Abstract
Memory, the ability to retain learned information, is necessary for survival. Thus far, molecular and cellular investigations of memory formation and storage have mainly focused on neuronal mechanisms. In addition to neurons, however, the brain comprises other types of cells and systems, including glia and vasculature. Accordingly, recent experimental work has begun to ask questions about the roles of non-neuronal cells in memory formation. These studies provide evidence that all types of glial cells (astrocytes, oligodendrocytes, and microglia) make important contributions to the processing of encoded information and storing memories. In this review, we summarize and discuss recent findings on the critical role of astrocytes as providers of energy for the long-lasting neuronal changes that are necessary for long-term memory formation. We focus on three main findings: first, the role of glucose metabolism and the learning- and activity-dependent metabolic coupling between astrocytes and neurons in the service of long-term memory formation; second, the role of astrocytic glucose metabolism in arousal, a state that contributes to the formation of very long-lasting and detailed memories; and finally, in light of the high energy demands of the brain during early development, we will discuss the possible role of astrocytic and neuronal glucose metabolisms in the formation of early-life memories. We conclude by proposing future directions and discussing the implications of these findings for brain health and disease.
Keywords: glucose, metabolism, glia, glycolysis, glycogenolysis, emotional arousal, development
Long-term memory and its underlying neuron-centric biological mechanisms
Memories can be classified according to their duration as short-term memories, which last for seconds to minutes, or long-term memories, which last for hours to years, and potentially throughout the entire lifetime. Formation of long-term memories is key for survival because it allows an organism to benefit from previous experience and respond adaptively to its changing environment. Short-term memories are distinct from long-term memories in terms of their underlying biological mechanisms and circuitry. Although long-term memories generally require de novo gene expression, short-term memories rely on post-translational protein modifications (Alberini 2009; Alberini and Kandel 2014; Squire and Dede 2015).
Memories can also be divided into different categories on the basis of the type of information encoded and stored. For example, one major distinction classifies memories as explicit (also known as declarative in humans) or implicit (non-declarative) (Squire 2004). Explicit memories retain information about facts, people, places, and things (also known as memories of what, where, who, and when, or wwww memories), and include episodic and semantic memories. Implicit memories, which are recalled in an unconscious/automatic manner, retain information about learned automatic responses, and include priming, procedural memories (memories of how to do things), and simple reflexes (Tulving 1972; Squire and Wixted 2011). Explicit and implicit memories recruit distinct systems (network of regions) for their encoding, consolidation, and storage. Both clinical and animal studies have revealed that explicit memories are processed by the medial temporal lobe, within which one critical region is the hippocampus, whereas implicit memories are processed elsewhere and can operate in the absence of an intact explicit system (Eichenbaum 2006; Kim and Fanselow 1992; Scoville and Milner 1957; Squire and Wixted 2011). Thus, explicit memories are also referred to as hippocampus-dependent memories. Although implicit and explicit memory systems can be functionally dissociated, under normal healthy conditions they cooperate to process and store complex information (Kim and Baxter 2001; McDonald et al. 2004).
Studies aimed at elucidating the biological bases of long-term memories have mainly focused on hippocampus-dependent memories. However, most of our understanding of the cellular and molecular mechanisms underlying memory formation and storage initially arose from investigations of simple forms of learning, such as the gill-withdrawal reflex of Aplysia californica and olfactory learning in Drosophila melanogaster (Yin et al. 1994; Dubnau and Tully 1998; Davis 2011; Kandel 2012). In Aplysia, these studies uncovered a great deal of information about the molecular and cellular pathways activated and recruited to implement long-term modifications of synaptic strength or long-term synaptic plasticity. These data converged with genetic and behavioral results obtained in Drosophila. Guided by this knowledge from these two invertebrate systems, studies on mammalian memory paradigms revealed that similar molecular pathways are also necessary in the more complex mammalian memory, including hippocampus-dependent memories. Ultimately, numerous studies in the last 30 years on many species converged on the conclusion that evolutionarily conserved biological mechanisms underlie long-term synaptic plasticity and long-term memory formation (Alberini 2009; Kandel 2012; Kandel et al. 2014). One classical example, which has been extensively investigated, is the evolutionarily conserved role of the cyclic adenosine monophosphate (cAMP)-dependent pathway and the functionally linked activation of the cAMP response element-binding protein (CREB)-dependent cascade of gene expression (Kida and Serita 2014; Lonze and Ginty 2002; Silva et al. 1998) (Figure 1).
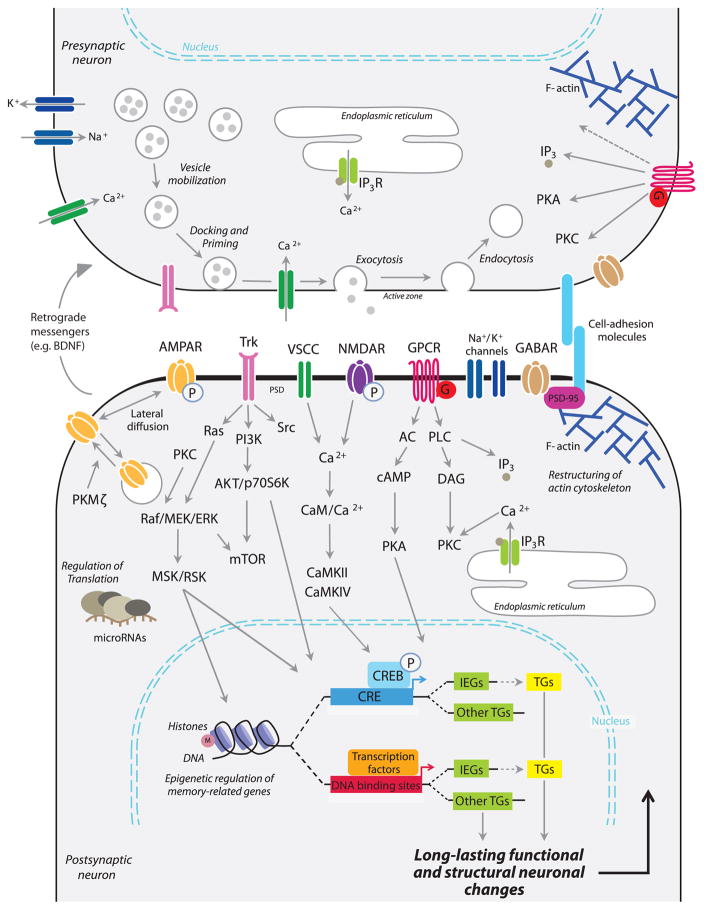
Figure 1. Schematic example of neuron-centric molecular pathways underlying long-term memory formation.
Most literature’s figures available thus far illustrating molecular mechanisms underlying learning and memory depict only pre and postsynaptic neurons and relative mechanisms of interest. One example is the following: learning-induced release of neurotransmitters (e.g. glutamate) and of neuronal growth factors (e.g. BDNF) activate different families of receptors, enabling the recruitment of various intracellular signaling pathways involving second messengers (e.g. Ca2+, cAMP) and protein kinases (e.g. CamKII, PKA). These signaling pathways regulate: 1) post-translational modifications [e.g., phosphorylation (P) of postsynaptic glutamatergic receptors]; 2) activation of a CREB-regulated gene cascade leading to the expression of target genes, including IEGs (e.g., C/EBP, c-Fos, Zif268), which in turn regulate the expression of late response genes critical for long-lasting functional (e.g., membrane translocation of new receptors) and structural neuronal changes (e.g., dendritic spine morphological changes). This gene expression is regulated by epigenetic mechanisms [e.g. histone acetylation and/or methylation (M), DNA methylation] as well as by several post-transcriptional and translational mechanisms including the mTOR pathway and microRNAs. Abbreviations: AC (adenylyl cyclase); AKT (protein kinase B or Akt); AMPAR (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor); BDNF (brain-derived neurotrophic factor); CaM (calmodulin); CaMKII/IV (Ca++-calmodulin kinase II/IV); cAMP (cyclic adenosine monophosphate); C/EBP (CCAAT/enhancer binding protein); CRE (cAMP response element); CREB (cAMP response element binding protein); DAG (diacylglycerol); GPCR (G protein–coupled receptors); IEGs (immediate early genes); IP3 (inositol trisphosphate); IP3R (inositol trisphosphate receptor); MSK (mitogen and stress activated protein kinase); mTOR (mammalian target of rapamycin); NMDAR (N-methyl-D-aspartate receptor); p70S6K (ribosomal protein S6 kinase beta-1); PI3K (phosphoinositide 3-kinase); PKA (protein kinase A); PKC (protein kinase C); PKMζ(protein kinase M zeta); PLC (phospholipase C); PSD-95 (post-synaptic density 95); RSK (ribosomal s6 kinase family); Src (proto-oncogene tyrosine-protein kinase); TGs (target genes); Trk (tyrosine receptor kinase); VSCC (voltage-sensitive calcium channel). Notably, multiple cell types in the brain express many of these mechanisms.
Numerous mammalian models of different types of short- and long-term memory, particularly in rodents, have been employed to investigate the complexity of mammalian memory processing in a variety of brain regions. These studies revealed that the expression and post-translational regulation of many classes of genes, RNAs, and proteins are required for long-term memory formation and storage; these include immediate-early genes (e.g., c-Fos, Zif268, NPAS4 and Arc/Arg3.1) (Bramham et al. 2008; Guzowski 2002; Loebrich and Nedivi 2009; Sun and Lin 2016; Veyrac et al. 2014), metabotropic and ionotropic receptors for various neurotransmitters (e.g., AMPA, NMDA, Kainate, GABA, and metabotropic glutamate receptors) and neuromodulators (e.g., dopaminergic and serotoninergic receptors), neurotrophic factors (e.g. tyrosine receptor kinase) (Fanselow et al. 1994; Gonzalez-Burgos and Feria-Velasco 2008; Kandel 2001; Makkar et al. 2010; Morris 2013; Purcell and Carew 2003; Riedel 1996; Riedel et al. 2003), kinases (e.g., ERK, CamKIIα, PKA, PKC, PKMζ, and MAPK) (Bejar et al. 2002; Kandel 2012; Lisman et al. 2002; Mayford 2007; Pastalkova et al. 2006; Rahn et al. 2013), transcription factors (e.g., CREB, C/EBP, NFkB, AP1, NPAS4, Zif268, NR4a, and SRF) (Alberini 2009; Alberini and Kandel 2014; Jones et al. 2001; Sun and Lin 2016), epigenetic regulators (e.g., MSK1, RSK2, NFkB, DNMT, HATs, and HDACs) (Day and Sweatt 2011; de la Fuente et al. 2015; Franklin and Mansuy 2010; Rudenko and Tsai 2014), microRNAs (e.g., miR-124, miR-132, miR-128b, and miR-134) (Bredy et al. 2011; Nudelman et al. 2010; Saab and Mansuy 2014), and a number of effector proteins engaged in structural changes, such as cell-adhesion molecules (e.g., neurexin and neuroligin) (Murase and Schuman 1999; Rose 1996; Ye et al. 2017; Bailey et al. 2015) (Figure 1).
These molecular investigations have been paralleled by electrophysiological studies, which showed that the cellular mechanisms underlying long-term memory involve long-term synaptic functional changes, and in particular long-term increases or decreases in synaptic transmission known as long-term potentiation (LTP) and long-term depression (LTD), respectively (Bliss and Collingridge 1993; Malenka and Bear 2004). Additional electrophysiological changes in the brain that have been implicated in long-term memory formation include electroencephalogram (EEG) coherence, i.e., phase synchronization of field potential oscillations, which coordinates the timing of neuronal spiking to promote synaptic plasticity across distributed brain regions (Corcoran et al. 2016; Zanto et al. 2011). Notably, this system-level communication among brain regions is controlled by sharp wave ripples (SPW-Rs) (Buzsáki 2015), a synchronous population pattern in the hippocampus that engages in crosstalk with a wide area of the cortex and several subcortical nuclei. SPW-Rs occur in “off-line” states of the brain during waking and in non-REM sleep, and are believed to consolidate episodic memories across the hippocampal-cortical system (Buzsáki 2015; Inostroza and Born 2013). These system-wide activities provide a possible mechanistic explanation for why hippocampus-dependent memories, which are fragile during the initial period when they are engaging a network of both hippocampal and cortical regions, become more stable and exclusively hippocampus-independent over time. This redistribution of memory representations and storage is known as system-level consolidation (Dudai et al. 2015; Squire et al. 2015; Frankland and Bontempi 2005).
Although these studies provided a great deal of information about the biological bases of learning and memory, they focused on neuronal mechanisms, and consequently generated conclusions mostly limited to neurons and neuronal functions. However, in addition to neurons, the brain comprises many types of cells and systems, including glia and vascular systems. Recent investigations have begun to assess the role of non-neuronal cells in long-term memory, and provided clear evidence that all glial cell types (i.e. astrocytes, oligodendrocytes and microglia) play critical roles in memory processing (Adamsky and Goshen 2017; Fields 2008; Gibbs et al. 2008; Lee et al. 2014; Moraga-Amaro et al. 2014; Parkhurst et al. 2013; Suzuki et al. 2011).
Astrocytes are particularly well equipped to influence neuronal functions involved in memory formation (Haydon and Nedergaard 2014; Moraga-Amaro et al. 2014): they are excitable through calcium fluctuations and respond to neurotransmitters released at synapses; they synchronize via calcium waves and release their own gliotransmitters, which are essential for synaptic plasticity; they communicate with blood vessels thus coupling circulation (blood flow) to local brain activity; and finally, they regulate energy metabolism in support of neuronal functions, including those required for memory formation (Henneberger et al. 2010; Pannasch and Rouach 2013; Perea et al. 2009; Bazargani and Attwell 2016). In regard to this metabolic role, astrocytes are perfectly positioned to balance the metabolism of glucose in the brain: on one side, the astrocytic endfeet directly contact the layers of the blood vessel that import glucose from the blood via the selective glucose transporter GLUT1, and on the other side, these cells extend processes that wrap around the pre- and post-synaptic compartments of neurons (Falkowska et al. 2015; Morgello et al. 1995) (Figure 2).
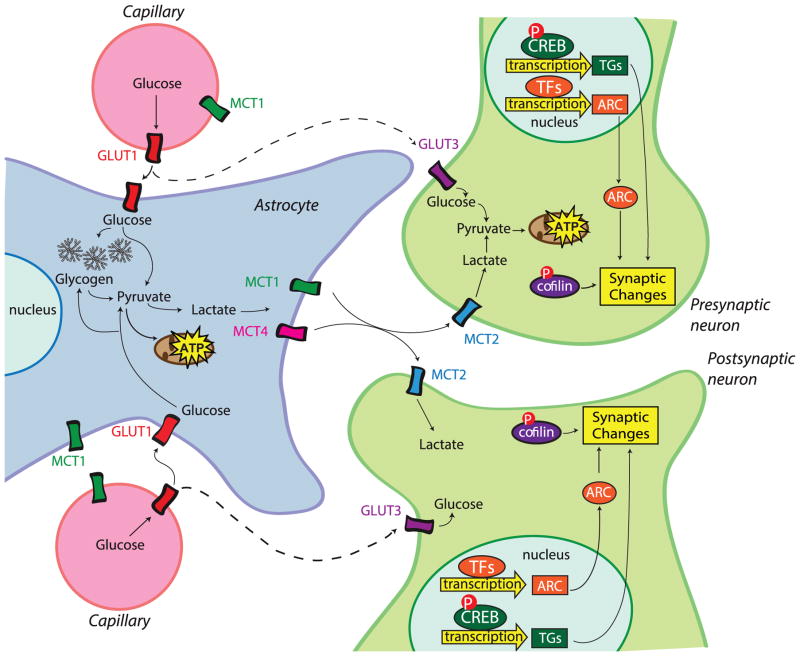
Figure 2. Astrocyte–neuron lactate coupling in long-term memory formation.
Glucose is taken up by astrocytes from surrounding capillaries via glucose transporters (GLUT1). Glucose can then be stored as glycogen in astrocytes or undergo glycolysis to become pyruvate. In astrocytes, pyruvate can be transported into the mitochondria or converted to lactate, which can be exported out of the astrocyte by the monocarboxylate transporter 1 or 4 (MCT1/4) and transported into neurons via MCT2. In neurons, astrocytic-derived lactate is converted back into pyruvate and transported into the mitochondria to generate ATP. Glucose may also be transported from the capillaries into neurons through the glucose transporter (GLUT3). In Suzuki et al. 2011, we showed that the astrocytic-derived lactate from glycogenolysis is critical for long-term memory formation in rats and for the underlying regulation of molecular changes required for long-term memory formation. These changes include the phosphorylation of the transcription factor CREB, the expression of target genes (TGs), the expression of immediate early genes such as Arc, and the phosphorylation of the actin-binding protein cofilin. Whether glucose transport into neurons through GLUT3 is important for memory formation remains to be determined.
In this review, we will specifically discuss the critical contribution of astrocytes, acting as regulators of glucose metabolism, to memory formation and storage.
Glycogen and glucose metabolisms play critical roles in memory formation
Studies by Paul Gold and colleagues identified systemic glucose as an intermediary of the memory-enhancing effect of norepinephrine (Gold and Korol 2012). Memories encoded in arousal states are remembered better (i.e., for longer periods and with greater detail), and arousal is well known to regulate the release of epinephrine from the adrenal glands. Epinephrine binds adrenergic receptors (ARs) on hepatocytes and initiates the breakdown of glycogen, a polymer of glucose stored in the liver (Sutherland and Rall 1960), leading to release of glucose into the bloodstream. Systemic glucose injections at doses comparable to those found in the blood after epinephrine treatment are sufficient to enhance memory, whereas low liver glycogen storage, as in food-deprived or aged rats, is associated with a lack of memory enhancement following epinephrine treatment (Morris et al. 2010; Talley et al. 2000). Conversely, peripherally blocking adrenergic receptors blocks the ability of epinephrine to enhance memory and increase blood glucose. Collectively, these studies support the conclusion that a major mechanism underlying the actions of epinephrine released by arousal is the increase in blood glucose.
The effect of glucose as a memory enhancer has been observed with both systemic and intracerebral injections, and it has been linked to the regulation of either norepinephrine or acetylcholine release. Ragozzino and colleagues showed that both systemic and intra-hippocampal injections of glucose, like injections of epinephrine, enhance spontaneous alternation, a form of spatial working memory, and increase the release of acetylcholine in the hippocampus (Ragozzino et al. 1998; Ragozzino et al. 1996).
The understanding of the role of glucose on memory modulation was considerably advanced by the observation that when rats are tested on a spontaneous alternation task, the levels of extracellular glucose in the hippocampus decrease significantly. Hence, it was suggested that learning and memory consume glucose, presumably to support the energy demands of the brain as it processes the new experience and stores the important information (McNay et al. 2000; McNay et al. 2001; McNay and Sherwin 2004).
Indeed, the brain consumes high levels of energy: the adult brain uses on average about 20% of total body energy, despite accounting for only 2% of total body weight. Glucose, the major source of energy entering the brain from the circulation, can either be directly metabolized or stored in the form of glycogen. In the mature brain, glycogen is stored mostly in astrocytes (Brown et al. 2004; Brunet et al. 2010; Cali et al. 2016; Cataldo and Broadwell, 1986; Maxwell and Kruger 1965; Petersen 1969; Pfeiffer-Guglielmi et al. 2003; reviewed in Waitt et al. 2017), and, under conditions of high energy demand such as glucose deprivation or intense neural activity, can be catabolized to rapidly deliver metabolic substrates (i.e., pyruvate and lactate) (Brown and Ransom 2015). Although neurons possess the enzymatic machinery to store and break down glycogen, under physiological conditions they suppress glycogen storage through a series of mechanisms. In fact, glycogen storage in neurons is observed only in severe neurological diseases such as progressive myoclonus epilepsy or Lafora disease, a brain disorder characterized by recurrent seizures (epilepsy) and a decline in intellectual function (Vilchez et al. 2007). Thus, glucose, either directly metabolized via glycolysis or supplied by astrocytic glycogenolysis, may fuel the high energy demands associated with the cellular changes underlying learning, memory formation, and memory storage.
One long-debated question is whether neurons directly import glucose entering the brain from the blood and use it immediately to provide the energy required to support their functions. An alternative model, suggested by Pellerin and Magistretti (Pellerin and Magistretti 1994), proposes that the high energy demands of stimulated neurons are supported by astrocytes, which supply the neurons with lactate produced via aerobic glycolysis, thereby providing the energy required for the activity-induced neuronal functions; hence, in the case of learning, for the changes involved in processing and storing memories. It is also possible that both mechanisms are utilized, perhaps in response to specific conditions.
The model proposed by Magistretti and Pellerin has been highly debated. These debates are complex and likely reflect the intricacy of metabolic regulations in different conditions. Given the variety of these conditions and systems, we will not be able discuss the points of the debate in this manuscript, thus we refer to several reviews reporting them (Chih et al., 2001; Chih and Roberts, 2003; Dienel and Hertz, 2001; Pellerin and Magistretti, 2003, 2012; Aubert et al., 2005; Dienel, 2010, 2017; DiNuzzo et al., 2010; Steinman et al. 2016). We will, however, discuss the literature important for the findings about the roles of glycogen, glucose and lactate in learning and memory as well as in brain plasticity.
Several studies reported that stimulation of brain areas increases glycogenolysis and glycolysis, as well as glucose uptake, in astrocytes, consistent with the idea that astrocytic glycogen and glucose metabolism are needed to sustain activity-dependent processes. For example, NMR spectroscopy, which allows measurement of lactate in vivo, revealed an elevation of lactate in the human visual cortex during physiologic photic stimulation (Prichard et al. 1991), and microsensor-based measures revealed an increase in extracellular lactate concentration in the dentate gyrus of the rat hippocampus after electrical stimulation of the perforant pathway (Hu and Wilson 1997). Moreover, whisker stimulation in the awake rat leads to rapid glycogen breakdown in layer IV of the somatosensory cortex (Swanson et al. 1992) and results in preferential increase in glucose uptake into astrocytes in comparison with neurons in the somatosensory cortex in vivo (Chuquet et al., 2010), although more mechanistic details need to be understood (Dienel and Cruz 2015). The physical position of astrocytes, between the blood flow on one side and neurons on the other, further supports the idea that astrocytic regulation of glucose metabolism subsidizes the energy requirements of activity, plasticity, learning, and memory formation.
In accordance with this view, metabolic profiling of astrocytes and neurons revealed distinct features indicating that glycolysis occurs mainly in astrocytes. For example, cultured neurons produce CO2 at a much higher rate than astrocytes, and their respective enzymatic profiles are consistent with the relative predominance of glycolysis in glial cells and oxidation in neurons (Bélanger et al. 2011; Hamberger and Hydén 1963; Hydén and Lange 1962). In addition, acutely isolated, FACS-purified astrocytes exhibit a primarily glycolytic profile (Lovatt et al. 2007; Zhang et al. 2014). Finally, the enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (Pfkfb3), which promotes glycolysis, is active in astrocytes but constantly subjected to proteasomal degradation in neurons (Bolaños et al. 2010; Herrero-Mendez et al. 2009), once again supporting the idea that astrocytes are the primary sites of glycolysis. Thus, a large body of evidence converges on the conclusion that astrocytes are predominantly glycolytic cells, whereas neurons are not, and instead exhibit high oxidative activity.
The first demonstration that astrocytic glycolysis is critical for learning and memory came from studies performed by Leif Hertz, Marie Gibbs, and colleagues, who showed that glycogenolysis is necessary for memory formation. Using taste avoidance training in a day-old chick, they showed that intracranial injection of an inhibitor of glycogen phosphorylase, 1,4-Dideoxy-1,4-imino-d-arabinitol (DAB), impaired memory in a dose-dependent manner, and concluded that glycogenolysis is a critical requirement for long-term memory storage (Gibbs et al. 2006). In agreement with this conclusion, breakdown of glycogen in the brain increases significantly during sensory activation in rats (Cruz and Dienel 2002; Swanson et al. 1992), and later studies detailed below demonstrated that glycogen contributes to several types of memory formation in rats and mice. In addition to glycogenolysis, aerobic glycolysis may also be necessary for memory formation, as revealed by experiments in which the glycolysis inhibitor 2-deoxyglucose was injected into the brains of 1 day-old chicks at training, resulting in long-term memory impairment (Gibbs et al. 2007). Thus, several studies have converged on the conclusion that glycogenolysis and aerobic glycolysis, resulting in the production of lactate, are critically linked to memory formation. This raises several questions: How exactly does this regulation occur? How are astrocytes functionally coupled to neurons? What are the target mechanisms that consume high levels of energy upon learning and allow memory consolidation to occur?
Astrocytic glycogenolysis, aerobic glycolysis, and lactate are critical for long-term memory formation in several brain regions
A model proposed by Pellerin and Magistretti (Pellerin and Magistretti 1994), known as the astrocyte–neuron lactate shuttle (ANLS), suggests that astrocyte glycolysis and neuronal oxidation play coordinated roles in long-term memory formation via transport of lactate. This model predicts that excitation, and hence glutamate release, stimulates the uptake of glutamate by astrocytes, which is converted into glutamine (glutamate–glutamine cycle), eventually sustaining synaptic release of glutamate. This cycle requires energy from astrocytes, which would therefore activate glucose uptake from the blood and metabolize it into lactate. Lactate, released by astrocytes via monocarboxylate transporters (MCTs), can enter other types of cells using similar transporters, which operate on the basis of concentration gradients of protons and monocarboxylate across the plasma membrane (Halestrap 2013; Pierre and Pellerin 2005). MCTs are proton-linked plasma membrane transporters that carry molecules containing one carboxylate group (hence the term monocarboxylates), such as lactate, pyruvate, and ketone bodies, across plasma membranes. MCT1 is expressed in astrocytes, ependymocytes, oligodendrocytes, and endothelial cells of blood vessels, whereas MCT4 is selectively expressed by astrocytes and enriched at synaptic sites (Pierre and Pellerin 2005; Rinholm et al. 2011; Suzuki et al. 2011). MCT2, on the other hand, is selectively expressed by neurons (Debernardi et al. 2003).
Thus, lactate, released by astrocytes via MCT4 and MCT1 is transported by MCT2 into neurons, where it is converted to pyruvate that is subsequently metabolized through oxidative phosphorylation in mitochondria to produce 14–17 ATPs per lactate molecule (Figure 2). This lactate supply from astrocytes to neurons provides an explanation for how neurons might handle the high-energy requirements evoked by active processes in response to stimuli.
The first studies that described the ANLS were performed in vitro, and questions were raised about whether these mechanisms occurred in vivo (Chih and Roberts 2003; Dienel and Cruz 2004; Gjedde et al. 2002). However, studies by Hertz and Gibbs in the chick described above suggested that glycogenolysis is involved in memory formation (for review see Gibbs 2016). In these studies, the chicks were exposed to two beads, one red and one blue, and trained to avoid pecking the red bead by association with an aversive taste. During the retention test, the ratio between the number of pecks of red and blue beads was measured, revealing an increase in avoidance of pecking red beads; the change in the discrimination ratio was indicative of memory (Hertz et al. 1996). The initial results showed that glycogen levels in the forebrain decreased 30 minutes after learning, concomitant with an elevation of glutamate, suggesting de novo synthesis of glutamate from glycogen to support memory consolidation (Hertz et al. 2003; O’Dowd et al. 1994). A few years later, the same group showed that DAB impairs taste aversion memory in day-old chicks when infused into the multimodal forebrain association region, the intermediate medial mesopallium (IMM), a brain region required for memory consolidation (Gibbs et al. 2006; Gibbs and Hertz 2008). They then found that glutamine was sufficient to rescue memory, and hence proposed that glycogenolysis was critical for the glutamate/glutamine shuttle, which may also be affected by DAB. A subsequent study from the same authors demonstrated that L-lactate is also sufficient to rescue chick taste aversion memory after treatment with an inhibitor of either glycogenolysis (DAB) or glycolysis (2-deoxyglucose) (Gibbs et al. 2007). Furthermore, administration of D-lactate, the competitive non-biologically active form of lactate, impaired chick taste aversion memory with a time delay that suggested it was inhibiting L-lactate metabolism and not uptake, leading the authors to conclude that astrocytic metabolism through glycogenolysis and lactate metabolism is critical for memory formation (Gibbs and Hertz 2008). These findings supported the idea that learning in the neonatal chick relies on the breakdown of glycogen for glutamate synthesis in astrocytes (Gibbs et al. 2007).
However, an additional interpretation is that lactate produced by glycogenolysis is transported into neurons for their use, thus contributing to support neuronal modifications critical for memory formation. We tested this hypothesis in vivo in mammalian brains, focusing specifically on whether mechanisms of glycogenolysis, astrocytic lactate release and transport into neurons are involved in memory consolidation, the process that stabilizes a newly formed, initially fragile memory into a long-lasting stable representation (Alberini 2009, Dudai 2004).
Using adult rats trained in an inhibitory avoidance (IA) task, in which the animals learn to avoid a context previously paired with a foot-shock (a contextual response to threat), we demonstrated that lactate transported from astrocytes to neurons in the hippocampus plays a critical role in long-term memory consolidation (Suzuki et al. 2011). Specifically, we found that hippocampal astrocytic glycogenolysis is required for memory consolidation, in vivo hippocampal long-term potentiation, and learning-induced increases in synaptic and cellular macromolecular changes, including expression of the immediate early gene (IEG) activity–regulated cytoskeleton-associated protein (Arc or Arg3.1) and phosphorylation of the transcription factor CREB and of the actin-severing protein cofilin, all of which are markers of long-term synaptic plasticity. In fact, DAB injected bilaterally in the dorsal hippocampus before or immediately after IA training persistently disrupted memory retention, and this disruption was prevented by co-injection of L-lactate, but not equicaloric concentrations of glucose. In addition, after IA training the hippocampal extracellular concentration of lactate, measured by in vivo microdialysis, significantly increased and remained elevated for more than 1 hour, returning to baseline by approximately 90 minutes post-training. This increase in lactate was completely abolished by bilateral DAB injection into the hippocampus, suggesting that it was the result of astrocytic glycogenolysis.
Furthermore, we found that hippocampal injection of the inactive isomer D-lactate before training also blocks long-term memory retention, suggesting that lactate metabolism is critical for long-term memory formation. Similar effects on memory retention were observed following knockdown of the lactate transporters (MCTs). Notably, although the memory impairments induced by the knockdown of lactate transporters expressed in astrocytes (MCT1 and MCT4) was rescued by addition of L-lactate, the impairment induced by knockdown of the transporter expressed in neurons (MCT2) was not, consistent with the idea that the transport of lactate out of astrocytes and into neurons is critical for memory formation. In accordance with this interpretation, a lactate gradient between astrocytes and neurons was recently observed and characterized at high resolution in vivo using two-photon microscopy (Machler et al. 2016). Therefore, we concluded that glycogenolysis and astrocyte–neuron lactate transport critically supports neuronal functions required for long-term memory formation. A more recent investigation further supported the role of astrocytic lactate in memory formation by showing that IA training induces hippocampal expression of molecules involved in astrocytic-neuronal transport, such as MCTs and the expression of lactate dehydrogenase (LDH) A and B, the enzymes that catalyze the interconversion of lactate and pyruvate (Tadi et al. 2015).
Similar conclusions were reached by Newman et al. (2011), who employed sensitive bioprobes to measure brain glucose and lactate levels in the hippocampus of rats while they underwent a spatial working memory task. They found that while extracellular glucose decreased, lactate levels increased during task performance, and intrahippocampal infusions of L-lactate enhanced memory in this task. In addition, pharmacological inhibition of astrocytic glycogenolysis with DAB impaired memory, and this impairment was reversed by either L-lactate or glucose, both of which can provide lactate to neurons in the absence of glycogenolysis. In this study, as in ours, blockade of the MCTs responsible for lactate uptake into neurons impaired memory, and this impairment was not reversed by either glucose or L-lactate, again supporting the idea that lactate uptake by neurons is necessary to support memory formation. The authors concluded, as we did, that astrocytes regulate memory formation by controlling the provision of lactate to sustain neuronal functions.
Additional studies based on genetic approaches support these conclusions. Delgado-Garcia and colleagues found that knockout of glycogen synthase in the nervous system of mice impairs both hippocampal LTP and associative learning (Duran et al. 2013). In addition, Boury-Jamot et al. (2016) and Zhang et al. (2016) reported that the consolidation and reconsolidation of appetitive conditioning using drugs of abuse (i.e., cocaine-conditioned place preference or self-administration) are also dependent on glycogenolysis and the directional transport of lactate from astrocytes to neurons via MCTs in the basolateral amygdala (BLA) of rats. Furthermore, extracellular lactate, as measured by in vivo microdialysis, is elevated in the BLA after IA training and retrieval (Sandusky et al. 2013).
Consistent with the results of these studies, we found that BLA glycogenolysis is critical for IA memory formation, as demonstrated by the fact that bilateral injection of DAB into the BLA 15 minutes prior to IA training severely and persistently disrupted memory retention in rats. This impairment was not rescued by a reminder shock delivered in a different context, a protocol that re-instates extinguished memories (Inda et al. 2011), suggesting that blocking glycogenolysis in the amygdala before training disrupts the consolidation process. Co-administration of L-lactate with DAB in the amygdala rescued the memory impairment, confirming the importance of the roles of glycogenolysis and lactate in diverse brain areas for IA memory consolidation (Figure 3).
Figure 3. Blocking glycogenolysis in the basolateral amygdala impairs long-term memory.
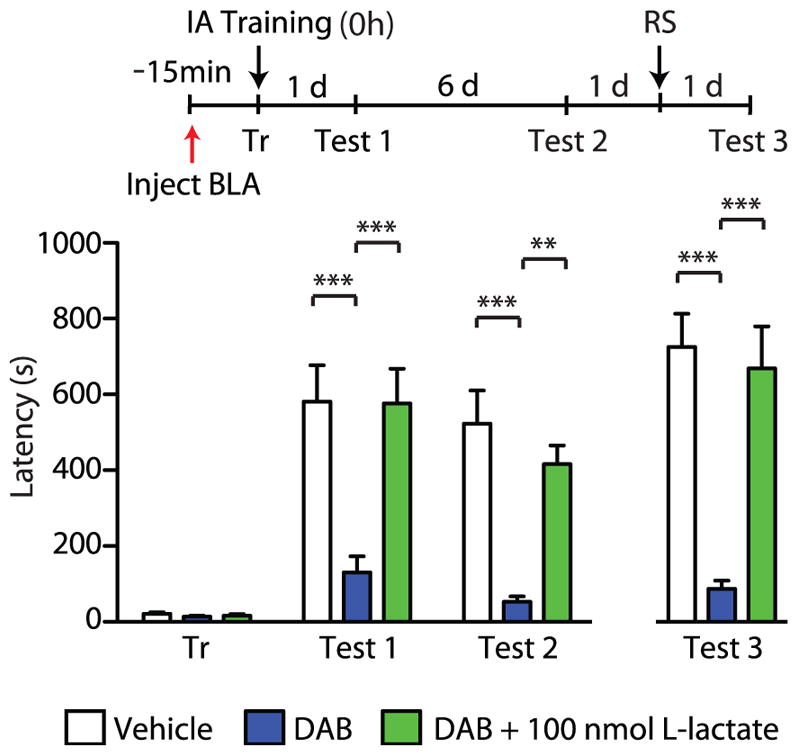
Long-term memory retention, expressed as mean latency values ± SEM in seconds (s), tested 1 day (d) after training (Test 1), 6 d later (Test 2), and after a reminder shock given in a distinct context (RS, Test 3). Vehicle, DAB (300 pmol), or DAB (300 pmol) + L-lactate (100 nmol) was injected bilaterally into the basolateral amygdala (BLA) 15 min prior to inhibitory avoidance (IA) training (red arrow). Statistical significance was assessed by two-way ANOVA followed by Bonferroni’s post hoc tests compared to vehicle (**p < 0.01; ***p < 0.001; n = 7–8/group).
The target functions fueled by lactate and/or glucose metabolism are still largely unknown. Brain energy is needed to support the electrical pulses required for neuronal communication, and for many housekeeping activities, including protein synthesis, phospholipid metabolism, neurotransmitter cycling, and transport of ions across cellular membranes (Du et al. 2008). As shown by the studies described above, lactate metabolism supports long-term memory formation and the training-dependent increase in expression of several molecules related to activity and plasticity, including Arc, cFos, and Zif268 (Gao et al. 2016; Suzuki et al. 2011; Yang et al. 2014). These effects are NMDA receptor–dependent, implying that lactate-dependent changes are linked to activity and/or plasticity (Yang et al. 2014). In vivo, lactate is sufficient to maintain neuronal activity (Wyss et al. 2011) and recent data showed that interstitial K+ elevations can activate a channel on the astrocyte membrane through which astrocytic lactate can flow into the interstitium, in parallel with the established transport via MCTs (Sotelo-Hitschfeld et al., 2015). This route for astrocytic lactate release is coupled to the membrane potential and allows lactate release against a concentration gradient, whereas the MCT is electro-neutral and net flux is governed by the trans-membrane concentrations of H+ and lactate. Furthermore, an astrocytic mechanism via bicarbonate-responsive soluble adenylyl cyclase leading to glycogen breakdown, enhanced glycolysis, and the release of lactate into the extracellular space, which is subsequently taken up by neurons for use as an energy substrate has been demonstrated (Choi et al. 2012). Collectively these studies support the conclusion that lactate delivery by astrocytes to neurons can be regulated in many ways in response to activity and studies are needed to understand whether parallel or selective mechanisms occur in vivo upon learning. Nevertheless it emerges that lactate is needed to support not only ionic membrane homeostasis after depolarization, but also numerous other neuronal functions required for long-term modifications associated with memory formation and storage.
Glycogenolysis and lactate are important in arousal/stress-mediated memory consolidation
An event is well remembered for a long time if it is embedded in emotions. Emotional arousal leads to more detailed and long-lasting memories, and the stress hormones adrenaline (also known as epinephrine) and cortisol (corticosterone in rodents) are sufficient to mediate and modulate memory consolidation (McGaugh 2015).
Because epinephrine does not freely pass the blood–brain barrier (BBB), it may modulate memory consolidation by activating β-adrenergic receptors located peripherally on vagal afferents projecting to the nucleus of the solitary tract in the brainstem. Noradrenergic projections from this region influence neuronal activity in other brain regions, including the amygdala and hippocampus (Miyashita and Williams 2004; Williams et al. 2000). Consistent with this, increases in noradrenaline (NA) levels in the hippocampus have been observed in vivo in rats as early as 20 minutes after peripheral epinephrine injection, peaking one hour after application, and this effect is completely blocked by lidocaine injection into the nucleus of the solitary tract (Miyashita and Williams 2004). Importantly, neurons arising from the nucleus of the solitary tract project to the locus coeruleus, an area that releases NA upon stimulation and arousal (Gibbs et al. 2010; Lopes et al. 2016; Sara 2009; Sara et al. 1994).
In mammalian brains as well as in cultured astrocytes, NA stimulates glycogenolysis (Magistretti 1988; Quach et al. 1988; Subbarao and Hertz 1990). Application of NA is sufficient to modulate memory consolidation through the activation of α- and β-adrenergic receptors (βARs) present in brain regions, such as the amygdala and the hippocampus (Roozendaal and McGaugh 2011). Because βAR blockers such as propranolol have been reported to interfere with memory consolidation and strengthening in rodents as well as in humans (Cahill et al. 1994; Dornelles et al. 2007; Przybyslawski et al. 1999), and have also been proposed as potential treatments for anxiety disorders, such as panic disorder and post-traumatic stress disorder (Ravaris et al. 1991; Vaiva et al. 2003), the action of NA through these receptors is of particular interest.
In the rat hippocampus, which is required for the consolidation of explicit/episodic memories, β1ARs predominate; both β2ARs and β3ARs are also present, although they are distributed differently (Milner et al. 2000; Rainbow et al. 1984; Summers et al. 1995), suggesting that the two types of receptors may have distinct roles. A variety of experiments with NA or βAR antagonists (e.g., propranolol) suggested that βARs play roles in memory encoding, modulation, and retrieval in humans and in rodents (Cahill et al. 1994; Przybyslawski et al. 1999; Summers et al. 1995). Studies of mice lacking β1ARs or treated with selective β1AR agonists or antagonists revealed critical roles of this receptor subtype in both synaptic plasticity and memory, with particular emphasis on memory retrieval (Murchison et al. 2004; Ramos et al. 2005; Winder et al. 1999). On the other hand, genetic deletion of β2ARs in mice impairs memory modulation by stress or corticosterone, and also impairs hippocampal plasticity, consistent with a role of β2ARs in the amygdala and its modulatory effects on the hippocampus and prefrontal cortex (Roozendaal and McGaugh 2011; Schutsky et al. 2011; Zhou et al. 2013).
In general, the functional contributions of βARs to memory processes are thought to result mainly from their effects on neurons, and have therefore been studied largely in neuronal/synaptic models (O’Dell et al. 2015). However, in addition to being expressed in pre- and postsynaptic compartments of neurons, βARs are also found in other cell types, particularly in astrocytes (Catus et al. 2011; Mantyh et al. 1995; Shao and Sutin 1992; Zhu and Kimelberg 2004). More specifically, it has been suggested that β2ARs in the nervous system are expressed predominantly in glia (Cash et al. 1986; Catus et al. 2011; Mantyh et al. 1995; Waeber et al. 1991), whereas β1ARs are found primarily in neurons, at synaptic junctions (Cash et al. 1986; Mantyh et al. 1995; Waeber et al. 1991). This suggestion raises the questions of which βAR subtype (β1AR or β2AR) and which βAR-expressing cells mediate hippocampal memory consolidation.
Moreover, β2adrenergic receptors (β2AR) play critical roles in memory consolidation in the avian cortex, and pharmacological experiments combining antagonists or agonists of βARs led Hertz and Gibbs to conclude that glycogenolysis stimulated by β2ARs is necessary for memory formation (reviewed in Gibbs 2016).
In recent studies, we sought to determine whether the contribution of βARs in the hippocampus to memory consolidation involves mechanisms related to glycogenolysis and astrocytic metabolism. Using our IA paradigm in young adult rats, we found that bilateral injections of propranolol into the dorsal hippocampus significantly impaired long-term memory, and that this impairment was not reversed following a reminder shock delivered in a different context, indicating that βARs in the hippocampus are required for memory consolidation. Propranolol also blunted the learning-evoked induction of plasticity mechanisms such as induction of pCREB, pCamKIIα, and Arc. Both the behavioral and molecular disruptions were rescued by L-lactate, but not by an equicaloric concentration of glucose. These results suggested that production of astrocyte-derived lactate (i.e., lactate derived from glycogenolysis in astrocytes) is targeted by propranolol. To determine which of the β1- and β2ARs might be involved in the training-dependent behavioral and molecular changes, we systemically injected either the β2AR-selective antagonist ICI-118,551 or the β1AR-selective antagonist betaxolol before training, and then conducted hippocampal microdialysis experiments. The results revealed that both training-dependent lactate release and memory retention were significantly blocked by antagonists of β2ARs, but not β1ARs. Likewise, bilateral injections of the same antagonists into the dorsal hippocampus confirmed that antagonists of β2ARs, but not β1ARs, impaired memory retention. The effects on memory retention persisted. Collectively, these data indicated that β2ARs, rather than β1ARs, in the hippocampus contribute to IA memory consolidation, and that they do so by engaging mechanisms that control elevation of lactate levels in the extracellular space of the hippocampus upon training. Our previous experiments showing that learning-evoked lactate increase is blocked by DAB (Suzuki et al. 2011) led us to conclude that the β2AR-dependent increase in lactate level evoked by training derives from glycogenolysis.
Using autoradiography of ligand binding (in the absence of specific antibodies, for which we extensively screened, but could not find), we found that the distribution of β2AR in the hippocampus overlapped to a great extent with the distribution of the astrocyte marker glial fibrillary acidic protein (GFAP) visualized by immunohistochemistry, again supporting the idea that β2ARs are enriched in astrocytes rather than neurons (Gao et al. 2016). Employing astrocyte- or neuron-selective adeno-associated virus (AAV) under the control of either a modified GFAP or a synapsin-specific promoter, we expressed antisense or short hairpin (shRNA) sequences to significantly downregulate β2AR expression in either astrocytes or neurons. We found that astrocytic, but not neuronal, knockdown of β2AR led to persistent memory disruption. This disruption was fully rescued by L-lactate, providing evidence for the conclusion that β2ARs expressed in hippocampal astrocytes -via lactate production-, but not β2ARs expressed neurons, are critical for long-term memory formation.
It is interesting to note that also in chicks β2ARs had been previously described as the critical βAR subtype involved in glycogenolysis, despite the fact that the distribution of βAR subtypes does not correlate in chick and mammalian brains (Hutchinson et al. 2007), suggesting that β2ARs expressed by astrocytes may have a conserved role in regulating glycogenolysis. In agreement with the general role of β2ARs in glycogenolysis, lactate production from aerobic glycolysis coupled to β2AR stimulation has also been identified in muscle during shock states associated with reduced or maintained blood flow (Levy et al. 2008).
It remains to be determined whether the lactate is produced solely from glycogenolysis and/or also from glucose import and directly entering glycolysis. Regardless, our data implicating hippocampal β2ARs, rather than β1ARs, in memory consolidation, in conjunction with the observation that supplying lactate rescues the IA memory impairment caused by hippocampal astrocytic β2AR knockdown, strengthens the conclusion that lactate produced by astrocytes is a key mediator of memory formation under arousal conditions (Suzuki et al. 2011).
Similar conclusions were provided by Jensen et al. (2016) who generated inducible astrocyte-specific β2AR knock-out mice by crossing homozygous β2AR floxed mice (Adrb2flox) and mice with heterozygous tamoxifen-inducible Cre recombinase-expression driven by the astrocyte specific L-glutamate/L-aspartate transporter promoter (GLAST-CreERT2). This study did not find any differences in physical health or motor functions between the knock-out mice and controls; however, it reported deficits in the ability of aged, but not young adult mice, in the water maze task. The study also found LTP impairment in hippocampal brain slices of aged knock-out mice maintained in low glucose media. The lack of effects on young mice may be due to the level of arousal of the task employed and future studies are needed to address this question.
In light of all these findings, we agree with Hertz and Gibbs (Hertz and Gibbs 2009) in suggesting that the level of arousal evoked by an experience may dictate whether glycogenolysis and/or glycolysis are recruited to promote memory consolidation. Further, we propose that if only memories of arousing experiences recruit the astrocytic β2AR mechanism, this may represent a distinct mechanistic signature of this type of memories. This would suggest that under physiological conditions glycogen acts to provide supplemental substrates when glucose is unable to support function during increased energy demand (Waitt et al. 2017). Again, future experiments should seek to address these important issues.
Notably, reminiscent of the effects of the glycogenolysis inhibitor DAB (Suzuki et al. 2011), direct supply of equicaloric concentrations of glucose into the hippocampus along with pharmacological blockers of β2AR failed to replicate the effect of lactate, and only at higher glucose concentrations were the memory impairments caused by β2ARs disruption transiently rescued. Although further experiments are needed to interpret this result, one possible explanation is that activity-dependent processes promote blood glucose entry into astrocytes rather than directly into neurons. Glucose then would be metabolized by astrocytes and, at least in part, converted into lactate, which would ultimately be transported from astrocytes to neurons (Magistretti and Pellerin 1999).
Mounting evidence indicates that astrocytic regulation of metabolism plays a role in many neurodegenerative diseases and brain-related pathologies, including Alzheimer’s disease, Huntington disease, multiple sclerosis, and amyotrophic lateral sclerosis (Maragakis and Rothstein 2006). For example, patients with multiple sclerosis exhibit dysregulated expression of glycolytic enzymes in both active and inactive lesions, and elevated levels of the lactate-producing enzyme LDHA in astrocytes within inactive lesions (Nijland et al. 2015). Furthermore, brain samples from patients with Alzheimer’s disease exhibit increases in both β2- and β1ARs, and β2 antagonists have yielded promising potential therapeutic results in mouse models (Fu and Jhamandas 2014; Dong et al. 2012). Further study will be required to fully understand the involvement of astrocytic β2ARs in neurodegenerative diseases. We suggest that disruption of the metabolic activation via astrocytic β2ARs in regions supporting cognitive functions may contribute to the pathological features of those diseases. Hence, targeting astrocytic β2AR mechanisms may help prevent or repair these disorders and/or prevent their precipitation by stress.
How is lactate used to promote learning and memory?
The involvement of astrocyte-derived lactate in long-term memory formation shifts the view within the field from a neurocentric model of learning and memory to one in which multi-cell type cooperation plays a key role. An important question that remains to be addressed is: What does the lactate do?
Lactate, as found in other systems (i.e. muscle) (Brooks 2007; Brooks 2011; Brooks 2016) can be used as energy substrate. Based on the evidence for a critical role of MCT2 in long-term memory formation (Newman et al. 2011; Suzuki et al. 2011), our studies and those of several other laboratories mentioned above suggested that the major role of lactate might be metabolic, in support of the energy-intensive processes induced by learning. MCT2s are enriched in synapses, as showing by synaptoneurosomal (Suzuki et al. 2011) and electron microscopy studies which revealed their localization in both axons and spines (Bergersen et al. 2001; Bergersen et al. 2005). The idea that lactate entering activated synapses plays a metabolic role would be consistent with the high energy demands of membrane and cellular processes essential for long-term synaptic plasticity and memory, which include—to mention only a few—cytoskeletal remodeling, receptor trafficking, synaptic release, and local mRNA translation (Basu et al. 2007; Fukazawa et al. 2003; Harris et al. 2012; Lamprecht and LeDoux 2004; Malinow and Malenka 2002; Santini et al. 2014; Shi et al. 1999; Sudhof 2013; Xu et al. 2012).
In addition to its role as a metabolite and energy substrate, lactate has been shown to signal via changes in the NADH/NAD+ ratio, and hence redox regulation (Brooks 2009), a mechanism found to be important in long-term plasticity and memory (Knapp and Klann 2002; Massaad and Klann 2011). In fact, using cultured cortical neurons, Yang et al., (2014) reported that L-lactate application enhances intracellular NADH levels, promotes plasticity-related gene expression, and potentiates NMDA receptor signaling. These authors also found that although application of NADH recapitulated the effects induced by L-lactate on NMDA activation, application of pyruvate was insufficient to induce these changes, leading them to conclude that lactate is more than just an energy substrate.
Several studies have focused on other types of lactate signaling mechanisms. For example, lactate also signals via prostaglandin action and contributes to vasomotor regulation (Gordon et al. 2008). In addition, lactate was recently shown to signal through a specific G-protein–coupled receptor, GPR81/HCAR1, which is distributed in neurons (enriched in synaptic compartments), astrocytes, and vasculature cells, possibly linking neurotransmission, neurovascular coupling, and brain energy metabolism (Lauritzen et al. 2014; Morland et al. 2015). As described and discussed in an excellent review by Linda Hildegard Bergersen (Bergersen 2015), HCAR1 is mostly concentrated in neurons, on the synaptic membrane and intravesicular organelles. This distribution led Bergersen to propose the intriguing hypothesis that intravesicular lactate could contribute to lactate signaling. Moreover, in C2C12 myotube muscle cells, lactate application induced the expression of the lactate receptor HCAR1 and of lactate transporters (MCT1/4), perhaps indicative of a positive feedback system, whereas pyruvate did not replicate this effect (Sun et al. 2016). In sum, our understanding of how lactate provides critical support for long-term memory formation is still in its infancy, and we cannot exclude the possibility that lactate plays multiple necessary roles in this process (Steinman et al. 2016).
Role of astrocyte–neuron metabolic coupling in memory formation during development
The metabolic demands of the developing brain are very high. Human brain glucose demand peaks during childhood (at an age of around 5 years) when glucose used by the brain represents approximately 60% of total body energy, compared to approximately 20% in adulthood (Kuzawa et al. 2014). This significant difference in energy requirements between the adult and developing brain may be reflected in differing roles of astrocyte and neuron metabolism in memory formation during development versus adulthood.
Astrocytes are critical for brain development. Due to their active contribution to several developmental processes, including neurogenesis, angiogenesis, axonal outgrowth, synaptogenesis, and synaptic maturation and pruning, dysfunction of astrocytes may contribute to the onset of neurodevelopment disorders, which could lead to learning and memory disabilities during adulthood (Clarke and Barres 2013). Although some astrogenesis has been reported in adults (Zhao et al. 2007), most astrocytes in the rodent brain are generated postnatally during the first 3 weeks of life, concurrently with the end of the initial neurogenic wave (Bandeira et al. 2009; Molofsky and Deneen 2015; Reemst et al. 2016). Astrocytes are distributed over all areas of the CNS, and likely migrate to their final destination shortly after their birth, leading to similar distributions between the neonatal and adult brains (Taft 2005; Tsai et al. 2012).
Therefore, given the extensive gliogenesis that greatly changes the cellular composition of the postnatal brain (Nedergaard et al. 2003), astrocytes in early development could either differentially influence memory processes, or remain uninvolved due to their functional immaturity. It would be interesting to determine whether astrocyte–neuron lactate metabolic coupling is necessary for memory formation during early development, as it is in adulthood.
Astrocyte–neuron metabolic interactions differ in several ways between the neonatal and adult brain (Brekke et al. 2015). For example, in the adult brain under normal conditions, glucose is the main energy substrate. By contrast, during the first three weeks following birth, the neonatal rat brain is characterized by reduced glucose utilization, coincident with lower expression of glucose transporters (GLUT) (Vannucci 1994; Vannucci et al. 1994; Vannucci and Simpson 2003) and the utilization of other substrates, such as ketone bodies, as energy sources (Hawkins et al. 1971; McKenna 2012). Indeed, during consumption of high-fat milk, ketone bodies provide a substantial fraction of the energy required by the neonatal brain (Dombrowski et al. 1989; Nehlig 2004). These nutrients are moved across the BBB through monocarboxylate transporters (MCTs) (Newman and Verdin 2014) whose expression decreases quickly after weaning, whereas expression of glucose transporters increases (Vannucci and Simpson 2003).
Even if the neonatal brain relies on alternative energy sources, glucose is indispensable for normal development. Using 13C-labeled glucose and 13C-labeled acetate, Morken and colleagues (2014) probed glycolysis, the pentose phosphate pathway (PPP), pyruvate carboxylation, and metabolic interactions between astrocytes and neurons in the 7-day-old rat brain, and compared these parameters to those of adult rats. In the immature brain, they observed lower transfer of glutamate from neurons to astrocytes, coupled to higher transfer of glutamine from astrocytes to neurons, indicating that much less glutamate-glutamine cycle is operating in the brain during early development. This reduction in the level of glutamate available for glutamine synthesis is partly compensated by an increase in pyruvate carboxylation in comparison with in the adult brain. Moreover, in the neonatal brain a relatively larger fraction of glucose is metabolized via the PPP and pyruvate carboxylation than by the glycolysis pathway, which is less active than the adult brain (Brekke et al. 2015; Morken et al. 2014).
The glucose, preferentially processed by the PPP, plays a key role during the rapid growth that occurs during the first postnatal weeks (Bandeira et al. 2009) by providing the substrates required for nucleotide and lipid synthesis, myelination, and production of NADPH, a crucial cofactor involved in cellular defenses against oxidative stress (Yager and Ashwal 2009). Thus, a high PPP-to-glycolysis ratio may explain the ability of the neonatal brain to more efficiently protect itself against oxidative stress.
Why is there a lower rate of glycolysis during early development? Moreover, does glycolysis play any role in memory formation at those early ages? Very few studies have described if and how glucose metabolism and astrocyte–neuron metabolic interactions contribute to the formation of long-term memory, and to cognitive functions more generally, during development. The first descriptions of such a contribution to memory formation during development were those of Gibbs and Hertz, described above, who used neonatal chicks in their studies and found that they require the breakdown of glycogen for glutamate synthesis in astrocytic memory mechanisms (Gibbs et al. 2007). Moreover, these authors reported mechanisms of glycogen-derived L-lactate (and/or pyruvate) transport between astrocytes and neurons in day-old chicks: in fact, either injection of D-lactate or pharmacological inhibition of MCT with α-cyano-4-hydroxycinnamate (4-CIN) inhibits their memory. Furthermore, glycogen breakdown and re-synthesis during learning and memory at this early developmental age were reported to be regulated by noradrenaline, serotonin, and ATP (Gibbs 2016). More specifically, it was shown that inhibition of β2-adrenergic, serotoninergic (5-HT2B), and purinergic P2Y1 receptors, all of which stimulate glycogenolysis, or of α2-adrenergic receptors, which favor glycogen synthesis, results in memory impairment in neonate chicks (Gibbs 2016).
In mammalian model systems (i.e., rodents) and humans, most studies have focused on the effect of glucose on memory. The essential finding of the last 20 years is that glucose, administered shortly before or after learning, or prior to memory retrieval, improves memory performance (Messier 2004). A similar effect has been observed at in 17- and 18-day-old rats, an age that corresponds to the period of infantile amnesia (Flint and Riccio 1999; Flint and Riccio 1997). Infantile amnesia refers to the inability of adults to remember early memories. In humans as in non-human animals memories formed during the age of infantile amnesia are very rapidly forgotten (Li et al. 2014; Madsen and Kim 2016).
Recently, we showed that IA experiences in infant rats, although apparently forgotten, are actually stored over the long-term in a latent form, as these memories can be reinstated later in life if reminders are experienced (Travaglia et al. 2016a). Contrary to what had been suggested previously, we found that the infant hippocampus (at postnatal day 17, PN17) is highly responsive and engaged in learning, and makes a necessary contribution to the formation of the latent, long-lasting memories by recruiting molecular mechanisms typical of critical periods (Travaglia et al 2016a and b). Hence, we suggested that, as in other brain functions and systems, the episodic learning system undergoes a critical period, during which the hippocampal system acquires functional competence through experience (Alberini and Travaglia 2017).
Two studies have shown that infantile amnesia in rats can be attenuated by administration of glucose at the time of memory retention testing (Flint and Riccio 1997) or the time of learning (Flint and Riccio 1999). In the first study, 17-day-old rats were subjected to IA conditioning; 24 hours later, the animals were injected systemically with either saline or glucose immediately before testing. The saline-injected rats exhibited weak memory performance, suggesting rapid forgetting, whereas this memory loss was significantly attenuated in glucose-injected rats (Flint and Riccio, 1997). In a following study, the same authors exposed 18-day-old-rats to an immediate post-training subcutaneous injection of glucose; these rats performed significantly better than saline-injected control animals on a retention test given 24 hours after training (Flint and Riccio, 1999). The mechanisms by which glucose attenuates infantile amnesia remain unknown, and a better understanding of the functional implication of glucose metabolism and astrocyte-neuron metabolic coupling in memory formation during development could provide important insights into how the memory system develops, as well as suggesting potential clinical applications for learning disabilities.
Conclusions
Although most previous studies on the biological mechanisms of memory formation and storage have centered on neuronal mechanisms, attention has recently been given to the roles of other brain cell types, and in particular glia. Here, we reviewed the evidence for the role of astrocytes in regulating glycogen and glucose metabolisms, and their functional coupling to neurons via lactate transfer, which is necessary for memory formation. Furthermore, we discussed studies performed in different species revealing the critical role of astrocytic βARs in regulating the consolidation and modulation of memory. We suggest that astrocytic glycogenolysis and/or glycolysis in conjunction with astrocytic–neuronal lactate shuttling, provide a mechanistic explanation for a critical role of astrocytes in memory formation and storage. These findings offer only an initial understanding of the metabolic cooperation between astrocytes and neurons in memory; future studies shall elucidate the functions of glycogenolysis and lactate in many other important and complex brain functions. Many questions remain to be addressed. In the context of memory research, the outstanding issues include whether the lactate-mediated metabolic coupling is a general mechanism engaged in different types of learning and memory, or only in memories encoded under arousal states. Other important outstanding questions include: Are these mechanisms common to any brain region in response to activity? Are they providing metabolic fuel or also cellular signaling? Which target mechanisms and cellular function do they support? How do these metabolic mechanisms mature during development, when the brain consumes the highest amount of energy? And finally, what are the implications when these mechanisms fail? These questions should be addressed in the near future, and the answers will greatly advance our understanding of the cooperative roles of different cell types in learning and memory processes.
Main Points.
Astrocyte glycogenolysis and lactate play a critical role in memory formation. Emotionally salient experiences form strong memories by recruiting astrocytic β2 adrenergic receptors and astrocyte-generated lactate. Glycogenolysis and astrocyte–neuron metabolic coupling may also play critical roles in memory formation during development, when the energy requirements of brain metabolism are at their peak.
Acknowledgments
This work was supported by NIH Grants MH100822 and MH065635 to CMA, and NIH Grant F30 MH098570 to VG.
Footnotes
The authors declare they have no conflicts of interest
References
- Adamsky A, Goshen I. Astrocytes in memory function: Pioneering findings and future directions. Neuroscience. 2017 doi: 10.1016/j.neuroscience.2017.05.033. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Transcription Factors in Long-Term Memory and Synaptic Plasticity. Physiological Reviews. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM, Kandel ER. The regulation of transcription in memory consolidation. Cold Spring Harb Perspect Biol. 2014;7:a021741. doi: 10.1101/cshperspect.a021741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM, Travaglia A. Infantile Amnesia: A Critical Period of Learning to Learn and Remember. J Neurosci. 2017;37(24):5783–5795. doi: 10.1523/JNEUROSCI.0324-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert A, Costalat R, Magistretti PJ, Pellerin L. Brain lactate kinetics: modeling evidence for neuronal lactate uptake upon activation. Proc Natl Acad Sci U S A. 2005;102:16448–16453. doi: 10.1073/pnas.0505427102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER, Harris KM. Structural Components of Synaptic Plasticity and Memory Consolidation. Cold Spring Harb Perspect Biol. 2015;7:a021758. doi: 10.1101/cshperspect.a021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KD, Edwards TM. D-Lactate inhibition of memory in a single trial discrimination avoidance task in the young chick. Neurobiol Learn Mem. 2007;88:269–276. doi: 10.1016/j.nlm.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Bandeira F, Lent R, Herculano-Houzel S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proceedings of the National Academy of Sciences. 2009;106:14108–14113. doi: 10.1073/pnas.0804650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J, Betz A, Brose N, Rosenmund C. Munc13-1 C1 domain activation lowers the energy barrier for synaptic vesicle fusion. J Neurosci. 2007;27:1200–10. doi: 10.1523/JNEUROSCI.4908-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazargani N, Attwell D. Astrocyte calcium signalling: the third wave. Nat Neurosci. 2016;19:182–189. doi: 10.1038/nn.4201. [DOI] [PubMed] [Google Scholar]
- Bejar R, Yasuda R, Krugers H, Hood K, Mayford M. Transgenic calmodulin-dependent protein kinase II activation: dose-dependent effects on synaptic plasticity, learning, and memory. J Neurosci. 2002;22:5719–26. doi: 10.1523/JNEUROSCI.22-13-05719.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger M, Allaman I, Magistretti Pierre J. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metabolism. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Bergersen L, Waerhaug O, Helm J, Thomas M, Laake P, Davies AJ, Wilson MC, Halestrap AP, Ottersen OP. A novel postsynaptic density protein: the monocarboxylate transporter MCT2 is co-localized with delta-glutamate receptors in postsynaptic densities of parallel fiber-Purkinje cell synapses. Exp Brain Res. 2001;136:523–34. doi: 10.1007/s002210000600. [DOI] [PubMed] [Google Scholar]
- Bergersen LH. Lactate transport and signaling in the brain: potential therapeutic targets and roles in body-brain interaction. J Cereb Blood Flow Metab. 2015;35:176–85. doi: 10.1038/jcbfm.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen LH, Magistretti PJ, Pellerin L. Selective postsynaptic co-localization of MCT2 with AMPA receptor GluR2/3 subunits at excitatory synapses exhibiting AMPA receptor trafficking. Cereb Cortex. 2005;15:361–70. doi: 10.1093/cercor/bhh138. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bolaños JP, Almeida A, Moncada S. Glycolysis: a bioenergetic or a survival pathway? Trends in Biochemical Sciences. 2010;35:145–149. doi: 10.1016/j.tibs.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Boury-Jamot B, Carrard A, Martin JL, Halfon O, Magistretti PJ, Boutrel B. Disrupting astrocyte-neuron lactate transfer persistently reduces conditioned responses to cocaine. Mol Psychiatry. 2016;21:1070–1076. doi: 10.1038/mp.2015.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28:11760–7. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Lin Q, Wei W, Baker-Andresen D, Mattick JS. MicroRNA regulation of neural plasticity and memory. Neurobiol Learn Mem. 2011;96:89–94. doi: 10.1016/j.nlm.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Brekke E, Morken TS, Sonnewald U. Glucose metabolism and astrocyte–neuron interactions in the neonatal brain. Neurochemistry International. 2015;82:33–41. doi: 10.1016/j.neuint.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Brooks GA. Lactate: link between glycolytic and oxidative metabolism. Sports Med. 2007;37:341–3. doi: 10.2165/00007256-200737040-00017. [DOI] [PubMed] [Google Scholar]
- Brooks GA. Cell-cell and intracellular lactate shuttles. J Physiol. 2009;587:5591–600. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA. Comprehensive Physiology. John Wiley & Sons, Inc; 2011. Bioenergetics of Exercising Humans. [DOI] [PubMed] [Google Scholar]
- Brooks GA. Energy Flux, Lactate Shuttling, Mitochondrial Dynamics, and Hypoxia. Adv Exp Med Biol. 2016;903:439–55. doi: 10.1007/978-1-4899-7678-9_29. [DOI] [PubMed] [Google Scholar]
- Brown AM, Baltan Tekkök S, Ransom BR. Energy transfer from astrocytes to axons: the role of CNS glycogen. Neurochemistry International. 2004;45:529–536. doi: 10.1016/j.neuint.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Brown AM, Ransom BR. Astrocyte glycogen as an emergency fuel under conditions of glucose deprivation or intense neural activity. Metab Brain Dis. 2015;30:233–9. doi: 10.1007/s11011-014-9588-2. [DOI] [PubMed] [Google Scholar]
- Brunet JF, Allaman I, Magistretti PJ, Pellerin L. Glycogen Metabolism as a Marker of Astrocyte Differentiation. Journal of Cerebral Blood Flow & Metabolism. 2010;30:51–55. doi: 10.1038/jcbfm.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25:1073–1188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–4. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Cali C, Baghabra J, Boges DJ, Holst GR, Kreshuk A, Hamprecht FA, et al. Three-dimensional immersive virtual reality for studying cellular compartments in 3D models from EM preparations of neural tissues. J Comp Neurol. 2016;524:23–38. doi: 10.1002/cne.23852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash R, Raisman R, Lanfumey L, Ploska A, Agid Y. Cellular localization of adrenergic receptors in rat and human brain. Brain Res. 1986;370:127–35. doi: 10.1016/0006-8993(86)91112-1. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Broadwell RD. Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. I. Neurons and glia. J Electron Microsc Tech. 1986;3:413–437. doi: 10.1007/BF01611733. [DOI] [PubMed] [Google Scholar]
- Catus SL, Gibbs ME, Sato M, Summers RJ, Hutchinson DS. Role of beta-adrenoceptors in glucose uptake in astrocytes using beta-adrenoceptor knockout mice. Br J Pharmacol. 2011;162:1700–15. doi: 10.1111/j.1476-5381.2010.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandley MJ, Szebeni A, Szebeni K, Crawford JD, Stockmeier CA, Turecki G, Kostrzewa RM, Ordway GA. Elevated gene expression of glutamate receptors in noradrenergic neurons from the locus coeruleus in major depression. Int J Neuropsychopharmacol. 2014;17:1569–78. doi: 10.1017/S1461145714000662. [DOI] [PubMed] [Google Scholar]
- Chih C-P, Lipton P, Roberts EL. Do active cerebral neurons really use lactate rather than glucose? Trends Neurosci. 2001;24:573–578. doi: 10.1016/s0166-2236(00)01920-2. [DOI] [PubMed] [Google Scholar]
- Chih C-P, Roberts EL. Energy Substrates for Neurons during Neural Activity: A Critical Review of the Astrocyte-Neuron Lactate Shuttle Hypothesis. Journal of Cerebral Blood Flow & Metabolism. 2003;23:1263–1281. doi: 10.1097/01.WCB.0000081369.51727.6F. [DOI] [PubMed] [Google Scholar]
- Choi HB, Gordon GR, Zhou N, Tai C, Rungta RL, Martinez J, et al. Metabolic communication between astrocytes and neurons via bicarbonate-responsive soluble adenylyl cyclase. Neuron. 2012;75:1094–1104. doi: 10.1016/j.neuron.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Frick BJ, Radulovic J, Kay LM. Analysis of coherent activity between retrosplenial cortex, hippocampus, thalamus, and anterior cingulate cortex during retrieval of recent and remote context fear memory. Neurobiology of Learning and Memory. 2016;127:93–101. doi: 10.1016/j.nlm.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz NF, Dienel GA. High Glycogen Levels in Brains of Rats with Minimal Environmental Stimuli: Implications for Metabolic Contributions of Working Astrocytes. Journal of Cerebral Blood Flow & Metabolism. 2002;22:1476–1489. doi: 10.1097/01.WCB.0000034362.37277.C0. [DOI] [PubMed] [Google Scholar]
- Davis Ronald L. Traces of Drosophila Memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day Jeremy J, Sweatt JD. Epigenetic Mechanisms in Cognition. Neuron. 2011;70:813–829. doi: 10.1016/j.neuron.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente V, Federman N, Zalcman G, Salles A, Freudenthal R, Romano A. NF-kappaB transcription factor role in consolidation and reconsolidation of persistent memories. Front Mol Neurosci. 2015;8:50. doi: 10.3389/fnmol.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debernardi R, Pierre K, Lengacher S, Magistretti PJ, Pellerin L. Cell-specific expression pattern of monocarboxylate transporters in astrocytes and neurons observed in different mouse brain cortical cell cultures. Journal of Neuroscience Research. 2003;73:141–155. doi: 10.1002/jnr.10660. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Hertz L. Glucose and lactate metabolism during brain activation. J Neurosci Res. 2001;66:824–838. doi: 10.1002/jnr.10079. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Nutrition during brain activation: does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought? Neurochem Int. 2004;45:321–51. doi: 10.1016/j.neuint.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Dienel GA. Astrocytes are ‘good scouts’: being prepared also helps neighboring neurons. J Cereb Blood Flow Metab. 2010;30:1893–1894. doi: 10.1038/jcbfm.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Contributions of glycogen to astrocytic energetics during brain activation. Metabolic Brain Disease. 2015;30:281–298. doi: 10.1007/s11011-014-9493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA. Lack of appropriate stoichiometry: Strong evidence against an energetically important astrocyte-neuron lactate shuttle in brain. J Neurosci Res. 2017 Feb 2; doi: 10.1002/jnr.24015. [DOI] [PubMed] [Google Scholar]
- DiNuzzo M, Mangia S, Maraviglia B, Giove F. Glycogenolysis in astrocytes supports blood-borne glucose channeling not glycogen-derived lactate shuttling to neurons: evidence from mathematical modeling. J Cereb Blood Flow Metab. 2010;30:1895–1904. doi: 10.1038/jcbfm.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski GJ, Jr, Swiatek KR, Chao KL. Lactate, 3-hydroxybutyrate, and glucose as substrates for the early postnatal rat brain. Neurochem Res. 1989;14:667–75. doi: 10.1007/BF00964877. [DOI] [PubMed] [Google Scholar]
- Dong JH, Chen X, Cui M, Yu X, Pang Q, Sun JP. beta2-adrenergic receptor and astrocyte glucose metabolism. J Mol Neurosci. 2012;48:456–63. doi: 10.1007/s12031-012-9742-4. [DOI] [PubMed] [Google Scholar]
- Dornelles A, de Lima MN, Grazziotin M, Presti-Torres J, Garcia VA, Scalco FS, Roesler R, Schroder N. Adrenergic enhancement of consolidation of object recognition memory. Neurobiol Learn Mem. 2007;88:137–42. doi: 10.1016/j.nlm.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Du F, Zhu X-H, Zhang Y, Friedman M, Zhang N, Uğurbil K, Chen W. Tightly coupled brain activity and cerebral ATP metabolic rate. Proceedings of the National Academy of Sciences. 2008;105:6409–6414. doi: 10.1073/pnas.0710766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau J, Tully T. Gene discovery in Drosophila: new insights for learning and memory. Annu Rev Neurosci. 1998;21:407–44. doi: 10.1146/annurev.neuro.21.1.407. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Karni A, Born J. The Consolidation and Transformation of Memory. Neuron. 2015;88:20–32. doi: 10.1016/j.neuron.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Duran J, Saez I, Gruart A, Guinovart JJ, Delgado-García JM. Impairment in Long-Term Memory Formation and Learning-Dependent Synaptic Plasticity in Mice Lacking Glycogen Synthase in the Brain. Journal of Cerebral Blood Flow & Metabolism. 2013;33:550–556. doi: 10.1038/jcbfm.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Remembering: functional organization of the declarative memory system. Curr Biol. 2006;16:R643–5. doi: 10.1016/j.cub.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Falkowska A, Gutowska I, Goschorska M, Nowacki P, Chlubek D, Baranowska-Bosiacka I. Energy Metabolism of the Brain, Including the Cooperation between Astrocytes and Neurons, Especially in the Context of Glycogen Metabolism. International Journal of Molecular Sciences. 2015;16:25939. doi: 10.3390/ijms161125939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Kim JJ, Yipp J, De Oca B. Differential effects of the N-methyl-D-aspartate antagonist DL-2-amino-5-phosphonovalerate on acquisition of fear of auditory and contextual cues. Behav Neurosci. 1994;108:235–40. doi: 10.1037//0735-7044.108.2.235. [DOI] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends in Neurosciences. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint RW, Jr, Riccio DC. Post-training Glucose Administration Attenuates Forgetting of Passive-Avoidance Conditioning in 18-Day-Old Rats. Neurobiology of Learning and Memory. 1999;72:62–67. doi: 10.1006/nlme.1998.3906. [DOI] [PubMed] [Google Scholar]
- Flint RW, Jr, Riccio DC. Pretest administration of glucose attenuates infantile amnesia for passive avoidance conditioning in rats. Dev Psychobiol. 1997;31:207–16. doi: 10.1002/(sici)1098-2302(199711)31:3<207::aid-dev5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Mansuy IM. The prevalence of epigenetic mechanisms in the regulation of cognitive functions and behaviour. Curr Opin Neurobiol. 2010;20:441–9. doi: 10.1016/j.conb.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Fu W, Jhamandas JH. Role of astrocytic glycolytic metabolism in Alzheimer’s disease pathogenesis. Biogerontology. 2014;15:579–86. doi: 10.1007/s10522-014-9525-0. [DOI] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–60. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- Gao V, Suzuki A, Magistretti PJ, Lengacher S, Pollonini G, Steinman MQ, Alberini CM. Astrocytic β2-adrenergic receptors mediate hippocampal long-term memory consolidation. Proceedings of the National Academy of Sciences. 2016;113:8526–8531. doi: 10.1073/pnas.1605063113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs ME. Role of Glycogenolysis in Memory and Learning: Regulation by Noradrenaline, Serotonin and ATP. Frontiers in Integrative Neuroscience. 2016:9. doi: 10.3389/fnint.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs ME, Anderson DG, Hertz L. Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia. 2006;54:214–222. doi: 10.1002/glia.20377. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Hertz L. Inhibition of astrocytic energy metabolism by d-lactate exposure impairs memory. Neurochemistry International. 2008;52:1012–1018. doi: 10.1016/j.neuint.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Hutchinson D, Hertz L. Astrocytic involvement in learning and memory consolidation. Neurosci Biobehav Rev. 2008;32:927–44. doi: 10.1016/j.neubiorev.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Hutchinson DS, Summers RJ. Noradrenaline release in the locus coeruleus modulates memory formation and consolidation; roles for alpha- and beta-adrenergic receptors. Neuroscience. 2010;170:1209–22. doi: 10.1016/j.neuroscience.2010.07.052. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Lloyd HGE, Santa T, Hertz L. Glycogen is a preferred glutamate precursor during learning in 1-day-old chick: Biochemical and behavioral evidence. Journal of Neuroscience Research. 2007;85:3326–3333. doi: 10.1002/jnr.21307. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Marrett S, Vafaee M. Oxidative and Nonoxidative Metabolism of Excited Neurons and Astrocytes. Journal of Cerebral Blood Flow & Metabolism. 2002;22:1–14. doi: 10.1097/00004647-200201000-00001. [DOI] [PubMed] [Google Scholar]
- Gold P, Korol D. Making Memories Matter. Frontiers in Integrative Neuroscience. 2012:6. doi: 10.3389/fnint.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos I, Feria-Velasco A. Serotonin/dopamine interaction in memory formation. Prog Brain Res. 2008;172:603–23. doi: 10.1016/S0079-6123(08)00928-X. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–9. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF. Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus. 2002;12:86–104. doi: 10.1002/hipo.10010. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. The SLC16 gene family – Structure, role and regulation in health and disease. Molecular Aspects of Medicine. 2013;34:337–349. doi: 10.1016/j.mam.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Hamberger A, Hydén H. Inverse enzymatic changes in neurons and glia during increased function and hypoxia. The Journal of Cell Biology. 1963;16:521–525. doi: 10.1083/jcb.16.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris Julia J, Jolivet R, Attwell D. Synaptic Energy Use and Supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Hawkins RA, Williamson DH, Krebs HA. Ketone-body utilization by adult and suckling rat brain in vivo. Biochemical Journal. 1971;122:13–18. doi: 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Nedergaard M. How do astrocytes participate in neural plasticity? Cold Spring Harb Perspect Biol. 2014;7:a020438. doi: 10.1101/cshperspect.a020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–6. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero-Mendez A, Almeida A, Fernandez E, Maestre C, Moncada S, Bolanos JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- Hertz L, Gibbs ME. What learning in day-old chickens can teach a neurochemist: focus on astrocyte metabolism. J Neurochem. 2009;109(Suppl 1):10–6. doi: 10.1111/j.1471-4159.2009.05939.x. [DOI] [PubMed] [Google Scholar]
- Hertz L, Gibbs ME, O’Dowd BS, Sedman GL, Robinson SR, SykovÁ EVA, Hajek I, Heritz E, Liang P, Rong H, et al. Astrocyte-Neuron Interaction During One-trial Aversive Learning in the Neonate Chick. Neuroscience & Biobehavioral Reviews. 1996;20:537–551. doi: 10.1016/0149-7634(95)00020-8. [DOI] [PubMed] [Google Scholar]
- Hertz L, O’Dowd BS, Ng KT, Gibbs ME. Reciprocal changes in forebrain contents of glycogen and of glutamate/glutamine during early memory consolidation in the day-old chick. Brain Research. 2003;994:226–233. doi: 10.1016/j.brainres.2003.09.044. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wilson GS. A Temporary Local Energy Pool Coupled to Neuronal Activity: Fluctuations of Extracellular Lactate Levels in Rat Brain Monitored with Rapid-Response Enzyme-Based Sensor. Journal of Neurochemistry. 1997;69:1484–1490. doi: 10.1046/j.1471-4159.1997.69041484.x. [DOI] [PubMed] [Google Scholar]
- Hutchinson DS, Summers RJ, Gibbs ME. Beta2- and beta3-adrenoceptors activate glucose uptake in chick astrocytes by distinct mechanisms: a mechanism for memory enhancement? J Neurochem. 2007;103:997–1008. doi: 10.1111/j.1471-4159.2007.04789.x. [DOI] [PubMed] [Google Scholar]
- Hydén H, Lange PW. A kinetic study of the neuron-glia relationship. The Journal of Cell Biology. 1962;13:233–237. doi: 10.1083/jcb.13.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inda MC, Muravieva EV, Alberini CM. Memory Retrieval and the Passage of Time: From Reconsolidation and Strengthening to Extinction. The Journal of Neuroscience. 2011;31:1635–1643. doi: 10.1523/JNEUROSCI.4736-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inostroza M, Born J. Sleep for preserving and transforming episodic memory. Annu Rev Neurosci. 2013;36:79–102. doi: 10.1146/annurev-neuro-062012-170429. [DOI] [PubMed] [Google Scholar]
- Isingrini E, Perret L, Rainer Q, Amilhon B, Guma E, Tanti A, Martin G, Robinson J, Moquin L, Marti F, et al. Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nat Neurosci. 2016;19:560–3. doi: 10.1038/nn.4245. [DOI] [PubMed] [Google Scholar]
- Jensen CJ, Demol F, Bauwens R, Kooijman R, Massie A, Villers A, Ris L, De Keyser J. Astrocytic β2 Adrenergic Receptor Gene Deletion Affects Memory in Aged Mice. PLoS One. 2016;11:e0164721. doi: 10.1371/journal.pone.0164721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, Charnay P, Bozon B, Laroche S, Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4:289–96. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–8. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Molecular Brain. 2012;5:14. doi: 10.1186/1756-6606-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel Eric R, Dudai Y, Mayford Mark R. The Molecular and Systems Biology of Memory. Cell. 2014;157:163–186. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Kida S, Serita T. Functional roles of CREB as a positive regulator in the formation and enhancement of memory. Brain Research Bulletin. 2014;105:17–24. doi: 10.1016/j.brainresbull.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Baxter MG. Multiple brain-memory systems: the whole does not equal the sum of its parts. Trends in Neurosciences. 2001;24:324–330. doi: 10.1016/s0166-2236(00)01818-x. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Knapp LT, Klann E. Potentiation of hippocampal synaptic transmission by superoxide requires the oxidative activation of protein kinase C. J Neurosci. 2002;22:674–83. doi: 10.1523/JNEUROSCI.22-03-00674.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW, Chugani HT, Grossman LI, Lipovich L, Muzik O, Hof PR, Wildman DE, Sherwood CC, Leonard WR, Lange N. Metabolic costs and evolutionary implications of human brain development. Proc Natl Acad Sci U S A. 2014;111:13010–5. doi: 10.1073/pnas.1323099111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- Lauritzen KH, Morland C, Puchades M, Holm-Hansen S, Hagelin EM, Lauritzen F, Attramadal H, Storm-Mathisen J, Gjedde A, Bergersen LH. Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex. 2014;24:2784–95. doi: 10.1093/cercor/bht136. [DOI] [PubMed] [Google Scholar]
- Lee HS, Ghetti A, Pinto-Duarte A, Wang X, Dziewczapolski G, Galimi F, Huitron-Resendiz S, Pina-Crespo JC, Roberts AJ, Verma IM, et al. Astrocytes contribute to gamma oscillations and recognition memory. Proc Natl Acad Sci U S A. 2014;111:E3343–52. doi: 10.1073/pnas.1410893111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B, Desebbe O, Montemont C, Gibot S. Increased aerobic glycolysis through beta2 stimulation is a common mechanism involved in lactate formation during shock states. Shock. 2008;30:417–21. doi: 10.1097/SHK.0b013e318167378f. [DOI] [PubMed] [Google Scholar]
- Li S, Callaghan BL, Richardson R. Infantile amnesia: forgotten but not gone. Learning & Memory. 2014;21:135–139. doi: 10.1101/lm.031096.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Loebrich S, Nedivi E. The Function of Activity-Regulated Genes in the Nervous System. Physiological Reviews. 2009;89:1079–1103. doi: 10.1152/physrev.00013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–23. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lopes LT, Patrone LG, Li KY, Imber AN, Graham CD, Gargaglioni LH, Putnam RW. Anatomical and functional connections between the locus coeruleus and the nucleus tractus solitarius in neonatal rats. Neuroscience. 2016;324:446–68. doi: 10.1016/j.neuroscience.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH-C, Han X, Takano T, Wang S, Sim FJ, et al. The Transcriptome and Metabolic Gene Signature of Protoplasmic Astrocytes in the Adult Murine Cortex. The Journal of Neuroscience. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machler P, Wyss MT, Elsayed M, Stobart J, Gutierrez R, von Faber-Castell A, Kaelin V, Zuend M, San Martin A, Romero-Gomez I, et al. In Vivo Evidence for a Lactate Gradient from Astrocytes to Neurons. Cell Metab. 2016;23:94–102. doi: 10.1016/j.cmet.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Madsen HB, Kim JH. Ontogeny of memory: An update on 40 years of work on infantile amnesia. Behavioural Brain Research. 2016;298(Part A):4–14. doi: 10.1016/j.bbr.2015.07.030. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Regulation of glycogenolysis by neurotransmitters in the central nervous system. Diabete Metab. 1988;14:237–46. [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Astrocytes Couple Synaptic Activity to Glucose Utilization in the Brain. News Physiol Sci. 1999;14:177–182. doi: 10.1152/physiologyonline.1999.14.5.177. [DOI] [PubMed] [Google Scholar]
- Makkar SR, Zhang SQ, Cranney J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology. 2010;35:1625–52. doi: 10.1038/npp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–26. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Allen CJ, Catton MD, Ghilardi JR, Levin LA, Maggio JE, Vigna SR. Beta 2-adrenergic receptors are expressed by glia in vivo in the normal and injured central nervous system in the rat, rabbit, and human. J Neurosci. 1995;15:152–64. doi: 10.1523/JNEUROSCI.15-01-00152.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2:679–89. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Massaad CA, Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal. 2011;14:2013–54. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DS, Kruger L. The fine structure of astrocytes in the cerebral cortex and their response to focal injury produced by heavy ionizing particles. The Journal of Cell Biology. 1965;25:141–157. doi: 10.1083/jcb.25.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M. Protein kinase signaling in synaptic plasticity and memory. Curr Opin Neurobiol. 2007;17:313–7. doi: 10.1016/j.conb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, Hong NS, Devan BD. The challenges of understanding mammalian cognition and memory-based behaviours: an interactive learning and memory systems approach. Neuroscience & Biobehavioral Reviews. 2004;28:719–745. doi: 10.1016/j.neubiorev.2004.09.014. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Consolidating memories. Annu Rev Psychol. 2015;66:1–24. doi: 10.1146/annurev-psych-010814-014954. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Dienel GA, Sonnewald U, Waagepetersen HS, Schousboe A. Energy metabolism of the brain. In: Brady SGJ, Siegel G, Albers RW, Price DL, editors. Basic Neurochemistry: Principles of Molecular, Cellular, and Medical Neurobiology. Oxford, UK: Elsevier Academic Press; 2012. pp. 200–231. [Google Scholar]
- McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proceedings of the National Academy of Sciences. 2000;97:2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, McCarty RC, Gold PE. Fluctuations in Brain Glucose Concentration during Behavioral Testing: Dissociations between Brain Areas and between Brain and Blood. Neurobiology of Learning and Memory. 2001;75:325–337. doi: 10.1006/nlme.2000.3976. [DOI] [PubMed] [Google Scholar]
- McNay EC, Sherwin RS. Effect of Recurrent Hypoglycemia on Spatial Cognition and Cognitive Metabolism in Normal and Diabetic Rats. Diabetes. 2004;53:418–425. doi: 10.2337/diabetes.53.2.418. [DOI] [PubMed] [Google Scholar]
- Messier C. Glucose improvement of memory: a review. Eur J Pharmacol. 2004;490:33–57. doi: 10.1016/j.ejphar.2004.02.043. [DOI] [PubMed] [Google Scholar]
- Milner TA, Shah P, Pierce JP. beta-adrenergic receptors primarily are located on the dendrites of granule cells and interneurons but also are found on astrocytes and a few presynaptic profiles in the rat dentate gyrus. Synapse. 2000;36:178–93. doi: 10.1002/(SICI)1098-2396(20000601)36:3<178::AID-SYN3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Williams CL. Peripheral arousal-related hormones modulate norepinephrine release in the hippocampus via influences on brainstem nuclei. Behav Brain Res. 2004;153:87–95. doi: 10.1016/j.bbr.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Deneen B. Astrocyte development: A Guide for the Perplexed. Glia. 2015;63:1320–1329. doi: 10.1002/glia.22836. [DOI] [PubMed] [Google Scholar]
- Moraga-Amaro R, Jerez-Baraona JM, Simon F, Stehberg J. Role of astrocytes in memory and psychiatric disorders. Journal of Physiology-Paris. 2014;108:240–251. doi: 10.1016/j.jphysparis.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Morgello S, Uson RR, Schwartz EJ, Haber RS. The human blood-brain barrier glucose transporter (GLUT1) is a glucose transporter of gray matter astrocytes. Glia. 1995;14:43–54. doi: 10.1002/glia.440140107. [DOI] [PubMed] [Google Scholar]
- Morken TS, Brekke E, Haberg A, Wideroe M, Brubakk AM, Sonnewald U. Neuron-astrocyte interactions, pyruvate carboxylation and the pentose phosphate pathway in the neonatal rat brain. Neurochem Res. 2014;39:556–69. doi: 10.1007/s11064-013-1014-3. [DOI] [PubMed] [Google Scholar]
- Morland C, Lauritzen KH, Puchades M, Holm-Hansen S, Andersson K, Gjedde A, Attramadal H, Storm-Mathisen J, Bergersen LH. The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: Expression and action in brain. J Neurosci Res. 2015;93:1045–55. doi: 10.1002/jnr.23593. [DOI] [PubMed] [Google Scholar]
- Morris KA, Chang Q, Mohler EG, Gold PE. Age-related memory impairments due to reduced blood glucose responses to epinephrine. Neurobiology of Aging. 2010;31:2136–2145. doi: 10.1016/j.neurobiolaging.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG. NMDA receptors and memory encoding. Neuropharmacology. 2013;74:32–40. doi: 10.1016/j.neuropharm.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Murase S, Schuman EM. The role of cell adhesion molecules in synaptic plasticity and memory. Curr Opin Cell Biol. 1999;11:549–53. doi: 10.1016/s0955-0674(99)00019-8. [DOI] [PubMed] [Google Scholar]
- Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–43. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: Redefining the functional architecture of the brain. Trends in Neurosciences. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Nehlig A. Brain uptake and metabolism of ketone bodies in animal models. Prostaglandins Leukot Essent Fatty Acids. 2004;70:265–75. doi: 10.1016/j.plefa.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends in Endocrinology & Metabolism. 2014;25:42–52. doi: 10.1016/j.tem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, Korol DL, Gold PE. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS One. 2011;6:e28427. doi: 10.1371/journal.pone.0028427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijland PG, Molenaar RJ, van der Pol SM, van der Valk P, van Noorden CJ, de Vries HE, van Horssen J. Differential expression of glucose-metabolizing enzymes in multiple sclerosis lesions. Acta Neuropathol Commun. 2015;3:79. doi: 10.1186/s40478-015-0261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman AS, DiRocco DP, Lambert TJ, Garelick MG, Le J, Nathanson NM, Storm DR. Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus. 2010;20:492–8. doi: 10.1002/hipo.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell TJ, Connor SA, Guglietta R, Nguyen PV. beta-Adrenergic receptor signaling and modulation of long-term potentiation in the mammalian hippocampus. Learn Mem. 2015;22:461–71. doi: 10.1101/lm.031088.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dowd BS, Gibbs ME, Ng KT, Hertz E, Hertz L. Astrocytic glycogenolysis energizes memory processes in neonate chicks. Brain Res Dev Brain Res. 1994;78:137–41. doi: 10.1016/0165-3806(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Pannasch U, Rouach N. Emerging role for astroglial networks in information processing: from synapse to behavior. Trends Neurosci. 2013;36:405–17. doi: 10.1016/j.tins.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Parkhurst Christopher N, Yang G, Ninan I, Savas Jeffrey N, Yates John R, III, Lafaille Juan J, Hempstead Barbara L, Littman Dan R, Gan W-B. Microglia Promote Learning-Dependent Synapse Formation through Brain-Derived Neurotrophic Factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–4. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–9. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Food for thought: challenging the dogmas. J. Cereb. Blood Flow Metab. 2003;23:1282–1286. doi: 10.1097/01.WCB.0000096064.12129.3D. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012;32:1152–1166. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–31. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Petersen KU. Fine structure of neuroglia cells in the cerebellar cortex of mammals. Z Zellforsch Mikrosk Anat. 1969;100:616–33. [PubMed] [Google Scholar]
- Pfeiffer-Guglielmi B, Fleckenstein B, Jung G, Hamprecht B. Immunocytochemical localization of glycogen phosphorylase isozymes in rat nervous tissues by using isozyme-specific antibodies. Journal of Neurochemistry. 2003;85:73–81. doi: 10.1046/j.1471-4159.2003.01644.x. [DOI] [PubMed] [Google Scholar]
- Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. Journal of Neurochemistry. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- Prichard J, Rothman D, Novotny E, Petroff O, Kuwabara T, Avison M, Howseman A, Hanstock C, Shulman R. Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci U S A. 1991;88:5829–31. doi: 10.1073/pnas.88.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci. 1999;19:6623–8. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell AL, Carew TJ. Tyrosine kinases, synaptic plasticity and memory: insights from vertebrates and invertebrates. Trends Neurosci. 2003;26:625–30. doi: 10.1016/j.tins.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Quach TT, Duchemin AM, Rose C, Schwartz JC. [3H]glycogenolysis in brain slices mediated by beta-adrenoceptors: comparison of physiological response and [3H]dihydroalprenolol binding parameters. Neuropharmacology. 1988;27:629–35. doi: 10.1016/0028-3908(88)90185-2. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Pal SN, Unick K, Stefani MR, Gold PE. Modulation of Hippocampal Acetylcholine Release and Spontaneous Alternation Scores by Intrahippocampal Glucose Injections. The Journal of Neuroscience. 1998;18:1595–1601. doi: 10.1523/JNEUROSCI.18-04-01595.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Unick KE, Gold PE. Hippocampal acetylcholine release during memory testing in rats: augmentation by glucose. Proc Natl Acad Sci U S A. 1996;93:4693–8. doi: 10.1073/pnas.93.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn EJ, Guzman-Karlsson MC, David Sweatt J. Cellular, molecular, and epigenetic mechanisms in non-associative conditioning: implications for pain and memory. Neurobiol Learn Mem. 2013;105:133–50. doi: 10.1016/j.nlm.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc Natl Acad Sci U S A. 1984;81:1585–9. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos BP, Colgan L, Nou E, Ovadia S, Wilson SR, Arnsten AF. The beta-1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biol Psychiatry. 2005;58:894–900. doi: 10.1016/j.biopsych.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Ravaris CL, Friedman MJ, Hauri PJ, McHugo GJ. A controlled study of alprazolam and propranolol in panic-disordered and agoraphobic outpatients. J Clin Psychopharmacol. 1991;11:344–50. [PubMed] [Google Scholar]
- Reemst K, Noctor SC, Lucassen PJ, Hol EM. The Indispensable Roles of Microglia and Astrocytes during Brain Development. Front Hum Neurosci. 2016;10:566. doi: 10.3389/fnhum.2016.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G. Function of metabotropic glutamate receptors in learning and memory. Trends Neurosci. 1996;19:219–24. doi: 10.1016/0166-2236(96)20012-8. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behavioural Brain Research. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Rinholm JE, Hamilton NB, Kessaris N, Richardson WD, Bergersen LH, Attwell D. Regulation of Oligodendrocyte Development and Myelination by Glucose and Lactate. The Journal of Neuroscience. 2011;31:538–548. doi: 10.1523/JNEUROSCI.3516-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Memory modulation. Behav Neurosci. 2011;125:797–824. doi: 10.1037/a0026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SP. Cell adhesion molecules and the transition from short- to long-term memory. J Physiol Paris. 1996;90:387–91. doi: 10.1016/s0928-4257(97)87926-0. [DOI] [PubMed] [Google Scholar]
- Rudenko A, Tsai LH. Epigenetic regulation in memory and cognitive disorders. Neuroscience. 2014;264:51–63. doi: 10.1016/j.neuroscience.2012.12.034. [DOI] [PubMed] [Google Scholar]
- Saab BJ, Mansuy IM. Neuroepigenetics of memory formation and impairment: the role of microRNAs. Neuropharmacology. 2014;80:61–9. doi: 10.1016/j.neuropharm.2014.01.026. [DOI] [PubMed] [Google Scholar]
- Santini E, Huynh TN, Klann E. Mechanisms of translation control underlying long-lasting synaptic plasticity and the consolidation of long-term memory. Prog Mol Biol Transl Sci. 2014;122:131–67. doi: 10.1016/B978-0-12-420170-5.00005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–23. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Vankov A, Herve A. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res Bull. 1994;35:457–65. doi: 10.1016/0361-9230(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Schutsky K, Ouyang M, Castelino CB, Zhang L, Thomas SA. Stress and glucocorticoids impair memory retrieval via beta2-adrenergic, Gi/o-coupled suppression of cAMP signaling. J Neurosci. 2011;31:14172–81. doi: 10.1523/JNEUROSCI.2122-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Sutin J. Expression of adrenergic receptors in individual astrocytes and motor neurons isolated from the adult rat brain. Glia. 1992;6:108–17. doi: 10.1002/glia.440060205. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–6. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–48. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Sotelo-Hitschfeld T, Niemeyer MI, Mächler P, Ruminot I, Lerchundi R, Wyss MT, et al. Channel-mediated lactate release by K+-stimulated astrocytes. J Neurosci. 2015;35:4168–4178. doi: 10.1523/JNEUROSCI.5036-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: A brief history and current perspective. Neurobiology of Learning and Memory. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Squire LR, Dede AJO. Conscious and Unconscious Memory Systems. Cold Spring Harbor Perspectives in Biology. 2015:7. doi: 10.1101/cshperspect.a021667. [DOI] [PMC free article] [PubMed]
- Squire LR, Genzel L, Wixted JT, Morris RG. Memory consolidation. Cold Spring Harb Perspect Biol. 2015;7:a021766. doi: 10.1101/cshperspect.a021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 2011;34:259–88. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao KV, Hertz L. Effect of adrenergic agonists on glycogenolysis in primary cultures of astrocytes. Brain Res. 1990;536:220–6. doi: 10.1016/0006-8993(90)90028-a. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013;80:675–90. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers RJ, Papaioannou M, Harris S, Evans BA. Expression of beta 3-adrenoceptor mRNA in rat brain. Br J Pharmacol. 1995;116:2547–8. doi: 10.1111/j.1476-5381.1995.tb17205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ye X, Xie M, Ye J. Induction of triglyceride accumulation and mitochondrial maintenance in muscle cells by lactate. Sci Rep. 2016;6:33732. doi: 10.1038/srep33732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Lin Y. Npas4: Linking Neuronal Activity to Memory. Trends Neurosci. 2016;39:264–75. doi: 10.1016/j.tins.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandusky L, Flint RW, McNay EC. Elevated glucose metabolism in the amygdala during an inhibitory avoidance task. Behavioural Brain Resarch. 2013;245:83–87. doi: 10.1016/j.bbr.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Gao V, Alberini CM. The Role of Lactate-Mediated Metabolic Coupling between Astrocytes and Neurons in Long-Term Memory Formation. Front Integr Neurosci. 2016;10:10. doi: 10.3389/fnint.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland EW, Rall TW. Formation of adenosine-3,5-phosphate (cyclic adenylate) and its relation to the action of several neurohormones or hormones. Acta Endocrinol Suppl (Copenh) 1960;34(Suppl 50):171–4. doi: 10.1530/acta.0.xxxivs171. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Stern Sarah A, Bozdagi O, Huntley George W, Walker Ruth H, Magistretti Pierre J, Alberini Cristina M. Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Morton MM, Sagar SM, Sharp FR. Sensory stimulation induces local cerebral glycogenolysis: demonstration by autoradiography. Neuroscience. 1992;51:451–61. doi: 10.1016/0306-4522(92)90329-z. [DOI] [PubMed] [Google Scholar]
- Tadi M, Allaman I, Lengacher S, Grenningloh G, Magistretti PJ. Learning-Induced Gene Expression in the Hippocampus Reveals a Role of Neuron -Astrocyte Metabolic Coupling in Long Term Memory. PLoS One. 2015;10:e0141568. doi: 10.1371/journal.pone.0141568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taft VR, JR, Perry GW. Distribution of GFAP+ astrocytes in adult and neonatal rat brain. International Journal of Neuroscience. 2005;115:1333–1343. doi: 10.1080/00207450590934570. [DOI] [PubMed] [Google Scholar]
- Talley CEP, Kahn S, Alexander LJ, Gold PE. Epinephrine Fails to Enhance Performance of Food-Deprived Rats on a Delayed Spontaneous Alternation Task. Neurobiology of Learning and Memory. 2000;73:79–86. doi: 10.1006/nlme.1999.3920. [DOI] [PubMed] [Google Scholar]
- Travaglia A, Bisaz R, Sweet ES, Blitzer RD, Alberini CM. Infantile amnesia reflects a developmental critical period for hippocampal learning. Nat Neurosci. 2016a;19:1225–33. doi: 10.1038/nn.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglia A, Bisaz R, Cruz E, Alberini CM. Developmental changes in plasticity, synaptic, glia and connectivity protein levels in rat dorsal hippocampus. Neurobiol Learn Mem. 2016b;135:125–138. doi: 10.1016/j.nlm.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SP, Merkle F, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–62. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Donaldson ETaW., editor. Organization of Memory. New York: Academic Press; 1972. pp. 381–402. [Google Scholar]
- Vaiva G, Ducrocq F, Jezequel K, Averland B, Lestavel P, Brunet A, Marmar CR. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54:947–9. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ. Developmental expression of GLUT1 and GLUT3 glucose transporters in rat brain. J Neurochem. 1994;62:240–6. doi: 10.1046/j.1471-4159.1994.62010240.x. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Seaman LB, Brucklacher RM, Vannucci RC. Glucose transport in developing rat brain: glucose transporter proteins, rate constants and cerebral glucose utilization. Mol Cell Biochem. 1994;140:177–84. doi: 10.1007/BF00926756. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Simpson IA. Developmental switch in brain nutrient transporter expression in the rat. Am J Physiol Endocrinol Metab. 2003;285:E1127–34. doi: 10.1152/ajpendo.00187.2003. [DOI] [PubMed] [Google Scholar]
- Veyrac A, Besnard A, Caboche J, Davis S, Laroche S. The transcription factor Zif268/Egr1, brain plasticity, and memory. Prog Mol Biol Transl Sci. 2014;122:89–129. doi: 10.1016/B978-0-12-420170-5.00004-0. [DOI] [PubMed] [Google Scholar]
- Vilchez D, Ros S, Cifuentes D, Pujadas L, Valles J, Garcia-Fojeda B, Criado-Garcia O, Fernandez-Sanchez E, Medrano-Fernandez I, Dominguez J, et al. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat Neurosci. 2007;10:1407–13. doi: 10.1038/nn1998. [DOI] [PubMed] [Google Scholar]
- Waeber C, Rigo M, Chinaglia G, Probst A, Palacios JM. Beta-adrenergic receptor subtypes in the basal ganglia of patients with Huntington’s chorea and Parkinson’s disease. Synapse. 1991;8:270–80. doi: 10.1002/syn.890080405. [DOI] [PubMed] [Google Scholar]
- Waitt AE, Reed L, Ransom BR, Brown AM. Emerging Roles for Glycogen in the CNS. Front Mol Neurosci. 2017;10:73. doi: 10.3389/fnmol.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Men D, Clayton EC. The effects of noradrenergic activation of the nucleus tractus solitarius on memory and in potentiating norepinephrine release in the amygdala. Behav Neurosci. 2000;114:1131–44. doi: 10.1037//0735-7044.114.6.1131. [DOI] [PubMed] [Google Scholar]
- Winder DG, Martin KC, Muzzio IA, Rohrer D, Chruscinski A, Kobilka B, Kandel ER. ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by beta-adrenergic receptors. Neuron. 1999;24:715–26. doi: 10.1016/s0896-6273(00)81124-1. [DOI] [PubMed] [Google Scholar]
- Wyss MT, Jolivet R, Buck A, Magistretti PJ, Weber B. In Vivo Evidence for Lactate as a Neuronal Energy Source. The Journal of Neuroscience. 2011;31:7477–7485. doi: 10.1523/JNEUROSCI.0415-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Morishita W, Buckmaster PS, Pang ZP, Malenka RC, Sudhof TC. Distinct neuronal coding schemes in memory revealed by selective erasure of fast synchronous synaptic transmission. Neuron. 2012;73:990–1001. doi: 10.1016/j.neuron.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager JY, Ashwal S. Animal models of perinatal hypoxic-ischemic brain damage. Pediatr Neurol. 2009;40:156–67. doi: 10.1016/j.pediatrneurol.2008.10.025. [DOI] [PubMed] [Google Scholar]
- Yang J, Ruchti E, Petit J-M, Jourdain P, Grenningloh G, Allaman I, Magistretti PJ. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proceedings of the National Academy of Sciences. 2014;111:12228–12233. doi: 10.1073/pnas.1322912111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Kapeller-Libermann D, Travaglia A, Inda MC, Alberini CM. Direct dorsal hippocampal-prelimbic cortex connections strengthen fear memories. Nat Neurosci. 2017;20:52–61. doi: 10.1038/nn.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JC, Wallach JS, Del Vechhio M, Wilder EL, Zhou H, Quimm QG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci. 2011;14:656–661. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. The Journal of Neuroscience. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xue Y, Meng S, Luo Y, Liang J, Li J, Ai S, Sun C, Shen H, Zhu W, et al. Inhibition of Lactate Transport Erases Drug Memory and Prevents Drug Relapse. Biological Psychiatry. 2016;79:928–939. doi: 10.1016/j.biopsych.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Zhao S, Chai X, Frotscher M. Balance between neurogenesis and gliogenesis in the adult hippocampus: role for reelin. Dev Neurosci. 2007;29:84–90. doi: 10.1159/000096213. [DOI] [PubMed] [Google Scholar]
- Zhou HC, Sun YY, Cai W, He XT, Yi F, Li BM, Zhang XH. Activation of beta2-adrenoceptor enhances synaptic potentiation and behavioral memory via cAMP-PKA signaling in the medial prefrontal cortex of rats. Learn Mem. 2013;20:274–84. doi: 10.1101/lm.030411.113. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Kimelberg HK. Cellular expression of P2Y and beta-AR receptor mRNAs and proteins in freshly isolated astrocytes and tissue sections from the CA1 region of P8–12 rat hippocampus. Brain Res Dev Brain Res. 2004;148:77–87. doi: 10.1016/j.devbrainres.2003.10.014. [DOI] [PubMed] [Google Scholar]