Abstract
Terbutaline and dexamethasone are used in the management of preterm labor, often for durations of treatment exceeding those recommended, and both have been implicated in increased risk of neurodevelopmental disorders. We used a variety of cell models to establish the critical stages at which neurodifferentiation is vulnerable to these agents and to determine whether combined exposures produce a worsened outcome. Terbutaline selectively promoted the initial emergence of glia from embryonic neural stem cells (NSCs). The target for terbutaline shifted with developmental stage: at later developmental stages modeled with C6 and PC12 cells, terbutaline had little effect on glial differentiation (C6 cells) but impaired the differentiation of neuronotypic PC12 cells into neurotransmitter phenotypes. In contrast to the specificity shown by terbutaline, dexamethasone affected both neuronal and glial differentiation at all stages, impairing the emergence of both cell types in NSCs but with a much greater impairment for glia. At later stages, dexamethasone promoted glial cell differentiation (C6 cells), while shifting neuronal cell differentiation so as to distort the balance of neurotransmitter phenotypes (PC12 cells). Finally, terbutaline and dexamethasone interacted synergistically at the level of late stage glial cell differentiation, with dexamethasone boosting the ability of terbutaline to enhance indices of glial cell growth and neurite formation while producing further decrements in glial cell numbers. Our results support the conclusion that terbutaline and dexamethasone are directly-acting neuroteratogens, and further indicate the potential for their combined use in preterm labor to worsen neurodevelopmental outcomes.
Keywords: C6 cells, Developmental neurotoxicity, Dexamethasone, Embryonic neural stem cells, PC12 cells, Preterm labor, Terbutaline
1. INTRODUCTION
Preterm delivery is a leading cause of perinatal morbidity and mortality, and of lasting neurobehavioral deficits. In the U.S., approximately 10% of live births occur preterm, and the rates are rising (Martin et al. 2017), reversing a longstanding trend. Glucocorticoids, notably dexamethasone, are the consensus treatment for preterm labor occurring between 24 and 34 weeks of gestation, so as to prevent neonatal respiratory distress syndrome (Gilstrap et al. 1995). β-Adrenergic receptor (βAR) agonists, particularly β2AR-selective drugs like terbutaline, are also typically given for up to 48 hr, with the intent of inhibiting uterine contractions for a sufficient period to enable the glucocorticoids to act (Haas et al. 2014).
In clinical practice, both glucocorticoids and β2AR agonists are often given for much longer durations than those that are recommended, with dexamethasone administered in repeated courses (Dammann and Matthews 2001) and terbutaline given continuously over a period of many weeks (Elliott and Morrison 2013; Perna et al. 2014). It is increasingly clear that there are harmful consequences to these therapies. The developmental neurotoxicity of glucocorticoids is well established, as these agents disrupt neural cell replication and differentiation, producing synaptic deficiencies that ultimately result in neurobehavioral, endocrine and cardiovascular disorders (Cavalieri and Cohen 2006; Drake et al. 2007; Meyer 1985; Moritz et al. 2005; Pryce et al. 2011; Rokyta et al. 2008; Tegethoff et al. 2009); adverse outcomes have now been verified in children prenatally exposed to glucocorticoids (Crowther et al. 2007; Hirvikoski et al. 2007; Needelman et al. 2008; Newnham 2001; Peltoniemi et al. 2011). However, the potential for neurobehavioral deficits resulting from terbutaline treatment are less well recognized. βARs, including the β2 subtype, are expressed prominently throughout the developing brain (Harden et al. 1977; Lorton et al. 1988) and control the balance between neural cell replication and apoptosis (Garofolo et al. 2003; Hodges-Savola et al. 1996; Slotkin et al. 1988; Zhu et al. 1998, 1999). In animal models, developmental exposure to terbutaline leads to glial activation (Rhodes et al. 2004; Zerrate et al. 2007), a hallmark of neurotoxicity (O’Callaghan 1993), culminating in abnormalities of brain structure, impaired synaptic function and behavioral deficits (Aldridge et al. 2005; Meyer et al. 2005; Rhodes et al. 2004; Slotkin et al. 1989; Slotkin and Seidler 2007; Zerrate et al. 2007). In clinical studies, prolonged use of terbutaline to prevent preterm delivery increases the risk of autism, learning disabilities and neuropsychiatric disorders in the offspring (Connors et al. 2005; Croen et al. 2011; Perna et al. 2014; Pitzer et al. 2001; Witter et al. 2009), particularly in individuals with β2AR polymorphisms that impair the ability to desensitize the receptors (Connors et al. 2005). Indeed, many of the structural, synaptic functional and behavioral features of autism are recapitulated by developmental exposure to terbutaline in animal models (Bercum et al. 2015; Slotkin 2008; Slotkin and Seidler 2013; Zerrate et al. 2007). In acknowledgment of these and other problems, and in light of the lack of efficacy of terbutaline for maintenance tocolysis, the U.S. Food and Drug Administration issued a “black box” warning against terbutaline use beyond an initial 48 hr period (U.S. Food and Drug Administration 2011a, b).
In the current study, we explored two aspects of the developmental neurotoxicity resulting from pharmacotherapies of preterm labor: (1) the mechanism of action and targets for adverse effects of terbutaline compared to dexamethasone, and (2) the effects of combination therapy, specifically the potential for these two agents to produce synergistic effects on neural cell development. We made use of in vitro models that encompass two “decision nodes” that define critical stages of neurodifferentiation that are vulnerable to toxicant injury (Slotkin et al. 2016, 2017a). First, we used neural stem cells (NSCs) derived from rat neuroepithelium on embryonic day 14, when separation into neurons and glia is determined, so as to examine how the treatments divert neural fate toward or away from neuronal and glial phenotypes (Slotkin et al. 2016, 2017a). In addition to this early decision node, we also examined a later node after the commitment to glial or neuronal phenotypes, characterized by effects on neuronotypic PC12 cells or gliotypic C6 cells, both of which are derived from the same species (rat) as the NSCs. PC12 cells are already committed to a neuronal phenotype and their subsequent differentiation involves selection of one of two neurotransmitters, dopamine or acetylcholine (Teng and Greene 1994). C6 cells closely mimic later stage differentiation of astroglia, including the transition from cell replication to cell enlargement and neurite formation (Garcia et al. 2005).
Importantly, βARs, the target for terbutaline, are present on neural precursor cells and regulate their proliferation and differentiation (Ishizuka et al. 2012; Jhaveri et al. 2010, 2014; Masuda et al. 2012). Likewise, βARs, including the β2AR subtype, are present in both PC12 and C6 cells (Haack et al. 2010; Jia et al. 2015; Neve and Molinoff 1986; Zhong and Minneman 1993). We have already used these cell models to elucidate the mechanism of action and critical stages of vulnerability to a variety of developmental neurotoxicants (Qiao et al. 2001; Slotkin et al. 2016, 2017a, b) and here, we used the same approach for terbutaline alone and in combination with dexamethasone.
2. MATERIALS & METHODS
2.1 NSC cultures and treatments
The techniques for NSC preparation, culturing and assays have all appeared previously (Slotkin et al. 2016). Primary neural stem cells (passage zero; MTI-GlobalStem, Gaithersburg, MD) were isolated from rat cortical neuroepithelium on embryonic day 14 and were frozen in DMEM/F-12 medium with N2 supplement (MTI-GlobalStem) and 10% dimethylsulfoxide. Cells were thawed and plated at 35,000 cells/cm2 on 12 mm coverslips pre-coated with poly-L-ornithine, contained in 24-well culture plates. The culture medium consisted of DMEM/F-12, GlutaMAX™ with N2 Supplement, 20 ng/ml human fibroblast growth factor and 20 ng/ml epidermal growth factor (all from MTI-GlobalStem). Cultures were maintained in a humidified incubator at 37° C with 5% CO2. Twenty-four hours later, the medium was changed to initiate spontaneous differentiation by eliminating the two growth factors, with the addition of 200 μM ascorbic acid and the test compounds, terbutaline hemisulfate, dexamethasone phosphate and d,l-propranolol HCl (all from Sigma-Aldrich, St. Louis, MO). After 3 days, half the medium was replaced, including the indicated treatment agents, and the exposures were continued for another 3 days (6 days total exposure). Concentration ranges for all agents were chosen based on prior work with in vitro models (Haack et al. 2010; Jameson et al. 2006a; Jia et al. 2015; Lemmens et al. 2017; Markus et al. 2010; Slotkin et al. 2016; Zhong and Minneman 1993).
2.2 NSC assays
At the end of the exposure period, the medium was removed and the coverslips washed with Dulbecco’s phosphate-buffered saline, fixed with 4% paraformaldehyde and washed three times with Dulbecco’s phosphate-buffered saline containing additional Ca2+ and Mg2+. Cells were permeabilized for 30 min in phosphate buffered saline containing 0.2% Triton X-100, washed three times with phosphate buffered saline (without Triton), followed by a 30 min incubation in BlockAid™ solution. Cells expressing a neuronal or astroglial phenotype were identified by immunocytochemistry according to manufacturers’ instructions, using microtubule-associated protein 2 (MAP2) for neurons and glial fibrillary acidic protein (GFAP) for astroglia. After permeabilization, the coverslips were incubated for 1 hr at room temperature using rabbit anti-MAP2 (1:200) and rat anti-GFAP (1:20) in BlockAid™. Coverslips were rinsed four times with phosphate-buffered saline and then incubated for 1 hr at room temperature with the appropriate fluor-conjugated secondary antibodies (donkey anti-rabbit IgG Alexa Fluor 647 and goat anti-rat IgG Alexa Fluor 555) diluted 1:400 in BlockAid™. After an additional five rinses with phosphate buffered saline, coverslips were incubated for 5 min with 300 nM 4′,6-diamidino-2-phenylindole (DAPI) nucleic acid stain to label individual cells. Coverslips were rinsed three times with phosphate buffered saline and mounted onto glass slides using ProLong Diamond Antifade mountant. All reagents for the NSC assays were obtained from MTI-GlobalStem except for rabbit anti-MAP2 (EMD Millipore, Billerica MA) and antifade mountant (Thermo Fisher Scientific, Waltham, MA).
Images of 3 to 4 fields/slide (each field = 3.22 × 105 μm2) were captured using a Zeiss Axio Imager widefield fluorescence microscope with 200× magnification and quantified for total cells (DAPI-positive stain for nuclei); across the multiple fields in a given culture, thousands of cells were counted. Each cell was then examined to see if it expressed a neuronal phenotype (MAP2-positive) or a glial phenotype (GFAP-positive). A cell was counted as positive only when the stain for a given phenotype coincided with a DAPI-stained nucleus. Values were averaged across the fields to render a single value for each culture.
2.3 PC12 cells
Because of the clonal instability of the PC12 cell line (Fujita et al. 1989), the experiments were performed on cells that had undergone fewer than five passages. As described previously (Qiao et al. 2003; Song et al. 1998), PC12 cells (American Type Culture Collection CRL-1721, obtained from the Duke Comprehensive Cancer Center, Durham, NC) were seeded (3 × 106 cells) onto 100 mm poly-D-lysine-coated plates in RPMI-1640 medium (Sigma-Aldrich) supplemented with 10% horse serum (Sigma-Aldrich), 5% fetal bovine serum (Sigma-Aldrich), and 50 μg/ml penicillin streptomycin (Invitrogen, Carlsbad, CA). Incubations were carried out with 5% CO2 at 37°C, standard conditions for PC12 cells. Twenty-four hours after plating, we initiated neurodifferentiation (Jameson et al. 2006b; Slotkin et al. 2007; Teng and Greene 1994) by changing the medium to include 50 ng/ml of 2.5 S murine nerve growth factor (Promega Corporation, Madison, WI). Test substances were added simultaneously so as to be present throughout neurodifferentiation. The medium was changed every 48 hr with the continued inclusion of nerve growth factor and test substances, and continued for 6 days, parallel to the NSC studies. At the end of each study, the cultures were examined under a microscope to verify the outgrowth of neurites.
Cells were harvested, washed, and analyzed for protein as described previously (Slotkin et al. 2007). To assess neurodifferentiation into dopamine and acetylcholine phenotypes, we assayed the enzymatic activities of tyrosine hydroxylase (TH) and choline acetyltransferase (ChAT), respectively, using established techniques (Jameson et al. 2006a, b), with values calculated as enzyme activity per mg protein.
2.4 C6 cells
C6 cells (American Type Culture Collection CCL-107, Duke Comprehensive Cancer Center) were seeded onto 100 mm polystyrene culture dishes in Kaighn’s modification of Ham’s F-12 medium (Thermo Fisher) supplemented with 15% horse serum (Sigma-Aldrich) and 2.5% fetal bovine serum (Sigma-Aldrich). Incubations were carried out with 5% CO2 at 37 °C. Forty eight hours after plating, we initiated treatments by changing the medium to include test substances, and the medium, including the test substances, was changed after 48 hr.
C6 cells undergo more rapid cell division than NSCs or PC12 cells, so we conducted studies with different combinations of plating density and incubation time, as necessitated to prevent the cells from reaching confluence: 0.6 × 106 cells/dish for 96 hr, 0.75 × 106 cells/dish for 90 hr, or 1.5 × 106 cells/dish for 72 hr. The results under all three conditions were the same, so these were combined for presentation.
Cells were harvested, washed, and the DNA and protein fractions were isolated and analyzed as described previously (Slotkin et al. 2007). Measurements of DNA, total protein and membrane protein were used as biomarkers for cell number, cell growth and neurite growth (Qiao et al. 2003; Song et al. 1998). Effects on cell number were determined by measuring DNA content, since each neural cell contains only a single, diploid nucleus (Winick and Noble 1965). DNA per cell is constant, so that cell growth entails an obligatory increase in total protein per cell (protein/DNA ratio) whereas neurite formation entails an additional rise in membrane protein per cell (membrane protein/DNA ratio).
2.5 Data analysis
Each study was performed using multiple, separate batches of cells, with 3–6 independent cultures for each treatment in each batch. Each batch of cells comprised a separately prepared, frozen and thawed preparation. Cultures within each batch were considered independent samples because each represented a separate plating, on a separate cover slip (NSCs) or culture dish (PC12 cells, C6 cells), each one individually treated and cultured. Results are presented as mean ± SE, with treatment comparisons carried out by analysis of variance (ANOVA) followed by Fisher’s Protected Least Significant Difference Test for post-hoc comparisons of individual treatments. The initial comparisons included factors of treatment and cell batch, and in each case, we found that the treatment effects for each type of experiment were the same across the different batches of cells, although the absolute values differed from batch to batch. Accordingly, we normalized the results across batches prior to combining them for presentation, so that multiple treatments could be compared readily on the same graph; however statistical comparisons were carried out on the original data, retaining the batch factor, so that treatment groups would be compared only to their contemporaneous controls. Significance for all tests was assumed at p < 0.05 (two-tailed).
3. RESULTS
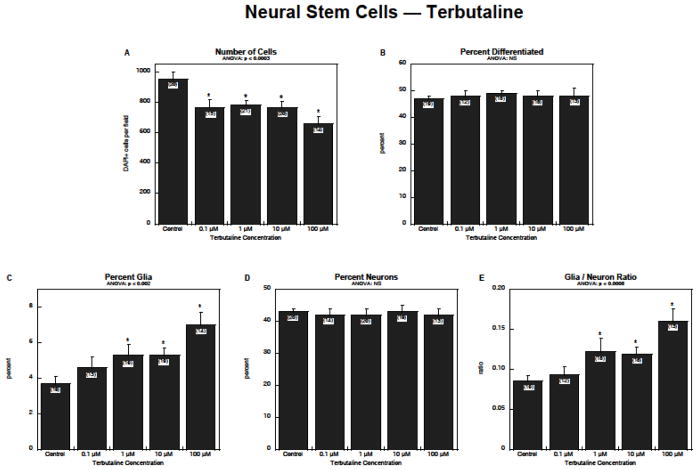
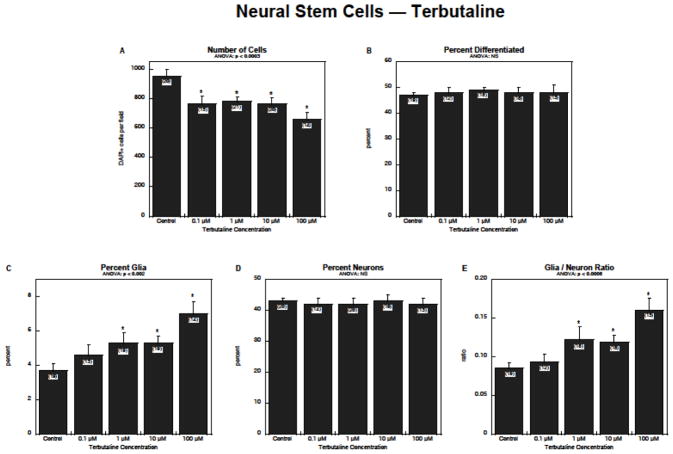
Exposure of NSCs to terbutaline produced a concentration-dependent reduction in the number of cells, with significant effects detected even at 0.1 μM (Figure 1A). Although there was no detectable change in the total proportion of cells undergoing neurodifferentiation (Figure 1B), terbutaline stimulated formation of glia by 50–100% (Figure 1C). Neurons, the majority of the differentiated cells, were unaffected (Figure 1D), and consequently, there was a substantial rise in the glia/neuron ratio (Figure 1E).
Figure 1.
Effects of terbutaline on NSC differentiation: (A) numbers of cells, (B) percentage of cells showing differentiation into neurons or glia, (C) percentage of glia, (D) percentage of neurons, (E) glia/neuron ratio. Data represent means and standard errors of the number of determinations shown in parentheses. ANOVA appears at the top of each panel and asterisks denote individual values that differ from the corresponding control. Abbreviation: NS = not significant.
To determine if the effects of terbutaline on NSCs were reflective of the drug’s actions atβARs, we co-treated the cells with 10 μM propranolol, a concentration shown to block the receptor in vitro (Jia et al. 2015). Before examining the interaction of terbutaline and propranolol on individual parameters, we performed a repeated-measures ANOVA on the three main outcome measures (cell number, percent glia, percent neurons), using log-transformed data because of heterogeneous variance among the measures. This global test indicated a significant interaction of terbutaline × propranolol × measurement type (p < 0.02), justifying separation of the values with vs. without propranolol, for each of the dependent measures.
Propranolol completely prevented the reduction in cell numbers caused by 1 μM terbutaline and prevented about 2/3 of the loss caused by 10 μM terbutaline (Figure 2A). Again, terbutaline was ineffective in changing the overall percentage of cells undergoing neurodifferentiation, a situation that was unchanged by propranolol alone or in combination with terbutaline (Figure 2B). However, propranolol completely blocked the promotional effect of terbutaline on formation of glia (Figure 2C). Either drug alone or in combination had no effect on formation of neurons (Figure 2D). Consequently, propranolol blocked the rise in glia/neuron ratio evoked by terbutaline (Figure 2E).
Figure 2.
Effects of terbutaline ± propranolol on NSC differentiation: (A) numbers of cells, (B) percentage of cells showing differentiation into neurons or glia, (C) percentage of glia, (D) percentage of neurons, (E) glia/neuron ratio. Data represent means and standard errors of the number of determinations shown in parentheses. ANOVA appears at the top of each panel and asterisks denote individual values that differ from the corresponding control. Abbreviation: NS = not significant.
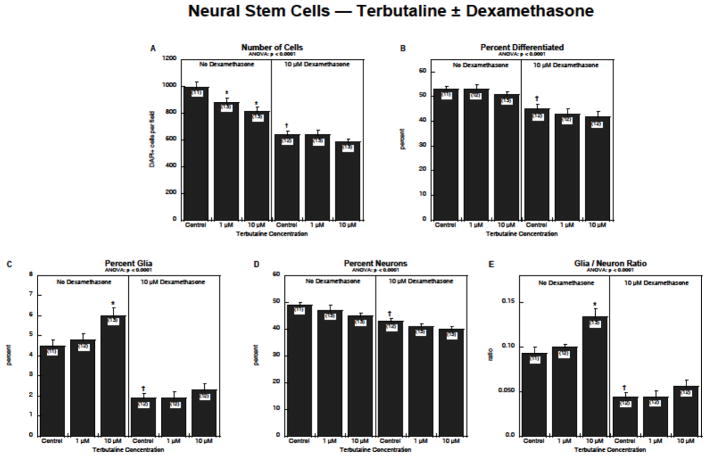
We then evaluated the interaction of terbutaline with dexamethasone in NSCs, using 10 μM dexamethasone, a concentration known to suppress NSC neurodifferentiation (Slotkin et al. 2016). Again, before examining each output variable, we performed a global, repeated-measures ANOVA for the three main outcome measures. In this case, there was no interaction of terbutaline × dexamethasone, or of terbutaline × dexamethasone × measurement type, indicating that dexamethasone did not significantly shift the response of the cells to terbutaline (i.e. responses to the combination treatment were not significantly distinguishable from additive effects of the two individual treatments). As before, terbutaline alone significantly reduced cell numbers but dexamethasone had an even greater effect, which then masked the response to terbutaline (Figure 3A). Whereas terbutaline did not affect the overall percentage of cells undergoing neurodifferentiation, dexamethasone elicited a significant reduction, with no further effect evident with addition of terbutaline in the presence of dexamethasone (Figure 3B). Terbutaline and dexamethasone had opposite effects on formation of glia, with terbutaline causing an increase and dexamethasone a decrease (Figure 3C); again dexamethasone’s effects were so massive that they masked those of terbutaline when the treatments were combined. Likewise, dexamethasone, but not terbutaline, caused a decrease in differentiation into neurons (Figure 3D), without any significant interaction between the two treatments. Because of these underlying differences, terbutaline increased the glia/neuron ratio whereas dexamethasone massively reduced the ratio, again obtunding the effects of additional terbutaline treatment (Figure 3E).
Figure 3.
Effects of terbutaline ± dexamethasone on NSC differentiation: (A) numbers of cells, (B) percentage of cells showing differentiation into neurons or glia, (C) percentage of glia, (D) percentage of neurons, (E) glia/neuron ratio. Data represent means and standard errors of the number of determinations shown in parentheses. ANOVA appears at the top of each panel. Asterisks denote individual values that differ from the corresponding control and daggers indicate differences between control values with vs. without dexamethasone.
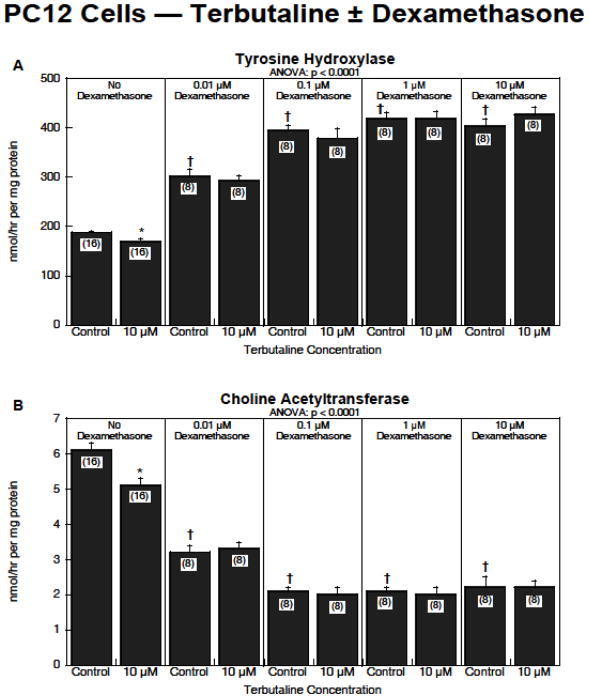
For studies in PC12 and C6 cells, after preliminary studies of dose-response curves to terbutaline and dexamethasone, we settled on a regimen of 10 μM terbutaline and dexamethasone concentrations ranging from 0.01–10 μM. In the PC12 cell model, global repeated-measures ANOVA across the two dependent measures (tyrosine hydroxylase and choline acetyltransferase activities) indicated a significant interaction of terbutaline × dexamethasone (p < 0.05), reflecting an overall reduction in the effects of terbutaline when dexamethasone was present. For tyrosine hydroxylase, terbutaline alone caused a small, but significant reduction in activity, whereas dexamethasone by itself produced a substantial increase (Figure 4A). Progressively higher dexamethasone concentrations eventually interfered with the effects of terbutaline, rendering them nonsignificant, or even shifting terbutaline’s effect so as to produce a slight increase over the corresponding control values. For choline acetyltransferase, both terbutaline and dexamethasone alone produced a significant reduction in activity instead of acting in opposite directions as they did for tyrosine hydroxylase (Figure 4B). Again, however, dexamethasone masked the effects of terbutaline, such that in the presence of dexamethasone, terbutaline no longer elicited any significant change.
Figure 4.
Effects of terbutaline ± dexamethasone on PC12 cell differentiation: (A) tyrosine hydroxylase activity, (B) choline acetyltransferase activity. Data represent means and standard errors of the number of determinations shown in parentheses. ANOVA appears at the top of each panel. Asterisks denote individual values that differ from the corresponding control and daggers indicate differences between control values with vs. without dexamethasone.
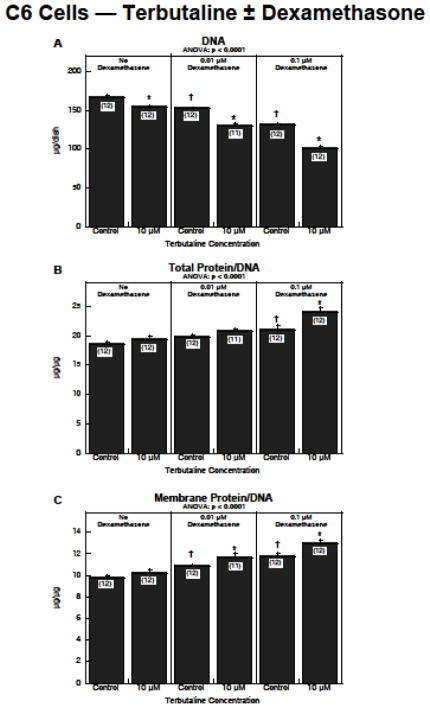
In C6 cells, the global repeated-measures ANOVA indicated a significant interaction of terbutaline × dexamethasone × measurement type (p < 0.0005), reflecting synergistic effects between the two treatments and justifying separate examination of the interaction for each of the dependent measures. For DNA content (Figure 5A), either terbutaline or dexamethasone alone elicited a significant decrease, but in this case, dexamethasone enhanced the effects of terbutaline (terbutaline × dexamethasone interaction, p < 0.0001); in the presence of dexamethasone, the reduction in DNA evoked by terbutaline was doubled or tripled. The same synergism was seen for the two cell growth parameters, total protein/DNA (Figure 5B) and membrane protein/DNA (Figure 5C). For both parameters, terbutaline alone evoked a small, nonsignificant increase, whereas dexamethasone produced a more robust and statistically significant rise. In the presence of dexamethasone, the increase caused by terbutaline was substantially enhanced, achieving statistical significance over the corresponding control values (p < 0.05 for the terbutaline × dexamethasone interaction for either parameter).
Figure 5.
Effects of terbutaline ± dexamethasone on C6 cell differentiation: (A) DNA content, (B) total protein/DNA ratio, (C) membrane protein/DNA ratio. Data represent means and standard errors of the number of determinations shown in parentheses. ANOVA appears at the top of each panel. Asterisks denote individual values that differ from the corresponding control and daggers indicate differences between control values with vs. without dexamethasone.
Because of the high rate of cell division in the C6 model, we did an additional study to evaluate whether the drop in DNA content caused by terbutaline reflected cell loss as opposed to impaired cell division. In this case, we prolonged the treatment period to allow the cells in the control plates to grow to confluence, at which point their replication rate slows. If reduced DNA in the terbutaline group reflected cytotoxic cell loss, the prolonged treatment would have a greater effect, whereas if it represented a slowing of mitosis, the longer period would enable the terbutaline-exposed cultures to “catch up” to the cell number in the controls. We found the latter to be true — prolonging the treatment to allow the control cells to grow to confluence, resulted in no significant difference between terbutaline and control samples (data not shown).
4. DISCUSSION
There are three main findings from our results. First, terbutaline and dexamethasone both act directly on neural cell differentiation, over and above any indirect effects on brain development that may be mediated via their myriad actions on maternal/fetal physiology, endocrine status, or other systemic effects. Second, the two agents target different events and stages of neurodifferentiation. Terbutaline specifically promotes the formation of glia from NSCs, but after cells are committed to the glial phenotype, their sensitivity declines, as characterized at the later stages by C6 cells. For the neuronal phenotype, the sensitivity to terbutaline is low at the NSC stage but after the commitment is made, terbutaline effectively impairs further neurodifferentiation into specific neurotransmitter subtypes, as characterized by PC12 cells. In contrast to the specificity shown by terbutaline, dexamethasone affects both neuronal and glial differentiation at all stages. In NSCs, dexamethasone impairs the emergence of both neurons and glia but with a much greater specificity for the latter cell type. At later stages, dexamethasone instead promotes glial cell differentiation as characterized in the C6 model, while shifting neuronal cell differentiation so as to distort the balance of neurotransmitter phenotypes (increasing the dopamine phenotype at the expense of the acetylcholine phenotype in the PC12 model). The third important finding is that terbutaline and dexamethasone interact synergistically specifically at the level of late stage glial cell differentiation, not for neuronal cell differentiation and not at earlier stages for either cell type. This indicates the potential for worsened neurodevelopmental outcomes when both drugs are used for inappropriately prolonged periods, a common clinical practice (Dammann and Matthews 2001; Elliott and Morrison 2013; Perna et al. 2014).
By itself, terbutaline decreased cell numbers in the NSC model but specifically promoted neurodifferentiation into the glial phenotype, effects that were partially (cell numbers) or completely (glial phenotype) blocked by a βAR antagonist, confirming a receptor-based mechanism of action. Since terbutaline promoted glial differentiation, the cell decrement is not likely to be reflective of cytotoxicity, which typically results in inhibition of neurodifferentiation into both neurons and glia (Slotkin et al. 2016). Indeed, neural precursor cells contain βARs that mediate the balance between mitotic activity and differentiation (Ishizuka et al. 2012; Jhaveri et al. 2010, 2014; Masuda et al. 2012) and β2AR stimulation is actually antiapoptotic in developing neural cells (Zhu et al. 1998). NSCs are still undergoing rapid cell replication in culture, increasing their numbers by nearly 10-fold in the 6-day treatment period studied here (Slotkin et al. 2016), so that even a modest impairment of mitotic activity or a shift toward differentiation (as found for glial cells) would be sufficient to reduce cell numbers (Slotkin et al. 2017b). Indeed, there was a similar reduction in cell numbers caused by terbutaline in the C6 model, and we demonstrated conclusively that this was not reflective of cytotoxicity, since the effect could be offset by prolonging the incubation period to allow mitotic “catch up” with the controls. At the high concentration of terbutaline, there was a small component of cell loss in the NSC model that was not blocked by propranolol, suggesting that other mechanisms may come into play; terbutaline has been shown to cause oxidative stress in the developing brain (Slotkin et al. 2005), an effect that may rely on actions distinct from specific receptor-mediated effects. In any case, the most notable finding with the NSC model is that terbutaline raised the glia/neuron ratio, a result entirely in keeping with glial activation found in the developing brain after terbutaline exposure in vivo (Zerrate et al. 2007). The present findings thus reinforce the conclusion that terbutaline acts directly as neuroteratogen (Rhodes et al. 2004; Zerrate et al. 2007).
The sensitivity of glial cell differentiation to terbutaline fell off after the commitment to the glial phenotype. In contrast to the high sensitivity seen at the NSC stage (precommitment), this drug had little effect on C6 cells (postcommitment), other than eliciting a small decline in cell numbers as noted above. In contrast, terbutaline affected late-stage neuronal cell differentiation. Whereas differentiation into the neuronal phenotype was unaffected at the NSC stage, terbutaline suppressed differentiation of neuronotypic PC12 cells into both dopamine and acetylcholine neurotransmitter phenotypes. Terbutaline thus exhibits stage-specific effects on development of glia vs. neurons, targeting glia primarily at the early decision node when NSCs differentiate into glial and neuronal phenotypes, whereas after that commitment, it specifically impairs further differentiation or the neuronal phenotype.
In general, the effects of dexamethasone alone were greater than those of terbutaline, but more importantly, differed substantially in terms of type of effect, specificity for developmental stage and selectivity for neurons vs. glia. In agreement with earlier studies with the NSC model (Slotkin et al. 2016), dexamethasone reduced cell numbers and retarded differentiation into the glial phenotype, the latter effect opposite to that of terbutaline. At the later decision node, dexamethasone enhanced neurodifferentiation of PC12 cells into the dopamine phenotype while suppressing emergence of the acetylcholine phenotype, as reported earlier (Jameson et al. 2006a); terbutaline suppressed both neurotransmitter types. In C6 cells, dexamethasone promoted differentiation, resulting in a decline in cell numbers but increases in the indices of cell growth and neurite formation. Again, the reduced cell numbers with dexamethasone are unlikely to reflect cytotoxicity, since this agent actually reduces apoptosis in this cell line (Gorman et al. 2000).
The dichotomy between the effects of terbutaline and dexamethasone is not surprising, given their entirely separate mechanisms of action. But what happens when the two are combined, as often used in preterm labor (Haas et al. 2014)? At the NSC stage, the more robust effects of dexamethasone masked those of terbutaline, so that the net effect of combined treatment was indistinguishable from that of dexamethasone alone. Likewise, our results with the PC12 model indicated that the effects of dexamethasone on later-stage neuronal cell differentiation overrode those of terbutaline, in this case showing a significant interaction reflecting loss of terbutaline’s effects in the presence of dexamethasone. However, for C6 cells, we found a synergistic effect of the two agents. In the presence of dexamethasone, terbutaline caused a greater cell loss and significant increases in indices of cell growth and neurite formation. The enhanced effect of terbutaline likely reflects the fact that dexamethasone increases β2AR expression (Zhong and Minneman 1993). Again, our findings point to stage-specific effects of these drugs, focusing in this case primarily on later stages of glial cell differentiation.
5. CONCLUSION
In summary, our results show that both terbutaline and dexamethasone have direct effects on neurodifferentiation, altering the balance between emergence of neurons and glia, and then further affecting later differentiation of both cell types. Terbutaline selectively promotes the emergence of glia from NSCs, whereas dexamethasone suppresses it. As modeled in PC12 cells, terbutaline impairs later neuronal differentiation into specific neurotransmitter phenotypes, whereas dexamethasone shifts phenotypic preference toward dopamine and away from acetylcholine. For later stage glial cell differentiation (C6 model), terbutaline had only minor effects but dexamethasone robustly promoted cell differentiation at the expense of reduced cell numbers. Notably, at this stage, the two drugs showed a synergistic interaction, with dexamethasone boosting the ability of terbutaline to enhance indices of glial cell growth and neurite formation while producing further decrements in cell numbers. Our results support the conclusion that terbutaline is a directly-acting neuroteratogen, and further indicate the potential for the clinically-used combination of terbutaline and dexamethasone to worsen neurodevelopmental outcomes. These findings reinforce the rectitude of the U.S. Food and Drug Administration’s decision to move terbutaline from pregnancy category B to category C, and to issue a warning against its prolonged use in the management of preterm labor (U.S. Food and Drug Administration 2011a, b). Additionally, our results point to the need to examine the potential long-term impact of dexamethasone, alone or in combination with terbutaline, on neurodevelopmental outcomes in the large number of children exposed to these agents in the management of preterm labor.
Acknowledgments
Funding: This work was supported by the National Institutes of Health [grant number ES10356].
Abbreviations
- ANOVA
analysis of variance
- βAR
β-adrenergic receptor
- DAPI
4′,6-diamidino-2-phenylindole
- GFAP
glial fibrillary acidic protein
- MAP2
microtubule-associated protein 2
- NSC
neural stem cell
Footnotes
Conflict of interest statement: TAS has received consultant income in the past three years from Pardieck Law (Seymour, IN) and Walgreen Co. (Deerfield, IL).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Developmental exposure to terbutaline and chlorpyrifos: pharmacotherapy of preterm labor and an environmental neurotoxicant converge on serotonergic systems in neonatal rat brain regions. Toxicol Appl Pharmacol. 2005;203:134–144. doi: 10.1016/j.taap.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Bercum FM, Rodgers KM, Benison AM, Smith ZZ, Taylor JK, Kornreich E, Grabenstatter HL, Dudek FE, Barth DS. Maternal stress combined with terbutaline leads to comorbid autistic-like behavior and epilepsy in a rat model. J Neurosci. 2015;35:15894–15902. doi: 10.1523/JNEUROSCI.2803-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri RL, Cohen WR. Antenatal steroid therapy: have we undervalued the risks? J. Matern Fetal Neonat Med. 2006;19:265–269. doi: 10.1080/14767050600676075. [DOI] [PubMed] [Google Scholar]
- Connors SL, Crowell DE, Eberhart CG, Copeland J, Newschaffer CJ, Spence SJ, Zimmerman AW. β2-Adrenergic receptor activation and genetic polymorphisms in autism: data from dizygotic twins. J Child Neurol. 2005;20:876–884. doi: 10.1177/08830738050200110401. [DOI] [PubMed] [Google Scholar]
- Croen LA, Connors SL, Matevia M, Qian Y, Newschaffer C, Zimmerman AW. Prenatal exposure to β 2-adrenergic receptor agonists and risk of autism spectrum disorders. J Neurodev Disord. 2011;3:307–315. doi: 10.1007/s11689-011-9093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther CA, Doyle LW, Haslam RR, Hiller JE, Harding JE, Robinson J Actords Study Group. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. New Eng J Med. 2007;357:1179–1189. doi: 10.1056/NEJMoa071152. [DOI] [PubMed] [Google Scholar]
- Dammann O, Matthews SG. Repeated antenatal glucocorticoid exposure and the developing brain. Pediatr Res. 2001;50:563–564. doi: 10.1203/00006450-200111000-00004. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Tang JI, Nyirenda MJ. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin Sci. 2007;113:219–232. doi: 10.1042/CS20070107. [DOI] [PubMed] [Google Scholar]
- Elliott JP, Morrison JC. The evidence regarding maintenance tocolysis. Obstet Gynecol Intl. 2013;2013:708023. doi: 10.1155/2013/708023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Lazarovici P, Guroff G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ Health Perspect. 1989;80:127–142. doi: 10.1289/ehp.8980127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia SJ, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: targeting glial cells. Environ Toxicol Pharmacol. 2005;19:455–461. doi: 10.1016/j.etap.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Garofolo MC, Seidler FJ, Cousins MM, Tate CA, Qiao D, Slotkin TA. Developmental toxicity of terbutaline: critical periods for sex-selective effects on macromolecules and DNA synthesis in rat brain, heart, and liver. Brain Res Bull. 2003;59:319–329. doi: 10.1016/s0361-9230(02)00925-5. [DOI] [PubMed] [Google Scholar]
- Gilstrap LC, Christensen R, Clewell WH, D’Alton ME, Davidson EC, Escobedo MB, Gjerdingen DK, Goddard-Finegold J, Goldenberg RL, Grimes DA, Hansen TN, Kauffman RE, Keeler EB, Oh W, Susman EJ, Vogel MG, Avery ME, Ballard RA, Crowley P, Garite T, Hankins GDV, Jobe AH, Koppe JG, Maher JE, Merkatz IR, Shankaran S, Simpson KN, Sinclair JC, Slotkin TA, Taeusch HW, Wright LL. Effect of corticosteroids for fetal maturation on perinatal outcomes. J Am Med Assoc. 1995;273:413–418. [Google Scholar]
- Gorman AM, Hirt UA, Orrenius S, Ceccatelli S. Dexamethasone pre-treatment interferes with apoptotic death in glioma cells. Neuroscience. 2000;96:417–425. doi: 10.1016/s0306-4522(99)00565-5. [DOI] [PubMed] [Google Scholar]
- Haack KKV, Tougas MR, Jones KT, El-Dahr SS, Radhakrishna H, McCarty NA. A novel bioassay for detecting GPCR heterodimerization: transactivation of beta 2 adrenergic receptor by bradykinin receptor. J Biomol Screen. 2010;15:251–260. doi: 10.1177/1087057109360254. [DOI] [PubMed] [Google Scholar]
- Haas DM, Benjamin T, Sawyer R, Quinney SK. Short-term tocolytics for preterm delivery - current perspectives. Intl J Womens Health. 2014;6:373–349. doi: 10.2147/IJWH.S44048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden TK, Wolfe BB, Sporn JR, Perkins JP, Molinoff PB. Ontogeny of β-adrenergic receptors in rat cerebral cortex. Brain Res. 1977;125:88–108. doi: 10.1016/0006-8993(77)90362-6. [DOI] [PubMed] [Google Scholar]
- Hirvikoski T, Nordenstrom A, Lindholm T, Lindblad F, Ritzen EM, Wedell A, Lajic S. Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. J Clin Endocrinol Metab. 2007;92:542–548. doi: 10.1210/jc.2006-1340. [DOI] [PubMed] [Google Scholar]
- Hodges-Savola C, Rogers SD, Ghilardi JR, Timm DR, Mantyh PW. β-Adrenergic receptors regulate astrogliosis and cell proliferation in the central nervous system in vivo. Glia. 1996;17:52–62. doi: 10.1002/(SICI)1098-1136(199605)17:1<52::AID-GLIA5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Goshima H, Ozawa A, Watanabe Y. β1-adrenoceptor stimulation enhances the differentiation of mouse induced pluripotent stem cells into neural progenitor cells. Neurosci Lett. 2012;525:60–65. doi: 10.1016/j.neulet.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Jameson RR, Seidler FJ, Qiao D, Slotkin TA. Adverse neurodevelopmental effects of dexamethasone modeled in PC12 cells: identifying the critical stages and concentration thresholds for the targeting of cell acquisition, differentiation and viability. Neuropsychopharmacology. 2006a;31:1647–1658. doi: 10.1038/sj.npp.1300967. [DOI] [PubMed] [Google Scholar]
- Jameson RR, Seidler FJ, Qiao D, Slotkin TA. Chlorpyrifos affects phenotypic outcomes in a model of mammalian neurodevelopment: critical stages targeting differentiation in PC12 cells. Environ Health Perspect. 2006b;114:667–672. doi: 10.1289/ehp.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri DJ, Mackay EW, Hamlin AS, Marathe SV, Nandam LS, Vaidya VA, Bartlett PF. Norepinephrine directly activates adult hippocampal precursors via β3-adrenergic receptors. J Neurosci. 2010;30:2795–2806. doi: 10.1523/JNEUROSCI.3780-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri DJ, Nanavaty I, Prosper BW, Marathe S, Husain BF, Kernie SG, Bartlett PF, Vaidya VA. Opposing effects of α2- and β-adrenergic receptor stimulation on quiescent neural precursor cell activity and adult hippocampal neurogenesis. PLoS One. 2014;9:e98736. doi: 10.1371/journal.pone.0098736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia JJ, Zeng XS, Yang LH, Bai J. The epinephrine increases tyrosine hydroxylase expression through upregulating thioredoxin-1 in PC12 cells. Biochimie. 2015;115:52–58. doi: 10.1016/j.biochi.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Lemmens S, Kusters L, Bronckaers A, Geurts N, Hendrix S. The β2-adrenoceptor agonist terbutaline stimulates angiogenesis via Akt and ERK signaling. J Cell Physiol. 2017;232:298–308. doi: 10.1002/jcp.25483. [DOI] [PubMed] [Google Scholar]
- Lorton D, Bartolome J, Slotkin TA, Davis JN. Development of brain beta-adrenergic receptors after neonatal 6-hydroxydopamine treatment. Brain Res Bull. 1988;21:591–600. doi: 10.1016/0361-9230(88)90198-0. [DOI] [PubMed] [Google Scholar]
- Markus T, Hansson SR, Cronberg T, Cilio C, Wieloch T, Ley D. β-Adrenoceptor activation epresses brain inflammation and is neuroprotective in lipopolysaccharide-induced sensitization to oxygen-glucose deprivation in organotypic hippocampal slices. J Neuroinflamm. 2010;7:94. doi: 10.1186/1742-2094-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Mathews TJ. Births: final data for 2015. Natl Vital Stat Rep. 2017;66:1–70. [PubMed] [Google Scholar]
- Masuda T, Nakagawa S, Boku S, Nishikawa H, Takamura N, Kato A, Inoue T, Koyama T. Noradrenaline increases neural precursor cells derived from adult rat dentate gyrus through β2 receptor. Prog Neuropsychopharmacol Biol Psychiat. 2012;36:44–51. doi: 10.1016/j.pnpbp.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Aldridge JE, Slotkin TA. Developmental exposure to terbutaline alters cell signaling in mature rat brain regions and augments the effects of subsequent neonatal exposure to the organophosphorus insecticide, chlorpyrifos. Toxicol Appl Pharmacol. 2005;203:154–166. doi: 10.1016/j.taap.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Meyer JS. Biochemical effects of corticosteroids on neural tissues. Physiol Rev. 1985;65:946–1020. doi: 10.1152/physrev.1985.65.4.946. [DOI] [PubMed] [Google Scholar]
- Moritz KM, Boon WM, Wintour EM. Glucocorticoid programming of adult disease. Cell Tissue Res. 2005;322:81–88. doi: 10.1007/s00441-005-1096-6. [DOI] [PubMed] [Google Scholar]
- Needelman H, Evans M, Roberts H, Sweney M, Bodensteiner JB. Effects of postnatal dexamethasone exposure on the developmental outcome of premature infants. J Child Neurol. 2008;23:421–424. doi: 10.1177/0883073807309232. [DOI] [PubMed] [Google Scholar]
- Neve KA, Molinoff PB. Effects of chronic administration of agonists and antagonists on the density of beta-adrenergic receptors. Am J Cardiol. 1986;57:17F–22F. doi: 10.1016/0002-9149(86)90883-0. [DOI] [PubMed] [Google Scholar]
- Newnham JP. Is prenatal glucocorticoid administration another origin of adult disease? Clin. Exp Pharmacol Physiol. 2001;28:957–961. doi: 10.1046/j.1440-1681.2001.03559.x. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP. Quantitative features of reactive gliosis following toxicant-induced damage of the CNS. Ann NY Acad Sci. 1993;679:195–210. doi: 10.1111/j.1749-6632.1993.tb18299.x. [DOI] [PubMed] [Google Scholar]
- Peltoniemi OM, Kari MA, Hallman M. Repeated antenatal corticosteroid treatment: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2011;90:719–727. doi: 10.1111/j.1600-0412.2011.01132.x. [DOI] [PubMed] [Google Scholar]
- Perna R, Loughan A, Perkey H, Tyson K. Terbutaline and associated risks for neurodevelopmental disorders. Child Dev Res. 2014;2014 Article 358608, http://dx.doi.org/358610.351155/352014/358608. [Google Scholar]
- Pitzer M, Schmidt MH, Esser G, Laucht M. Child development after maternal tocolysis with β-sympathomimetic drugs. Child Psychiat Hum Dev. 2001;31:165–182. doi: 10.1023/a:1026419720410. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Aubert Y, Maier C, Pearce PC, Fuchs E. The developmental impact of prenatal stress, prenatal dexamethasone and postnatal social stress on physiology, behaviour and neuroanatomy of primate offspring: studies in rhesus macaque and common marmoset. Psychopharmacology. 2011;214:33–53. doi: 10.1007/s00213-010-1989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos modeled in vitro: comparative effects of metabolites and other cholinesterase inhibitors on DNA synthesis in PC12 and C6 cells. Environ Health Perspect. 2001;109:909–913. doi: 10.1289/ehp.01109909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Violin JD, Slotkin TA. Nicotine is a developmental neurotoxicant and neuroprotectant: stage-selective inhibition of DNA synthesis coincident with shielding from effects of chlorpyrifos. Dev Brain Res. 2003;147:183–190. doi: 10.1016/s0165-3806(03)00222-0. [DOI] [PubMed] [Google Scholar]
- Rhodes MC, Seidler FJ, Abdel-Rahman A, Tate CA, Nyska A, Rincavage HL, Slotkin TA. Terbutaline is a developmental neurotoxicant: effects on neuroproteins and morphology in cerebellum, hippocampus and somatosensory cortex. J Pharmacol Exp Ther. 2004;308:529–537. doi: 10.1124/jpet.103.060095. [DOI] [PubMed] [Google Scholar]
- Rokyta R, Yamamotova A, Slamberova R, Franek M, Vaculin S, Hruba L, Schutova B, Pometlova M. Prenatal and perinatal factors influencing nociception, addiction and behavior during ontogenetic development. Physiol Res. 2008;57(Suppl 3):S79–S88. doi: 10.33549/physiolres.931602. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Institute of Medicine, editor. Autism and the Environment. National Academies Press; Washington, DC: 2008. How may animal models be used to examine potential environmental-based mechanisms? pp. 107–114. [Google Scholar]
- Slotkin TA, Baker FE, Dobbins SS, Eylers JP, Lappi SE, Seidler FJ. Prenatal terbutaline exposure in the rat: selective effects on development of noradrenergic projections to cerebellum. Brain Res Bull. 1989;23:263–265. doi: 10.1016/0361-9230(89)90206-2. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Ryde IT, Tate CA, Seidler FJ. Screening for developmental neurotoxicity using PC12 cells: comparisons of organophosphates with a carbamate, an organochlorine and divalent nickel. Environ Health Perspect. 2007;115:93–101. doi: 10.1289/ehp.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Oliver CA, Seidler FJ. Critical periods for the role of oxidative stress in the developmental neurotoxicity of chlorpyrifos and terbutaline, alone or in combination. Dev Brain Res. 2005;157:172–180. doi: 10.1016/j.devbrainres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Developmental exposure to terbutaline and chlorpyrifos, separately or sequentially, elicits presynaptic serotonergic hyperactivity in juvenile and adolescent rats. Brain Res Bull. 2007;73:301–309. doi: 10.1016/j.brainresbull.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Terbutaline impairs the development of peripheral noradrenergic projections: implications for autism spectrum disorders and pharmacotherapy of preterm labor. Neurotoxicol Teratol. 2013;36:91–96. doi: 10.1016/j.ntt.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Skavicus S, Card J, Giulio RTD, Seidler FJ. In vitro models reveal differences in the developmental neurotoxicity of an environmental polycylic aromatic hydrocarbon mixture compared to benzo[a]pyrene: neuronotypic PC12 cells and embryonic neural stem cells. Toxicology. 2017a;377:49–56. doi: 10.1016/j.tox.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Skavicus S, Card J, Levin ED, Seidler FJ. Diverse neurotoxicants target the differentiation of embryonic neural stem cells into neuronal and glial phenotypes. Toxicology. 2016;372:42–51. doi: 10.1016/j.tox.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Skavicus S, Stapleton HM, Seidler FJ. Brominated and organophosphate flame retardants target different neurodevelopmental stages, characterized with embryonic neural stem cells and neuronotypic PC12 cells. Toxicology. 2017b;390:32–42. doi: 10.1016/j.tox.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Windh R, Whitmore WL, Seidler FJ. Adrenergic control of DNA synthesis in developing rat brain regions: effects of intracisternal administration of isoproterenol. Brain Res Bull. 1988;21:737–740. doi: 10.1016/0361-9230(88)90040-8. [DOI] [PubMed] [Google Scholar]
- Song X, Violin JD, Seidler FJ, Slotkin TA. Modeling the developmental neurotoxicity of chlorpyrifos in vitro: macromolecule synthesis in PC12 cells. Toxicol Appl Pharmacol. 1998;151:182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- Tegethoff M, Pryce CR, Meinlschmidt G. Effects of intrauterine exposure to synthetic glucocorticoids on fetal, newborn, and infant hypothalamic-pituitary-adrenal axis function in humans: a systematic review. Endocrine Rev. 2009;30:753–789. doi: 10.1210/er.2008-0014. [DOI] [PubMed] [Google Scholar]
- Teng KK, Greene LA. Cultured PC12 cells: a model for neuronal function and differentiation. In: Celis JE, editor. Cell Biology: A Laboratory Handbook. Academic Press; San Diego: 1994. pp. 218–224. [Google Scholar]
- U.S. Food and Drug Administration. [accessed 21 February 2017];FDA Drug Safety Communication: New warnings against use of terbutaline to treat preterm labor. 2011a http://www.fda.gov/drugs/drugsafety/ucm243539.htm.
- U.S. Food and Drug Administration. [accessed 21 February 2017];FDA response to citizen petition on terbutaline. 2011b https://www.fda.gov/Drugs/DrugSafety/ucm243539.htmafety/UCM243797.pdf.
- Winick M, Noble A. Quantitative changes in DNA, RNA and protein during prenatal and postnatal growth in the rat. Dev Biol. 1965;12:451–466. doi: 10.1016/0012-1606(65)90009-6. [DOI] [PubMed] [Google Scholar]
- Witter F, Zimmerman A, Reichmann J, Connors S. In utero β2 adrenergic agonist exposure and adverse neurophysiologic and behavioral outcomes. Am J Obstet Gynecol. 2009;201:553–559. doi: 10.1016/j.ajog.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Zerrate MC, Pletnikov M, Connors SL, Vargas DL, Seidler FJ, Zimmerman AW, Slotkin TA, Pardo CA. Neuroinflammation and behavioral abnormalities after neonatal terbutaline treatment in rats: implications for autism. J Pharmacol Exp Ther. 2007;322:16–22. doi: 10.1124/jpet.107.121483. [DOI] [PubMed] [Google Scholar]
- Zhong HY, Minneman KP. Close reciprocal regulation of β1- and β2-adrenergic receptors by dexamethasone in C6 glioma cells: effects on catecholamine responsiveness. Mol Pharmacol. 1993;44:1085–1093. [PubMed] [Google Scholar]
- Zhu Y, Culmsee C, Semkova I, Krieglstein J. Stimulation of β2-adrenoceptors inhibits apoptosis in rat brain after transient forebrain ischemia. J Cerebral Blood Flow Metab. 1998;18:1032–1039. doi: 10.1097/00004647-199809000-00013. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Prehn JHM, Culmsee C, Krieglstein J. The β2-adrenoceptor agonist clenbuterol modulates Bcl-2, Bcl-xl and Bax protein expression following transient forebrain ischemia. Neuroscience. 1999;90:1255–1263. doi: 10.1016/s0306-4522(98)00564-8. [DOI] [PubMed] [Google Scholar]