Summary
Context
Exogenous testosterone administration may affect blood clotting, polycythemia, and may increase atherosclerosis, though any association with cardiovascular events is unclear. While the literature is inconclusive, some studies have suggested testosterone use may increase short-term risk of cardiovascular events and stroke, and injection testosterone may convey higher risks than other dosage forms.
Objective
We sought to evaluate the short-term cardiovascular risk of receiving injection testosterone.
Design
We conducted a case-crossover analysis comparing injection testosterone exposure in the seven days prior to an outcome event to referent windows in the past to estimate the acute association of cardiovascular outcomes with the receipt of testosterone injections.
Patients
We identified adult male testosterone users hospitalized with myocardial infarction (MI), stroke, or a composite of MI, stroke, or unstable angina in US commercial claims (2000–2013) or Medicare (2007–2010) databases.
Measurements
We identified testosterone use for the patients from pharmacy dispensing claims or in-office procedure codes in the insurance billing data.
Results
We identified 2,898 commercially-insured men with events and recent testosterone use, and 339 from Medicare. Injected testosterone was associated with an increased risk of adverse events (composite outcome of myocardial infarction, stroke, or unstable angina) in the immediate post-injection period for the older, Medicare population only: commercial insurance, OR=0.98 (95% CI: 0.86–1.12); Medicare, OR=1.45 (1.07, 1.98). This association was either greatly attenuated or not present when evaluating receipt of any testosterone dosage forms (injection, gel, patch, implant): commercial insurance, OR=1.01 (0.92, 1.11); Medicare, OR=1.26 (95% CI: 0.98–1.63).
Conclusions
Testosterone injections were uniquely associated with short-term risk of acute cardio- and cerebrovascular events in older adult men following injection receipt.
Keywords: Androgens, testosterone, case-crossover studies, adverse drug event, pharmacoepidemiology, Medicare, cardiovascular disease
Introduction
There has been considerable debate and disagreement about the cardiovascular effects and safety of testosterone supplementation in the wake of its increasing use around the world,1,2 particularly among men with unclear indications for its use.1,3 There have been concerns about testosterone’s association with increased cardiovascular events, although there is substantial inconsistency in the literature;4 many studies have suggested no increased cardiovascular risk associated with testosterone.5–7 However, some studies have suggested increased risk of myocardial infarction, stroke, and mortality in testosterone users8–11—particularly short-term increases12 in risk in older men13 and those with pre-existing cardiovascular disease.10 Testosterone treatment has been associated with various cardiovascular effects, such as increased coronary artery plaque volume,14 increased red blood cell count15 and the polycythemia,16,17 and testosterone product labels carry warnings of venous thromboembolism.18 While some regulatory agencies have warned of a potential for increased cardiovascular risk associated with testosterone use19,20 and taken steps to clarify/narrow approved indications and limit expanding use of testosterone products,20–22 conclusive agreement on the cardiovascular safety is still lacking. Concerns of unmeasured confounding and inappropriate comparator groups in prior non-randomized studies, differences in the included populations in trials and non-randomized studies, and wide variation in testosterone use outside of clinical guidelines have all contributed to the current inconsistency and disagreement in the published studies and commentaries.
Testosterone is available in different dosage forms, including injections, transdermal patches and gels, and implants—each dosage form has unique pharmacodynamics, which may result in different safety profiles. Depending on the dose and dosing frequency, testosterone injections may result in immediate spikes in serum testosterone levels23 compared to more subtle increases caused by transdermal applications.24 These pharmacokinetic differences offer opportunities to study the impact of shorter-term exposure to higher levels of testosterone exposure. In a previous retrospective cohort study, we observed higher cardiovascular risks in injection testosterone users11 relative to gel or patch users; these observed differences prompted further exploration of the potential risks associated with testosterone injections, particularly acute risks. These safety signals deserve further investigation to understand the risks associated with testosterone treatment.
The objective of this study was to estimate the short-term association of injection testosterone with acute cerebrovascular and cardiovascular events using large US data sources. Using a self-controlled case-crossover design, we evaluated the timing of acute events relative to testosterone injections.
Materials and Methods
We undertook a self-controlled case-crossover analyses of injection testosterone safety in two large US healthcare databases. The symptoms leading men to be tested for and receive testosterone treatment are subtle (e.g. fatigue, malaise, loss of libido, etc.) and rarely recorded in insurance billing diagnosis claims; furthermore, laboratory test values are unavailable in insurance billing claims, resulting in difficulty adjusting for differences between baseline testosterone values or cardiovascular markers of testosterone users and non-users. Self-controlled designs assume intermittent exposures and transient effects of exposure, and by comparing exposed time to unexposed time within individuals, they inherently control for time-fixed confounding, making them well-suited for studying the acute effects of testosterone injections.
Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). The project was approved by the University of North Carolina at Chapel Hill Institutional Review Board.
Data sources
We used the MarketScan™ (Copyright © 2016 Truven Health Analytics Inc. All Rights Reserved).) employer-based commercial insurance databases, including the Commercial Claims and Encounters, and the Medicare Supplementary and Coordination of Benefit databases, which contain healthcare insurance billing claims from large employers throughout the United States for employees, spouses, and dependents with employer-sponsored commercial insurance plans, as well as retirees aged 65+ years with employer-sponsored Medicare supplementary plans. We considered adult (18+ years) males during the years 2000 – 2013. We also utilized a 20% national random sample of US Medicare beneficiaries with primary fee-for-service coverage, aged 65+, with Parts A, B and D coverage from the years 2007–2010. These insurance claims databases contain individual-level information about plan enrollment, and adjudicated, paid claims for inpatient and outpatient procedures and diagnoses, and pharmacy dispensing of prescription medications. Direct clinical measurements and laboratory measurements are not available in either data source.
Outcome assessment
Case-crossover designs first identify men experiencing the outcome, and then evaluate exposures preceding the outcome to determine if exposure was more likely to occur immediately before the as compared to periods of time in the more distant past. We identified men experiencing myocardial infarction (MI), stroke, or a composite of MI, stroke, or unstable angina in the databases through diagnoses in hospitalization claims, with the day of hospital admittance as the index date. We identified MI as an inpatient claim with an ICD-9-CM diagnosis code of 410 or any subcode in the first or second position without any requirement for length of stay.25,26 Stroke was defined as an inpatient discharge ICD-9 codes 433.x1, 434.x1, 435.x, 436.x, 437.1x, or 437.9x in any position.27,28 The composite was a combination of either MI or stroke, or unstable angina (discharge ICD-9 code 411.xx in the first or second position).29 As the case-crossover is a case-only analysis, each outcome necessitated a separate cohort of men experiencing that outcome; a man could contribute to multiple outcome-specific analyses.
Exposure assessment
Testosterone injections preceding the outcome were identified from both in-office procedure claims and pharmacy dispensing codes. Included formulations included testosterone cypionate, enanthate, propionate, and suspensions; testosterone undecanoate was not included, as it was not approved in the US during the study period.
For sensitivity analyses, we considered all testosterone dosage forms as a class: testosterone gels and patches were identified with pharmacy dispensing claims; implants were identified from procedure claims. In both analyses, for all dosage forms, the stated service date of the procedure or pharmacy dispensing date was considered the day of exposure onset.
Descriptive Characteristics
To describe the clinical characteristics of the included men, we searched insurance claims occurring up to one year preceding the index outcome date to identify relevant diagnosis and procedure claims. Each outcome-specific analysis is restricted to only those who experienced the current outcome of interest, so for the overall descriptive analysis, we used the composite outcome cohort which contains the other, outcome-specific cohorts. We identified symptoms and explicit diagnoses of hypogonadism, cardiovascular conditions and recent cardiovascular events, other comorbidities, and preventive care and screening received (see Table 1 for complete list). These characteristics are for descriptive purposes only, and they are not included in the case-crossover analysis, which implicitly controls for within-person characteristics.
Table 1.
Characteristics of injection testosterone-using men in the year before experiencing one of the composite of outcomes of myocardial infarction, stroke, or unstable angina, by data source
| Characteristic | Commercial Insurance N=1,262 |
Medicare N=208 |
|---|---|---|
| Age, mean (SD) | 56.1(6.96) | 75.9(6.91) |
| Hypogonadism symptoms and diagnostics | ||
| Diagnosis of hypogonadism / testicular dysfunction | 751 (59.5%) | 175 (84.1%) |
| Received a blood testosterone test | 720 (57.1%) | 136 (65.4%) |
| Anemia | 65 (5.2%) | 50 (24.0%) |
| Fatigue | 382 (30.3%) | 127 (61.1%) |
| Osteoporosis | 86 (6.8%) | 34 (16.3%) |
| Sexual dysfunction | 274 (21.7%) | 86 (41.3%) |
| Cardiovascular conditions and events | ||
| Arrhythmia | 303 (24.0%) | 130 (62.5%) |
| Heart failure | 166 (13.2%) | 92 (44.2%) |
| Hypertension | 778 (61.6%) | 195 (93.8%) |
| Ischemic heart disease | 711 (56.3%) | 165 (79.3%) |
| Myocardial infarction | 539 (42.7%) | 91 (43.8%) |
| Stroke | 459 (36.4%) | 111 (53.4%) |
| Unstable angina | 325 (25.8%) | 69 (33.2%) |
| Venous thromboembolism | 36 (2.9%) | NTSR |
| Other heart disease | 362 (28.7%) | 124 (59.6%) |
| Other comorbidities | ||
| Asthma | 70 (5.5%) | 20 (9.6%) |
| Cancer | 123 (9.7%) | 78 (37.5%) |
| Chronic obstructive pulmonary disease | 189 (15.0%) | 95 (45.7%) |
| Diabetes mellitus | 426 (33.8%) | 100 (48.1%) |
| Depression and other psychiatric disorders | 115 (9.1%) | 23 (11.1%) |
| Obesity | 119 (9.4%) | 21 (10.1%) |
| Substance abuse | 171 (13.5%) | 28 (13.5%) |
| Preventive care and screening | ||
| Colonoscopy | 44 (3.5%) | NTSR |
| Fecal occult blood test | 19 (1.5%) | NTSR |
| Flu vaccination | 40 (3.2%) | 129 (62.0%) |
| Lipid test | 740 (58.6%) | 155 (74.5%) |
| Pneumonia vaccination | 36 (2.9%) | 24 (11.5%) |
| Prostate exam | 617 (48.9%) | 150 (72.1%) |
Definitions: SD, standard deviation; NTSR, number too small to report
Statistical Analysis
We performed a case-crossover analysis to assess outcomes occurring immediately after receiving testosterone injections, as testosterone injections may result in acute spikes in serum testosterone levels23. Case-crossover designs are outcome-anchored analysis which identify patients at the time of the outcome, then, within the same individual, compare the use of the medication of interest in a focal period of time immediately preceding the event to referent windows further in the past. Men were identified at the admission date for the outcome of interest, which served as the index date for the case-crossover analysis. The 7 days prior to the admission date served as the focal window, during which receipt of testosterone was assessed. We defined six adjacent 7-day referent periods in each individual prior to the risk period to assess previous receipt of testosterone (Figure). In the primary analysis, a 30-day gap separated the last referent window and the beginning of the focal window, but we varied the length from 14 to 90 days in sensitivity analyses to test the effects of different gaps between the focal and referent windows. We wanted gaps long enough to allow for the resolution of short-term effects of previous injections; however, case-crossover designs may be influenced by overall increasing time trends in medication usage, where all later time periods may be more likely to be exposed simply due to increasing use, so we also wanted gaps short enough to not be influenced by long-term trends. We estimated the association of testosterone receipt with each outcome separately using conditional logistic regression, estimating odds ratios (OR) and 95% confidence intervals (CI).
Figure 1.
Case-crossover study schematic
As a sensitivity analysis, we additionally performed another case-crossover analysis considering the receipt of any testosterone product (pharmacy dispensing, injection, or implant) as an exposure event, allowing for switching between dosage forms in the focal and referent windows. We sought to evaluate whether acute cardiovascular outcomes were associated with the receipt of any testosterone or simply due to overall, increasing testosterone usage trends across the study period, rather than unique to testosterone injections. Lastly, we performed an ad hoc sensitivity analysis restricting to only in-office administered injections; in the primary analysis, injections could be identified from both in-office procedure claims with exact administration dates, or pharmacy dispensing claims where the dispensing date is known, but the actual use by the patient is unknown.
Results
We identified 284,218 eligible commercially-insured men who experienced the composite outcome, and 91,348 in Medicare. Only those with discrepant testosterone exposure between the focal and referent windows prior to the event were retained for analysis, resulting in 1,266 included commercially-insured patients (mean age=57.3, SD 7.7) and 208 in Medicare (mean age=75.4, SD 6.9) for our primary analysis of testosterone injections. Of all the injections received in the commercial insurance population, 85% were testosterone cypionate, 11% testosterone enanthate, 3% testosterone suspensions, and 1% testosterone propionate; in Medicare, 85% were testosterone cypionate, 13% testosterone enanthate, and other counts were too small to report, per Medicare privacy rules. The prevalence of cardiovascular conditions, recent cardiovascular events, and other comorbidities was high in these populations of testosterone users, and the characteristics of the injection testosterone users differed greatly by data source; the older, Medicare population had higher levels of most comorbidities (Table 1). Additionally, the older, Medicare population had higher frequencies of recorded diagnoses of hypogonadism and serum testosterone tests, as well as more frequent diagnoses associated with hypogonadism, including fatigue, osteoporosis, and sexual dysfunction.
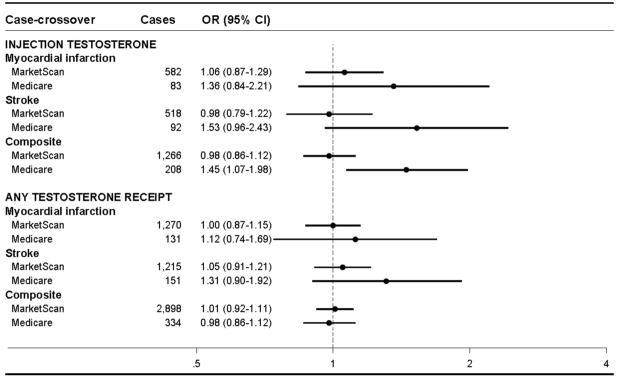
In the younger, commercially-insured population, the new receipt of testosterone injections was not associated with short-term increased risk of MI, stroke, or the composite outcome (Figure, Table 2); composite OR=0.98 (95% CI: 0.86, 1.12). While there were fewer eligible patients in Medicare, resulting in less precise effect measure estimates, we observed elevated short-term risks associated with testosterone injections in the older Medicare population.; the odds ratios for injections were consistently increased for all outcomes: composite outcome, OR=1.45 (95% CI: 1.07, 1.98).
Figure 2.
Case-crossover analysis of testosterone receipt and risk of acute events
Abbreviations: OR, odds ratio; CI, confidence interval
*Composite outcome consists of myocardial infarction, stroke, or unstable angina
Table 2.
Case-crossover analysis of testosterone injections and all testosterone receipt with acute cardiovascular and cerebrovascular events, 30-day gap between exposure and control periods
| Testosterone injections | Any testosterone receipt** | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dataset | Outcome | Injections during focal window, N | Injections during referent windows, N | OR | (95% CI) | Receipt during focal window, N | Receipt during referent windows, N | OR | (95% CI) |
| MarketScan | MI | 150 | 857 | 1.06 | (0.87, 1.29) | 266 | 1,602 | 1.00 | (0.87, 1.15) |
| Stroke | 115 | 700 | 0.98 | (0.79, 1.22) | 251 | 1,444 | 1.05 | (0.91, 1.21) | |
| Composite* | 296 | 1,805 | 0.98 | (0.86, 1.12) | 601 | 3,579 | 1.01 | (0.92, 1.11) | |
| Medicare | MI | 26 | 123 | 1.36 | (0.84, 2.21) | 32 | 175 | 1.12 | (0.74, 1.69) |
| Stroke | 28 | 120 | 1.53 | (0.96, 2.43) | 39 | 186 | 1.31 | (0.90, 1.92) | |
| Composite* | 65 | 291 | 1.45 | (1.07, 1.98) | 86 | 426 | 1.26 | (0.98, 1.63) | |
Abbreviations: OR, odds ratio; CI, confidence interval
Composite outcome consists of myocardial infarction, stroke, or unstable angina
Testosterone injection, implantation, or pharmacy dispensing
In the sensitivity analysis considering all testosterone products, we identified 2,898 men in commercial-insured (mean age=57.6, SD 7.5) and 334 in Medicare (mean age=75.0, SD 7.1) experiencing an outcome with former testosterone use. The characteristics of the overall sample were very similar to the primary cohort of injection users, and the results of the case-crossover analysis in Medicare were attenuated somewhat toward the null, OR=1.26 (95% CI: 0.98, 1.63). The any testosterone analysis in the commercial insurance population was similarly null as the injection analysis; OR=1.01 (95% CI: 0.92, 1.11).
In sensitivity analyses when the gap between the risk and control periods was varied, the conclusions drawn from the resulting effect estimates were essentially unchanged across the different analyses (Table 3). However, for some outcomes, the ORs were slightly elevated in the analysis using longer gaps.
Table 3.
Case-crossover results, varying length of gap between the focal window and referent windows
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Testosterone injections | Any testosterone receipt | ||||||||
|
| |||||||||
| Commercial Insurance | Medicare | Commercial Insurance | Medicare | ||||||
| Outcome | Gap, days | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) |
| MI | 14 | 1.01 | (0.83, 1.23) | 1.38 | (0.85, 2.24) | 0.96 | (0.84, 1.10) | 1.09 | (0.72, 1.65) |
| 30 | 1.06 | (0.87, 1.29) | 1.36 | (0.84, 2.21) | 1.00 | (0.87, 1.15) | 1.12 | (0.74, 1.69) | |
| 60 | 1.11 | (0.92, 1.35) | 1.41 | (0.87, 2.28) | 1.04 | (0.91, 1.20) | 1.15 | (0.76, 1.73) | |
| 90 | 1.16 | (0.96, 1.41) | 1.48 | (0.91, 2.41) | 1.07 | (0.93, 1.23) | 1.16 | (0.76, 1.75) | |
| Stroke | 14 | 1.00 | (0.81, 1.25) | 1.47 | (0.92, 2.35) | 1.04 | (0.90, 1.20) | 1.24 | (0.85, 1.80) |
| 30 | 0.98 | (0.79, 1.22) | 1.53 | (0.96, 2.43) | 1.05 | (0.91, 1.21) | 1.31 | (0.90, 1.92) | |
| 60 | 1.05 | (0.85, 1.31) | 1.44 | (0.92, 2.27) | 1.07 | (0.93, 1.24) | 1.27 | (0.88, 1.85) | |
| 90 | 1.05 | (0.84, 1.30) | 1.28 | (0.82, 2.01) | 1.05 | (0.91, 1.21) | 1.21 | (0.83, 1.76) | |
| Composite | 14 | 0.97 | (0.84, 1.11) | 1.41 | (1.04, 1.92) | 0.99 | (0.90, 1.09) | 1.19 | (0.92, 1.54) |
| 30 | 0.98 | (0.86, 1.12) | 1.45 | (1.07, 1.98) | 1.01 | (0.92, 1.11) | 1.26 | (0.98, 1.63) | |
| 60 | 1.06 | (0.92, 1.21) | 1.45 | (1.07, 1.96) | 1.05 | (0.96, 1.15) | 1.26 | (0.98, 1.63) | |
| 90 | 1.08 | (0.95, 1.24) | 1.42 | (1.05, 1.93) | 1.06 | (0.97, 1.16) | 1.24 | (0.96, 1.61) | |
Abbreviations: OR, odds ratio; CI, confidence interval
Composite outcome consists of myocardial infarction, stroke, or unstable angina
In the commercial insurance, 33.4% of all injections received during the study windows were pharmacy-dispensed prescriptions, and the rest were administered in-office; in Medicare, 15.0% were pharmacy-dispensed. In a sensitivity analysis considering only in-office administered injections, the commercial insurance estimate for the composite outcome was similarly null to the primary analysis, OR=1.03 (95% CI: 0.87, 1.23), and the Medicare result was elevated above the primary analysis, OR=1.52 (95% CI: 1.08, 2.13).
Discussion
In this large, multi-database study of testosterone use, we observed acute increases in cardiovascular events associated with injections, although these increases were not consistent across all outcomes and databases. The case-crossover analysis suggested that receiving an injection is associated with increased risk of the composite outcome in the older, Medicare population. Within Medicare, the OR for the stroke and MI outcomes were consistent with the elevated composite estimate, though they are imprecise due to small numbers of cases. These increased associations were not observed in the younger, commercially-insured population. Our work is consistent with some previous investigations of the cardiovascular safety of testosterone which have demonstrated relatively acute increases in cardiovascular risk,10,13 and stronger associations between testosterone use and cardiovascular events in the elderly10,11,13 and those with pre-existing cardiovascular disease.10
There has been considerable debate about testosterone’s role in cardiovascular disease and events as many cardiovascular risk factors are associated with reduced testosterone levels, obscuring whether cardiovascular events are associated with testosterone treatment itself, or rather the underlying hypogonadism or related conditions;30,31 some studies have even demonstrated protective associations of testosterone treatment against cardiovascular events.6,7 Potential pathways between testosterone treatment and cardiovascular events are unknown, although evidence has suggested associations between testosterone treatment and cardiovascular conditions, including atherosclerosis progression,14 increased hematocrit,15,32,33 polycythemia,34 and thrombosis.35 Additionally, testosterone has been used widely by men with unclear indications, further obscuring whether potential risk is modified by the presence of true underlying hypogonadism.
This study used two separate populations of men; utilizing both data sources allowed us to evaluate potential risk in different age groups and testosterone usage patterns, as commercially-insured and Medicare populations have unique distributions of risk factors, clinical indications, and patterns of testosterone use,11 with injections being more commonly used in the older, Medicare population.
The case-crossover analysis allowed us to investigate the initial, post-injection period where i serum testosterone spikes may occur, rather than entire period of testosterone use. Self-controlled designs estimate the treatment effect in the treated, answering the question: among testosterone-using men who experience the outcome, what is the likelihood of experiencing the outcome immediately after an injection? Self-controlled designs should not be confounded by time-stable personal characteristics, such as race, family history, chronic comorbidities, or other factors which may be difficult to accurately measure in secondary healthcare data; many indications for testosterone use are infrequently captured through diagnosis coding, and self-controlled designs allow us to avoid confounding by these unmeasured characteristics.
Using insurance claims has many advantages, mainly with respect to detailed medication and outcome information, yet they are limited in their lack of information on important personal characteristics for confounding control–to address the latter, we used a self-controlled design which does not require measuring these variables; however, case-crossover designed make important assumptions about the timing of exposure effects, and violations of these assumptions may result in biased estimates. To test our assumptions about the timing, we performed sensitivity analyses varying the length of gaps in the case-crossover analysis; while the results were consistent for the composite outcome across all gap lengths, the small increases in OR estimates observed in some of the 60- and 90-day gap analyses may be due to the increasing prevalence of testosterone across the study period,1 making events more likely to be in periods of testosterone use. To assess this potential bias, we used multiple referent windows within individuals, and multiple gap lengths between the focal and referent windows, which generally were consistent, although some outcome estimates were slightly elevated when using the longer gaps.. Additionally, the “all testosterone” sensitivity analysis resulted in null or attenuated estimates; although the composite OR remained somewhat elevated (OR=1.26, 95% CI: 0.98, 1.63), the reduced or null results in these analyses do not suggest that the simple time trends of increasing testosterone use across the study period explain the entirety of the observed injection associations; furthermore, injection use was generally decreasing during the study period, further not explaining the observed injection associations.
Valid estimates require accurately identifying true exposure times; injections and implants identified through in-office procedures are very reliable as the exact date of receipt is known, but we don’t have information about whether or how pharmacy-dispensed prescriptions are actually used; we assigned the dispensing date as the exposure date, and we used 7-day focal and referent windows to allow for some variability in patients’ use of dispensed product after pharmacy dispensing; however, not all dispensed product may actually be used or used later, leaving the potential for exposure misclassification. To further test the potential influence of exposure timing misclassification, we performed a sensitivity analysis restricted to only in-office injections with more precise administration dates, and the results were robust across these analyses, showing a very similar null result for the commercial insurance population, and a similarly elevated estimate for Medicare. Additionally, self-payments not reimbursed by insurance, free samples, and illicit non-prescription use may result in an underestimate of testosterone exposure. 36,37 Insurance procedure claims for testosterone injections lack the granularity necessary to assess the exact testosterone dose received, so we considered all testosterone injections together as a class. We may be missing some cases of cardiovascular events or stroke which did not result in a hospitalization, limiting the generalizability of our results somewhat. While the large size and national coverage of the databases allows for inclusion of a wide variety of patient populations and treatment patterns, ultimately the absolute number of cases among testosterone users available for analysis was small, resulting in imprecision around some estimates. Additionally, insurance claims and diagnoses lack the same level of granularity as clinical records, and the absence of laboratory measurements makes it difficult to differentiate men using testosterone for approved indications or treating to normalized serum testosterone levels from those not, limiting our ability to measure potential modification by indicated use vs. off-label use, as risk may vary based on the normalization of testosterone values.38
While there continues to be inquiry into and debate about the role of testosterone in cardiovascular disease and acute events, we demonstrated an acute association between injectable testosterone and increased risk of acute cardiovascular events. Considerable disagreement between studies remains, and larger controlled trials may be needed to fully establish testosterone’s relative benefits and risks. However, in a time of widespread use, particularly among those with unclear indications for use, the potential risks of testosterone treatment should be carefully considered along with the clinical necessity of its use.
Supplementary Material
Acknowledgments
The authors wish to acknowledge Virginia Pate from the University of North Carolina at Chapel Hill for her programming and statistical analysis assistance.
This project was funded by the US National Institute on Aging (grant number 5 R01 AG042845). The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The database infrastructure used for this project was funded by the Pharmacoepidemiology Gillings Innovation Lab (PEGIL) for the Population-Based Evaluation of Drug Benefits and Harms in Older US Adults (GIL 200811.0010), the Center for Pharmacoepidemiology, Department of Epidemiology, UNC Gillings School of Global Public Health; the CER Strategic Initiative of UNC’s Clinical & Translational Science Award (UL1TR001111); the Cecil G. Sheps Center for Health Services Research, UNC; and the UNC School of Medicine.
Footnotes
Preliminary results of this work were presented at the 31st Annual International Convention on Pharmacoepidemiology and Therapeutic Risk Management in Boston, MA, August 24, 2015.
Conflicts of Interest: JBL performed this work while an employee of UNC where he received salary support from the Center for Pharmacoepidemiology in the UNC Department of Epidemiology; current member companies include GlaxoSmithKline, Merck, and UCB Biosciences. JBL is currently an employee of RTI International, an independent nonprofit research organization that does work for government agencies and pharmaceutical companies. MAB has received research support from Amgen and AstraZeneca and has served as a scientific advisor for Merck, Amgen, and RxAnte; he also owns equity in NoviSci, LLC, a data sciences company. TS receives investigator-initiated research funding and support as Principal Investigator (R01/R56 AG023178) from the National Institute on Aging (NIA), and as Co-Investigator (R01 CA174453; R01 HL118255), National Institutes of Health (NIH). He also receives salary support as Director of the Comparative Effectiveness Research (CER) Strategic Initiative, NC TraCS Institute, UNC Clinical and Translational Science Award (UL1TR001111) and as Director of the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Merck) and research support from pharmaceutical companies (Amgen, AstraZeneca) to the Department of Epidemiology, University of North Carolina at Chapel Hill; Dr. Stürmer does not accept personal compensation of any kind from any pharmaceutical company. He owns stock in Novartis, Roche, BASF, AstraZeneca, and Novo Nordisk. CRM has current research collaborations with Roche, Novartis and Merck, none of which are relevant to this study.
References
- 1.Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014 Mar;99(3):835–842. doi: 10.1210/jc.2013-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the united states, 2001 to 2011. JAMA Internal Medicine. 2013:1–2. doi: 10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Layton JB, Kim Y, Alexander GC, Emery SL. Association Between Direct-to-Consumer Advertising and Testosterone Testing and Initiation in the United States, 2009–2013. JAMA. 2017 Mar 21;317(11):1159–1166. doi: 10.1001/jama.2016.21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onasanya O, Iyer G, Lucas E, Lin D, Singh S, Alexander GC. Association between exogenous testosterone and cardiovascular events: an overview of systematic reviews. Lancet Diabetes Endocrinol. 2016 Nov;4(11):943–956. doi: 10.1016/S2213-8587(16)30215-7. [DOI] [PubMed] [Google Scholar]
- 5.Alexander GC, Iyer G, Lucas E, Lin D, Singh S. Cardiovascular Risks of Exogenous Testosterone Use Among Men: A Systematic Review and Meta-Analysis. Am J Med. 2017 Mar;130(3):293–305. doi: 10.1016/j.amjmed.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Cheetham TC, An J, Jacobsen SJ, et al. Association of Testosterone Replacement With Cardiovascular Outcomes Among Men With Androgen Deficiency. JAMA Intern Med. 2017 Feb 21; doi: 10.1001/jamainternmed.2016.9546. [DOI] [PubMed] [Google Scholar]
- 7.Baillargeon J, Urban RJ, Kuo Y-F, et al. Risk of Myocardial Infarction in Older Men Receiving Testosterone Therapy. Annals of Pharmacotherapy. 2014 Jul 2;2014 doi: 10.1177/1060028014539918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108. doi: 10.1186/1741-7015-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 10.Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9(1):e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Layton JB, Meier CR, Sharpless JL, Sturmer T, Jick SS, Brookhart MA. Comparative Safety of Testosterone Dosage Forms. JAMA Intern Med. 2015 Jul;175(7):1187–1196. doi: 10.1001/jamainternmed.2015.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etminan M, Skeldon SC, Goldenberg SL, Carleton B, Brophy JM. Testosterone Therapy and Risk of Myocardial Infarction: A Pharmacoepidemiologic Study. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2015 doi: 10.1002/phar.1534. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 13.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010 Jul 8;363(2):109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budoff MJ, Ellenberg SS, Lewis CE, et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317(7):708–716. doi: 10.1001/jama.2016.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy CN, Snyder PJ, Stephens-Shields AJ, et al. Association of Testosterone Levels With Anemia in Older Men: A Controlled Clinical Trial. JAMA Intern Med. 2017 Apr 01;177(4):480–490. doi: 10.1001/jamainternmed.2016.9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010 Jun;95(6):2560–2575. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 17.Jones SD, Jr, Dukovac T, Sangkum P, Yafi FA, Hellstrom WJ. Erythrocytosis and Polycythemia Secondary to Testosterone Replacement Therapy in the Aging Male. Sex Med Rev. 2015 Apr;3(2):101–112. doi: 10.1002/smrj.43. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Food and Drug Administration. [Accessed April 17, 2017];FDA adding general warning to testosterone products about potential for venous blood clots. 2014 https://www.fda.gov/Drugs/DrugSafety/ucm401746.htm.
- 19.Canada H. [Accessed 19 April, 2016];Information Update - Possible cardiovascular problems associated with testosterone products. 2014 http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2014/40587a-eng.php.
- 20.U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use. [Accessed 19 April, 2016];2015 doi: 10.1016/j.juro.2015.06.058. http://www.fda.gov/Drugs/DrugSafety/ucm436259.htm. [DOI] [PubMed]
- 21.Australian Government; Health Do, editor. Schedule of Pharmaceutical Benefits: Summary of Changes. 2015. [Google Scholar]
- 22.European Medicines Agency. [Accessed 19 April, 2016];No consistent evidence of an increased risk of heart problems with testosterone medicines. 2014 http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Testosterone-containing_medicines/human_referral_prac_000037.jsp&mid=WC0b01ac05805c516f.
- 23.Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab. 1999 Oct;84(10):3469–3478. doi: 10.1210/jcem.84.10.6078. [DOI] [PubMed] [Google Scholar]
- 24.Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000 Dec;85(12):4500–4510. doi: 10.1210/jcem.85.12.7045. [DOI] [PubMed] [Google Scholar]
- 25.Hlatky MA, Ray RM, Burwen DR, et al. Use of Medicare Data to Identify Coronary Heart Disease Outcomes in the Women’s Health Initiative. Circulation: Cardiovascular Quality and Outcomes. 2014 Jan 1;7(1):157–162. doi: 10.1161/CIRCOUTCOMES.113.000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. American heart journal. 2004;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Lakshminarayan K, Larson JC, Virnig B, et al. Comparison of Medicare Claims Versus Physician Adjudication for Identifying Stroke Outcomes in the Women’s Health Initiative. Stroke. 2014 Mar 1;45(3):815–821. doi: 10.1161/STROKEAHA.113.003408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke. 2002 Oct;33(10):2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 29.Varas-Lorenzo C, Castellsague J, Stang MR, Tomas L, Aguado J, Perez-Gutthann S. Positive predictive value of ICD-9 codes 410 and 411 in the identification of cases of acute coronary syndromes in the Saskatchewan Hospital automated database. Pharmacoepidemiol Drug Saf. 2008;17(8):842–852. doi: 10.1002/pds.1619. [DOI] [PubMed] [Google Scholar]
- 30.Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011 Nov;165(5):687–701. doi: 10.1530/EJE-11-0447. [DOI] [PubMed] [Google Scholar]
- 31.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002 Feb;87(2):589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 32.Basaria S, Harman S, Travison TG, et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: A randomized clinical trial. JAMA. 2015;314(6):570–581. doi: 10.1001/jama.2015.8881. [DOI] [PubMed] [Google Scholar]
- 33.Bachman E, Travison TG, Basaria S, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci. 2014 Jun;69(6):725–735. doi: 10.1093/gerona/glt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wheeler KM, Smith RP, Kumar RA, Setia S, Costabile RA, Kavoussi PK. A Comparison of Secondary Polycythemia in Hypogonadal Men Treated with Clomiphene Citrate versus Testosterone Replacement: A Multi-Institutional Study. J Urol. 2017 Apr;197(4):1127–1131. doi: 10.1016/j.juro.2016.10.068. [DOI] [PubMed] [Google Scholar]
- 35.Martinez C, Suissa S, Rietbrock S, et al. Testosterone treatment and risk of venous thromboembolism: population based case-control study. BMJ. 2016 Nov 30;355:i5968. doi: 10.1136/bmj.i5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lauffenburger JC, Balasubramanian A, Farley JF, et al. Completeness of prescription information in US commercial claims databases. Pharmacoepidemiol Drug Saf. 2013 Aug;22(8):899–906. doi: 10.1002/pds.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Sturmer T, Brookhart MA. Evidence of sample use among new users of statins: implications for pharmacoepidemiology. Med Care. 2014 Sep;52(9):773–780. doi: 10.1097/MLR.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015 Oct 21;36(40):2706–2715. doi: 10.1093/eurheartj/ehv346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.