Abstract
Microglia have diverse actions, ranging from synapse pruning in development to cytotoxic effects in disease. Brain energy metabolism and substrate availability vary under normal and disease states, but how these variations influence microglial function is relatively unknown. Microglia, like most other cell types, express the full complement of gene products required for both glycolytic and oxidative metabolism. Evidence suggests that microglia increase aerobic glycolysis and decrease respiration when activated by various stimuli. Mitochondrial function, glucose availability, and glycolytic rate influence pro-inflammatory gene expression at both transcriptional and post-translational levels. These effects are mediated through CtBP, an NADH - sensitive transcriptional co-repressor; through effects on NLRP3 inflammasome assembly and caspase-1 activation; through formation of advanced glycation end-products; and by less well-defined mechanisms. In addition to these transcriptional effects, microglial glucose metabolism is also required for superoxide production by NADPH oxidase, as glucose is the obligate substrate for regenerating NADPH in the hexose monophosphate shunt. Microglia also metabolize acetoacetate and beta-hydroxybutyrate, which are generated during fasting or ketogenic diet, and respond to these ketones as metabolic signals. Beta-hydroxybutyrate inhibits histone de-acetylases and activates microglial GRP109A receptors. These actions suppress microglia activation after brain injury and promote neuroprotective microglia phenotypes. As our understanding of microglial activation matures, additional links between energy metabolism and microglial function are likely to be identified.
Keywords: inflammasome, glycolysis, superoxide, ketone, TREM2
Main points
Microglial activation induces pro-inflammatory gene transcription and release of reactive oxygen species (ROS). Both of these events are modulated by bioenergetic factors, including cytosolic NADH levels, ketones, glucose availability, and glycolytic rate.
Introduction
Microglia are resident immune cells of the central nervous system (CNS), closely related to dendritic cells found in other tissues and to circulating macrophages. Microglia express an array of immune molecules, including toll like receptors (TLRs), integrins such as CD11b, nucleotide-binding oligomerization domains (NODs), and triggering receptor expressed on myeloid cells (TREM) (Derecki et al. 2014; Hsieh et al. 2009; Nimmerjahn et al. 2005; Parkhurst and Gan 2010). Microglia secrete trophic factors, release proteases required for remodelling extracellular matrix, and phagocytize cells or cell processes destined for destruction (Fourgeaud et al. 2016; Nakanishi 2003; Schafer et al. 2012; Tremblay et al. 2010; Wake et al. 2009). Consistent with their immune cell lineage, microglia also act as detectors of infection and injury, and rapidly respond to these events. This patterned response is generally termed “microglial activation” and involves retraction of processes, migration to sites of injury, and the release of proteases, reactive oxygen species (ROS) and cytokines (Davalos et al. 2012; Ghosh and Geahlen 2015; Kettenmann et al. 2011; Vasek et al. 2016). These responses serve as an innate first line response to infection, but in conditions such as stroke and neurodegenerative disorders the cytotoxic aspects of this innate response may have a net negative effect on outcome (Banati et al. 1993; Kobayashi et al. 2015; Lehnardt 2010).
Mechanisms by which inflammation affects cell energy metabolism are now well established (Hotamisligil 2006), but much less is known about how energy metabolism affects inflammatory responses. The issue is important because many disorders are accompanied by changes in brain energy metabolism (Beal 1995; Bergsneider et al. 1997; Ding et al. 2013; Dusick et al. 2007; Orth and Schapira 2002; Robbins and Swanson 2014). Here we review current understanding of microglial energy metabolism and how energy metabolism influences microglial function.
Energy substrates in normal and injured CNS
The central nervous system is unique in requiring a continuous supply of glucose for normal function. All other tissues, including the heart, can readily metabolize fatty acids, amino acids, and ketones in place of glucose substrate, but the blood brain barrier prevents rapid influx of these alternative substrates under most conditions (Siesjö 1978). Glucose is metabolized almost completely to CO2 in normal brain. There can be transient increases in glucose metabolism to lactate in response to local brain activity, but the lactate thus produced is subsequently metabolized oxidatively, or else escapes into the venous system (Dienel 2012). Blood glucose concentrations are normally 4 – 6 mMol/L, essentially all of which can be extracted by brain. Normal, fully oxygenated blood carries approximately 9 mmol/L O2, of which only a portion can be extracted (Siesjö 1978). There is a glucose concentration gradient between blood and brain, with normal CSF glucose values in the range of 2 – 3 mMol/L, and normal extracellular glucose values being about 1.5 mMol/L. There is similarly a gradient to oxygen, with brain parenchyma containing about 2 mMol/L O2. Since 6 moles of O2 are required to oxidize each mole of glucose, this leaves a large excess of glucose available for anaerobic metabolism to lactate when blood delivery of oxygen falls short of demand. This can occur during hypoxia, but also occurs in conditions such as stroke, brain trauma, and increased intracranial pressure. Under these conditions, the rate of glucose utilization in the ischemic brain can be increased by elevated blood glucose concentration (hyperglycemia). The resulting anaerobic glucose metabolism can generate substantial amounts of ATP, but also produces lactic acid (as occurs normally in exercising muscle).
With reductions in blood glucose, as occur during fasting or strenuous exercise, the ketones acetoacetate and beta-hydroxybutyrate produced by peripheral fat metabolism can be used by brain to supplement glucose as an energy source. Ketones enter brain through monocarboxylic transporters (MCTs) at the blood brain barrier at a rate influenced by both blood ketone concentration and MCT expression level (Halestrap and Price 1999). Fasting increases MCT expression at the blood-brain barrier, and ketones can provide the majority of brain energy substrate under starvation conditions. MCTs also transport lactate and pyruvate. Blood-derived lactate provides supports approximately 10% of brain metabolism under basal plasma lactate conditions of ~ 1.0 mmol/L, and higher percentages at supra-physiological plasma lactate concentrations (Boumezbeur et al. 2010).
Microglia utilize both oxidative and glycolytic energy metabolism
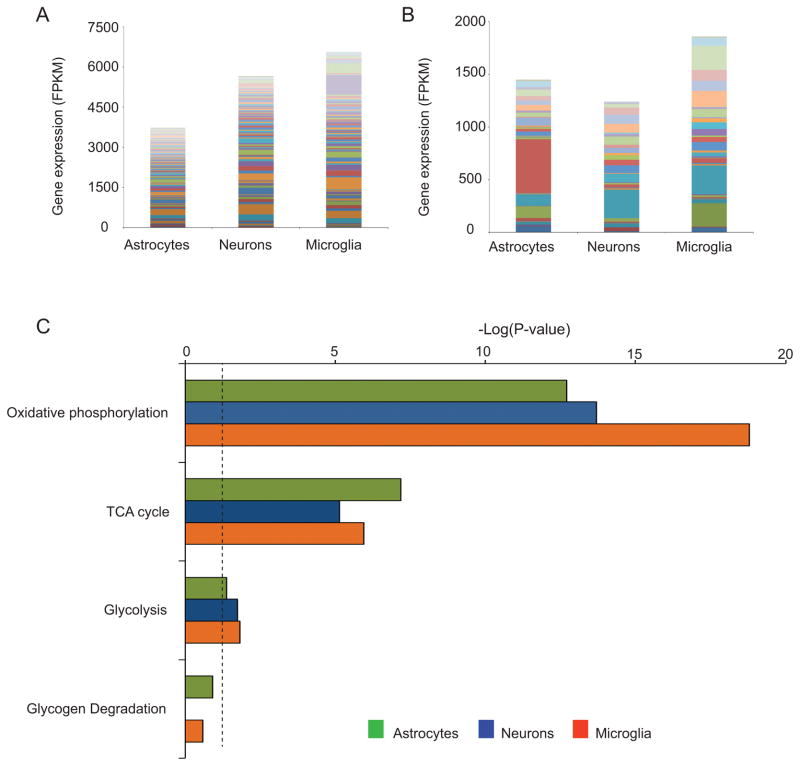
Direct data on the relative contributions of glycolytic and oxidative ATP production in microglia in situ are not available. As an indirect assessment, we compared the mRNA levels of genes related to energy metabolism in neurons, microglia and astrocytes, using a database of cell-type specific gene expression generated from acutely dissociated mouse brain (Zhang et al. 2014), (Fig. 1, Tables 1 and 2). This analysis shows that microglia express the full complement of genes required for both glycolytic and oxidative energy metabolism. Perhaps surprisingly, the quantitative expression of genes involved in oxidative metabolism is, in aggregate, comparable or higher in microglia than in neurons and astrocytes (Fig. 1). These gene expression data do not necessarily indicate the relative rates and routes of energy metabolism in these cell types, but they do confirm the capacity of microglia in situ to generate ATP by both glycolytic and oxidative pathways.
Figure 1. Comparative expression profile analysis of genes related to oxidative and glycolytic metabolism in astrocytes, neurons and microglia.
The FPKM (Fragments Per Kilobase of transcript sequence per Million mapped fragments) data, previously reported in (Zhang et al. 2014) and available at http://web.stanford.edu/group/barres_lab/brain_rnaseq.html, was exported and analysed using a cut-off value of 5 FPKM in at least one sample. Stacked bar columns show the expression of oxidative (A) and glycolytic (B) metabolism-related genes in astrocytes, neurons and microglia. Colors represent individual genes, listed in Tables 1 and 2. (C) Canonical pathways related to the oxidative and glycolytic metabolism in each cell type as assigned by Ingenuity Pathway Analysis software (IPA) (http://www.ingenuity.com/index.html). Horizontal axis shows the −log(P-value) for the pathways in each cell type, based on the probability that genes in each cell type were included in the predefined IPA canonical pathways by true association as opposed to random chance. Dotted line shows P = 0.05.
Table 1.
Expression of genes related to oxidative metabolism in different cell types
| Function | Gene | Expression (FPKM) | ||
|---|---|---|---|---|
|
| ||||
| Astrocytes | Neurons | Microglia | ||
| Acetyl-CoA Biosynthesis | Acaa2 | 32.1 | 3.5 | 18.1 |
| Acox1 | 53.7 | 35.2 | 17.9 | |
| Acox3 | 7.1 | 4.4 | 31.8 | |
| Cpt1a | 49.5 | 11.7 | 19.5 | |
| Cpt1c | 15.8 | 26.4 | 2.5 | |
| Cs | 94.1 | 61.5 | 28.4 | |
| Pdha1 | 49.6 | 74.4 | 13.2 | |
| Pdhb | 19.0 | 27.5 | 11.1 | |
| Pdhx | 12.8 | 11.8 | 4.1 | |
| Slc25a20 | 14.2 | 4.7 | 7.5 | |
|
| ||||
| Oxidative Phosphorylation | Atp5a1 | 121.1 | 231.8 | 195.3 |
| Atp5b | 215.6 | 384.8 | 280.6 | |
| Atp5c1 | 52.0 | 81.5 | 80.7 | |
| Atp5d | 51.4 | 105.1 | 127.3 | |
| Atp5e | 63.4 | 90.2 | 146.4 | |
| Atp5f1 | 24.2 | 44.1 | 15.8 | |
| Atp5g1 | 3.1 | 11.1 | 5.1 | |
| Atp5g2 | 21.9 | 31.5 | 80.8 | |
| Atp5g3 | 143.3 | 243.0 | 110.0 | |
| Atp5h | 4.7 | 7.7 | 8.2 | |
| Atp5j | 43.5 | 75.8 | 36.4 | |
| Atp5j2 | 34.5 | 83.1 | 97.8 | |
| Atp5l | 8.4 | 19.0 | 9.9 | |
| Atp5o | 25.0 | 58.6 | 42.0 | |
| Atp5s | 4.5 | 6.6 | 1.4 | |
| Atpaf2 | 9.1 | 7.7 | 7.0 | |
| Cox10 | 11.8 | 4.0 | 3.4 | |
| Cox11 | 7.8 | 6.2 | 1.1 | |
| Cox17 | 13.5 | 12.4 | 26.5 | |
| Cox4i1 | 120.8 | 255.3 | 446.3 | |
| Cox5a | 53.2 | 109.8 | 61.4 | |
| Cox6a1 | 83.7 | 160.6 | 189.2 | |
| Cox6a2 | 0.1 | 1.2 | 7.2 | |
| Cox6b1 | 89.7 | 187.9 | 210.8 | |
| Cox6c | 49.8 | 74.9 | 62.8 | |
| Cox7a2 | 40.4 | 85.5 | 58.7 | |
| Cox7a2l | 20.6 | 49.4 | 140.0 | |
| Cox7b | 11.5 | 29.5 | 10.3 | |
| Cox8a | 123.0 | 191.0 | 228.0 | |
| Ndufa1 | 21.7 | 49.5 | 34.5 | |
| Ndufa10 | 21.6 | 31.8 | 30.3 | |
| Ndufa11 | 5.4 | 12.0 | 7.5 | |
| Ndufa12 | 8.8 | 13.3 | 10.6 | |
| Ndufa13 | 17.7 | 32.5 | 36.2 | |
| Ndufa2 | 37.5 | 54.7 | 80.0 | |
| Ndufa3 | 46.7 | 60.6 | 100.7 | |
| Ndufa4 | 75.4 | 167.3 | 64.6 | |
| Ndufa5 | 16.7 | 37.0 | 11.0 | |
| Ndufa6 | 35.2 | 60.5 | 78.4 | |
| Ndufa7 | 35.3 | 53.4 | 74.2 | |
| Ndufa8 | 18.2 | 37.4 | 32.5 | |
| Ndufa9 | 28.3 | 42.2 | 40.9 | |
| Ndufb10 | 31.6 | 62.5 | 45.5 | |
| Ndufb11 | 23.6 | 32.1 | 42.9 | |
| Ndufb2 | 29.6 | 80.8 | 37.1 | |
| Ndufb3 | 21.8 | 52.5 | 25.5 | |
| Ndufb4 | 6.7 | 10.4 | 12.1 | |
| Ndufb5 | 27.0 | 43.6 | 34.1 | |
| Ndufb6 | 33.8 | 65.4 | 25.6 | |
| Ndufb7 | 23.4 | 45.1 | 68.8 | |
| Ndufb8 | 30.3 | 74.9 | 79.7 | |
| Ndufb9 | 46.3 | 70.1 | 60.4 | |
| Ndufs1 | 31.8 | 22.3 | 7.5 | |
| Ndufs2 | 43.8 | 56.6 | 67.7 | |
| Ndufs3 | 8.0 | 9.5 | 8.0 | |
| Ndufs4 | 14.0 | 25.1 | 16.0 | |
| Ndufs6 | 14.4 | 18.8 | 16.5 | |
| Ndufs7 | 31.2 | 44.3 | 46.7 | |
| Ndufs8 | 21.0 | 27.9 | 76.3 | |
| Ndufv1 | 27.3 | 44.8 | 37.7 | |
| Ndufv2 | 22.7 | 37.4 | 16.6 | |
| Ndufv3 | 23.1 | 58.7 | 51.3 | |
| Slc25a14 | 5.4 | 9.4 | 1.7 | |
| Slc25a27 | 4.2 | 27.0 | 0.3 | |
| Uqcrb | 8.4 | 11.6 | 6.1 | |
| Uqcrc1 | 43.7 | 64.6 | 92.4 | |
| Uqcrc2 | 50.2 | 62.8 | 36.2 | |
| Uqcrfs1 | 49.6 | 63.3 | 46.2 | |
| Uqcrh | 56.6 | 94.1 | 116.7 | |
| Uqcrq | 52.3 | 75.2 | 89.2 | |
|
| ||||
| Oxidative Phosphorylation, TCA cycle | Sdha | 46.1 | 45.6 | 36.1 |
| Sdhb | 34.6 | 55.0 | 82.7 | |
| Sdhc | 51.8 | 56.2 | 66.3 | |
| Sdhd | 43.3 | 51.7 | 54.0 | |
|
| ||||
| Radicals detoxification | Gsr | 7.7 | 7.6 | 7.3 |
| Nfe2l2 | 11.9 | 2.1 | 55.7 | |
| Nfkb1 | 7.3 | 1.9 | 51.0 | |
| Nfkbia | 49.6 | 13.1 | 720.1 | |
| Sod1 | 22.6 | 33.4 | 33.5 | |
| Sod2 | 14.0 | 21.6 | 14.0 | |
| Sod3 | 9.1 | 0.4 | 0.8 | |
| Txn2 | 28.8 | 42.6 | 45.8 | |
| Txnip | 27.5 | 5.0 | 358.5 | |
|
| ||||
| TCA cycle | Aco1 | 23.3 | 7.4 | 11.6 |
| Aco2 | 31.6 | 38.5 | 29.8 | |
| Idh1 | 82.8 | 38.6 | 8.4 | |
| Idh2 | 39.5 | 10.3 | 77.2 | |
| Mdh1 | 44.7 | 105.3 | 59.8 | |
| Mdh2 | 62.0 | 120.3 | 85.4 | |
| Ogdh | 15.4 | 16.9 | 16.6 | |
| Ogdhl | 13.6 | 4.3 | 1.1 | |
| Mut | 10.2 | 3.4 | 1.3 | |
| Pck2 | 2.6 | 4.7 | 10.9 | |
| Pcx | 32.8 | 6.4 | 4.2 | |
| Slc25a1 | 24.6 | 43.7 | 30.4 | |
| Slc25a10 | 4.0 | 2.4 | 19.5 | |
| Slc25a11 | 19.8 | 38.1 | 55.6 | |
| Slc25a12 | 17.0 | 30.9 | 4.9 | |
Table 2.
Expression of genes related to glycolytic metabolism in different cell types
| Function | Gene | Expression (FPKM) | ||
|---|---|---|---|---|
|
| ||||
| Astrocytes | Neurons | Microglia | ||
| Glucose transporters | Slc2a1 | 56.4 | 11.4 | 46.6 |
| Slc2a3 | 1.7 | 32.9 | 5.4 | |
| Slc2a5 | 0.2 | 0.3 | 223.0 | |
|
| ||||
| Glycogen Degradation | Agl | 13.7 | 4.0 | 1.2 |
| Gaa | 25.6 | 33.6 | 38.6 | |
| Mtap | 5.0 | 2.1 | 2.8 | |
| Pgm1 | 6.1 | 6.5 | 6.0 | |
| Pgm2 | 27.0 | 10.7 | 8.5 | |
| Pygb | 111.0 | 31.7 | 19.8 | |
| Pygm | 5.6 | 0.7 | 9.6 | |
|
| ||||
| Glycolysis | Aldoa | 109.8 | 270.5 | 271.6 |
| Aldoart1 | 6.2 | 13.0 | 14.6 | |
| Aldoart2 | 3.8 | 8.7 | 11.9 | |
| Aldoc | 512.1 | 28.2 | 42.6 | |
| Bpgm | 18.0 | 13.3 | 4.6 | |
| Eno1 | 2.4 | 6.8 | 14.5 | |
| Eno2 | 5.0 | 82.6 | 35.3 | |
| Eno3 | 6.1 | 5.5 | 19.6 | |
| Gapdh | 40.2 | 73.4 | 79.9 | |
| Gpi1 | 26.6 | 53.6 | 49.1 | |
| Hk1 | 14.2 | 41.6 | 13.4 | |
| Hk2 | 4.5 | 0.4 | 62.3 | |
| Hk3 | 0.1 | 0.2 | 64.0 | |
| Pfkl | 12.9 | 20.0 | 37.5 | |
| Pfkm | 73.9 | 47.9 | 8.9 | |
| Pfkp | 8.9 | 31.6 | 2.4 | |
| Pgam1 | 40.1 | 79.1 | 75.8 | |
| Pgk1 | 18.4 | 22.1 | 18.3 | |
| Pgm2l1 | 1.1 | 14.6 | 0.7 | |
| Pkm2 | 53.8 | 83.6 | 153.6 | |
| Tpi1 | 39.7 | 84.2 | 95.1 | |
|
| ||||
| Glycolysis (anaerobic) | Ldha | 42.4 | 68.6 | 105.9 |
| Ldhb | 62.1 | 34.7 | 228.4 | |
|
| ||||
| Glycolysis (regulators) | Pfkfb2 | 23.5 | 14.6 | 1.5 |
| Pfkfb3 | 60.3 | 5.3 | 77.2 | |
| Pfkfb4 | 12.5 | 4.4 | 9.7 | |
Microglia express several glucose transporters, with GLUT3 (SLC2A3) being the major isoform (Kalsbeek et al. 2016). GLUT3 is a passive, facilitated glucose transporter, and it is also the dominate isoform expressed by neurons (Vannucci et al. 1997). Unlike other cell types in brain, microglia also express GLUT5 (SLC2A5) (Payne et al. 1997; see also Table 2). GLUT5 is classified as a fructose transporter because it has a high affinity for fructose and relatively low affinity for glucose. As fructose is present only at low concentrations in brain, the physiological role of this transporter on microglia remains obscure (Douard and Ferraris 2008). Microglia are also able to take up ketones and lactate, through the monocarboxylic transporters MCT1 and MCT2 (Moreira et al. 2009). The extent to which these non-glucose substrates fuel microglial function is unknown, but ketones have been shown to influence microglial function (as further described below).
Quiescent microglia are thought to rely primarily upon oxidative phosphorylation for ATP production (Bernhart et al. 2010; Chenais et al. 2002; Moss and Bates 2001; Won et al. 2012). Activated microglia in culture increase lactate production, decrease mitochondrial oxygen consumption, and decrease mitochondrial ATP production, indicative of an increased reliance on glycolysis (Gimeno-Bayon et al. 2014; Voloboueva et al. 2013). A switch from oxidative phosphorylation to aerobic glycolysis has likewise been shown to occur in macrophages as they transition between resting and activated states (Galvan-Pena and O’Neill 2014). However, the concept that activated microglia shift to glycolytic energy production does not cleanly fit with observations of microglial mitochondrial density. In cultured rat microglia, stimulation by LPS and IFN-γ increases the abundance of microglial mitochondria (Banati et al. 2004; Ferger et al. 2010). Microglial activation is also associated with de novo synthesis of mitochondrial translocator protein (Venneti et al. 2006), suggesting that the increased mitochondrial number is attributable to mitochondrial biogenesis rather than mitochondrial fission.
While it seems reasonable to assume that impaired capacity for energy production would influence microglial function, there only limited information available in this area. Reduced substrate availability has been shown to induce stress granule formation in cultured microglia. Cytosolic stress granules are irregular complexes of RNA and RNA binding proteins(Hanson and Mair 2014; Krisenko et al. 2015), and in microglia they stimulate the production of reactive oxygen and nitrogen species by activation of spleen tyrosine kinase (SYK) and increased expression of its substrates such as NF-κB (Ghosh and Geahlen 2015). The mitochondrial toxins carbon monoxide, rotenone, and 3-nitroproprionate each influence LPS-induced cytokine production by cultured microglia (Ferger et al. 2010; Wilson et al. 2017); however, glycolytic capacity can often fully compensate for impaired mitochondrial ATP production in cultured cells, and consequently the effects of these toxins may be indirect. More direct evidence that bioenergetic capacity may directly influence microglial function comes from studies of mice deficient in Triggering Receptor Expressed On Myeloid Cells -2 (TREM2). TREM2 is a microglial surface receptor that is required for normal microglial responses to dead and damaged cells. TREM2 deficient microglia in mouse brain exhibit, in addition to functional impairment, a reduced mitochondrial mass, increased AMPK phosphorylation (indicating low energy status), and increased autophagy (Ulland et al. 2017). Manipulations that reconstitute TREM2 intracellular signaling or support ATP synthesis in these mice microglia reverse all of these abnormalities, suggesting that the functional impairment stems from bioenergetic compromise.
Glucose and microglial ROS production
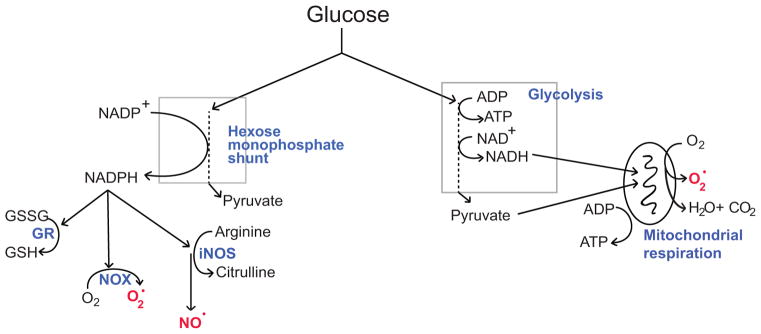
Independent of its function role in energy metabolism, glucose is also required for the quenching of reactive oxygen species (ROS) and for repairing oxidative damage to proteins through glutathione and thioredoxin - coupled pathways. This is because glucose is the exclusive substrate for the hexose monophosphate shunt (Fig. 2). NADPH produced by the hexose monophosphate shunt is required by glutathione reductase to convert oxidized glutathione (GSSG) back to reduced glutathione (GSH), the major thiol anti-oxidant in brain, and likewise required for reducing thioredoxin to its active form (Mustacich and Powis 2000).
Figure 2. Glucose fuels both ROS scavenging and ROS production.
Glucose metabolized through the hexose monophosphate shunt provides reducing equivalents for regenerating NADPH from NADP+. NADPH in turn is used by glutathione reductase (GR) to regenerate glutathione (GSH) from glutathione disulfide (GSSG). GSH is used for enzymatic and non-enzymatic scavenging of ROS, and for repair of proteins that have been oxidized by ROS. However, NADPH derived from the hexose monophosphate shunt is also a requisite cofactor for the production of nitric oxide (NO·) by nitric oxide synthase and superoxide (O2·) by NADPH oxidase (NOX). Glucose metabolized through glycolysis provides reducing equivalents for regenerating NADH from NAD+. These reducing equivalents (electrons) normally transit the mitochondrial electron transport chain in the process of generating CO2 and ATP, but under some conditions a substantial fraction of these electrons are instead captured by oxygen to form superoxide. (Adapted from (Robbins and Swanson 2014).
Glucose is also used by cells to generate ROS. One way this occurs is by fueling the electron transport chain of mitochondria. Glucose is normally the origin of the vast majority of reducing equivalents (electrons) passing through the mitochondrial electron transport chain in CNS tissues (Fig. 2). Reducing equivalents carried by NADH enter the mitochondrial electron transport chain and normally pass all the way to mitochondrial complex IV (cytochrome C oxidase) during oxidative ATP production, where they reduce molecular oxygen (O2) to H2O. However, it is possible for electrons to escape from the electron transport chain or reduced mitochondrial enzymes to form superoxide (O2·) (Adam-Vizi 2005; Nicholls and Ferguson 2013; Starkov et al. 2004). The rate at which this process generates superoxide in healthy cells is likely very low, far below the early estimate of ~2% of respiratory oxygen flux obtained using isolated (cell-free) mitochondria (Boveris 1977). However, this rate becomes appreciable during ischemia-reperfusion (Niatsetskaya et al. 2012), in damaged or defective mitochondria (Hayashi and Cortopassi 2015), and in the presence of mitochondrial toxins (Adam-Vizi 2005; Nicholls and Ferguson 2013).
A second way that glucose fuels ROS production is through generating NADPH (via the hexose monophosphate shunt) for enzymatic production of nitric oxide and superoxide (Fig. 2). Microglial inducible nitric oxide synthase (iNOS) uses NADPH and L-arginine as substrates to generate nitric oxide (Possel et al. 2000). Nitric oxide is non-polar, lipid permeable, and has a relatively long half-life and diffusion distance in brain (Pacher et al. 2007). Superoxide, by contrast, is polar, largely lipid impermeable, and has a relatively short half-life and diffusion distance (Pacher et al. 2007). Neither of these ROS is intrinsically highly cytotoxic, but both can be further metabolized to produce highly reactive and cytotoxic species such as hypochlorous acid, peroxynitrite, and hydroxyl radical.
Superoxide is enzymatically produced by NADPH oxidase, a multi-subunit protein complex with several isoforms distinguished on the basis of their catalytic subunits: NOX1 – NOX5, DUOX 1, and DUOX2. Most cell types express one or more forms of NADPH oxidase and generate superoxide as a local signaling molecule (Bedard and Krause 2007). Cells of immune lineage, including microglia, express predominately the NOX2 isoform and use superoxide (in conjunction with nitric oxide or HCl to kill invading pathogens (Kauppinen et al. 2008; Schieber and Chandel 2014). NOX2 generates superoxide by the transfer of electrons to molecular oxygen on one side of a membrane, while oxidizing NADPH to NADP+ and H+ on the other side. In stimulated phagocytes, the rate of O2 consumption by NOX2 can exceed the normal rate of O2 consumption by mitochondria, a process termed as “respiratory burst”. This rapid superoxide production requires the continuous metabolism of glucose by the hexose monophosphate shunt for supply of NADPH substrate (Cohen and Chovaniec 1978; Decoursey and Ligeti 2005).
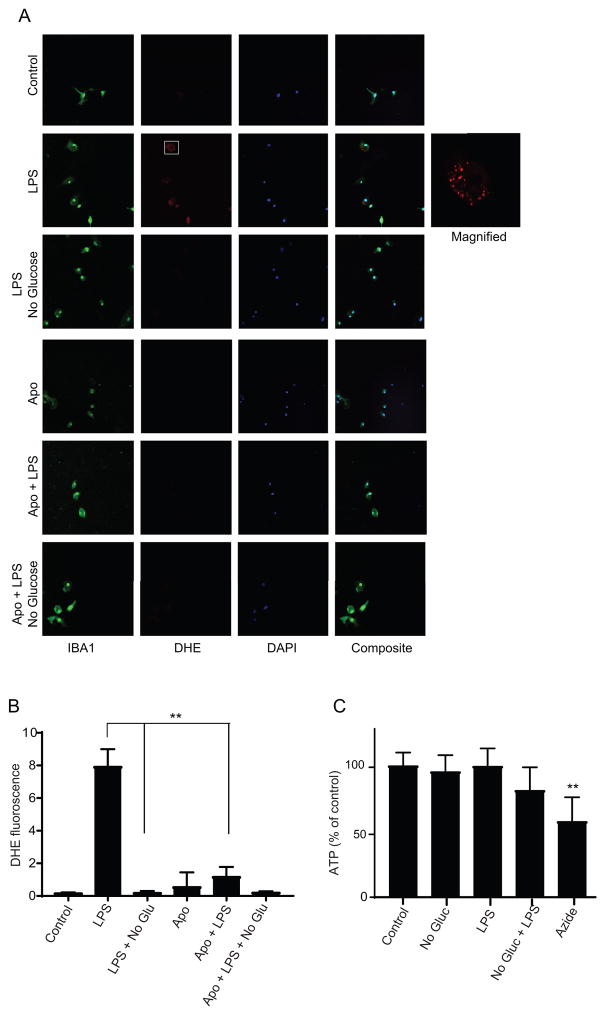
Whether microglia are likewise capable of very rapid superoxide production has not been established, but it is nevertheless likely that the ability of microglia to generate superoxide is dependent upon glucose availability. To directly test this idea, we treated primary mouse microglial cultures with bacterial lipopolysaccharide (LPS) to activate NOX2, and evaluated superoxide production in the presence and absence of glucose in the medium (Fig. 3). The medium was supplemented with glutamine as an energy source to prevent ATP depletion. LPS induced a robust superoxide production in medium containing glucose (4 mM), as detected by dihydroethidium oxidation. By contrast, superoxide production was negligible in medium that did not contain glucose (Fig. 3).
Figure 3. Superoxide production by microglia requires glucose.
(A) LPS induced a robust superoxide production in the microglia in control medium, but not in glucose-free medium. The fluorescent red signal is produced by oxidized dihydroethidium. Lack of signal in cultures co-treated with NOX inhibitor apocynin (Apo, 10 μM) confirms superoxide as the oxidant species. (B) Quantified data. n = 3, **p < 0.01; means ± s.e.m. (C) Measures of cellular ATP in cells treated as in A showed no significant decrement in the glucose-free conditions. The mitochondrial inhibitor azide (10 μM) was used as a positive control in presence of glucose. n = 3, **p < 0.01 v. control; means ± s.e.m. Primary microglia were prepared as described (Saura et al. 2003). Experiments were done using DMEM containing no phenol red, 4 mM glutamine, and either 4 or 0 mM glucose. LPS was used at 500 ng/ml. Cells were incubated with dihydroethidium (DHE; 10 μM) for 30 minutes, then fixed with 4% formaldehyde. Microglia were identified using antibody to Iba1 (ab10709, Abcam) and green fluorescent secondary antibody. Cell nuclei are labelled with DAPI (blue). Superoxide production was quantified as described (Brennan et al. 2009). For ATP measurements, cells were lysed after 30 minute intervals using detergent supplied by the Abcam luminescence ATP detection assay kit (ab113849). Luminescence was measured in parallel with ATP standards treated identically. Cultures were visually inspected to confirm no significant well-to-well variation in cell number. Data were obtained from 3 independent experiments, each with 2–3 internal replicates. Statistical comparisons were made by AOVA with Bonferroni’s test. All studies were approved by the San Francisco Veterans Affairs Medical Center animal studies committee.
The link between glucose and ROS production may also be relevant to the deleterious effects of elevated blood glucose (hyperglycemia) in brain ischemia-reperfusion (Bruno et al. 1999; Martini and Kent 2007; Myers and Yamaguchi 1977; Parsons et al. 2002; Ribo et al. 2005). A widely cited mechanism by which hyperglycemia can exacerbate brain injury is by increasing lactic acid production and thereby reducing pH in hypoxic tissue (Plum 1983); however, elevated glucose exacerbates ischemic injury even when acidosis is minimal (D’Alecy et al. 1986; Park et al. 2001; Venables et al. 1985) and in hypoxic brain slices where pH is tightly controlled (Newell et al. 1995; Rytter et al. 2003). Hyperglycemia increases superoxide production from neurons during ischemia-reperfusion, and NOX2 inhibition prevents both the increased superoxide production and increased neuronal death that would otherwise occur (Suh et al. 2008; Won et al. 2011). It remains to be established if the superoxide production by microglia in the later, inflammatory stage of ischemic injury is likewise increased by high blood glucose concentrations.
Energy metabolism affects pro-inflammatory gene expression by microglia
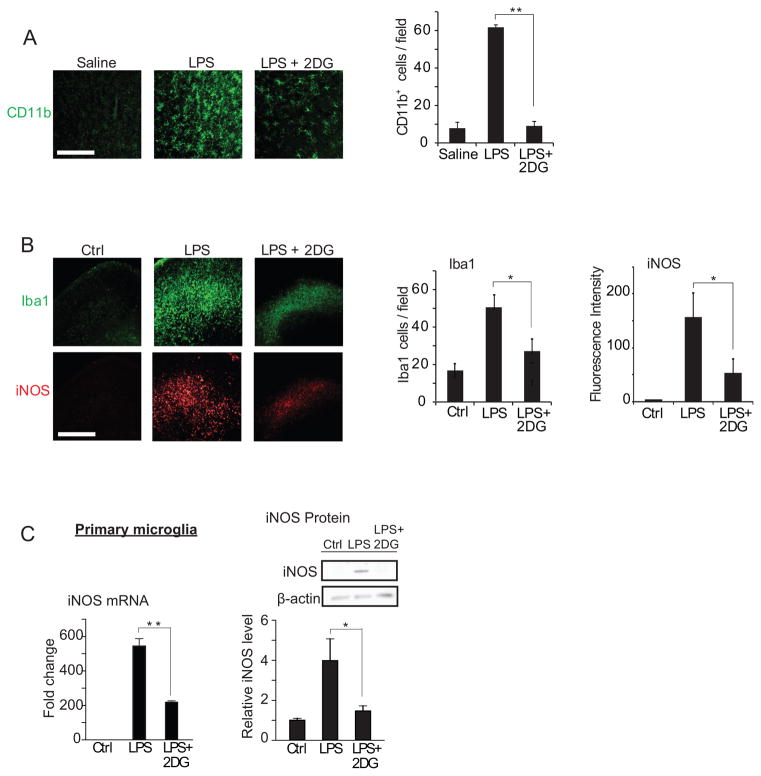
Inflammatory responses are influenced at the gene transcription level by factors that affect cellular bioenergetic state, such as caloric restriction, ketogenic diet, and the glycolytic inhibitor 2-deoxyglucose (2DG) (Goth 1959; Longo and Mattson 2014). Caloric restriction, ketogenic diet, and 2DG each produce a ketogenic state in which glucose utilization is suppressed, and they also reduce inflammation, tissue loss, and functional impairment after brain injury (Appelberg et al. 2009; Gasior et al. 2006; Loncarevic-Vasiljkovic et al. 2012; Tu et al. 2012; Wang et al. 2009; Yu and Mattson 1999). Examples of this are shown in Figure 4. In these studies, lipopolysaccharide (LPS) was used as an inflammatory stimulus, and the reduced glycolytic flux resulting from caloric restriction was mimicked by the glycolytic inhibitor 2DG (Garriga-Canut et al. 2006; Yao et al. 2011). Systemic (intraperitoneal) administration of LPS to rats induced a robust activation of brain microglia, and this was attenuated by co-administration of 2DG (Fig. 4A). In organotypic brain slice cultures (Fig. 4B), 2DG likewise suppressed the effect of LPS on microglial activation and on the expression of inducible nitric oxide synthase (iNOS), a hallmark of inflammatory activation in microglia and macrophages (Forstermann and Kleinert 1995). Primary microglial cultures similarly showed an attenuated response to LPS in the presence of 2DG (Fig. 4C), thus confirming that the effects of LPS and 2DG on microglia are not dependent upon indirect, systemic effects of these agents.
Figure 4. Glucose metabolism affects microglial activation and ROS production at the transcriptional level.
(A) Immunostaining for CD11b identifies activated microglia in rat hippocampus. CD11b expression was increased 24 hours after intraperitoneal injection with LPS (10 mg/kg). The increase was attenuated by co-injection with the glycolytic inhibitor 2-deoxyglucose (2DG; 100mg/kg). Scale bar = 100 μm. ** p < 0.01, n = 6. (B) Immunostaining for Iba1 and iNOS identify activated microglia in mouse hippocampal slice cultures after 24 hours incubation with LPS (10 μg/ml) or LPS +2DG (1 mM). Scale bar = 100 μm; n ≥ 3, * p < 0.05. Culture medium for contained 6 mM glutamine and 5 mM glucose. (C) Effects of 1mM 2DG on LPS - induced iNOS transcript and protein expression in microglial cultures. n = 5; * p < 0.05, ** p < 0.01. Data are means ± s.e.m. Figure adapted from (Shen et al. 2017).
Recent studies have identified several mechanisms by which glucose levels can influence pro-inflammatory gene transcription. One of these mechanisms involves the formation of advanced glycation end products (AGE), which are modifications of proteins and lipids resulting from non-enzymatic reactions with sugars. AGE formation is generally a slow process, but is accelerated by high glucose levels. Microglia express receptors for advanced glycation end-products, which stimulate pro-inflammatory signaling pathways when activated (Bianchi et al. 2010; Chen et al. 2017). Glycolytic rate (though not necessarily glucose levels per se) can also influence formation of NLRP1 and NLRP3 inflammasomes. Inflammasomes are responsible for caspase 1 activation, which is required for maturation and secretion of IL-1β and other cytokines (Franchi et al. 2009). Increased glycolytic rate facilitates formation of NLRP3 activation in macrophages, (Moon et al. 2015; Xie et al. 2016), by a mechanism that remains to be established. In brain, NLRP3 inflammasomes are found in microglia and not in astrocytes (Gustin et al. 2015). Intermittent fasting was shown to suppress post-ischemic expression of inflammasomes, and this effect was associated with reduced Il1-beta and reduced NF-κB signalling in the post-ischemic brain (Fann et al. 2014).
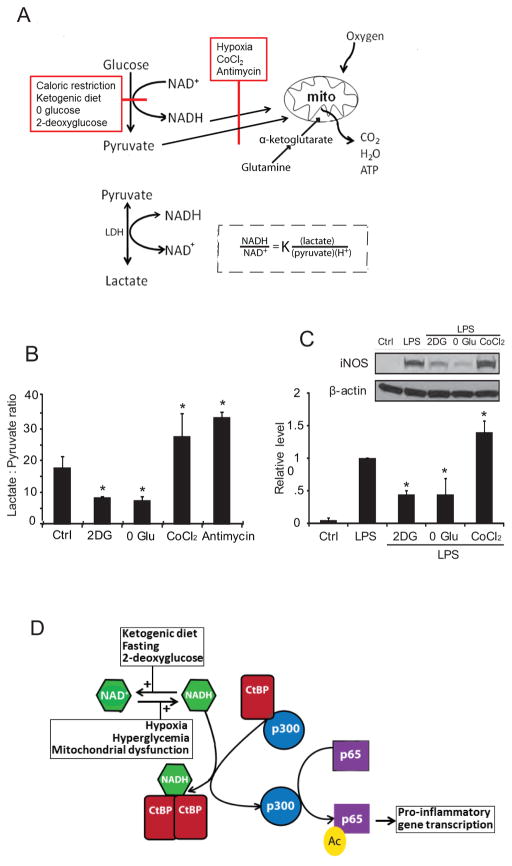
An additional, more direct way that glucose metabolism exerts transcriptional control over microglial activation is through an NADH-sensitive co-repressor termed C-terminal binding protein (CtBP). Factors that slow glucose flux through glycolysis, such as reduced glucose availability or glycolytic inhibitors, reduce NADH levels and thereby reduce NADH:NAD+ ratio. By contrast, factors that slow oxidative metabolism, such as hypoxia and mitochondrial inhibitors, have the opposite effect (Fig. 5A). The resulting relative changes in NADH are several hundred-fold greater than the reciprocal changes in NAD+ because the cytosolic NADH : NAD+ ratio is normally in the range of 1:700 (Ying 2008). For this reason, changes in cytosolic NADH levels provide a highly sensitive measure of changes in glycolytic flux.
Figure 5. NADH and CtBP mediate coupling between glucose metabolism and gene transcription.
(A) Factors that slow glucose flux through glycolysis, such as reduced glucose availability or glycolytic inhibitors, reduce NADH levels and thereby reduce NADH:NAD+ ratio, whereas factors that slow oxidative metabolism, such as hypoxia and mitochondrial inhibitors, have the opposite effect. Lactate dehydrogenase (LDH) maintains the lactate:pyruvate ratio in equilibrium with the cytosolic NADH:NAD+ ratio. In the experiments shown, glutamine provided α-ketoglutarate to fuel mitochondrial ATP production in the absence of glycolysis. (B) The lactate:pyruvate ratio provides an index of the cytosolic NADH:NAD+ ratio in cells treated with glycolytic inhibition (2DG, 1 mM 2-deoxyglucose; 0 Glu, glucose-free medium) or mitochondrial inhibitors (CoCl2, 200 μM cobalt chloride; antimycin, 1 μM antimycin A). n = 4; *p < 0.05 v. control. (C) LPS-induced iNOS expression was suppressed in RAW267.4 cells treated with 1 mM 2DG or glucose-free medium, and was increased in cells treated with the CoCl2. n = 4; * p < 0.05 v. control. Error bars show s.e.m. (D) CtBP normally binds and inhibits p300 acetylase. NADH promotes CtBP dimerization and resultant release of p300 from CtBP. Unbound p300 acetylates the p65 subunit of NF-κB to promote NF-κB transcriptional activity. Fasting, ketogenic diet, and glycolytic inhibition reduce NADH levels and thereby reduce CtBP dimerization and increase CtBP repression of p300 activity. Hypoxia, hyperglycemia, and mitochondrial dysfunction have the reverse effect. Panels A, B, and C are from (Shen et al. 2017).
The CtBPs are among a very limited number of NADH - sensitive proteins known to influence gene transcription (Ghosh et al. 2010). CtBP in its monomeric form suppresses the activity of the acetyltransferase p300/CBP (Kim et al. 2005), which acetylates both histones and the pro-inflammatory transcription factor, NF-κB (Chen et al. 2002). Increased NADH levels promote the formation of CtBP dimers, and thereby modulate CtBP association with its binding partners (Zhang et al. 2002). In microglia and the macrophage RAW264.7 cell line, glucose flux was shown to regulate the expression of iNOS and other pro-inflammatory genes by a mechanism involving CtBP sensing of cytosolic NADH levels (Shen et al. 2017). This mechanism may more generally couple glucose metabolism to innate immune reactivity (Fig. 5C).
Direct effects of ketones on microglial activation
Sustained reductions in blood glucose levels lead to a rise in circulating ketones as peripheral fat is metabolized to acetoacetate, beta-hydroxybutyrate, and their breakdown product acetone. A ketogenic state is common in animals in the wild, and occurs in humans during fasting and extended exercise. Fasting and ketogenic diet have well-established anti-inflammatory effects, including suppression of microglial activation (Fann et al. 2014; Gasior et al. 2006; Longo and Mattson 2014). These effects are mediated in part by direct signaling by ketones on microglia and macrophages. Beta-hydroxybutyrate activates G-protein-coupled receptors 109A (GPR109A) and inhibits histone de-acetylases (Huang et al. 2017; Newman and Verdin 2014; Rahman et al. 2014). In microglia, the activation of GPR109A by beta-hydroxybutyrate attenuates NF-kB signaling and pro-inflammatory cytokine production (Fu et al. 2015). Activation of GPR109A also promotes a neuroprotective phenotype in microglia/macrophages, and in a mouse stroke model the beneficial and anti-inflammatory effects of ketogenic diet are lost in mice lacking the GPR109A receptor. Microglia and macrophages also express other metabolite receptors in addition to GPR109A, but the effects on these on microglia function remain to be established.
Conclusions
As “sentinels” of the nervous system, it is fitting that microglia respond to changes in brain metabolism. It has been recognized for decades that bioenergetic factors influence innate immune responses (Goth, 1959), but only recently have some the mechanisms that underlie these effects been identified. As our understanding of microglial activation becomes more nuanced and complete, we can likely expect to find additional links between bioenergetic factors and specific aspects of activation, such as specific gene expression patterns or morphological changes. However, microglia are normally in a state that is “quiescent” but not inert, as they perform trophic, signaling, and other functions important to normal brain homeostasis, and virtually nothing is yet known about how bioenergetic factors may influence these basal microglial functions. Moreover, most of what is now known about bioenergetic influences on microglia comes from studies performed with dissociated cultures and thus require validation in more complex systems. Microglial cultures do not always reflect the properties of microglia in situ, and do not account for the complexity inherent in multiple interacting cell types simultaneously experiencing and responding to bioenergetic changes.
Acknowledgments
This work was supported by the NIH (RO1 NS081149) and by the Dept. of Veterans Affairs (1IO1 BX003249).
References
- Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal. 2005;7:1140–9. doi: 10.1089/ars.2005.7.1140. [DOI] [PubMed] [Google Scholar]
- Appelberg KS, Hovda DA, Prins ML. The effects of a ketogenic diet on behavioral outcome after controlled cortical impact injury in the juvenile and adult rat. J Neurotrauma. 2009;26:497–506. doi: 10.1089/neu.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati RB, Egensperger R, Maassen A, Hager G, Kreutzberg GW, Graeber MB. Mitochondria in activated microglia in vitro. J Neurocytol. 2004;33:535–41. doi: 10.1007/s11068-004-0515-7. [DOI] [PubMed] [Google Scholar]
- Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993;7:111–8. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38:357–66. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bergsneider M, Hovda DA, Shalmon E, Kelly DF, Vespa PM, Martin NA, Phelps ME, McArthur DL, Caron MJ, Kraus JF, et al. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J Neurosurg. 1997;86:241–51. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- Bernhart E, Kollroser M, Rechberger G, Reicher H, Heinemann A, Schratl P, Hallstrom S, Wintersperger A, Nusshold C, DeVaney T, et al. Lysophosphatidic acid receptor activation affects the C13NJ microglia cell line proteome leading to alterations in glycolysis, motility, and cytoskeletal architecture. Proteomics. 2010;10:141–58. doi: 10.1002/pmic.200900195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi R, Giambanco I, Donato R. S100B/RAGE-dependent activation of microglia via NF-kappaB and AP-1 Co-regulation of COX-2 expression by S100B, IL-1beta and TNF-alpha. Neurobiol Aging. 2010;31:665–77. doi: 10.1016/j.neurobiolaging.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Boumezbeur F, Petersen KF, Cline GW, Mason GF, Behar KL, Shulman GI, Rothman DL. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2010;30:13983–91. doi: 10.1523/JNEUROSCI.2040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A. Mitochondrial production of superoxide radical and hydrogen peroxide. Adv Exp Med Biol. 1977;78:67–82. doi: 10.1007/978-1-4615-9035-4_5. [DOI] [PubMed] [Google Scholar]
- Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–63. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno A, Biller J, Adams HP, Jr, Clarke WR, Woolson RF, Williams LS, Hansen MD. Acute blood glucose level and outcome from ischemic stroke. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Neurology. 1999;52:280–4. doi: 10.1212/wnl.52.2.280. [DOI] [PubMed] [Google Scholar]
- Chen J, Sun Z, Jin M, Tu Y, Wang S, Yang X, Chen Q, Zhang X, Han Y, Pi R. Inhibition of AGEs/RAGE/Rho/ROCK pathway suppresses non-specific neuroinflammation by regulating BV2 microglial M1/M2 polarization through the NF-kappaB pathway. J Neuroimmunol. 2017;305:108–114. doi: 10.1016/j.jneuroim.2017.02.010. [DOI] [PubMed] [Google Scholar]
- Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002;21:6539–48. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenais B, Morjani H, Drapier JC. Impact of endogenous nitric oxide on microglial cell energy metabolism and labile iron pool. J Neurochem. 2002;81:615–23. doi: 10.1046/j.1471-4159.2002.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HJ, Chovaniec ME. Superoxide production by digitonin-stimulated guinea pig granulocytes. The effects of N-ethyl maleimide, divalent cations; and glycolytic and mitochondrial inhibitors on the activation of the superoxide generating system. J Clin Invest. 1978;61:1088–96. doi: 10.1172/JCI109008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alecy LG, Lundy EF, Barton KJ, Zelenock GB. Dextrose containing intravenous fluid impairs outcome and increases death after eight minutes of cardiac arrest and resuscitation in dogs. Surgery. 1986;100:505–11. [PubMed] [Google Scholar]
- Davalos D, Ryu JK, Merlini M, Baeten KM, Le Moan N, Petersen MA, Deerinck TJ, Smirnoff DS, Bedard C, Hakozaki H, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227. doi: 10.1038/ncomms2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decoursey TE, Ligeti E. Regulation and termination of NADPH oxidase activity. Cell Mol Life Sci. 2005;62:2173–93. doi: 10.1007/s00018-005-5177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Katzmarski N, Kipnis J, Meyer-Luehmann M. Microglia as a critical player in both developmental and late-life CNS pathologies. Acta Neuropathol. 2014;128:333–45. doi: 10.1007/s00401-014-1321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab. 2012;32:1107–38. doi: 10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, Yao J, Rettberg JR, Chen S, Brinton RD. Early decline in glucose transport and metabolism precedes shift to ketogenic system in female aging and Alzheimer’s mouse brain: implication for bioenergetic intervention. PLoS One. 2013;8:e79977. doi: 10.1371/journal.pone.0079977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douard V, Ferraris RP. Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab. 2008;295:E227–37. doi: 10.1152/ajpendo.90245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusick JR, Glenn TC, Lee WN, Vespa PM, Kelly DF, Lee SM, Hovda DA, Martin NA. Increased pentose phosphate pathway flux after clinical traumatic brain injury: a [1,2–13C2]glucose labeling study in humans. J Cereb Blood Flow Metab. 2007;27:1593–602. doi: 10.1038/sj.jcbfm.9600458. [DOI] [PubMed] [Google Scholar]
- Fann DY, Santro T, Manzanero S, Widiapradja A, Cheng YL, Lee SY, Chunduri P, Jo DG, Stranahan AM, Mattson MP, et al. Intermittent fasting attenuates inflammasome activity in ischemic stroke. Exp Neurol. 2014;257:114–9. doi: 10.1016/j.expneurol.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Ferger AI, Campanelli L, Reimer V, Muth KN, Merdian I, Ludolph AC, Witting A. Effects of mitochondrial dysfunction on the immunological properties of microglia. J Neuroinflammation. 2010;7:45. doi: 10.1186/1742-2094-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstermann U, Kleinert H. Nitric oxide synthase: expression and expressional control of the three isoforms. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:351–64. doi: 10.1007/BF00172772. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Traves PG, Tufail Y, Leal-Bailey H, Lew ED, Burrola PG, Callaway P, Zagorska A, Rothlin CV, Nimmerjahn A, et al. TAM receptors regulate multiple features of microglial physiology. Nature. 2016;532:240–244. doi: 10.1038/nature17630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–7. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SP, Wang JF, Xue WJ, Liu HM, Liu BR, Zeng YL, Li SN, Huang BX, Lv QK, Wang W, et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J Neuroinflammation. 2015;12:9. doi: 10.1186/s12974-014-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan-Pena S, O’Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, Morrison JF, Ockuly J, Stafstrom C, Sutula T, et al. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9:1382–7. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol. 2006;17:431–9. doi: 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Geahlen RL. Stress Granules Modulate SYK to Cause Microglial Cell Dysfunction in Alzheimer’s Disease. EBioMedicine. 2015;2:1785–98. doi: 10.1016/j.ebiom.2015.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, George S, Roy U, Ramachandran D, Kolthur-Seetharam U. NAD: a master regulator of transcription. Biochim Biophys Acta. 2010;1799:681–93. doi: 10.1016/j.bbagrm.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Gimeno-Bayon J, Lopez-Lopez A, Rodriguez MJ, Mahy N. Glucose pathways adaptation supports acquisition of activated microglia phenotype. J Neurosci Res. 2014;92:723–31. doi: 10.1002/jnr.23356. [DOI] [PubMed] [Google Scholar]
- Goth A. Inhibition of anaphylactoid edema in the rat by 2-deoxyglucose. Am J Physiol. 1959;197:1056–8. doi: 10.1152/ajplegacy.1959.197.5.1056. [DOI] [PubMed] [Google Scholar]
- Gustin A, Kirchmeyer M, Koncina E, Felten P, Losciuto S, Heurtaux T, Tardivel A, Heuschling P, Dostert C. NLRP3 Inflammasome Is Expressed and Functional in Mouse Brain Microglia but Not in Astrocytes. PLoS One. 2015;10:e0130624. doi: 10.1371/journal.pone.0130624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343(Pt 2):281–99. [PMC free article] [PubMed] [Google Scholar]
- Hanson KK, Mair GR. Stress granules and Plasmodium liver stage infection. Biol Open. 2014;3:103–7. doi: 10.1242/bio.20136833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi G, Cortopassi G. Oxidative stress in inherited mitochondrial diseases. Free Radic Biol Med. 2015;88:10–7. doi: 10.1016/j.freeradbiomed.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, Seaman WE. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem. 2009;109:1144–56. doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang P, Xu X, Zhang Y, Gong Y, Hu W, Gao M, Wu Y, Ling Y, Zhao X, et al. The ketone body metabolite beta-hydroxybutyrate induces an antidepression-associated ramification of microglia via HDACs inhibition-triggered Akt-small RhoGTPase activation. Glia. 2017 doi: 10.1002/glia.23241. [DOI] [PubMed] [Google Scholar]
- Kalsbeek MJ, Mulder L, Yi CX. Microglia energy metabolism in metabolic disorder. Mol Cell Endocrinol. 2016;438:27–35. doi: 10.1016/j.mce.2016.09.028. [DOI] [PubMed] [Google Scholar]
- Kauppinen TM, Higashi Y, Suh SW, Escartin C, Nagasawa K, Swanson RA. Zinc triggers microglial activation. J Neurosci. 2008;28:5827–35. doi: 10.1523/JNEUROSCI.1236-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kim JH, Cho EJ, Kim ST, Youn HD. CtBP represses p300-mediated transcriptional activation by direct association with its bromodomain. Nat Struct Mol Biol. 2005;12:423–8. doi: 10.1038/nsmb924. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Konishi H, Takai T, Kiyama H. A DAP12-dependent signal promotes pro-inflammatory polarization in microglia following nerve injury and exacerbates degeneration of injured neurons. Glia. 2015;63:1073–82. doi: 10.1002/glia.22802. [DOI] [PubMed] [Google Scholar]
- Krisenko MO, Higgins RL, Ghosh S, Zhou Q, Trybula JS, Wang WH, Geahlen RL. Syk Is Recruited to Stress Granules and Promotes Their Clearance through Autophagy. J Biol Chem. 2015;290:27803–15. doi: 10.1074/jbc.M115.642900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–63. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- Loncarevic-Vasiljkovic N, Pesic V, Todorovic S, Popic J, Smiljanic K, Milanovic D, Ruzdijic S, Kanazir S. Caloric restriction suppresses microglial activation and prevents neuroapoptosis following cortical injury in rats. PLoS One. 2012;7:e37215. doi: 10.1371/journal.pone.0037215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–92. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini SR, Kent TA. Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab. 2007;27:435–51. doi: 10.1038/sj.jcbfm.9600355. [DOI] [PubMed] [Google Scholar]
- Moon JS, Hisata S, Park MA, DeNicola GM, Ryter SW, Nakahira K, Choi AM. mTORC1-Induced HK1-Dependent Glycolysis Regulates NLRP3 Inflammasome Activation. Cell Rep. 2015;12:102–15. doi: 10.1016/j.celrep.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Moreira TJ, Pierre K, Maekawa F, Repond C, Cebere A, Liljequist S, Pellerin L. Enhanced cerebral expression of MCT1 and MCT2 in a rat ischemia model occurs in activated microglial cells. J Cereb Blood Flow Metab. 2009;29:1273–83. doi: 10.1038/jcbfm.2009.50. [DOI] [PubMed] [Google Scholar]
- Moss DW, Bates TE. Activation of murine microglial cell lines by lipopolysaccharide and interferon-gamma causes NO-mediated decreases in mitochondrial and cellular function. Eur J Neurosci. 2001;13:529–38. doi: 10.1046/j.1460-9568.2001.01418.x. [DOI] [PubMed] [Google Scholar]
- Mustacich D, Powis G. Thioredoxin reductase. Biochem J. 2000;346(Pt 1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Myers RE, Yamaguchi S. Nervous system effects of cardiac arrest in monkeys. Preservation of vision. Arch Neurol. 1977;34:65–74. doi: 10.1001/archneur.1977.00500140019003. [DOI] [PubMed] [Google Scholar]
- Nakanishi H. Microglial functions and proteases. Mol Neurobiol. 2003;27:163–76. doi: 10.1385/MN:27:2:163. [DOI] [PubMed] [Google Scholar]
- Newell DW, Barth A, Papermaster V, Malouf AT. Glutamate and non-glutamate receptor mediated toxicity caused by oxygen and glucose deprivation in organotypic hippocampal cultures. J Neurosci. 1995;15:7702–11. doi: 10.1523/JNEUROSCI.15-11-07702.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab. 2014;25:42–52. doi: 10.1016/j.tem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niatsetskaya ZV, Sosunov SA, Matsiukevich D, Utkina-Sosunova IV, Ratner VI, Starkov AA, Ten VS. The oxygen free radicals originating from mitochondrial complex I contribute to oxidative brain injury following hypoxia-ischemia in neonatal mice. J Neurosci. 2012;32:3235–44. doi: 10.1523/JNEUROSCI.6303-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Ferguson SJ. Bioenergetics. London: Academic Press; 2013. [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Orth M, Schapira AH. Mitochondrial involvement in Parkinson’s disease. Neurochem Int. 2002;40:533–41. doi: 10.1016/s0197-0186(01)00124-3. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WS, Chang YS, Lee M. Effects of hyperglycemia or hypoglycemia on brain cell membrane function and energy metabolism during the immediate reoxygenation-reperfusion period after acute transient global hypoxia-ischemia in the newborn piglet. Brain Res. 2001;901:102–8. doi: 10.1016/s0006-8993(01)02295-8. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Gan WB. Microglia dynamics and function in the CNS. Curr Opin Neurobiol. 2010;20:595–600. doi: 10.1016/j.conb.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, Tress BM, Davis SM. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52:20–8. doi: 10.1002/ana.10241. [DOI] [PubMed] [Google Scholar]
- Payne J, Maher F, Simpson I, Mattice L, Davies P. Glucose transporter Glut 5 expression in microglial cells. Glia. 1997;21:327–31. doi: 10.1002/(sici)1098-1136(199711)21:3<327::aid-glia7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Plum F. What causes infarction in ischemic brain?: The Robert Wartenberg Lecture. Neurology. 1983;33:222–223. doi: 10.1212/wnl.33.2.222. [DOI] [PubMed] [Google Scholar]
- Possel H, Noack H, Putzke J, Wolf G, Sies H. Selective upregulation of inducible nitric oxide synthase (iNOS) by lipopolysaccharide (LPS) and cytokines in microglia: in vitro and in vivo studies. Glia. 2000;32:51–9. doi: 10.1002/1098-1136(200010)32:1<51::aid-glia50>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Rahman M, Muhammad S, Khan MA, Chen H, Ridder DA, Muller-Fielitz H, Pokorna B, Vollbrandt T, Stolting I, Nadrowitz R, et al. The beta-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:3944. doi: 10.1038/ncomms4944. [DOI] [PubMed] [Google Scholar]
- Ribo M, Molina C, Montaner J, Rubiera M, Delgado-Mederos R, Arenillas JF, Quintana M, Alvarez-Sabin J. Acute hyperglycemia state is associated with lower tPA-induced recanalization rates in stroke patients. Stroke. 2005;36:1705–9. doi: 10.1161/01.STR.0000173161.05453.90.9f. [DOI] [PubMed] [Google Scholar]
- Robbins NM, Swanson RA. Opposing effects of glucose on stroke and reperfusion injury: acidosis, oxidative stress, and energy metabolism. Stroke. 2014;45:1881–6. doi: 10.1161/STROKEAHA.114.004889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytter A, Cronberg T, Asztely F, Nemali S, Wieloch T. Mouse hippocampal organotypic tissue cultures exposed to in vitro “ischemia” show selective and delayed CA1 damage that is aggravated by glucose. J Cereb Blood Flow Metab. 2003;23:23–33. doi: 10.1097/01.WCB.0000034361.37277.1B. [DOI] [PubMed] [Google Scholar]
- Saura J, Tusell JM, Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia. 2003;44:183–9. doi: 10.1002/glia.10274. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–62. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Kapfhamer D, Minnella AM, Kim J-E, Won SW, Chen Y, Huang Y, Low LH, Massa SM, Swanson RA. Bioenergetic state regulates innate inflammatory responses through the transcriptional co-repressor factor CtBP. Nat Commun. 2017;8:624–31. doi: 10.1038/s41467-017-00707-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjö BK. Brain Energy Metabolism. New York: John Wiley & Sons; 1978. [Google Scholar]
- Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci. 2004;24:7779–88. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SW, Shin BS, Ma H, Van Hoecke M, Brennan AM, Yenari MA, Swanson RA. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64:654–63. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu YF, Lu PJ, Huang CC, Ho CJ, Chou YP. Moderate dietary restriction reduces p53-mediated neurovascular damage and microglia activation after hypoxic ischemia in neonatal brain. Stroke. 2012;43:491–8. doi: 10.1161/STROKEAHA.111.629931. [DOI] [PubMed] [Google Scholar]
- Ulland TK, Song WM, Huang SC, Ulrich JD, Sergushichev A, Beatty WL, Loboda AA, Zhou Y, Cairns NJ, Kambal A, et al. TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell. 2017;170:649–663. e13. doi: 10.1016/j.cell.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci Sj, Maher, Simpson Ia. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia. 1997;21:2–21. doi: 10.1002/(sici)1098-1136(199709)21:1<2::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Vasek MJ, Garber C, Dorsey D, Durrant DM, Bollman B, Soung A, Yu J, Perez-Torres C, Frouin A, Wilton DK, et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534:538–43. doi: 10.1038/nature18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables GS, Miller SA, Gibson G, Hardy JA, Strong AJ. The effects of hyperglycaemia on changes during reperfusion following focal cerebral ischaemia in the cat. J Neurol Neurosurg Psychiatry. 1985;48:663–9. doi: 10.1136/jnnp.48.7.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S, Lopresti BJ, Wiley CA. The peripheral benzodiazepine receptor (Translocator protein 18kDa) in microglia: from pathology to imaging. Prog Neurobiol. 2006;80:308–22. doi: 10.1016/j.pneurobio.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloboueva LA, Emery JF, Sun X, Giffard RG. Inflammatory response of microglial BV-2 cells includes a glycolytic shift and is modulated by mitochondrial glucose-regulated protein 75/mortalin. FEBS Lett. 2013;587:756–62. doi: 10.1016/j.febslet.2013.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–80. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, van Hoecke M, Tang XN, Lee H, Zheng Z, Swanson RA, Yenari MA. Pyruvate protects against experimental stroke via an anti-inflammatory mechanism. Neurobiol Dis. 2009;36:223–31. doi: 10.1016/j.nbd.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JL, Bouillaud F, Almeida AS, Vieira HL, Ouidja MO, Dubois-Rande JL, Foresti R, Motterlini R. Carbon monoxide reverses the metabolic adaptation of microglia cells to an inflammatory stimulus. Free Radic Biol Med. 2017;104:311–323. doi: 10.1016/j.freeradbiomed.2017.01.022. [DOI] [PubMed] [Google Scholar]
- Won SJ, Tang XN, Suh SW, Yenari MA, Swanson RA. Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by Increasing superoxide production. Ann Neurol. 2011;70:583–90. doi: 10.1002/ana.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won SJ, Yoo BH, Kauppinen TM, Choi BY, Kim JH, Jang BG, Lee MW, Sohn M, Liu J, Swanson RA, et al. Recurrent/moderate hypoglycemia induces hippocampal dendritic injury, microglial activation, and cognitive impairment in diabetic rats. J Neuroinflammation. 2012;9:182. doi: 10.1186/1742-2094-9-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, Yu Y, Kang R, Zhu S, Yang L, Zeng L, Sun X, Yang M, Billiar TR, Wang H, et al. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat Commun. 2016;7:13280. doi: 10.1038/ncomms13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Chen S, Mao Z, Cadenas E, Brinton RD. 2-Deoxy-D-glucose treatment induces ketogenesis, sustains mitochondrial function, and reduces pathology in female mouse model of Alzheimer’s disease. PLoS One. 2011;6:e21788. doi: 10.1371/journal.pone.0021788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57:830–9. [PubMed] [Google Scholar]
- Zhang Q, Piston DW, Goodman RH. Regulation of corepressor function by nuclear NADH. Science. 2002;295:1895–7. doi: 10.1126/science.1069300. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–47. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]