417 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Rudel T. K., The national determinants of deforestation in sub-Saharan Africa. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120405 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sloan S., Goosem M., Laurance S. G., Tropical forest regeneration following land abandonment is driven by primary rainforest distribution in an old pastoral region. Landsc. Ecol. 31, 601–618 (2016). [Google Scholar]
- 33.Gilroy J. J., Woodcock P., Edwards F. A., Wheeler C., Baptiste B. L. G., Uribe C. A. M., Haugaasen T., Edwards D. P., Cheap carbon and biodiversity co-benefits from forest regeneration in a hotspot of endemism. Nat. Clim. Change 4, 503–507 (2014). [Google Scholar]
- 34.Jantz P., Goetz S., Laporte N., Carbon stock corridors to mitigate climate change and promote biodiversity in the tropics. Nat. Clim. Change 4, 138–142 (2014). [Google Scholar]
- 35.P. Gilson, A. VanWambeke, R. Gutzweiler, Notice Explicative de la Carte des Sols et de la Végétation, N6: Yangambi, planchette 2: Yangambi (INEAC, 1956). [Google Scholar]
- 36.Kearsley E., de Haulleville T., Hufkens K., Kidimbu A., Toirambe B., Baert G., Huygens D., Kebede Y., Defourny P., Bogaert J., Beeckman H., Steppe K., Boeckx P., Verbeeck H., Conventional tree height–diameter relationships significantly overestimate aboveground carbon stocks in the Central Congo Basin. Nat. Commun. 4, 2269 (2013). [DOI] [PubMed] [Google Scholar]
- 37.O. L. Phillips, T. R. Baker, T. R. Feldpausch, R. Brienen, Field manual for plot establishment and remeasurement (Rainfor, 2010); http://www.rainfor.org/en/manuals/in-the-field. [Google Scholar]
- 38.Chave J., Andalo C., Brown S., Cairns M. A., Chambers J. Q., Eamus D., Fölster H., Fromard F., Higuchi N., Kira T., Lescure J.-P., Nelson B. W., Ogawa H., Puig H., Riéra B., Yamakura T., Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145, 87–99 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Letcher S. G., Chazdon R. L., Rapid recovery of biomass, species richness, and species composition in a forest chronosequence in northeastern Costa Rica. Biotropica 41, 608–617 (2009). [Google Scholar]
- 40.Dent D. H., Wright S. J., The future of tropical species in secondary forests: A quantitative review. Biol. Conserv. 142, 2833–2843 (2009). [Google Scholar]
- 41.Naeem S., Emmett Duffy J., Zavaleta E., The functions of biological diversity in an age of extinction. Science 336, 1401–1406 (2012). [DOI] [PubMed] [Google Scholar]
- 42.J. Asta, W. Erhardt, M. Ferretti, F. Fornasier, U. Kirschbaum, P. L. Nimis, O. W. Purvis, S. Pirintsos, C. Scheidegger, C. Van Haluwyn, V. Wirth, Mapping lichen diversity as an indicator of environmental quality, in Monitoring with Lichens—Monitoring Lichens, P. Nimis, C. Scheidegger, P. A. Wolseley, Eds. (Springer Netherlands, 2002), vol. 7, pp. 273–279.
- 43.M. Leponce, A. Dejean, How to assess rapidly the spatial distribution of numerically dominant ants in the canopy? in Anais XX Simposio de Mirmecologia I Encuentro de Mirmecologistas de las Americas (Universidade Federal Rural do Rio de Janeiro, 2011), pp. 49–50. [Google Scholar]
- 44.R. E. Martin, R. H. Pine, A. F. Blase, A Manual for Mammalogy with Keys to Families of the World (McGraw-Hill Higher Education, 2001), vol. 3. [Google Scholar]
- 45.C. E. Shannon, W. Weaver, The Mathematical Theory of Communication (University of Illinois Press, 1949). [Google Scholar]
- 46.Simpson E. H., Measurement of diversity. Nature 163, 688 (1949). [Google Scholar]
- 47.Jost L., Entropy and diversity. Oikos 113, 363–375 (2006). [Google Scholar]
- 48.Chao A., Gotelli N. J., Hsieh T. C., Sander E. L., Ma K. H., Colwell R. K., Ellison A. M., Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 84, 45–67 (2014). [Google Scholar]
- 49.Chao A., Jost L., Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 93, 2533–2547 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Sørensen T., A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. Biol. Skr. 5, 1–34 (1948). [Google Scholar]
- 51.Morisita M., Measuring of interspecific association and similarity between communities. Mem. Fac. Sci. Kyushu Univ. Ser. E 3, 65–80 (1959). [Google Scholar]
- 52.L. Jost, A. Chao, R. L. Chazdon, Compositional similarity and β (beta) diversity, in Biological Diversity: Frontiers in Measurement and Assessment, A. E. Magurran, B. McGill, Eds. (Oxford Univ. Press, 2011), pp. 66–84.
- 53.T. C. Hsieh, K. H. Ma, A. Chao, iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers) (2016); http://chao.stat.nthu.edu.tw/wordpress/software_download/.
- 54.A. Chao, K. H. Ma, T. C. Hsieh, SpadeR: Species Prediction and Diversity Estimation with R (2015); http://chao.stat.nthu.edu.tw/wordpress/software_download/. [Google Scholar]
- 55.Dutka A. F., Ewens F. J., A method of improving the accuracy of polynomial regression analysis. J. Qual. Technol. 3, 149–155 (1971). [Google Scholar]
- 56.Mitchell-Olds T., Shaw R. G., Regression analysis of natural selection: Statistical inference and biological interpretation. Evolution 41, 1149–1161 (1987). [DOI] [PubMed] [Google Scholar]
- 57.J. Oksanen, F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs, H. Wagner, vegan: Community Ecology Package (2015); http://cran.r-project.org/package=vegan.
- 58.Mantel N., The detection of disease clustering and generalized regression approach. Cancer Res. 27, 209–220 (1967). [PubMed] [Google Scholar]
- 59.Edwards D. P., Magrach A., Woodcock P., Ji Y., Lim N. T.-L., Edwards F. A., Larsen T. H., Hsu W. W., Benedick S., Vun Khen C., Chung A. Y. C., Reynolds G., Fisher B., Laurance W. F., Wilcove D. S., Hamer K. C., Yu D. W., Selective-logging and oil palm: Multitaxon impacts, biodiversity indicators, and trade-offs for conservation planning. Ecol. Appl. 24, 2029–2049 (2014). [PubMed] [Google Scholar]
- 60.Goslee S. C., Urban D. L., The ecodist package for dissimilarity-based analysis of ecological data. J. Stat. Softw. 22, 1–19 (2007). [Google Scholar]
- 61.Feest A., The quantitative ecology of soil Mycetozoa. Prog. Protistol. 2, 331–361 (1987). [Google Scholar]
- 62.Eyi Ndong H., Degreef J., De Kesel A., Les champignons comestibles de l’Afrique centrale. Taxonomie et identification. Abc Taxa 10, 253 (2011). [Google Scholar]
- 63.F. Rose, S. Coppins, Site assessment of epiphytic habitats using lichen indices, in Monitoring with Lichens—Monitoring Lichens, P. L. Nimis, C. Scheidegger, P. Wolseley, Eds. (Kluwer, Dordrecht-Boston-London, NATO Science, 2002), pp. 343–348. [Google Scholar]
- 64.Lücking R., The use of foliicolous lichens as bioindicators in the tropics, with special reference to the micriclimate. Abstr. Bot. 21, 99–116 (1997). [Google Scholar]
- 65.Folgarait P. J., Ant biodiversity and its relationship to ecosystem functioning: A review. Biodivers. Conserv. 7, 1221–1244 (1998). [Google Scholar]
- 66.Del Toro I., Ribbons R. R., Pelini S. L., The little things that run the world revisited: A review of ant-mediated ecosystem services and disservices (Hymenoptera: Formicidae). Myrmecol. News 17, 133–146 (2012). [Google Scholar]
- 67.Cooleman S., Bapeamoni F., Louette M., Lens L., Angenong’a U., Bird functional diversity in the Yangambi Biosphere Reserve, DR Congo. Bull. African Bird Club 22, 171–182 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/3/eaar6603/DC1
fig. S1. AGC increases from regrowth to old-growth forest (mixed or monodominant).
fig. S2. Orthogonal polynomial regression between each aspect of taxonomic biodiversity at the α level and carbon stock for each group.
fig. S3. Relationships between compositional dissimilarity (Sørensen and Morisita-Horn indices) and difference in carbon stocks (in Mg ha−1) between plots.
table S1. Overview of the sampled groups.
table S2. Parameter estimates for orthogonal polynomial regression between each of three measures of taxonomic biodiversity at the α-level and carbon storage, separately for each organismal group.
table S3. For most groups, community composition differs more between forests with larger differences in carbon stock.
table S4. Compositional dissimilarity (Sørensen and Morisita-Horn) is unrelated to geographic distance between pairs of plots, except for trees based on Sørensen dissimilarity in old-growth forests.
table S5. Number of observed individuals and species in regrowth and old-growth forests for each organismal group.
Biodiversity data