Abstract
Background
The current study used recombinant herpes simplex virus type I to increase expression of mu opiate receptors and the opioid ligand preproenkephalin in peripheral nerve fibers in a mouse model of neuropathic pain. We predicted that viral vector delivery of a combination of genes encoding the mu opioid receptor and preproenkephalin would attenuate neuropathic pain and enhance opioid analgesia. The behavioral effects would be paralleled by changes in response properties of primary afferent neurons.
Methods
Recombinant herpes simplex virus type 1 containing cDNA sequences of the mu opioid receptor, human preproenkephalin, a combination, or E. coli lacZ gene marker (as a control) was used to investigate the role of peripheral opioids in neuropathic pain behaviors.
Results
Inoculation with the mu opioid receptor viral vector (n = 13) reversed mechanical allodynia and thermal hyperalgesia, and produced leftward shifts in loperamide (ED50 = 0.6 ± 0.2 mg/kg versus ED50 = 0.9 ± 0.2 mg/kg for control group, n = 8, mean ± SD) and morphine dose-response curves (ED50 = 0.3 ± 0.5 mg/kg versus ED50 = 1.1 ± 0.1 mg/kg for control group). In mu opioid receptor viral vector inoculated C-fibers, heat evoked responses (n = 12) and ongoing spontaneous activity (n = 18) were decreased after morphine application. Inoculation with both mu opioid receptor and preproenkephalin viral vectors did not alter mechanical and thermal responses.
Conclusions
Increasing primary afferent expression of opioid receptors can decrease neuropathic pain-associated behaviors and increase systemic opioid analgesia through inhibition of peripheral afferent fiber activity.
Introduction
Neuropathic pain is a debilitating condition that affects more than 4 million individuals in the United States alone1. Chronic neuropathic pain is often refractory to current analgesic therapies or requires escalating doses of opioids that result in treatment-limiting side effects. Driven by the characterization of peripheral opioid ligands and receptors2,3, peripheral opioid receptor-mediated analgesia has been demonstrated in experimental models of inflammatory pain4, neuropathic pain5–7, and possibly clinical settings7–9. Recognition is growing of an important role for peripheral opioid analgesia in neuropathic pain, suggesting that the peripheral opioid system may yield novel analgesic approaches with a more limited side-effect profile4. Currently available peripherally active opioid agonists have limited utility as analgesics because they decrease intestinal motility. An alternative strategy is to directly modify the peripheral opioid system in the tissues of interest for the modulation of pain, namely the peripheral nervous system, using virally mediated gene transfer.
Virally mediated gene transfer with HSV-1 vectors has been shown to effectively increase the expression of the mu opioid receptor (mOR)10 or the endogenous opioid ligand enkephalin in afferent neurons11 with concomitant behavioral changes in nociception and analgesia12,13. Virally mediated expression of mOR increases analgesia produced by systemic morphine6,8 or the peripherally active opioid agonist loperamide10. In animal models, virally mediated expression of enkephalin is antinociceptive in capsaicin-induced thermal hyperalgesia14–16, trigeminal neuralgia17, spinal nerve ligation (SNL) models of neuropathic pain18. Several of these studies have demonstrated reversal of antinociception with the nonspecific opioid antagonist naloxone, suggesting that enkephalin-induced antinociception is dependent upon signaling through opioid receptors14,17,19. This raises the possibility for an additive antinociceptive and analgesic effect when HSV-1 is used to simultaneously overexpress both enkephalin and mOR to alleviate chronic neuropathic pain.
This study is a side by side comparison of recombinant HSV-1 driven over expression of mOR, the endogenous opioid ligand preproenkephalin, or a combination of the two viral vectors in a mouse L5 SNL model of chronic neuropathic pain. We defined the time course of post-nerve injury over which HSV vectors that encode the mOR (hsvMOR) and/or preproenkephalin (hsvPPE) can (1) increase mOR and/or preproenkephalin in primary afferent neurons, (2) modulate mechanical allodynia and thermal hyperalgesia, and (3) enhance both systemic (morphine) and peripheral (loperamide) analgesia. In addition, (4) we determined if any of these behavioral changes could be established at the primary afferent level by myelinated or unmyelinated fibers as evidence of peripherally mediated opioid analgesia in neuropathic pain.
Materials and methods
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of South Carolina School of Medicine, Columbia, South Carolina, and by the Johns Hopkins Animal Care and Use Committee, Baltimore, Maryland. Efforts were made throughout the experiment to minimize animal discomfort and to reduce the number of animals used. Female and male Swiss-Webster mice (5–6 weeks old, 20–30 g; Charles River, Wilmington, MA) were used. To date no sex differences have been reported regarding the effects of inoculation, and this hypothesis was not directly tested in this study. Animals were housed in a standard 12–12 hour light–dark environment with food and water ad libitum. Animals were randomly assigned to treatment groups using simple randomization.
Timeline
The experimental timeline is shown in Fig. 1. For immunohistochemistry, mice underwent an L5 SNL followed by intraplantar inoculation with hsvCON, hsvMOR, hsvPPE, or hsvMOR+PPE on day 7 post-surgery (n=4/group/time point). A separate group of mice underwent sham surgery followed by intraplantar inoculation with hsvCON on day 7 post-surgery (n=4/time point). On days 1, 9, and 16 post-inoculation, hind paw skin, L3-L6 dorsal root ganglia (DRG), and lumbar spinal cord were collected for immunohistochemical analysis of mOR and enkephalin immunoreactivity. Track tracing of peripheral nerve fibers using wheat germ agglutinin-Biotin and Cholera B Toxin-Biotin, indicate that at least 70% of dorsal root ganglion neurons become infected with the HSV-1 virus (data not shown).
Figure 1.
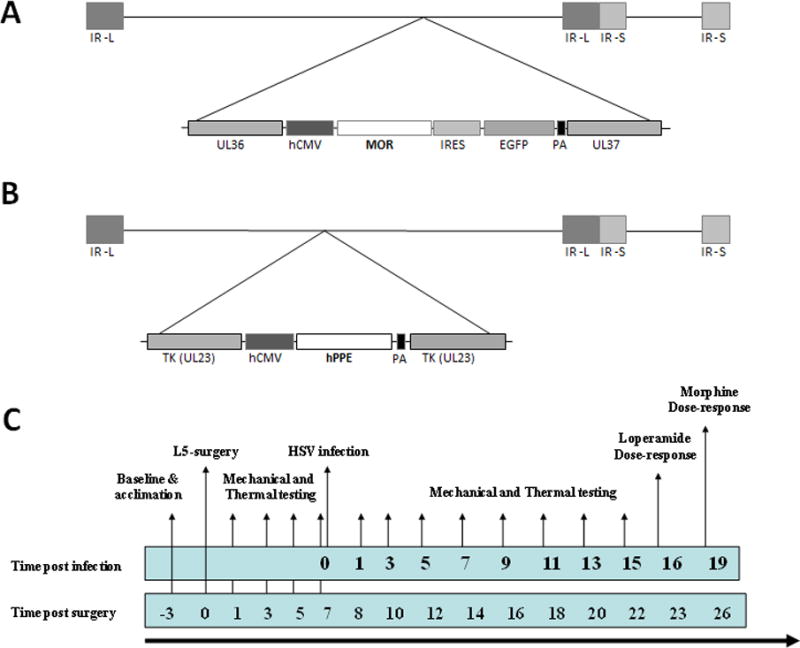
Viral constructs and study timeline. Schematic diagram of hsvMOR recombinant herpes vector (A), expression of β-galactosidase (E. coli lacZ), and preproenkephalin (PPE) (B) are driven by the hCMV immediate-early enhancer-promoter as previously described in 10. (C) Before spinal nerve ligation surgery, mice were acclimated to the blinded experimenter and test environment. Animals were tested for basal paw mechanical withdrawal thresholds and heat threshold latencies. All groups were assessed for mechanical allodynia and thermal hyperalgesia on days 1, 5, 7, 8, 10, 12, 14, 16, 18 and 22 after L5 ligation surgery,. Viruses were administered at day 7 post-surgery. Cumulative loperamide dose-response curves were generated at 16 days post-viral administration. Cumulative morphine dose-response curves were generated at 19 days post-viral administration. Electrophysiology studies were carried out in separate groups of animals with the same injury and inoculation timeline. Tissue was harvested at 7 to 14 days post-inoculation in correlation with behavioral studies. PA, polyadenylation signal; IR, internal repeat; L, long; S, short. hCMV = human cytomegalovirus; HSV = herpes simplex virus; IR = internal repeat; IRES = internal ribosomal entry site; EGFP = enhanced green flourescent protein; hPPE = human preproenkephalin; L = long; MOR = mu-opioid receptor; TK = tymadine kinase; PA = polyadenylation signal; S = short; UL36 and UL37 = herpes simplex virus genes for tegument proteins.
For behavioral assessment, baseline mechanical paw withdrawal thresholds (PWTs) and thermal paw withdrawal latencies (PWLs) were obtained prior to nerve ligation. Development of thermal hyperalgesia and mechanical allodynia was confirmed on day 7 post-L5 SNL, prior to intraplantar injection with hsvCON (n=8), hsvMOR (n=13), hsvPPE (n=9), hsvMOR+PPE (n=8), buffer that was used to harvest the viral vectors from the infected cells (n=8), or buffer that was used to harvest the viral vectors, which included cell debris from the culture (n=8). Paw volume was measured on days 1, 3, 5, and 7 post-infection using a Plethysmometer Meter (IITC Life Science Inc.) according to the manufacturer’s instructions. An experimenter blinded to group assessed thermal hyperalgesia and mechanical allodynia for 15 days post-injection. Cumulative loperamide and morphine dose-response curves were generated on days 16 and 19 post-injection, respectively.
For ex-vivo recordings, mice underwent an L5 SNL followed by either no injection or intraplantar inoculation with hsvCON or hsvMOR on day 7 post-surgery. Recordings were done to correspond with behavioral experiments (Fig. 1), 7–14 days after inoculation. Experimenters were blinded to virus infection throughout all behavioral and histochemical experiments and analysis.
Recombinant HSV-1 vector infection
As previously described10,15, recombinant KOS strain HSV-1 viruses encoding the human preproenkephalin (hsvPPE) and rat mu-opioid receptor gene (hsvMOR) in the sense orientation under control of the human cytomegalovirus immediate-early promoter/enhancer were used (Fig. 1). Two similarly constructed vectors encoding the Escherichia coli lac Z gene served as controls (hsvCON). In hsvMOR and one of the control virus constructs (hsvCON [with GFP]), the cDNA for green fluorescent protein (GFP) was included as a reporter gene. Herpes simplex viruses are ideal for targeting nerve fibers, because they are neurotrophic and establish latent infection mainly in the trigeminal and dorsal root ganglia20. On the day of infection, mice were anesthetized with isoflurane for intraplantar administration of virus into the left hind paw. Vehicle that was used to isolate virus from cell culture (10% sucrose/PBS-D), vehicle applied to a mock-infected culture (MOCK), or vehicle containing hsvPPE, hsvMOR, or hsvCON (2 × 107 pfu/μl) was administered subcutaneously in the left plantar hind paw (10 μl). In all experiments, the virus suspensions were prepared to contain 1 × 107 pfu/μl virus encoding GFP + 1 × 107 pfu/μl virus not encoding GFP. For instance, hsvPPE alone was balanced with hsvCON (with GFP) and hsvMOR alone was balanced with hsvCON (without GFP). The control group included both hsvCON (with GFP) and hsvCON (without GFP).
L5 Spinal Nerve Ligation [SNL]
Mice were anesthetized with isoflurane in O2 carrier (3% induction, 1.5% maintenance). The fur overlying L3-S3 was shaved, and the skin was cleaned with povidone iodine (Betadine). A small incision was made to the skin and fascia overlaying L5-S1. The paravertebral musculature was retracted from the vertebral transverse processes. The L6 transverse process was partially removed, exposing the L4 and L5 spinal nerves. The L5 spinal nerve was lifted slightly and transected. The wound was irrigated with saline and closed in two layers with 3–0 polyester suture (fascial plane) and surgical skin staples or vicryl sutures. The sham surgical procedure was identical, but the spinal nerve was not ligated.
Immunohistochemistry: mu-opioid receptor (mOR) and Enkephalin (Enk) immunoreactivity
On days 1, 9, and 16 post-infection, mice were deeply anesthetized for transcardiac perfusion with 0.1 M phosphate-buffered saline (pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate buffer. Lumbar spinal cords and ipsilateral L3–6 DRG were removed and post-fixed in 4% paraformaldehyde for 24 hours followed by cryoprotection in 30% sucrose in 0.1 M phosphate buffer. Left plantar hind paw skin was removed and post-fixed in 4% paraformaldehyde for 4 hours followed by cryoprotection in 30% sucrose in 0.1 M phosphate buffer. For assessment of paw edema, skin samples were collected on day 1 post-infection for hematoxylin & eosin histochemistry.
Lumbar spinal cords (30 μm), DRG (12 μm), and skin (12 μm) were serially sectioned. DRG and skin were thaw-mounted onto slides for slide-mounted immunohistochemistry. Spinal cords were placed into Tris-buffered saline (TBS) with 1% Tween 80 (TBST) in 24-well plates for free-floating immunohistochemistry as previously described. Spinal cord sections were rinsed in TBS and quenched for endogenous peroxidase activity and antigen recovery in mentholic peroxide for 15 minutes. Samples were rinsed three times (10 minutes each) and then blocked for 20 minutes at room temperature with normal donkey serum (1.5%) in TBST. This was followed by a 24-hour incubation at 4°C with polyclonal rabbit anti-mOR (1:1,000; Neuromics, Minneapolis, MN) or polyclonal rabbit anti-met-enkephalin (1:750; Immunostar) primary antibody in 1% normal donkey serum in TBST. The next day, samples were rinsed three times (10 minutes each) with TBST. Samples were incubated for 1.5 hour at room temperature with biotinylated affinity-purified donkey anti-rabbit immunoglobulin G secondary antibody (1:1000; Jackson Immunoresearch) in 1.5% normal donkey serum in TBST. Samples were washed three times (10 minutes each) and incubated for 1 hour at room temperature with horseradish peroxidase–conjugated streptavidin (1:1600, Jackson ImmunoResearch) in TBST. Samples were washed four times (10 minutes each) and developed with diaminobenzidine. After substrate–chromogen reaction, spinal cord tissues were mounted on slides. All slides were then dehydrated and coverslipped.
DRG and skin were processed as described above, but the biotinylated secondary antibody was replaced with a Texas Red–conjugated affinity-purified donkey anti-rabbit immunoglobulin G (1:200; Jackson ImmunoResearch). After a 2-hour incubation at room temperature in the dark, the slides were washed three times (10 minutes each) in TBS, coverslipped with VectaShield (Vector Laboratories, Burlingame, CA), and stored at 4°C until analysis.
Immunohistochemistry of DRG, skin, or spinal cords was performed in a single run. Tissues across serial sections were analyzed and averaged by an investigator blinded to the experimental conditions and virus infections. Spinal cord section imaging and analysis were carried out as described previously10. Briefly, digital images were obtained for each spinal cord slice. Image J version 1.26 (National Institutes of Health, Bethesda, MD) was used to determine the optical density of mOR immunoreactivity (mOR-ir) and met-enkephalin immunoreactivity (Enk-ir) within lamina I–III of ipsilateral L3-6 spinal cords. Density was averaged across animals in a treatment group (n=4 tissues per spinal level/mouse). The mOR-ir was variable between neurons, versus the epidermal nerve fibers and spinal cord, which is not surprising given that mOR staining differences with respect to localization have been reported in previous studies21.
In DRG, mOR-ir and Enk-ir cell bodies were counted and categorized into small (4-20 μm), medium (22-40 μm), and large (42-77 μm) diameter. Only cells in which the nucleolus was present were sized. Similarly, cell bodies expressing GFP were counted by size. Because the hsvMOR virus included the cDNA for GFP, we normalized the number of the co-labeled mOR-ir- and GFP-expressing cell bodies as a percentage of total cell bodies within each DRG. Data are presented as a percentage of the total number of cell bodies positive for mOR + GFP or enkephalin across L3-6 DRG.
In skin sections, we counted the number of mOR-ir, Enk-ir, and GFP-positive dermal nerve innervations. For hsvMOR-infected mice, we quantified the percentage of mOR + GFP co-labeled nerve innervations. Because the hsvPPE construct did not encode for GFP, we did not count the number of GFP+ epidermal nerve terminals.
Capsaicin-induced C-fos expression
Two hours post-capsaicin (intraplantar, 20 μl of 0.15% capsaicin dissolved in vehicle containing 10% ethanol, 10% Tween 80, and 80% saline, Sigma, St. Louis, MO) administration, mice were deeply anesthetized and perfused transcardially with 0.1 M phosphate buffered saline (pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate buffer. After perfusion, spinal cords were removed and post-fixed in 4% paraformaldehyde for 24 h and then placed in 30% sucrose in 0.1 M phosphate buffer for cryoprotection (n=8 mice/virus treatment).
Spinal cords were sectioned (20μm) and free-floating c-fos immunohistochemistry was performed as detailed above for the mOR and Enk immunohistochemistry. The primary antibody (polyclonal rabbit anti-fos at a 1:5,000 dilution in TBS+, Sigma) and the secondary antibody (biotin-SP-conjugated affini-pure donkey anti-rabbit IgG at a 1:1,000 dilution in TBS+, Jackson Immunoresearch) were used. The number of c-fos positive neurons in laminas I and II of L3-L6 spinal cord sections were counted by an investigator blinded to infection (5 sections per level/animal).
Behavioral studies
Mechanical allodynia
Animals were acclimated to the experimenter and testing environment prior to behavioral testing. Mice were placed in plastic boxes over a mesh floor, and their basal responses to mechanical stimuli were quantified as the total number of paw withdrawals to an ascending series of calibrated von Frey filaments (Stoelting Company, Wood Dale, IL; 0.16, 0.4, 1.0, 2.0, and 4.0 g) applied to the plantar surface of the ipsilateral hind paw. Each von Frey filament was applied five consecutive times, and the maximum possible number of withdrawal responses was 25. The filament force that caused more than one withdrawal incidence out of five stimulations was considered the PWT. %PWT was calculated by dividing the measured PWT by the pre-surgery basal PWT. Allodynia was characterized as withdrawal of the paw to these normally non-noxious stimuli (i.e., a decrease in the %PWT).
Thermal hyperalgesia
Thermal PWL to radiant heat stimuli was measured using a plantar stimulator analgesia meter (IITC model 390). Animals were placed under separate plastic boxes on a heated glass floor. Radiant heat was applied from below to the plantar surface of the ipsilateral hind paw, and an electronic timer measured the time until the animal removed its paw from the thermal stimulus. PWL was defined as the average latency in three sets of a single stimulation. We waited at least 10 minutes between each set to prevent sensitization. We used a maximum exposure of 20 seconds to prevent any potential tissue damage. %PWL was calculated by dividing the measured PWL by the pre-surgery basal PWL. Hyperalgesia was characterized as an enhanced withdrawal of the paw (i.e., shorter PWL) to this the radiant heat.
Pharmacologic studies: Opioid dose-response curve
Cumulative dose-response curves were generated for subcutaneous loperamide (20% Cremophor EL in sterile saline) and subcutaneous morphine (in sterile saline). First, PWLs were measured at 10 minutes after vehicle administration. Cumulative doses of loperamide (0.17, 0.5, 1, 1.5, 2, 3, and 4 mg/kg) or morphine (0.25, 0.5, 1, 1.5, 2, 3, and 4 mg/kg) were then administered in 15-minute intervals, and PWLs were measured 10 minutes after each administration. Loperamide and morphine were administered subcutaneously in the ipsilateral flank through a 29-gauge insulin syringe.
Spontaneous Behavioral Assessment: Capsaicin injection
TRPV1 expression and activity has been implicated in multiple chronic pain states, including neuropathic pain22. Since intradermal capsaicin-injection protocols have been used to as neuropathic pain models in humans23, we chose to monitor spontaneous nocifensive behaviors and thermal withdrawal thresholds in mice post capsaicin-injection. At four weeks post-virus infection, baseline paw withdrawal latencies were measured. Capsaicin (20 μl of 0.15% capsaicin dissolved in vehicle containing 10% ethanol, 10% Tween 80, and 80% saline, Sigma, St. Louis, MO) was subcutaneously administered into the left plantar hind paw of mice (n=10 mice/treatment). Mice were returned back to the plastic boxes and the first 5 minutes post-capsaicin were video-taped using a digital camcorder. Thermal paw withdrawal latencies were measured in 10 minute intervals post-capsaicin for 60 minutes. Videos were scored by a blinded observer who analyzed the time spent licking, flicking and holding the hind paw. Pain scores were calculated using the formula: [2(seconds spent in licking/flicking of the paw) + seconds spent holding the paw] /(60 · n), where n= recorded time in minute.
Ex vivo electrophysiologic studies
One- to two-weeks after inoculation, mice were euthanized with pentobarbital (50 mg/kg, i.p.), and the hairy skin of the hind paw, together with the saphenous or sural nerve, was carefully dissected and transferred to an in vitro system described in detail previously24. The skin was mounted corium side up in an organ bath and superfused (750 ml/h) with synthetic interstitial fluid (SIF) heated to ~32°C and continuously bubbled with carbogen to obtain a pH of 7.4. The nerve was threaded through a hole from the organ bath into a mineral-oil-filled recording chamber containing a splitting platform. Under a microscope and with fine watchmaker forceps, epi- and perineurium were carefully removed and the nerve teased into smaller bundles that were placed onto a recording electrode positioned above the splitting platform. Nerve bundles were teased into smaller filaments until single fiber activity could be recorded. Cutaneous receptive fields (RFs) were first localized by applying mechanical stimuli to the skin with a blunt glass rod. After localizing the RF, the investigator applied electrical stimuli at the RF with a concentric electrode (up to 1 mA, 100 μs) to determine conduction latency, which, together with the conduction distance, was used to calculate conduction velocity (CV). Fibers with a CV < 1 m/s were classified as C-fibers, those with a CV between 1 and 10 m/s were classified as Aδ-fibers, and those with CVs >10 m/s were classified as Aβ-fibers.
Mechanical thresholds were determined with an ascending series of von Frey filaments. The smallest filament to produce a response in two out of four trials was regarded as threshold. RFs were isolated with a 10-mm steel ring from the surrounding preparation to restrict the effect of the thermal stimuli and drug effects to the area of interest. SIF was removed from within the ring, and heat sensitivity was assessed by using superfused heated SIF at the receptive field in a resistive heating perfusion system24. Immediately after heat testing, the ring was refilled with SIF and the unit allowed to rest for 10 minutes before being tested for cold sensitivity. Cold SIF was superfused at the RF through a perfusion system as described in detail previously24. Mechanical sensitivity was tested by applying an ascending series of suprathreshold mechanical stimuli (0.1–8 g or 0.98–78.5 mN for 2 seconds, separated by 1 minute) to the RF with a blunt probe (1-mm diameter) connected to a computer-controlled mechanical stimulator (Model 305C, Aurora Scientific, Aurora, ON, Canada).
After baseline mechanical and thermal testing was completed, SIF within the ring was replaced by a 2 μM morphine SIF solution, which was maintained for 5 minutes. This concentration of morphine (morphine sulfate, Sigma Aldrich, St Louis, MO) was equivalent to the systemic 1mg/kg dose that had antinociceptive effects in previous behavioral experiments. and is accepted in the range of opiate concentrations for use in a skin nerve preparation25. Five minutes after morphine application, we retested the responsiveness to mechanical and thermal stimuli using the same sequence and timing as described for baseline testing with the exception that morphine SIF solution was applied in the rest periods between stimuli.
Data analysis
All action potentials were filtered, amplified, digitized, and stored on a computer using DAPSYS (Brian Turnquist, Bethel University, St. Paul, MN; see www.dapsys.net, retrieved November 1st, 2017). DAPSYS was also used to control the mechanical stimulator and to record temperatures from heat and cold stimulation. Fibers were recorded from skin in an hsvMOR-inoculated mouse under SIF conditions and under morphine conditions. These afferents responded to mechanical and heat stimuli or only to mechanical stimuli, and according to previously established criteria would be classified as CMH and CM fibers, respectively. Data were analyzed only from the units where the thermal and mechanical stimulation protocol was completed.
The total number of evoked action potentials to a given mechanical or thermal stimulus, the instantaneous peak frequency, and thermal thresholds (defined as the temperature at which the first action potential was recorded, or a 30% increase in activity if the unit had spontaneous activity) were used for data analysis. Evoked responses for thermal and mechanical stimuli included a post-stimulus period of 5 and 3 seconds, respectively. The peak instantaneous frequency data were median smoothed to account for data variance introduced by a single action potential. Spontaneous activity was also measured and compared between SNL groups after morphine application by using mean evoked action potentials per minute under SIF and morphine conditions.
Statistical analysis
We performed the appropriate one-way, two-way, or repeated measure analysis of variance (ANOVA) followed by post hoc analysis (Bonferroni) to determine the significance of virus-mediated changes in behavior, immunohistochemistry, and histochemistry. Differences in dose-response curves were determined by using repeated-measures ANOVA. ED50 was calculated by using nonlinear regression to generate a sigmoidal dose-response curve. The goodness of fit of the curve was determined by R2 values between 0.91 and 0.96. P<0.05 was considered significant. All statistical analyses were carried out with Prism 4.0 (GraphPad Software Inc., San Diego, CA). Data are presented as mean ± SD, and P < 0.05 was considered statistically significant. Sample sizes were based on previous behavioral and immunohistochemistry studies10,26.
To compare pre- and post-morphine electrophysiology data, and to compare results between different virus-treatment groups, we carried out statistical tests where appropriate with Statistica software. Fibers from mice that were non-inoculated or hsvCON inoculation demonstrated similar mechanical and thermal thresholds (data not shown) and were collapsed post-hoc into a Control group (“No treatment and hsvCON”). We used factorial ANOVA to compare data in uninjured and injured animals (2 levels) and vector treatment (2 levels). Data are presented as mean ± SD, and P < 0.05 was considered statistically significant. Since the evoked responses to thermal and mechanical stimuli vary considerably across fibers, we normalized the data for analysis. For example, the number of evoked action potentials and peak instantaneous frequency to a given stimulus at baseline (pre-morphine) or post-morphine was normalized to: [Post/(Pre + Post)] = fraction of the response stimulus (i.e., if morphine had no effect, responses at baseline and after morphine would each contribute approximately 50% to the total response). Thresholds are presented as post-morphine – pre-morphine in °C (temperature data) or grams (mechanical data). The percent change in spontaneous activity is represented as: [(Post – Pre)/Pre] × 100.
Results
Enkephalin (Enk)-ir and mu-opioid receptor (mOR)-ir in plantar hind paw skin
Hindpaw infection with viral vectors produced mild to moderate short-term inflammation which resolved within 3 days post-infection (See Figure, Supplemental Digital Content 1, paw volume and inflammation data). Nerve injury did not alter the number of Enk-ir innervations in plantar hind paw skin compared with that of sham surgery controls (Fig. 2). Infection with hsvMOR, hsvPPE and hsvMOR + PPE increased the number of Enk-ir innervations on day 16 post-infection (ANOVA; Fig. 2A). Nerve injury decreased the number of co-localized mOR-ir and GFP-positive innervations in the plantar hind paw skin of hsvCON-infected mice compared with that of sham mice (Fig. 2B). Infection with hsvMOR, hsvPPE and hsvMOR + PPE increased the number of mOR-ir and GFP co-localized innervations compared with that of sham and hsvCON (ANOVA; Fig. 2B), 16 days post-inoculation.
Figure 2.
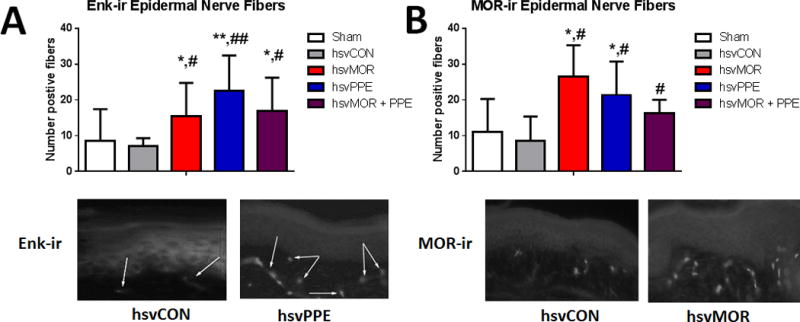
The number of Enkephalin (Enk-ir) and co-labeled mu-opioid receptor (mOR-ir) and GFP-positive epidermal nerve fibers in plantar hind paw skin increases after hsvMOR, hsvPPE and hsvMOR + PPE inoculation. A. Top: Quantification of Enk-ir positive nerve fibers of epidermis on Day 16 post viral inoculation. There is a significant increase in Enk-ir in hsvMOR, hsvPPE and hsvMOR + PPE treatment groups compared to sham and control virus (ANOVA, F(4, 19) = 2.3, P = 0.01; hsvCON). Bottom: Histology images of Enk+ epidermal nerve fibers from forepaw footpad skin following treatment with control virus (left) and hsvPPE (right). B. Top: Quantification of MOR-ir positive nerve fibers of epidermis on Day 16 post viral inoculation. Bottom: Histology images of MOR+ epidermal nerve fibers from the forepaw footpad skin following treatment with control virus (left) and hsvMOR (right). There is a significant increase in MOR-ir in hsvMOR, hsvPPE and hsvMOR + PPE treatment groups compared to sham (ANOVA, F(4, 19) = 3.5, P = 0.032). # vs. sham; # significant at P<0.05; ## significant at P<0.01. * vs. control virus- treated (hsvCON);* significant at P<0.05; ** significant at P<0.01. Bonferroni post-hoc for multiple comparisons.
Enkephalin (Enk)-ir and mu-opioid receptor (mOR)-ir in L3-L6 Dorsal Root Ganglia (DRG)
Nerve injury did not change the percentage of medium, large, or total Enk-ir or mOR-ir DRG cells compared with that of sham surgery controls (Fig. 3). The number of large, medium, small and total Enk-ir DRG cells was higher in hsvPPE and hsvMOR+PPE mice than in sham or hsvCON mice. hsvMOR mice also had increased the number of small Enk-ir DRG cells on day 16 post-infection compared with sham and hsvCON (two-way ANOVA, factors: group × neuron size, Fig. 3A). Infection with hsvMOR produced a large increase in the number of large and medium DRG cells that co-localized mOR-ir and GFP compared with the number in sham and hsvCON-inoculated animals. hsvPPE and hsvMOR + PPE also produced a large increase in the number of medium and large DRG cells that co-localized mOR-ir and GFP 16 days post-infection compared with that of sham and hsvCON-inoculated animals (two-way ANOVA, factors: group × neuron size, Fig. 3B). Due to the large decrease in mOR-ir in small diameter DRG after nerve injury, the total number of mOR-ir DRGs did not significantly differ across treatment groups (two-way ANOVA, factors: group × neuron size, P = 0.064).
Figure 3.
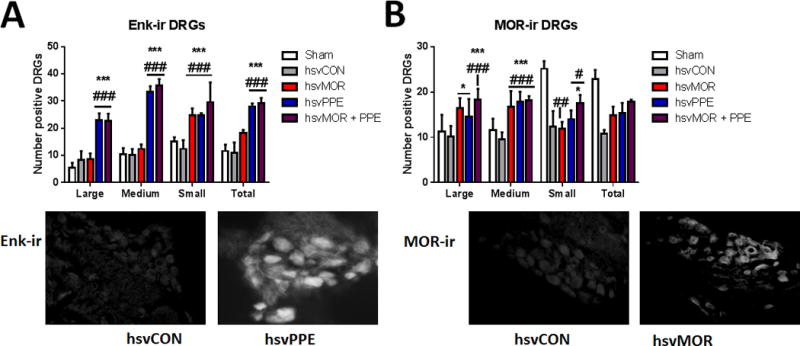
Inoculation with hsvMOR, hsvPPE or hsvMOR + PPE increases the number of Enkephalin (Enk-ir) and co-labeled mu-opioid receptor (mOR)-ir and green fluorescent protein-positive dorsal root ganglion cells. A. Quantification of Enk-ir positive DRGs on Day 16 post viral inoculation. There is a significant increase in Enk-ir in hsvPPE and hsvMOR + PPE treatment groups compared to sham and control virus (hsvCON, hsvCON (two-way ANOVA, factors: group × neuron size, F(12, 60) = 7.5, P <0.001). Bottom: Histology images of Enk+ dorsal root ganglion with control virus (left) and hsvPPE (right). B. Quantification of MOR-ir positive DRGs on Day 16 post viral inoculation. Bottom: Histology images of MOR+ dorsal root ganglion with control virus (left) and hsvMOR (right). There is a significant increase in MOR-ir in large and medium sized DRGs of hsvMOR, hsvPPE and hsvMOR + PPE treatment groups compared to sham and control virus (hsvCON, two-way ANOVA, factors: group × neuron size, F(12, 60) = 10.5, P < 0.001). # vs. sham; # significant at P<0.05; ## significant at P<0.01; ### significant at P<0.001. * vs. control virus-treated (hsvCON);* significant at P<0.05; ** significant at P<0.01; *** significant at P<0.001.
Bonferroni post-hoc for multiple comparisons.
Enkephalin (Enk)-ir and mu-opioid receptor (mOR)-ir in lamina I–III of the lumbar spinal cord
Nerve injury produced dynamic changes in Enk-ir in laminae I–III of the lumbar spinal cord across the time course (Fig. 4A). Overall, hsvPPE and hsvMOR+PPE increased Enk-ir in laminae I–III on day 16 post-infection, which was significantly higher than in sham or hsvCON-infected mice (two-way ANOVA, factors: group × lamina, Fig. 4A). Nerve-injured mice exhibited an increase in mOR-ir in laminae I–III on day 16 post-infection compared with that of sham mice (two-way ANOVA, factors: group × lamina, Fig. 4B). However, hsvMOR markedly increased, mOR-ir in laminae I–III when compared to that of sham and hsvCON mice. hsvPPE and hsvMOR+PPE caused an increase in mOR-ir compared with that of sham, and hsvCON-infected mice (Fig. 4B).
Figure 4.
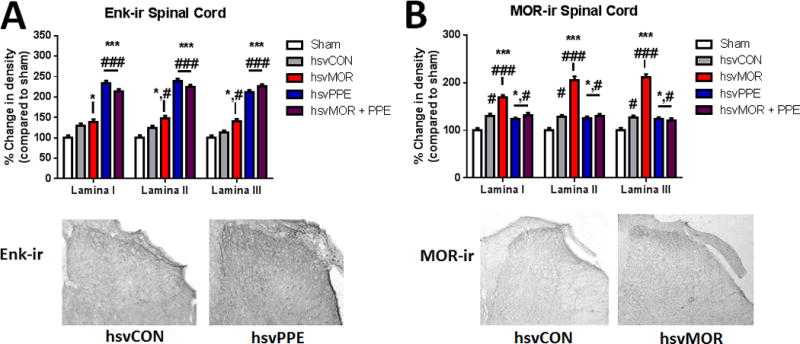
The optical density of Enkephalin (Enk-ir) and co-labeled mu-opioid receptor (mOR-ir) and green fluorescent protein-positive areas of lamina I–III of the dorsal lumbar spinal cord increases after hsvMOR, hsvPPE and hsvMOR + PPE inoculation. A. Quantification of Enk-ir positive areas of the dorsal horn on Day 16 post viral inoculation. There is a significant increase in Enk-ir in hsvPPE and hsvMOR + PPE treatment groups compared to sham and control virus (two-way ANOVA, factors: group × lamina, F(8, 45) = 7.1, P < 0.001, hsvCON). Below: Histology images of Enk+ areas of the dorsal horn of the spinal cord with control virus (left) and hsvPPE (right). B. Quantification of MOR-ir positive areas of the dorsal horn on Day 16 post viral inoculation. Below: Histology images of MOR+ areas of the dorsal horn of the spinal cord with control virus (left) and hsvMOR (right). There is a significant increase in MOR-ir in all lamina of hsvMOR, hsvPPE and hsvMOR + PPE treatment groups compared to sham and control virus (two-way ANOVA, factors: group × lamina, F(8, 45) = 18.7, P < 0.001; hsvCON). # vs. sham; # significant at P<0.05; ## significant at P<0.01; ### significant at P<0.001. * vs. control virus-treated (hsvCON);* significant at P<0.05; ** significant at P<0.01; *** significant at P<0.001. Bonferroni post-hoc for multiple comparisons.
Mechanical allodynia and thermal hyperalgesia
Our previous studies and preliminary data indicate that mechanical and thermal sensitivity four weeks after hsvMOR, hsvPPE and hsvMOR+ PPE inoculation result in similar evoked thresholds (data not shown). The focus of this study was to investigate the efficacy of elevated opiate receptors and/or opiate peptides after nerve injury, and therefore SNL was used as a model for neuropathic pain in humans. All mice developed equivalent mechanical allodynia and thermal hyperalgesia by day 7 post-L5 SNL, as evidenced by decreases in percent paw withdrawal thresholds and latencies from baseline (day 0). Mice inoculated with vehicle, MOCK, or hsvCON demonstrated similar allodynia across the entire time course of the study (data not shown) and were collapsed into a Control group. hsvMOR reversed mechanical allodynia to pre-ligation PWTs by day 5 post-infection (experimental day 12; vs. Control at the same time point, Fig. 5A). For the remainder of the study, PWTs remained significantly higher in the hsvMOR group than in the Control group (repeated measures ANOVA; Fig. 5A). By day 9 post-infection (experimental day 16) with hsvMOR, PWTs exceeded pre-ligation thresholds, showing the development of analgesia (Fig. 5A). By day 9 post-infection with hsvPPE, mechanical allodynia was reversed to levels equivalent to pre-ligation PWTs (vs. Control at the same time point and vs. hsvPPE). In contrast, hsvMOR+PPE did not change mechanical allodynia compared with that of control (repeated measures ANOVA, Fig. 5A). By experimental day 22, hsvMOR paw withdrawal thresholds exceeded preligation thresholds (fig. 5B). In contrast, hsvPPE did not reverse mechanical allodynia (fig. 5A) or thermal hyperalgesia (fig. 5C) as compared to control (repeated measures ANOVA, p = 0.16 and p = 0.99, respectively). Furthermore, hsvMOR + PPE had no effect on the duration of thermal hyperalgesia compared with that of the control group (repeated measures ANOVA, p = 0.12; fig. 5D).
Figure 5.
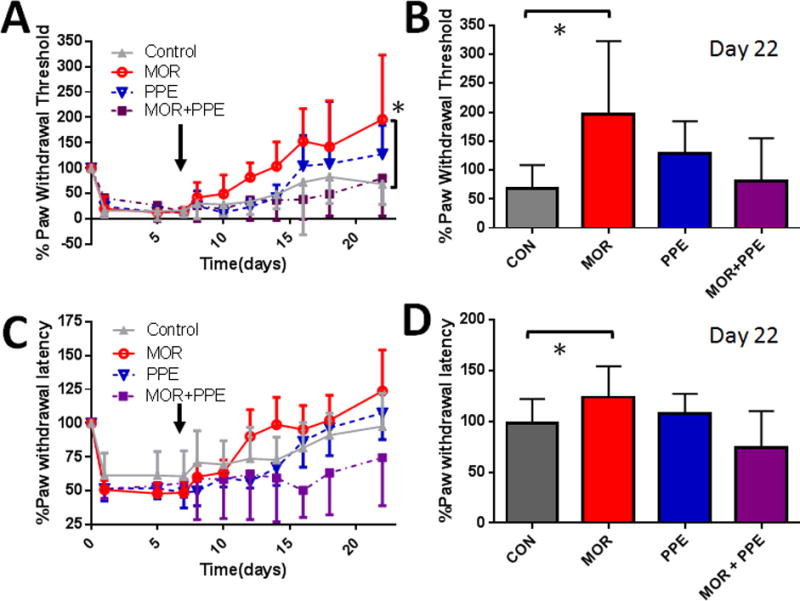
Mechanical allodynia and thermal hyperalgesia after L5 spinal nerve ligation surgery are attenuated in hsvMOR inoculated mice. At 7 days after nerve injury (arrow), MOR, PPE, MOR+PPE combination, or control (CON) virus was administered into the intraplantar aspect of the ipsilateral hind paw; mechanical allodynia and thermal hyperalgesia were then assessed over 15 days. (A) MOR and PPE virus infections reversed mechanical allodynia after 12 and 16 days, respectively. The MOR+PPE virus-infected mice had an allodynia reversal time course comparable to that of control virus-infected mice. Paw withdrawal threshold increased significantly over time for MOR virus-inoculated animals (repeated measures ANOVA, F(30, 500) = 3.9, *P<0.001 vs. control group. (B) The paw withdrawal threshold after MOR infection was significantly increased compared to control virus infection at the conclusion of the behavioral study (Day 22, ANOVA, F(3, 50) = 8.3, *P<0.001 vs. control group. (C) MOR and PPE virus infections reversed thermal allodynia after 12 and 16 days, respectively, compared to 21 days for control virus infection (CON). Mice administered the MOR+PPE virus combination did not have allodynia reversal during the time course of the experiment. (D) The thermal threshold after MOR infection was significantly increased compared with that after control virus infection at the conclusion of the behavioral study (Day 22, ANOVA, F(3, 50) = 5.9, *P = 0.022 vs. control group). Bonferroni post-hoc for multiple comparisons.
Peripheral and systemic opioid analgesia
To examine virus-mediated changes in opioid analgesia, we tested the peripherally active opioid agonist, loperamide, and the peripherally and centrally active opioid agonist, morphine, on SNL-induced thermal hyperalgesia at 16 days post-infection. Mice inoculated with vehicle, MOCK, or hsvCON demonstrated similar loperamide and morphine dose-response curves and were collapsed into a single Control group for statistical analysis (data not shown). hsvMOR produced a leftward shift in the loperamide dose-response curve compared with that of the Control group (Fig. 6A). The ED50 for loperamide was 0.9 ± 0.2 mg/kg in Control mice and 0.6 ± 0.2 mg/kg in hsvMOR-infected mice (Table 1). In contrast, loperamide was not analgesic at any of the doses tested in mice infected with hsvPPE or hsvMOR+PPE. An ED50 for loperamide could not be calculated for hsvPPE or hsvMOR+PPE.
Figure 6.
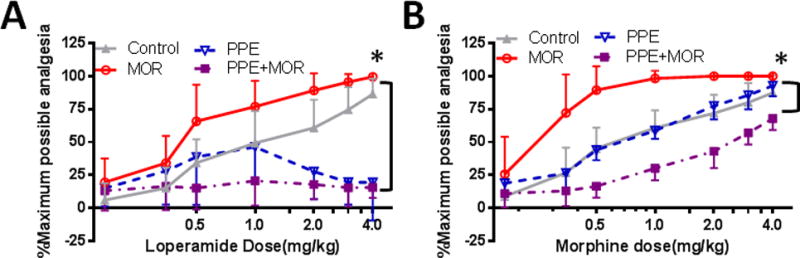
Loperamide and morphine dose response effect on paw withdrawal latency to heat is significantly shifted after intraplantar administration of hsvMOR versus hsvPPE, hsvMOR+PPE in nerve-injured mice. (A) The loperamide dose-response curve was significantly shifted to the left in animals infected with MOR virus; day 16 post-infection, *P<0.001 compared with control. The loperamide dose-response curve in PPE-infected mice was comparable to that of control virus-infected mice at low doses (0.17-1.0 mg/kg); however, at higher cumulative doses, the PPE-infected mice were nonresponsive to loperamide. MOR+PPE virus-infected mice were largely nonresponsive to loperamide. (B) The morphine dose-response curve was shifted to the left in animals infected with MOR; day 19 post infection, *P<0.001 compared with control. The morphine dose-response curve was shifted to the right in animals infected with MOR+PPE as compared to that in controls. In contrast, the morphine dose-response curve of PPE-infected mice was comparable that of control virus-infected mice. Percent maximum analgesia (%MPA) was calculated based on the formula: %MPA= (measured PWL (s) – pre-drug PWL (s)) / (20 – pre- drug PWL (s)) × 100. Bonferroni post-hoc for multiple comparisons.
Table 1.
Effective doses (ED50s) of morphine and loperamide (mg/kg) heat latency paw withdrawal 16 days after intraplantar administration of virus (mean ± SD).
| Morphine ED50 ± SD (95% CI) |
Loperamide ED50 ± SD (95% CI) |
|
|---|---|---|
| hsvCON (n=8) | 1.1 ± 0.1 (0.9-1.3) |
0.9 ± 0.2 (0.6-1.2) |
| hsvMOR (n=13) | 0.3 ± 0.5** (0.3-1.2) |
0.6±0.2** (0.1-1.1) |
| hsvPPE (n=9) | 0.9 ±0.1 (0.7-1.2) |
N/A |
| hsvMOR+PPE (n=8) | 1.7 ± 0.2** (1.3-2.0) |
N/A |
EC50s were compared to those of the control group (hsvCON);
significant at P<0.001
CI, confidence interval; N/A, not applicable; SD, standard deviation
Similarly, hsvMOR produced a leftward shift in the morphine dose-response curve compared with that of the Control group (Fig. 6B). The ED50 for morphine was 1.1 ± 0.1 mg/kg in Control mice and 0.3 ± 0.5 mg/kg in hsvMOR-infected mice (Table 1). hsvPPE had no effect on morphine analgesia, as shown by an overlapping dose-response curve (P>0.05) and equivalent ED50 with Control. hsvMOR+PPE produced a rightward shift in the morphine dose-response curve compared to that of Control and increased the ED50 for morphine to 1.7 ± 0.2 mg/kg (Table 1).
Capsaicin-induced spontaneous behaviors, thermal hyperalgesia, and neuronal activity
HsvCON infected mice had a pain score of 0.58 ± 0.15 (average ± SD) calculated from spontaneous pain-associated behaviors exhibited during the first 5 minutes after intraplantar capsaicin administration (Fig. 7A). A statistically significant decrease in pain score was observed in mice infected with hsvMOR (0.38 ± 0.11), hsvPPE (0.27 ± 0.09), or hsvMOR+PPE (0.35 ± 0.11) as compared to hsvCON (Fig. 7A). Ten minutes post capsaicin, all groups developed heat hyperalgesia evidenced by the decrease in paw withdrawal latency (Fig. 7B). Heat hyperalgesia resolved more quickly and maximal heat hyperalgesia was significantly attenuated in animals infected with hsvMOR, hsvPPE and hsvMOR+PPE as compared to hsvCON.
Figure 7.
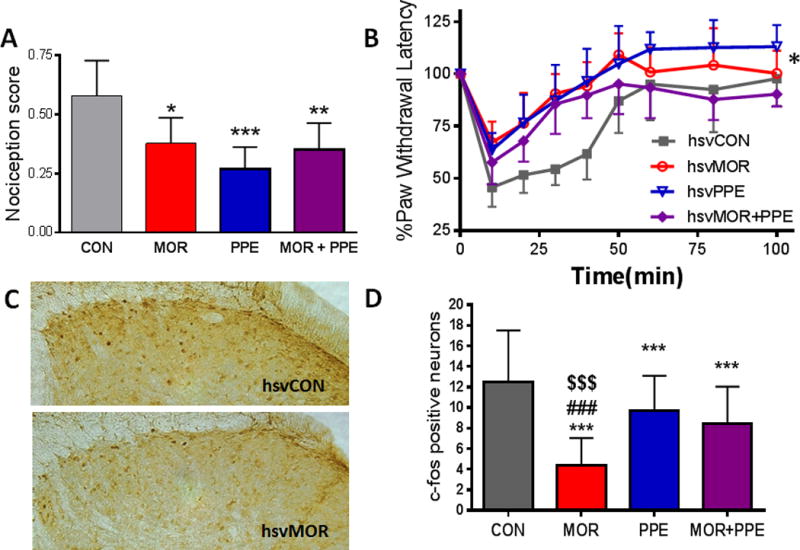
HsvMOR, hsvPPE, and hsvMOR+PPE decreased capsaicin-induced nociception and neuronal activation at 4 weeks after topical hindpaw infection. (A). HsvMOR, hsvPPE, and hsvMOR+PPE produced lower capsaicin-induced nociception scores five minutes post subcutaneous capsaicin administration in the left hindpaw, in comparison to hsvCON (*,**,*** p<0.05, 0.01, 0.001 vs. hsvCON). (B). HsvMOR, hsvPPE, and hsvMOR+PPE attenuated capsaicin-induced thermal hyperalgesia compared to hsvCON (**,*** P< 0.01,0.001 hsvMOR vs hsvCON. #,### P<0.05,0.001 hsvPPE versus hsvCON. $$$ P<0.001 hsvMOR+PPE versus hsvCON). (C). C-fos expression in the dorsal horn of the lumbar spinal cord at two hours post capsaicin administration was greater in hsvCON as compared to hsvMOR infection. (D). HsvMOR, hsvPPE, and hsvMOR+PPE infection decreased the number of c-fos positive neurons compared to HSV-CON (*** P<0.001 compared to hsvCON, ### P<0.001 compared to HSV-PPE, $$$ P<0.001 compared to hsvMOR+PPE).
C-fos was used as a marker of neuronal activation and was assayed in separate animals two hours after capsaicin, a time point when c-fos should be maximally induced (Fig. 7C). There was a statistically significant reduction in the number of c-fos positive neurons in hsvMOR, hsvPPE and hsvMOR+PPE inoculated animals, as compared to hsvCON infected mice. hsvMOR infected mice had significantly fewer c-fos positive neurons compared to hsvPPE and hsvMOR+PPE inoculated mice (Fig. 7D).
Changes in physical properties/responsiveness of primary afferent fibers after Spinal Nerve Ligation (SNL)
Neuropathic hyperalgesia was reversed (Fig. 5) and behavioral responses after opioid administration (Fig. 6) were shifted significantly to the left in hsvMOR-inoculated animals compared with those in hsvCON-inoculated animals. Animal groups inoculated with hsvPPE and hsvMOR+PPE did not show such effects. Therefore, we did not include them in the subsequent electrophysiological experiments that focused on the effects of hsvMOR-inoculation on receptive properties of afferent fibers.
Sensitization in primary afferent fibers has been postulated to contribute to the pain phenotype seen in many peripheral neuropathies, especially after nerve injury. We evaluated baseline properties and evoked activity of C-, Aδ - and Aβ-fibers recorded from animals with or without nerve injury that were either not inoculated, or inoculated with control or hsvMOR virus (Table 2). Compared to fibers recorded from uninjured animals, C-fibers recorded from nerve-injured animals had significantly higher heat thresholds, lower cold thresholds, and slower CVs (Table 3) with no significant effects due to vector. Additionally, we observed no significant baseline changes in Aδ- or Aβ-fibers after nerve injury or with respect to viral vector inoculation (data not shown).
Table 2.
Populations of units based on response properties
| Unit Type | No Treatment | hsvCON | hsvMOR | SNL | SNL hsvCON | SNL hsvMOR |
|---|---|---|---|---|---|---|
| C-fibers | n = 11 | n = 23 | n = 18 | n = 20 | n = 23 | n = 16 |
| CMH | 4 | 2 | 3 | 6 | 4 | 2 |
| CMHC | 2 | 12 | 6 | 6 | 3 | 2 |
| CMC | 0 | 1 | 6 | 2 | 6 | 3 |
| CM | 2 | 3 | 0 | 4 | 2 | 2 |
| CH/CC/CHC | 3 | 5 | 3 | 2 | 8 | 7 |
| Aδ-fibers | n = 11 | n = 8 | n = 7 | n = 14 | n = 11 | n = 17 |
| ADM | 9 | 7 | 7 | 10 | 8 | 11 |
| ADMC | 0 | 1 | 0 | 0 | 1 | 1 |
| ADMHC | 0 | 0 | 0 | 0 | 0 | 1 |
| ADMH | 2 | 0 | 0 | 4 | 2 | 4 |
| ADH | 0 | 0 | 0 | 0 | 0 | 0 |
| Aβ-fibers | n = 13 | n = 7 | n = 14 | n = 16 | n = 17 | n = 17 |
SNL, spinal nerve ligation; CMH, Mechano-Heat Sensitive C-fiber; CMHC, Mechano-Heat-Cold Sensitive C-fiber; CMC, Mechano-Cold Sensitive C-fiber; CM, Mechano-Sensitive C-fiber; CH, Heat and Cold Sensitive C-fiber; CC, Cold Sensitive C-fiber; CHC, Heat and Cold Sensitive C-fiber; ADM, Mechano-Sensitive Aδ-fibers; ADMC, Mechano-Cold Sensitive Aδ-fibers; ADMHC, Mechano-Heat and Cold Sensitive Aδ-fibers; ADMH, Mechano-Heat Sensitive Aδ-fibers; ADH, Heat-Sensitive Aδ-fibers.
Table 3.
Baseline properties of C-fibers (mean ± SD).
| Group | Von Frey threshold (mbars) | Heat threshold (°C)1 | Cold threshold (°C)2 | Mechanical threshold (g) | Conduction Velocity (m/s)3 |
|---|---|---|---|---|---|
| No vector and hsvCON (n =34) | 1.8 ± 0.6 | 40.1 ± 4.5 | 14.0 ± 6.4 | 1.8 ± 0.8 | 0.65 ± 0.2 |
| hsvMOR (n = 18) | 1.4 ± 0.8 | 38.2 ± 5.9 | 12.9 ± 6.4 | 1.9 ± 1.7 | 0.74 ± 0.25 |
| SNL no vector and hsvCON (n = 43) | 1.5 ± 0.7 | 41.2 ± 3.9* | 17.3 ± 9.8† | 3.0 ± 2.3 | 0.65 ± 0.26‡ |
| SNL hsvMOR (n = 16) | 1.6 ± 0.4 | 41.8 ± 5.6* | 17.2 ± 6.0† | 1.6 ± 0.5 | 0.48 ± 0.08‡ |
SNL, spinal nerve ligation.
Significant increase in SNL (SNL no vector and hsvCON; SNL hsvMOR) vs. uninjured fibers (no vector and hsvCON; hsvMOR).
ANOVA: F(1, 74) = 5.7, P<0.01
Significant decrease in SNL (SNL no vector and hsvCON; SNL hsvMOR) vs. uninjured fibers (no vector and hsvCON; hsvMOR).
ANOVA: F(1, 64) = 6.2, P<0.01.
Significant decrease in SNL (SNL no vector and hsvCON; SNL hsvMOR) vs. uninjured fibers (no vector + hsvCON and hsvMOR).
ANOVA: F(1, 107) = 6.4, P<0.05.
1 Millibar (mbar) = 0.0001 mN force.
Upon mechanical stimulation, Aβ-and Aδ-fibers from animals in the nerve-injured control groups (SNL) exhibited significantly increased responses to suprathreshold stimulation compared with those of the uninjured controls (no treatment and hsvCON); the difference became more apparent across larger forces (repeated measures ANOVA, factors: injury status × force, Fig. 8A and See Figure, Supplemental Digital Content 2, Aβ receptive field responses to mechanical stimulation data, respectively). Suprathreshold mechanical responses did not differ between Aδ-fibers recorded from lesioned or uninjured animals that were inoculated with hsvMOR after injury (Fig. 8B), and the responses in these groups were not statistically different from those observed in uninjured control animals (compare to Fig. 8A). Interestingly, C-fibers from lesioned animals did not show more robust responses to suprathreshold mechanical stimuli than did fibers from uninjured controls (Fig. 8C and 8D).
Figure 8.
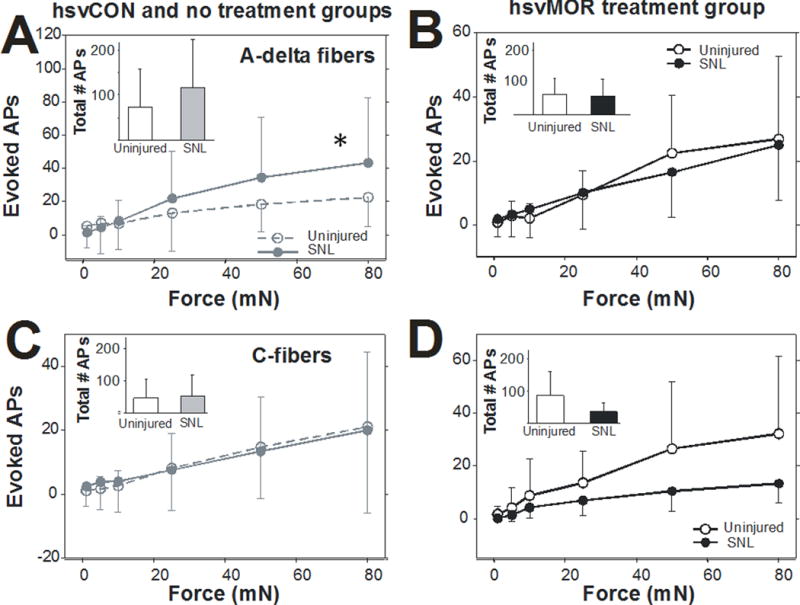
Nerve injury enhances mechanically evoked responses of myelinated primary afferent fibers. Evoked mechanical stimuli were delivered to the receptive fields of nerve fibers by a feedback-controlled mechanical stimulator in naïve mice and mice after spinal nerve ligation (SNL). (A) Aδ-fiber responses in control fibers (no treatment and hsvCON) to mechanical stimulation were increased after SNL compared with those of uninjured fibers across increasing forces of mechanical stimulation (repeated measures ANOVA: F(5, 215) = 3.3, P = 0.0014).
Inset: The total number of mechanically evoked action potentials was slightly higher in nerve- injured Aδ-fibers than in uninjured fibers. (B) Aδ-fiber responses in hsvMOR-inoculated mice did not differ between injured and uninjured fibers across increasing forces of mechanical stimulation. (C) C-fiber mechanical responses in control fibers (no treatment and hsvCON) were not increased after nerve injury across forces (D) C-fiber mechanical responses in from hsvMOR inoculated fibers were not increased after nerve injury.
Decrease in evoked mechanical and thermal responses in hsvMOR fibers after morphine application
The sensitivity to evoked stimulation was measured in primary afferent nerve fibers before and after morphine application in uninjured and nerve-injured animals. Regardless of injury status, Aβ-fibers recorded from animals in the hsvMOR inoculation group displayed a significant decrease in the mechanically evoked responses across forces after morphine application (See Figure, Supplemental Digital Content 3, hsvMOR inoculated Aβ-fiber responses data), and total responses post-morphine were significantly smaller than their corresponding pre-morphine responses (paired t-test). Mechanical responses recorded from Aβ-fibers in the control groups (no treatment and hsvCON) were similar to those after morphine application. We observed no significant decrease in mechanically evoked responses for Aδ-fibers or C-fibers post-morphine, regardless of vector treatment or injury status (data not shown).
Compared to C-fibers from uninoculated animals or animals inoculated with control virus, heat-sensitive C-fibers from animals inoculated with hsvMOR showed a significant decrease in the fraction of evoked action potentials post-morphine (ANOVA, Factor: vector, Fig. 9A). The heat threshold post-morphine was significantly greater in C-fibers from hsvMOR-inoculated animals than in control fibers (ANOVA, Factor: vector, Fig. 9B). Although the fraction of evoked potentials and heat thresholds before and after morphine application did change with statistical significance in hsvMOR-inoculated fibers compared with that in controls, the numerical difference was very small, suggesting that some fibers may be affected more than others, or that the peripheral terminals of heat-sensitive C-fibers are not readily affected by hsvMOR inoculation. Overall, spontaneous activity was found in over 50% of C-fibers after nerve injury, and this activity was significantly decreased after morphine application in hsvMOR fibers but not in control fibers (Fig. 9C and D). Only one fiber in the SNL hsvMOR group had spontaneous activity above 10 action potentials/minute, whereas seven of the fibers in the no treatment and hsvCON groups had spontaneous activities above that rate. After morphine incubation, the remaining C-fiber spontaneous activity was significantly decreased in the hsvMOR-inoculated fibers compared to that in fibers recorded from nerve-lesioned, uninoculated, or control virus-inoculated animals (% Change = [(Post – Pre)/Pre] × 100, unpaired t-test, P<0.05; Fig. 9D; example traces shown in Fig. 9E and 9F).
Figure 9.
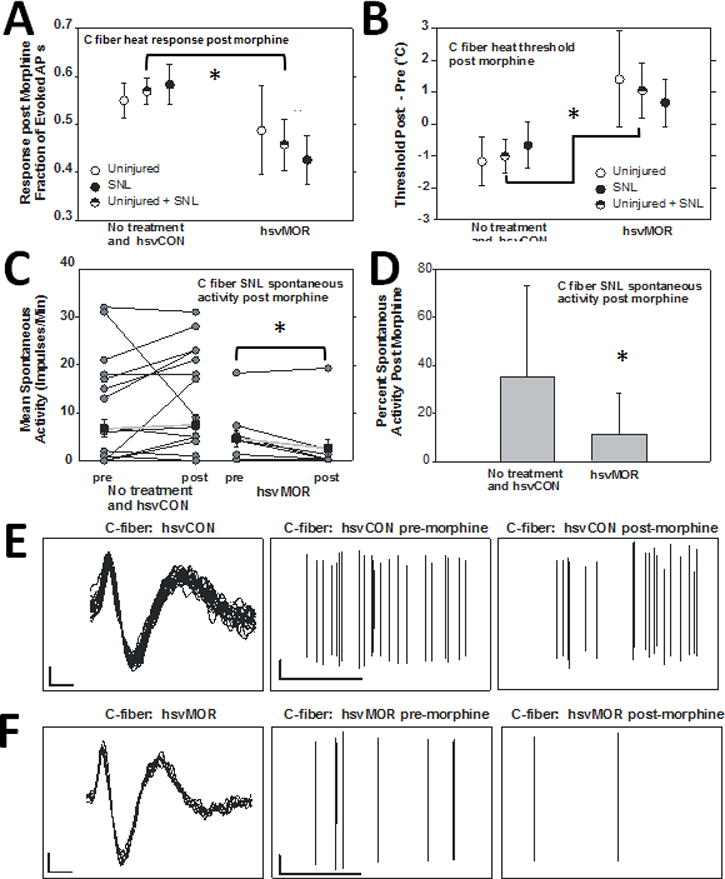
Morphine application causes a significant decrease in evoked and spontaneous C-fiber activity in mu-opioid receptor (hsvMOR) viral vector inoculated fibers. (A) The fraction of heat-evoked action potentials (APs) after morphine application was lower in hsvMOR-inoculated fibers than in un- inoculated and hsvCON-inoculated fibers (F(1, 69)=4.2, *P = 0.044). Data are presented as the fraction change after morphine application compared to pre-morphine values. Overall the heat responses in C-fibers were quite variable and data were pooled in order to reduce variance, especially in uninjured animals. “Uninjured + SNL” = units combined from “Uninjured” and “SNL”. (B) Heat threshold in hsvMOR-inoculated fibers increased significantly after morphine application compared with that in un-inoculated and hsvCON-inoculated fibers (ANOVA, factor: vector, F(1, 71)=4.5, *P = 0.038). (C) The mean spontaneous activity (impulses/min) was significantly less in C-fibers from hsvMOR-inoculated mice with spinal nerve ligation (SNL) after morphine application (post) than before morphine application (pre) (paired t-test, *P = 0.028). Averaged data are shown in black squares. (D) Spontaneous activity decreased significantly after morphine application in hsvMOR-inoculated fibers (unpaired t-test, *P = 0.048). E. Example traces of C-fiber spontaneous activity from hsvCON infected animal. Left: Waveform traces, Vertical bar: 2mV, Horizontal bar: 0.5 msecs. Middle: before morphine application, Vertical bar: 2mV, Horizontal bar: 20msecs. Right: Five minutes post morphine application. F. As in E for example traces of C-fiber spontaneous activity from hsvMOR infected animal. Left: Waveform traces, Middle: before morphine application, Right: Five minutes post morphine application.
Discussion
Expression of mu-opioid receptor (mOR) and Enkephalin following nerve injury
Immunoreactivity for mOR in epidermal nerve fibers in the plantar hindpaw skin, and small-diameter DRG cells decreased in hsvCON nerve injured mice compared to sham mice, in agreement with previous reports of a nerve-injury induced loss of MOR27–29. MOR expression is regulated by several factors during chronic pain, including DNA methylation at the MOR promotor and histone deacetylation which are both involved in MOR gene silencing30,31. Whether HSV-1 inoculation has any effect on these epigenetic factors in the peripheral nervous system is unknown, but regulatory mechanisms for the control of MOR expression could be a future target for gene therapy.
In contrast, enkephalin expression was not changed in the plantar hindpaw skin, and DRG cells in hsvCON nerve injured mice compared to sham mice. While exogenous administration of enkephalins32 and virally-mediated expression of preproenkephalin15,16,19,33,34 reduces pain in experimental models, the role for endogenous preproenkephalin in the initiation and maintenance of neuropathic pain has not been well studied17.
Effect of nerve lesion on receptive properties of cutaneous afferents
SNL increased the response to suprathreshold mechanical stimulation in Aδ-fibers, while SNL increased the response only at the highest force for Aβ- fibers (Supplemental Digital Content 2), and responses in C-fibers did not differ between unlesioned- and SNL- animals treated with control virus. Changes in peripheral nerve sensitivity have also been found in A delta- and C-fibers in a spared nerve injury mouse model35 indicating that mechanical allodynia may be mediated by both myelinated and unmyelinated fibers. Since we only found modest changes in heat sensitivity after SNL, peripheral sensitization of C-fibers to heat stimuli is unlikely to explain behavioral signs of heat hyperalgesia that has been observed following SNL in mice. In contrast, cold thresholds in C-fibers were increased by about 3°C (Table 3), and this change may contribute to signs of cold hyperalgesia that develops following SNL36 and in neuropathic pain patients37. Following SNL, we observed a considerable number of C-fibers with spontaneous activity. Spontaneous activity in intact C-fibers following SNL has previously been observed in nonhuman primate38 and rat39,40, and such activity may induce spontaneous pain behavior and contribute to the initiation and maintenance of central sensitization.
Overexpression of mu-opioid receptor (mOR)
The attenuation of evoked responses indicate that tactile and heat sensitivity are decreased after hsvMOR inoculation. Future studies utilizing conditioned place preference, gait analysis, and innate behavioral quantification (e.g. digging, locomotion) could help provide further insight on the effects of enhanced MORs with the viral vector on ongoing, spontaneous pain; although negative results have been reported for multiple types of these behavioral assays, making data interpretation somewhat challenging41.
Similar to previous studies10, virus driven MOR expression was observed in large and medium afferents and led to significant increases in MOR expression in lamina I–III. This presence of MOR in large diameter afferents coupled with the more diffuse MOR immunoreactivity in the spinal cord dorsal horn suggested that hsvMOR may allow for expression of MOR in myelinated fibers. Interestingly, hsvMOR animals also had increased levels of Enk-ir in epidermal nerve fibers, DRG cells, and dorsal horn of the spinal cord, which could indicate changes in PPE production in keratinocytes and immune cells after infection.
Effects of hsvMOR inoculation on properties of cutaneous afferents
Inoculation with hsvMOR in Aδ-fibers appears to prevent the SNL- induced increase to suprathreshold stimulation in this fiber class (compare Fig 8A and B). This effect may contribute to the ‘spontaneous’ reversal in paw withdrawal von Frey thresholds that was observed in animals inoculated with hsvMOR (Fig. 5A). Furthermore, the incidence of C-fibers with spontaneous activity was reduced in lesioned animals inoculated with hsvMOR (Fig. 9C). This decrease may in turn reduce central sensitization and thereby contribute to the reversal in mechanical paw withdrawal thresholds that was observed in nerve-lesioned, hsvMOR inoculated animals (Fig. 5A). The mechanically evoked activity in C fibers was increased in uninjured hsvMOR animals versus animals with SNL (Fig. 8D). This increase is surprising, as hsvCON animals do not show an increased sensitivity to mechanical stimulation (Fig. 8C). It is possible that overall neuronal sensitivity and changes in evoked responses are more pronounced in recordings from hsvMOR animals than hsvCON animals, due to increased neuroinflammation and changes in intracellular signaling after nerve injury42,43.
Opioid Analgesia
HsvMOR inoculation produced a leftward shift in loperamide- and morphine analgesia, and these data are similar to previous hsvMOR studies in uninjured10 and injured mice26. Previous studies have demonstrated that peripheral opioid receptors, endogenous or virally inserted, are a key site of action for opioid analgesia, as their efficacy can be reduced via naloxone17,19,44 and methyl-naltrexone7,45. Taken together, these results suggest that increased expression of MOR on peripheral and spinal afferent nerve terminals may be important sites of action for opioid analgesia following nerve injury.
Spontaneously evoked behaviors and c-fos expression after capsaicin exposure
In the present study, infection with hsvMOR, hsvPPE, or hsvMOR+PPE decreased capsaicin-induced behaviors. This outcome is not unexpected, since peripheral administration of opiates attenuates capsaicin pain in humans46. hsvMOR produced maximal inhibition of capsaicin-induced c-fos expression as compared to hsvPPE and hsvMOR+PPE (Fig. 7D). The decrease in c-fos activation of hsvMOR could be directly due to attenuation of nociceptor activation, or a decrease in paw flinching/licking, and/or animal movement after capsaicin injection. Regardless, the c-fos data suggests that excitatory inputs and presynaptic modulation/postsynaptic hyperpolarization of secondary sensory neurons are likely mediated by mu-opiate receptors after noxious stimulation (e.g.capsaicin injection).
Effects of morphine on properties of cutaneous afferents
The effects of morphine on heat-evoked responses in C-fibers (Fig. 9) were minimal, while in contrast, the increased behavioral effects of morphine following hsvMOR inoculation (Fig. 6) may instead be due to an increased effect in the dorsal horn of the spinal cord as hsvMOR inoculation led to an increased, time-dependent expression of MOR in the dorsal horn (Fig. 5). Morphine also reduced the responses to suprathreshold mechanical stimulation in Aβ-fibers recorded from animals inoculated with hsvMOR (Supplemental Digital Content 3). The behavioral effects of morphine inhibition of Aβ- fibers mechanosensitivity in the SNL model are not clear; however, such inhibition may be beneficial in patients with postherpetic neuralgia or traumatic nerve injury that present with dynamic mechanical allodynia.37
Overexpression of Preproenkephalin (PPE)
Opioid peptides, enkephalin in particular, stimulate cytokine release and immunocyte chemotaxis, and can induce changes that signal for immunocyte activation47. The effects of opioid peptides that are locally released from immune cells48 could explain the increase in Enk-ir in hsvMOR infected animals as soon as day 1 post-infection (See Tables, Supplemental Digital Content 4–6, tables for Enk-ir and mOR-ir in epidermal nerve fibers, dorsal root ganglia, and lumbar spinal cord, respectively). These data suggest that trafficking of enkephalin vesicles in primary afferent neurons may be initially enhanced3 and that expression observed at day 1 post-infection is likely from non-neuronal cells.
Previous studies using an HSV proenkephalin sequence showed reductions in mechanical allodynia in a L5 spinal nerve ligation model in rats18. Surprisingly, in this study, infection with hsvPPE did not reverse thermal hyperalgesia and had no effect on morphine analgesia in nerve injured mice. This is different from previous studies showing a reduction of neuropathic pain in rodent models of nerve injury18,49, and capsaicin induced pain15. It is noteworthy that our behavioral studies were conducted at least two weeks post intradermal injection of the virus and nerve injury, and experiments included female mice.
Overexpression of Preproenkephalin (PPE) and mu-opioid receptor (mOR)
Surprisingly, co-infection with hsvPPE and hsvMOR decreased enkephalin and MOR expression in nerve fibers and did not reverse mechanical allodynia or thermal hyperalgesia. The analgesic effects hsvMOR + PPE was predicted to be additive to those of hsvMOR and hsvPPE. It is possible that an upregulation of an inhibitory G-protein coupled receptor and an inhibitory peptide in nerve fibers could alter receptor activity and downstream signaling pathways50 and/or increase expression of pro-nociceptive signaling molecules not investigated in this study. Furthermore, co-infection shifted loperamide and morphine dose-response curves to the right suggestive of opioid receptor desensitization, which could produce a physiological state similar to opioid tolerance.
Potential Clinical implications
Our preclinical findings suggest that enhancing peripheral mOR expression after nerve injury, using viral vectors, attenuates mechanical allodynia and thermal hyperalgesia through their effects on primary afferent signaling. These data suggest that important factors such as the transgene sequence, species, timing post inoculation, biological sex, route of administration and etiology of chronic pain are all important factors to consider in preclinical studies as this therapy moves closer to the clinic. Previous preclinical studies using hsvPPE have been recently tested in clinical trials for intractable cancer pain51,52, indicating that there is some translational potential for this viral vector system using hsvMOR. The peripheral application of hsvMOR presents a novel, translatable therapeutic strategy for the treatment of neuropathic pain with minimal CNS adverse effects that may result from enhanced endogenous opioid analgesia and a decrease in the dose of exogenous opioids.
Supplementary Material
Acknowledgments
The authors thank Claire Levine, M.S., E.L.S. (Scientific Editor, Department of Anesthesiology and Critical Care Medicine, The Johns Hopkins University), for her editorial help.
Financial Support
Supported by United States National Institutes of Neurological Disease and Stroke Grant NS-26363 (S.N. Raja) and by the University of South Carolina Department of Pharmacology, Physiology, Neuroscience (S. M. Sweitzer, S. P. Wilson). A.H. Klein was supported by an NIH F32 fellowship grant (DA-036991).
Footnotes
Competing Interests
The authors declare no competing interests.
Meeting Presentation
Meeting Presentation (in part): Mohammad H, Raja S, Wilson SP, Sweitzer SM. Title: Herpes simplex virus driven preproenkephalin and mu opioid receptor but not delta opioid receptor expression in primary afferents reverses neuropathic pain-associated behaviors and enhances peripheral opioid analgesia. Society for Neuroscience 38th Annual Meeting, 774.12, USA, November, 2008.
References
- 1.Pasero C. Pathophysiology of neuropathic pain. Pain Manag Nurs. 2004;5:3–8. doi: 10.1016/j.pmn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Elde R, Arvidsson U, Riedl M, Vulchanova L, Lee JH, Dado R, Nakano A, Chakrabarti S, Zhang X, Loh HH, Law PY, H ö kfelt T, Wessendorf M. Distribution of neuropeptide receptors. New views of peptidergic neurotransmission made possible by antibodies to opioid receptors. Ann N Y Acad Sci. 1995;757:390–404. doi: 10.1111/j.1749-6632.1995.tb17497.x. [DOI] [PubMed] [Google Scholar]
- 3.Antunes bras J, Becker C, Bourgoin S, Lombard M, Cesselin F, Hamon M, Pohl M. Met-enkephalin is preferentially transported into the peripheral processes of primary afferent fibres in both control and HSV1-driven proenkephalin A overexpressing rats. Neuroscience. 2001;103:1073–83. doi: 10.1016/s0306-4522(01)00034-3. [DOI] [PubMed] [Google Scholar]
- 4.Janson W, Stein C. Peripheral opioid analgesia. Curr Pharm Biotechnol. 2003;4:270–4. doi: 10.2174/1389201033489766. [DOI] [PubMed] [Google Scholar]
- 5.Obara I, Przewlocki R, Przewlocka B. Local peripheral effects of mu-opioid receptor agonists in neuropathic pain in rats. Neurosci Lett. 2004;360:85–9. doi: 10.1016/j.neulet.2004.01.056. [DOI] [PubMed] [Google Scholar]
- 6.Truong W, Cheng C, Xu QG, Li XQ, Zochodne DW. Mu opioid receptors and analgesia at the site of a peripheral nerve injury. Ann Neurol. 2003;53:366–75. doi: 10.1002/ana.10465. [DOI] [PubMed] [Google Scholar]
- 7.Guan Y, Johanek LM, Hartke TV, Shim B, Tao YX, Ringkamp M, Meyer RA, Raja SN. Peripherally acting mu-opioid receptor agonist attenuates neuropathic pain in rats after L5 spinal nerve injury. Pain. 2008;138:318–29. doi: 10.1016/j.pain.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu Y, Xu Y, Li GW, Huang LY. Remote nerve injection of mu opioid receptor adeno-associated viral vector increases antinociception of intrathecal morphine. J Pain. 2005;6:447–54. doi: 10.1016/j.jpain.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Kolesnikov YA, Jain S, Wilson R, Pasternak GW. Peripheral morphine analgesia: synergy with central sites and a target of morphine tolerance. J Pharmacol Exp Ther. 1996;279:502–6. [PubMed] [Google Scholar]
- 10.Zhang G, Mohammad H, Peper BD, Raja S, Wilson SP, Sweitzer SM. Enhanced peripheral analgesia using virally mediated gene transfer of the mu-opioid receptor in mice. Anesthesiology. 2008;108:305–13. doi: 10.1097/01.anes.0000299836.61785.79. [DOI] [PubMed] [Google Scholar]
- 11.Wilson SP, Yeomans DC. Genetic therapy for pain management. Curr Rev Pain. 2000;4:445–50. doi: 10.1007/s11916-000-0068-5. [DOI] [PubMed] [Google Scholar]
- 12.Glorioso JC, Fink DJ. Herpes vector-mediated gene transfer in the treatment of chronic pain. Mol Ther. 2009;17:13–8. doi: 10.1038/mt.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guedon JM, Wu S, Zheng X, Churchill CC, Glorioso JC, Liu CH, Liu S, Vulchanova L, Bekker A, Tao YX, Kinchington PR, Goins WF, Fairbanks CA, Hao S. Current gene therapy using viral vectors for chronic pain. Mol Pain. 2015;11:27. doi: 10.1186/s12990-015-0018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson SP, Yeomans DC. Virally mediated delivery of enkephalin and other neuropeptide transgenes in experimental pain models. Ann N Y Acad Sci. 2002;971:515–21. doi: 10.1111/j.1749-6632.2002.tb04516.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilson SP, Yeomans DC, Bender MA, Lu Y, Goins WF, Glorioso JC. Antihyperalgesic effects of infection with a preproenkephalin-encoding herpes virus. Proc Natl Acad Sci U S A. 1999;96:3211–6. doi: 10.1073/pnas.96.6.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeomans DC, Lu Y, Laurito CE, Peters MC, Vota-Vellis G, Wilson SP, Pappas GD. Recombinant herpes vector-mediated analgesia in a primate model of hyperalgesia. Mol Ther. 2006;13:589–97. doi: 10.1016/j.ymthe.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Meunier A, Latrémolière A, Mauborgne A, Bourgoin S, Kayser V, Cesselin F, Hamon M, Pohl M. Attenuation of pain-related behavior in a rat model of trigeminal neuropathic pain by viral-driven enkephalin overproduction in trigeminal ganglion neurons. Mol Ther. 2005;11:608–16. doi: 10.1016/j.ymthe.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Hao S, Mata M, Goins W, Glorioso JC, Fink DJ. Transgene-mediated enkephalin release enhances the effect of morphine and evades tolerance to produce a sustained antiallodynic effect in neuropathic pain. Pain. 2003;102:135–42. doi: 10.1016/s0304-3959(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama H, Sasaki K, Franks ME, Goins WF, Goss JR, de Groat WC, Glorioso JC, Chancellor MB, Yoshimura N. Gene therapy for bladder overactivity and nociception with herpes simplex virus vectors expressing preproenkephalin. Hum Gene Ther. 2009;20:63–71. doi: 10.1089/hum.2008.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steiner I, Kennedy PG, Pachner AR. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol. 2007;6:1015–28. doi: 10.1016/S1474-4422(07)70267-3. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt Y, Gavériaux-Ruff C, Machelska H. μ-Opioid receptor antibody reveals tissue-dependent specific staining and increased neuronal μ-receptor immunoreactivity at the injured nerve trunk in mice. PLoS One. 2013;8:e79099. doi: 10.1371/journal.pone.0079099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Neill J, Brock C, Olesen AE, Andresen T, Nilsson M, Dickenson AH. Unravelling the mystery of capsaicin: a tool to understand and treat pain. Pharmacol Rev. 2012;64:939–71. doi: 10.1124/pr.112.006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustafsson H, Akesson J, Lau CL, Williams D, Miller L, Yap S, Rolan P. A comparison of two formulations of intradermal capsaicin as models of neuropathic pain in healthy volunteers. Br J Clin Pharmacol. 2009;68:511–7. doi: 10.1111/j.1365-2125.2009.03489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann K, Hein A, Hager U, Kaczmarek JS, Turnquist BP, Clapham DE, Reeh PW. Phenotyping sensory nerve endings in vitro in the mouse. Nat Protoc. 2009;4:174–96. doi: 10.1038/nprot.2008.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moshourab R, Schmidt Y, Machelska H. Skin-nerve preparation to assay the function of opioid receptors in peripheral endings of sensory neurons. Methods Mol Biol. 2015;1230:215–28. doi: 10.1007/978-1-4939-1708-2_17. [DOI] [PubMed] [Google Scholar]
- 26.Smith SN, Paige C, Velazquez KT, Smith TP, Raja SN, Wilson SP, Sweitzer SM. Injury-specific promoters enhance herpes simplex virus-mediated gene therapy for treating neuropathic pain in rodents. J Pain. 2015;16:283–90. doi: 10.1016/j.jpain.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansikka H, Zhao C, Sheth RN, Sora I, Uhl G, Raja SN. Nerve injury induces a tonic bilateral mu-opioid receptor-mediated inhibitory effect on mechanical allodynia in mice. Anesthesiology. 2004;100:912–21. doi: 10.1097/00000542-200404000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Kohno T, Ji RR, Ito N, Allchorne AJ, Befort K, Karchewski LA, Woolf CJ. Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain. 2005;117:77–87. doi: 10.1016/j.pain.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 29.Porreca F, Tang QB, Bian D, Riedl M, Elde R, Lai J. Spinal opioid mu receptor expression in lumbar spinal cord of rats following nerve injury. Brain Res. 1998;795:197–203. doi: 10.1016/s0006-8993(98)00292-3. [DOI] [PubMed] [Google Scholar]
- 30.Hwang CK, Song KY, Kim CS, Choi HS, Guo XH, Law PY, Wei LN, Loh HH. Evidence of endogenous mu opioid receptor regulation by epigenetic control of the promoters. Mol Cell Biol. 2007;27:4720–36. doi: 10.1128/MCB.00073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun L, Zhao JY, Gu X, Liang L, Wu S, Mo K, Feng J, Guo W, Zhang J, Bekker A, Zhao X, Nestler EJ, Tao YX. Nerve injury-induced epigenetic silencing of opioid receptors controlled by DNMT3a in primary afferent neurons. Pain. 2017;158:1153–1165. doi: 10.1097/j.pain.0000000000000894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong Y, Abbott FV. Peripheral opioid modulation of pain and inflammation in the formalin test. Eur J Pharmacol. 1995;277:21–8. doi: 10.1016/0014-2999(95)00045-m. [DOI] [PubMed] [Google Scholar]
- 33.Kang W, Wilson MA, Bender MA, Glorioso JC, Wilson SP. Herpes virus-mediated preproenkephalin gene transfer to the amygdala is antinociceptive. Brain Res. 1998;792:133–5. doi: 10.1016/s0006-8993(98)00194-2. [DOI] [PubMed] [Google Scholar]
- 34.Yeomans DC, Jones T, Laurito CE, Lu Y, Wilson SP. Reversal of ongoing thermal hyperalgesia in mice by a recombinant herpesvirus that encodes human preproenkephalin. Mol Ther. 2004;9:24–9. doi: 10.1016/j.ymthe.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Smith AK, O’Hara CL, Stucky CL. Mechanical sensitization of cutaneous sensory fibers in the spared nerve injury mouse model. Mol Pain. 2013;9:61. doi: 10.1186/1744-8069-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katsura H, Obata K, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Sakagami M, Noguchi K. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp Neurol. 2006;200:112–23. doi: 10.1016/j.expneurol.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 37.Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13:924–35. doi: 10.1016/S1474-4422(14)70102-4. [DOI] [PubMed] [Google Scholar]
- 38.Ali Z, Ringkamp M, Hartke TV, Chien HF, Flavahan NA, Campbell JN, Meyer RA. Uninjured C-fiber nociceptors develop spontaneous activity and alpha-adrenergic sensitivity following L6 spinal nerve ligation in monkey. J Neurophysiol. 1999;81:455–66. doi: 10.1152/jn.1999.81.2.455. [DOI] [PubMed] [Google Scholar]
- 39.Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci. 2001;21:RC140. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Djouhri L, Fang X, Koutsikou S, Lawson SN. Partial nerve injury induces electrophysiological changes in conducting (uninjured) nociceptive and nonnociceptive DRG neurons: Possible relationships to aspects of peripheral neuropathic pain and paresthesias. Pain. 2012;153:1824–36. doi: 10.1016/j.pain.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheahan TD, Siuda ER, Bruchas MR, Shepherd AJ, Mohapata DP, Gereau RW, IV, Golden JP. Inflammation and nerve injury minimally affect mouse voluntary behaviors proposed as indicators of pain. Neurobiology of Pain. 2017;2:1–12. doi: 10.1016/j.ynpai.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenk HN, Brederson JD, Honda CN. Morphine directly inhibits nociceptors in inflamed skin. J Neurophysiol. 2006;95:2083–97. doi: 10.1152/jn.00394.2005. [DOI] [PubMed] [Google Scholar]
- 43.Brederson JD, Honda CN. Primary afferent neurons express functional delta opioid receptors in inflamed skin. Brain Res. 2015;1614:105–11. doi: 10.1016/j.brainres.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goss JR, Harley CF, Mata M, O’Malley ME, Goins WF, Hu X, Glorioso JC, Fink DJ. Herpes vector-mediated expression of proenkephalin reduces bone cancer pain. Ann Neurol. 2002;52:662–5. doi: 10.1002/ana.10343. [DOI] [PubMed] [Google Scholar]
- 45.Tiwari V, Yang F, He SQ, Shechter R, Zhang C, Shu B, Zhang T, Wang Y, Dong X, Guan Y, Raja SN. Activation of Peripheral μ-opioid Receptors by Dermorphin [D-Arg2, Lys4] (1-4) Amide Leads to Modality-preferred Inhibition of Neuropathic Pain. Anesthesiology. 2016;124:706–20. doi: 10.1097/ALN.0000000000000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinnman E, Nygårds EB, Hansson P. Peripherally administrated morphine attenuates capsaicin-induced mechanical hypersensitivity in humans. Anesth Analg. 1997;84:595–9. doi: 10.1097/00000539-199703000-00024. [DOI] [PubMed] [Google Scholar]
- 47.Stefano GB, Kream RM. Opioid peptides and opiate alkaloids in immunoregulatory processes. Arch Med Sci. 2010;6:456–60. doi: 10.5114/aoms.2010.14271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cabot PJ, Carter L, Gaiddon C, Zhang Q, Schäfer M, Loeffler JP, Stein C. Immune cell-derived beta-endorphin. Production, release, and control of inflammatory pain in rats. J Clin Invest. 1997;100:142–8. doi: 10.1172/JCI119506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma F, Wang C, Yoder WE, Westlund KN, Carlson CR, Miller CS, Danaher RJ. Efficacy of Herpes Simplex Virus Vector Encoding the Human Preproenkephalin Gene for Treatment of Facial Pain in Mice. J Oral Facial Pain Headache. 2016;30:42–50. doi: 10.11607/ofph.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saloman JL, Scheff NN, Snyder LM, Ross SE, Davis BM, Gold MS. Gi-DREADD Expression in Peripheral Nerves Produces Ligand-Dependent Analgesia, as well as Ligand-Independent Functional Changes in Sensory Neurons. J Neurosci. 2016;36:10769–10781. doi: 10.1523/JNEUROSCI.3480-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gene Transfer for Cancer Pain. ClinicalTrials.gov. Bethesda, MD: National Library of Medicine (US); 2014. https://clinicaltrials.gov/show/NCT00804076, Retrieved October 27th, 2017. [Google Scholar]
- 52.NP2 Enkephalin For Treatment of Intractable Cancer Pain. ClinicalTrials.gov. Bethesda, MD: National Library of Medicine (US); 2014. https://clinicaltrials.gov/show/NCT01291901, Retrieved October 27th, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


