Abstract
History of psychosis or mania, if uncontrolled, both represent relative contraindications for kidney transplantation. We examined 3,680 US veterans who underwent kidney transplantation. The diagnosis of history of psychosis/mania was based on a validated algorithm. Measured confounders were used to create a propensity score-matched cohort (n=442). Associations between pre-transplantation psychosis/mania and death with functioning graft, all-cause death, graft loss and rejection were examined in survival models and logistic regression models. Post-transplant medication non-adherence was assessed using proportion of days covered (PDC) for tacrolimus and mycophenolic acid in both groups. The mean±SD age of the cohort at baseline was 61±11 years, 92% were male, 66% and 27% of patients were white and African-American, respectively. Compared to patients without history of psychosis/mania, patients with a history of psychosis/mania had similar risk of death with functioning graft [Sub-Hazard Ratio(SHR)(95%Confidence Interval (CI)): 0.94(0.42–2.09)], all-cause death[Hazard Ratio(95%CI): 1.04(0.51–2.14)], graft loss [SHR(95%CI): 1.07(0.45–2.57)] and rejection [Odds Ratio(95%CI): 1.23(0.60–2.53)]. Moreover, there was no difference in immunosuppresive drug PDC in patients with and without history of psychosis/mania (PDC: 76±21% vs 78±19%, p=0.529 for tacrolimus; PDC: 78±17% vs 79±18%, p=0.666 for mycophenolic acid). After careful selection, pre-transplantation psychosis/mania are not associated with adverse outcomes in kidney transplant recipients.
Keywords: bipolar disorder, kidney transplantation, mortality, schizophrenia, survival
Introduction
The prevalence of bipolar disorder is around 3% and schizophrenia is around 1% in the general population.[1, 2] These disorders are more frequently found in US veterans compared to the general population.[3] Both schizophrenia and bipolar disorder showed associations with common and strong risk factors of chronic kidney disease (CKD) such as diabetes mellitus, hypertension, hyperlipidemia and cardiovascular disease.[4] In addition, treatment of bipolar disorder with lithium has a strong association with the development and worsening of CKD.[5]
There are very few absolute contraindications for kidney transplantation. Psychiatric disorders, especially a history of psychosis and/or mania, which are the cardinal symptoms of schizophrenia and bipolar disorder, remains a relative contraindication endorsed by most organ transplant societies.[6–9] There are several reasons for this, including concerns about relapse of psychiatric illness, medication and other post-transplant treatment adherence, inadequate social support, emotional and cognitive capability, and potential drug interactions between psychotropic and immunosuppressant medications.[10, 11] However, there are very few data to support these concerns and most of them stem from assessment during the post-transplant period.[12]
Published data on post-transplant outcomes in patients with history of pre-transplant history of psychosis/mania are extremly limited, and consists mainly of case reports and very small observational studies.[13–19] These observational studies, [11, 13, 16, 17, 19] have shown the feasibility of transplantation in patients with history of psychiatric disorders with an excellent patient and allograft survival rate. One of the largest studies examined 164 veteran organ transplant recipients (40 with a kidney graft), and reported excellent outcomes in the first three years after transplantation.[17] Similar results were reported from the Irish National Renal Transplant Programme.[11] Comparing 15 patients with diagnosis of bipolar affective disorder and 6 patients with schizophrenia with the rest of the recipients, there were no significant differences in patient survival, graft survival, and graft function.[11] Well-known risk factors of allograft loss were antisocial behavior, associated depression, medical non-compliance, history of psychotic episodes more than one year before transplantation, homelessness, and isolation.[4, 20] A recent study from Europe included 47 patients with history of bipolar disorder and schizophrenia, and found similar graft and patient survival in recipients with history of these disorder versus others.[13, 21] All of these previous studies are small, focusing primarily on patient and allograft survival and have severe methodological limitations such as low number of events, lack of considering competing risks in transplant outcomes and unmeasured confounders such as medical comorbidities, medications and laboratory data and none of these studies assessed medication adherence in these patients. Consequently, the association between history of pre-transplantation psychosis/mania and graft and patient outcomes post-transplantation is still uncertain. In addition, these studies did not assess associations between the history of psychosis/mania and risk of rejection or medication non-adherence after transplantation.
To address this knowledge gap, we aimed to investigate the association of history of pre-transplantation psychosis/mania with post-transplant all-cause mortality and death with functioning graft, graft loss, rejection and medication adherence using a large nationally representative cohort of US veterans with pre- and post-transplantation data. We hypothesized that the history of pre-transplantation psychosis/mania is associated with higher risk of death, graft loss, rejection and medication non-adherence.
Materials and Methods
Data Source and Cohort Definition
We analyzed longitudinal data of kidney transplant recipients from the Transition of Care in CKD (TC-CKD) study, a retrospective cohort study examining US veterans with late-stage non dialysis dependent chronic kidney disease (NDD-CKD) transitioning to renal replacement therapy from October 1, 2007 through March 31, 2014.[22–24] A total of 85,505 US veterans were identified from the US Renal Data System as a source population. Only individuals who received preemptive kidney transplantation or transitioned to receive renal replacement therapy and then subsequently received kidney transplantation were included in the source population. The algorithm for the cohort definition is shown in Figure 1. We excluded patients, who were never transplanted (n=81,294) and those without any available information on comorbid conditions including history of psychosis/mania (n=531), which resulted in a study population of 3,680 patients. From this 3,680 patients a propensity score-matched cohort was created including 442 kidney transplant recipients.
Figure 1.
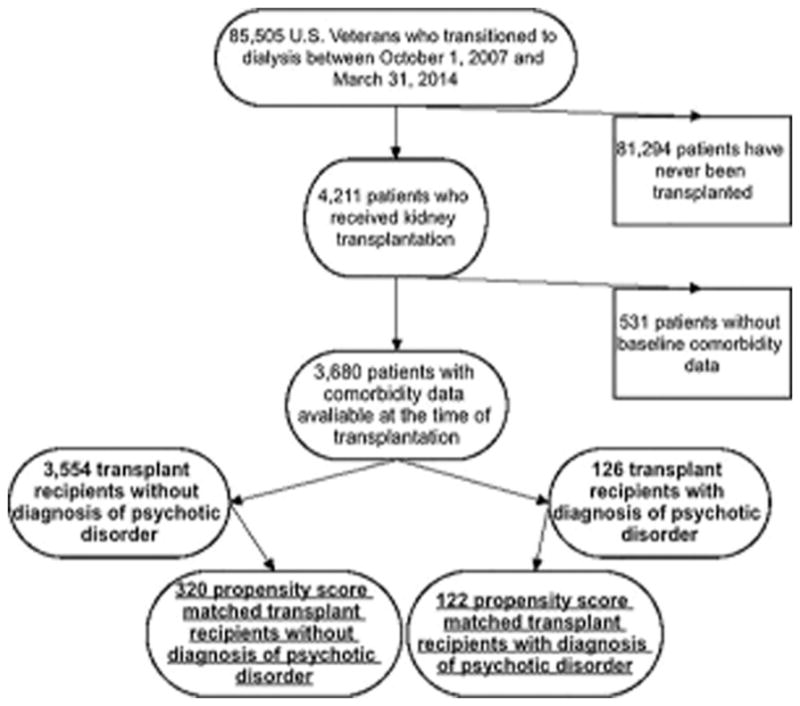
Flow Chart of Selection of the Patients
Exposure Variable
Information on history of psychosis/mania before transplantation was extracted from Veterans Affairs (VA) Inpatient and Outpatient Medical SAS Datasets, using the ICD-9-CM diagnostic codes as well as from VA/Centers for Medicare and Medicaid Services data. We used the validated algorithm described by Frayne et al.[25] to define history of psychosis/mania using outpatient or inpatient medical records prior to kidney transplantation.
Covariates
Data from the United States Renal Data System (USRDS) Patient and Medical Evidence files were used to determine patients’ baseline demographic characteristics at the time of kidney transplantation. Information on comorbidities at the time of kidney transplantation was extracted from VA Inpatient and Outpatient Medical SAS Datasets, using the ICD-9-CM diagnostic and Current Procedural Terminology codes, as well as from VA/Centers for Medicare and Medicaid Services data. Medication data was collected from both Centers for Medicare and Medicaid Services Data (Medicare Part D) and VA pharmacy dispensation records. Patients who received at least one dispensation of medication within the 12 months pre-transplantation period were recorded as having been treated with these medications. Laboratory data was obtained from VA research databases as previously described, [26, 27] and their baseline values were defined as the average of each covariate during the 12 months pre-transplantation period.
Assessment of Medication Adherence and Persistence
Detailed information about each tacrolimus and mycophenolic acid prescription was collected during the first year after kidney transplantation in a subcohort of propensity score-matched patients (n=149 for tacrolimus and n=144 for mycophenolic acid), who received these prescriptions through a VA pharmacy. Only 7 patients received cyclosporin in the propensity matched cohort, hence this data has not been analyzed. Proportion of days covered (PDC) and medication persistence were calculated. The detailed description of PDC has been published previously.[24] Figure S1 shows the graphical description of the calculations ofr the different adherence methods.
Briefly, PDC was defined as the proportion of days when the drug was available in the measurement period, capped at 100%.[28, 29] The index date was the date of the first available prescription after transplantation. The last prescription had to be dispensed before the first-year transplantation anniversary, and the full prescription period was included in the denominator, regardless whether the supply lasted until after the date of the first-year transplantation anniversary. Only outpatient prescriptions were taken into account. Any inpatient time period was added to the denominator. For medication persistence the following algorithm was used: persistence was coded as being 1 (present) if a patient refilled each subsequent prescription with gaps not exceeding 30 or 60 days; otherwise, it was coded as 0 (absent, or non-persistent).[29]
Outcome Assessment
The primary outcomes of interest were death, graft loss, rejection and adherence to immunosuppressive drugs after kidney transplantation. All-cause mortality data, censoring events, and associated dates were obtained from VA and USRDS data sources.
These outcomes were defined as follows:
For the all-cause death analysis the start of the follow-up period was the date of kidney transplantation, and patients were followed up until death or other censoring events including loss to follow-up, or end of follow-up period.[22–24] For this analysis we used Cox proprotional hazards regression.
For the death with functioning graft analysis the start of the follow-up period was the date of kidney transplantation, and patients were followed up until death or other events including graft loss, loss to follow-up, or end of follow-up period (September 30th, 2014).[22–24] For this analysis we used competing risks regression, where the primary outcome was death and the competing outcome was graft loss. Data was censored for loss to follow-up, or end of follow-up period.
For the graft loss analysis, the start of the follow-up period was the date of kidney transplantation, and patients were followed up until graft loss or other events including death, loss to follow-up, or end of follow-up period.[22–24] For this analysis we used competing risks regression, where the primary outcome was graft loss and the competing outcome was death. Data was censored for loss to follow-up, or end of follow-up period.
For rejection analyses unfortunately, we did not have data about the time of rejection, hence we were not able to run any type of time-to-event analysis for this outcome. For the rejection data derived from USRDS we used logistic regression analyses.
Finally for immunosuppressive medication adherence we calculated proportion of days covered (PDC) and medication persistence for tacrolimus and mycophenolic acid. The detalied description of the PDC calculations are described above.
Statistical analysis
Baseline patient characteristics were summarized according to the presence or absence of history of psychosis/mania prior to kidney transplantation, and presented as percent for categorical variables and mean ± standard deviation (SD) or median and interquartile range (IQR) for continuous variables. Differences between patients with and without history of psychosis and mania were assessed using standardized differences before and after propensity score matching.
The propensity score method was used to account for baseline differences arising from dissimilarities in clinical and demographic characteristics of patients with and without history of psychosis/mania. Variables associated with history of psychosis/mania were identified using logistic regression and were used to calculate propensity scores. STATA’s “psmatch2” command suite was used to generate the propensity score-matched cohorts by 1-to-4 nearest neighbor matching with replacement. The following variables were included in the logistic regression model to create the propensity score: age, gender, race/ethnicity, postal code of the patient’s address, preemptive transplantation, type of transplant donor (deceased vs living), type of dialysis modality, duration of dialysis before transplantation, presence of comorbidities (myocardial infarction, diabetes, hypertension, heart failure, ischemic heart disease, cerebrovascular disease, paraplegia/hemiplegia, renal disease, peripheral vascular disease, lung disease, peptic ulcer disease, connective tissue disease, anemia, hyperlipidemia, liver disease, malignancy, depression) and medication use (phosphorous binders, active vitamin D (native or active), renin-angiotensin-aldosterone system inhibitors, alpha-blockers, β-blockers, calcium channel blockers, vasodilators, insulin, diuretics, statins, antianginals, anticoagulants, thrombolytics, aspirin, digitalis and erythropoietin stimulating agents). Figure S2 shows the distribution of the propensity score in the two groups pre- and post-matching.
The associations between pre-transplantation history of psychosis/mania and post-transplantation outcomes were assessed in the propensity matched cohort using competing risks regression (Fine and Gray)[30] for death with functioning graft and graft loss, and Kaplan-Meier method and Cox proportional hazard models for all-cause mortality. Logistic regression analysis was used for rejection risk assessment. The mean ± standard deviation (SD) of PDC for immunosuppresive drugs were compared using t-test, while chi2-tests were used to compare medication non-persistance and categorical PDC (group 1: PDC=100% vs group 2: PDC<100%) for different immunosuppressive drugs.
We conducted several sensitivity analyses to evaluate the robustness of our main findings. Associations were examined in subgroups of patients stratified by sex, race, marital status and presence/absence of diabetes, presence/absence of ischemic heart disease and preemptive transplantation. Potential interactions were formally tested by including relevant interaction terms. We adjusted for income and marital status as sensitivity analysis to assess whether these variables have any effect on the examined association. These variables have not been selected in the main model due to significant missingness (19% for income and 10% for marital status). Finally, we also performed all analyses in the entire cohort after adjustment for propensity score.
Reported P values were two-sided and reported as significant at <0.05 for all analyses. All analyses were conducted using STATA/MP Version 15 (STATA Corporation, College Station, TX). The study was approved by the Institutional Review Boards of the Memphis and Long Beach VA Medical Centers, with exemption from informed consent.
Results
Baseline characteristics
The mean±SD age of the cohort at baseline was 61±11 years, 92% were male, 66% and 27% of patients were white and African-American, respectively. 19% of the transplants were preemptive, 72% were married, 48% of the patients were diabetic. In the entire cohort, we identified 126 and 3,554 patients with and without a history of psychosis/mania, respectively. 24 (0.65%) patients had a history of mania,106 (2.88%) patients had a history of psychosis, and 4 (0.11%) patients had a history of both. Baseline characteristics of patients categorized by history of psychosis/mania status are shown in Table 1. In the original cohort (n=3,680) patients with history of psychosis/mania were more likely to be African-American and unmarried, had higher prevalence of diabetes mellitus, peripheral vascular disease, chronic lung disease, liver disease, depression, hypertension, and were more likely to receive anti-hypertensive medications. These differences disappeared after matching by propensity score (Table 1).
Table 1.
Baseline characteristics of the study population
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| No history of psychosis/mania (n=3,554) | History of psychosis/mania (n=126) | Std.Diff. | No history of psychosis/mania (n=320) | History of psychosis/mania (n=122) | Std. Diff. | |
| Demographics: | ||||||
| Age (years) | 61±11 | 59±9 | 0.072 | 59±10 | 59±9 | −0.007 |
| Gender (male), n (%) | 3,262 (92) | 114 (90) | 0.334 | 291 (91) | 110 (90) | 0.026 |
| Race, n (%) | −0.615 | 0.056 | ||||
| White | 2,343 (66) | 75 (60) | 212 (66) | 73 (60) | ||
| African-American | 933 (26) | 47 (37) | 92 (29) | 45 (37) | ||
| Others | 58 (2) | 0 (0) | 3 (1) | 0 (0) | ||
| Unknown | 220 (6) | 4 (3) | 13 (4) | 4 (3) | ||
| Comorbidities: | ||||||
| Myocardial Infarction, n (%) | 234 (7) | 13 (10) | 0.334 | 25 (8) | 12 (10) | 0.071 |
| Congestive Heart Failure, n (%) | 540 (15) | 23 (18) | 0.083 | 56 (18) | 23 (19) | 0.035 |
| Peripheral Vascular Disease, n (%) | 370 (10) | 19 (15) | −0.112 | 38 (12) | 16 (13) | 0.037 |
| Cerebrovascular Disease, n (%) | 273 (8) | 12 (10) | 0.160 | 32 (10) | 12 (10) | −0.005 |
| Chronic Pulmonary Disease, n (%) | 457 (13) | 33 (26) | 0.432 | 74 (23) | 30 (25) | 0.034 |
| Connective Tissue Disease, n (%) | 71 (2) | 4 (3) | 0.147 | 7 (2) | 4 (3) | 0.067 |
| Peptic Ulcer Disease, n (%) | 40 (1) | 4 (3) | 0.230 | 7 (2) | 4 (3) | 0.067 |
| Paraplegia and Hemiplegia, n (%) | 19 (0.5) | 1 (0.8) | 0.000 | 1 (0) | 1 (1) | 0.067 |
| Diabetes, n (%) | 1,681 (47) | 78 (62) | 0.080 | 175 (55) | 74 (61) | 0.121 |
| Liver Disease, n (%) | 394 (11) | 24 (19) | 0.366 | 63 (20) | 22 (18) | −0.042 |
| Malignancy, n (%) | 221 (6) | 12 (10) | 0.291 | 32 (10) | 12 (10) | −0.005 |
| Anemia, n (%) | 1,764 (50) | 72 (57) | 0.195 | 183 (57) | 71 (58) | 0.020 |
| Depression, n (%) | 182 (5) | 71 (56) | 1.353 | 104 (33) | 67 (55) | 0.463 |
| Hyperlipidemia, n (%) | 1,403 (40) | 54 (43) | −0.020 | 136 (43) | 53 (43) | 0.019 |
| Hypertension, n (%) | 2,823 (79) | 113 (90) | −0.160 | 273 (85) | 109 (89) | 0.121 |
| Ischemic Heart Disease, n (%) | 863 (24) | 40 (32) | 0.480 | 95 (30) | 38 (31) | 0.032 |
| Preemptive transplantation, n (%) | 696 (20) | 21 (17) | 0.144 | 59 (18) | 20 (16) | −0.054 |
| Living donor transplantation, n (%) | 1,160 (33) | 33 (26) | −0.301 | 84 (26) | 32 (26) | 0 |
| Dialysis modality: hemodialysis, n (%) | 2,253 (82) | 89 (87) | 0.212 | 209 (65) | 86 (70) | −0.078 |
| Duration of dialysis (days), median (IQR) | 551 (145–1,062) | 690 (298–1,335) | −0.157 | 630 (174–1,209) | 670 (298–1,335) | 0.110 |
| Medications: | ||||||
| ESAs, n (%) | 252 (7) | 16 (13) | −0.228 | 40 (13) | 15 (12) | −0.006 |
| Native Vitamin D, n (%) | 295 (8) | 25 (20) | 0.051 | 65 (20) | 24 (20) | −0.016 |
| Active Vitamin D, n (%) | 505 (14) | 33 (26) | 0.110 | 73 (23) | 30 (25) | 0.042 |
| Sevelamer, n (%) | 771 (22) | 45 (36) | −0.207 | 113 (35) | 43 (35) | −0.001 |
| Lanthanum, n (%) | 210 (6) | 11 (9) | 0.065 | 19 (6) | 11 (9) | 0.117 |
| Calcium acetate, n (%) | 660 (19) | 33 (26) | −0.729 | 87 (27) | 32 (26) | −0.022 |
| Anticoagulants, n (%) | 260 (7) | 24 (19) | 0.272 | 53 (17) | 20 (16) | −0.005 |
| Thrombolytics, n (%) | 19 (0.5) | 1 (0.8) | 0.284 | 6 (2) | 1 (1) | −0.091 |
| Aspirin, n (%) | 385 (11) | 47 (37) | 0.233 | 96 (30) | 44 (36) | 0.129 |
| Digitalis, n (%) | 29 (1) | 1 (0.8) | −0.162 | 3 (1) | 1 (1) | −0.013 |
| β-blockers, n (%) | 1,343 (38) | 80 (64) | 0.115 | 187 (58) | 77 (63) | 0.096 |
| α-blockers, n (%) | 458 (13) | 27 (21) | 0.069 | 57 (18) | 26 (21) | 0.088 |
| Calcium channel blockers, n (%) | 1,187 (33) | 68 (54) | 0.283 | 163 (51) | 64 (52) | 0.030 |
| Antianginals, n (%) | 209 (6) | 16 (13) | 0.088 | 39 (12) | 15 (12) | 0.003 |
| Statins, n (%) | 1,220 (34) | 77 (61) | 0.063 | 185 (58) | 74 (61) | 0.058 |
| Vasodilators, n (%) | 477 (13) | 26 (21) | −0.637 | 63 (20) | 25 (20) | 0.020 |
| Thiazides diuretics, n (%) | 128 (4) | 7 (6) | −0.088 | 17 (5) | 7 (6) | 0.019 |
| Loop diuretics, n (%) | 855 (24) | 42 (330 | 0.020 | 109 (34) | 40 (33) | −0.027 |
| Potassium sparing diuretics, n (%) | 93 (3) | 4 (3) | 0.077 | 12 (4) | 4 (3) | −0.026 |
| RAASi, n (%) | 994 (28) | 55 (44) | −0.044 | 139 (43) | 51 (42) | −0.033 |
| Insulin, n (%) | 765 (22) | 47 (37) | 0.025 | 114 (36) | 45 (37) | 0.026 |
| Other variables NOT included propensity score: | ||||||
| Marital status, n (%) | 0.485 | 0.500 | ||||
| Married | 2,328 (72) | 77 (61) | 210 (71) | 75 (62) | ||
| Single | 246 (8) | 5 (4) | 17 (6) | 5 (4) | ||
| Divorced | 550 (17) | 39 (31) | 58 (20) | 38 (31) | ||
| Widowed | 100 (3) | 5 (4) | 10 (3) | 4 (3) | ||
| Income (USD), median (IQR) | 20,874 (1,585–38,646) | 19,626 (4,524–34,260) | −0.030 | 22,020 (5,040–35,028) | 19,626 (3,435–35,028) | 0.389 |
| Service Connection, % | 90 (30–100) | 100 (60–100) | 0.161 | 100 (60–100) | 100 (60–100) | 0.096 |
| Charlson Comorbidity Index, median (IQR) | 2 (0–3) | 2 (1–4) | 0.474 | 2 (1–3) | 2 (1–4) | 0.268 |
| Serum albumin (g/dL), mean±SD | 3.7±0.5 | 3.7±0.5 | −0.394 | 3.7±0.6 | 3.8±0.5 | −0.213 |
| Serum AST, (g/dL), median (IQR) | 20 (16–26) | 19 (15–26) | 0.237 | 21 (17–28) | 19 (15–26) | 0.621 |
| Serum ALT, (g/dL), median (IQR) | 20 (15–28) | 20 (15–29) | 0.297 | 21 (15–29) | 19 (15–29) | 0.235 |
| Blood hemoglobin, (g/dL), mean±SD | 11.5±1.3 | 11.5±1.4 | 0.156 | 11.4±1.3 | 11.5±1.4 | 0.418 |
| Serum phosphorus, (g/dL), mean±SD | 4.9±1.2 | 4.9±1.9 | −0.282 | 4.9±1.2 | 5.0±1.1 | −0.410 |
| Serum PTH, (g/dL), median (IQR) | 260 (184–428) | 303 (152–419) | −0.142 | 253 (152–401) | 296 (150–404) | −0.143 |
| Systolic BP (mmHg), mean±SD | 137±18 | 133±17 | −0.374 | 138±17 | 132±18 | −0.652 |
| Diastolic BP (mmHg), mean±SD | 75±11 | 74±9 | −0.381 | 75±10 | 74±10 | −0.549 |
| Body mass index (kg/m2), mean±SD | 28±4 | 29±4 | −0.481 | 28±4 | 29±4 | −0.372 |
Abbreviations: ALT: alanine aminotransferase; AST: aspartate aminotransferase; BP: blood pressure; ESAs: erythropoietin stimulating agents; IQR: inter-quartile range; PTH: parathyroid hormone; RAASi: renin-angiotensin-aldosterone system inhibitors; SD: standard deviation; Std. Diff.: Standardized differences; USD: United States dollar.
Predictors of Psychotic Disorders
In our multivariable logistic regression model, presence of chronic lung disease, depression, as well as aspirin and statin usage were associated with history of psychosis/mania (Table S1).
Death with Functioning Graft
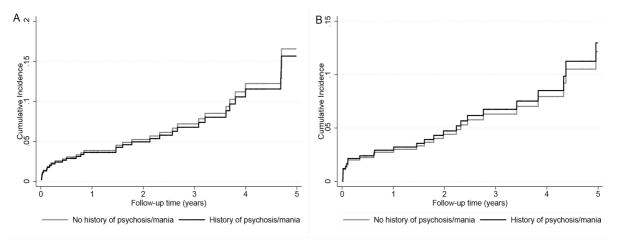
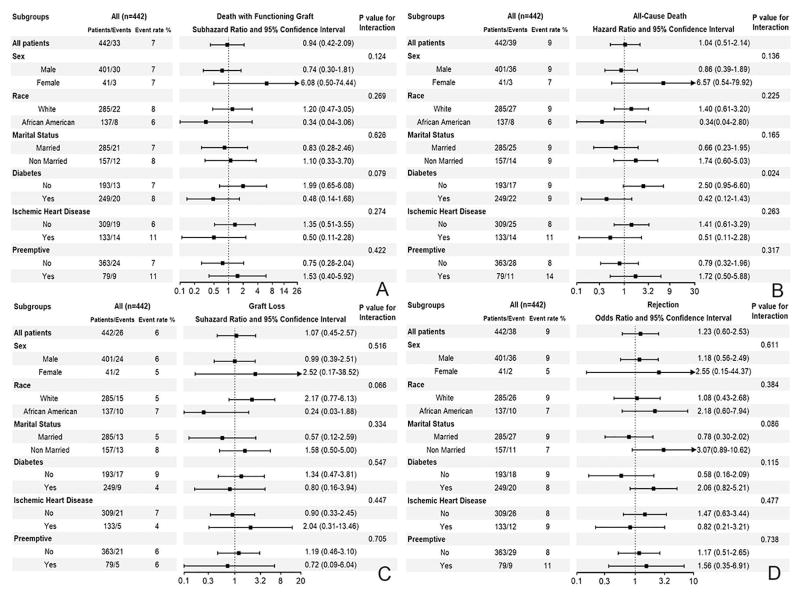
During a median follow-up period of 2 years, a total of 39 (9%) deaths occurred (crude incidence rate, 38 per 1000 patient-years; 95% confidence interval [CI]: 28–52). The crude mortality rate was similar in patients with history of psychosis/mania (10 (8%) deaths, 37 per 1000 patient-years, 95% CI: 20–70) versus patients without history of psychosis/mania (29 (9%) deaths, 38 per 1000 patient-years, 95% CI: 26–54) as shown in Figure 2A. Compared to patients without history of psychosis/mania, patients with history of psychosis/mania had similar risk of death with functioning graft in competing risks regression [SubHazard Ratio (SHR) (95% CI): 0.94 (0.42–2.09)] (Table 2). Similar results were found after further adjustment for marital status and income (SHR (95% CI): 0.82 (0.36–1.85) in our sensitivity analysis. Moreover, there was no association between history of psychosis (SHR (95% CI): 1.23 (0.29–5.27), history of mania (SHR (95% CI): 0.83 (0.34–2.00) and risk of death with functioning graft when the two disorders were analyzed separately. Additonally, there was a lack of association between history of psychosis/mania and risk of death with functioning graft in different subgroups (Figure 3A). Moreover, similar results were found in the entire cohort after adjustment for propensity score (SHR (95% CI): 0.53 (0.19–1.45) in our sensitivity analysis.
Figure 2.
Cumulative incidence of death with functioning graft (panel A) and graft loss (panel B) using competing risks regression models in the propensity-matched cohort
Table 2.
Association between history of psychosis and/or mania and post-transplantation outcomes using Cox proportional regression, competing risks regression and logistic regression models in the propensity-matched cohort (n=442)
| History of psychosis and/or mania vs no of history of psychosis and/or mania (ref.) | Hazard Ratios (HRs) | 95% Confidence Interval of HRs | p-value |
| All Cause Death | 1.04 | 0.51–2.14 | 0.913 |
| History of psychosis and/or mania vs no of history of psychosis and/or mania (ref.) | SubHazard Ratios (SHRs) | 95% Confidence Interval of SHRs | p-value |
| Death with Functioning Graft | 0.94 | 0.42–2.09 | 0.881 |
| Graft Loss | 1.07 | 0.45–2.57 | 0.874 |
| History of psychosis and/or mania vs no of history of psychosis and/or mania (ref.) | Odds Ratios (ORs) | 95% Confidence Interval of ORs | p-value |
| Rejection | 1.23 | 0.60–2.53 | 0.567 |
| Immunosuppressive Adherence: Proportion of days covered for | History of psychosis and/or mania | No of history of psychosis and/or mania | p-value* |
| Tacrolimus (%) (mean±SD) | 76±21 | 78±19 | 0.529 |
| Mycophenolic acid (%) (mean±SD) | 78±17 | 79±18 | 0.666 |
| Immunosuppressive Persistence: 30 days gap | |||
| Tacrolimus | 54% | 54% | 0.998 |
| Mycophenolic acid | 49% | 48% | 0.949 |
Abbreviations: HR: Hazard Ratio; OR: Odds Ratio; SHR: SubHazard Ratio
: P values for adherence are result of t-test and chi-square test
Figure 3.
Association between history of psychosis or mania and death with functioning graft (panel A), all-cause death (panel B), graft loss (panel C) and rejection (panel D) in the propensity-matched cohort in different subgroups
All-cause Death
The survival probabilty was similar in patients with and without history of psychosis/mania as shown in Figure S3. Compared to patients without history of psychosis/mania, patients with history of psychosis/mania had similar all-cause mortality risk [HR (95% CI): 1.04 (0.51–2.14)] (Table 2). Similar results were found after additional adjustment for marital status and income (HR (95% CI): 0.89 (0.43–1.86)). Moreover, there was no association of history of psychosis (HR (95% CI): 0.82 (0.36–1.87) and history of mania (HR (95% CI): 1.70 (0.52–5.52) with all-cause death when the two disorders were analyzed separately. Additonally, there was a lack of association between history of psychosis/mania and all-cause mortality risk in different subgroups (Figure 3B). Moreover, similar results were found in the entire cohort after adjustment for propensity scores (HR (95% CI): 0.60 (0.23–1.54) in our sensitivity analysis.
Graft Loss
A total of 56 (13%) graft losses occurred (crude incidence rate, 54 per 1000 patient-years; 95% CI: 41–70). The crude graft loss rate was similar in patients with history of psychosis/mania (14 (11%) graft loss, 52 per 1000 patient-years, 95% CI: 31–88) versus patients without history of psychosis/mania (42 (13%) graft loss, 55 per 1000 patient-years, 95% CI: 40–74) as shown in Figure 2B. Compared to patients without a history of psychosis/mania, patients with history of psychosis/mania had similar graft loss risk in competing risks regression [SHR (95% CI): 1.07 (0.45–2.57)] (Table 2). Similar result was found after additional adjustment for marital status and income (SHR (95% CI): 0.84 (0.36–1.98). Moreover, there was no association of history of psychosis (SHR (95% CI): 0.87 (0.33–2.32) and history of mania (SHR (95% CI): 1.64 (0.37–7.20) with risk of graft loss when the two disorders were analyzed separately. Additonally, there was a lack of association between history of psychosis/mania and graft loss risk in different subgroups (Figure 3C). Moreover, similar results were found in the entire cohort after adjustment for propensity score (SHR (95% CI): 1.33 (0.43–4.09) in our sensitivity analysis.
Risk of Rejection
Compared to patients without history of psychosis/mania, patients with history of psychosis/mania had similar risk of rejection [Odds Ratio (OR) (95% CI): 1.23 (0.60–2.53)] (Table 2). Similar results were found after additional adjustment for marital status and income (OR (95% CI): 1.30 (0.62–2.75)). Moreover, there was no association of history of psychosis (OR (95% CI): 1.04 (0.47–2.27) and history of mania (OR (95% CI): 2.26 (0.73–6.99) with risk of rejection when the two disorders have been analyzed separately. Additonally, there was a lack of association between history of psychosis/mania and risk for rejection in different subgropus (Figure 3D). Moreover, similar results were found in the entire cohort after adjustment for propensity score (OR (95% CI): 1.31 (0.60–2.88) in our sensitivity analysis.
Medication non-adherence
Of the 442 patients in the propensity matched cohort, 149 patients received tacrolimus prescriptions from a VA pharmacy after transplantation. The average proportion of days covered (PDC) for tacrolimus in the first year after transplantation was 77±20. There was no difference in PDC in patients with and without history of psychosis/mania (PDC: 76±21 vs 78±19, p=0.529). In addition, the proportion of patients with PDC<100% was also similar between these groups (89% with history of psychosis/mania versus 87% without history of psychosis/mania, p=0.762). Finally, the 30- and 60 days persistence with drug therapy (duration of time from initial drug dispensation to “unauthorized” discontinuation) was also similar in patients with and without history of psychosis/mania (30 days: 54% vs 54%, p=0.998; 60 days: 39% vs 28%, p=0.183).
Of the 442 patients in the propensity matched cohort, 144 patients received mycophenolic acid prescriptions from a VA pharmacy after transplantation. The average PDC for mycophenolic acid in the first year after transplantation was 79±17%. There was no difference in PDC in patients with and without history of psychosis/mania (PDC: 78±17% vs 79±18%, p=0.666). In addition, the proportion of patients with PDC<100% was also similar between these groups (96% with history of psychosis/mania versus 93% without history of psychosis/mania, p=0.440). Finally, the 30- and 60 days persistence with drug therapy was also similar in patients with and without history of psychosis/mania (30 days: 49% vs 48%, p=0.949; 60 days: 20% vs 20%, p=0.954).
Discussion
In this large national cohort of incident kidney transplant US veterans, we found that recipients with history of psychosis/mania have similar survival, graft loss, and rejection risk compared to recipients without these diagnoses. In addition, we showed that these selected recipients with history of psychosis/mania have similar post-transplantation immunosuppressive medication adherence compared to their counterparts without these diagnoses.
Very few previous studies assess the association between history of psychosis/mania and post-transplant outcomes. Most of them are small observational trials with very few patients, and have several methodological flaws.[13–19] There are many potential reasons why these studies, including our own, have not shown any differences in outcome. A main reason is good medication adherence after transplantation. Our results show that the adherence to anti-rejection medications in the post-transplant period was similar in recipients with history of psychosis/mania versus the ones without. A previous study involving United States Renal Data System (USRDS) patients showed that medication non-adherence is associated with higher risk of graft loss and death in transplant recipients who were hospitalized with a diagnosis of psychosis after transplantation.[12] Some of these patients might have had new psychotic diagnoses after transplantation secondary to several factors such as high dose steroid use, drug interactions or surgery. Another potential explanation is the free access to health care in the VA system. A recent study showed that the quality of care of mental disorders was better in the VA healthcare system compared to the private sector, [31] which could explain both a better selection process and also better quality of care after transplantation.
Our study suggests that transplantation can be safe even in patients with a history of psychosis/mania. However, it is important to note that while all these recipients have been transplanted, they likely underwent very careful selection prior to being listed for transplantation. Our study does not suggest that all end stage renal disease (ESRD) patients with history of psychosis/mania should be eligible for transplantation. Almost 9% of dialysis patients are hospitalized with a mental disorder in a year, [32] but only 3.5% of kidney transplant recipients have history of psychosis/mania, which might suggests that many ESRD patients with history of psychosis/mania are not transplanted. Our study demonstrates that the selection process in VA medical centers is successful and results in similar graft and patient outcomes. While these results are encouraging, we need more data from outside the VA system and from other countries confirming our results.
Our study is notable for its relatively large sample size and event numbers, and for being representative of veterans who received care in the VA system across the entire US. In addition, we used a validated method to diagnose the history of psychosis/mania from an administrative dataset.[25] To our knowledge, this is the largest study to assess the association of history of psychosis/mania before kidney transplantation with transplantation outcomes. In addition, this is the first study which assessed medication adherence after kidney transplantation in recipients with these diagnoses.
This study also has several limitations that need to be acknowledged. Patients were mostly male US veterans; hence, the results may not be generalizable to women or other patient populations, in particular to those outside the US. Our study is also limited by the use of an administrative database and by diagnoses being based on ICD codes instead of clinical evaluation. We did not have details about the clinical care and evaluation of patients pre- and post-transplantation, or about any special care of guidance they may have received pre- and post-transplantation from medical professionals or caretakers. Additionally, we do not have data about the type of rejection, therefore more granular analyses cannot be performed in our dataset. However, we used a definition based on a validated algorithm [25] to eliminate this potential bias. We did not include other psychiatric problems in our analyses as the reliability of the ICD codes for these problems is questionable. Moreover, we did not have information listing and transplantation data for patients who did not undergo kidney transplantation, hence we do not know how many of them were assessed for transplantation and found to be eligible or ineligible. Finally, as with all observational studies, we cannot eliminate the effect of unmeasured confounders.
Conclusion
In conclusion, this large national cohort of US transplant recipients with history of psychosis/mania shows similar medication adherence and survival, graft loss and rejection risk compared to recipients without these diagnoses. This demonstrates that the transplant candidate selection process can be successful. Further studies are needed to define how we can safely select even more transplant candidates from the dialysis patient population with history of psychosis/mania.
Supplementary Material
Acknowledgments
CPK and KKZ are employees of the Department of Veterans affairs. Opinions expressed in this paper are those of the author’s and do not necessarily represent the opinion of the Department of Veterans Affairs. The results of this paper have not been published previously in whole or part.
Funding Support:
This study is supported by grant 5U01DK102163 from the National Institute of Health (NIH) to KKZ and CPK, and by resources from the US Department of Veterans Affairs. The data reported here have been supplied in part by the United States Renal Data System (USRDS). Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
Disclosures
None of the authors have relevant conflicts of interest.
References
- 1.Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351:476–86. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- 2.Freedman R. Schizophrenia. N Engl J Med. 2003;349:1738–49. doi: 10.1056/NEJMra035458. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi RB, Post EP, Sun H, et al. Prevalence, Comorbidity, and Prognosis of Mental Health Among US Veterans. Am J Public Health. 2015;105:2564–9. doi: 10.2105/AJPH.2015.302836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price A, Whitwell S, Henderson M. Impact of psychotic disorder on transplant eligibility and outcomes. Curr Opin Organ Transplant. 2014;19:196–200. doi: 10.1097/MOT.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 5.Alsady M, Baumgarten R, Deen PM, de Groot T. Lithium in the Kidney: Friend and Foe? J Am Soc Nephrol. 2016;27:1587–95. doi: 10.1681/ASN.2015080907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudley C, Harden P. Renal Association Clinical Practice Guideline on the assessment of the potential kidney transplant recipient. Nephron Clin Pract. 2011;118(Suppl 1):c209–24. doi: 10.1159/000328070. [DOI] [PubMed] [Google Scholar]
- 7.Ebpg European Renal A, European Society for Organ T. European Best Practice Guidelines for Renal Transplantation (part 1) Nephrol Dial Transplant. 2000;15(Suppl 7):1–85. [PubMed] [Google Scholar]
- 8.Kalble T, Lucan M, Nicita G, et al. EAU guidelines on renal transplantation. Eur Urol. 2005;47:156–66. doi: 10.1016/j.eururo.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Kasiske BL, Cangro CB, Hariharan S, et al. The evaluation of renal transplantation candidates: clinical practice guidelines. Am J Transplant. 2001;1(Suppl 2):3–95. [PubMed] [Google Scholar]
- 10.Klapheke MM. The role of the psychiatrist in organ transplantation. Bull Menninger Clin. 1999;63:13–39. [PubMed] [Google Scholar]
- 11.Butler MI, McCartan D, Cooney A, et al. Outcomes of Renal Transplantation in Patients With Bipolar Affective Disorder and Schizophrenia: A National Retrospective Cohort Study. Psychosomatics. 2017;58:69–76. doi: 10.1016/j.psym.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Abbott KC, Agodoa LY, O’Malley PG. Hospitalized psychoses after renal transplantation in the United States: incidence, risk factors, and prognosis. J Am Soc Nephrol. 2003;14:1628–35. doi: 10.1097/01.asn.0000069268.63375.4a. [DOI] [PubMed] [Google Scholar]
- 13.Kofman T, Pourcine F, Canoui-Poitrine F, et al. Safety of renal transplantation in patients with bipolar or psychotic disorders: a retrospective study. Transpl Int. 2017 doi: 10.1111/tri.13078. [DOI] [PubMed] [Google Scholar]
- 14.Dave V, Mulley W, Kanellis J, Summers S. Managing psychosis in a renal transplant recipient with bipolar affective disorder and allograft rejection. Nephrology (Carlton) 2015;20(Suppl 1):2–5. doi: 10.1111/nep.12419. [DOI] [PubMed] [Google Scholar]
- 15.Power RE, Hayanga AJ, Little DM, Hickey DP. Outcome of cadaveric renal transplantation in patients with psychiatric disorders. Ir Med J. 2002;95:172–4. [PubMed] [Google Scholar]
- 16.Carrasco FR, Moreno A, Ridao N, et al. Kidney transplantation complications related to psychiatric or neurological disorders. Transplant Proc. 2009;41:2430–2. doi: 10.1016/j.transproceed.2009.06.166. [DOI] [PubMed] [Google Scholar]
- 17.Evans LD, Stock EM, Zeber JE, et al. Posttransplantation Outcomes in Veterans With Serious Mental Illness. Transplantation. 2015;99:e57–65. doi: 10.1097/TP.0000000000000616. [DOI] [PubMed] [Google Scholar]
- 18.DiMartini A, Twillman R. Organ transplantation and paranoid schizophrenia. Psychosomatics. 1994;35:159–61. doi: 10.1016/S0033-3182(94)71790-4. [DOI] [PubMed] [Google Scholar]
- 19.Zimbrean P, Emre S. Patients with psychotic disorders in solid-organ transplant. Prog Transplant. 2015;25:289–96. doi: 10.7182/pit2015296. [DOI] [PubMed] [Google Scholar]
- 20.Corbett C, Armstrong MJ, Parker R, Webb K, Neuberger JM. Mental health disorders and solid-organ transplant recipients. Transplantation. 2013;96:593–600. doi: 10.1097/TP.0b013e31829584e0. [DOI] [PubMed] [Google Scholar]
- 21.Molnar MZ. Is kidney transplantation safe after careful selection of the recipients with a history of psychotic disorder? Transpl Int. 2017 doi: 10.1111/tri.13082. [DOI] [PubMed]
- 22.Sumida K, Molnar MZ, Potukuchi PK, et al. Association of Slopes of Estimated Glomerular Filtration Rate With Post-End-Stage Renal Disease Mortality in Patients With Advanced Chronic Kidney Disease Transitioning to Dialysis. Mayo Clin Proc. 2016;91:196–207. doi: 10.1016/j.mayocp.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumida K, Molnar MZ, Potukuchi PK, et al. Association between vascular access creation and deceleration of estimated glomerular filtration rate decline in late-stage chronic kidney disease patients transitioning to end-stage renal disease. Nephrol Dial Transplant. 2016 doi: 10.1093/ndt/gfw220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molnar MZ, Gosmanova EO, Sumida K, et al. Predialysis Cardiovascular Disease Medication Adherence and Mortality After Transition to Dialysis. Am J Kidney Dis. 2016 Apr 12; doi: 10.1053/j.ajkd.2016.02.051. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frayne SM, Berg E, Holmes TH, et al. Mental illness-related disparities in length of stay: algorithm choice influences results. J Rehabil Res Dev. 2010;47:709–18. doi: 10.1682/jrrd.2009.08.0112. [DOI] [PubMed] [Google Scholar]
- 26.Kovesdy CP, Norris KC, Boulware LE, et al. Association of Race With Mortality and Cardiovascular Events in a Large Cohort of US Veterans. Circulation. 2015;132:1538–48. doi: 10.1161/CIRCULATIONAHA.114.015124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovesdy CP, Alrifai A, Gosmanova EO, et al. Age and Outcomes Associated with BP in Patients with Incident CKD. Clin J Am Soc Nephrol. 2016;11:821–31. doi: 10.2215/CJN.08660815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choudhry NK, Shrank WH, Levin RL, et al. Measuring concurrent adherence to multiple related medications. Am J Manag Care. 2009;15:457–64. [PMC free article] [PubMed] [Google Scholar]
- 29.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–7. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 30.Fine J, Gray R. A proportional hazards model for subdisribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 31.Watkins KE, Smith B, Akincigil A, et al. The Quality of Medication Treatment for Mental Disorders in the Department of Veterans Affairs and in Private-Sector Plans. Psychiatr Serv. 2016;67:391–6. doi: 10.1176/appi.ps.201400537. [DOI] [PubMed] [Google Scholar]
- 32.Kimmel PL, Thamer M, Richard CM, Ray NF. Psychiatric illness in patients with end-stage renal disease. Am J Med. 1998;105:214–21. doi: 10.1016/s0002-9343(98)00245-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.