Abstract
Background
Cigarette smoking is the strongest risk factor for chronic obstructive pulmonary disease (COPD). Smoking burden is frequently measured in pack-years, but the relative contribution of cigarettes smoked per day versus duration toward the development of structural lung disease, airflow obstruction and functional outcomes is not known.
Methods
We analyzed cross-sectional data from a large multicenter cohort (COPDGene) of current and former smokers. Primary outcome was airflow obstruction (FEV1/FVC); secondary outcomes included five additional measures of disease: FEV1, CT emphysema, CT gas trapping, functional capacity (six-minute walk distance, 6MWD), and respiratory morbidity (St. George’s Respiratory Questionnaire, SGRQ). Generalized linear models were estimated to compare the relative contribution of each smoking variable to the outcomes, after adjustment for age, race, sex, body-mass-index, CT scanner, center, age of smoking onset and current smoking status. We also estimated adjusted means of each outcome by categories of pack-years and combined groups of categorized smoking duration and cigarettes/day, and estimated linear trends of adjusted means for each outcome by categorized cigarettes/day, smoking duration and pack-years.
Results
10,187 subjects were included. For FEV1/FVC, standardized beta co-efficient for smoking duration was greater than for cigarettes/day and pack-years (p<0.001). After categorization, there was a linear increase in adjusted means FEV1/FVC with increase in pack-years (regression co-efficient β=−0.023±SE0.003;p=0.003) and duration over all ranges of smoking cigarettes/day (β=−0.041±0.004;p<0.001) but a relatively flat slope for cigarettes/day across all ranges of smoking duration (β=−0.009±0.0.009;p=0.34). Strength of association of duration was similarly greater than pack-years for emphysema, gas trapping, FEV1, 6MWD and SGRQ.
Conclusion
Smoking duration alone provides stronger risk estimates of COPD than the composite index of pack-years.
Keywords: Smoking, pack-years, emphysema
Background
Chronic obstructive pulmonary disease (COPD) is characterized by partially reversible airflow obstruction and is usually associated with structural changes on lung imaging.1 Although the demonstration of airflow obstruction on spirometry is sine qua non for the diagnosis of COPD, the pre-test probability of a correct diagnosis relies on accurate quantification of risk factors as well as symptom burden. Cigarette smoking is the strongest risk factor for COPD,1 and although no precise estimate of a threshold effect is available, in those smokers who develop COPD there is a dose-effect relationship. Various parameters of tobacco exposure have been proposed including urinary and salivary cotinine estimations; however, these are expensive and do not provide accurate estimates of the cumulative exposure.2 COPD is a disease with a long latency period and hence questionnaires are best suited for assessing exposure over time.3
Smoking burden is frequently measured in pack-years, a product of the average number of packs of cigarettes smoked a day and smoking duration in years. The exposure-time risk profile for some diseases with a clear onset of disease has been delineated. For example, lung cancer is associated more strongly with the duration of smoking than with the average cigarettes/day.4–6 This delineation not only provides mechanistic insights, it also has implications for tobacco cessation strategies. The pack-years product assumes equal weightage for both cigarettes/day and duration of smoking, and the relative contribution of cigarettes/day versus duration toward the development of COPD is not known. Although ideally this is best answered by studying onset of smoking to onset of disease, COPD remains undiagnosed in the majority of patients long after the onset of disease and hence we adapted the design of comparing disease severity across a range of smoking burden. We hypothesized that cigarettes smoked per day and smoking duration differentially impact the occurrence and severity of COPD disease components including airflow obstruction, structural lung disease, and functional outcomes.
Methods
Study population
We analyzed cross-sectional data from a large multicenter cohort (COPDGene),the details of this study have been previously published.7 Briefly, participants in COPDGene were of self-identified non-Hispanic White or African-American racial/ethnic category, and included current and former smokers (age 45–80 years) with at least ten pack-year smoking history. Participants with other known lung diseases other than asthma were excluded; for example, pulmonary fibrosis, extensive bronchiectasis, and cystic fibrosis, previous surgical excision of at least one lung lobe (or lung volume reduction procedure), active cancer under treatment, suspected lung cancer (large or highly suspicious lung mass), metal in the chest, recent exacerbation of COPD treated with antibiotics or steroids, recent eye surgery, myocardial infarction, other cardiac hospitalization, recent chest or abdominal surgery, inability to use albuterol, history of chest radiation therapy, and first or second degree relative already enrolled in the study. The institutional review boards of all 21 participating centers approved the COPDGene study. Written informed consent was obtained from all participants prior to enrollment in the cohort. Those with known lung disease except for COPD and asthma were excluded. Detailed assessments of smoking history were recorded as reported by the participants at the time of enrollment including age at which participants started smoking, duration of smoking (calculated as the difference between the age at time of quitting or time of enrollment and the age at smoking initiation) and the average number of cigarettes a day. Smoking burden was also assessed using the traditional metric of pack-years, which is the product of the average number of packs of cigarettes a day and smoking duration in years. To lessen the effect of early onset smoking on the developing lung, we also separately analyzed participants who started smoking at or after the age of 18 years.
Measurement of disease
The primary outcome was airflow obstruction measured by the ratio of the forced expiratory volume in the first second to the forced vital capacity (FEV1/FVC). Secondary outcomes included five additional measures of disease: FEV1, CT emphysema, CT gas trapping, functional capacity using six minute walk distance (6MWD), and respiratory morbidity using St. George’s Respiratory Questionnaire (SGRQ). Presence of COPD was primarily assessed by spirometric and imaging metrics, and disease burden quantitated using respiratory quality of life and functional capacity measurements. COPD was defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria of post-bronchodilator FEV1/FVC <0.70. Participants with normal ratio but FEV1<80% predicted were categorized to have GOLD unclassifiable disease or Preserved Ratio Impaired Spirometry (PRISm).8
Computed tomographic scans were performed at full inspiration (total lung capacity, TLC) and end-tidal exhalation (functional residual capacity, FRC). After segmentation and extrusion of the large and medium sized airways, emphysema was quantified as the percentage of lung volume at TLC with attenuation < −950 Hounsfield Units (HU) by density mask analyses using 3D Slicer software (http://airwayinspector.acil-bwh.org/).7 CT measures of emphysema correlate well with emphysema measured on histopathology.9 Gas trapping was quantified as the percentage of lung volume at FRC with attenuation < −856 HU. Respiratory-related quality of life was assessed using SGRQ; SGRQ ranges from 0 to 100 with higher scores indicating worse respiratory-related quality of life.10 We assessed functional capacity using the 6MWD according to ATS guidelines.11
Statistical Analyses
We analyzed the data using two methods. Please see Supplement for details. First, using general linear models, we estimated association of each outcome with the smoking variables (duration, cigarettes/day and pack-years) as continuous variables in separate fixed-effects models after adjustment for age, race, sex, body-mass-index, CT scanner type, center, age of smoking onset and current smoking status.12 To estimate relative effect sizes, we compared the standardized regression coefficients of the smoking variables for associations with the outcome. FEV1/FVC was the primary outcome, and we repeated these analyses for each of the secondary outcomes (CT emphysema, CT gas trapping, FEV1, 6MWD and SGRQ). Results were determined to be statistically significant when the accompanying statistical test yielded a probability of <0.05.
Second, to improve clinical interpretability and applicability across a range of combination of smoking duration and cigarettes/day, we categorized the smoking variables by increments of 10 units to create consistent intervals for all three of the smoking variables. The six metrics of disease described above were treated as outcome variables and their adjusted means were calculated for each grouping of 23 combinations of smoking duration and cigarettes/day using a 5 X 5 table (25 groups were designed and two groups are not evaluable due to zero or insufficient cases). Similarly, adjusted means of outcomes were estimated for 5 groups of smoking pack-years. Covariates used for adjusting means of the outcomes were age, race, sex, body-mass-index, CT scanner type, center, age of smoking onset and current smoking status. Linear trends of the adjusted means of outcomes over categorized smoking duration and cigarettes/day were then drawn to illustrate the trends of the 23 adjusted means of the outcomes over cigarettes/day and duration respectively (Supplemental Table 1). These trends provide information on the relative contribution of one smoking variable towards COPD when the other smoking variable is held constant. In addition, linear trends were estimated for the adjusted means of outcomes over 5 groups of pack-years. Steepness of linear slope over pack-years was graphically illustrated for comparison with linear slopes over duration and cigarettes/day. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).).
Results
Participant Characteristics
10,300 subjects were enrolled in the COPDGene study. We excluded 108 participants who were non-smokers. 10,187 subjects were included in the current analyses after excluding 5 subjects who were placed in the category with smoking duration 0 to 10 years and smoking cigarettes/day average of ≥40.1 cigarettes per day, due to insufficient number of cases. Characteristics of participants included are shown in Table 1. The mean age at enrollment was 59.6 (9.0) years. 5446 (53.5%) were male and 3407 (33.4%) were African American. The cohort included 5414 (53.2%) current smokers and 4773 (46.8 %) former smokers. The mean pack-years of smoking was 44.2 (25.0) (interquartile range IQR 27.2 to 54.9). The average duration of smoking was 36.4 (10.0) years (IQR 30.9 to 43.0 years), and mean cigarettes/day was 24.3 (11.3) (IQR 20.0 to 30.0). There were weak correlations between age and pack-years of smoking (r = 0.266), smoking duration (r=0.342), and cigarettes/day cigarettes/day(r=0.134). Supplemental Table 2 shows smoking burden by cigarettes/day and duration across GOLD stages.
Table 1.
Characteristics of participants
| Parameters | n=10,187 |
|---|---|
| Age (years) | 59.6 (9.0) |
| Sex, Female (%) | 4741 (46.5%) |
| Race, African-American (%) | 3407 (33.4%) |
| Body-mass-index (kg/m2) | 28.8 (6.3) |
| Smoking Pack-years | 44.2 (25.0) |
| Smoking duration (years) | 36.4 (10.0) |
| Cigarettes per day | 24.3 (11.3) |
| Current Smokers (%) | 5414 (53.2%) |
| FEV1 (L) | 2.24 (0.92) |
| FEV1 % predicted | 76.4 (25.6) |
| FVC (L) | 3.30 (1.01) |
| FVC % predicted | 87.0 (18.3) |
| FEV1/FVC | 0.67 (0.16) |
| CT Emphysema (%) | 6.2 (9.6) |
| CT Gas Trapping (%) | 21.9 (19.9) |
| Six-minute walk distance (m) | 411.5 (121.6) |
| St. George’s Respiratory Questionnaire Score (units) | 27.4 (22.9) |
All values are expressed as mean (standard deviation) unless otherwise indicated.
FEV1 = forced expiratory volume in the first second. FVC = forced vital capacity. CT = computed tomography.
Smoking and airflow obstruction
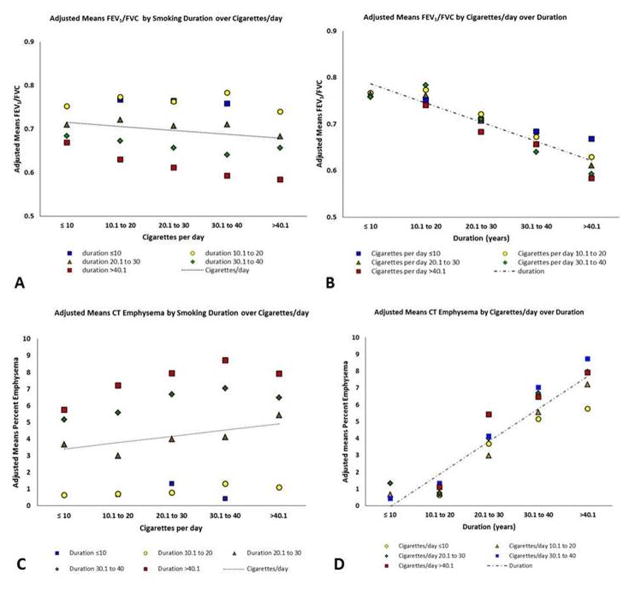
We also found that for the degree of airflow obstruction (FEV1/FVC), the adjusted effect size was greater for smoking duration than for cigarettes/day and pack-years. After categorization, we found a linear increase in FEV1/FVC with increase in pack-years (β=−0.023±SE0.003;p=0.003). With increasing cigarettes/day, the gradient remained relatively flat across all ranges of smoking duration (β=−0.009±0.009;p=0.34) (Figure 1 Panel A). Smoking duration also had a linear inverse relationship with FEV1/FVC (β=−0.041±0.004;p<0.001), and its slope was again steeper than pack-years for association with FEV1/FVC (Figure 1 Panel B and Figure 2 Panel A). Similar results were seen for FEV1 (Figure 2 Panel B).
Figure 1.
Panel A shows linear slopes for adjusted means of FEV1/FVC over categorized Cigarettes per day. Panel B shows linear slopes for adjusted means of FEV1/FVC over categorized Duration. Color-coded data points represent estimated adjusted means of FEV1/FVC by categorized Duration (A) or Cigarettes/day (B). All categorization is based on 10 unit increments.
Panel C shows linear slopes for adjusted means of CT Emphysema over categorized Cigarettes per day. Panel D shows linear slopes for adjusted means of CT Emphysema over categorized Smoking Duration. Color-coded data points represent estimated adjusted means of CT emphysema by categorized Duration (C) or Cigarettes/day (D). All categorization is based on 10 unit increments.
Least square means of FEV1/FVC and CT emphysema are adjusted for age, race, sex, body-mass-index, scanner type, center, age of smoking onset and current smoking status.
FEV1/FVC = Ratio of forced expiratory volume in the first second to the forced vital capacity.
CT = Computed Tomography.
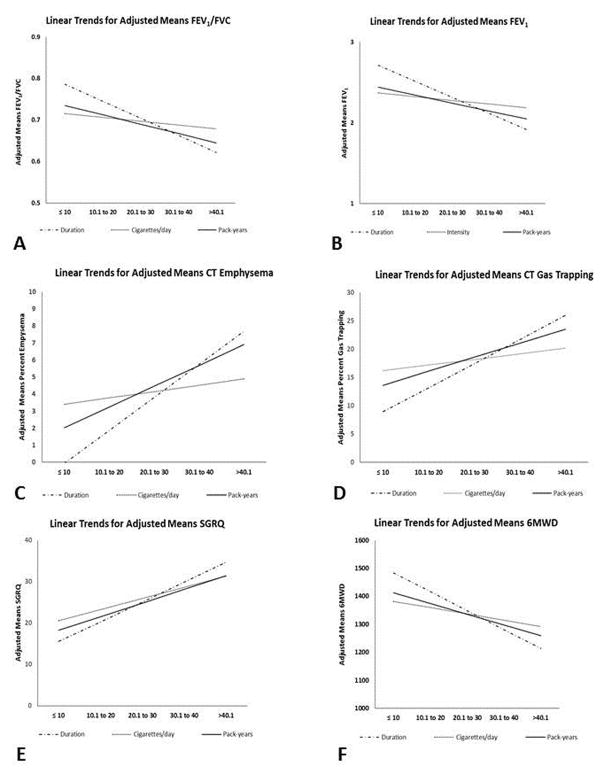
Figure 2.
shows summary linear slopes for lung function (A. FEV1/FVC and B. FEV1), structural lung disease (C. CT emphysema and D. CT gas trapping), and quality of life (E. SGRQ) and exercise capacity (F. 6MWD) over categorized Cigarettes per day, categorized Smoking Duration and over categorized Pack-years. All categorization is based on 10 unit increments.
All outcome least square means adjusted for age, race, sex, body-mass-index, scanner type, center, age of smoking onset and current smoking status.
FEV1 = forced expiratory volume in the first second. FVC = forced vital capacity. CT = computed tomography. SGRQ = St George’s Respiratory Questionnaire. 6MWD = six minute walk distance.
Smoking and structural lung disease on CT
For CT emphysema, the adjusted effect size was greater for smoking duration than for cigarettes/day and pack-years (Table 2). The root-mean-square error (RMSE) for the model with duration was less than RMSE for cigarettes/day and pack-years. On categorization of the data, there was a linear increase in adjusted means percent emphysema with increase in pack-years (regression co-efficient β=1.219±SE0.099;p=0.001) (Figure 2 Panel C and Figure 3). With increasing cigarettes/day, the slope remained relatively flat across all ranges of smoking duration (β=0.377±0.437;p=0.40) (Figure 1 Panel C and Figure 3). Smoking duration also had a linear relationship with percent emphysema (β=1.937±0.151;p<0.001), and its slope was steeper than pack-years for association with emphysema (Figure 1 Panel C and Figure 2 Panel C).
Table 2.
Generalized linearmodels comparing adjusted effect size of Smoking Variables on Outcomes
| Outcome | Predictor | Standardized b | SE of Standardized b | Z value | P value |
|---|---|---|---|---|---|
| FEV1/FVC | Duration | −0.310 | 0.012 | ||
| Cigarettes/day | −0.080 | 0.009 | −15.442 | <0.001 | |
| Pack-years | −0.183 | 0.009 | −8.353 | <0.001 | |
| FEV1 | Duration | −0.253 | 0.011 | ||
| Cigarettes/day | −0.077 | 0.008 | −12.853 | <0.001 | |
| Pack-years | −0.165 | 0.009 | −6.32 | <0.001 | |
| CT Emphysema | Duration | 0.219 | 0.012 | ||
| Cigarettes/day | 0.058 | 0.009 | 10.385 | <0.001 | |
| Pack-years | 0.128 | 0.010 | 5.754 | <0.001 | |
| CT Gas trapping | Duration | 0.247 | 0.012 | ||
| Cigarettes/day | 0.077 | 0.009 | 11.009 | <0.001 | |
| Pack-years | 0.159 | 0.010 | 5.566 | <0.001 | |
| 6MWD | Duration | −0.186 | 0.012 | ||
| Cigarettes/day | −0.082 | 0.009 | −7.055 | <0.001 | |
| Pack-years | −0.150 | 0.009 | −2.387 | 0.008 | |
| SGRQ | Duration | 0.227 | 0.013 | ||
| Cigarettes/day | 0.148 | 0.010 | 4.907 | <0.001 | |
| Pack-years | 0.230 | 0.010 | 0.042 | 0.483 |
CT = computed tomography. FEV1 = forced expiratory volume in the first second. FVC = forced vital capacity. 6MWD = six minute walk distance. SGRQ = St George’s Respiratory Questionnaire.
Estimated standardized regression co-efficient b is adjusted for age, race, sex, body-mass-index, scanner type, center, age of smoking onset and current smoking status.
P value obtained by Z-test for comparing standardized b of smoking duration with that of cigarettes/day and pack-years of smoking.
Figure 3.
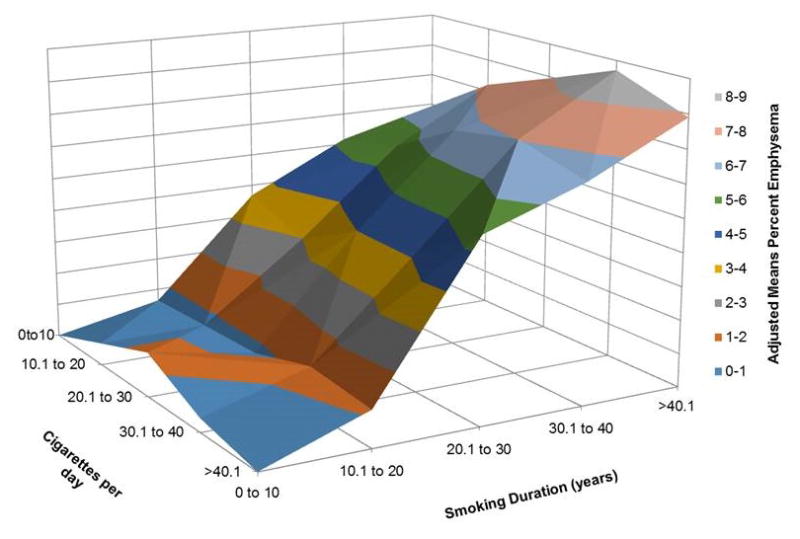
Three dimensional surface plots demonstrate the relationships between linear slopes for the adjusted means of CT emphysema over categorized Cigarettes/day and over categorized Smoking Duration (years). The adjusted means CT emphysema slopes for Cigarettes/day are relatively flat across all duration categories whereas adjusted means CT emphysema increases linearly with increasing Smoking Duration across all categories of cigarettes/day. All categorization is based on 10 unit increments. Color panel on the right shows adjusted means of CT emphysema adjusted for age, race, sex, body-mass-index, CT scanner type, center, age of smoking onset and current smoking status.
CT = Computed Tomography. Please note that surface plots were drawn for 23 of the 25 combinations of smoking cigarettes/day and smoking duration, and the two combinations with insufficient participants were treated as zero to smoothen the surface plots.
Similarly for gas trapping, the effect size was greater for smoking duration than for cigarettes/day and pack-years (Table 2). After categorization, there was a linear increase in the percent gas trapping with increase in pack-years (β=2.484±SE0.291;p=0.003) (Figure 2, Panel D). The slope remained comparatively flat across all ranges of smoking duration with increasing cigarettes/day (β=1.001±0.960;p=0.31). Smoking duration also had a linear relationship with percent gas trapping (β=4.254±0.352;p<0.001), and its slope was again steeper than pack-years for association with gas trapping (Figure 2 Panel D).
Smoking and respiratory morbidity
The effect size was greater for smoking duration than for cigarettes/day and pack-years for association with SGRQ (Table 2). SGRQ also linearly worsened with increase in pack-years (β=3.292±SE0.418;p=0.004). Unlike the relationship with other disease measures, cigarettes/day was associated with worse respiratory quality of life (β=2.696±1.111;p=0.024). SGRQ linearly worsened with greater smoking duration (β=4.781±0.720;p<0.001), and this slope was steeper than pack-years for association with SGRQ (Figure 2 Panel E).
6MWD decreased in a linear fashion with increase in pack-years (β=−38.392±SE4.500;p=0.003). With increasing cigarettes/day, the slope remained relatively flat across all ranges of smoking duration (β=−22.361±15.903;p=0.17). 6MWD also decreased linearly with smoking duration (β=−67.617±7.979;p<0.001), and this slope was steeper than that for pack-years (Figure 2 Panel F). The effect size was greater for smoking duration than for cigarettes/day and pack-years for association with 6MWD. Figure 2 shows a comparison of slopes for outcomes per each smoking variable.
Secondary analyses
To examine whether the age of smoking onset impacted the results, we performed sensitivity analyses by repeating these analyses to test the relationship between smoking variables and disease metrics, in participants who started smoking at or after the age of 18 (Supplemental Table 3), as well as for the entire cohort without adjustment for age of smoking onset (Supplemental Table 4). We found that for both these analyses, the linear trends for smoking duration was steeper for COPD outcomes than pack-years, and that the slope for cigarettes/day is relatively flat.
Discussion
In a cohort of current and former smokers, we showed that smoking duration alone is more strongly associated with estimates of COPD disease components than cigarettes smoked per day, and the composite index of pack-years. Although cigarettes/day is also associated with COPD, using pack-years as measured currently may lower the strength of association between cigarette smoking and COPD metrics in epidemiologic studies.
Measuring smoking burden using pack-years has long been recognized to be imprecise. The relative contributions of smoking duration and cigarettes/day have been examined in other smoking related diseases, but with relatively easily recognized disease onset such as lung cancer and esophageal cancer.4–6,13 For both these cancers, smoking duration was more strongly associated with disease than cigarettes/day, but no comparisons were made to assess the strength of these associations with pack-years. Similar results have been reported for cardiovascular disease for which smoking duration appears to be more important than cigarettes/day, without direct comparisons with pack-years.14 Results from the Lung Health Study showed that increasing cigarettes/day is associated with greater lung function decline in mild to moderate COPD, but no direct comparisons were made between cigarettes/day and duration of smoking.15,16 We found that smoking duration is not only more important for presence of disease in a chronic slowly evolving disease, but also that cigarettes/day as currently recorded may attenuate the strength of association between cigarette smoking and disease measures. The reasons for this finding could be biologic or epidemiologic. It is plausible that a longer duration of smoking is associated with increasing accumulation of genetic and epigenetic changes, especially in this polygenic condition.17 There may also be alterations in the lung microbiome over time with continued cigarette smoke exposure;18 there is lesser microbial diversity with continued cigarette smoking and this may contribute to disease progression.19 Epidemiologically, smoking burden has been estimated in several ways, including prospective direct methods such as personal monitoring in smokers’ microenvironments and biochemical assays such as plasma, urinary and salivary cotinine levels.3 These are limited by feasibility and costs especially in COPD which has a long latency period, as well as non-availability of measurements of time of exposure. Inherent to the long duration of smoking, smoking history is almost always self-reported and hence subject to recall bias. We believe that the duration of smoking is more easily recalled than the average cigarettes/day of smoking which tends to fluctuate over time. Cigarettes smoked per day is also harder to quantify accurately and cigarettes/day measurements correlate poorly with biochemical assessments of smoking exposure.2 This could be due to different formulations of cigarettes and different amounts of exposure that depend on mainstream and sidestream smoke, with the latter likely more dangerous.2 Smoking topography is heterogeneous and many smokers adjust the level of smoke inhaled, further affecting measurement.20 It is also possible that the “bandwidth” of cigarettes smoked per day is constrained by increments of number of cigarettes by half to one pack, although we did have participants reporting increments of single units of average cigarettes smoked per day, and hence cigarettes/day has a narrower range, resulting in lower statistical strength and misclassification bias. Not recognizing the most accurate exposure profile might result in under or overestimation of risk and also biased estimates of effect size.
The duration of smoking is also likely impacted by the age of onset of smoking. Early onset smoking is likely to impact lung growth and maturation and result in a lower baseline peak lung function which has been shown to be a risk factor for COPD.21 We performed a sensitivity analyses with and without adjustment for age of onset and found that adjustment for age of smoking onset strengthened the relationship between smoking duration and COPD but not that for cigarettes/day and pack-years. Although smoking duration is more likely to be greater with older age, this is also true for the composite of pack-years; we also adjusted for age at enrollment and age of smoking onset.
Our findings have several significant public health implications. First, information on smoking duration alone for estimating risk of COPD is easier to obtain and likely less affected by recall bias and hence is more pragmatic. This is not to say that the number of cigarettes smoked per day does not matter. Although cigarettes/day is likely important and our study was not designed to assess mechanistic relationships, the current methods of measurement with average number of cigarettes per day over time appear to provide inferior risk assessment to that provided by smoking duration. The threshold effect for disease occurrence in COPD may not lie in the cigarettes smoked per day but in the duration of smoking. Our findings reinforce the importance of complete smoking cessation rather than decreasing the number of cigarettes smoked to reduce the risk of lung disease.
Our study has several strengths. This is a large well characterized cohort of current and former smokers with diversity of race and sex; data collection was blinded to the outcomes, minimizing the potential bias in comprehensiveness of exposure assessments which can be impacted by ascertainment of knowledge of disease status. Data on smoking duration and cigarettes/day was meticulously collected by investigators to determine risk, but this level of detail may not be feasible in a busy clinical practice. This is more likely to impact smoking cigarettes/day measurements than duration. The study has some limitations. Participants enrolled in the COPDGene study had to have at least a 10 pack-year smoking history, and the cohort contains a large proportion of participants with COPD with heavy smoking burden and the range of smoking cigarettes/day is likely narrower and more uniform than in a general population sample. Smoking exposure history is subject to recall bias; however in a disease with a long latency, validated questionnaires are the most accepted method of measuring exposure. We did not include non-smoking controls because using non-smokers as a reference standard tends to overestimate the dose-response relationship as any smoking is likely to be worse than never smoking. Compared to base models, all models with smoking measures contributed incrementally to the COPD outcomes, and this could be due to the inclusion of participants with at least a 10 pack-year smoking history which establishes a baseline smoking exposure. For this reason, we performed additional analyses to study the differential effects of cigarettes/day and duration across the range of each smoking measure. We calculated smoking duration as the difference between the age at smoking onset and the age at smoking cessation, and did not account for possible short periods of smoking cessation; however our methodology is less subject to recall bias, and is consistent with the methods used in large epidemiologic studies.16,22 Findings from the Lung Health Study also showed that the rate of decline of lung function over 11 years was similar between sustained and intermittent smokers, suggesting that periods of intermittent cessation do not significantly impact outcomes.22
Conclusions
In a large cohort of smokers with and without COPD, smoking duration provides stronger risk estimates of COPD components than cigarettes smoked per day and the composite index of pack-years. Given the limitations of measuring cigarettes/day, giving equal weightage to smoking cigarettes/day and duration might attenuate the measured strength of association between smoking and COPD, and result in misclassification and biased estimates of disease risk.
Supplementary Material
What is the key question
What is the relative contribution of cigarettes smoked per day versus duration toward the development of COPD?
What is the bottom line?
In a population of current and former smokers, we found that although the average number of cigarettes smoked per day is important, smoking duration alone is more strongly associated with COPD disease components than pack-years, and that cigarettes/day may lower the strength of association between cigarette smoking and COPD metrics in epidemiologic studies. Our findings have significant public health implications and might make smoking history ascertainment easier as well as more likely to be accurate.
Why read on?
We show that due to difficulties in ascertainment of cigarettes/day, smoking duration is more strongly associated with COPD than the composite of pack-years.
Acknowledgments
Funding
The project was supported by Award R01 HL089897 and R01 HL089856 from the National Heart, Lung and Blood Institute. The COPDGene project is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprised of AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, Siemens, Sunovion and GlaxoSmithKline. Dr. Bhatt is supported by NIH K23 Grant K23HL133438.
Footnotes
Descriptor: Smoking Burden and COPD
Author Contributions: Dr. Bhatt had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bhatt, Kim and Bailey
Acquisition, analysis, or interpretation of data: All authors
Drafting of the manuscript: Bhatt
Critical revision of the manuscript for important intellectual content: All authors
Statistical analysis: Kim and Bhatt
Study supervision: All authors
Trial Registration: ClinicalTrials.gov: Identifier: NCT00608764
References
- 1.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Etter JF, Perneger TV. Measurement of self reported active exposure to cigarette smoke. J Epidemiol Community Health. 2001;55(9):674–80. doi: 10.1136/jech.55.9.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaakkola MS, Jaakkola JJ. Assessment of exposure to environmental tobacco smoke. Eur Respir J. 1997;10(10):2384–97. doi: 10.1183/09031936.97.10102384. [DOI] [PubMed] [Google Scholar]
- 4.Doll R, Peto R. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. J Epidemiol Community Health. 1978;32(4):303–13. doi: 10.1136/jech.32.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flanders WD, Lally CA, Zhu BP, et al. Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: results from Cancer Prevention Study II. Cancer Res. 2003;63(19):6556–62. [PubMed] [Google Scholar]
- 6.Lubin JH, Caporaso NE. Cigarette smoking and lung cancer: modeling total exposure and intensity. Cancer Epidemiol Biomarkers Prev. 2006;15(3):517–23. doi: 10.1158/1055-9965.EPI-05-0863. [DOI] [PubMed] [Google Scholar]
- 7.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. Copd. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan ES, Castaldi PJ, Cho MH, et al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15:89. doi: 10.1186/s12931-014-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madani A, Zanen J, de Maertelaer V, et al. Pulmonary emphysema: objective quantification at multi-detector row CT--comparison with macroscopic and microscopic morphometry. Radiology. 2006;238(3):1036–43. doi: 10.1148/radiol.2382042196. [DOI] [PubMed] [Google Scholar]
- 10.Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–7. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 11.Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 12.Filion KB, Azoulay L, Platt RW, et al. A Multicenter Observational Study of Incretin-based Drugs and Heart Failure. N Engl J Med. 2016;374(12):1145–54. doi: 10.1056/NEJMoa1506115. [DOI] [PubMed] [Google Scholar]
- 13.Pandeya N, Williams GM, Sadhegi S, et al. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol. 2008;168(1):105–14. doi: 10.1093/aje/kwn091. [DOI] [PubMed] [Google Scholar]
- 14.Lubin JH, Couper D, Lutsey PL, et al. Risk of Cardiovascular Disease from Cumulative Cigarette Use and the Impact of Smoking Intensity. Epidemiology. 2016;27(3):395–404. doi: 10.1097/EDE.0000000000000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tashkin DP, Altose MD, Connett JE, et al. Methacholine reactivity predicts changes in lung function over time in smokers with early chronic obstructive pulmonary disease. The Lung Health Study Research Group. Am J Respir Crit Care Med. 1996;153(6 Pt 1):1802–11. doi: 10.1164/ajrccm.153.6.8665038. [DOI] [PubMed] [Google Scholar]
- 16.Scanlon PD, Connett JE, Waller LA, et al. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med. 2000;161(2 Pt 1):381–90. doi: 10.1164/ajrccm.161.2.9901044. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura H. Genetics of COPD. Allergol Int. 2011;60(3):253–8. doi: 10.2332/allergolint.11-RAI-0326. [DOI] [PubMed] [Google Scholar]
- 18.Sze MA, Dimitriu PA, Hayashi S, et al. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(10):1073–80. doi: 10.1164/rccm.201111-2075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erb-Downward JR, Thompson DL, Han MK, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One. 2011;6(2):e16384. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scherer G. Smoking behaviour and compensation: a review of the literature. Psychopharmacology (Berl) 1999;145(1):1–20. doi: 10.1007/s002130051027. [DOI] [PubMed] [Google Scholar]
- 21.Lange P, Celli B, Agusti A, et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med. 2015;373(2):111–22. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 22.Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166(5):675–9. doi: 10.1164/rccm.2112096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.