Abstract
HEPA filtration in combination with an electrostatic precipitator (ESP) can be a cost-effective approach to reducing indoor particulate exposure, but ESPs produce ozone. The health effect of combined ESP-HEPA filtration has not been examined. We conducted an intervention study in 89 volunteers. At baseline, the air handling units of offices and residences for all subjects were comprised of coarse, ESP, and HEPA filtration. During the 5-week long intervention, the subjects were split into two groups, one with just the ESP removed and the other with both the ESP and HEPA removed. Each subject was measured for cardiopulmonary risk indicators once at baseline, twice during the intervention, and once two weeks after baseline conditions were restored. Measured indoor and outdoor PM2.5 and ozone concentrations, coupled with time-activity data, were used to calculate exposures. Removal of HEPA filters increased 24-hour mean PM2.5 exposure by 38 (95% CI: 31, 45) µg/m3. Removal of ESPs decreased 24-hour mean ozone exposure by 2.2 (2.0, 2.5) ppb. No biomarkers were significantly associated with HEPA filter removal. In contrast, ESP removal was associated with a −16.1% (−21.5%, −10.4%) change in plasma soluble P-selectin and a −3.0% (−5.1%, −0.8%) change in systolic blood pressure, suggesting reduced cardiovascular risks.
Keywords: Indoor air intervention, HEPA, electrostatic precipitator, particulate matter, ozone, biomarker
INTRODUCTION
In cities with high outdoor air pollution, people often use filtration technologies to reduce indoor levels of particulate matter (PM). High efficiency particulate air (HEPA) filtration is a type of particle filter that must remove no less than 99.97% of particles of 0.3 µm in diameter.[1] However, soiled HEPA filters are hard to clean and costly to replace. Soiled HEPA filters increase the pressure drop and hence increase the operating (electricity) cost of the ventilation/filtration system. Another PM removal technology is an electrostatic precipitator (ESP) that uses an electrical discharge to charge particles so that particles can be readily deposited onto grounded metal plates. Although less efficient in removing PM, ESPs are easy to clean and less costly to operate compared to HEPA filtration. Utilizing an ESP upstream of a HEPA filter can substantially reduce the pressure drop resulting from particle accumulation on the HEPA, and can also reduce operating costs by extending the lifetime of HEPA filters. However, ESPs produce incidental ozone (O3) as a byproduct during the process of electrically charging the air. O3 can react with materials in the air handling system and chemicals in the indoor environment to produce secondary pollutants.[2–4] The net health impact from the combined use of ESP-HEPA filtration has not been examined.
Studies evaluating the health effects of HEPA filtration have reported inconsistent results, with some observing beneficial changes in several biomarkers reflecting cardiorespiratory health indicators and others observing no effects (see Discussion). The use of ESP alone has previously been associated with some improved lung function measures.[5] The present study, is the first, to the best of our knowledge, to examine the health impacts of combined HEPA filtration and ESP in participants who worked and mainly resided in a working campus. Specifically, the indoor air in study subjects’ offices and living quarters was purified either by coarse filter (F8)-ESP-HEPA filtration, F8-HEPA filtration, or only F8 filtration of mainly coarse particles. The similarity in participants’ daily activity patterns and the fact that they spent most of their time in only a few filtered locations allowed for detailed exposure assessments.
Based on the same field study, we previously evaluated associations between pollutant exposures and biomarker outcomes.[6] We found significant associations of either 24-hour O3 exposure or 2-week O3 exposure with a biomarker of platelet activation (plasma soluble P-selectin) and systolic blood pressure, suggesting that O3 exposure may increase cardiovascular disease risk via blood pro-coagulation and blood pressure increases.. The aim of the present study is to examine the impact of temporarily removing either ESPs alone or both ESPs and HEPA filters from the three part (F8-ESP-HEPA) air handling systems on cardiorespiratory function and biomarkers reflecting cardiorespiratory pathophysiology.
METHODS
Study Participants
This study was conducted on the 1 km2 Broad Group campus known as Broad Town in Changsha City, Hunan Province, China from December 1st, 2014 to January 31st, 2015. We recruited 89 office workers with the requirements that all study participants must work in one of two Broad Town offices, spend at least four nights a week in the Broad Town dormitories, be over 18 years old, and be free from major self-reported chronic diseases. In addition, individuals with abnormal results for a blood test of lipids and biomarkers of liver and kidney dysfunction were excluded. The study protocol was approved by the Ethics Committee of the Shanghai First People’s Hospital and the Campus IRB of Duke University; and explicit written consent was obtained from all participants.
Intervention Conditions
Table 1 shows the details of the study design and intervention conditions. At baseline, all participant’s offices and dorms had a single-pass central air handling unit (AHU) with an F8 (MERV 12) pre-filter, an ESP, and a HEPA filter installed in that order immediately downstream of the air intake. Participants were assigned into two intervention groups, Group A (n = 36) and Group B (n = 53). Beginning on December 6, 2014 and continued for 5 weeks, the ESP was turned off and the HEPA filter removed in both offices and dorms for Group A (F8 remained only), whereas the ESP was turned off, but the HEPA filter remained in place (F8 + HEPA) for Group B. In order to give separate dormitory conditions for each group, Group A subjects were moved from their original dormitory rooms into a single similar dormitory building (“Intervention A Dorm”) during and following the intervention, whereas Group B subjects remained in their original dormitories. The F8 filter was not removed for either group due to concerns that large dust particles would damage the AHU ventilation equipment. The intervention for both groups ended on January 13th, 2015, when baseline filtration conditions were restored to the offices and dorms.
Table 1.
Study design and intervention conditions
| Condition | Pre-intervention (Dec. 1–5, 2014) |
Intervention (Dec. 6, 2014 – Jan. 13, 2015) |
Post-intervention (Jan. 14–31, 2015) |
|---|---|---|---|
| Duration | 5 days | 39 days | 18 days |
| Biomarker sampling visits | Visit 1 (Dec. 2–5, 2014) | Visit 2 (Dec. 23–26 & 30, 2014) & Visit 3 (Jan. 6–8 & 13, 2015) | Visit 4 (Jan. 27–30, 2015) |
| Group A | |||
| Filtration | F8 + ESP + HEPA | F8 | F8 + ESP + HEPA |
| Office Location | Office A | Office A | Office A |
| Living Quarters | Dorm Buildings 1–6 | Intervention A Dorm | Intervention A Dorm |
| Group B | |||
| Filtration | F8 + ESP + HEPA | F8 + HEPA | F8 + ESP + HEPA |
| Office Location | Office B | Office B | Office B |
| Living Quarters | Dorm Buildings 1–6 | Dorm Buildings 1–6 | Dorm Buildings 1–6 |
Air Pollutant Monitoring
The pollutants to be affected by HEPA and ESP were PM and O3. Hourly outdoor concentrations of PM2.5 and O3, as well as potentially confounding co-pollutants NO2 and SO2, were obtained from a government monitoring station 4.5 km from Broad Town. Only 3.8% of these data were missing for the study period, and linear interpolation was used to fill in those data gaps. There were few local air pollution sources or major roadways between Broad Town and the monitoring station, which was primarily upwind of the study site. Least square linear regressions comparing outdoor concentrations measured simultaneously at the both sites showed a slope of 1.03 and R2 of 0.998 for PM2.5 and a slope of 0.97 and R2 of 0.988 for O3.
Indoor PM2.5 and O3 concentrations were continuously measured in the center of each group’s office during the day and in two dormitory rooms per night using nephelometers (SidePak AM510, TSI Inc.) and a UV absorption monitor (Model 205, 2B Tech), respectively. All the monitors were field-calibrated using a primary method at the study location. Indoor measurements were used to establish indoor/outdoor (I/O) ratios accounting for filtration conditions and the presence of indoor smoking, which was only detected in the dormitory of the 13 active smokers in this study.[6] These ratios were used to estimate hourly mean PM2.5 and O3 concentrations outside of the monitoring times. No cooking or incense burning was detected in any of the offices and dorms. Because indoor NO2 and SO2 were not expected to be affected by the filtration conditions, we used established I/O ratios (0.8 for NO2 and 0.5 for SO2) and measured outdoor concentrations to estimate indoor concentrations.[7, 8] Time-activity questionnaires were administered at each biomarker sampling visit and included detailed accounts down to the minute of where the participants were for the past 24 hours, total times subjects estimated that they spent at Broad Town and in other locations over the previous seven days, and general questions regarding potential health confounders, such as whether participants had a respiratory infection at the time of sampling. Time-activity questionnaire data were integrated with indoor and outdoor pollutant hourly average concentrations to calculate cumulative exposure concentrations. In these calculations, the I/O ratios for unknown indoor environments were assumed to be 0.8 for PM2.5 and 0.35 for O3 based on previous findings in lightly sealed indoor spaces[9, 10] and expectations for structures in the Changsha region, which tended to be poorly insulated. As the study was organized with approximately 2-week breaks between biomarker measurements and changes in filtration conditions, two-week exposure concentrations were estimated as representative of ‘sub-chronic’ exposure effects, as opposed to 24-hour exposure for acute effects.
Biomarker Measurements
Each participant was assessed for pulmonary and cardiovascular function and pathophysiologic biomarkers four times over the study period, once before the intervention as a baseline measurement, once approximately two weeks into the intervention period, once approximately four weeks into the intervention period, and once approximately two weeks after the intervention period ended and baseline conditions were restored (see Table 1). Each sampling period took about four days to complete, and efforts were made to conduct all sessions for each subject on the same day of week and at the same time of day, though work schedules necessitated some rescheduling. No sessions were scheduled within a day following a trip away from Broad Town.
As biomarkers of pulmonary pathophysiology, we measured exhaled breath condensate (EBC) malondialdehyde (MDA) for oxidative stress;[11] fractional exhaled nitric oxide (FeNO),[12] EBC pH, and EBC nitrite and nitrate (EBCNN) for inflammation;[11] and FEV1, FVC, and FEV1/FVC ratio for lung function. Measured biomarkers of systemic inflammation and oxidative stress included C-reactive protein (CRP),[13] urinary 8-hydroxy-2’-deoxyguanosine (8-OHdG),[14] and urinary MDA (UMDA).[15] Vascular tone was assessed with brachial systolic and diastolic blood pressure (SBP and DBP) and pulse pressure (PP).[16] Arterial stiffness was assessed using brachial AI and carotid-femoral pulse wave velocity (PWV).[17] Myocardial oxygen supply relative to demand was evaluated with the subendocardial viability ratio (SEVR).[18] Finally, plasma sCD62P and von Willebrand factor (VWF) were evaluated as biomarkers of platelet activation[19] and endothelial cell dysfunction,[20] respectively.
Each sampling day began with the collection of venous blood and urine at 8:00 AM, followed throughout the day by any order of breath and EBC collection and pulse wave analysis (PWA) for AI and PWV, with spirometry always being conducted last. Urine samples were immediately frozen for later solid phase extraction and LC-MS analysis for 8-OHdG.[21] Blood samples were centrifuged; and plasma aliquots were stored at −30 °C before analysis for sCD62P, CRP, and VWF using ELISA methods (R&D Systems for CRP and sCD62P; RayBiotech for VWF). Breath sample collection and analyses, as well as spirometry and PWA measurements, were conducted using standard procedures.[6]
Statistical Analyses
A number of statistical methods were used to test whether subject characteristics and time-activity patterns were balanced between the subject groups, as potential imbalances could confound biomarker associations with the intervention. Mann-Whitney U tests, Chi-squared tests of independence, and Student’s t-tests were used to examine cross-sectional differences between groups for non-Gaussian data, frequency data, and Gaussian data, respectively. For the longitudinal time-activity patterns, between-group comparisons were made using linear mixed effects models with group as a fixed effect and subject-specific intercepts as random effects to account for correlation in the within-subject repeated measures for the repeated time-activity measures. Measured air pollutant levels in different locations between the intervention periods were compared using Kruskal-Wallis non-parametric tests for global period effect and Dunn post-hoc tests for period-specific differences due to the heavily right-skewed distributions of the air pollutant data. Significance tests for between-group differences in calculated pollutant exposure for each sampling visit were performed with linear mixed models with subject-specific intercepts and a visit by group interaction fixed effect.
To examine the relationship between filtration and biomarkers, we formulated the model as a hierarchical linear mixed effects model, Formula 1, with aj being subject j-specific intercepts. The fixed effects in this model for subject j and observation i included dummy variables for HEPA and ESP presence (e.g., ESPs were not present during the intervention period), either 24-hour or 2-week cumulative exposure in all unfiltered locations (i.e., anywhere not in the Broad Town offices or dorms) for pollutant p (PM2.5 or O3) over time length t (UEptij) to control for those exposures unaffected by filtration conditions, 24-hour or 2-week mean total exposures to NO2 and SO2 (TEptij), 24-hour mean ambient temperature (TEMP24hij) and relative humidity (RH24hij), the time spent in a room with an active smoker in the past 24 hours as a measure of secondhand smoke (TSHS24h), respiratory infection status (RIS), menstruation status (MS), day of the week (WD), and the hours since a subject last ate (LA). In addition to controlling for variations in outdoor concentrations, the cumulative unfiltered PM2.5 and O3 exposure variables also capture information on variations in time spent away from the offices and dorms. The model selection for 24-hour or 2-week pollutant covariates is described in the Supplementary Materials.
| Formuls 1) |
We assume that the subject-specific random effects αj are independently distributed along a Gaussian distribution with mean µα and variance σα2. Concerns over collinearity between the exposure and filtration predictors prompted the use of a Bayesian generalized mixed effects ridge regression to shrink collinearity-associated variance inflation. This model used a penalized Cauchy distribution prior for the mean slope estimates and a penalized half-Cauchy prior for the standard deviation of the subject-specific intercepts as “shrinkage priors” previously shown to improve maximum likelihood estimation (MLE) when there is high correlation between predictors[22] and also shown to have improved simulation MLE results using our own data. All calculations were made using JAGS,[23] version 4.2.0, and the “R2jags”,[24] “R2WinBUGS”,[25] “nlme”,[26] and “FSA”[27] packages in R, version 3.3.3.[28] Additional details and code for this model can be found in the Supplementary Materials.
RESULTS
Participant Characteristics
Table 2 shows participant characteristics between the study groups. Almost all characteristics are comparable between the groups except for small but statistically significant differences in 24-hour time-activity patterns. Namely, Group A spent about 0.7 hours more in the office and 0.4 less hours outdoors than Group B. Fifteen subjects (17%) were active smokers during the study period: 4 in Group A and 11 in Group B. The mean total time in the 24 hours before each sampling visit spent in the offices and dorms was 20.4 h for Group A and 19.1 h for Group B. In terms of the estimated time spent in all filtered locations in the 2 weeks before each sampling visit, Group A spent a mean of 225.9 h or 67.2% of the total time, and Group B spent a mean of 210.9 h or 62.8% of the total time.
Table 2.
Study Participant Characteristics by Experimental Group
| Group A (n=34) |
Group B (n=52) |
p-value | |
|---|---|---|---|
| Age (Mean ± SD)a | 31.7 ± 8.4 | 31.5 ± 7.3 | 0.87 |
| Age Range | 22–52 | 22–52 | — |
| Female (Number (%))b | 9 (26.5%) | 16 (30.8%) | 0.76 |
| BMI (Mean ± SD)c | 22.0 ± 3.1 | 22.5 ± 2.5 | 0.39 |
| Number of Current Smokers (Number (%))b | 4 (11.8%) | 11 (21.2%) | 0.40 |
| Number of Ex-Smokers (Number (%))b | 5 (14.7%) | 1 (1.9%) | 0.06 |
| Pack-years for Current and Ex-Smokers (Mean ± SD)a | 0.9 ± 2.8 | 0.9 ± 2.3 | 0.86 |
| Time spent in the dorms over the past 24 hours (Mean ± SD)d | 11.9 ± 2.5 | 11.3 ± 3.5 | 0.21 |
| Time spent in the offices over the past 24 hours (Mean ± SD)d | 8.5 ± 1.9 | 7.8 ± 1.6 | 0.006* |
| Time spent in other indoor spaces over the past 24 hours (Mean ± SD)d | 2.7 ± 2.7 | 3.6 ± 3.6 | 0.08 |
| Time spent outdoors over the past 24 hours (Mean ± SD)d | 0.8 ± 0.9 | 1.2 ± 1.3 | 0.02* |
Depending on the type of data, the significance tests evaluating group differences in each characteristic were either
Mann-Whitney U test,
Chi-squared test of independence,
Student’s t-test, or
linear mixed effects model.
p < 0.05
Eighty-one out of the 89 subjects (91%) completed all four visits, 5 subjects completed 3 visits, and 3 subjects withdrew from the study after the first visit. No subjects were excluded from analyses if they had completed fewer than 4 visits. Out of 343 observations, 2 (0.6%) were omitted because participants had taken medications that might affect outcomes, and an additional 8 (2.3%) were omitted due to insufficient time-activity data. Of the remaining 333 observations, 37 (11%) included self-reported respiratory infections and 16 (4.8%) included self-reported menstruation.
Indoor PM2.5 and O3 Concentrations
The changes in hourly PM2.5 concentrations monitored at all locations between intervention periods are shown in Table 3. Outdoor PM2.5 concentrations remained above the World Health Organization (WHO) 24-hour PM2.5 guideline of 25 µg/m3[29] throughout the study and generally increased as the study progressed. In the offices and dorms with HEPA filtration, the indoor PM2.5 concentrations were much lower than outdoors, with median hourly concentrations ranging from 5.5 to 14.6 µg/m3. After both the ESP and HEPA filters were removed for Group A, Office A and the Intervention A Dorm had significant increases in mean indoor PM2.5 concentrations of 29.9 and 39.8 µg/m3, respectively. After the ESP and HEPA filters were restored, the indoor concentrations in Office A and the Intervention A Dorm decreased by an average of 22.1 and 32.4 µg/m3, respectively. Office B had a small, but statistically significant, reduction of about 0.99 µg/m3 in indoor PM2.5 concentrations after the ESP was turned off, followed by a significant mean increase of 1.85 µg/m3 in the post-intervention period. In the main dorms housing Group B during and after the intervention, the only significant change in indoor PM2.5 concentrations occurred from the during- to post-intervention periods when there was a significant mean increase of 17.1 µg/m3. In the dorms in which smoking, either by the subjects themselves or by their neighbors, occurred, the indoor PM2.5 concentrations did not change significantly during the study, and their concentrations during the intervention period were similar to those of the dorm rooms without HEPA filters (Intervention A Dorm). There was a strict no-smoking policy in the Intervention A Dorm that prevented indoor smoking there.
Table 3.
PM2.5 Concentrations in Different Locations during the Study Period
| Location | Pre median, mean (SD), range |
During median, mean (SD), range |
Post median, mean (SD), range |
During – Pre | Post – During | ||
|---|---|---|---|---|---|---|---|
| Mean difference (95% CI) |
p-value | Mean difference (95% CI) |
p-value | ||||
| Outdoors | 53.0, 70.3 (53.2), 17–344 | 79.0, 90.1 (46.3), 19–268 | 100.0, 109.5 (55.8), 14–264 | 16.5 (10.0, 23.0) | <0.001 | 19.4 (12.4, 26.4) | <0.001 |
| Office A | 9.3, 9.6 (5.6), 2.8–21 | 38.3, 39.5 (17.4), 15–101 | 14.2, 17.3 (11.4), 0.8–73 | 29.9 (19.6, 40.1) | <0.001 | −22.1 (−26.5, −17.8) | <0.001 |
| Office B | 6.9, 6.6 (2.2), 1.5–9.8 | 5.5, 5.6 (3.1), 0.0–15 | 6.3, 7.4 (2.8), 2.6–18 | −0.99 (−2.18, 0.20) | 0.003 | 1.85 (1.05, 2.65) | <0.001 |
| Dorms | 7.9, 11.7 (8.2), 1.3–29 | 7.6, 15.8 (18.4), 0.0–72 | 14.6, 33.0 (27.1), 7.0–85 | 4.13 (−6.63, 14.9) | 0.95 | 17.1 (9.86, 24.4) | <0.001 |
| Dorms (Smoking) | 33.2, 46.0 (30.2), 22–156 | 39.8, 44.9 (32.2), 1.1–199 | 70.9, 58.5 (39.3), 3.1–136 | −1.12 (−19.2, 16.7) | 0.77 | 13.6 (−0.38, 27.6) | 0.11 |
| Intervention A Dorm | — | 38.4, 51.4 (31.3), 8.7–160 | 10.2, 19.0 (28.0), 3.0–142 | 39.8 (25.2, 54.3)a | <0.001a | −32.4 (−40.6, −24.2) | <0.001 |
Significance tests for between-period differences in PM2.5 concentrations were performed with Kruskal-Wallis non-parametric tests for global period effect and Dunn post-hoc tests for period-specific differences. Bolded p-values are < 0.05.
Since Group A subjects only moved from their dorms to the hotel at the beginning of the “During” period, the “During – Pre” comparison here is comparing the PM2.5 concentrations in dorms during the “Pre” period to the hotel during the “During” period. Indoor smoking did not occur among smokers in Intervention A Dorm, so “Dorms (Smoking)” only refers to Group B dorms in the during and post-intervention periods.
Hourly O3 concentrations in different locations are shown in Table 4. In keeping with seasonal trends of decreasing daylight and concomitant decreases in O3 formation, outdoor O3 was the highest at the beginning of the study, and it declined as the study progressed. Only 8 days, 3 during the pre-intervention period and 5 during the first two weeks of the intervention period, had O3 daily maximum 8-hour rolling averages that exceeded the WHO guideline of 50 ppb.[29] The mean value for these maximum daily 8-hour averages was 33.0 ppb (range = 4.3 – 60.5 ppb). Each indoor location experienced an initial decline in indoor O3 as the ESPs were switched off and the outdoor concentrations fell, though this was not significant for the dorms. During the post-intervention period, each location experienced a small, non-significant increase in indoor O3. Monitoring at the supply air ducts and breathing zone of Office B revealed that the ESP increased supply air O3 by about 13.5 ppb and the steady state breathing zone concentration by about 3 ppb, as we reported previously.[30]
Table 4.
O3 Concentrations in Different Locations during the Study Period
| Location | Pre median, mean (SD), range |
During median, mean (SD), range |
Post median, mean (SD), range |
During – Pre | Post – During | ||
|---|---|---|---|---|---|---|---|
| Mean difference (95% CI), p-value |
p- value |
Mean difference (95% CI) |
p- value |
||||
| Outdoors | 34.0, 29.9 (14.8), 0.5–64.5 | 21.0, 24.4 (13.9), 0.5–65.0 | 7.5, 11.7 (10.3), 0.5–44.5 | −5.5 (−7.3, −3.8) | <0.001 | −12.6 (−14.5, −10.7) | <0.001 |
| Office A | 12.8, 13.8 (3.0), 10.5–18.2 | 5.0, 5.8 (3.2), 1.9–14.3 | 4.3, 6.7 (5.1), 2.4–21.4 | −8.0 (−11.8, −4.2) | <0.001 | 0.9 (−1.1, 2.9) | 0.90 |
| Office B | 9.1, 10.9 (4.6), 5.8–21.1 | 4.6, 5.0 (2.1), 1.5–13.2 | 4.5, 6.0 (3.6), 1.9–16.8 | −5.9 (−7.6, −4.2) | <0.001 | 1.0 (−0.2, 2.1) | 0.51 |
| Dorms | 4.9, 6.5 (4.4), 3.3–16.0 | 3.9, 4.3 (2.9), 0.5–14.4 | 3.4, 4.9 (3.0), 2.6–10.5 | −2.2 (−5.6, 1.2) | 0.25 | 0.6 (−2.3, 3.5) | 1.00 |
| Intervention A Dorm | — | 3.3, 3.9 (2.4), 0.8–14.4 | 3.9, 4.2 (1.5), 1.8–7.5 | −2.7 (−5.3, −0.07)a | 0.01a | 0.4 (−1.8, 2.6) | 0.90 |
Significance tests for between-period differences in O3 concentrations were performed with Kruskal-Wallis non-parametric tests for global period effect and Dunn post-hoc tests for period-specific differences. Bolded p-values are < 0.05.
Since Group A subjects only moved from their dorms to the hotel at the beginning of the “During” period, the “During – Pre” comparison here is comparing the O3 concentrations in dorms during the “Pre” period to the hotel during the “During” period.
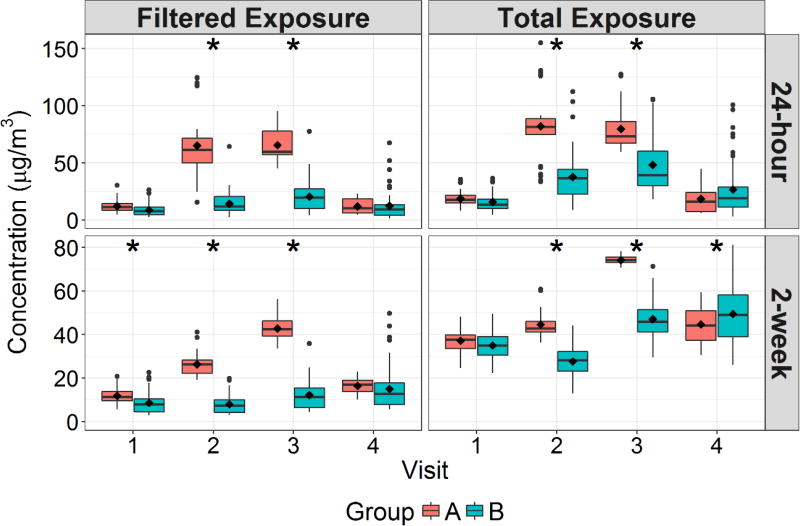
PM2.5 and O3 Exposures
Figure 1 shows the group-specific 24-hour and 2-week calculated PM2.5 exposures in filtered locations (offices and dorms) only and total exposure in all locations prior to each biomarker sampling visit. The 24-hour mean total exposures to PM2.5 for all subjects ranged from 3.2 to 155 µg/m3. During Visits 2 and 3, Group A had significantly higher exposures than Group B in all exposure categories, though this difference was smaller for the total exposure. For 24-hour filtered location exposure, Group A was 50.9 and 45.2 µg/m3 higher for Visits 2 and 3, respectively, whereas in terms of total exposure these differences were 44.2 and 31.4 µg/m3 for Visits 2 and 3, respectively. For 2-week filtered exposure, Group A was 18.5 and 30.6 µg/m3 higher for Visits 2 and 3, respectively, which were reduced to 17.0 and 26.9 µg/m3 for Visits 2 and 3, respectively, when evaluating total exposures. These Visit 2 and 3 differences were highly significant between the groups. In addition, the 2-week filtered location exposure for Visit 1 for Group A was 3.0 µg/m3 higher than for Group B, and the 2-week total exposure for Visit 4 for Group B was 5.1 µg/m3 higher than for Group A. These small differences were the only significant differences in PM2.5 exposure outside of the intervention period.
Figure 1.
Boxplots of 24-hour and 2-week Average Filtered Environment and Total PM2.5 Exposure Stratified Between Study Groups and Biomarker Sampling Visits
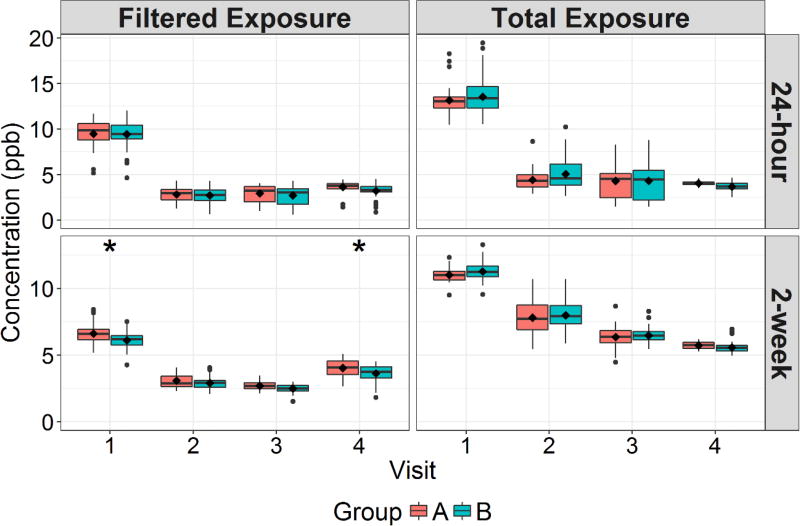
Figure 2 shows the 24-hour and 2-week total and filtered location exposures between groups for O3. The 24-hour total exposures to O3 for all subjects ranged from 1.4 to 19.4 ppb. There were only slight between-group differences in O3 exposure for each sampling visit. The only of these differences that were statistically significant were for 2-week filtered location exposure, for which Group A was 0.5 and 0.4 ppb higher than Group B for Visits 1 and 4, respectively. In linear mixed models controlling for outdoor O3 concentrations, ESP use was estimated to have only contributed 2.5 and 2.2 ppb to 24-hour filtered location exposure and total exposure, respectively.
Figure 2.
Boxplots of 24-hour and 2-week Average Filtered Environment and Total O3 Exposure Stratified Between Study Groups and Biomarker Sampling Visits
Biomarker and Physiology Outcomes
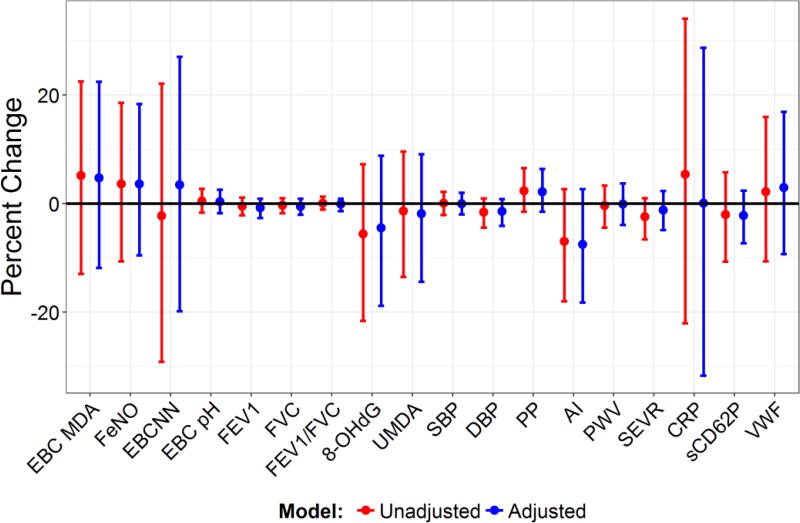
Biomarker concentrations and physiologic outcome values (together simply called biomarkers) are summarized in Table S2 in the Supplement. Figure 3 shows the Bayesian Generalized Ridge Regression results for the effect of HEPA removal on biomarkers in unadjusted models, only containing the dummy variables for HEPA and ESP filtration, or in the fully adjusted models. There were no significant associations between any of the biomarkers measured and HEPA filtration, despite the marked reductions in PM2.5 exposure that occurred with HEPA usage.
Figure 3.
Mean Percent Change in Biomarkers and 95% Credible Intervals Associated with HEPA Filter Removal in Unadjusted and Fully Adjusted Models
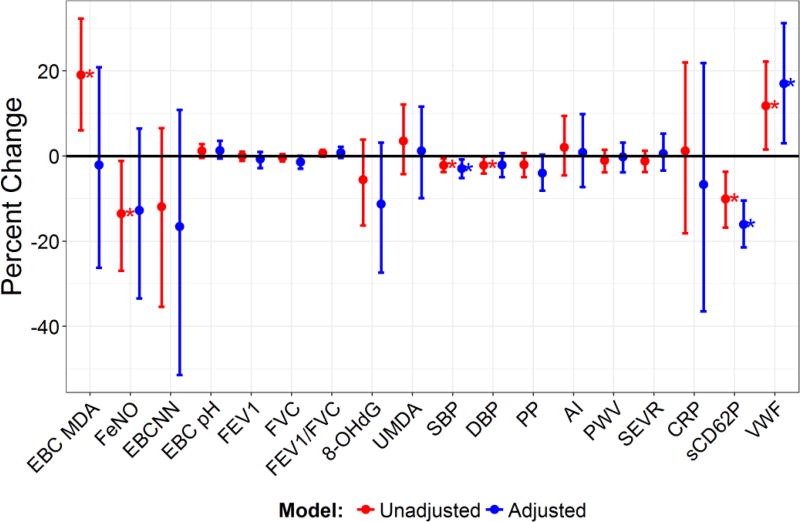
As is shown in Figure 4, in the adjusted models, ESP removal was associated with several significant biomarker changes. These included significant decreases in sCD62P (−16.1% (95% CI: −10.4%, −21.5%)) and SBP (−3.0% (95% CI: −0.8%, −5.1%)), and a significant increase in VWF (17.0% (95% CI: 2.5%, 30.8%)). Several significant associations in the unadjusted models became nonsignificant in the adjusted models, including an increase in EBC MDA and decreases in FeNO and DBP.
Figure 4.
Mean Percent Change in Biomarkers and 95% Credible Intervals Associated with ESP Removal in Unadjusted and Fully Adjusted Models
As a measure of biomarker associations with pollutant exposures encountered in the relatively short time spent away from the filtered office and dorm environments, 2-week cumulative “unfiltered” (i.e., away from the office and dorm) O3 exposure was associated with increased sCD62P. Each interquartile range (IQR) increase (793 ppb-hr) was associated with a 16.1% (95% CI: 10.4%, 21.5%) increase in sCD62P. No other biomarkers were associated with cumulative O3 or PM2.5 exposures that occurred away from the office and dorm.
When excluding active smokers from the analysis, there were several changes in biomarker associations (see Figures S1–S2). In association with ESP removal, the increase in VWF became nonsignificant (16.8% (95% CI: −0.1%, 31.1%)); the increase in EBC pH became significant (2.9% (95% CI: 0.5%, 5.2%)); and the decrease in FVC became significant (1.9% (95% CI: 0.1%, 3.7%)). In addition, the sensitivity analysis excluding active smokers changed the association between sCD62P and 2-week cumulative unfiltered O3 exposure from significant (see above) to nonsignificant.
DISCUSSION
The principal finding for this study is that the removal of central AHU HEPA filtration for the timeframe tested was associated with large increases in indoor PM2.5 concentrations and personal exposure, but not with concomitant changes in biomarker levels. In contrast, ESP removal was associated with small decreases in O3 concentrations and statistically significant decreases in several cardiovascular biomarkers, particularly sCD62P and SBP, suggesting possible adverse effects of ESP-associated exposure to O3 (and its associated secondary pollutants). ESP removal was also associated with an adverse change in VWF, though this association was less robust to sensitivity analyses than those suggesting beneficial effects of ESP removal. Furthermore, the exposures to O3 that occurred during the relatively small amount of time, in a two week span, when the subjects were away from the filtered offices and dorms were also adversely associated with sCD62P. However, this association became nonsignificant when active smokers were excluded in the analysis. When active smokers are excluded from the analysis, ESP removal is associated with a small adverse change in FVC and beneficial changes in sCD62P, SBP, and EBC pH.
The lack of a difference in biomarker response between the group receiving HEPA filtration during the intervention period (Group B) and the one without HEPA filtration (Group A) is surprising, especially given that the mean reduction in total 24-hour personal exposure by the HEPA filtration during the intervention was 37.8 µg/m3 or about 47%. None of our analyses revealed any significant association between the HEPA intervention and any biomarkers.
Firstly, the F8 filter remained in all AHUs throughout the study, and it is possible that, although this pre-filter only had a PM2.5 filtration efficiency of about 50% and there was still a large difference in exposure between Groups A (F8) and B (F8 and HEPA), this F8 caused enough of an exposure reduction that the HEPA filter provided no additional benefit. This is unlikely, however, as the mean ± SD 24-hour PM2.5 exposure concentration for Group A during the period in which they only had F8 filtration was 80.8 ± 24.7 µg/m3, corresponding with an outdoor 24-hour concentration of 126.4 ± 37.1 µg/m3, far exceeding the WHO air quality guideline of 25 µg/m3. Even the minimum ambient and exposure values for Group A during the intervention period exceeded this guideline, and so these levels are expected to be high enough to elicit a biomarker response. However, a longer intervention of one year with a MERV11/F6-rated filter, which removed even less PM than the F8 filter, led to reductions in several cardiovascular and inflammatory biomarkers,[31] which suggests that biomarkers can respond to small reductions in PM over a longer timeframe.
Secondly, variations in the chemical composition of PM2.5 may have influenced biomarker response. In a study evaluating college students moving from a suburban campus to one in urban Beijing, PM2.5 mass concentration was found to be a less reliable predictor of biomarker response than specific PM2.5 components. In that study significant blood pressure increases were observed after the move to the urban campus despite the fact that the PM2.5 mass concentrations were lower there.[32] Further analysis showed that PM2.5 sources and constituents associated with the urban campus, in particular coal combustion-related constituents, were more strongly and likely associated with increases in blood pressure than the sources and constituents related to the suburban campus.[33] Another study evaluating biomarker associations with PM2.5 sources in Beijing found that PM2.5 related to vehicle and industrial combustion, oil combustion, and vegetative burning had many more consistent associations with biomarker outcomes than PM2.5 related to soil and road dust or secondary aerosols.[34]
Thirdly, the lack of biomarker associations with the removal of HEPA filtration may be due to the possibility that it could take longer than the study period for the pathophysiologic mechanisms explored in this study to change in association with a prolonged increase in PM2.5 exposure. To the best of our knowledge, this is the first study to evaluate an indoor air filtration intervention in terms of removing rather than introducing filtration. Chamber studies of controlled, acute PM2.5 exposures in the form of wood smoke or diesel exhaust have primarily observed small increases in pulmonary inflammation markers and inconsistent increases in cardiovascular pathophysiologic biomarkers.[35–38] Few studies have evaluated the time course of biomarker responses to prolonged increases in exposure. A study of biomarker changes in response to ambient air quality improvements during the Beijing Olympics found several of the same biomarkers measured in our study, including SBP and sCD62P, increase after the Olympics ended and PM2.5 concentrations rose again.[39] However, the Olympic-period interventions affected both indoor and outdoor air quality, whereas our filtration intervention had no effect on outdoor air quality.
In terms of biomarker responses to the addition of filtration (as opposed to the subtraction of HEPA in our study), previous studies evaluating the biomarker effects of HEPA filtration in healthy adults have shown mixed results, with studies evaluating interventions lasting either a short time (up to 48 hours) or a long time (a year) finding more associations between filtration and changes in biomarkers of cardiorespiratory disease pathophysiology than interventions lasting a few weeks. Table 5 summarizes the previous studies that have evaluated biomarker responses in healthy adults to air purification interventions. Each of these seven studies utilized a crossover design of some type with studies of various durations, interventions, and exposure and biomarker measurements. Remarkably, no study has ever found a biomarker association with a filtration intervention that lasted more than 7 days but less than a year after the filtration began. The study that showed the most associations between filtration in healthy adults and biomarker outcomes, Chen et al., involved confining young adults in their dorms for 48-hours with a portable air purifier containing a non-HEPA electret air filter. Another study that showed several biomarker associations with filtration also involved a non-HEPA electret air filter, this time over an entire year of constant use in three in-home air conditioning units.[31] The other studies showing biomarker associations with a filtration intervention have only shown slight improvements in 1–2 biomarkers, such as the microvascular endothelial function marker reactive hyperemia index (RHI) in both Bräuner et al. and Allen et al. Weichenthal et al. did find slight improvements in lung function after 7 days, but these became nonsignificant when excluding two outlying subjects, suggesting that these results may not be generalizable. A 14-day central home AHU HEPA intervention was also associated with biomarker outcomes, but then it was only at 2 days, not 7 or 14 days, into the intervention period that a significant change was observed in monocyte surface CD62L, a selectin implicated in inflammation.[40]
Table 5.
Summary of Previous Studies Evaluating Filtration Effects on Biomarker Outcomes in Healthy Adults
| Study | Intervention Duration |
Purifier Type | Pollution Decrease |
Biomarkers Improved with Intervention |
|---|---|---|---|---|
| Chuang et al. 2017[31] | 2 × 1y; no washout | House AC electret filter (MERV11/F6; non-HEPA) | PM2.5: 8.6 µg/m3 (40%) | SBP, DBP, 8-OHdG, & CRP |
| Pádro-Martínez et al. 2015[41] | 2 × 21d; no washout | Window-mounted HEPA | PNC: 4900 #/cm3 (42%) | None |
| Chen et al. 2015[42] | 2 × 48h; 2w washout | Portable electret filter (non-HEPA) | PM2.5: 55 µg/m3 (57%) | SBP, DBP, sCD40L, FeNO, MCP-1, IL-1β, & MPO |
| Kajbafzadeh et al. 2015[43] | 2 × 7d; no washout | Portable HEPA + activated carbon | PM2.5: 2.8 µg/m3 (40%) | None |
| Karottki et al. 2013[44] | 2 × 14d; no washout | House AHU H11 HEPA | PM2.5: 3.8 µg/m3 (50%) | Monocyte CD62L (only on Day 2 of intervention) |
| Weichenthal et al. 2013[45] | 2 × 7d; 1w washout | Portable electret filter (non-HEPA) | PM2.5: 37 µg/m3 (~60%)a | FEV1 & PEFR (both dependent on two outlying subjects) |
| Allen et al. 2011[46] | 2 × 7d; no washout | Portable HEPA | PM2.5: 6.2 µg/m3 (~60%)a | Reactive hyperemia index (RHI); Males only: CRP, IL-6, & band cell counts |
| Bräuner et al. 2008[47] | 2 × 48h; no washout | Portable HEPA | PM2.5: 7.9 µg/m3 (~60%)a | RHI & hemoglobin |
Exact percentage not given, but was instead approximated from the published data
It is possible that these biomarkers respond and remain changed over a few days after an air pollutant exposure intervention, but as the body adapts to the lower exposure levels, these biomarkers return to some baseline level over the first few weeks of the intervention. After this intermediate period, chronic biomarker levels may begin to change in response to filtration, as was shown in the year-long intervention study. This is supported by a study examining the time lag day associations between biomarkers of different cardiorespiratory pathophysiologic pathways and ambient PM2.5, which showed that these pathways tend to be associated with PM2.5 concentrations over the previous 0–3 days, but these associations drop off and reverse direction when evaluating earlier PM2.5 concentrations.[48] Another study found several significant biomarker associations with size-fractionated PM averaged over the past day or less, with associations decreasing as the averaging time increased and only VWF and the fibrinolysis inhibitor plasminogen activator inhibitor-1 (PAI-1) showing any associations with 3-day mean PM.[49] In contrast, 30-day mean PM2.5 has been associated with increased SBP and Pulse Pressure (PP), though this effect was only significant in warmer months and for people near high road densities, conditions which were not present in our filtration study.[50] When evaluating long-term pollutant effects, several studies have shown associations between annual mean PM2.5 and inflammatory biomarkers (IL-6 and CRP) and markers of plasminogen activity.[51, 52] This evidence suggests a short-term biomarker response detectable a few days following a change in pollutant exposure, an intermediate-term return to baseline biomarker levels, and a long-term change in biomarker levels in response to changes in pollutant exposure lasting a few days, a few weeks, or approximately a year, respectively.
This does not appear to be the case for the ESP intervention, however, which may suggest different timing for biomarker responses to O3 and its related pollutants than to PM2.5. When evaluating exposures over the past year, both O3 and PM2.5 have been associated with increases in biomarkers related to cardiovascular disease risk.[51] There is a dearth of studies evaluating biomarker responses in association with O3 and PM2.5 at an intermediate time range of two weeks to a month, though it has been shown that both 2-week and 1-month O3 have been associated with elevations in an oxidative stress marker plasma 8-iso-PGF2α,[53] which has known platelet-activating properties.[54] Ultimately the present study cannot reveal differences in the time courses of biomarker responses to interventions lasting for weeks, but this is a possible explanation for the lack of HEPA-associated biomarker results seen in this and other studies with a similar duration between intervention and biological assessment.
This study is the first to evaluate ESP use in the context of cardiovascular pathophysiology. The only other study to evaluate ESP associations with health outcomes involved a 3-week ESP intervention in office AHUs that reduced total airborne particle concentrations by about 30 µg/m3 or 46% and sub-5 µm particle concentrations by 5 µg/m3 or 55%. The intervention was associated with slight improvements in peak expiratory flow (a lung function measure), nasal volume, and nasal symptoms in the intervention group, but improvements were also observed in the control group and “there was no improvement in the intervention group, compared with the control group.”[5] It is important to note that the cited study used a charcoal filter downstream of the ESP to remove O3 from the supply air. In our study, the only decrement in lung function measures observed in association with ESP removal was a slight decrease in FVC that was only significant when omitting smokers, which may be explained by the observation that smokers have less of a lung function response to air pollutants such as PM and O3.[55, 56] In the main analysis, there was also an increase in the endothelial dysfunction marker VWF in association with ESP removal that became nonsignificant when omitting smokers, which may suggest some beneficial effects of ESP use. The strengthening of this association when including smokers may be influenced by acute smoking inducing increases in VWF, which has been previously observed,[57] but in this study VWF levels were not significantly different in smokers. Compared to HEPA filtration, the only measured “beneficial” changes in exposure the ESP-HEPA filtration caused was about a 1 µg/m3 decrease in PM2.5 exposure in Office B, suggesting that whatever potential beneficial effects the ESP-HEPA combination has over HEPA filtration alone may be related to unmeasured pollutants.
There was also a robust, albeit small decrease in EBC pH associated with ESP-HEPA when omitting smokers, possibly indicating some influence of ESP-associated O3 and O3 reaction products on increasing airway inflammation and subsequent acidification that is not evident in active smokers. Active smokers show reduced EBC pH under normal conditions[58], and this may have blunted any response to ESP-associated pollutant induced lung inflammation. Our previously published analysis of pollutant exposure associations with biomarkers for this study found a significant and robust association between O3 exposure and sCD62P and less robust associations with DBP, SBP, FeNO, and EBCNN, which supports the ESP associations with sCD62P and SBP being related to increased exposure to O3 and O3-associated reaction products.[6]
As part of this overall study, and as reported in another earlier paper[30], we compared particle number concentrations between the intervention and post-intervention periods for Office B with the intent of isolating and identifying the effect of ESP use on UFP formation. We found that particle number concentration, an approximate surrogate for UFP, increased by about 22,000 particles/cm3 when the ESP was in use, and that most of this increase could be attributed to indoor secondary particle formation within the room, since supply air particle number concentrations downstream of the HEPA filter were very low.[30] In the present study, it is not possible to determine which factor associated with ESP use was driving the increases in blood pressure and platelet activation: O3, O3-derived products (e.g., UFP, peroxides, ozonides, stable Criegee biradicals), or a combination of these species. There exists a physiological basis for a possible link between O3, sCD62P, and blood pressure, which has been previously discussed.[6] These biomarker effects seem to be responding in an opposing fashion to endothelial cell dysfunction as indicated by VWF, perhaps due to different mechanisms that have been suggested in a study showing a clear VWF response to tobacco smoke but no changes in sCD62P or other thrombotic markers.[57]
There are several limitations to this study. All of the previous filtration intervention studies have been crossover trials, in which each subject receives treatment at some point during the study in a random order. This has the benefit of each subject serving as his or her own control while receiving all possible treatments in different orders to avoid bias arising from time-varying confounders. Our study examined subjects longitudinally, and so every subject acted as their own control, but only one group had the HEPA filter removed given that both groups were evaluated in parallel. As a result, it is more difficult to control for the influence of time-varying confounders. Due to the limited size of the subject pool, we were not able to have another group with no manipulation of the baseline filtration conditions (i.e, ESP-HEPA). However, the fact that there was a pre- and post-intervention period means that uncontrolled time-varying confounders that would tend to change with season would not have the same effect in both non-intervention periods, reducing the chance that these would be relevant confounders. Furthermore, our analysis used several different methods and sets of covariates to control for factors that change over time, and there was little change in the results. Given how outdoor pollutant and weather trends, co-pollutant trends, and differences in time-activity were controlled in our models, we believe that the intervention estimates reflect the observed biomarker effects attributable to each intervention.
Although the combined use of an ESP and HEPA filter, as opposed to HEPA use alone, offers economic benefits by reducing operational costs, it may increase cardiovascular disease risk for the occupants. This study presents the first evidence for changes in biomarkers indicative of negative health effects resulting from ESP-HEPA use, indicating that the concomitant low-level increases in indoor O3 and O3-derived products may impact cardiovascular health. Depending on whether smokers were included in the analysis, either VWF or FVC improved in association with ESP-HEPA use, though these results were less robust than those for the biomarkers indicative of adverse health effects. Our results also showed that biomarker responses were not detectable weeks after the removal of HEPA filters even though this intervention resulted in substantial increases in indoor PM2.5 concentrations.
Supplementary Material
PRACTICAL IMPLICATIONS.
This study is the first to examine the effects of combined ESP-HEPA filtration on cardiorespiratory disease risk indicators. It adds to only a handful of studies evaluating HEPA filtration effects on these indicators of air pollution toxicity pathways. The results of this study support previous literature suggesting limited physiological responses to PM reductions associated with HEPA filtration, particularly weeks into filtration interventions. Our findings suggest that ESP use may increase cardiovascular health risk through increasing blood pressure and thrombosis markers.
Acknowledgments
This research was funded by a grant from the National Natural Science Foundation of China (51420105010). Dr. Day’s research was also supported by a training grant from the National Institute of Environmental Health Sciences (NIEHS; T32-ES021432) and from the Doctoral Scholars Program of the Duke Global Health Institute.
We thank the Broad Group for providing access to their workers, filtration devices, and facilities. The company, however, had no involvement in study design nor in interpretation of the results. We also thank Zhongnan Pu and Yiwen Di of Tsinghua University for their assistance in sample collection.
Footnotes
The authors declare no conflicts of interest. Drs. Y Zhang and J Zhang had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Dr. Day and Mr. Xiang conducted and are responsible for the data analysis.
References
- 1.American Society of Mechanical Engineers, ASME AG-1a–2004. Addenda to ASME AG-1–2003 Code on Nuclear Air and Gas Treatment. 2004 [Google Scholar]
- 2.Boelter KJ, Davidson JH. Ozone generation by indoor, electrostatic air cleaners. Aerosol Sci Technol. 1997;27:689–708. [Google Scholar]
- 3.Waring MS, Siegel JA, Corsi RL. Ultrafine particle removal and generation by portable air cleaners. Atmos Environ. 2008;42:5003–5014. [Google Scholar]
- 4.Weschler CJ, Shields HC. Indoor ozone/terpene reactions as a source of indoor particles. Atmos Environ. 1999;33:2301–2312. [Google Scholar]
- 5.Skulberg KR, Skyberg K, Kruse K, Eduard W, Levy F, Kongerud J, Djupesland P. The effects of intervention with local electrostatic air cleaners on airborne dust and the health of office employees. Indoor Air. 2005;15:152–9. doi: 10.1111/j.1600-0668.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 6.Day DB, Xiang J, Mo J, Li F, Chung M, Gong J, Weschler CJ, Ohman-Strickland PA, Sundell J, Weng W, Zhang Y, Zhang JJ. Association of Ozone Exposure With Cardiorespiratory Pathophysiologic Mechanisms in Healthy Adults. JAMA Intern Med. 2017;177:1–10. doi: 10.1001/jamainternmed.2017.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nazaroff WW, Gadgil AJ, Weschler CJ. Critique of the Use of Deposition Velocity in Modeling Indoor Air-Quality. Modeling of Indoor Air Quality and Exposure, ASTM STP 1205. 1993;1205:81–104. [Google Scholar]
- 8.Yocom JE. Indoor-Outdoor Air-Quality Relationships - a Critical-Review. J Air Pollut Control Assoc. 1982;32:500–520. doi: 10.1080/00022470.1971.10469525. [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Zhao B. Review of relationship between indoor and outdoor particles: I/O ratio, infiltration factor and penetration factor. Atmos Environ. 2011;45:275–288. [Google Scholar]
- 10.Weschler CJ. Ozone in indoor environments: Concentration and chemistry. Indoor Air. 2000;10:269–288. doi: 10.1034/j.1600-0668.2000.010004269.x. [DOI] [PubMed] [Google Scholar]
- 11.Liang Y, Yeligar SM, Brown LAS. Exhaled Breath Condensate: A Promising Source for Biomarkers of Lung Disease. Scientific World J. 2012;2012:7. doi: 10.1100/2012/217518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones SL, Kittelson J, Cowan JO, Flannery EM, Hancox RJ, McLachlan CR, Taylor DR. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am J Respir Crit Care Med. 2001;164:738–743. doi: 10.1164/ajrccm.164.5.2012125. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 14.Ren CZ, Fang SN, Wright RO, Suh H, Schwartz J. Urinary 8-hydroxy-2 '-deoxyguanosine as a biomarker of oxidative DNA damage induced by ambient pollution in the Normative Aging Study. Occup Environ Med. 2011;68:562–569. doi: 10.1136/oem.2010.056358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong J, Zhu T, Kipen H, Wang G, Hu M, Ohman-Strickland P, Lu SE, Zhang L, Wang Y, Zhu P, Rich DQ, Diehl SR, Huang W, Zhang JJ. Malondialdehyde in exhaled breath condensate and urine as a biomarker of air pollution induced oxidative stress. J Expo Sci Environ Epidemiol. 2013;23:322–7. doi: 10.1038/jes.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sesso HD, Stampfer MJ, Rosner B, Hennekens CH, Gaziano JM, Manson JE, Glynn RJ. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in men. Hypertension. 2000;36:801–807. doi: 10.1161/01.hyp.36.5.801. [DOI] [PubMed] [Google Scholar]
- 17.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 18.Xia JJ, Liao S. Pulse Wave Analysis for Cardiovascular Disease Studies Using Subendocardial Viability Ratio. Can Con El Comp En. 2014 [Google Scholar]
- 19.Blann AD, Nadar SK, Lip GYH. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J. 2003;24:2166–2179. doi: 10.1016/j.ehj.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Sonneveld MAH, Cheng JM, Oemrawsingh RM, de Maat MPM, Kardys I, Garcia-Garcia HM, van Geuns RJ, Regar E, Serruys PW, Boersma E, Akkerhuis KM, Leebeek FWG. Von Willebrand factor in relation to coronary plaque characteristics and cardiovascular outcome Results of the ATHEROREMO-IVUS study. Thromb Haemost. 2015;113:577–584. doi: 10.1160/TH14-07-0589. [DOI] [PubMed] [Google Scholar]
- 21.Wang CJ, Yang NH, Chang CC, Liou SH, Lee HL. Rapid and simple one-step membrane extraction for the determination of 8-hydroxy-2 '-deoxyguanosine in human plasma by a combination of on-line solid phase extraction and LC-MS/MS. J Chromatogr B. 2011;879:3538–3543. doi: 10.1016/j.jchromb.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 22.James G, Witten D, Hastie T. An Introduction to Statistical Learning: With Applications in R. Springer Texts in Statistics; New York: 2014. [Google Scholar]
- 23.Plummer M. Proceedings of the 3rd International Workshop on Distributed Statistical Computing. Vienna, Austria: 2003. JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. [Google Scholar]
- 24.Su Y-S, Yajima M. R2jags: Using R to Run 'JAGS'. R package version 0.5–7 2015 [Google Scholar]
- 25.Sturtz S, Ligges U, Gelman A. R2WinBUGS: A package for running WinBUGS from R. J Stat Softw. 2005;12:1–16. [Google Scholar]
- 26.Pinheiro J, Bates D, Debroy S, Sarkar D, R Core Team nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–131 2017 [Google Scholar]
- 27.Ogle DH. FSA: Fisheries Stock Analysis. R package version 0.8.12 2017 [Google Scholar]
- 28.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2017. [Google Scholar]
- 29.WHO. WHO Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide: global update 2005: summary of risk assessment. 2006 [Google Scholar]
- 30.Xiang J, Weschler CJ, Mo J, Day D, Zhang J, Zhang Y. Ozone, Electrostatic Precipitators, and Particle Number Concentrations: Correlations Observed in a Real Office during Working Hours. Environ Sci Technol. 2016;50:10236–44. doi: 10.1021/acs.est.6b03069. [DOI] [PubMed] [Google Scholar]
- 31.Chuang HC, Ho KF, Lin LY, Chang TY, Hong GB, Ma CM, Liu IJ, Chuang KJ. Long-term indoor air conditioner filtration and cardiovascular health: A randomized crossover intervention study. Environ Int. 2017;106:91–96. doi: 10.1016/j.envint.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Wu S, Deng F, Huang J, Wang H, Shima M, Wang X, Qin Y, Zheng C, Wei H, Hao Y, Lv H, Lu X, Guo X. Blood pressure changes and chemical constituents of particulate air pollution: results from the healthy volunteer natural relocation (HVNR) study. Environ Health Perspect. 2013;121:66–72. doi: 10.1289/ehp.1104812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu S, Deng F, Wei H, Huang J, Wang X, Hao Y, Zheng C, Qin Y, Lv H, Shima M, Guo X. Association of cardiopulmonary health effects with source-appointed ambient fine particulate in Beijing, China: a combined analysis from the Healthy Volunteer Natural Relocation (HVNR) study. Environ Sci Technol. 2014;48:3438–48. doi: 10.1021/es404778w. [DOI] [PubMed] [Google Scholar]
- 34.Altemose B, Robson MG, Kipen HM, Ohman Strickland P, Meng Q, Gong J, Huang W, Wang G, Rich DQ, Zhu T, Zhang J. Association of air pollution sources and aldehydes with biomarkers of blood coagulation, pulmonary inflammation, and systemic oxidative stress. J Expo Sci Environ Epidemiol. 2016 doi: 10.1038/jes.2016.38. [DOI] [PubMed] [Google Scholar]
- 35.Mills NL, Tornqvist H, Gonzalez MC, Vink E, Robinson SD, Soderberg S, Boon NA, Donaldson K, Sandstrom T, Blomberg A, Newby DE. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. The New England journal of medicine. 2007;357:1075–82. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 36.Nightingale JA, Maggs R, Cullinan P, Donnelly LE, Rogers DF, Kinnersley R, Chung KF, Barnes PJ, Ashmore M, Newman-Taylor A. Airway inflammation after controlled exposure to diesel exhaust particulates. Am J Respir Crit Care Med. 2000;162:161–6. doi: 10.1164/ajrccm.162.1.9908092. [DOI] [PubMed] [Google Scholar]
- 37.Salvi S, Blomberg A, Rudell B, Kelly F, Sandstrom T, Holgate ST, Frew A. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med. 1999;159:702–9. doi: 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- 38.Stockfelt L, Sallsten G, Almerud P, Basu S, Barregard L. Short-term chamber exposure to low doses of two kinds of wood smoke does not induce systemic inflammation, coagulation or oxidative stress in healthy humans. Inhalation toxicology. 2013;25:417–425. doi: 10.3109/08958378.2013.798387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Zhu T, Kipen H, Wang G, Huang W, Rich D, Zhu P, Wang Y, Lu SE, Ohman-Strickland P, Diehl S, Hu M, Tong J, Gong J, Thomas D. Cardiorespiratory biomarker responses in healthy young adults to drastic air quality changes surrounding the 2008 Beijing Olympics. Res Rep Health Eff Inst. 2013:5–174. [PMC free article] [PubMed] [Google Scholar]
- 40.Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–8. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 41.Pádro-Martínez LT, Owusu E, Reisner E, Zamore W, Simon MC, Mwamburi M, Brown CA, Chung M, Brugge D, Durant JL. A Randomized Cross-over Air Filtration Intervention Trial for Reducing Cardiovascular Health Risks in Residents of Public Housing near a Highway. Int J Environ Res Public Health. 2015;12:7814–38. doi: 10.3390/ijerph120707814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen R, Zhao A, Chen H, Zhao Z, Cai J, Wang C, Yang C, Li H, Xu X, Ha S, Li T, Kan H. Cardiopulmonary benefits of reducing indoor particles of outdoor origin: a randomized, double-blind crossover trial of air purifiers. J Am Coll Cardiol. 2015;65:2279–87. doi: 10.1016/j.jacc.2015.03.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kajbafzadeh M, Brauer M, Karlen B, Carlsten C, van Eeden S, Allen RW. The impacts of traffic-related and woodsmoke particulate matter on measures of cardiovascular health: a HEPA filter intervention study. Occup Environ Med. 2015;72:394–400. doi: 10.1136/oemed-2014-102696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karottki DG, Spilak M, Frederiksen M, Gunnarsen L, Brauner EV, Kolarik B, Andersen ZJ, Sigsgaard T, Barregard L, Strandberg B, Sallsten G, Moller P, Loft S. An indoor air filtration study in homes of elderly: cardiovascular and respiratory effects of exposure to particulate matter. Environ Health. 2013;12:116. doi: 10.1186/1476-069X-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weichenthal S, Mallach G, Kulka R, Black A, Wheeler A, You H, St-Jean M, Kwiatkowski R, Sharp D. A randomized double-blind crossover study of indoor air filtration and acute changes in cardiorespiratory health in a First Nations community. Indoor Air. 2013;23:175–84. doi: 10.1111/ina.12019. [DOI] [PubMed] [Google Scholar]
- 46.Allen RW, Carlsten C, Karlen B, Leckie S, Eeden Sv, Vedal S, Wong I, Brauer M. An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am J Respir Crit Care Med. 2011;183:1222–1230. doi: 10.1164/rccm.201010-1572OC. [DOI] [PubMed] [Google Scholar]
- 47.Bräuner EV, Forchhammer L, Moller P, Barregard L, Gunnarsen L, Afshari A, Wahlin P, Glasius M, Dragsted LO, Basu S, Raaschou-Nielsen O, Loft S. Indoor particles affect vascular function in the aged: an air filtration-based intervention study. Am J Respir Crit Care Med. 2008;177:419–25. doi: 10.1164/rccm.200704-632OC. [DOI] [PubMed] [Google Scholar]
- 48.Roy A, Gong J, Thomas DC, Zhang J, Kipen HM, Rich DQ, Zhu T, Huang W, Hu M, Wang G, Wang Y, Zhu P, Lu SE, Ohman-Strickland P, Diehl SR, Eckel SP. The cardiopulmonary effects of ambient air pollution and mechanistic pathways: a comparative hierarchical pathway analysis. PLoS One. 2014;9:e114913. doi: 10.1371/journal.pone.0114913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen R, Zhao Z, Sun Q, Lin Z, Zhao A, Wang C, Xia Y, Xu X, Kan H. Size-fractionated particulate air pollution and circulating biomarkers of inflammation, coagulation, and vasoconstriction in a panel of young adults. Epidemiology. 2015;26:328–36. doi: 10.1097/EDE.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 50.Auchincloss AH, Roux AVD, Dvonch JT, Brown PL, Barr RG, Davigius ML, Goff DC, Kaufman JD, O'Neill MS. Associations between recent exposure to ambient fine particulate matter and blood pressure in the Multi-Ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2008;116:486–491. doi: 10.1289/ehp.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Green R, Broadwin R, Malig B, Basu R, Gold EB, Qi L, Sternfeld B, Bromberger JT, Greendale GA, Kravitz HM, Tomey K, Matthews K, Derby CA, Jackson EA, Green R, Ostro B. Long- and Short-term Exposure to Air Pollution and Inflammatory/Hemostatic Markers in Midlife Women. Epidemiology. 2016;27:211–20. doi: 10.1097/EDE.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hajat A, Allison M, Diez-Roux AV, Jenny NS, Jorgensen NW, Szpiro AA, Vedal S, Kaufman JD. Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation: a repeat-measures analysis in the Multi-Ethnic Study of Atherosclerosis (MESA) Epidemiology. 2015;26:310–20. doi: 10.1097/EDE.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen C, Arjomandi M, Balmes J, Tager I, Holland N. Effects of chronic and acute ozone exposure on lipid peroxidation and antioxidant capacity in healthy young adults. Environ Health Perspect. 2007;115:1732–7. doi: 10.1289/ehp.10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ting HJ, Khasawneh FT. Platelet function and Isoprostane biology. Should Isoprostanes be the newest member of the Orphan-ligand family? J Biomed Sci. 2010;17 doi: 10.1186/1423-0127-17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abbey DE, Burchette RJ, Knutsen SF, McDonnell WF, Lebowitz MD, Enright PL. Long-term particulate and other air pollutants and lung function in nonsmokers. Am J Respir Crit Care Med. 1998;158:289–98. doi: 10.1164/ajrccm.158.1.9710101. [DOI] [PubMed] [Google Scholar]
- 56.Frampton MW, Morrow PE, Torres A, Voter KZ, Whitin JC, Cox C, Speers DM, Tsai Y, Utell MJ. Effects of ozone on normal and potentially sensitive human subjects. Part II: Airway inflammation and responsiveness to ozone in nonsmokers and smokers. Res Rep Health Eff Inst. 1997:39–72. discussion 81–99. [PubMed] [Google Scholar]
- 57.Blann AD, Kirkpatrick U, Devine C, Naser S, McCollum CN. The influence of acute smoking on leucocytes, platelets and the endothelium. Atherosclerosis. 1998;141:133–9. [PubMed] [Google Scholar]
- 58.MacNee W, Rennard SI, Hunt JF, Edwards LD, Miller BE, Locantore NW, Tal-Singer R. Evaluation of exhaled breath condensate pH as a biomarker for COPD. Resp Med. 2011;105:1037–1045. doi: 10.1016/j.rmed.2011.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.