Abstract
Japanese gentians are the most important ornamental flowers in Iwate Prefecture and their breeding and cultivation have been actively conducted for half a century. With its cool climate and large hilly and mountainous area, more than 60% of gentian production in Japan occurs in Iwate Prefecture. Recent advances in gentian breeding and cultivation have facilitated the efficient breeding of new cultivars; disease control and improved cultivation conditions have led to the stable production of Japanese gentians. Molecular biology techniques have been developed and applied in gentian breeding, including the diagnosis of viral diseases and analysis of physiological disorders to improve gentian production. This review summarizes such recent approaches that will assist in the development of new cultivars and support cultivation. More recently, new plant breeding techniques, including several new biotechnological methods such as genome editing and viral vectors, have also been developed in gentian. We, therefore, present examples of their application to gentians and discuss their advantages in future studies of gentians.
Keywords: breeding, cultivation, DNA marker, Gentiana scabra, Gentian triflora, metabolome, new plant breeding techniques
Introduction
Japanese cultivated gentians have been mainly bred from two endemic gentian species, including Gentiana triflora Pall. var. japonica and G. scabra Bunge var. buergeri. Molecular lineage analysis has indicated that these two species are very closely related within the Gentiana genus (Mishiba et al. 2009). Interspecific hybrids have, therefore, also been produced and used in gentian breeding by conventional hybridization. In this review, we define “Japanese gentians” as cultivated gentians derived from these two Gentiana species, although some other Gentiana species are also used for breeding and cultivation. Gentians are mainly sold as cut flowers and potted plants from early summer to late autumn in Japan. The breeding history of gentian was recently summarized in Japanese by Dr. T. Hikage of the Hachimantai City Floricultural Research and Development Center (Hikage 2016), who showed that there is an increasing tendency to breed novel cultivars of Japanese gentians in accordance with supply and demand. Gentian cultivation in New Zealand and Chile started decades ago, and Japanese gentians are exported as cut flowers to Japan, the EU, and the USA. The international delivery of gentians means that they are becoming internationally recognized crops. Therefore, development of new cultivars with novel flower traits becomes more important to appeal to foreign flower markets. To this end, molecular breeding techniques for gentians have been developed over the last decade (Nishihara et al. 2015). For example, a dwarf potted gentian cultivar has been developed using the Agrobacterium rhizogenes wild A4 strain (Mishiba et al. 2006). Nevertheless, Japanese gentians have less variation compared with other major flowers such as carnations and roses.
Cultivated gentian production is affected in the field by disease and the incident of physiological disorders (Nishihara et al. 2015). Furthermore, the most noteworthy difficulty associated with gentian cultivation is the inability to control their flowering time. This is because Japanese cultivated gentians are perennial plants and there is little information available regarding their physiological ecology in terms of flowering and overwintering responses. Recently, the molecular mechanisms underlying flowering and dormancy have been characterized in several plants, and the importance of photoperiod and temperature has been elucidated (Maurya and Bhalerao 2017, Song et al. 2013). In Japanese gentians, several genes regulating flowering time, such as GtFTs, GtTFL1 and GtSVPs, have been cloned and analyzed (Imamura et al. 2011, Yamagishi et al. 2016). Dehydrin genes (GtDHNs) and W14/15-encoding novel esterase genes involved in drought and freezing tolerance have also been characterized (Imamura et al. 2013, Tsutsumi and Hikage 2014). Recently, gentio-oligosaccharide-mediated modulation of bud dormancy was first identified in Japanese gentian (Takahashi et al. 2014), although the complete picture of flowering and overwintering remains unknown. This knowledge could, in the future, be applied to the artificial regulation of gentian growth and flowering.
In this review, we focus on the recent development of basic technologies for the improvement of Japanese gentian breeding and cultivation.
Gentian production in Japan
Gentian breeding and cultivation
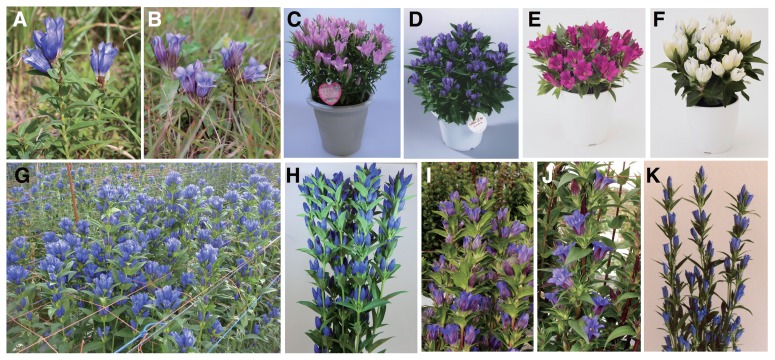
Before the advent of gentian breeding, native wild gentian flowers were gathered by farmers for sale. However, the persistent demand for commercial use led the Iwate Prefecture to breed elite gentian cultivars to provide a consistent supply of cultivated gentian plants (Yoshiike 1992). Thus, extensive gentian breeding and cultivation studies started in the mid-1960’s, with the first F1 cultivar ‘Iwate’ developed in 1977. Since then, more than twenty cultivars have been bred for use in Iwate Prefecture as cut flowers and potted plants from early July to early November (Table 1). Unlike rice, wheat, and soybean, Japanese gentian plants show strong inbreeding depression traits making it difficult to breed elite fixed cultivars. The population breeding strategy of mass selection (Kuckuck et al. 1991) was, therefore, predominantly used for the breeding of all gentian cultivars except those propagated vegetatively. Namely, F1 seeds were obtained from maternal and paternal populations with selected phenotypes to retain a certain level of heterozygosity. As vegetatively propagated cultivars are usually more expensive, F1 seed cultivars are preferable. At present, 328 Gentiana cultivars, including expired cultivars, have been registered in the Plant Variety database (Ministry of Agriculture, Forestry and Fisheries, Japan). In fact, there are likely to be more gentian cultivars because vegetative or personally bred lines are sometimes unregistered. Gentian flower colors are predominantly blue because of the accumulation of polyacylated anthocyanins (Goto et al. 1982, Hosokawa et al. 1997), but original cultivars with specific characteristics such as pink and white flowers are also found in local areas such as Hachmantai City (old name Ashirocho), Nishiwaga-town (Waga-gun) and Koromogawa (Oshu City). Among them, Hachimantai city, which is located in the northwestern part of Iwate Prefecture, is the most popular gentian production area and has bred many interesting cultivars. For example, the cultivar ‘New Hybrid Ashiro’ was bred there using foreign species that shows light blue-colored stylish flowers suitable for floral arrangements. More recently, a red flowered gentian cultivar ‘Koibeni’, which is used as a potted plant, was also bred there using foreign species (Hikage 2016). Some wild and cultivated gentians are shown in Fig. 1. In gentians, however, variations in the color, style, and shape of the flowers are not as pronounced as in other ornamental flowers. In lisianthus (Eustoma grandiflorum, known as prairie gentian), which belongs to the family Gentianaceae like gentians, a wide variety of cultivars have been bred from a single purple wild flower, allowing consumers to select their optimal flowers. Gentian breeding is, therefore, considered to have reached a midpoint on the path to obtaining optimal breeding results. Further research and development are, therefore, needed.
Table 1.
List of Gentiana cultivars bred by Iwate Prefecturea
| Cultivar name | Species | Propagation | Flower color | Purpose | Registration year | Flowering period | Registration number |
|---|---|---|---|---|---|---|---|
| Iwate | G. triflora | F1 seeds | Blue | Cut flower | 1977 | Late Aug.–middle Sept. | NAb |
| Iwate Otome | G. triflora | F1 seeds | Blue | Cut flower | 1984 | Late Aug.–early Sept. | 544 |
| Ihatov | G. triflora | F1 seeds | Blue | Cut flower | 1986 | Late Aug.–middle Aug. | 1100 |
| Giovanni | G. triflora | F1 seeds | Blue | Cut flower | 1986 | Late Sept.–middle Sept. | 1101 |
| Albireo | G. triflora × G. scabra | F1 seeds | Blue | Cut flower | 1990 | Late Oct.–middle Oct. | 2553 |
| Maciry | G. triflora | F1 seeds | Blue | Cut flower | 1992 | Late July–early Aug. | 3073 |
| Homoi | G. triflora | F1 seeds | White | Potted flower | 1992 | Late Aug.–early Sept. | 3074 |
| Alta | G. scabra | Vegetative | Blue | Cut flower | 1994 | Late Oct.–early Nov. | 4085 |
| Polarno White | G. scabra × G. triflora | Vegetative | White | Cut flower | 1996 | Middle Sept.–late Sept. | 4999 |
| Aokorin | G. scabra | Vegetative | Blue | Potted flower | 2000 | Late Sept.–early Oct. | 7713 |
| Momokorin | G. scabra | Vegetative | Pink | Potted flower | 2000 | Late Sept.–early Oct. | 7714 |
| Polarno Blue | G. scabra × G. triflora | Vegetative | Blue | Cut flower | 2000 | Late Sept.–early Oct. | 7715 |
| Majel | G. triflora | F1 seeds | Blue | Cut flower | 2008 | Late July–early Aug. | 16725 |
| Qust | G. triflora | F1 seeds | Blue | Cut flower | 2008 | Middle July–late July | 16726 |
| Iwate-VEB6 | G. triflora | F1 seeds | Blue | Cut flower | 2010 | Early July | 18762 |
| Iwate-DPB1 | G. scabra × G. triflora | Vegetative | Blue | Potted flower | 2011 | Late Aug.–Sept. | 20345 |
| Momozukinchan | (G. triflora × G. scabra) × G. scabra | Vegetative | Pink | Potted flower | 2011 | Late Aug.–Sept. | 20210 |
| Iwate Yumeminori | G. triflora | F1 seeds | Blue | Cut flower | 2014 | Late Sept.–early Oct. | 22946 |
| Iwate Ymemitsuki | G. triflora | F1 seeds | Blue | Cut flower | 2015 | Early Oct. | 24342 |
| Iwate-LB3 | G. triflora | F1 seeds | Blue | Cut flower | 2017 | Early Sept. | 25770 |
| Iwate-LB4 | G. triflora | F1 seeds | Blue | Cut flower | 2017 | Early Sept.–middle Sept. | 25771 |
Plant Variety database of PVP Office MAFF in Japan was searched in Oct, 2017.
Not available because this variety was registered under the old Seeds and Seedings Law.
Fig. 1.
Wild and cultivated Japanese gentians. Native gentians found in the mountains: (A) Gentiana triflora and (B) Gentiana scabra; Gentian cultivars: (C) ‘Momozukin-chan’, (D) ‘Iwate Otome’, (E) ‘Koibeni’, and (F) ‘Crystal Ashiro’; (G) Cultivated gentians grown in agricultural fields: (H) ‘Iwate Umenozomi’, (I) ‘Albireo’, (J) ‘Alta’, and (K) ‘New hybrid Ashiro’. C, D, H, I, and J were developed by Iwate Prefecture. E, F, and K were developed by Hachimantai City.
The regular supply of seeds and seedlings enables us to make advances in gentian production in Iwate Prefecture. A breakthrough in gentian cultivation was the discovery that the application of gibberellin (GA) treatment to seedlings could enhance their growth and promote bolting (Kodama 2006). GA treatment is known to promote seed germination and plant growth in various plant species (Davies 2010). In Gentiana lutea, it has also been reported that GA pregermi-native treatment was effective for increasing seed germination rate (Gonzalez-Lopez and Casquero 2014). In Japanese gentians, enhanced growth of the plants in their first year by GA treatment shortens the nursery time for these seedlings and consequently promotes flowering and permits harvesting in the next year. Through this technique, the breeding and cultivation time has been shortened significantly, allowing more rapid gentian cultivation. As the effects of GA treatment are not well understood in gentian at the molecular level, further analysis of the mechanisms underlying this response is now under way. Along with gentian breeding, gentian cultivation is another important issue as there are many problems associated with gentian cultivation such as viral, fungal and bacterial pathogens and phytoplasmas. Recently, several unknown symptoms that are not caused by pathogens have also become problematic for gentian cultivation in some areas. Since Japanese cultivated gentians are perennial and are usually cultivated for more than five years, efficient overwinter survival is also important to maintain high productivity. As gentian flowering time is significantly influenced by environmental factors, methods of controlling flowering time need to be developed. In addition to the issues described here, there are many other challenges that need to be overcome during gentian cultivars, with field farmers and breeders eagerly awaiting solutions to these challenges. Researchers have been studying these problems at the molecular level using the latest metabolomics analysis techniques as discussed below.
Biotechnological approach for efficient gentian breeding
Production of pure gentian lines by in vitro culture techniques
To promote gentian breeding, the development of pure lines such as anther and unfertilized ovule cultures was recently achieved. As the details are summarized in a recent review (Doi and Takahata 2015), we discuss them only briefly here. Using anther culture, it first became possible to produce doubled haploid (DH) lines (Doi et al. 2010). Using one pure line (Aki6PS) obtained using anther culture as a parent, a cultivar named ‘Ashirono Akizora’ was bred and registered in 2012. This was not a completely pure cultivar because one parent was a standard line bred through normal population hybridization. Nevertheless, this cultivar is very uniform compared with other cultivars. Anther culture is, therefore, a promising approach to produce pure gentian lines with homogeneous genetic backgrounds that can be used as parental lines for novel uniform cultivars. While this approach has potential for the production of pure lines, the efficiency was not exceptionally high and later experiments have shown that it was somewhat difficult to apply this technique to various gentian breeding lines. Additionally, the female gametes of G. triflora have been reported to be a better material for obtaining regenerated haploid and doubled haploid plants with a higher frequency (Pathirana et al. 2011). Thus, another approach using unfertilized ovule culture has also been attempted. This method was shown to be more efficient and applicable to various gentian materials when compared with anther culture (Doi et al. 2011) and can also be applied to a wide range of gentian genotypes (Doi et al. 2013) including non-Japanese gentians. Optimization of the culture conditions and chromosome doubling has been studied in a breeding program supported by the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry (project number 27030C). In that study, the unfertilized ovule culture technique was applied to other gentian resources including G. asclepiada, G. gracilipes, and G. septemfida (Takamura et al. 2016). We have also obtained hybrids between Japanese gentians and G. acaulis or G. oschtenica by embryo culture. Although commercial cultivars have not been bred yet, interspecific hybrids between G. triflora and G. lutea were successfully produced by embryo culture (Morgan 2004). Given that the Gentiana genus contains more than 360 species, it is important to use the available genetic resources for the breeding program to increase the variation in different Japanese gentian traits (Ho and Liu 2001). As hybrids are generally infertile, amphidiploids are usually produced and used for hybridization. To eliminate the crossing process and reduce breeding time, the unfertilized ovule culture of amphidiploids is also considered effective for production of progeny and cultivars. For these reasons, an understanding of the characteristics of gentian DH lines, including the degree of inbreeding depression and fertility, is essential. Taken together, the use of unfertilized ovule culture is one of the most promising approaches for the production of doubled haploid plants (pure lines), and interspecific hybrids by embryo culture may also represent a method of increasing gentian genetic variation. This would allow novel cultivars with uniform and novel traits to enter the market in the near future.
Application of DNA markers for gentian breeding
Marker assisted selection (MAS) has been applied to a range of different crops (Henry 2012). Compared with conventional plant breeding, MAS is particularly useful because it can be performed at the early stages of seedling development and can replace time-consuming field cultivations or bioassays. It helps by providing information about the parental lines before crossing and allows us to design a logical breeding strategy to achieve various objectives. Previously, DNA markers linked to several agronomically important traits, including flower color, flower style, flowering time and overwinter survival rate, have been developed in Japanese gentian (Nishihara et al. 2015). These are summarized in Table 2. The molecular markers can also be used for cultivar identification and linkage analysis (Shimada et al. 2009, Ushiku et al. 2011). In fact, the first genetic linkage map for Japanese gentian was generated using simple sequence repeat (SSR) and several DNA markers (Nakatsuka et al. 2012). Japanese gentians originally had blue flowers, but some mutant lines have arisen with pink or white flowers that can be used as breeding materials. We have identified the mutations that cause these changes in flower color at the molecular level and have developed DNA markers that allow for the selection of a desired flower color phenotype based on the banding pattern of linked molecular (DNA) markers. These PCR-based markers can successfully distinguish plants that will produce pink and white flowers from those that will produce blue flowers at the seedling stage (Kakizaki et al. 2009, Nakatsuka et al. 2011, Nishihara et al. 2011). There are, however, no molecular markers available for flower color intensity, even though this is an important flower trait in gentian. High temperatures or low light conditions frequently result in low flower color intensity. This affects flower quality and sales as consumers prefer flowers with more intense colors. As only limited information is available for flower color intensity in Japanese gentian, we are attempting to analyze the mechanism(s) that regulate flower color intensity using RNA-Seq and metabolomic analysis through a study entitled “Breeding of floricultural plants adapted for high practical needs and development of low cost cultivation technique”, funded by the Ministry of Agriculture, Forestry, and Fisheries (project number 15653424). Thus far, analysis of the effects of environmental conditions, examined using crossing populations, has indicated that flower color intensity is affected by both genetic background and environmental conditions. Typically, the expression levels of several flavonoid-biosynthesis-related genes such as CHS, CHI, and GST differ significantly with low light conditions at young flower stage 2 (Tasaki et al. 2017a). As transcriptome analysis has previously revealed the involvement of transcription factor genes in flower color intensity in tree peony (Paeonia osti) (Gao et al. 2016), we are focusing on transcription factors such as MYBs, bHLHs, and WDRs in gentian and are now in the process of developing DNA markers to predict flower color intensity in gentian.
Table 2.
Developed DNA markers in Japanese gentians
| Traits | Genes | Marker types | Remarks | References |
|---|---|---|---|---|
| Flower color | ||||
| Pink vs. blue | F3′5′H | SCAR | Insertion in exon | Kakizaki et al. 2009 |
| F3′5′H | SCAR | Insertion in intron | Nishihara et al. 2011 | |
| White vs. blue | MYB3, ANS | SCAR | Insertions or deletions | Nakatsuka et al. 2011 |
| Flower type | ||||
| Single vs. double | AG1 | SCAR | Insertion in intron | Tasaki et al. 2017b |
| Flowering time | TFL1 | SCAR | Insertion in promoter | Imamura et al. 2011 |
| Overwinter survival | W14/15 | SCAR | Sequence variation | Hikage et al. 2016 |
| Cultivar identification | ANS, CHI, F3H, F3′5′H | SCAR | Variations in intron | Shimada et al. 2009 |
| Unknown | SSR | Unknown | Ushiku et al. 2011 | |
| Lineage analysis | Unknown | RAPD, SCAR | Unknown | Jomori et al. 2000 |
| trnL, rpL16 | PS-ID, trn | Sequence variation | Mishiba et al. 2009 | |
| CHS, CHI, ANS, F3′5′H1/2, MYB3, bHLH1, FT1/2, TFL1 | AFLP, RAPD, SSR, REMAP, SCAR | Unknown | Nakatsuka et al. 2012 | |
| W14/15 | SCAR, SNP | Sequence variation, Dot blot hybridization | Hikage et al. 2011 | |
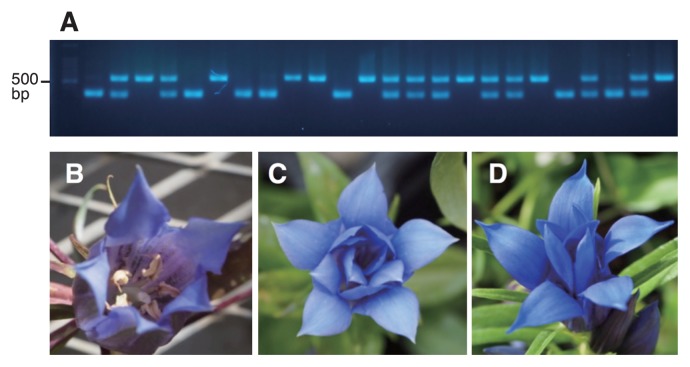
Recently, a DNA marker to detect the insertion mutation of the GsAG1 gene was developed for distinguishing single-and double-flowers in Japanese gentian (Tasaki et al. 2017b). This marker can now be used to identify double flower individuals in many crossing populations. An example of the application of this marker in an F2 population is shown in Fig. 2. During May and June of 2016 and 2017 we screened ca. 4,000 seedlings and ca. 10,000 seedlings, respectively. These seedlings mainly comprised individuals from F2 populations derived from selfing F1 plants that were themselves crosses between double-flower mutants and breeding lines. F3 and BC1 populations were also subjected to marker analyses. Such screening can largely reduce the number of planted seedlings, thereby reducing the effort and cost associated with gentian cultivation. Other DNA markers for disease-resistance, flowering time, and overwinter survival rate are also necessary to improve the efficiency of gentian breeding. As the Japanese gentian genome is relatively large at approximately 5 Gb (Mishiba et al. 2009), reduced-representation sequencing approaches such as RAD-Seq and RNA-Seq-based mapping are considered to be more practical and realistic in these species than whole-genome sequencing. Recent advances in next generation sequencing and novel genotyping-by-sequencing technologies (Scheben et al. 2017) will be also helpful for marker development in gentian in the future.
Fig. 2.
Molecular DNA marker analysis of an F2 population for the selection of double- flowered gentian individuals. (A) Twenty-four F2 individuals derived from selfing of an F1 (single-flower × double-flower) population were subjected to multiplex PCR analysis. Left lane, 100-bp ladder marker with the 500-bp band indicated. Fragments of ca. 530 bp indicate the AG1 mutated allele while those of ca. 350 bp indicate the normal allele. An example of a typical single-flower (B) and two examples of double-flowers (C, D).
Mutation breeding in gentian
Many attempts have been made to create new Japanese gentian cultivars, including culture techniques and MAS as discussed above. Cultivars with novel traits in relation to flower color, flower shape, and flowering time, are necessary to provide additional choice for consumers and to overcome current issues in cultivation. At present, only two gentian species, G. triflora and G. scabra, are generally used for breeding, with these species having only a limited number of natural mutants available. Therefore, the introduction of mutations to expand the range of available genetic resources is desirable. Several techniques, including chemical treatment and gamma and X-ray irradiation, have long been used to induce mutations in a range of crops. Mutagenesis using heavy ion beam irradiation was developed relatively recently and has been shown to be effective in the induction of mutations for crop improvement in several facilities in Japan. Indeed, in some ornamental flowers such as carnation, dahlia, and verbena (Kazama et al. 2008a, Okamura et al. 2003), cultivars produced using this technique have already reached the market. Ion beam irradiation can induce a wide variety of mutants in terms of flower color and shape (Okamura et al. 2003), making it ideal for use in gentian breeding. Japanese gentians are perennial plants, meaning that it can take significant time to obtain M2 populations. Therefore, while seeds are typically used for ion-beam irradiation, we have used cultured gentian plantlets as the irradiation materials to shorten the time required for the acquisition of mutants. The Heavy Ion Medical Accelerator facility in Chiba (HIMAC) was available through the Heavy Ions research project at NIRS-HIMAC. The ion beams accelerated at HIMAC have high energy that allows equal linear energy transfer (LET) irradiation through thick plant tissues. The irradiation conditions, including the ion species, irradiation doses, and the plant materials, are important for the effective acquisition of mutant lines. For this reason, the mutation rates induced by various combinations of ion species and irradiation doses have been evaluated in several facilities (Kazama et al. 2008b, Yamaguchi et al. 2009, Yamaguchi et al. 2010). However, irradiation conditions should be determined independently at each ion beam facility because the particle energy is dependent on each individual accelerator. We, therefore, began to determine suitable irradiation conditions for cultured gentians using NIRS-HIMAC in 2013. We have tested the biological effects of irradiation with several ion species, including C, Ne, Ar, Si, and Fe, and various irradiation doses on cultured gentians. Representative results are shown in Fig. 3. We found that growth was repressed by C ion beam at irradiation doses of more than 4 Gy and was inhibited strongly by doses of greater than 15 Gy. This result was similar to previous results obtained using chrysanthemum lateral buds irradiated with C ion beams (Yamaguchi et al. 2008). The phenotypes of the resulting gentians are currently being assessed and detailed results will be published elsewhere.
Fig. 3.
Assessment of heavy ion-beam irradiation in in vitro cultured gentian. Cultured gentian cv. ‘Albireo’ plantlets were exposed to a carbon ion beam (calculated linear energy transfer = 13 kev/μm) in the range of 1–15 Gy. After a few weeks, the node segments were cut out and transferred to new solid media. Photos of the plantlets grown from lateral shoots were taken ca. 3 months after irradiation. Scale unit shows cm.
Use of virus vectors for gentian research
Plant virus vectors are important biotechnological tools in plant research and there are many advantages associated with using viral vectors compared with other vector systems. For example, viral vectors can be used for the expression of heterologous proteins and the induction of gene silencing in plant species recalcitrant to stable transformation. Viral vectors can also accelerate basic research in ornamental plants with long growth times. Virus-induced gene silencing has been used in several plant species, including some ornamental species. For example, the Tobacco rattle virus has been used for California poppy (Eschscholzia californica) in the family Papaveraceae (Wege et al. 2007) and Thalictrum (Di Stilio et al. 2010) and Aquilegia coerulea (Gould and Kramer 2007) in the family Ranunculaceae and the Cucumber mosaic virus for petunia in the family Solanaceae (Koseki et al. 2005) and Lilium leichtlinii in the family Liliaceae (Tasaki et al. 2016a). In gentian, a virus-induced gene silencing system using Apple latent spherical virus (ALSV) vector has been successfully used for the functional analysis of genes like the class C MADS-box gene, GsAG1, which is associated with the morphological change from single to double flowers (Nakatsuka et al. 2015), and W14/15, which is associated with winter hardiness required for overwintering (Hikage et al. 2016). Furthermore, infection with the ALSV vector harboring FLOWERING LOCUS T (FT) has been shown to accelerate flowering in gentian and lisianthus plants (Fekih et al. 2016). These results indicate that the ALSV vector is a powerful tool for the study of basic gene function and/or promoting flowering. The ALSV vector is not integrated into the plant host genome. Notably, the virus is rarely transmitted to the offspring via pollen. Furthermore, an elimination technique such as heat treatment can also be used to remove ALSV. These facts mean that the use of ALSV may reduce gentian generation time and accelerate gentian breeding while circumventing the GMO regulations associated with New Plant Breeding Techniques (NPBT).
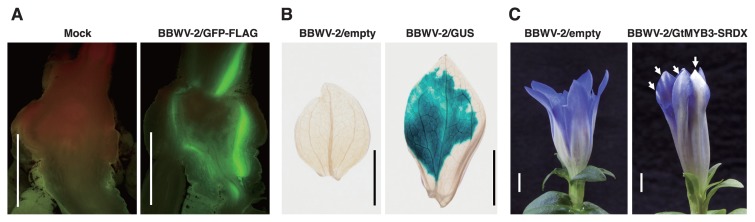
Recently, we developed a new viral gene-expression system in gentian using the Broad bean wilt virus 2 (BBWV-2) (Tasaki et al. 2016b) that included the two infectious cDNAs, pBBR1 and pBBR2, that harbor the full-length RNA1 and RNA2 sequences of the BBWV-2 Ty isolate, respectively (Atsumi et al. 2013). This virus did not induce disease symptoms in gentian under our experimental conditions. Notably, BBWV inoculation is easily performed by rub-inoculation of gentian leaves using crude sap of upper symptomatic leaves of N. benthamiana infected with viral constructs by co-agroinfiltration. This is in contrast with the particle bombardment that is required for ALSV inoculation of gentian. Examples of exogenous gene expression in gentian using the BBWV-2 vector are shown in Fig. 4. This vector can systemically infect flower, crown, root, and overwinter buds for considerable periods, leading to the expression of exogenous GFP-3FLAG (819 nucleotides) in these organs. The BBWV-2 vector can also express long genes such as the 1,809- nucleotides long GUS gene in inoculated leaves. A chimeric repressor (GtMYB3-SRDX, 960 nucleotides) can induce a partial reduction in pigmentation in flowers at 70 days post inoculation, indicating that endogenous genes can also be functionally analyzed in a relatively short period of time. The BBWV-2 vector is the first example of a viral vector derived from the genus Fabavirus in the family Secoviridae. This vector is, therefore, likely to also be useful in other plants that are susceptible to BBWV.
Fig. 4.
Use of the BBWV-2 vector for gene expression and functional analysis in gentian. (A) Fluorescence produced by the green fluorescent protein (GFP-3FLAG, 819 nucleotides) in crowns at 162 days post inoculation (dpi). (B) Detection of β-glucuronidase (GUS, 1,809 nucleotides) activity by histochemical staining with X-Gluc in inoculated leaves at 17 dpi. (C) Reduced pigmentation in flower petals with a chimeric repressor responsible for flower pigmentation (GtMYB3-SRDX, 960 nucleotides) at 70 dpi. White arrows indicate the partial change of petal pigmentation from blue to white. Scale bar = 5 mm.
Various issues associated with gentian cultivation
Important issues affecting the gentian life cycle
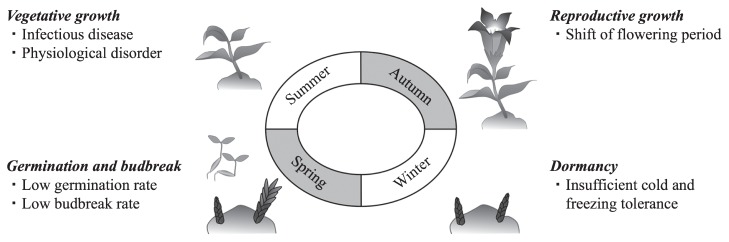
Japanese gentians are ornamental perennials that bloom from early summer to late autumn in Japan. After flowering, gentians produce overwintering buds (OWBs) that are dormant during winter and begin to grow when spring comes. While replicating the cycle of these processes is necessary for stable cultivation and production, there are various difficulties associated with doing so as summarized in Fig. 5. One of the fundamental issues in generating stable cultivation and production practices is that gentian cultivation to date has been exclusively performed according to the experiences of farmers, with little scientific information available regarding the various physiological and ecological aspects. To overcome this, investigation of causal mechanisms from an academic point of view is needed. For example, as discussed above, most Japanese cultivars are F1 hybrids, so poor seed germination rates are a major problem for gentian production. Comparison of metabolite levels among cultivars with different seed germination rates suggested that aberrant accumulation of specific amino acids and decreases in energy metabolites repressed seed germination (Takahashi et al. 2016). These identified metabolites can be used to screen potential new cultivars in advance for poor germination ability. Furthermore, dormancy during overwintering is required to survive until spring as OWBs with insufficient dormancy levels did not have sufficient freezing tolerance, leading to plant death. Takahashi et al. (2014) revealed that the metabolite profile of gentians was drastically altered during dormancy and that gentiobiose, which is a major gentio-oligosaccharide in gentian, served as a dormancy regulator. A more recent report showed that gentian OWBs abundantly expressed the highly polymorphic gene W14/15 and that a particular W14/15 genotype was strongly associated with the winter survival rate of OWBs (Hikage et al. 2016). As little is known about the mechanisms underlying bud dormancy in perennials, and particularly in gentians, these findings will help to shed light on the regulation of dormancy in the OWBs. Although methods of improving the rate of seed germination and overwintering have not yet been established, understanding the regulatory mechanisms underlying the gentian life cycle should provide clues for stable gentian cultivation.
Fig. 5.
Various problems encountered in gentian production throughout the year. These include issues encountered at various growth stages. More scientific data are required to assure stable continuous production of Japanese gentians.
Problems associated with flowering time
The highest demand for gentian flowers occurs in mid-August and late September in Japan, leading to the development and cultivation of cultivars that bloom at these times. However, the gentian flowering period varies significantly according to the weather (annual variation and likely temperature and sunshine conditions), with such year-to-year shifts in the flowering period representing a serious problem in gentian production. Until now, very little research has been done on the flowering characteristics of gentians and the flowering mechanisms remain relatively uncharacterized. Previous studies using Arabidopsis thaliana have reported that FLOWERING LOCUS T (FT) promotes flowering initiation and that TERMINAL FLOWER1 (TFL1) represses it (reviewed in Wickland and Hanzawa 2015). Imamura et al. (2011) first reported that gentian orthologues of these genes, GtFT1 and GtTFL1, are key factors for regulating the flowering period in gentians. They showed that overexpression of GtFT1 or suppression of GtTFL1 promoted flowering in gentian plantlets, suggesting that GtFT1 and GtTFL1 acted as an activator and repressor, respectively, of flowering, in consistent with the pattern observed in Arabidopsis (Ratcliffe et al. 1998, Shannon and Meeks-Wagner 1991). In leaves, GtFT1 expression increased from the vegetative to the reproductive stage in both early- and late-flowering lines, whereas GtTFL1 was highly expressed only in the late-flowering line. These results suggest that the timing and intensity of GtFT1 and GtTFL1 expression contribute to differences in the flowering period in gentians. It remains unclear, however, which environmental factors regulate the expression of GtFT1 and GtTFL1 and induce flowering. Although Japanese gentians have been predominantly used for grave flowers in Japan to date, new cultivars with novel flower colors and shapes are being developed as previously described. For example, a new red-flowered cultivar ‘Koibeni’ has been developed by breeding using foreign Gentiana species (Fig. 1). However, this cultivar flowers from November to December under normal Japanese field conditions. Characterization of the mechanism(s) regulating flowering time in foreign gentian species and the development of DNA markers for flowering time are, therefore, required. The ability to control gentian flowering time will allow for further diversification of their intended use and expand the worldwide market for gentians.
Infectious diseases and physiological disorders in field cultivated gentians
Japanese gentians survive several years of growth and dormancy cycling, allowing farmers to usually harvest flowers for more than five years. Plant death and morphological aberrations caused by pathogens or physiological disorders can cause significant economic damage to gentian producers. It is, therefore, important to identify the pathogens or environmental factors responsible for these diseases. In gentians, pest control against common diseases such as leaf blight, leaf spot, and witch’s broom is well established (Yoshiike 2003), but unexplained diseases are discovered occasionally. A yellowed and dwarf-like phenotype, known as the gentian kobu-sho syndrome, is a disease that results in leaf yellowing and shortening of stem internodes (Takahashi et al. 2009). The cause of this disease was unknown for a long time until the causal virus was identified recently using double stranded-RNA isolation, exhaustive amplification, cloning, and sequence (DECS) analysis (Kobayashi et al. 2013). DECS analysis, a powerful tool for comprehensive detection of double stranded-RNA viruses, also detected other novel viral pathogens in gentians (Atsumi et al. 2015). Generally, viruses cannot be easily detected and plant viral diseases are particularly difficult to distinguish from the symptoms of nutritional deficiency or other abiotic stresses. Therefore, identification of the causal virus and early removal of infected plants are important to prevent the disease from spreading. Additionally, the identification of viral vectors and the pathogenic mechanism of causal viruses will contribute to the development of removal methods and the breeding of improved disease-resistant cultivars. This may also lead to the development of further viral vectors useful for basic research as discussed above.
If the causal pathogens are not found in a diseased plant, there is a high possibility that the disease is a type of physiological disorder caused by unfavorable environmental stresses. For example, it is known that various stresses such as nutrient deficiency, high or low temperature, excess light, and drought induce morphological changes in plants (Ashraf and Harris 2013, Waraich et al. 2012). Although these stresses induce characteristic phenotypes in plants, detailed physiological analyses are necessary for absolute diagnosis. Recently, systems-biology approaches such as transcriptomics, proteomics and metabolomics have been developed and used to reveal global biological responses in plants exposed to abiotic stresses (reviewed in Cramer et al. 2011). The information in these studies outlines specific components that respond to each stress; these components will be helpful in identifying the underlying causes of physiological disorders. In gentians, metabolome analysis identified the cause of gentian spotted bleaching disease (GSBD), a novel disease of unknown etiology showing leaf etiolation, necrotic leaf spots, and a delay in flowering (Takahashi et al. 2017). In the leaves of GSBD-affected plants, fluctuations of intermediate metabolites in hypoxia-responsive metabolic pathways, including the Calvin cycle, sucrose cleavage, and alanine synthesis pathways, were observed. Waterlogging stress induced similar symptoms and metabolic changes as those observed in GSBD-affected leaves, suggesting that root hypoxia induced by waterlogged soil conditions is the main cause of GSBD. Notably, traditional methods of characterizing unexplained diseases are very time-consuming. As there is often a considerable lapse between infection and the appearance of disease symptoms in gentians, infections can become serious before a disease management strategy can be developed and implemented. Conversely, DECS and metabolome analyses can shorten the time required to identify the cause of new diseases and require only small amounts of diseased plant tissue. The introduction of such advanced technologies in gentian cultivation would accelerate the resolution of disease-related problems and facilitate cultivation management.
Conclusions and future prospects
As we have discussed, several new technologies have been recently developed and applied to gentian breeding. Using these techniques, new cultivar(s), including a cultivar with more vividly red flowers, or those with double flowers, are expected to be released in the coming years. Along with cultivar development, cultivation practices are an important aspect of gentian production. Given that molecular biology-related tools can be used to solve a number of problems associated with gentian cultivation, we intend to continue to use and develop these techniques. In this review, we have not discussed transgenic strategies to dramatically alter traits such as flower-color, style, flowering time, and disease resistance. While this is also an attractive approach, it is not likely to be widely used in the near future because of the restraints associated with GMO usage in Japan. Recent advances in NPBT (Lusser and Davies 2013, Schaart et al. 2016) represent breakthrough technologies in plant breeding; we are, therefore, beginning to develop some of these techniques in gentian. For example, genome editing using the CRISPR/Cas9 system has been shown to work well in gentian (Fig. 6) and will shortly be available for the modification of important traits. The use of viral vectors will also continue, particularly for the reduction of variation in flowering time and to accelerate plant breeding. Application of these NPBT approaches will depend on alterations of the existing biosafety laws in Japan. Currently, it is difficult to release plants modified in such ways into the field without strict biosafety assessments, such as those required for GMOs. If NPBT-derived products are certified as non-GMO, however, gentian breeding practices will be significantly more advanced than previously. In preparation for such a time, further basic research should be conducted for the practical development and assessment of NPBT.
Fig. 6.
Application of the CRISPR/Cas9 system for genome editing of gentian. Phytoene desaturase (PDS) was used as a target gene by the CRISPR/Cas9 system. A single gRNA for the PDS gene from gentian and Cas9 from Streptococcus pyogenes were co-expressed by Agrobacterium tumefaciens-mediated transformation. (A) Regenerated white shoots from infected leaf sections. (B) A white shoot transferred to rooting medium. (C) PDS-mutated gentian. Genome editing was confirmed in this plant by sequencing analysis. Scale bar = 1 cm.
Acknowledgments
We thank Drs. Tsutsumi Ken-ichi (Iwate University, Japan), Takashi Hikage (Hachimantai City Floricultural Research and Development Center), and Mr. Hiromi Kawamura (Iwate Agricultural Research Center) for providing us with the gentian plant materials and photographs of gentians. We also thank members of the Department of Horticultural Sciences, Iwate Biotechnology Research Center, Iwate Agricultural Research Center, and Hachimantai City Floricultural Research and Development Center for technical assistance and helpful discussions. We also thank Drs. Takashi Shimokawa and Yoshiya Furusawa associated with the heavy ion research project at NIRS-HIMAC. The Cas9 gene was kindly provided by Prof. Holger Puchta, Botanical Institute II, Karlsruhe Institute of Technology, Karlsruhe, Germany. The work described here was financially supported by Iwate Prefecture and also in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science under the programs “Breeding of floricultural plants adapted for high practical needs and development of low cost cultivation technique” and “Science and technology research promotion program for agriculture, forestry, fisheries and food industry” for the Ministry of Agriculture, Forestry, and Fisheries, Japan.
Literature Cited
- Ashraf, M. and Harris, P.J.C. (2013) Photosynthesis under stressful environments: An overview. Photosynthetica 51: 163–190. [Google Scholar]
- Atsumi, G., Tomita, R., Kobayashi, K. and Sekine, K.T. (2013) Establishment of an agroinoculation system for broad bean wilt virus 2. Arch. Virol. 158: 1549–1554. [DOI] [PubMed] [Google Scholar]
- Atsumi, G., Tomita, R., Yamashita, T. and Sekine, K.T. (2015) A novel virus transmitted through pollination causes ring-spot disease on gentian (Gentiana triflora) ovaries. J. Gen. Virol. 96: 431–439. [DOI] [PubMed] [Google Scholar]
- Cramer, G.R., Urano, K., Delrot, S., Pezzotti, M. and Shinozaki, K. (2011) Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biol. 11: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, P.J. (2010) Plant Hormones Biosynthesis, Signal Transduction, Action! Springer, Netherlands. [Google Scholar]
- Di Stilio, V.S., Kumar, R.A., Oddone, A.M., Tolkin, T.R., Salles, P. and McCarty, K. (2010) Virus-induced gene silencing as a tool for comparative functional studies in Thalictrum. PLoS ONE 5: e12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi, H., Takahashi, R., Hikage, T. and Takahata, Y. (2010) Embryogenesis and doubled haploid production from anther culture in gentian (Gentiana triflora). Plant Cell Tissue Organ Cult. 102: 27–33. [Google Scholar]
- Doi, H., Yokoi, S., Hikage, T., Nishihara, M., Tsutsumi, K.I. and Takahata, Y. (2011) Gynogenesis in gentians (Gentiana triflora, G. scabra): production of haploids and doubled haploids. Plant Cell Rep. 30: 1099–1106. [DOI] [PubMed] [Google Scholar]
- Doi, H., Hoshi, N., Yamada, E., Yokoi, S., Nishihara, M., Hikage, T. and Takahata, Y. (2013) Efficient haploid and doubled haploid production from unfertilized ovule culture of gentians (Gentiana spp.). Breed. Sci. 63: 400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi, H. and Takahata, Y. (2015) Haploid and doubled haploid plant production in gentian (Gentiana spp.). In: Rybczyński, J.J., Michael R. and Mikuła D.A. (eds.) The Gentianaceae—Volume 2: Biotechnology and Applications, Springer, pp. 187–197. [Google Scholar]
- Fekih, R., Yamagishi, N. and Yoshikawa, N. (2016) Apple latent spherical virus vector-induced flowering for shortening the juvenile phase in Japanese gentian and lisianthus plants. Planta 244: 203–214. [DOI] [PubMed] [Google Scholar]
- Gao, L., Yang, H., Liu, H., Yang, J. and Hu, Y. (2016) Extensive transcriptome changes underlying the flower color intensity variation in Paeonia ostii. Front. Plant Sci. 6: 1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Lopez, O. and Casquero, P.A. (2014) Effects of GA3 pre-germinative treatment on Gentiana lutea L. var. aurantiaca germination and seedlings morphology. Sci. World J. 2014: 751279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, T., Kondo, T., Tamura, H., Imagawa, H., Iino, A. and Takeda, K. (1982) Structure of gentiodelphin, an acylated anthocyanin isolated from Gentiana makinoi, that is stable in dilute aqueous solution. Tetrahedron Lett. 23: 3695–3698. [Google Scholar]
- Gould, B. and Kramer, E.M. (2007) Virus-induced gene silencing as a tool for functional analyses in the emerging model plant Aquilegia (columbine, Ranunculaceae). Plant Methods 3: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, R.J. (2012) Molecular markers in plants. Wiley-Blackwell, Hoboken, NJ. [Google Scholar]
- Hikage, T., Kogusuri, K., Tanaka-Saito, C., Watanabe, S., Chiba, S., Kume, K., Doi, H., Saitoh, Y., Takahata, Y. and Tsutsumi, K. (2011) W14/15 esterase gene haplotype can be a genetic landmark of cultivars and species of the genus Gentiana L. Mol. Genet. Genomics 285: 47–56. [DOI] [PubMed] [Google Scholar]
- Hikage, T. (2016) Gentian. In: Shibata, M. (ed.) Hana no Hinsyukairyo no Nihonshi. Yushokan, Tokyo, pp. 109–126. [Google Scholar]
- Hikage, T., Yamagishi, N., Takahashi, Y., Saitoh, Y., Yoshikawa, N. and Tsutsumi, K. (2016) Allelic variants of the esterase gene W14/15 differentially regulate overwinter survival in perennial gentian (Gentiana L.). Mol. Genet. Genomics 291: 989–997. [DOI] [PubMed] [Google Scholar]
- Ho, T.N. and Liu, S.W. (2001) A worldwide monograph of Gentiana. Science Press, Beijing, China. [Google Scholar]
- Hosokawa, K., Fukushi, E., Kawabata, J., Fujii, C., Ito, T. and Yamamura, S. (1997) Seven acylated anthocyanins in blue flowers of Gentiana. Phytochemistry 45: 167–171. [DOI] [PubMed] [Google Scholar]
- Imamura, T., Nakatsuka, T., Higuchi, A., Nishihara, M. and Takahashi, H. (2011) The gentian orthologs of the FT/TFL1 gene family control floral initiation in Gentiana. Plant Cell Physiol. 52: 1031–1041. [DOI] [PubMed] [Google Scholar]
- Imamura, T., Higuchi, A. and Takahashi, H. (2013) Dehydrins are highly expressed in overwintering buds and enhance drought and freezing tolerance in Gentiana triflora. Plant Sci. 213: 55–66. [DOI] [PubMed] [Google Scholar]
- Jomori, H., Nakamura, I., Kameya, N. and Takahata, Y. (2000) RAPD analysis of Gentiana scabra and G. triflora and detection of species-specific SCAR markers. Breed. Res. 2: 81–87. [Google Scholar]
- Kakizaki, Y., Nakatsuka, T., Kawamura, H., Abe, J., Abe, Y., Yamamura, S. and Nishihara, M. (2009) Development of codominant DNA marker distinguishing pink from blue flowers in Gentiana scabra. Breed. Res. 11: 9–14. [Google Scholar]
- Kazama, Y., Saito, H., Miyagai, M., Takehisa, H., Ichida, H., Miyazawa, Y., Mishiba, K., Kanaya, T., Suzuki, K., Bae, C.H. et al. (2008a) Effect of heavy ion-beam irradiation of plant growth and mutation induction in Nicotiana tabacum. Plant Biotechnol. 25: 105–111. [Google Scholar]
- Kazama, Y., Saito, H., Yamamoto, Y., Hayashi, Y., Ichida, H., Ryuto, H., Fukunishi, N. and Abe, T. (2008b) LET-dependent effects of heavy-ion beam irradiation in Arabidopsis thaliana. Plant Biotechnol. 25: 113–117. [Google Scholar]
- Kobayashi, K., Atsumi, G., Iwadate, Y., Tomita, R., Chiba, K.-I., Akasaka, S., Nishihara, M., Takahashi, H., Yamaoka, N., Nishiguchi, M. et al. (2013) Gentian Kobu-sho-associated virus: a tentative, novel double-stranded RNA virus that is relevant to gentian Kobu-sho syndrome. J. Gen. Plant Pathol. 79: 56–63. [Google Scholar]
- Kodama, K. (2006) IV-9. Gentian. In: Japanese Society of Hortuculture Science (ed.) Horticulture in Japan 2006. Shoukadoh Publishing, Kyoto, pp. 248–253. [Google Scholar]
- Koseki, M., Goto, K., Masuta, C. and Kanazawa, A. (2005) The star-type color pattern in Petunia hybrida ‘red Star’ flowers is induced by sequence-specific degradation of chalcone synthase RNA. Plant Cell Physiol. 46: 1879–1883. [DOI] [PubMed] [Google Scholar]
- Kuckuck, H., Kobabe, G. and Wenzel, G. (1991) Fundamentals of plant breeding. Springer-Verlag, Berlin, Heidelberg. [Google Scholar]
- Lusser, M. and Davies, H.V. (2013) Comparative regulatory approaches for groups of new plant breeding techniques. Nat. Biotechnol. 30: 437–446. [DOI] [PubMed] [Google Scholar]
- Maurya, J.P. and Bhalerao, R.P. (2017) Photoperiod- and temperature-mediated control of growth cessation and dormancy in trees: a molecular perspective. Ann. Bot. 120: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishiba, K., Nishihara, M., Abe, Y., Nakatsuka, T., Kawamura, H., Kodama, K., Takesawa, T., Abe, J. and Yamamura, S. (2006) Production of dwarf potted gentian using wild-type Agrobacteriun rhizogenes. Plant Biotechnol. 23: 33–38. [Google Scholar]
- Mishiba, K., Yamane, K., Nakatsuka, T., Nakano, Y., Yamamura, S., Abe, J., Kawamura, H., Takahata, Y. and Nishihara, M. (2009) Genetic relationships in the genus Gentiana based on chloroplast DNA sequence data and nuclear DNA content. Breed. Sci. 59: 119–127. [Google Scholar]
- Morgan, E.R. (2004) Use of in ovulo embryo culture to produce interspecific hybrids between Gentiana triflora and Gentiana lutea. N. Z. J. Crop Hortic. Sci. 32: 343–347. [Google Scholar]
- Nakatsuka, T., Saito, M., Sato-Ushiku, Y., Yamada, E., Nakasato, T., Hoshi, N., Fujiwara, K., Hikage, T. and Nishihara, M. (2011) Development of DNA markers that discriminate between white- and blue-flowers in Japanese gentian plants. Euphytica 184: 335–344. [Google Scholar]
- Nakatsuka, T., Yamada, E., Saito, M., Hikage, T., Ushiku, Y. and Nishihara, M. (2012) Construction of the first genetic linkage map of Japanese gentian (Gentianaceae). BMC Genomics 13: 672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka, T., Saito, M., Yamada, E., Fujita, K., Yamagishi, N., Yoshikawa, N. and Nishihara, M. (2015) Isolation and characterization of the C-class MADS-box gene involved in the formation of double flowers in Japanese gentian. BMC Plant Biol. 15: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara, M., Hikage, T., Yamada, E. and Nakatsuka, T. (2011) A single-base substitution suppresses flower color mutation caused by a novel miniature inverted-repeat transposable element in gentian. Mol. Genet. Genomics 286: 371–382. [DOI] [PubMed] [Google Scholar]
- Nishihara, M., Mishiba, K., Imamura, T., Takahashi, H. and Nakatsuka, T. (2015) Molecular breeding of Japanese gentians—Applications of genetic transformation, metabolome analyses, and genetic markers. In: Rybczyński, J.J., Michael R. and Mikuła D.A. (eds.) The Gentianaceae—Volume 2: Biotechnology and Applications, Springer, pp. 239–265. [Google Scholar]
- Okamura, M., Yasuno, N., Ohtsuka, M., Tanaka, A., Shikazono, N. and Hase, Y. (2003) Wide variety of flower-color and -shape mutants regenerated from leaf cultures irradiated with ion beams. Nucl. Instrum. Methods Phys. Res. B 206: 574–578. [Google Scholar]
- Pathirana, R., Frew, T., Hedderley, D., Timmerman-Vaughan, G. and Morgan, E. (2011) Haploid and doubled haploid plants from developing male and female gametes of Gentiana triflora. Plant Cell Rep. 30: 1055–1065. [DOI] [PubMed] [Google Scholar]
- Ratcliffe, O.J., Amaya, I., Vincent, C.A., Rothstein, S., Carpenter, R., Coen, E.S. and Bradley, D.J. (1998) A common mechanism controls the life cycle and architecture of plants. Development 125: 1609–1615. [DOI] [PubMed] [Google Scholar]
- Schaart, J.G., van de Wiel, C.C., Lotz, L.A. and Smulders, M.J. (2016) Opportunities for products of new plant breeding techniques. Trends Plant Sci. 21: 438–449. [DOI] [PubMed] [Google Scholar]
- Scheben, A., Batley, J. and Edwards, D. (2017) Genotyping-by-sequencing approaches to characterize crop genomes: choosing the right tool for the right application. Plant Biotechnol. J. 15: 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon, S. and Meeks-Wagner, D.R. (1991) A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell 3: 877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada, N., Nakatsuka, T., Nakano, Y., Kakizaki, Y., Abe, Y., Hikage, T. and Nishihara, M. (2009) Identification of gentian cultivars using SCAR markers based on intron-length polymorphisms of flavonoid biosynthetic genes. Sci. Hortic. 119: 292–296. [Google Scholar]
- Song, Y.H., Ito, S. and Imaizumi, T. (2013) Flowering time regulation: photoperiod- and temperature-sensing in leaves. Trends Plant Sci. 18: 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, H., Munemura, I., Nakatsuka, T., Nishihara, M. and Uchimiya, H. (2009) Metabolite profiling by capillary electrophoresis —mass spectrometry reveals aberrant putrescine accumulation associated with idiopathic symptoms of gentian plants. J. Hortic. Sci. Biotechnol. 84: 312–318. [Google Scholar]
- Takahashi, H., Imamura, T., Konno, N., Takeda, T., Fujita, K., Konishi, T., Nishihara, M. and Uchimiya, H. (2014) The gentio-oligosaccharide gentiobiose functions in the modulation of bud dormancy in the herbaceous perennial Gentiana. Plant Cell 26: 3949–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, H., Fujita, K., Yoshida, C. and Nishihara, M. (2016) Metabolite profiling reveals the involvement of aberrant metabolic changes in Gentiana triflora seed showing poor germination. J. Hortic. Sci. Biotechnol. 91: 148–155. [Google Scholar]
- Takahashi, H., Abe, H., Fujita, K. and Sekine, K.-T. (2017) The use of metabolome analysis to identify the cause of an unexplained disease of Japanese gentians (Gentiana triflora). Metabolomics 13: 51. [Google Scholar]
- Takamura, Y., Hikage, T., Hatakeyama, K. and Takahata, Y. (2016) Application of unfertilized ovule culture in doubled haploid production of foreign gentians. Breed. Res. 18 (Suppl. 1): 232. [Google Scholar]
- Tasaki, K., Terada, H., Masuta, C. and Yamagishi, M. (2016a) Virusinduced gene silencing (VIGS) in Lilium leichtlinii using the Cucumber mosaic virus vector. Plant Biotechnol. 33: 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki, K., Atsumi, G., Nishihara, M. and Sekine, K.T. (2016b) Development of a Broad bean wilt virus 2-based expression vector for gentian. Sci. Hortic. 201: 279–286. [Google Scholar]
- Tasaki, K., Higuchi, A., Watanabe, A., Kurokawa, Y., Washiashi, R., Takahashi, H., Sasaki, N. and Nishihara, M. (2017a) Analysis of factors responsible for flower color intensity in Japanese gentian. Abstr. Japan. Soc. Plant. Cell Mol. Biol. Annual Meet. 101. [Google Scholar]
- Tasaki, K., Higuchi, A., Fujita, K., Watanabe, A., Sasaki, N., Fujiwara, K., Abe, H., Naito, Z., Takahashi, R., Hikage, T. et al. (2017b) Development of molecular markers for breeding of double flowers in Japanese gentian. Mol. Breed. 37: 33. [Google Scholar]
- Tsutsumi, K. and Hikage, T. (2014) Genes expressed in the overwinter buds of gentian (Gentiana spp.): Application to taxonomic, phylogenetic, and phylogeographical analyses. In: Rybczyński, J.J., Michael R. and Mikuła D.A. (eds.) The Gentianaceae—Volume 1: Biotechnology and Applications, Springer, pp. 251–265. [Google Scholar]
- Ushiku, Y., Shimada, N., Saito, M., Yamada, E., Hikage, T., Nakatsuka, T. and Nishihara, M. (2011) Development of simple sequence repeat markers for identification of Japanese gentian cultivars. J. Japan. Soc. Hort. Sci. 80: 475–485. [Google Scholar]
- Waraich, E.A., Ahmad, R., Halim, A. and Aziz, T. (2012) Alleviation of temperature stress by nutrient management in crop plants: a review. J. Soil Sci. Plant Nutr. 12: 221–244. [Google Scholar]
- Wege, S., Scholz, A., Gleissberg, S. and Becker, A. (2007) Highly efficient virus-induced gene silencing (VIGS) in California poppy (Eschscholzia californica): an evaluation of VIGS as a strategy to obtain functional data from non-model plants. Ann. Bot. 100: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickland, D.P. and Hanzawa, Y. (2015) The FLOWERING LOCUS T/TERMINAL FLOWER 1 gene family: functional evolution and molecular mechanisms. Mol. Plant 8: 983–997. [DOI] [PubMed] [Google Scholar]
- Yamagishi, N., Kume, K., Hikage, T., Takahashi, Y., Bidadi, H., Wakameda, K., Saitoh, Y., Yoshikawa, N. and Tsutsumi, K. (2016) Identification and functional analysis of SVP ortholog in herbaceous perennial plant Gentiana triflora: Implication for its multifunctional roles. Plant Sci. 248: 1–7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, H., Shimizu, A., Hase, Y., Degi, K., Tanaka, A. and Morishita, T. (2008) Mutation induction with ion beam irradiation of lateral buds of chrysanthemum and analysis of chimeric structure of induced mutants. Euphytica 165: 97–103. [Google Scholar]
- Yamaguchi, H., Hase, Y., Tanaka, A., Shikazono, N., Degi, K., Shimizu, A. and Morishita, T. (2009) Mutagenic effects of ion beam irradiation on rice. Breed. Sci. 59: 169–177. [Google Scholar]
- Yamaguchi, H., Shimizu, A., Hase, Y., Tanaka, A., Shikazono, N., Degi, K. and Morishita, T. (2010) Effects of ion beam irradiation on mutation induction and nuclear DNA content in chrysanthemum. Breed. Sci. 60: 398–404. [Google Scholar]
- Yoshiike, T. (1992) Rindou (Gentiana). Seibundou Shinkousha, Tokyo, Japan. [Google Scholar]
- Yoshiike, T. (2003) Seisankatei to Gijyutu (Rindou). In: Nougyou Gijyutu Taikei Kaki: Vol. 9: Nousangyoson Bunka Kyokai, Tokyo, Japan, pp. 521–530. [Google Scholar]