Abstract
Flower color is the most important trait in the breeding of ornamental plants. In the floriculture industry, however, bluish colored flowers of desirable plants have proved difficult to breed. Many ornamental plants with a high production volume, such as rose and chrysanthemum, lack the key genes for producing the blue delphinidin pigment or do not have an intracellular environment suitable for developing blue color. Recently, it has become possible to incorporate a blue flower color trait through progress in molecular biological analysis of pigment biosynthesis genes and genetic engineering. For example, introduction of the F3′5′H gene encoding flavonoid 3′,5′-hydroxylase can produce delphinidin in various flowers such as roses and carnations, turning the flower color purple or violet. Furthermore, the world’s first blue chrysanthemum was recently produced by introducing the A3′5′GT gene encoding anthocyanin 3′,5′-O-glucosyltransferase, in addition to F3′5′H, into the host plant. The B-ring glucosylated delphinidin-based anthocyanin that is synthesized by the two transgenes develops blue coloration by co-pigmentation with colorless flavone glycosides naturally present in the ray floret of chrysanthemum. This review focuses on the biotechnological efforts to develop blue flowers, and describes future prospects for blue flower breeding and commercialization.
Keywords: anthocyanins, blue flower, chrysanthemum, co-pigmentation, delphinidin, genetic engineering, ornamental plant
Introduction
Flowers come in various colors, including bi-colored or multi-colored petals with patterns. Then again, the flower color of a single plant species is limited in many cases. In the flower industry, flower color is one of the key traits that is targeted to breed successful ornamental flowers. If there are no closely related wild species with the desired color, however, it is difficult to create flowers with that color by conventional breeding. Wild species with bluish flower colors, such as purple, violet and blue, account for about 15%–20% of all flower colors (Warren and Mackenzie 2001, Weevers 1952). Rose, chrysanthemum, lily, carnation and gerbera are the major ornamental plants grown for cut flowers in global flower markets. Until recently, however, none of these plants had bluish colored flowers because they have no related wild species with these colors that can be used for crossbreeding.
The blue coloration in plants is produced by photonic structures (Jacobs et al. 2016, Moyroud et al. 2017, Vignolini et al. 2012) and pigments. Anthocyanins are pigments that are involved in blue color development in flower organs such as petals and tepals. The anthocyanins responsible for blue color development are mostly delphinidin derivatives, while cyanidin derivatives are rare. The biosynthetic pathway that produces the chromophore anthocyanidin has been well elucidated, and the enzymatic genes responsible for its biosynthesis have been recently reviewed (Sasaki and Nakayama 2015, Zhang et al. 2014). Many studies have also been reported on subsequent modifications of anthocyanidin, such as methylation, glycosylation and acylation. Some mechanisms of blue color development involving anthocyanins have been elucidated. For example, the intramolecular association of polyacylated anthocyanin with aromatic organic acid, intermolecular association of anthocyanins with flavones, flavonols, or aromatic organic acid derivatives, or metalloanthocyanin complex formation with flavonoids and metal ions is necessary to stabilize blue coloration under suitable conditions of vacuolar pH (Yoshida et al. 2009).
In various flowers, biotechnology has been used for altering flower color and shape, increasing disease resistance and improving vase life (Azadi et al. 2016, Chandler and Sanchez 2012). Thirty years ago, Meyer et al. (1987) reported modification of petunia flower color using genetic engineering technology for the first time. Subsequently, various genes related to blue color development, as well as anthocyanin biosynthesis, have been isolated. In particular, isolation of F3′5′H encoding flavonoid 3′,5′-hydroxylase, a key gene for synthesis of the anthocyanidin delphinidin, which leads to blue coloration in flowers, was reported by Holton et al. (1993). Such genes were expected to lead to blue coloration in various flowers, such as roses and chrysanthemums; however, it has not proved so easy to achieve blue flower coloration.
In this review, I summarize the status of research and development aimed at using genetic engineering technologies to create blue flowers; in particular, I focus on flower color modification of chrysanthemum, in which a truly blue flower color was recently realized for the first time. I also discuss future prospects for molecular breeding and practical application of blue flower coloration.
Vacuolar pH determinant and metal ion transporter involved in blue color development
Delphinidin-type anthocyanidins include delphinidin, petunidin and malvidin derivatives in which the 3′ and 5′ positions of the B-ring are hydroxylated and/or methoxylated. Flowers containing these anthocyanins do not always produce a blue color, however, but instead are often purple, mauve, or pink. In such flowers, control of elevation of vacuolar pH and/or coexistence with metal ions is considered effective for blue coloration. In this section, I describe recent studies on vacuolar pH determinants and metal ion transporters involved in the color development of blue flowers.
Manipulation of genes related to vacuolar pH
In hydrangea, which develops blue coloration under relatively low pH conditions, pH 4 to 5 is suitable for blue color development due to the interaction of delphinidin 3-glucoside, 5-caffeoylquinic acid and Al3+, while a more acidic pH of 3 to 4 leads to red coloration (Yoshida et al. 2003). In petunia, methylated anthocyanins, and petunidinand malvidin-based anthocyanins accumulate in the corolla. Even for petunia flowers with the same anthocyanin composition, the color can differ greatly depending on the pH of the corolla.
In petunia, the PH1–PH7 gene loci are reported to be involved in determining the pH of petals (de Vlaming et al. 1983, van Houwelingen et al. 1998). PH5 encodes a H+ P3A-ATPase proton pump present in the vacuolar membrane. Mutation of PH5 causes the increased pH in the vacuole, resulting in the petals changing coloration from purplish red to bluish violet (Verweij et al. 2008). In addition, the H+ P3A-ATPase encoded by PH5 physically binds to the P3B-ATPase encoded by PH1, resulting in an increase in proton transport activity and a more acidic vacuolar pH (Faraco et al. 2014). In addition, the expression of PH5 is directly regulated by PH3 and PH4 transcription factors. The PH6 gene, also termed ANTHOCYANIN1 ( AN1), encodes a bHLH protein that interacts with PH3, PH4 and WD40 protein AN11. When a transposon is inserted into AN1, anthocyanin is not synthesized and the pH of petals rises (Spelt et al. 2002).
Although anthocyanins become unstable, flowers can develop a blue color under alkaline conditions. For example, if polyacylation with aromatic organic acids occurs, anthocyanins remain stable even under weakly alkaline conditions. In the corolla of morning glory, a rise in pH from 6.6 to 7.7 leads to blue coloration due to the presence of polyacylated peonidin glycoside (Yoshida et al. 1995). The genes involved in producing weakly alkaline vacuoles at the time of flowering are InNHX1 and InNHX2, which encode proteins responsible for transporting K+/Na+ into the vacuoles (Fukuda-Tanaka et al. 2000, Ohnishi et al. 2005, Yamaguchi et al. 2001).
Even if delphinidin-type anthocyanin accumulates, rose petals tend to express redness due to high the acidity of their vacuoles (Katsumoto et al. 2007). To express blue coloration, it is important to introduce genes that can create a weakly acidic pH of about 5.6 to 6.2, or to use strains or cultivars that naturally exhibit a relatively higher pH. Cyclamen spp. have several natural flower colors such as white, light yellow, pink, red, magenta, and purple. The anthocyanins responsible for cyclamen flower color include anthocyanidin 3,5-diglucoside; a peonidin-based anthocyanin that is 3′-methylated cyanidin in the reddish flower; and a malvidin-based anthocyanin in the magenta flower (Hase et al. 2012). In 2012, violet cyclamens were developed by Hokko Chemical Industry Co., Ltd., and are currently being sold as “Serenadia™” by Suntory Flowers Ltd. Violet cyclamens were produced through the mutation of cultured cells (Terakawa 2012). Takamura et al. (2015) reported that the major anthocyanin responsible for the violet coloration in cyclamen is malvidin 3,5-diglucoside, and that a change toward a blue color can be achieved by a recessive mutation that increases the pH in petal cells. Multi-petaled cyclamens produced by suppression of the AGAMOUS gene using Chimeric REpressor gene-Silencing Technology (CRES-T) have also been reported (Tanaka et al. 2013), and the development of blue cyclamens with multi-petal blooms is expected in the near future.
Manipulation of genes encoding metal transporters
Tulip (Tulipa gesneriana) has varieties with purple flowers in addition to white, red and yellow. Delphinidin 3-rutinoside accumulates as a major pigment in the purple perianths (Shoji et al. 2007). No tulips have blue coloration throughout the whole perianth; however, the inner bottom portion may develop a blue color due to a local accumulation of iron ions (Shoji et al. 2007). A vacuolar ion transporter of iron, TgVit1, facilitates the coexistence of iron ions and delphinidin 3-rutinoside (Momonoi et al. 2009).
When TgVit1 is transiently overexpressed in the cells of purple perianths, and the ferritin synthesis gene TgFER is suppressed to prevent an accumulation of ferritin bound to iron ions, the cell color becomes blue (Momonoi et al. 2009, Shoji et al. 2010). There have been attempts to produce a blue tulip by expressing TgVit1 in whole perianths under the control of a petal-specific TgMYB1 promoter (Shoji 2015). We expect that the flowering of these blue tulips will be reported in the near future. In addition, attempts have been made to introduce TgVit1 into the mutant of the interspecific hybrid of Cyclamen persicum and C. purpurascens, which accumulates delphinidin 3,5-diglucoside in petals (Kondo et al. 2009), to create blue cyclamens (Kurihara et al. 2015). The blue coloration was observed by transient TgVit1 expression in the petal cells, and blue flower colored cyclamen is expected to bloom due to stable expression.
Via metal complex formation, iron ions are involved in the blue coloration of Nemophila (Yoshida et al. 2015), cornflower (Shiono et al. 2005, Takeda et al. 2005) and blue poppy (Yoshida et al. 2006). A TgVit1 homologue, CcVIT, has been reported in cornflower (Yoshida and Negishi 2013). The HmVALT and HmPALT1 genes, which encode Al3+-transporters localized in vacuolar and cytoplasmic membranes respectively, have been reported in hydrangea, which develops blue coloration through the involvement of Al3+ (Negishi et al. 2012, 2013).
Thus, it is expected that blue flowers might be produced in other plants by introducing a gene encoding a metal ion transporter known to be involved in the blue color development of petals.
Breeding of blue flowers by genetic engineering
In many plants species, cyanidin-based anthocyanins lead to red color development; however, some plants with cyanidin-based anthocyanin as the main anthocyanin may develop blue flowers. As mentioned above, blue color develops due to the formation of metal complexes in cornflowers and blue poppies containing cyanidin, and due to an increase in vacuolar pH during flowering in morning glory containing peonidin. However, many other flowers develop blue coloration through an accumulation of delphinidin-based anthocyanins.
The key enzyme of delphinidin biosynthesis is F3′5′H, which belongs to the CYP75A or CYP75B subfamily of cytochrome P450 enzymes. cDNAs encoding F3′5′H were initially isolated from petunia (Holton et al. 1993) and eggplant (Toguri et al. 1993) and have now been isolated from various plant species (Tanaka and Brugliera 2013). However, the production of delphinidin-based anthocyanins alone is not sufficient for blue color development. To develop blue coloration, it is necessary to modify the environment (e.g., vacuolar pH) where the delphinidin-based anthocyanin is present, to add aromatic organic acids to the glycosyl moieties of the anthocyanin, or to facilitate an interaction between the anthocyanin and coexisting copigments and/or metal ions. In this section, I describe the creation of blue flowers by introduction of the delphinidin biosynthetic pathway and anthocyanin modification gene.
Carnation and rose
Violet carnations and roses that accumulate delphinidin-based anthocyanins through the introduction of F3′5′H have been bred by the research group of Suntory and Florigene, and both Florigene Mooncarnation™ and Suntory blue rose Applause™ have been successfully commercialized worldwide (Tanaka and Brugliera 2013).
The anthocyanin responsible for the flower color of carnation is the 3,5-diglucoside-6″-4,6‴-1 cyclic malate of pelargonidin or cyanidin (Bloor 1998, Nakayama et al. 2000). The violet-colored Mooncarnation was initially created by introducing the petunia genes F3′5′H and DFR into the white flower of a DFR mutant carnation. The F3′5′H gene from Viola has also been introduced into Mooncarnations. The violet color is expressed by co-pigmentation, and the bluest Mooncarnation uses C-glucosyl flavones as co-pigments (Fukui et al. 2003).
The main anthocyanin responsible for color development in roses is cyanidin 3,5-diglucoside. In most plant species, anthocyanidin is first glycosylated at the 3-hydroxyl group. In roses, however, the hydroxyl groups at positions 5 and 3 are sequentially glycosylated by a single enzyme: UDP-glucose: anthocyanidin 5,3-O-glucosyltransferase (Ogata et al. 2005).
For a long time, it was considered that blue roses are synonymous with “impossible”. The petals of some roses contain cyanidin-based anthocyanins named rosacyanins, in which gallic acid and ellagitannin are combined (Fukui et al. 2002, 2006). Therefore, “blue roses” with a grayish purple or mauve flower color have been produced by cross-breeding roses containing rosacyanins. Two Japanese rose breeders, Mr. Moriji Komori and Ms. Junko Kawamoto, have successfully bred blue roses, including ‘Seiryu’ and ‘Misty Purple’, respectively.
By contrast, the research group of Suntory and Florigene developed blue roses by genetic engineering (Katsumoto et al. 2007). Roses that have petals with a high flavonol content and relatively high pH—traits that are considered to be suitable for blue color development—were selected for gene introduction. Among various F3′5′H genes, the pansy F3′5′H gene was found to be effective for producing delphinidin-based anthocyanins in roses. In addition, a Torenia gene encoding anthocyanin 5-aromatic acyltransferase was introduced with pansy F3′5′H, which enabled acylation of anthocyanin with an aromatic organic acid, and the world’s first blue rose, Suntory blue rose Applause was created.
Delphinidin-derived anthocyanins include petunidin- and malvidin-based anthocyanins in which the hydroxyl groups at the 3′ and/or 5′ positions are methylated. This methylation reaction depends on S-adenosylmethionine:anthocyanin 3′,5′-O-methyltransferase (A3′5′OMT). It has been reported that malvidin-based anthocyanins can accumulate in rose petals through the co-expression of Torenia A3′5′OMT and pansy F3′5′H (Nakamura et al. 2015). The resulting malvidin-producing roses have brilliant magenta flowers as compared with roses that accumulate delphinidin-based anthocyanin.
Dahlia, Phalaenopsis and lily
The major anthocyanins responsible for flower color in dahlia are 3-(6-malonyl)glucoside-5-glucosides of pelargonidin and cyanidin (Takeda et al. 1986, Yamaguchi et al. 1999). The transformation system of the dahlia plant has been reported for the Dahlia × pinnata ‘Yamatohime’ (Otani et al. 2013). In addition, a “blue dahlia” was created in a collaboration between Prof. Mii (Chiba University) and Ishihara Sangyo Kaisha Ltd. The F3′5′H gene derived from dayflower, Commelina communis L., was introduced under the control of the 35S promoter of CaMV to produce delphinidin derivatives, and the resultant transgenic dahlia turned violet (Mii 2013, Nakano et al. 2016). Various blue dahlias have been subsequently created by using the violet transgenic Yamatohime as a parent for hybridization (Mii 2013).
Prof. Mii and collaborators have also succeeded in creating a “blue Phalaenopsis orchid” (Mii 2012). The major anthocyanins of pink-colored Phalaenopsis are polyacylated cyanidin-based anthocyanins in which the 7- and 3′-positions are glycosylated and acylated with aromatic organic acids (Tatsuzawa et al. 1997). For this reason, Phalaenopsis is able to produce delphinidin-based anthocyanin by the introduction of dayflower F3′5′H, and the flowers are the closest to blue that have been achieved by the expression of only F3′5′H.
The F3′5′H gene derived from Phalaenopsis has also been transiently expressed in lily to make the cells of pink petals turn blue (Qi et al. 2013). In addition, purple lilies have been obtained by expressing the Campanula F3′5′H gene under the control of the CaMV 35S promoter (Nakano et al. 2016, Tanaka et al. 2012).
Chrysanthemum
The anthocyanins responsible for color development in chrysanthemums are cyanidin 3-(6″-malonyl)glucoside and cyanidin 3-(3″,6″-dimalonyl)glucoside (Nakayama et al. 1997). To introduce the blue flower color trait into chrysanthemums, it is first necessary to establish a system of regeneration and transformation. Shinoyama et al. (2012) have summarized and reviewed various transformation systems of chrysanthemums. Some promoters, including chrysanthemum ubiquitin extension protein (UEP1) promoter (Annadana et al. 2002) and tobacco elongation factor 1α (EF1α) promoter (Aida et al. 2005), have been reported as effective for the expression of transgenes in chrysanthemum. So far, however, there are no successful examples of chrysanthemums that produce delphinidin through the expression of F3′5′H using these promoters.
The Florigene and Suntory group have obtained purpleand violet-colored chrysanthemums containing a high proportion of delphinidin by suppressing the endogenous F3′H gene and expressing pansy F3′5′H under the control of the rose CHS promoter (Brugliera et al. 2013). When this gene construct was introduced and selected via the NPTII gene, however, the highest delphinidin content achieved was less than 6% (Noda et al. 2013). Noda et al. also examined delphinidin production in chrysanthemum petals using pansy F3′5′H expressed under the control of other promoters, including CaMV 35S, Viola F3′5′H, rugosa rose F3H, Gerbera CHS, and rugosa rose DFR. In all of these cases, however, only individual plants with a low percentage of delphinidin (~1%–2%) were obtained (Noda et al. 2013). Furthermore, delphinidin was not produced when cineraria (Senecio cruentus) F3′5′H (He et al. 2013) or petunia F3′5′H (Seo et al. 2007) was expressed with the CaMV 35S promoter.
The most effective promoter for accumulating delphinidin in chrysanthemum ray florets is the chrysanthemum F3H promoter. Using this promoter to express Campanula F3′5′H, Noda et al. (2013) obtained a delphinidin content of almost 100%. The accumulation of delphinidin can also be effectively improved by using the 5′-untranslated region of the alcohol dehydrogenase gene (ADH) of tobacco, Arabidopsis or rice. Even with the chrysanthemum F3H promoter, the content of delphinidin is about 30% for other F3′5′H genes. Together, these results suggest that selection of the optimal promoter depends on both the host and the transgene used for expression. Synthesis of delphinidin-based anthocyanins via the F3′5′H gene was shown to modify the ray floret color to purple or violet, a color that had not been produced in chrysanthemum previously. However, the flower color was not generally a color that would be said to be “blue”. Further ingenuity was necessary to create truly “blue chrysanthemums”.
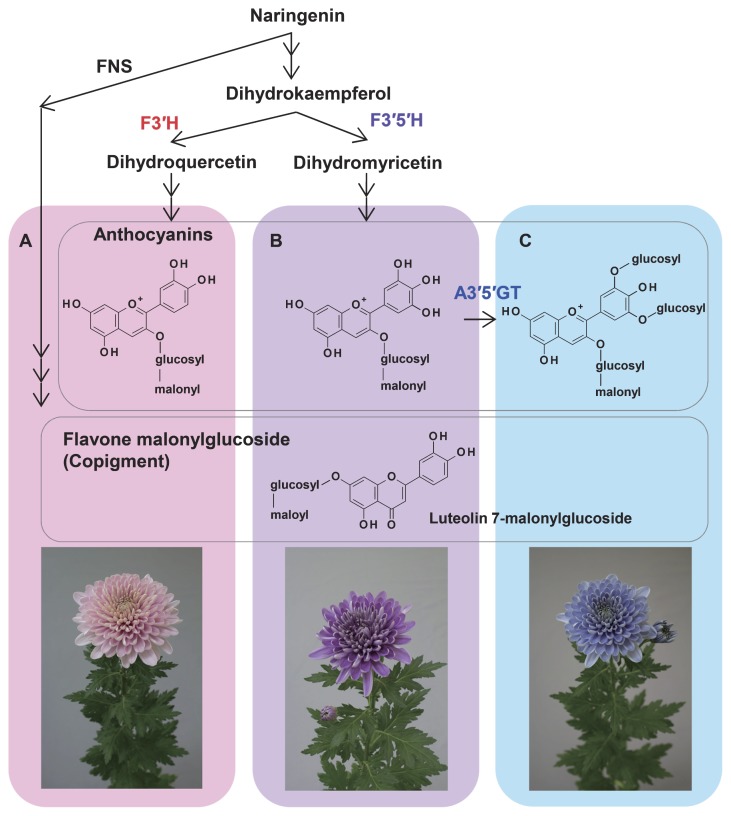
In addition to changing the anthocyanidin structure from cyanidin to delphinidin, it was considered that modification with multiple aromatic organic acids or the presence of interactive co-pigments and/or metal ions would be required for blue color development of chrysanthemum. Therefore, Noda et al. (2017) attempted to generate plants that could synthesize delphinidin 3-(6″-malonyl)glucoside 3′,5′-di-p-coumaroyglucoside (ternatin D3), one of blue polyacylated anthocyanins in butterfly pea (Terahara et al. 1998). The resulting F3′5′H-expressing chrysanthemums were found to accumulate delphinidin 3-(6″-malonyl)glucoside. To modify this to ternatin D3, glucosylation at the 3′ and 5′ positions of the B-ring and further acylation with aromatic organic acid were necessary. Therefore, the genes required for glucosylation and acylation were introduced, and blue-colored transformants were obtained. Notably, however, the blue petals of transgenic chrysanthemums did not contain ternatin D3, but rather ternatin C5 glucosylated at the 3′ and 5′-hydroxyl groups. Thus, it seemed that the gene introduced for aromatic acylation did not work well in chrysanthemum and the blue color development was due to an accumulation of ternatin C5. This idea was confirmed by analyzing recombinants in which only two genes were introduced: namely, campanula F3′5′H and butterfly pea A3′5′GT (Fig. 1). This method was found to be applicable to the creation of blue flowers in various chrysanthemums (Fig. 2). Furthermore, it was the first time that such a truly blue color flower has been generated by using genetic engineering technology.
Fig. 1.
Anthocyanins and co-pigments responsible for blue color development in chrysanthemum. A, Pink color of host plant due to the interaction between cyanidin 3-malonylglucoside and flavone 7-malonylglucosides (co-pigments); B, Purple or violet color of transgenic plant due to the interaction between delphinidin 3-malonylglucoside and co-pigments; C, Blue color of transgenic plant due to the interaction between ternatin C5 and co-pigments. FNS, flavone synthase; F3′H, flavonoid 3′-hydroxylase; F3′5′H, flavonoid 3′,5′-hydroxylase; A3′5′GT, UDP-glucose:anthocyanin 3′,5′-O-glucosyltransferase.
Fig. 2.
Different types of blue chrysanthemum flower. Host plants are shown on the left; transgenic plants are shown on the right. A, Decorative type of ‘Sei Arabella’; B, Anemone type of S25 line; C, Pompons type of T12 line.
The fact that the blue coloration was developed only through the glycosylation of delphinidin-based anthocyanin was an unexpected discovery. Glycosylation of the hydroxyl group of the B-ring has been reported to be responsible for red color development (Andersen and Jordheim 2010). Furthermore, when purified ternatin C5 was dissolved in a buffer solution of petal juice at pH 5.6, the color of the solution was violet. Thus, other factors were thought to be involved in the blue coloration of chrysanthemum, in addition to ternatin C5 production. Noda et al. (2017) searched for co-pigments that interact with ternatin C5 to develop blue coloration. Analysis of an extract from blue petals by the cross-TLC method revealed that bands of different substances intersected in the blue- and violet-colored parts of the anthocyanin band. The substances contained in these bands were identified as candidates for co-pigments, and their structures were investigated. As a result, flavone 7-malonyl glucosides were identified to interact with ternatin C5 and to develop blue coloration (Fig. 1). In addition, solutions of 3,3′,5′-triglucosyl delphinidins such as ternatin C5 and flavone 7-malonylglucoside mixed at a molar ratio of 1:5 or more were shown to have absorption spectra similar to those of blue petals. Therefore, the blue coloration of the transgenic chrysanthemum was shown to be caused by intermolecular co-pigmentation.
Formation of metal complexes, another blue color development mechanism, requires the transport of metal ions into vacuoles, as well as the acylation and glycosylation of complex constituents such as anthocyanins and flavones. In addition to controlling multiple glycosylation and acylation, anthocyanin polyacylation requires a vacuolar trafficking system to transport the necessary enzymes to the appropriate cellular organelles (Poustka et al. 2007, Sasaki et al. 2014, Xiang et al. 2013). Notably, the method of creating blue flowers in chrysanthemum does not require these trafficking systems. Thus, introduction of the butterfly pea A3′5′GT gene to synthesize anthocyanin, which then develops blue coloration by interacting with endogenous substances in the host petal, represents an effective method of blue flower generation. Furthermore, it raises the possibility of that bestowing the blue trait on various flower species will be much easier than it had been previously thought.
Efficient production of blue flowers
Techniques for plant regeneration and transformation have been established for various plant species. However, the efficiency of regeneration and transformation varies depending on the breeding line or cultivar. For many plant species, therefore, it is often labor-intensive to identify lines or cultivars suitable for blue coloration.
In chrysanthemum, for example, only about 30 of 150 breeding lines or varieties tested showed efficient plant regeneration. Of these, only 15 showed altered flower color to violet-blue or blue by transformation; furthermore, the efficiency of blue flower production was understandably varied among them. For some strains, more than 50 transformants can be obtained from 3000 leaflets, while for others only a few are obtained. Individual plants expressing the target blueness are further limited. To efficiently produce blue flowers of high commercial value, therefore, it is necessary to select hosts for which recombinants can be efficiently obtained. Furthermore, optimization of the culture system is required to improve recombination efficiency; in recent years, however, not many researchers are conducting such studies.
Lastly, it is desirable to use a gene construct that can reliably obtain transformants with the target flower color. By combining the appropriate transgene, promoter and terminator, the object metabolite can be synthesized without needing to suppress endogenous competing biosynthetic pathways. In the production of the blue chrysanthemum, for example, the proportion of transformants containing a high proportion of delphinidin-based anthocyanins was increased by using the AtHSP terminator (Nagaya et al. 2010) in the expression cassette of F3′5′H (Noda et al. 2017).
Commercialization of transgenic blue flowers
Genetically modified flowers have to pass the risk assessments mandated in international protocols (Biosafety Clearing-House 2017) and/or governmental regulations and policies for cultivation, distribution and retail. In order to grow and sell genetically modified flowers in the open environment, it is necessary to prove that the risk to the ecology, including the possibility for gene flow into related wild species and influence on unspecified organisms, is minimized (Chandler et al. 2013, Kikuchi et al. 2008, Nakamura et al. 2011a, 2011b, Shinoyama et al. 2008). In order to use genetically modified flowers for commercial purposes in Japan, it is necessary to obtain approval from the Ministry of Agriculture, Forestry and Fisheries and the Ministry of the Environment and (Japan Biosafety Clearing House 2017). Regulatory approval requires several years of examination and costs. Therefore, any genetically modified flowers aimed at commercialization must have both unprecedented marketability and various agronomic traits suitable for production and distribution.
The transgenic flowers that are so far approved in Japan are roses and carnations. Roses are currently the only genetically modified crops that are cultivated in Japan. To commercialize blue chrysanthemums both domestically and abroad, it will be necessary to obtain approval in accordance with the regulations of each target country. It will be necessary to confirm that there is no competitive advantage or hazardous substance productivity in the blue chrysanthemum. Wild species of chrysanthemums are widely distributed in Eastern Asia including Japan. Thus, it will be necessary to impart traits such as male and female sterility into the transgenic plants to prevent effects on biodiversity. By applying “New Plant Breeding Techniques” such as genome editing and other modern biotechnology, blue chrysanthemum varieties with sterility traits will be developed. This will facilitate the commercialization of blue chrysanthemums in Japan, contribute to the development of the flower industry, and meet the demands of farmer, florist and consumer.
Acknowledgements
I thank all collaborators who helped in the generation of blue chrysanthemums. I also thank all flower color researchers for their excellent work.
Literature Cited
- Aida, R., Nagaya, S., Yoshida, K., Kishimoto, S., Shibata, M. and Ohmiya, A. (2005) Efficient transgene expression in chrysanthemum, Chrysanthemum morifolium Ramat., with the promoter of a gene for tobacco elongation factor 1α protein. JARQ 39: 269–274. [Google Scholar]
- Andersen, Ø.M. and Jordheim, M. (2010) Chemistry of flavonoid-based colors in plants. In: Mander, L. and Liu H.W. (eds.) Comprehensive Natural Products II: Chemistry and Biology, Elsevier Science, pp. 547–614. [Google Scholar]
- Annadana, S., Beekwilder, M.J., Kuipers, G., Visser, P.B., Outchkourov, N., Pereira, A., Udayakumar, M., De Jong, J. and Jongsma, M.A. (2002) Cloning of the chrysanthemum UEP1 promoter and comparative expression in florets and leaves of Dendranthema grandiflora. Transgenic Res. 11: 437–445. [DOI] [PubMed] [Google Scholar]
- Azadi, P., Bagheri, H., Nalousi, A.M., Nazari, F. and Chandler, S.F. (2016) Current status and biotechnological advances in genetic engineering of ornamental plants. Biotechnol. Adv. 34: 1073–1090. [DOI] [PubMed] [Google Scholar]
- Biosafety Clearing-House (2017) https://bch.cbd.int/
- Bloor, S.J. (1998) A macrocyclic anthocyanin from red/mauve carnation flowers. Phytochemistry 49: 225–228. [Google Scholar]
- Brugliera, F., Tao, G.-Q., Tems, U., Kalc, G., Mouradova, E., Price, K., Stevenson, K., Nakamura, N., Stacey, I., Katsumoto, Y. et al. (2013) Violet/blue chrysanthemums—Metabolic engineering of the anthocyanin biosynthetic pathway results in novel petal colors. Plant Cell Physiol. 54: 1696–1710. [DOI] [PubMed] [Google Scholar]
- Chandler, S.F. and Sanchez, C. (2012) Genetic modification; the development of transgenic ornamental plant varieties. Plant Biotechnol. J. 10: 891–903. [DOI] [PubMed] [Google Scholar]
- Chandler, S.F., Senior, M., Nakamura, N., Tsuda, S. and Tanaka, Y. (2013) Expression of flavonoid 3′,5′-hydroxylase and acetolactate synthase genes in transgenic carnation: Assessing the safety of a nonfood plant. J. Agric. Food Chem. 61: 11711–11720. [DOI] [PubMed] [Google Scholar]
- de Vlaming, P., Schram, A.W. and Wiering, H. (1983) Genes affecting flower colour and pH of flower limb homogenates in Petunia hybrida. Theor. Appl. Genet. 66: 271–278. [DOI] [PubMed] [Google Scholar]
- Faraco, M., Spelt, C., Bliek, M., Verweij, W., Hoshino, A., Espen, L., Prinsi, B., Jaarsma, R., Tarhan, E., de Boer, A.H. et al. (2014) Hyperacidification of vacuoles by the combined action of two different P-ATPases in the tonoplast determines flower color. Cell Rep. 6: 32–43. [DOI] [PubMed] [Google Scholar]
- Fukuda-Tanaka, S., Inagaki, Y., Yamaguchi, T., Saito, N. and Iida, S. (2000) Colour-enhancing protein in blue petals. Nature 407: 581. [DOI] [PubMed] [Google Scholar]
- Fukui, Y., Kusumi, T., Masuda, K., Iwashita, T. and Nomoto, K. (2002) Structure of rosacyanin B, a novel pigment from the petals of Rosa hybrida. Tetrahedron Lett. 43: 2637–2639. [Google Scholar]
- Fukui, Y., Tanaka, Y., Kusumi, T., Iwashita, T. and Nomoto, K. (2003) A rationale for the shift in colour towards blue in transgenic carnation flowers expressing the flavonoid 3′,5′-hydroxylase gene. Phytochemistry 63: 15–23. [DOI] [PubMed] [Google Scholar]
- Fukui, Y., Nomoto, K., Iwashita, T., Masuda, K., Tanaka, Y. and Kusumi, T. (2006) Two novel blue pigments with ellagitannin moiety, rosacyanins A1 and A2, isolated from the petals of Rosa hybrida. Tetrahedron 62: 9661–9670. [Google Scholar]
- Hase, Y., Akita, Y., Kitamura, S., Narumi, I. and Tanaka, A. (2012) Development of an efficient mutagenesis technique using ion beams: Toward more controlled mutation breeding. Plant Biotechnol. 29: 193–200. [Google Scholar]
- He, H., Ke, H., Keting, H., Qiaoyan, X. and Silan, D. (2013) Flower colour modification of chrysanthemum by suppression of F3′H and overexpression of the exogenous Senecio cruentus F3′5′H gene. PLoS ONE 8: e74395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton, T.A., Brugliera, F., Lester, D.R., Tanaka, Y., Hyland, C.D., Menting, J.G.T., Lu, C.-Y., Farcy, E., Stevenson, T.W. and Cornish, E.C. (1993) Cloning and expression of cytochrome P450 genes controlling flower colour. Nature 366: 276–279. [DOI] [PubMed] [Google Scholar]
- Jacobs, M., Lopez-Garcia, M., Phrathep, O.-P., Lawson, T., Oulton, R. and Whitney, H.M. (2016) Photonic multilayer structure of Begonia chloroplasts enhances photosynthetic efficiency. Nat. Plants 2: 16162. [DOI] [PubMed] [Google Scholar]
- Japan Biosafety Clearing House (2017) http://www.biodic.go.jp/bch/english/e_index.html
- Katsumoto, Y., Fukuchi-Mizutani, M., Fukui, Y., Brugliera, F., Holton, T.A., Karan, M., Nakamura, N., Yonekura-Sakakibara, K., Togami, J., Pigeaire, A. et al. (2007) Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant Cell Physiol. 48: 1589–1600. [DOI] [PubMed] [Google Scholar]
- Kikuchi, A., Watanabe, K., Tanaka, Y. and Kamada, H. (2008) Recent progress on environmental bio-safety assessment of genetically modified trees and floricultural plants in Japan. Plant Biotechnol. 25: 9–15. [Google Scholar]
- Kondo, E., Nakayama, M., Kameari, N., Tanikawa, N., Morita, Y., Akita, Y., Hase, Y., Tanaka, A. and Ishizaka, H. (2009) Red-purple flower due to delphinidin 3,5-diglucoside, a novel pigment for Cyclamen spp., generated by ion-beam irradiation. Plant Biotechnol. 26: 565–569. [Google Scholar]
- Kurihara, C., Hosoi, S., Kondo, E., Shoji, K. and Akita, Y. (2015) Genetic approach for breeding of the blue-flowered fragrant cyclamen. J. Fac. Eng., Saitama Inst. Technol. 25: 17–21. [Google Scholar]
- Meyer, P., Heidmann, I., Forkmann, G. and Saedler, H. (1987) A new petunia flower colour generated by transformation of a mutant with a maize gene. Nature 330: 677–678. [DOI] [PubMed] [Google Scholar]
- Mii, M. (2012) Aoi Kochoran/daria no kaihatsu (Development of blue Phalenopsis and blue Dahlia). Noukou to Engei (Agriculture and Horticulture) 67: 42–46. [Google Scholar]
- Mii, M. (2013) Aoi Dahlia hinshu no Ikusei (Breeding of blue dahlia cultivars). Saishin Nogyo Gijutsu Kaki 5: 43–50. [Google Scholar]
- Momonoi, K., Yoshida, K., Mano, S., Takahashi, H., Nakamori, C., Shoji, K., Nitta, A. and Nishimura, M. (2009) A vacuolar iron transporter in tulip, TgVit1, is responsible for blue coloration in petal cells through iron accumulation. Plant J. 59: 437–447. [DOI] [PubMed] [Google Scholar]
- Moyroud, E., Wenzel, T., Middleton, R., Rudall, P.J., Banks, H., Reed, A., Mellers, G., Killoran, P., Westwood, M.M., Steiner, U. et al. (2017) Disorder in convergent floral nanostructures enhances signalling to bees. Nature 550: 469–474. [DOI] [PubMed] [Google Scholar]
- Nagaya, S., Kawamura, K., Shinmyo, A. and Kato, K. (2010) The HSP terminator of Arabidopsis thaliana increases gene expression in plant cells. Plant Cell Physiol. 51: 328–332. [DOI] [PubMed] [Google Scholar]
- Nakamura, N., Fukuchi-Mizutani, M., Katsumoto, Y., Togami, J., Senior, M., Matsuda, Y., Furuichi, K., Yoshimoto, M., Matsunaga, A., Ishiguro, K. et al. (2011a) Environmental risk assessment and field performance of rose (Rosa × hybrida) genetically modified for delphinidin production. Plant Biotechnol. 28: 251–261. [Google Scholar]
- Nakamura, N., Tems, U., Fukuchi-Mizutani, M., Chandler, S., Matsuda, Y., Takeuchi, S., Matsumoto, S. and Tanaka, Y. (2011b) Molecular based evidence for a lack of gene-flow between Rosa × hybrida and wild Rosa species in Japan. Plant Biotechnol. 28: 245–250. [Google Scholar]
- Nakamura, N., Katsumoto, Y., Brugliera, F., Demelis, L., Nakajima, D., Suzuki, H. and Tanaka, Y. (2015) Flower color modification in Rosa hybrida by expressing the S-adenosylmethionine: anthocyanin 3′,5′-O-methyltransferase gene from Torenia hybrida. Plant Biotechnol. 32: 109–117. [Google Scholar]
- Nakano, M., Mii, M., Kobayashi, H., Otani, M. and Yagi, M. (2016) Molecular approaches to flower breeding. Breed. Res. 18: 34–40. [Google Scholar]
- Nakayama, M., Koshioka, M., Shibata, M., Hiradate, S., Sugie, H. and Yamaguchi, M. (1997) Identification of cyanidin 3-O-(3″,6″-O- dimalonyl-β-glucopyranoside) as a flower pigment of chrysanthemum (Dendranthema grandiflorum). Biosci. Biotechnol. Biochem. 61: 1607–1608. [Google Scholar]
- Nakayama, M., Koshioka, M., Yoshida, H., Kan, Y., Fukui, Y., Koike, A. and Yamaguchi, M. (2000) Cyclic malyl anthocyanins in Dianthus caryophyllus. Phytochemistry 55: 937–939. [DOI] [PubMed] [Google Scholar]
- Negishi, T., Oshima, K., Hattori, M., Kanai, M., Mano, S., Nishimura, M. and Yoshida, K. (2012) Tonoplast- and plasma membrane-localized aquaporin-family transporters in blue Hydrangea sepals of aluminum hyperaccumulating plant. PLoS ONE 7: e43189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi, T., Oshima, K., Hattori, M. and Yoshida, K. (2013) Plasma membrane-localized Al-transporter from blue hydrangea sepals is a member of the anion permease family. Genes Cells 18: 341–352. [DOI] [PubMed] [Google Scholar]
- Noda, N., Aida, R., Kishimoto, S., Ishiguro, K., Fukuchi-Mizutani, M., Tanaka, Y. and Ohmiya, A. (2013) Genetic engineering of novel bluer-colored chrysanthemums produced by accumulation of delphinidin-based anthocyanins. Plant Cell Physiol. 54: 1684–1695. [DOI] [PubMed] [Google Scholar]
- Noda, N., Yoshioka, S., Kishimoto, S., Nakayama, M., Douzono, M., Tanaka, Y. and Aida, R. (2017) Generation of blue chrysanthemums by anthocyanin B-ring hydroxylation and glucosylation and its coloration mechanism. Sci. Adv. 3: e1602785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata, J., Kanno, Y., Itoh, Y., Tsugawa, H. and Suzuki, M. (2005) Plant biochemistry: Anthocyanin biosynthesis in roses. Nature 435: 757–758. [DOI] [PubMed] [Google Scholar]
- Ohnishi, M., Fukada-Tanaka, S., Hoshino, A., Takada, J., Inagaki, Y. and Iida, S. (2005) Characterization of a novel Na+/H+ antiporter gene InNHX2 and comparison of InNHX2 with InNHX1, which is responsible for blue flower coloration by increasing the vacuolar pH in the Japanese morning glory. Plant Cell Physiol. 46: 259–267. [DOI] [PubMed] [Google Scholar]
- Otani, Y., Chin, D.P. and Mii, M. (2013) Establishment of Agrobacterium-mediated genetic transformation system in Dahlia. Plant Biotechnol. 30: 135–139. [Google Scholar]
- Poustka, F., Irani, N.G., Feller, A., Lu, Y., Pourcel, L., Frame, K. and Grotewold, E. (2007) A trafficking pathway for anthocyanins overlaps with the endoplasmic reticulum-to-vacuole protein-sorting route in Arabidopsis and contributes to the formation of vacuolar inclusions. Plant Physiol. 145: 1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, Y., Lou, Q., Quan, Y., Liu, Y. and Wang, Y. (2013) Flower-specific expression of the Phalaenopsis flavonoid 3′,5′-hydoxylase modifies flower color pigmentation in Petunia and Lilium. Plant Cell Tissue Organ Cult. 115: 263–273. [Google Scholar]
- Sasaki, N., Nishizaki, Y., Ozeki, Y. and Miyahara, T. (2014) The role of acyl-glucose in anthocyanin modifications. Molecules 19: 18747–18766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, N. and Nakayama, T. (2015) Achievements and perspectives in biochemistry concerning anthocyanin modification for blue flower coloration. Plant Cell Physiol. 56: 28–40. [DOI] [PubMed] [Google Scholar]
- Seo, J., Kim, S.W., Kim, J., Cha, H.W. and Liu, J.R. (2007) Co-expression of flavonoid 3′,5′-hydroxylase and flavonoid 3′-hydroxylase accelerates decolorization in transgenic chrysanthemum petals. J. Plant Biol. 50: 626–631. [Google Scholar]
- Shinoyama, H., Mochizuki, A., Nomura, Y. and Kamada, H. (2008) Environmental risk assessment of genetically modified chrysanthemums containing a modified cry1Ab gene from Bacillus thuringiensis. Plant Biotechnol. 25: 17–29. [Google Scholar]
- Shinoyama, H., Aida, R., Ichikawa, H., Nomura, Y. and Mochizuki, A. (2012) Genetic engineering of chrysanthemum (Chrysanthemum morifolium): Current progress and perspectives. Plant Biotechnol. 29: 323–337. [Google Scholar]
- Shiono, M., Matsugaki, N. and Takeda, K. (2005) Phytochemistry: Structure of the blue cornflower pigment. Nature 436: 791. [DOI] [PubMed] [Google Scholar]
- Shoji, K., Miki, N., Nakajama, N., Momonoi, K., Kato, C. and Yoshida, K. (2007) Perianth bottom-specific blue color development in tulip cv. Murasakizuisho requires ferric ions. Plant Cell Physiol. 48: 243–251. [DOI] [PubMed] [Google Scholar]
- Shoji, K., Momonoi, K. and Tsuji, T. (2010) Alternative expression of vacuolar ion transporter and ferritin genes leads to blue/purple coloration of flowers in Tulip cv. ‘Murasakizuisho’. Plant Cell Physiol. 51: 215–224. [DOI] [PubMed] [Google Scholar]
- Shoji, K. (2015) Identification of cis-element for tulip petal-specific TgMYB1 promoter and its application for modifying a flower color. Proceedings of the Annual Meeting of Japan Society for Bioscience, Biotechnology, and Agrochemistry. 4D23a03. [Google Scholar]
- Spelt, C., Quattrocchio, F., Mol, J. and Koes, R. (2002) ANTHOCYANIN1 of petunia controls pigment synthesis, vacuolar pH, and seed coat development by genetically distinct mechanisms. Plant Cell 14: 2121–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura, T. and Matsuzaki, T. (2015) Characteristics of F1 progenies obtained by crosses between bluish-violet flowered cyclamen and other cyclamen cultivars. Hort. Res. (Japan) 14 (Supple. 1): 412. [Google Scholar]
- Takeda, K., Harborne, J.B. and Self, R. (1986) Identification and distribution of malonated anthocyanins in plants of the Compositae. Phytochemistry 25: 1337–1342. [Google Scholar]
- Takeda, K., Osakabe, A., Saito, S., Furuyama, D., Tomita, A., Kojima, Y., Yamadera, M. and Sakuta, M. (2005) Components of protocyanin, a blue pigment from the blue flowers of Centaurea cyanus. Phytochemistry 66: 1607–1613. [DOI] [PubMed] [Google Scholar]
- Tanaka, Y., Nakamura, N., Kobayashi, H., Okuhara, H., Kondo, M., Koike, Y., Hoshi, Y. and Nomizu, T. (2012) Method for cultivating lilies containing delphinidin in the petals thereof. PCT Publication No. WO2012036290. [Google Scholar]
- Tanaka, Y. and Brugliera, F. (2013) Flower colour and cytochromes P450. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 368: 20120432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, Y., Oshima, Y., Yamamura, T., Sugiyama, M., Mitsuda, N., Ohtsubo, N., Ohme-Takagi, M. and Terakawa, T. (2013) Multi-petal cyclamen flowers produced by AGAMOUS chimeric repressor expression. Sci. Rep. 3: 2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuzawa, F., Saito, N., Seki, H., Hara, R., Yokoi, M. and Honda, T. (1997) Acylated cyanidin glycosides in the red-purple flowers of Phalaenopsis. Phytochemistry 45: 173–177. [Google Scholar]
- Terahara, N., Toki, K., Saito, N., Honda, T., Matsui, T. and Osajima, Y. (1998) Eight new anthocyanins, ternatins C1−C5 and D3 and preternatins A3 and C4 from young Clitoria ternatea flowers. J. Nat. Prod. 61: 1361–1367. [DOI] [PubMed] [Google Scholar]
- Terakawa, T. (2012) Aoi Cyclamen (Blue Cyclamen). Saishin Nogyo Gijutsu Kaki 4: 153–158. [Google Scholar]
- Toguri, T., Umemoto, N., Kobayashi, O. and Ohtani, T. (1993) Activation of anthocyanin synthesis genes by white light in eggplant hypocotyl tissues, and identification of an inducible P-450 cDNA. Plant Mol. Biol. 23: 933–946. [DOI] [PubMed] [Google Scholar]
- van Houwelingen, A., Souer, E., Spelt, K., Kloos, D., Mol, J. and Koes, R. (1998) Analysis of flower pigmentation mutants generated by random transposon mutagenesis in Petunia hybrida. Plant J. 13: 39–50. [DOI] [PubMed] [Google Scholar]
- Verweij, W., Spelt, C., Di Sansebastiano, G.P., Vermeer, J., Reale, L., Ferranti, F., Koes, R. and Quattrocchio, F. (2008) An H+ P-ATPase on the tonoplast determines vacuolar pH and flower colour. Nat. Cell Biol. 10: 1456–1462. [DOI] [PubMed] [Google Scholar]
- Vignolini, S., Rudall, P.J., Rowland, A.V., Reed, A., Moyroud, E., Faden, R.B., Baumberg, J.J., Glover, B.J. and Steiner, U. (2012) Pointillist structural color in Pollia fruit. Proc. Natl. Acad. Sci. USA 109: 15712–15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, J. and Mackenzie, S. (2001) Why are all colour combinations not equally represented as flower-colour polymorphisms? New Phytol. 151: 237–241. [DOI] [PubMed] [Google Scholar]
- Weevers, T.H. (1952) Flower colours and their frequency. Acta Bot. Neerl. 1: 81–92. [Google Scholar]
- Xiang, L., Etxeberria, E. and Van den Ende, W. (2013) Vacuolar protein sorting mechanisms in plants. FEBS J. 280: 979–993. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, M., Oshida, N., Nakayama, M., Koshioka, M., Yamaguchi, Y. and Ino, I. (1999) Anthocyanidin 3-glucoside malonyltransferase from Dahlia variabilis. Phytochemistry 52: 15–18. [Google Scholar]
- Yamaguchi, T., Fukada-Tanaka, S., Inagaki, Y., Saito, N., Yonekura-Sakakibara, K., Tanaka, Y., Kusumi, T. and Iida, S. (2001) Genes encoding the vacuolar Na+/H+ exchanger and flower coloration. Plant Cell Physiol. 42: 451–461. [DOI] [PubMed] [Google Scholar]
- Yoshida, K., Kondo, T., Okazaki, Y. and Katou, K. (1995) Cause of blue petal colour. Nature 373: 291. [Google Scholar]
- Yoshida, K., Toyama-Kato, Y., Kameda, K. and Kondo, T. (2003) Sepal color variation of Hydrangea macrophylla and vacuolar pH measured with a proton-selective microelectrode. Plant Cell Physiol. 44: 262–268. [DOI] [PubMed] [Google Scholar]
- Yoshida, K., Kitahara, S., Ito, D. and Kondo, T. (2006) Ferric ions involved in the flower color development of the Himalayan blue poppy, Meconopsis grandis. Phytochemistry 67: 992–998. [DOI] [PubMed] [Google Scholar]
- Yoshida, K., Mori, M. and Kondo, T. (2009) Blue flower color development by anthocyanins: from chemical structure to cell physiology. Nat. Prod. Rep. 26: 884–915. [DOI] [PubMed] [Google Scholar]
- Yoshida, K. and Negishi, T. (2013) The identification of a vacuolar iron transporter involved in the blue coloration of cornflower petals. Phytochemistry 94: 60–67. [DOI] [PubMed] [Google Scholar]
- Yoshida, K., Tojo, K., Mori, M., Yamashita, K., Kitahara, S., Noda, M. and Uchiyama, S. (2015) Chemical mechanism of petal color development of Nemophila menziesii by a metalloanthocyanin, nemophilin. Tetrahedron 71: 9123–9130. [Google Scholar]
- Zhang, Y., Butelli, E. and Martin, C. (2014) Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 19: 81–90. [DOI] [PubMed] [Google Scholar]