Abstract
Background: Obesity, a global health problem and a chronic disease, is associated with increased risk of developing type 2 diabetes and coronary heart diseases. A wide variety of natural remedies have been explored for their obesity treatment potential.
Objective: The anti-adipogenic effect of ginsenoside Rg5:Rk1 (Rg5:Rk1) on 3T3-L1 mature adipocytes was investigated.
Materials and Methods: To elucidate the anti-obesity effect of Rg5:Rk1, a mixture of protopanaxadiol type ginsenosides isolated from Panax ginseng Meyer in a 3T3-L1 adipocytes. In order to determine the anti-obesity effect of Rg5:Rk1, based on oil Red O Staining, triglyceride (TG) content in adipose cells was assessed. Furthermore, to elucidate the possible mechanism of Rg5:RK1 effect on lipid accumulation, mRNA and protein expression analyses of adipocyte markers such as STAT3, PPARγ, CBEPα and ap2 were carried out.
Results: Rg5:Rk1 treatment showed an inhibition of lipid droplet accumulation and decrease of TG content. In addition, expression of STAT3, PPARγ, CEBPα and ap2 were decreased in a dose dependent manner. Similarly, the Rg5:Rk1 treatment reduced PPARγ and CEBPα protein expression.
Conclusion: Rg5:Rk1 treatment exhibits anti-adipogenic activity by down-regulation of the STAT3/ PPARg/CEBPa signaling pathway in 3T3-L1 adipocyte cell line.
Keywords: Adipogenesis, Ginsenosides, Obesity, PPAR-γ3T3-L1 cell
1. Background
Obesity is considered a formost health problem and major risk factor for serious metabolic diseases such as diabetes, hypertension, heart disease, dyslipidemia, atherosclerosis, stroke, nonalcoholic fatty liver disease, neuronal injury and cancer (1-4). Moreover, obesity is defined as the imbalance between energy intake and expenditure that eventually leads to the accumulation of the excess amount of lipids (triglycerides) in a major cellular component adipocytes cell. Adipocytes play an important role in regulating lipid metabolism (5, 6). However, adipogenic differentiation is a complex process where pre-adipocytes are converted into adipocytes by regulating multiple signaling pathways. In this process, genes such as peroxisome proliferator-activated receptor gamma (PPARγ) and CCAATT enhancer binding proteins (C/EBP) are required to be transcribed for adipogenic differentiation. These so-called master regulators of adipogenesis control the expression of a variety of transcriptional factor to the formation of mature adipocytes (7). In addition to these transcription factors, recent studies have shown that the signal transducer and activator of transcription 3 (STAT3) and Jak2 (Janus kinase 2) play important roles in adipogenic differentiation by regulating PPARγ (8). Therefore, suppression of this process is essential to control adipogenesis. Many studies have aimed to reduce obesity by focusing on decreasing pre-adipocyte differentiation, inhibiting adipogenesis and increasing lipolysis (9). Although, a lot of drugs are available in market to treat obesity, but their prolong exposure may cause severe side effects. Thus, scientists are looking forward for new natural sources which have a strong potential of anti-adipogenic activity.
Natural compounds from different sources have gained attention owing to safety and extended efficacies in the development of pharmaceutical agents against obesity. Several natural plants, have been shown to exert anti-adipogenic through the modulation of different signaling pathway (10). Panax ginseng is one of the ancient beneficial medicinal plant has reported for the various pharmacological effect such as anti-cancer, anti-inflammation, anti-obesity, bone osteoporosis, sexual enhancement and diabetics (11). Ginsenoside Rg5:Rk1 is a mixture of protopanaxadiol-type ginsenoside, isolated from P. ginseng root, via a nine-time repetitive steaming and drying process (black ginseng), and was shown to possess many biological effects such as osteoporosis and inflammation (12, 13). However, the effect of Rg5:Rk1 in adipogenesis and the related underlying mechanism have not yet been investigated.
2. Objective
In this study, the anti-adipogenic effect of ginsenoside Rg5:Rk1 was investigated. Cytotoxicity and lipid accumulation or TG content in 3T3-L1 mature adipocytes were evaluated. Furthermore, the ability of ginsenoside Rg5:Rk1 to reduce the expression of different adipogenic markers in 3T3-L1 mature adipocytes was assessed.
3. Materials and Methods
3.1. Chemicals
3T3-L1 fibroblast cells were purchased from the ATCC, American Type culture collection. The cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and 1% penicillin streptomycin (P/S). Cell culture media Dulbecco’s modified Eagle’s medium (DMEM) and newborn calf serum (BCS) was obtained from Welgene (Gyeongsangbuk-do, South Korea), the antibiotic solution was purchased from GeneDEPOT (Barker, TX, USA). Insulin, 3-isobutyl-1-methyxanthine (IBMX), dexamethasone were purchased from Wako (Tokyo, Japan), PPARγ (cat no: sc 7273, mouse monoclonal), CEBPα (cat no: sc-61) and Beta actin (cat no: sc-47778, mouse monoclonal) primary antibody were from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The ginsenoside Rg5:Rk1 mixture (95% purity) was obtained from Ginseng Genetic Resource Bank (Kyung Hee University, Korea) and dissolved in dimethyl sulfoxide (DMSO) and stored at -20 ºC.
3.2. Cell Culture and Differentiation of Adipocytes
3T3-L1 fibroblast was grown and differentiated into the adipocyte. The cells were maintained in DMEM supplemented with 10% BCS and 1 % penicillin-streptomycin (P/S) (complete medium) at 37 °C in a CO2 incubator. Cells were plated at density 1 ×104 in a 24 well plate complete medium. Two or three days after confluency (day 0) of the cells were stimulated with differentiation medium (MDI) containing 1 µM dexamethasone, 0.5 mM IBMX, and 10 µg.mL-1 insulin added into Complete media. After two days later, 3T3-L1 cells were supplemented with insulin in complete media defined as differentiation medium (DM) in the presence or absence of ginsenoside Rg5:Rk1 and further incubated for additional 5-8 days. Approximately the day 8 adipocyte cell displayed the characteristic of the differentiated cell (with lipid droplet) under the microscope.
3.3. Cytotoxicity Assay
Cytotoxicity of ginsenoside Rg5:Rk1 was determined by the MTT assay. 3T3-L1 pre-adipocytes were seeded in a 96 well plate at a density of 1x104cells/well. After confluency (24 h incubation), the media was fully removed and the cells were treated with various concentrations (1-100 μg.mL-1) of ginsenoside Rg5:Rk1 for 72 h. MTT assay solutions (20 μL) were added to the treated cells to stain the pre-adipocyte cell. Cell viability was determined using an ELISA reader (Bio-Tek Instruments, Inc., Vinooski, VT, USA) at 570 nm as previously described (14).
3.4. Oil Red O Staining and TG Content
To investigate the excess lipid accumulation in adipocyte cell Oil red O (ORO) staining assay was performed. After differentiation of 3T3-L1 pre-adipocyte to mature adipocyte on day 8, cells were washed with PBS and fixed with 3.7% (v/v) formaldehyde for 1 h. Cells were stained with 60% isopropanol in filtered ORO solution (6:4 of oil red stock solution: distilled water) for 30 min. The excess stain was removed by washing with distilled water 3 to 4 times before observation under an inverted light microscope (Nikon Instruments, Melville, NJ, USA). For the quantitative determination of excess lipid (TG content) in adipocytes, ORO staining eluted with 100% isopropanol and the absorbance was measured at 520 nm using Synergy™2 microplate reader (Bio-Tek Instruments, Inc., Vinooski, VT, USA).
3.5. RNA Isolation and RT-PCR
Total RNA was extracted from differentiated 3T3-L1 on day 8 using TriZol LS reagents (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s protocol. First strand cDNA was synthesized with 1 µg of total RNA by using Thermo Scientific cDNA synthesis kit (Onebio, Lithuania, EU) according to the protocol. The Reverse-transcription polymerase chain reaction (RT-PCR) was performed on the cDNA using gene-specific primers (Table 1). For amplification of PPAR-γ and STAT3 the PCR cycles (31) were 94 °C for 40 s; 58 °C; 72 °C for 5 min. PCR amplicons were loaded in 1% agarose gel for electrophoresis.
Table 1. Primers used for RT-PCR and quantative real-time RT-PCR.
| Primer Name | Sequence | Tm ( ◦ C) |
| PPAR-γ |
Forward: 5'-ATGGGTGAAACTCTGGGAGATT-3' Reverse: 5'-AGCTTCAATCGGATGGTTCTT-3' |
58.4 55.9 |
| CEBPα |
Forward: 5'-TTCATGGAGAATGGGGGCAC-3' Reverse: 5'-TAGACGTGCACACTGCCATT-3' |
59.3 57.3 |
| STAT3 |
Forward: 5'-GCCACGT TGGTGTTTCATAATC-3' Reverse: 5'-TTCGAAGGTTGTGCTGATAGAG-3' |
58.4 58.4 |
| ap2 |
Forward : 5'-AAAGACAGCTCCTCCTCGAAGGTT-3' Reverse: 5'-TGACCAAATCCCCATTTACGC-3' |
66.7 57.9 |
| β-actin |
Forward:5'-ATGAAGTGTGACGTTACGC-3' Reverse: 5'-CCTAGAAGCATTTGCGGTGCAC-3' |
52.94 64.75 |
3.6. Real-Time PCR
The gene expression levels were analyzed by quantitative real-time RT-PCR using a real-time rotary analyzer (Rotor-Gene 6000; Corbet Life Science, Sydney, Australia). The reaction was performed by using SYBER® Green SensiMix plus Master Mix (Quantace, England), with gene specific primers (Table 1). The thermo reaction (10-14 µL) was contained 1-2 µg of cDNA. Real-Time PCR was carried out for 40 cycles of 95 °C: 10 sec; 60 °C: 10 sec;72 °C: 20 sec. β-actin was used as housekeeping gene to normalize the data (15).
3.7. Western Blotting
Cells were seeded in 6 well plate at a density of 1×104 and adipocyte differentiation was induced as described above with the presence or absence of ginsenoside Rg5:Rk1 at concentration of 100 µg.mL-1. After differentiation on day 8, cells were rinsed twice with PBS and incubated with 2X sodium dodecyl sulfate (SDS) loading buffer containing 100 mM Tris-Cl (pH 6.8), 4% (w/v) SDS, 0.2% (w/v) bromophenol blue, 20% (v/v) glycerol and 200 mM β-mercaptoethanol for 5 min at 22 ºC (17). Cell lysates were collected and denatured at 95 ºC for 10 min. Protein samples were loaded to 10% SDS-polyacrylamide gel and electrophoresed. The separated proteins were transferred to PVDF membrane (ATTO Corporation, Tokyo, Japan) at 100 V for 2 h. Membranes were blocked with 5% skimmed milk for 2 h and incubated with specific primary antibodies such as PPARγ, CEBPα (Santa Cruz Biotechnology, Inc) for 16 h at 4 ºC. The memberanes were washed 7 times with Tris-buffered saline and Tween 20 (TBST). The blot was probed by anti-rabbit secondary antibodies (Santa Cruz Biotechnology, Inc) for 2 h at 22 ºC and immuno-reactivity was detected through Enhanced Chemiluminescence system (Amersham Bioscience Inc., Piscataway, NJ, USA) and exposed to X-ray film (Fuji photo Film Co. Ltd Minato, Tokyo, Japan). Band densities were measured using ImageJ software (16).
3.8. Statistical Analysis
Statistical analysis was performed using GraphPad 6.04 software (La Jolla, CA). Data are expressed as means ± standard error of mean (SEM) of three independent experiment. The statistical significance of the differences between values was evaluated by one-way ANOVA. Statistical significance was accepted at a level of p ≤ 0.05.
4. Results
Effects of Ginsenoside Rg5:Rk1 on Cell Viability
Ginsenoside Rg5:Rk1 exerted no cytotoxic effect at concentration up to 100 µg.mL-1whereas more than 98% cells were viable (Data not shown).
4.1. Effect of Ginsenoside Rg5:Rk1 on Intracellular Lipid Accumulation During Differentiation in 3T3-L1 Pre-adipocyte
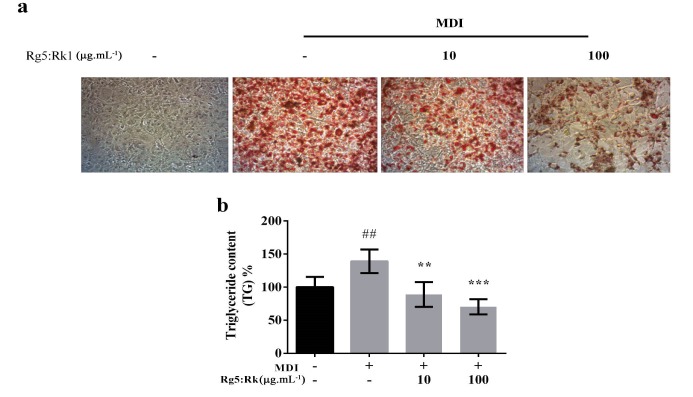
The effect of Rg5:Rk1 was tested to determine if it can suppress adipogenesis differentiation in 3T3-L1. Cells were incubated with adipocyte hormonal cocktail with the presence or absence of Rg5:Rk1 at different concentration. Inside fat droplet, formation was analyzed by ORO assay. As shown in Figure 1a, intracellular lipid accumulation reduced to 100 µg.mL-1compared with MDI-treated cells used as the positive control. In addition, TG content suppressed at same concentration (Fig. 1b).
Figure 1.
Effect of different concentration of ginsenoside Rg5:Rk1 on MDI-induced intracellular lipid accumulation in 3T3-L1 cells. The data shown are representatives of triplicate experiments. a: Demonstration of reduction of lipid accumulation in the cultured cells treated with ginsenoside Rg5:RK1, in a dose dependent manner.b: Comparative study of the Triglyceride content of the cells, treated with various amount of ginsenoside Rg5:RK1 Data are expressed as a percentage of control. ## (MDI treated cell) p < 0.01 compare with control (absence of MDI and only given complete media) and **: p < 0.01; ***: p < 0 .001 versus MDI treated cell (differentiated cell).
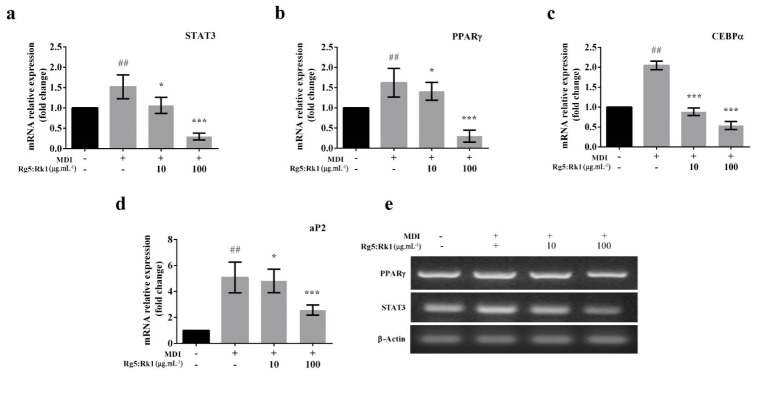
4.2. Effect of Ginsenoside Rg5:Rk1 on Adipogenic Markers STAT3, PPARγ, CEBPα, ap2
Adipocyte differentiation is inflicted by the master regulator PPARγ involved in triglyceride synthesis and pre-adipocyte to adipocytes. Therefore, 3T3-L1 cells were differentiated into mature adipocyte in the presence/absence of ginsenoside Rg5:Rk1. The expression of adipocyte markers, STAT3, PPARγ, CEBPα, and ap2, were analyzed by both RT-PCR and qRT-PCR. STAT3, PPARγ, CEBPα, and ap2 were significantly suppressed in a dose-dependent manner (Figs. 2a-2d). RT-PCR data showed that the expression of STAT3 and PPARγ were significantly decreased by the treatment with ginsenoside Rg5:Rk1 at the 100 µg.mL-1 Therefore, ginsenoside Rg5:Rk1 may have a natural anti-adipogenic compound.
Figure 2.
Effect of different concentration of ginsenoside Rg5:Rk1 on adipogenic transcriptional factors in 3T3-L1 adipocytes. Panels a-d: Th quantification of mRNA levels of STAT3, PPAR-γ, CEBPα and aP2 genes were analyzed by qRT-PCT and normalized with β-actin as house keeping gene. Each value is expressed as the mean ± standard error of three independent experiments. ## (MDI treated cell) p < 0.01 compare with control (absence of MDI and only given complete media) and **: p < 0.01; ***: p < 0 .001 versus MDI treated cell (differentiated cell). Panel e: ThemRNA expression levels of PPAR-γ and STAT3 by RT-PCR.
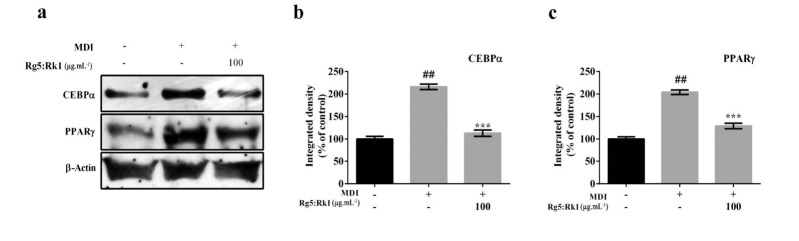
4.3. Inhibitory Effect of Ginsenoside Rg5:Rk1 on the Expression of Adipogenic Protein Expression
The effect of Rg5:Rk1 (100 µg.mL-1) on the expression of PPARγ and CEBPα involved in adipogenesis were examined. Western blot analysis revealed that a time-dependent increase in the protein expression levels of PPARγ and CEBPα in 3T3-L1 pre-adipocytes induced by the treatment of MDI in positive control compared with control (cell culturing complete media containing 10% BCS, 1% penicillin streptomycin in DMEM). Whereas, treatment with Rg5:Rk1 (100 µg.mL-1) significantly inhibited the adipogenic protein expression such as PPARγ and CEBPα (Fig. 3).
Figure 3.
Protein expression levels of PPAR and CEBP were measured by western blotting (panel a), and integrated density was analyzed by ImageJ software (panels b and c). Integrated density values were expressed as % of control. All experiment were performed in triplicate.
5. Discussion
This study exposed for the first time the anti-adipogenic effect of Rg5:Rk1 on the suppression of adipocyte differentiation through down-regulation of transcriptional factors such as PPARɣ, STAT3, aP2 and CEBPα. Obesity is characterized at the cellular level by an increase in the number and size of adipocytes differentiated from fibroblastic pre-adipocytes to mature adipocytes in adipose tissue (18). Therefore, in order to interpret the potential anti-obesity effect and the mechanisms involved, the optimal working concentration of ginsenoside Rg5:Rk1 (Rg5:Rk1) was checked by evaluating its cytotoxicity on 3T3-L1 pre-adipocytes. The result showed no significant cytotoxicity effect on 3T3-L1 up to 100 µg.mL-1.
The development of fat cells from pre-adipocyte to mature adipocyte includes morphological changes, increase in cell numbers, expansion of cell size, expression of lipogenic enzyme and extensive accumulation of lipid droplet (19-21). In addition, accumulation of lipid droplet is an index of adipogenesis and it can be confirmed by ORO staining assay, which stains TGs and lipids in the mature adipocytes (22). So far, many studies have been reported that ginsenosides from Panax ginseng Meyer can reduce lipid accumulation in mature adipocyte by up and/or down regulation of several signaling pathways at in vitro level (23-25). Our data showed that, Rg5:Rk1 effectively inhibited the adipogenic differentiation (reduced lipid droplet size) in 3T3-L1 pre-adipocytes at 100 µg.mL-1 (Fig. 1a). Similarly, the TG content in mature adipocytes significantly reduced at 100 µg.mL-1 (Fig. 1b). Taken together, these data suggest that Rg5:Rk1 may have anti-adipogenic effect by reducing lipid droplet accumulation in 3T3-L1 mature adipocyte.
Adipogenesis is the procession of the reflection of lipid accumulation of intracellular adipocytes through several signaling pathway by inducing overexpression of transcriptional factors such as STAT3, PPARɣ, CEBPα and ap2 (26, 27). Among them, PPARɣ and CEBPα are the major transcriptional factors regulating adipogenesis through modulating the expression of its target genes during early to middle stage of differentiation. Specially, PPARɣ (peroxisome proliferator-activated receptor-gamma) is highly expressed in adipocyte tissue compare with other tissues, and highly up-regulated when pre-adipocytes are converted into mature adipocyte (28). Besides, CEBPα (CCAAT/enhancer binding protein-alpha) primarily expressed in fat, liver and intestinal tissues, and is also reported as a key regulator of adipogenesis. Particularly, PPARɣ and CEBPα were reported to act as a cross-regulator on adipogenesis. These transcription factors control the adipogenic target gene such as ap2 (activating protein), which is directly implicated in lipogenic pathways in 3T3-L1 adipocytes (7). In addition, recent studies showed that signal transducer and activator of transcription 3 (STAT3) plays an imperative role in adipogenesis process (29). Furthermore, several studies have shown adipocyte differentiation is largely influenced by the expression of PPARγ and CEBPα (30-32). We found that transcript analysis of STAT3, PPARɣ, CEBPα and ap2 genes, were significantly enhanced by the stimulation of hormone cocktail (MDI) once compared with undifferentiated cells. On the other hand, mRNA expression levels of these genes were decreased in differentiated cells after treatment with Rg5:Rk1 at 100 µg.mL-1 (Figs. 2a-d). In addition, RT-PCR data showed that STAT3, PPARɣ genes were significantly blocked by the treatment of Rg5:Rk1 at concentration 100 µg.mL-1 (Fig. 2e). Therefore, these results support that Rg5:Rk1 is capable of blocking adipogenic transcriptional factors. Subsequently, we investigated the protein expression level of major adipogenic proteins, PPARɣ and CEBPα. It was found that Rg5:Rk1 significantly down-regulated protein expression levels at 100 µg.mL-1 (Figs. 3a-c). Results indicates that expression of mRNA level of adipogenic genes and adipogenic proteins are similarly downregulated by the treatment of Rg5:Rk1 at 100 µg.mL-1. Taken together, this data suggest that reduction of lipid accumulation in 3T3-l1 cells after Rg5:Rk1 treatment might be regulated by down-regulation of the STAT3/ PPARɣ/CEBPα signaling pathway. However, this study is limited by the nature of pre-adipocytes growth cycle that remains to be elucidated.
In conclusion, our results demonstrate that Rg5:Rk1 from Panax ginseng Meyer efficiently inhibited adipogenesis in 3T3-L1 adipocytes by a significant reduction in intracellular lipid accumulation without induction of cytotoxicity in a dose-dependent manner. Furthermore, the inhibition of adipogenesis by Rg5:Rk1 might be mediated by major transcriptional activators PPARγ and CEBPα. Therefore, ginsenoside Rg5:Rk1 may provide a possible therapeutic approach for the treatment of obesity.
Conflict of Interest
The authors have declared that there is no conflict of interest.
Acknowledgments
This research was supported by iPET (313038-03-1-SB010), Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries, Republic of Korea. The ginseng sample used in this study was provided by Ginseng Bank Kyung Hee University.
References
- 1.Giri S, Rattan R, Haq E, Khan M, Yasmin R, Won J-s, Key L, Singh AK, Singh I. AICAR inhibits adipocyte differentiation in 3T3L1 and restores metabolic alterations in diet-induced obesity mice model. Nutr Metab. 2006;3(1):1. doi: 10.1186/43-7075-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–43. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 3.Hursting SD, Hursting MJ. Growth Signals, Inflammation, and Vascular Perturbations Mechanistic Links Between Obesity, Metabolic Syndrome, and Cancer. Arterioscler Thromb Vasc Biol. 2012;32(8):1766–70. doi: 10.1161/ATVBAHA.111.241927. [DOI] [PubMed] [Google Scholar]
- 4.Choi JS, Kim J-H, Ali MY, Min B-S, Kim G-D, Jung HA. Coptis chinensis alkaloids exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBP-α and PPAR-γ. Fitoterapia. 2014;98:199–208. doi: 10.1016/j.fitote.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Frühbeck G, Gómez-Ambrosi J, Muruzábal FJ, Burrell MA. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab. 2001;0(6):E827–E47. doi: 10.1152/ajpendo.2001.0.6.E827. [DOI] [PubMed] [Google Scholar]
- 6.Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11(8):327–32. doi: 10.1016/S1043-2760(00)001-5. [DOI] [PubMed] [Google Scholar]
- 7.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14(11):13–307. doi: 10.1002/cphy.c160022. [DOI] [PubMed] [Google Scholar]
- 8.Wang D, Zhou Y, Lei W, Zhang K, Shi J, Hu Y, Shu G, Song J. Signal transducer and activator of transcription 3 (STAT3) regulates adipocyte differentiation via peroxisome-proliferator-activated receptor γ (PPARγ) Biol Cell. 2010;102(1):1–12. doi: 10.1042/BC20090070. [DOI] [PubMed] [Google Scholar]
- 9.Ono M, Fujimori K. Antiadipogenic effect of dietary apigenin through activation of AMPK in 3T3-L1 cells. J Agric Food Chem. 2011;59(24):13346–52. doi: 10.1021/jf203490a. [DOI] [PubMed] [Google Scholar]
- 10.Yun JW. Possible anti-obesity therapeutics from nature-A review. Phytochemistry. 2010;71(14):1625–41. doi: 10.1016/j.phytochem.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Helmes S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9(3):259–75. [PubMed] [Google Scholar]
- 12.Siddiqi MH, Siddiqi MZ, Kang S, Noh HY, Ahn S, Simu SY, Aziz MA, Sathishkumar N, Jiménez Pérez ZE, Yang DC. Inhibition of osteoclast differentiation by ginsenoside Rg3 in RAW264 7 cells via RANKL, JNK and p38 MAPK pathways through a modulation of Cathepsin K: an in silico and in vitro study. Phytother Re. s 20;29(9):16–94. doi: 10.1016/j.phytochem.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Ahn S, Siddiqi MH, Aceituno VC, Simu SY, Zhang J, Perez ZEJ, Kim Y-J, Yang D-C. Ginsenoside Rg5: Rk1 attenuates TNF-α/IFN-γ-induced production of thymus-and activation-regulated chemokine (TARC/CCL17) and LPS-induced NO production via downregulation of NF-κB/p38 MAPK/STAT1 signaling in human keratinocytes and macrophages. In Vitro Cell Dev Biol Anim. 2016;52(3):287–95. doi: 10.1007/s11626-015-9983-y. [DOI] [PubMed] [Google Scholar]
- 14.Siddiqi MH, Siddiqi MZ, Ahn S, Kim YJ, Yang DC. Ginsenoside Rh1 induces mouse osteoblast growth and differentiation through the bone morphogenetic protein 2/runt-related gene 2 signalling pathway. J Pharm Pharmacol. 2014;66(12):1763–73. doi: 10.1111/jphp.12306. [DOI] [PubMed] [Google Scholar]
- 15.Ahn S, Siddiqi MH, Aceituno VC, Simu SY, Zhang J, Perez ZEJ, Kim Y-J, Yang D-C. Ginsenoside Rg5: Rk1 attenuates TNF-α/IFN-γ-induced production of thymus-and activation-regulated chemokine (TARC/CCL17) and LPS-induced NO production via downregulation of NF-κB/p38 MAPK/STAT1 signaling in human keratinocytes and macrophages. In Vitro Cellular & Developmental Biology-Animal. 2015:1–9. doi: 10.1007/s11626-015-9983-y. [DOI] [PubMed] [Google Scholar]
- 16.collines TJ. ImageJ for microscopy. Biotechniques. 2007;43(1):25–30. doi: 10.2144/000112517. [DOI] [PubMed] [Google Scholar]
- 17.Dubon MJ, Park KS. Substance P enhances the proliferation and migration potential of murine bone marrow-derived mesenchymal stem cell-like cell lines. Exp Ther Med. 2015;9(4):1185–91. doi: 10.3892/etm.2015.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prins JB, O’rahilly S. Regulation of adipose cell number in man. Clin Sci. 1997;92(1):3–11. doi: 10.1042/cs0920003. [DOI] [PubMed] [Google Scholar]
- 19.Devlin MJ, Yanovski SZ, Wilson GT. Obesity: what mental health professionals need to know. Am J Psychiatry. 2000;157(6):854–66. doi: 10.1176/appi.ajp.157.6.854. [DOI] [PubMed] [Google Scholar]
- 20.Fujioka K. Management of obesity as a chronic disease: nonpharmacologic, pharmacologic, and surgical options. Obes Res. 2002;10(S12):116S–23S. doi: 10.1038/oby.2002.204. [DOI] [PubMed] [Google Scholar]
- 21.Choi H, Eo H, Park K, Jin M, Park E-J, Kim S-H, Park JE, Kim S. A water-soluble extract from Cucurbita moschata shows anti-obesity effects by controlling lipid metabolism in a high fat diet-induced obesity mouse model. Biochem Biophys Res Commun. 2007;359(3):419–25. doi: 10.1016/j.bbrc.2007.05.107. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez-Zacarias J, Castro-Munozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97(6):493–7. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 23.Park S, Ahn IS, Kwon DY, Ko BS, Jun WK. Ginsenosides Rb1 and Rg1 suppress triglyceride accumulation in 3T3-L1 adipocytes and enhance β-cell insulin secretion and viability in Min6 cells via PKA-dependent pathways. Biosci Biotechnol Biochem. 2008;72(11):2815–23. doi: 10.1016/j.bbrc.2007.05.107. [DOI] [PubMed] [Google Scholar]
- 24.Hwang J-T, Kim S-H, Lee M-S, Kim SH, Yang H-J, Kim M-J, Kim H-S, Ha J, Kim MS, Kwon DY. Anti-obesity effects of ginsenoside Rh2 are associated with the activation of AMPK signaling pathway in 3T3-L1 adipocyte. Biochem Biophys Res Commun. 2007;364(4):1002–8. doi: 10.1016/j.bbrc.2007.10.125. [DOI] [PubMed] [Google Scholar]
- 25.Siraj FM, Natarajan S, Huq MA, Kim YJ, Yang DC. Structural investigation of ginsenoside Rf with PPARγ major transcriptional factor of adipogenesis and its impact on adipocyte. J Ginseng Res. 2015;39(2):141–7. doi: 10.1016/j.jgr.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song N-J, Yoon H-J, Kim KH, Jung S-R, Jang W-S, Seo C-R, Lee YM, Kweon D-H, Hong J-W, Lee J-S. Butein is a novel anti-adipogenic compound. J Lipid Res. 2013;54(5):1385–96. doi: 10.1194/jlr.M035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobson NG, Szabo SJ, Weber-Nordt RM, Zhong Z, Schreiber RD, Darnell J, Murphy KM. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat) 3 and Stat4. J Exp Med. 1995;181(5):1755–62. doi: 10.1194/jlr.M035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farmer S. Regulation of PPARγ activity during adipogenesis. Int J Obes. 2005;29:S13–S6. doi: 10.1038/sj.ijo.0802907. [DOI] [PubMed] [Google Scholar]
- 29.Zhang K, Guo W, Yang Y, Wu J. JAK2/STAT3 pathway is involved in the early stage of adipogenesis through regulating C/EBPβ transcription. J Cell Biochem. 2011;112(2):488–97. doi: 10.1002/jcb.22936. [DOI] [PubMed] [Google Scholar]
- 30.Park HJ, Chung BY, Lee M-K, Song Y, Lee SS, Chu GM, Kang S-N, Song YM, Kim G-S, Cho J-H. Centipede grass exerts anti-adipogenic activity through inhibition of C/EBPβ, C/EBPα, and PPARγ expression and the AKT signaling pathway in 3T3-L1 adipocytes. BMC Complement Altern Med. 2012;12(1):1. doi: 10.1186/1472-6882-12-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang SJ, Park N-Y, Lim Y. Anti-adipogenic effect of mulberry leaf ethanol extract in 3T3-L1 adipocytes. Nutrition research and practice. 2014;8(6):613–7. doi: 10.4162/nrp.2014.8.6.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen ED, Hsu C-H, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes & development. 2002;16(1):22–6. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]