Abstract
Somatic cell nuclear transfer is a valuable technique for the generation of genetically engineered animals, however the efficiency of cloning in mammalian species is low (1–3%). Differentiated somatic cells commonly used in nuclear transfer utilize the tricarboxylic acid cycle and cellular respiration for energy production. Comparatively the metabolism of somatic cells contrasts that of the cells within the early embryos which predominately use glycolysis. Early embryos (prior to implantation) are evidenced to exhibit characteristics of a Warburg Effect (WE)-like metabolism. We hypothesized that pharmacologically driven fibroblast cells can become more blastomere-like and result in improved in vitro embryonic development after SCNT. The goals were to determine if subsequent in vitro embryo development is impacted by 1) cloning pharmacologically treated donor cells pushed to have a WE-like metabolism, or 2) culturing non-treated donor clones with pharmaceuticals used to push a WE-like metabolism. Additionally, we investigated early gestational survival of the donor-treated clone embryos. Here we demonstrate that in vitro development of clones is not hindered by pharmacologically treating either the donor cells or the embryos themselves with CPI, PS48, or the combination of these drugs. Furthermore, these experiments demonstrate that early embryos (or at least in vitro produced embryos) have a low proportion of mitochondria which have high membrane potential and treatment with these pharmaceuticals does not further alter the mitochondrial function in early embryos. Lastly, we show that survival in early gestation was not different between clones from pharmacologically induced WE-like donor cells and controls.
Keywords: somatic cell nuclear transfer, cloning, donor cell, metabolism, embryo, Warburg, embryonic development, gestation
Introduction
Advancing gene editing technologies has allowed the creation of pigs that exhibit phenotypes of pathological conditions such as breast cancer, spinal muscular atrophy, cystic fibrosis, and other maladies. These models are often more reliable and more phenotypically accurate than other animal models (reviewed in (Fan and Lai 2013; Prather et al. 2013a; Walters et al. 2012)). Moreover, genetically modified pigs are proving to be advantageous to production agriculture and animal welfare as well (Prather et al. 2013b; Whitworth et al. 2016; Whyte and Prather 2011). In light of these innovations, one limitation of the pig is the lack of porcine embryonic stem cell lines or validated pluripotency induction protocols which are readily available in other model species (Brevini et al. 2007; Hall 2008; Koh and Piedrahita 2014; Piedrahita et al. 1990). Due to this, the majority of genetic modifications for pig models are achieved through Somatic Cell Nuclear Transfer (SCNT), however the success rate of this process is currently only 1–3% in swine (Whitworth and Prather 2010). Nuclear transfer embryos which are derived from blastomere donor cells are demonstrated to have a high rate of development (Heyman et al. 2002; Mitalipov et al. 2002; Wilmut et al. 2002); however, others contend that the degree of differentiation of the donor cell may have little impact (Oback and Wells 2007; Wakayama and Yanagimachi 2001l).
Recent evidence suggests that early embryos exhibit a Warburg Effect (WE)-like metabolism prior to implantation (Krisher and Prather 2012; Redel et al. 2012). A WE hallmark is the predominate use of glycolysis to acquire energy where subsequently lactate is produced as opposed to the tricarboxylic acid cycle (TCA) as used by differentiated cells (Warburg 1956). This is a metabolic phenotype exhibited in various types of cancer cells. Increased signaling of the PI3K/AKT pathway is correlated with an increase in glycolytic metabolism through regulation of expression and activity of glucose transporters and the enzymes phosphofructokinase and hexokinase (reviewed in (Hatzivassiliou et al. 2005; Robey and Hay 2009)). The allosteric small molecule PS48 (5-(4-Chloro-phenyl)-3-phenyl-pent-2-enoic acid) is demonstrated to promote glycolysis and lactate production as well as enhance reprogramming efficiency of induced pluripotent stem cells by about 15-fold (Zhu et al. 2010). The pharmaceutical CPI-613 (6, 8-bis(benzylthio)octanoic acid: hereafter called CPI) inhibits pyruvate dehydrogenase which catalyzes the conversion of pyruvate to acetyl-CoA, a reaction necessary for entrance to the TCA cycle (Lee et al. 2011; Zachar et al. 2011). Both compounds should decrease mitochondrial use of the TCA cycle and promote the PI3K pathway, promoting resources toward the pentose phosphate pathway. In this study, we investigated embryonic development of clones as impacted by PS48, CPI, and the combination of drugs (MIX) on 1) embryos created from the treated donor cells, and 2) during culture of embryos created from non-treated donor cells. Embryos produced from these treatments were assessed for percentage of zygotic cleavage and blastocyst formation, total cell number within blastocysts, DNA damage or degradation within blastomeres, and mitochondrial membrane potential (Δψm) all of which have been previously implicated as indicators of developmental quality and competence.
Results
In vitro development of embryos derived from non-treated donor cells cultured with pharmaceuticals after reconstruction
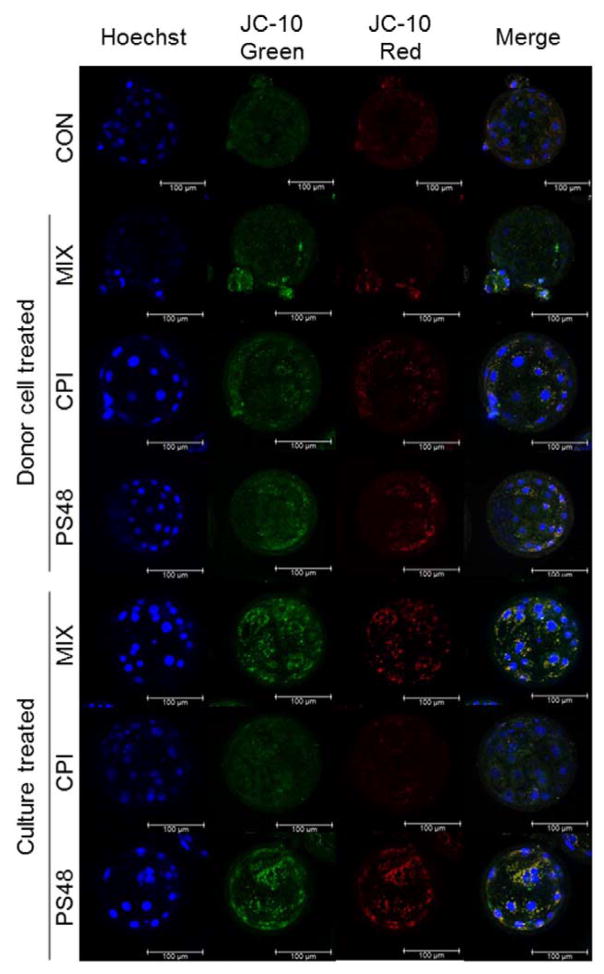
Drug treatments were tested in the culture of SCNT embryos from non-drug-treated fibroblasts where pharmaceuticals were added during the incubation period with scriptaid culture and again (16 hours later) when they were moved to new culture media for the remainder of development until the blastocyst stage (day 7; 168 hours after activation). Blastocyst development was impacted by drug culture treatment (P = 0.05): PS48 10 μM and CON (0 μM) had higher percentages (43.3 and 41.2%) compared to CPI 100 μM and MIX (33.6 and 32.7 ± 2.9%; Table 1). Compared to the other treatments, terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) positive cell number was increased in MIX embryos (P = 0.01; MIX = 2.1 vs. ≤ 1.4 ± 0.26 in other treatments; Table 1). Zygotic cleavage percentage and cell number within blastocysts were not altered with embryonic drug culture (P ≥ 0.07; Table 1). By the time blastocyst formation had occurred, mitochondrial membrane potential (as measured by JC-10 staining) was not different when treatment during culture (after cloning; P = 0.23; Table 1). Figure 3 depicts representative JC-10 stained clone embryos. For publication, embryos were imaged differently than the imaging of blastocysts for analysis (see methods section for further detail) therefore intensities depicted are not reflective of intensity values in Table 1.
Table 1.
Impacts of PS48 and CPI-613 treatment in culture media on subsequent development of clones.
| Treatment‡
|
SEM | P-value | ||||
|---|---|---|---|---|---|---|
| CON | CPI | PS48 | MIX | |||
| Cleavage rate§, % | 91.8 | 89.2 | 95.2 | 87.7 | 1.9 | 0.07 |
| Blastocyst rate*, % | 41.1 A | 33.6B | 43.3A | 32.7 B | 2.9 | 0.05 |
| Blastocyst cell number | 32.3 | 34.8 | 36.8 | 35.1 | 2.2 | 0.48 |
| TUNEL positive cell number≠ | 1.2A | 1.0A | 1.4A | 2.1B | 0.36 | 0.01 |
| Red Intensity, AU╪ | 15.1A | 11.4C | 12.6 BC | 13.9 AB | 0.74 | 0.0038 |
| Green Intensity, AU╪ | 79.6A | 52.5C | 60.7 BC | 73.6 AB | 5.8 | 0.0045 |
| Red/green ratio╪ | 0.26 | 0.29 | 0.24 | 0.21 | 0.02 | 0.23 |
Embryos treated with the pharmaceuticals CPI (100 μM), PS48 (10 μM), the mixture of the two (MIX), or as a vehicle control (CON; 100 μM DMSO) during scriptaid treatment and embryo culture.
Percentage of reconstructed embryos which cleaved after 40 hours; n ≥ 52 clones per treatment for each of the 5 replicates.
Percentage of reconstructed embryos which developed to the blastocyst stage; n ≥ 52 clones per treatment for each of the 5 replicates.
Number of cells in blastocysts which stained positive for terminal deoxynucleotidyl transferase dUTP nick-end labeling; n ≥ 10 clones per treatment for each of the 3 replicates.
Intensity of red or green fluorescence or the ratio of red/green fluorescence of blastocyst stage embryos stained with JC-10; n ≥ 10 clones per treatment for each of the 3 replicates.
Figure 3.
Representative CPI (100 μM), PS48 (10 μM), MIX (CPI 100 μM + PS48 10 μM) or CON (0 μM) donor-treated or culture-treated somatic cell nuclear transfer derived blastocysts stained with the biphasic dye JC-10, a measure for mitochondrial membrane potential (Δψm).
In vitro development of donor cell treated derived embryos
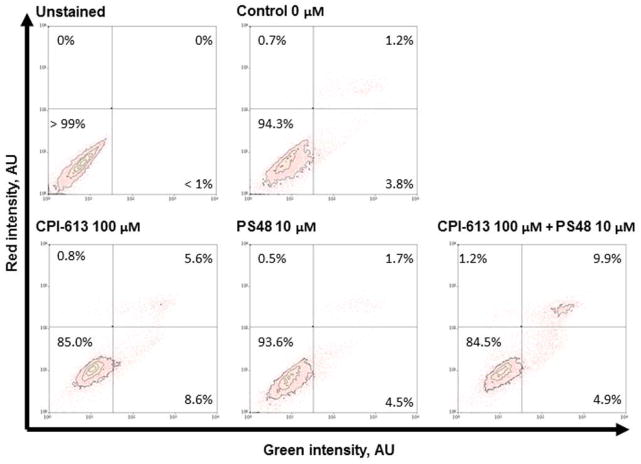
After 7 days of respective treatments, fibroblasts were analyzed for viability by Annexin-V-conjugated FITC and propidium iodide staining. All treatments yielded a large proportion of healthy cells for use in SCNT (Figure 1). Initially, we tested for impact of CPI and PS48 dosage on number of blastomeres and degree of DNA degradation within clones derived from pharmaceutically treated donor fibroblasts. The percentage of embryos which underwent zygotic cleavage, the percent which formed blastocysts, the number of blastomeres within blastocysts, and the number of blastomeres with detectable DNA damage (as measured by TUNEL) were not augmented with donor cell dosages of CPI (0, 50, 100 μM; P ≥ 0.33; Supplementary Table 1), nor by dosages of PS48 (0, 5, 10 μM; P ≥ 0.08; Supplementary Table 2). As the highest concentrations of CPI and PS48 did not negatively impact development, they were used in subsequent experiments; moreover the two compounds were also used in combination as per the goal to program a WE-like metabolic effect. Cleavage and blastocyst percentages, blastocyst cell number, and TUNEL positive cell number were not augmented by donor cell treatments (P > 0.14; Table 2.). Mitochondrial membrane potential of blastocysts was not impacted when donor cells received pharmacological treatment (P = 0.12; Table 1). Figure 3 depicts representative JC-10 stained clone embryos. For publication, embryos were imaged differently than the imaging of blastocysts for analysis (see methods section for further detail) therefore intensities depicted are not reflective of intensity values in Table 1.
Figure 1.
Cell viability population measures from annexin-V (FITC) and propidium iodide intensity of porcine fetal fibroblasts after 7 day pharmacological treatment with CPI (100 μM), PS48 (10 μM), the mixture of the two (MIX), or without drugs (CON; 0 μM).
Table 2.
Impacts of donor cell treatment with PS48 and CPI-613 for 7 days prior to nuclear transfer on subsequent development of clones.
| Treatment‡
|
SEM | P-value | ||||
|---|---|---|---|---|---|---|
| CON | CPI | PS48 | MIX | |||
| Cleavage rate§, % | 90.5 | 91.0 | 91.8 | 87.7 | 2.4 | 0.67 |
| Blastocyst rate*, % | 41.6 | 36.1 | 46.2 | 45.3 | 3.1 | 0.14 |
| Blastocyst cell numberΔ | 34.6 | 32.0 | 35.8 | 35.3 | 2.1 | 0.50 |
| TUNEL positive cell number≠ | 1.9 | 1.2 | 1.5 | 1.7 | 0.3 | 0.20 |
| Red Intensity, AU╪ | 10.4 A | 10.0 AB | 9.0 BC | 9.4C | 0.4 | 0.02 |
| Green Intensity, AU╪ | 56.0A | 48.7AB | 38.5B | 46.0AB | 4.6 | 0.05 |
| Red/green ratio╪ | 0.23 | 0.26 | 0.30 | 0.26 | 0.02 | 0.12 |
Donor fibroblasts treated for 7 days with the pharmaceuticals CPI (100 μM), PS48 (10 μM), the mixture of the two (MIX), or without drugs (CON; 0 μM).
Percentage of reconstructed embryos which cleaved after 40 hours; n ≥ 52 clones per treatment for each of the 7 replicates.
Percentage of reconstructed embryos which developed to the blastocyst stage; n ≥ 52 clones per treatment for each of the 7 replicates.
Number of cells in blastocysts which stained positive for terminal deoxynucleotidyl transferase dUTP nick-end labeling; n ≥ 12 clones per treatment for each of the 3 replicates.
Intensity of red or green fluorescence or the ratio of red/green fluorescence of blastocyst stage embryos stained with JC-10; n ≥ 10 clones per treatment for each of the 3 replicates.
During SCNT, the treatments were randomized so that reconstructed embryos from one treatment or another were not always created first or last. We kept track of the ‘order’ of reconstruction and activation during all SCNTs. The order in which SCNT embryos were produced in the same day did not affect the rate of zygotic cleavage (P = 0.80); however, it did impact the percentage which obtained blastocyst stage development (P = 0.03). The duration between SCNT groups was approximately 20 minutes across replicates. Embryos which were in the first 3 SCNT groups created had higher blastocyst production rates ≥ 41.5% whereas those which were produced last had a rate of 33.5% blastocyst formation (Error = 3.2%; Table 3).
Table 3.
Impact of nuclear transfer order on subsequent development of clones.
| Order of nuclear transfer and fusion‡
|
SEM | P-value | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
|
| ||||||
| Cleavage rate§*, % | 88.6 | 89.7 | 91.6 | 91.1 | 2.4 | 0.80 |
| Blastocyst rate*, % | 47.6 A | 41.6 A | 46.4 A | 33.5 B | 3.2 | 0.03 |
| Blastocyst cell number | 34.7 | 36.1 | 33.4 | 33.4 | 2.0 | 0.73 |
| TUNEL positive cell number≠ | 1.3 A | 1.3 A | 1.4 A | 2.2 B | 0.2 | 0.03 |
Order in which embryos were reconstructed, fused, and artificially activated.
Percentage of reconstructed embryos which cleaved after 40 hours.
Percentage of reconstructed embryos which developed to the blastocyst stage.
Number of cells in blastocysts which stained positive for terminal deoxynucleotidyl transferase dUTP nick-end labeling.
In utero early gestational survival of embryos based on transfer parameters
Embryos used for embryo transfer had been reconstructed either 5 or 6 days prior. Embryos which were transferred on day 6 of development had a higher probability of survival to day 35 of gestation than embryos transferred on day 5 of development (0.070 ± 0.013 vs. 0.036 ± 0.010; P = 0.017). There was also an impact of cell line on embryonic survival (P < 0.0001) where clones created from the green fluorescent protein cell line had higher survival probabilities than those from the tomato fluorescent line (0.081 vs. 0.025; Error = 0.010). There was not an interaction of cell line used for cloning and embryonic day of development on survival (P = 0.65). Gilts used as surrogates were either in day 3, 4, or 5 of their estrous cycles where day 0 of heat is considered the first day gilts were observed as ‘in heat’ or receptive to breeding. There was an interaction of gilt cycle day and day of embryonic development at the time of transfer (P < 0.0001) where day 6 developed embryos transferred into day 4 cyclic surrogates had higher probability of survival (0.119 ± 0.016) than the other combinations for transfer (P ≤ 0.054 ± 0.019; Figure 2).
Figure 2.
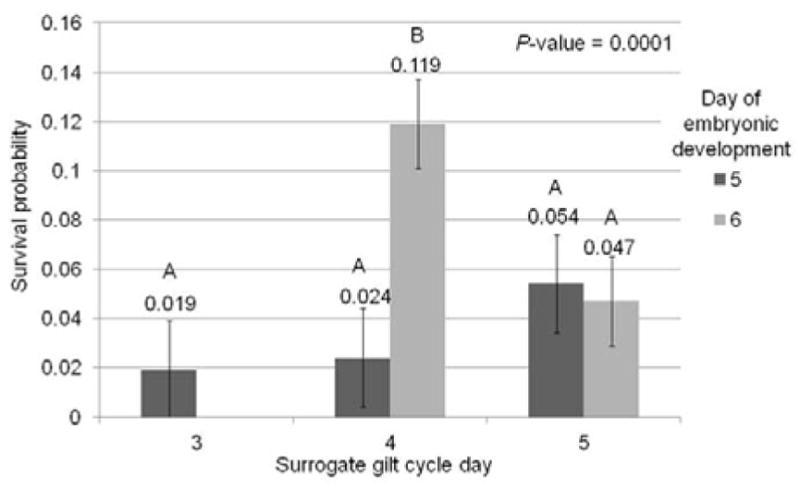
Survival probability (number of piglets collected at day 35 of gestation from total number of reconstructed embryos transferred) of developmental day 5 or 6 embryos transferred to surrogate gilts on 3, 4, and 5 days after observed heat.
In utero early gestational survival of embryos from CPI and PS48 treated donor cells
Five of the twelve surrogates became pregnant in experiments in which we transferred embryos created from SCNT of cells treated for 7 days with either CPI (100 μM) or PS48 (10 μM). For results of day 35 retrievals, see Table 4. One gilt was humanely euthanized shortly after embryo transfer for health and welfare concerns unrelated to the experiment and was not included in analysis. There was no main effect of donor cell treatment on survival (P = 0.85; Table 5). Moreover there was not an interaction of treatment and cell line on survival probability (P = 0.05; Table 5).
Table 4.
Gestational day 35 fetal retrievals from somatic cell nuclear transfer of CPI (100 μM CPI-613) and PS48 (10 μM) treated GFP (green fluorescent protein) and TOM (tomato fluorescent protein) fibroblasts.
| Gilt recipient cycle day | Blastocysts transferred | Developmental day of blastocysts | Total litter size | Number of CPI fetuses‡ | Number of PS48 fetuses‡ |
|---|---|---|---|---|---|
| 5 | 50 | 6 | 7 | 2 TOM | 5 GFP |
| 4 | 40 | 6 | 1* | 1 GFP | None |
| 4 | 40 | 6 | 5 | 5 GFP | None |
| 5 | 50 | 6 | 2 | 1 GFP | 1 TOM |
| 4 | 46 | 6 | 13 | 5 TOM | 8 GFP |
Donor fibroblasts treated for 7 days with the pharmaceuticals CPI (100 μM) or PS48 (10 μM) prior to somatic cell nuclear transfer.
Uterus contained 3 small regressing fetuses with no observable fluorescence.
Table 5.
Impact of 7 day donor cell treatment with 100 μM CPI-613 or 10 μM PS48 treated GFP (green fluorescent protein) and TOM (tomato fluorescent protein) fibroblasts on clone embryo transfer survival to day 35 of gestation.
| Treatment‡ | Cell line | Survival probability≠ | SE | P-value |
|---|---|---|---|---|
| CPI | GFP | 0.066 | 0.023 | 0.05 |
| PS48 | GFP | 0.105 | 0.021 | |
| CPI | TOM | 0.057 | 0.021 | |
| PS48 | TOM | 0.094 | 0.023 |
Donor fibroblasts treated for 7 days with the pharmaceuticals CPI (100 μM) or PS48 (10 μM) prior to somatic cell nuclear transfer.
Survival probability: number of piglets collected at day 35 of gestation from total number of reconstructed embryos transferred.
In utero early gestational survival of embryos from MIX and CON treated donor cells
Four of the fourteen surrogates became pregnant in experiments in which we transferred embryos created from SCNT of cells treated for 7 days with either MIX (CPI 100 μM and PS48 10 μM) or CON (0 μM). For results of day 35 retrievals see Table 6. In this study five gilts were detected to be pregnant by ultrasound on day 21, but had lost the pregnancies by day 35 when fetuses were retrieved. Donor cell treatment did not impact survival in early gestation (P = 0.91; Table 7), and there was not a significant interaction of treatment and cell line (P = 0.60; Table 7).
Table 6.
Gestational day 35 fetal retrievals from somatic cell nuclear transfer of CON (0 μM) and MIX (100 μM CPI-613 + 10 μM PS48) treated GFP (green fluorescent protein) and TOM (tomato fluorescent protein) fibroblasts.
| Gilt recipient cycle day | Blastocysts and morulas transferred | Developmental day of blastocysts | Total litter size | Number of CON fetuses | Number of MIX fetuses |
|---|---|---|---|---|---|
| 5 | 50§ | 5 | 7€ | 2 TOM | 5 GFP |
| 4 | 36‡ | 6 | 2 | 2 GFP | None |
| 4 | 40‡ | 6 | 8* | 1 TOM | 7 GFP |
| 3 | 40≠ | 5 | 8|| | 7 GFP | 1 TOM |
Twenty blastocysts and five morula of each treatment were transferred.
Uterus contained two small fetuses both in regression; one TOM and one GFP.
All blastocysts transferred.
Uterus contained 2 small fetuses 1 highly regressed and 1 with cranial bleeding; one TOM and 1 GFP.
Seventeen blastocysts and 3 morula of TOM MIX transferred and eighteen blastocysts and two morula of GFP CON transferred.
Uterus contained three additional highly regressed necrotic fetuses two were GFP and one was TOM.
Table 7.
Interaction of 7 day donor cell treatment with MIX (100 μM CPI-613 and 10 μM PS48) or CON (0 μM) on GFP (green fluorescent protein) or TOM (tomato fluorescent protein) fibroblasts on clone embryo transfer survival to day 35 of gestation.
| Treatment‡ | Cell line | Survival probability≠ | SE | P-value |
|---|---|---|---|---|
| CON | GFP | 0.070 | 0.019 | 0.60 |
| MIX | GFP | 0.077 | 0.017 | |
| CON | TOM | 0.020 | 0.017 | |
| MIX | TOM | 0.009 | 0.019 |
Donor fibroblasts treated for 7 days with the pharmaceuticals CPI (100 μM) and PS48 (10 μM) or as CON (0 μM) prior to somatic cell nuclear transfer.
Survival probability: number of piglets collected at day 35 of gestation from total number of reconstructed embryos transferred.
Discussion
In the current study we did not detect differences in cleavage, blastocyst formation, or DNA degradation amongst our treatments. The SCNT embryos produced in our experiments exhibited in vitro development typical of porcine clones. For example, 41.4% of SCNT embryos reached the blastocyst stage and had an average cell number of about 33 cells; of these, roughly 1- 2 cells were TUNEL positive. These parameters are similar to previous observations of porcine SCNT blastocysts (Liang et al. 2015; Samiec et al. 2015). During SCNT experiments for donor cell treatments, we noticed negative association between the length of time, or order, in which the embryos were produced, and development to the blastocyst stage, as well as with the amount of DNA degradation within those blastocysts. This occurred without differences in cleavage or total cell number of blastocysts. The impact of order was eliminated in the pharmaceutically culture-treated experiments as the fused groups of embryos were split evenly amongst treatments prior to addition of pharmaceuticals to culture media. We are currently exploring why this occurred; however, we recorded the time at which donor cell treatments were brought to the SCNT technicians. The average duration of time between treatments was about 20 minutes, which is due partially to the large number of clones created in this study. We speculate that perhaps the effect of order is due to an optimal oocyte maturation time having passed, or related to time-delay of fusion and activation, or the time interval between fusion and activation. In any event, these results necessitate additional research.
The developmental measures in this study are commonly used qualitative measures for testing embryonic competence. Recent literature indicates that rate of blastocyst formation and total cell number within blastocyst may not be the best predictors for in utero survival (Redel et al. 2016). For example, there are studies in which blastocyst rate of SCNT embryos was higher than that of IVF embryos (Cui et al. 2011; Iager et al. 2008) however the birth rate of IVF produced embryos in many species is currently higher than that of clones. Emerging technologies including use of time-lapse recording demonstrate that the timing of cleavage and blastocyst formation may be more useful predictors of embryonic survival than the actual rate of cleavage and blastocyst formation (Cruz et al. 2012; Isom et al. 2012; Kaith et al. 2015; Motato et al. 2016). In the present study however, in vitro development was reflective of in utero survival where none of the treatments out performed each other.
Contrary to our hypothesis, clone development during in vitro culture to the blastocyst stage and survival in early gestation were not largely impacted when donor cells were treated with pharmaceutical agents. Our objective was to determine if a drug-induced Warburg-like effect in donor cells prior to SCNT would improve clone survival, however we also tested CPI and PS48 in embryo culture media. Selected dosages of CPI (100 μM) and PS48 (10 μM) were based on prior research demonstrating a dosage-dependent loss of mitochondrial membrane potential in the same porcine fetal fibroblast line which was used for the in vitro experiments in this study (Mordhorst et al., 2018). The mixture of these drugs at these concentrations induced changes in expression of phosphoenolpyruvate carboxykinase 2, phosphoglycerate dehydrogenase, phosphoserine phosphatase as well as the amount of pyruvate, fructose, serine, and glutamine in conditioned media indicating changes in metabolic pathways including glycolysis and serine biosynthesis (Mordhorst et al., 2018).
In the current study the same non-fluorescent cell line was used for all experiments except for the embryo transfers. That cell line was created from a fetus within the tomato litter and therefore was a full sibling to the fetus from which the tomato cell line (used in embryo transfers) came from (see Figure 5). Furthermore, the CAG-promoter driven GFP-Proteasome Fusion Protein (PSMA1) transgenic boar used in experiments from Miles et al. (2013) and the transgenic Ubiquitin C (UBC) promoter driven tandem-dimer (td)Tomato fluorescent boar are both derived from the same original Minnesota Mini pig line. Therefore, all cell lines in these experiments are consanguineous. See Figure 4 for images of fluorescent cell lines and reconstructed embryos. The most profound effect observed in clone gestational survival was related to the line of cells used, not by gene expression induced by treatment within those cell lines. Interestingly, in this study the UBC driven tdTomato clone lines have decreased gestational survival. While there are numerous possible reasons why this may be occurring, no specific cause is obvious. This observation could be related to the fluorescent protein itself, for example there is another red fluorescent protein known as ‘KillerRed’ from the jellyfish Anthomedusa which has a mutated protein anm2CP and is highly phototoxic (Pletnev et al., 2009; Vegh et al., 2011). However tdTomato is widely used by many other laboratories so the issue at hand is likely related to the insertion site of the gene. Further investigations are warranted in determining why cell lines of the same cell type with similar genetics can impact the outcome of cloning to such a degree. We and other laboratories, have observed that some cell lines are more ‘clonable’ than others (Powell et al., 2004; Shi et al., 2007).
Figure 5.
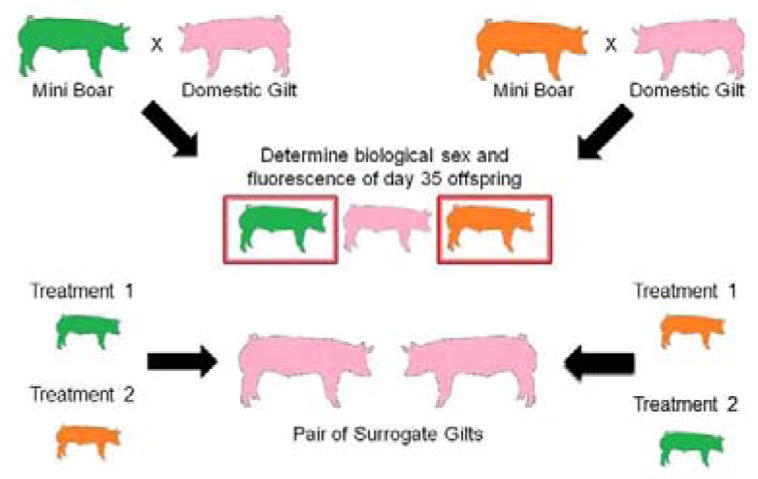
Design of cell line creation by breeding of CAG- promoter driven GFP-Proteasome Fusion Protein (PSMA1) and Ubiquitin C (UBC)- promoter driven tandem-dimer (td)Tomato fluorescent protein Minnesota miniature boars to domestic gilts.
Figure 4.
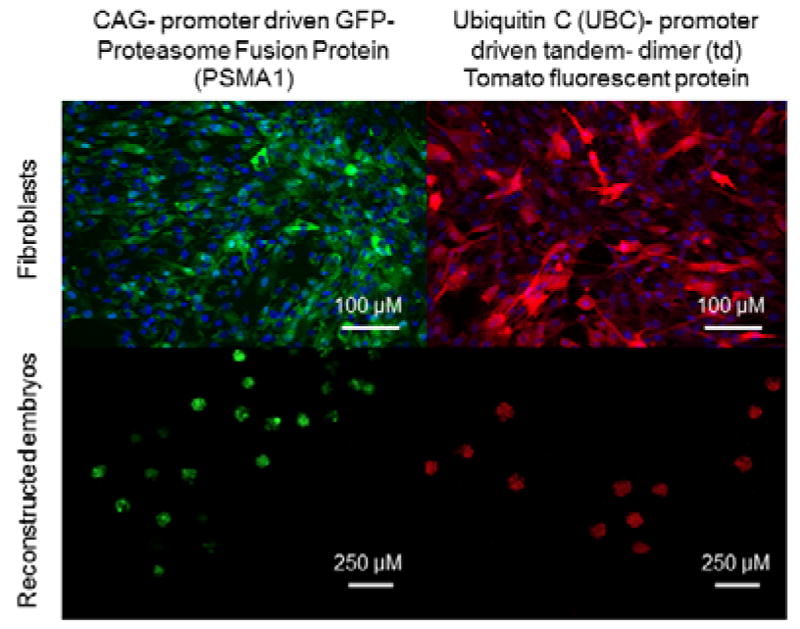
Green and tomato fluorescent cell lines used in somatic cell nuclear transfer for embryo transfer experiments and cleaving reconstructed embryos.
We did not observe developmental differences when the embryos from non-treated donors were exposed to the pharmaceutical agents. After Scriptaid treatment, drugs were added and were not added to the embryo media again during the week of culture. We tested CPI and PS48 in embryo culture to determine impacts of their exposure on clone embryos as pharmaceuticals were kept in the droplets with the cells during manipulation in our donor cell treatments.
There were no changes in mitochondrial membrane potential in clones derived from drug-treated donor cells. This is likely due to low contribution of donor-inherited mitochondria as the enucleated oocytes contain many of their own mitochondria. Moreover, pharmaceutical treatments applied initially during in vitro embryo culture treatment did not provide any long-term mitochondrial membrane potential impairment. In this study all embryos had a low red intensity to green intensity ratio (ratio of J-aggregates to monomers) averaging 0.26 regardless of treatment. Figure 3 depicts the lower amount of red JC-10 stain as well as the overall similarity of mitochondrial membrane potential amongst the clone embryo treatments in this study. Notably embryos in this figure were not imaged in the same way which was performed for the analysis, therefore the intensities seen may not reflect values which are shown. This can be attributed to imaging in dish wells using a confocal microscope with adjusted excitation/emission wavelengths whereas for analysis, embryos were imaged on slides via widefield microscope using standard FITC and Texas red filters. Please see our methods for further detail.
Our results indicate that in early clone embryos, mitochondrial membrane potential is low, and there is not a large amount of mitochondrial respiratory function occurring during this stage of pre-implantation development. This supports the observations from Leese et al. (2008) that embryo metabolism is ‘quiet’ and from Krisher and Prather (2012) that early embryonic metabolism is glycolytic and exhibits the ‘Warburg Effect’. In a study using oocytes from a polycystic ovarian syndrome gilt model, lower quality oocytes had lower red/green intensity ratios of JC-1 staining (Jia et al. 2016). The authors’ reported intensity ratios were higher than those observed in our study. While this may simply be due to method of staining and use of JC-1 vs. JC-10, this could be due to differences in mitochondrial function between oocytes and blastocysts or perhaps indicate that lower Δψm is an adverse impact of SCNT. Jia et al. (2016) demonstrated that the lower quality oocytes had abnormally activated one-carbon metabolism and mtDNA hypermethylation.
Phenotypically, mitochondria within oocytes and early pre-implantation embryos have few cristae and are spherical; however, mitochondrial morphology seems to mature as progressive embryonic development and differentiation occurs with mitochondrial elongation and cristae development (Houghton 2006; Hyttel and Niemann 1990; Van Blerkom 2009). A similar phenomenon occurs in cardiomyocytes and stem cells where differentiation is correlated to larger mitochondria with distinct cristae, increased mitochondrial mass or mtDNA content, and ATP production (Chen et al. 2010; Hom et al. 2011; Lonergan et al. 2007; Rehman 2010; St. John et al. 2005). Indeed many reviews have described the metabolism of early embryos to be largely glycolytic and many have discussed the mechanisms and motives for a Warburg-like metabolism (Krisher and Prather 2012; Redel et al. 2012; Rehman 2010; Smith and Sturmey 2013; Vander Heiden et al. 2009). Along with observations that suggest mitochondrial ‘maturation’ is associated with differentiation, there is evidence that glycolysis may facilitate reprogramming and pluripotency maintenance (Folmes et al. 2011; Kondoh et al. 2005; Moussaieff et al. 2015; Zhu et al. 2010). The compound PS48 is thought to enhance reprogramming efficiency mediated through activation of the PI3K/AKT pathway (Zhu et al. 2010). In this study SCNT embryos that were treated with PS48 in the culture media had a similar number of cells and percentage of blastocyst development to that of controls. Other research from our laboratory demonstrated that blastocyst rate and total cell number was improved when PS48 was added in culture media of IVF embryos (Spate et al. 2015); however percentage of blastocyst development and total cell number are similar between studies. Of note, however not reaching statistical significance, PS48-treated donor embryos had higher survival probabilities than CPI-treated donors (Table 5).
Future investigations as to what constitutes the ‘ideal donor cell’ for use in SCNT are lucrative and warranted. There have been numerous attempts to enhance synchrony or nuclear reprogramming of donor cells including but not limited to, investigations of serum starvation and cell cycle regulation, type of cell, age of animal from which cells were extracted, age of cells themselves (or passage number), epigenetic reprogramming, degree of pluripotency, and antioxidant treatment of cells (Bonk et al. 2007; Campbell et al. 1996; Chen et al. 2015; Dominko et al. 1999; Heyman et al. 2002; Iager et al. 2008; Kato et al. 2000; Mitalipov et al. 2002; Oback and Wells 2007; Powell et al. 2004; Tani et al. 2001; Wakayama and Yanagimachi 2001; Wells et al. 2003; Whitworth et al. 2011; Wilmut et al. 2002; Yang et al. 2012). Alas, none of these treatments have served as the breakthrough needed to dramatically improve the standstill efficiency of SCNT.
In the current study, all pregnancies resultant from embryo transfer were collected on day 35 of gestation. Therefore, there is the limitation that no term piglets have been delivered from these treatments. We chose this day because 30% of conceptus loss occurs in gestation days 10–30 in pigs (Wessels et al. 2007). From the CPI and PS48 donor-treated clone transfers (5 pregnancies) there was the same total number of CPI and PS48 fetuses collected (14) leading us to speculate that neither treatment had better enhanced in-utero viability over the other. Similarly, in the MIX and CON treated donor clones transferred, there were 12 CON fetuses and 13 MIX. In the first embryo transfer experiment we had a higher pregnancy rate 41.6% (5/12 surrogates) compared to the second experiment 28.6% (4/14 surrogates) which could be due to numerous factors including season. In the first experiment we were able to transfer all blastocysts however due to harvest facility complications in the second experiment, some morula stage embryos were also transferred which may have contributed to pregnancy success. In total we reconstructed 460 clone embryos for transfer in the first experiment and 590 for the second. Therefore the rate of success for cloning as per embryos transferred to fetus procurement was 6.1% (28 fetuses from 460 embryos) and 4.2% (25 fetuses from 590 embryos) in these experiments. This study has demonstrated that fibroblasts pharmacologically treated with the compounds CPI-613 and PS48 can be successfully used in SCNT and subsequently result in early pregnancy; however, the altered metabolism of donor cells we previously evidenced did not improve survival in early gestation.
Conflicts of Interest
Authors declare no conflicts of interest.
Materials and Methods
Chemicals and materials were purchased from Sigma-Aldrich, St. Louis, MO unless otherwise specified.
Animal care and compliance with ethical standards
All procedures performed in studies involving animals were in accordance with the ethical standards of the University of Missouri Institutional Animal Care and Use Committee at the University of Missouri in Columbia, MO. This study does not contain any experiments with human participants performed by any of the authors.
Fetal-derived fibroblast cell culture
Porcine fetal fibroblast cell lines used in the study were established from a day 35 pregnancy and utilized in previous experiments in our laboratory (Mordhorst et al., 2018). A cryogenic vial of fibroblasts (0.5 mL aliquots; 1.5 million/mL in media containing 85% FBS and 15% DMSO) was defrosted from liquid nitrogen storage for each replicate. Cells were thawed and cultured in DMEM (1 g/L glucose, glutamine, and pyruvate with phenol red) supplemented with 15% FBS (Corning, Manassas, VA, USA) for seven days in T25 flasks (Corning, Corning, NY, USA) with addition of respective treatment concentrations: 100 μM CPI-613 (CPI), 10 μM PS48 (PS48; Stemgent, Cambridge, MA, USA), both drug concentrations were mixed (MIX), or lacked pharmaceutical addition (CON). Incubators were maintained at 38.5°C with a humidified atmosphere of 5% oxygen, 5% carbon dioxide, and 90% nitrogen during experiments. Cells were cultured as per previously established protocol (Mordhorst et al., 2018) to maintain similar cell densities across treatments at passaging in an effort to keep drug exposure amongst treatments comparable. Using DMSO for suspension, CPI stocks were diluted to 100 mM and PS48 stocks were diluted to 10 mM to eliminate the confounding of DMSO concentration with pharmaceutical treatment. Briefly, after thawing, cells were plated at 1 × 105 cells/mL in T25 flasks. After 72 and 120 hours, cells were passaged to new T25 flasks and plated (approximately 5 × 105 cells) to achieve ~80% confluence (desired confluence prior to nuclear transfer). Media was changed daily on all flasks, i.e. those which received PS48, CPI, or MIX had new drugs applied daily (24± 2 hours). On day 7 (168 hours) flasks were briefly rinsed with PBS + 0.01 M EDTA, and fibroblast cells were dissociated from flasks by brief incubation (37°C) with 1X TrypLE Express (Gibco, Denmark). Fibroblasts were pelleted (5 minutes at 500 × g) and resuspended with their respective drug treatments and approximately 2,000 cells/treatment were collected to be used for selection during SCNT.
Annexin-V-FITC and propidium iodide cell viability
Fibroblast viability was measured based on positive fluorescence in a FL × FL2 (530/40 × 575/25 filters) channel plot by using a Beckman Coulter CyAN ADP Analyzer cytometer (Beckman Coulter, Inc, Fullerton, CA). Axis of plots were determined by using fibroblasts singly stained with either annexin-V (FITC conjugated) or propidium iodide as well as by using unstained control cells. Fibroblasts were defined as healthy and viable if they did not stain positively for annexin-V or propidium iodide.
Somatic cell nuclear transfer and surgical embryo transfer
Sow-derived oocytes were purchased from DeSoto Biosciences (Seymour, TN) and shipped overnight in maturation medium. After 40–42 hours of maturation, cumulus cells were removed from the oocytes by vortexing in the presence of 0.1% hyaluronidase. Average percentage of oocyte maturation across replicates in experiments was 82.1% (standard deviation 3.7%). Oocytes used for enucleation were selected based on presence of a polar body and uniform cytoplasm. Oocytes were placed in manipulation medium (Lai and Prather 2003) supplemented with 7.0 μg/mL cytochalasin B during oocyte manipulation. In all experiments, SCNT was performed by two technicians and each treatment was split evenly amongst the technicians. Order of treatment in which SCNT was performed first was selected randomly. A hand-tooled thin glass capillary was used to remove the polar body along with a portion of the adjacent cytoplasm (presumably containing the metaphase II plate) and a donor cell was placed in the perivitelline space. For donor cell treatments, fibroblasts which had been treated daily for 7 days with pharmaceuticals were used and had the compounds freshly added at the time they were placed in droplets to be used for SCNT.
Afterward, reconstructed embryos were fused in a fusion medium (0.3 M mannitol, 0.1 mM CaCl2, 0.1 mM MgCl2, 0.5 mM HEPES buffer, pH 7.2) by two DC pulses (1-sec interval) at 1.2 kV/cm for 30 μsec using a BTX Electro Cell Manipulator (Harvard Apparatus). After electric pulse fusion, fused embryos were fully activated with 200 μM thimerosal for 10 minutes in the dark and 8 mM dithiothreitol for 30 minutes. Embryos were then incubated in our in house culture media MU1 (Redel et al. 2015) with a histone deacetylase inhibitor 0.5 μM Scriptaid, for 14–16 hours in the normal (atmospheric 20%) oxygen incubator. The next day, the SCNT embryos were moved into new MU1 culture media (without Scriptaid) and placed in a low (5%) oxygen incubator. To investigate the impact of the pharmaceuticals on embryos themselves, clone embryos were also created from non-drug-treated fibroblast donor cells and cultured with CPI-613 (50 or 100 μM), PS48 (5 or 10 μM), or vehicle control (DMSO 100 μM) after artificial activation. Additionally, fresh CPI and PS48 for respective treatments were applied to MU1 culture media (after incubation with Scriptaid) and placed in low oxygen incubators. Blastocyst stage SCNT embryos were either stained and imaged (day 7 of development) or 40 to 50 were surgically transferred into the oviductal ampullary-isthmic junction of a surrogate gilt (day four or five of development). Surgical embryo transfer technique was conducted as previously established within our laboratory (Lai and Prather 2003). To establish which donor cell treatment the fetuses originated from, we bred CAG-promoter driven GFP- Proteasome Fusion Protein (PSMA1) and Ubiquitin C (UBC) promoter driven tandem-dimer (td) Tomato fluorescent protein Minnesota miniature boars to domestic (Yorkshire/Landrace cross) gilts to create fluorescent cell lines which were treated with pharmaceuticals (as per donor cell treatments of embryo transfer experiments; see Figure 4) and conducted embryo transfers using two surrogates each time; one surrogate received an even number of GFP expressing treatment ‘1’ fibroblast clones and Tomato expressing treatment ‘2’ fibroblast clones and another surrogate received an even number of the opposite fluorescent color by pharmaceutical combination of embryos. See Figure 5 for design of cell line development.
Embryo staining and imaging
Blastocyst cell number and chromosomal integrity
On day 7 of embryonic development, blastocysts from all treatments were collected, washed in TL HEPES, and zona pellucidae were removed by using a physiological saline lowered to a pH of 1.79. Blastocysts were fixed with 4% paraformaldehyde (96% TL HEPES) for 20 minutes, then permeablized with 0.1% Triton (in TL HEPES) for 15 minutes. Embryos were then incubated at 38.5°C with TUNEL stain for 30 minutes then with Hoechst 33342 (2 ng/mL) for an additional 15 minutes. Embryos which served as positive controls were treated with DNAse 25 μg/mL and embryos which served as negative controls were incubated without the TUNEL reaction enzyme. After staining, slides were immediately prepared using Vectashield mounting medium (Vectashield, Burlingame, CA) and embryos were imaged at 20X magnification using a Nikon Eclipse Ti-S (Nikon Instruments Inc., Tokyo, Japan) inverted microscope in FITC and UV filtered channels and cell numbers were collected.
Mitochondrial activity in blastocysts
To determine if our treatments impacted mitochondria respiratory capacity (and thereby TCA cycle capability) within embryos we measured mitochondrial membrane potential (Δψm) using a JC-10 a biphasic cationic dye. The properties of JC-10 allow the dye to infiltrate both the cytoplasm and mitochondria in a monomeric green emission form (525 nm); yet when mitochondrial membrane potential is elevated, the dye forms J-aggregates which have an orange emission (590 nm). An increased ratio of red to green fluorescence intensity (Texas red/FITC filters) is indicative of higher mitochondrial membrane potential. Blastocysts from each treatment were collected on day 7 of embryonic development and washed in TL HEPES. Embryos were then incubated at 38.5°C in TL HEPES with 2.5 μM JC-10 (5, 5′, 6, 6′-Tetrachloro-1, 1′, 3, 3′-tetraethylbenzimidazolylcarbocyanine iodide; Enzo Life Sciences, Farmingdale, NY) for 30 minutes. Positive control embryos were treated with 5 μM valinomycin to depolarize mitochondria during incubation with JC-10. Slides were prepared immediately using Vectashield mounting medium (Vectashield, Burlingame, CA). Embryos were imaged at 40X magnification using a Nikon Eclipse E600 Microscope with Y-FL EPI Fluorescence (Nikon Instruments Inc., Tokyo, Japan) in FITC and Texas red filter channels by fluorescence microscopy using NIS-Element software F 2.30 (Nikon Instruments Inc., Tokyo, Japan). Mean intensity of green and orange-red fluorescence was measured using Image J software (available from the National Institutes of Health webpage: https://imagej.nih.gov/ij/) by outlining each entire embryo as a region of interest first in the FITC micrograph then by applying the same ROI outline to the Texas red micrograph. For publication images, to visualize both the nuclei and mitochondria, embryos were incubated in TL HEPES with 2.5 μM JC-10 for 15 minutes then Hoechst 33342 (2 ng/mL) was added and incubation continued another 15 minutes in 38.5°C. Embryos were transported in an incubator to the Molecular Cytology Core at the University of Missouri (0.80 km distance; 15 minute travel duration). Afterward embryos were placed in TL HEPES in 35 mm petri dishes which have 20 mm microwells (MatTek corporation, Ashland, MA, USA) for imaging at 20X magnification using an inverted scanning confocal Leica TCS SP8 laser scanning microscope with a 405-nm diode laser and tunable supercontinuum white light laser to generate maximum projections. The following excitation/emission band-pass wavelengths were used: 405/430–480 nm (DAPI), 490/500–550 nm (green), and 540/580–615 nm (red). Supplier recommendations for excitation/emission of JC-10 are: low membrane potential (green; ~510 nm and 520 nm); high membrane potential (red; ~510 nm and 570 nm). The confocal microscope was equipped with an environmental box for live cell imaging.
Statistical Analysis
In all experiments, three or more biological replicates were collected for analysis and the average number of clone embryos created per treatment (for in vitro development) within each replicate was 68.9 ± 5.6. The average number of embryos per replicate for each treatment used for JC-10 staining and imaged in experiments was 14.9 ± 0.8 and the average number for TUNEL staining was 14.0 ± 3.4 embryos. Data were normally distributed as confirmed via Univariate procedure in SAS 9.4 (SAS, Cary NC) and therefore no transformations were made prior to statistical analysis. Analyzed variables were considered statistically different if the P-value was less than 0.05. All variables were compared using the generalized linear model procedure of SAS for main effect of treatment. Additionally, we analyzed for the effect of ‘order’ for data from the donor-treated fibroblast clones which we define as the order in which the groups of embryos were created (cells inserted to enucleated oocytes and fused) during nuclear transfer on each day for the variables of rate of embryonic cleavage and blastocyst formation.
Supplementary Material
Acknowledgments
Financial support was provided by the National Institute of Health (R01HD080636), Food for the 21st Century at the University of Missouri, and the University of Missouri Molecular Life Science Fellowship.
Abbreviations
- CPI-613
6, 8-bis(benzylthio)octanoic acid
- JC-10
5, 5′, 6, 6′-Tetrachloro-1, 1′, 3, 3′-tetraethylbenzimidazolylcarbocyanine iodide
- MIX
mixture of PS48 +CPI
- PI3K/AKT pathway
phosphatidylinositol-3-kinase/protein kinase B
- PS48
5-(4-Chloro-phenyl)-3-phenyl-pent-2-enoic acid
- SCNT
somatic cell nuclear transfer
- TCA
tricarboxylic acid
- WE
Warburg effect
- Δψm
mitochondrial membrane potential
Footnotes
Conference Presentation: This research was presented in part at the 50th Annual Meeting for the Society for the Study of Reproduction 13–16 July 2017 in Washington D.C., United States.
References
- Bonk AJ, Cheong H-T, Li R, Lai L, Hao Y, Liu Z, Samuel M, Fergason EA, Whitworth KM, Murphy CN. Correlation of developmental differences of nuclear transfer embryos cells to the methylation profiles of nuclear transfer donor cells in Swine. Epigenetics. 2007;2(3):179–186. doi: 10.4161/epi.2.3.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevini TAL, Antonini S, Cillo F, Crestan M, Gandolfi F. Porcine embryonic stem cells: Facts, challenges and hopes. Theriogenology 68, Supplement. 2007;1(0):S206–S213. doi: 10.1016/j.theriogenology.2007.05.043. [DOI] [PubMed] [Google Scholar]
- Campbell KH, McWhir J, Ritchie W, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380(6569):64–66. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- Chen C-T, Hsu S-H, Wei Y-H. Upregulation of mitochondrial function and antioxidant defense in the differentiation of stem cells. Biochimica et Biophysica Acta (BBA) - General Subjects. 2010;1800(3):257–263. doi: 10.1016/j.bbagen.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang L, Guo Z, Wang Y, He R, Qin Y, Quan F, Zhang Y. Improving the development of early bovine somatic-cell nuclear transfer embryos by treating adult donor cells with vitamin C. Molecular reproduction and development. 2015;82(11):867–879. doi: 10.1002/mrd.22531. [DOI] [PubMed] [Google Scholar]
- Cruz M, Garrido N, Herrero J, Pérez-Cano I, Muñoz M, Meseguer M. Timing of cell division in human cleavage-stage embryos is linked with blastocyst formation and quality. Reproductive biomedicine online. 2012;25(4):371–381. doi: 10.1016/j.rbmo.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Cui X-S, Xu Y-N, Shen X-H, Zhang L-Q, Zhang J-B, Kim N-H. Trichostatin A modulates apoptotic-related gene expression and improves embryo viability in cloned bovine embryos. Cellular Reprogramming (Formerly “Cloning and Stem Cells”) 2011;13(2):179–189. doi: 10.1089/cell.2010.0060. [DOI] [PubMed] [Google Scholar]
- Dominko T, Mitalipova M, Haley B, Beyhan Z, Memili E, McKusick B, First NL. Bovine Oocyte Cytoplasm Supports Development of Embryos Produced by Nuclear Transfer of Somatic Cell Nuclei from Various Mammalian Species. Biology of reproduction. 1999;60(6):1496–1502. doi: 10.1095/biolreprod60.6.1496. [DOI] [PubMed] [Google Scholar]
- Fan N, Lai L. Genetically Modified Pig Models for Human Diseases. Journal of Genetics and Genomics. 2013;40(2):67–73. doi: 10.1016/j.jgg.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Folmes Clifford DL, Nelson Timothy J, Martinez-Fernandez A, Arrell DK, Lindor Jelena Z, Dzeja Petras P, Ikeda Y, Perez-Terzic C, Terzic A. Somatic Oxidative Bioenergetics Transitions into Pluripotency-Dependent Glycolysis to Facilitate Nuclear Reprogramming. Cell Metabolism. 2011;14(2):264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall V. Porcine Embryonic Stem Cells: A Possible Source for Cell Replacement Therapy. Stem cell reviews. 2008;4(4):275–282. doi: 10.1007/s12015-008-9040-2. [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G, Andreadis C, Thompson CB. Akt-directed metabolic alterations in cancer. Drug Discovery Today: Disease Mechanisms. 2005;2(2):255–262. [Google Scholar]
- Heyman Y, Chavatte-Palmer P, LeBourhis D, Camous S, Vignon X, Renard JP. Frequency and Occurrence of Late-Gestation Losses from Cattle Cloned Embryos. Biology of reproduction. 2002;66(1):6–13. doi: 10.1095/biolreprod66.1.6. [DOI] [PubMed] [Google Scholar]
- Hom Jennifer R, Quintanilla Rodrigo A, Hoffman David L, de Mesy Bentley Karen L, Molkentin Jeffery D, Sheu S-S, Porter George A., Jr The Permeability Transition Pore Controls Cardiac Mitochondrial Maturation and Myocyte Differentiation. Developmental Cell. 2011;21(3):469–478. doi: 10.1016/j.devcel.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton FD. Energy metabolism of the inner cell mass and trophectoderm of the mouse blastocyst. Differentiation. 2006;74(1):11–18. doi: 10.1111/j.1432-0436.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- Hyttel P, Niemann H. Ultrastructure of porcine embryos following development in vitro versus in vivo. Molecular reproduction and development. 1990;27(2):136–144. doi: 10.1002/mrd.1080270208. [DOI] [PubMed] [Google Scholar]
- Iager AE, Ragina NP, Ross PJ, Beyhan Z, Cunniff K, Rodriguez RM, Cibelli JB. Trichostatin A improves histone acetylation in bovine somatic cell nuclear transfer early embryos. Cloning and stem cells. 2008;10(3):371–380. doi: 10.1089/clo.2007.0002. [DOI] [PubMed] [Google Scholar]
- Isom SC, Whitworth KM, Prather RS. Timing of first embryonic cleavage is a positive indicator of the in vitro developmental potential of porcine embryos derived from in vitro fertilization, somatic cell nuclear transfer and parthenogenesis. Molecular reproduction and development. 2012;79(3):197–207. doi: 10.1002/mrd.22013. [DOI] [PubMed] [Google Scholar]
- Jia L, Li J, He B, Jia Y, Niu Y, Wang C, Zhao R. Abnormally activated one-carbon metabolic pathway is associated with mtDNA hypermethylation and mitochondrial malfunction in the oocytes of polycystic gilt ovaries. Scientific reports. 2016:6. doi: 10.1038/srep19436. Article ID 19436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaith S, Saini M, Raja A, Sahare A, Jyotsana B, Madheshiya P, Palta P, Chauhan M, Manik R, Singla S. Early cleavage of handmade cloned buffalo (bubalus bubalis) embryos is an indicator of their developmental competence and quality. Reproduction in Domestic Animals. 2015;50(2):214–220. doi: 10.1111/rda.12472. [DOI] [PubMed] [Google Scholar]
- Kato Y, Tani T, Tsunoda Y. Cloning of calves from various somatic cell types of male and female adult, newborn and fetal cows. Journal of Reproduction and Fertility. 2000;120(2):231–237. [PubMed] [Google Scholar]
- Koh S, Piedrahita JA. From “ES-like” cells to induced pluripotent stem cells: A historical perspective in domestic animals. Theriogenology. 2014;81(1):103–111. doi: 10.1016/j.theriogenology.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh H, Lleonart ME, Gil J, Wang J, Degan P, Peters G, Martinez D, Carnero A, Beach D. Glycolytic enzymes can modulate cellular life span. Cancer research. 2005;65(1):177–185. [PubMed] [Google Scholar]
- Krisher RL, Prather RS. A role for the Warburg effect in preimplantation embryo development: metabolic modification to support rapid cell proliferation. Molecular reproduction and development. 2012;79(5):311–320. doi: 10.1002/mrd.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai L, Prather RS. Production of cloned pigs by using somatic cells as donors. Cloning & Stem Cells. 2003;5(4):233–241. doi: 10.1089/153623003772032754. [DOI] [PubMed] [Google Scholar]
- Lee K, Shorr R, Maturo C, Boteju LW, Sheldon A. Formation and anti-tumor activity of uncommon in vitro and in vivo metabolites of CPI-613, a novel anti-tumor compound that selectively alters tumor energy metabolism. Drug metabolism letters. 2011;5(3):163–182. doi: 10.2174/187231211796904991. [DOI] [PubMed] [Google Scholar]
- Leese HJ, Baumann CG, Brison DR, McEvoy TG, Sturmey RG. Metabolism of the viable mammalian embryo: quietness revisited. MHR: Basic science of reproductive medicine. 2008;14(12):667–672. doi: 10.1093/molehr/gan065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Zhao M-H, Choi J-w, Kim N-H, Cui X-S. Scriptaid treatment decreases DNA methyltransferase 1 expression by induction of microRNA-152 expression in porcine somatic cell nuclear transfer embryos. PloS one. 2015;10(8):e0134567. doi: 10.1371/journal.pone.0134567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan T, Bavister B, Brenner C. Mitochondria in stem cells. Mitochondrion. 2007;7(5):289–296. doi: 10.1016/j.mito.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles EL, O’Gorman C, Zhao J, Samuel M, Walters E, Yi Y-J, Sutovsky M, Prather RS, Wells KD, Sutovsky P. Transgenic pig carrying green fluorescent proteasomes. Proceedings of the National Academy of Sciences. 2013;110(16):6334–6339. doi: 10.1073/pnas.1220910110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitalipov SM, Yeoman RR, Nusser KD, Wolf DP. Rhesus Monkey Embryos Produced by Nuclear Transfer from Embryonic Blastomeres or Somatic Cells. Biology of reproduction. 2002;66(5):1367–1373. doi: 10.1095/biolreprod66.5.1367. [DOI] [PubMed] [Google Scholar]
- Mordhorst BR, Murphy SL, Ross RM, Samuel MS, Rojas Salazar S, Ji T, Behura SK, Wells KD, Green JA, Prather RS. Pharmacologic reprogramming designed to induce a Warburg Effect in porcine fetal fibroblasts alters gene expression and quantities of metabolites from conditioned media without increased cell proliferation. Cellular Reprogramming. 2018;20(1) doi: 10.1089/cell.2017.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motato Y, de los Santos MJ, Escriba MJ, Ruiz BA, Remohí J, Meseguer M. Morphokinetic analysis and embryonic prediction for blastocyst formation through an integrated time-lapse system. Fertility and sterility. 2016;105(2):376–384. e379. doi: 10.1016/j.fertnstert.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Moussaieff A, Rouleau M, Kitsberg D, Cohen M, Levy G, Barasch D, Nemirovski A, Shen-Orr S, Laevsky I, Amit M. Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell metabolism. 2015;21(3):392–402. doi: 10.1016/j.cmet.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Oback B, Wells D. Donor cell differentiation, reprogramming, and cloning efficiency: elusive or illusive correlation? Molecular reproduction and development. 2007;74(5):646–654. doi: 10.1002/mrd.20654. [DOI] [PubMed] [Google Scholar]
- Piedrahita JA, Anderson GB, BonDurant RH. On the isolation of embryonic stem cells: Comparative behavior of murine, porcine and ovine embryos. Theriogenology. 1990;34(5):879–901. doi: 10.1016/0093-691x(90)90559-c. [DOI] [PubMed] [Google Scholar]
- Pletnev S, Gurskaya NG, Pletneva NV, Lukyanov KA, Chudakov DM, Martynov VI, Popov VO, Kovalchuk MV, Wlodawer A, Dauter Z. Structural basis for phototoxicity of the genetically encoded photosensitizer KillerRed. Journal of Biological Chemistry. 2009;284(46):32028–32039. doi: 10.1074/jbc.M109.054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AM, Talbot NC, Wells KD, Kerr DE, Pursel VG, Wall RJ. Cell Donor Influences Success of Producing Cattle by Somatic Cell Nuclear Transfer. Biology of reproduction. 2004;71(1):210–216. doi: 10.1095/biolreprod.104.027193. [DOI] [PubMed] [Google Scholar]
- Prather RS, Lorson M, Ross JW, Whyte JJ, Walters E. Genetically engineered pig models for human diseases. Annual review of animal biosciences. 2013a;1:203, 203–219. doi: 10.1146/annurev-animal-031412-103715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather RS, Rowland RR, Ewen C, Trible B, Kerrigan M, Bawa B, Teson JM, Mao J, Lee K, Samuel MS. An intact sialoadhesin (Sn/SIGLEC1/CD169) is not required for attachment/internalization of the porcine reproductive and respiratory syndrome virus. Journal of virology. 2013b;87(17):9538–9546. doi: 10.1128/JVI.00177-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redel BK, Brown AN, Spate LD, Whitworth KM, Green JA, Prather RS. Glycolysis in preimplantation development is partially controlled by the Warburg Effect. Molecular reproduction and development. 2012;79(4):262–271. doi: 10.1002/mrd.22017. [DOI] [PubMed] [Google Scholar]
- Redel BK, Spate LD, Lee K, Mao J, Whitworth KM, Prather RS. Glycine supplementation in vitro enhances porcine preimplantation embryo cell number and decreases apoptosis but does not lead to live births. Molecular reproduction and development. 2016;83(3):246–258. doi: 10.1002/mrd.22618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redel BK, Tessanne KJ, Spate LD, Murphy CN, Prather RS. Arginine increases development of in vitro-produced porcine embryos and affects the protein arginine methyltransferase–dimethylarginine dimethylaminohydrolase–nitric oxide axis. Reproduction, Fertility and Development. 2015;27(4):655–666. doi: 10.1071/RD14293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman J. Empowering self-renewal and differentiation: the role of mitochondria in stem cells. Journal of molecular medicine. 2010;88(10):981–986. doi: 10.1007/s00109-010-0678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RB, Hay N. Is Akt the “Warburg kinase”?—Akt-energy metabolism interactions and oncogenesis. Seminars in cancer biology. 2009;19(1):25. doi: 10.1016/j.semcancer.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samiec M, Opiela J, Lipiński D, Romanek J. Trichostatin A-mediated epigenetic transformation of adult bone marrow-derived mesenchymal stem cells biases the in vitro developmental capability, quality, and pluripotency extent of porcine cloned embryos. BioMed research international. 2015 doi: 10.1155/2015/814686. Article ID 814686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Danielle G, Sturmey Roger G. Parallels between embryo and cancer cell metabolism. Biochemical Society Transactions. 2013;41(2):664–669. doi: 10.1042/BST20120352. [DOI] [PubMed] [Google Scholar]
- Spate LD, Brown A, Redel BK, Whitworth KM, Prather RS. PS48 can replace bovine serum albumin in pig embryo culture medium, and improve in vitro embryo development by phosphorylating AKT. Molecular reproduction and development. 2015;82(4):315–320. doi: 10.1002/mrd.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John JC, Ramalho-Santos J, Gray HL, Petrosko P, Rawe VY, Navara CS, Simerly CR, Schatten GP. The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells. Cloning and stem cells. 2005;7(3):141–153. doi: 10.1089/clo.2005.7.141. [DOI] [PubMed] [Google Scholar]
- Tani T, Kato Y, Tsunoda Y. Direct exposure of chromosomes to nonactivated ovum cytoplasm is effective for bovine somatic cell nucleus reprogramming. Biology of reproduction. 2001;64(1):324–330. doi: 10.1095/biolreprod64.1.324. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J. Mitochondria in early mammalian development. Elsevier; 2009. pp. 354–364. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegh RB, Solntsev KM, Kuimova MK, Cho S, Liang Y, Loo BL, Tolbert LM, Bommarius AS. Reactive oxygen species in photochemistry of the red fluorescent protein “Killer Red”. Chemical communications. 2011;47(17):4887–4889. doi: 10.1039/c0cc05713d. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Yanagimachi R. Mouse cloning with nucleus donor cells of different age and type. Molecular reproduction and development. 2001;58(4):376–383. doi: 10.1002/1098-2795(20010401)58:4<376::AID-MRD4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Walters EM, Wolf E, Whyte JJ, Mao J, Renner S, Nagashima H, Kobayashi E, Zhao J, Wells KD, Critser JK. Completion of the swine genome will simplify the production of swine as a large animal biomedical model. BMC medical genomics. 2012;5(1):1. doi: 10.1186/1755-8794-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Wells DN, Laible G, Tucker FC, Miller AL, Oliver JE, Xiang T, Forsyth JT, Berg MC, Cockrem K, L’Huillier PJ, Tervit HR, Oback B. Coordination between donor cell type and cell cycle stage improves nuclear cloning efficiency in cattle. Theriogenology. 2003;59(1):45–59. doi: 10.1016/s0093-691x(02)01273-6. [DOI] [PubMed] [Google Scholar]
- Wessels JM, Linton NF, Croy BA, Tayade C. A review of molecular contrasts between arresting and viable porcine attachment sites. American journal of reproductive immunology. 2007;58(6):470–480. doi: 10.1111/j.1600-0897.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- Whitworth KM, Prather RS. Somatic cell nuclear transfer efficiency: how can it be improved through nuclear remodeling and reprogramming? Molecular reproduction and development. 2010;77(12):1001–1015. doi: 10.1002/mrd.21242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth KM, Rowland RR, Ewen CL, Trible BR, Kerrigan MA, Cino-Ozuna AG, Samuel MS, Lightner JE, McLaren DG, Mileham AJ. Gene-edited pigs are protected from porcine reproductive and respiratory syndrome virus. Nature biotechnology. 2016;34(1):20–22. doi: 10.1038/nbt.3434. [DOI] [PubMed] [Google Scholar]
- Whitworth KM, Zhao J, Spate LD, Li R, Prather RS. Scriptaid corrects gene expression of a few aberrantly reprogrammed transcripts in nuclear transfer pig blastocyst stage embryos. Cellular Reprogramming (Formerly “Cloning and Stem Cells”) 2011;13(3):191–204. doi: 10.1089/cell.2010.0087. [DOI] [PubMed] [Google Scholar]
- Whyte JJ, Prather RS. Genetic modifications of pigs for medicine and agriculture. Molecular reproduction and development. 2011;78(10–11):879–891. doi: 10.1002/mrd.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmut I, Beaujean N, de Sousa PA, Dinnyes A, King TJ, Paterson LA, Wells DN, Young LE. Somatic cell nuclear transfer. Nature. 2002;419(6709):583–587. doi: 10.1038/nature01079. [DOI] [PubMed] [Google Scholar]
- Yang X, Mao J, Walters EM, Zhao M-T, Teson J, Lee K, Prather RS. Xenopus egg extract treatment reduced global DNA methylation of donor cells and enhanced somatic cell nuclear transfer embryo development in pigs. BioResearch open access. 2012;1(2):79–87. doi: 10.1089/biores.2012.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachar Z, Marecek J, Maturo C, Gupta S, Stuart SD, Howell K, Schauble A, Lem J, Piramzadian A, Karnik S, Lee K, Rodriguez R, Shorr R, Bingham PM. Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo. Journal of Molecular Medicine. 2011;89(11):1137–1148. doi: 10.1007/s00109-011-0785-8. [DOI] [PubMed] [Google Scholar]
- Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, Kim J, Zhang K, Ding S. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell stem cell. 2010;7(6):651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.