Abstract
Invasive fungal infections are a serious cause of morbidity and mortality in patients with hematologic malignancies. Conidiobolus species are molds within the order Entomophthorales and may disseminate to become rapidly fatal in immunocompromised individuals. This species of fungal infections are often multidrug resistant (MDR) and present unique therapeutic challenges. Reports of Conidiobolus infections are rare in pediatric oncology. We report the successful treatment of an adolescent male with B cell lymphoblastic leukemia and MDR invasive sinopulmonary Conidiobolus infection with emphasis on early and aggressive neutrophil support with surgical debridement. The strategies described could be applied to other MDR fungal infections.
Keywords: leukemia, Conidiobolus, granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte transfusions, multidrug resistance
Introduction
Invasive fungal infections are estimated to occur in 7.2% of children with cancer, and those with leukemia are disproportionately represented due to prolonged neutropenia and corticosteroid treatment.[1] Case-fatality rates in invasive fungal infections have been reported in published series to be between 20–70%. Poor outcomes are most often associated with disseminated disease, central nervous system (CNS) involvement or persistent neutropenia.[2] Aspergillus and Candida species (spp.) are the most common causes of invasive fungal infections in pediatric oncology patients but many organisms can cause opportunistic infections.[3] As advances are achieved in new antifungal agents, newly recognized resistant organisms causing serious infections are emerging. Conidiobolus spp. are multidrug resistant (MDR) molds that may cause lethal infections in immunocompromised patients.[4]
Conidiobolus spp. are MDR molds in the order Entomophthorales. Originally Conidiobolus and Basidiobolus spp. were categorized under the class Zygomycetes but now reside in a new subphylum called Entomophthoromycotina.[4] Conidiobolus spp. are typically found in tropical regions as a cause of disfiguring, indolent rhinofacial disease in immunocompetent individuals. In immunocompromised individuals, infection can disseminate widely to lung, skin, mediastinum, gastrointestinal tract, heart, liver, or brain.[4–6] Among the few cases of Conidiobolus infections in the oncology literature, rapid dissemination and mortality is commonly reported.[5,7] We report the successful treatment of an adolescent male who presented with B cell lymphoblastic leukemia (B-ALL) and invasive sinopulmonary Conidiobolus infection. The approaches used in this case may be applicable to other patients with hematologic malignancies and MDR fungal infections.
Case Description
A 15 year-old male presented with intermittent fever, fatigue, neck pain, cough, and petechiae. Chest radiograph at presentation showed no acute cardiopulmonary abnormalities, while laboratory evaluation revealed severe neutropenia with circulating blasts. Flow cytometry confirmed the diagnosis of B-ALL. Because his age was greater than or equal to 13 years-old, very high risk 4-drug induction chemotherapy was initiated with daunorubicin, vincristine, pegasparagase and prednisone. He was placed on cefepime for empiric antibacterial coverage due to fevers on presentation and fluconazole for antifungal prophylaxis. On day 5 of induction, he developed nasal congestion, left-sided facial pain, and worsening dry cough. Further history revealed that he recently worked on the family farm and had exposures to silage, hay, soil, and manure. Chest computerized tomography (CT) scan showed bilateral, diffuse, peripheral, nodular ground glass opacities (Fig. 1a). Sinus CT scan showed mucosal thickening of the left maxillary and ethmoid sinuses (Fig. 1b).
Figure 1.
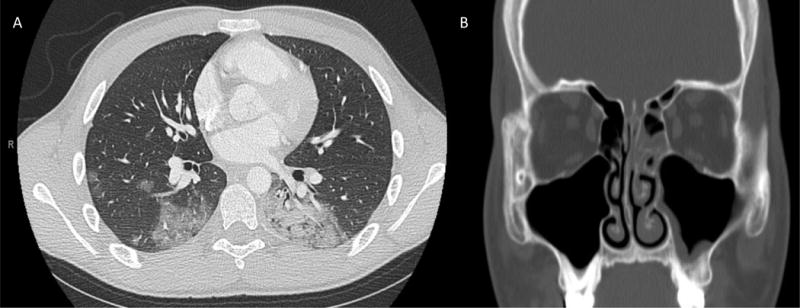
(A) Chest CT scan chest with contrast at day 5 of induction showing diffuse scattered ground-glass and dense airspace opacities in the right upper and middle lobe and left lower lobe. (B) Sinus CT with contrast showing mild to moderate mucosal thickening of left maxillary and ethmoid sinuses.
Otolaryngological examination showed pale insensate mucosa with necrosis of the left middle turbinate (Fig. 2a). This location is a disease-predictive variable for acute invasive fungal sinusitis with 97% specificity.[8] The patient underwent endoscopic resection of the left middle turbinate with opening of the left maxillary, ethmoid, sphenoid, and frontal sinuses. Biopsy demonstrated necrotic respiratory mucosa with angioinvasive hyphae that were broad but septate and branching (Fig. 2b). This histological appearance was suggestive of Aspergillus species, supporting initiation of voriconazole and micafungin. After 4 days of culture, zygospores with prominent beak and sporangiola characteristic of Conidiobolus species grew on fungal culture (Fig. 2c) phenotypically identified as Conidiobolus coronatus. Antimicrobial susceptibilities were performed in the Fungus Testing Laboratory of the University of Texas Health Science Center at San Antonio and demonstrated in vitro susceptibility to terbinafine, intermediate minimum inhibitory concentrations (MICs) to amphotericin B and anidulafungin, and resistance to all other tested antifungal agents (Table 1). Once susceptibilities were finalized, antifungal therapy was changed to liposomal amphotericin B (LAmB) 10 mg/kg intravenous (IV) daily, anidulafungin 1.5 mg/kg IV daily, and terbinafine 250 mg PO twice daily.
Figure 2.
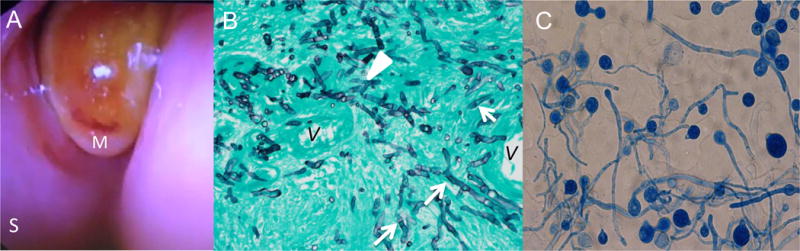
(A) Nasal endoscopy revealed pale insensate mucosa with necrotic tissue in the middle turbinate. M: middle turbinate; S: septum (B) Photomicrograph (Gomori methenamine silver stain, original magnification ×200) of left middle turbinate showed numerous hyphae invading vascular wall. The hyphae are relatively broad, appear septate (arrows), and have irregular contours. Rare branching is seen (white arrowhead) V: vessel. (C) Lactophenol cotton blue wet mount shows hyphae with septa but as the culture matured, zygospores formed each with a prominent beak and sporangiola, characteristic of Entomophthorales
TABLE 1.
Conidiobolus resistance pattern to antifungals
| Drug | MIC (μg/mL) |
|---|---|
| Amphotericin B | 2 |
| Anidulafungin | 4 |
| Caspofungin | > 8 |
| Micafungin | > 8 |
| Fluconazole | > 64 |
| Itraconazole | > 16 |
| Posaconazole | > 16 |
| Voriconazole | > 16 |
| Terbinafine | 0.125 |
| Isavuconazole | > 16 |
Chest CT scan showed evidence of progressive pulmonary lobar infiltration (Fig. 3a) and prompted bronchoscopy, which demonstrated extended-spectrum beta-lactamase (ESBL) producing Escherichia coli without fungal organisms. His clinical course was further complicated by Klebsiella pneumoniae and Pseudomonas aeruginosa sepsis on day 11 of induction requiring intensive care. His clinical deterioration led to temporary discontinuation of leukemia therapy on day 17 of induction coincident with a bone marrow evaluation revealing morphologic remission but with 1% residual leukemia by flow cytometry.
Figure 3.
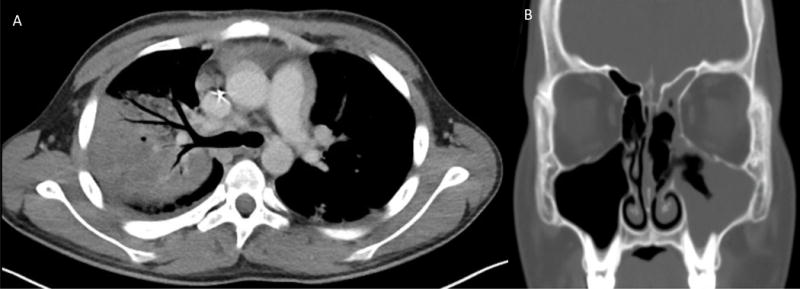
(A) Chest CT scan with contrast at day 36 after initiation of induction chemotherapy showing right upper lobe consolidation, ground-glass airspace disease in the left lower lobe and right sided pleural effusion. (B) Sinus CT without contrast showing worsened mucosal thickening of left maxillary and ethmoid sinuses.
In order to contain his multidrug resistant (MDR) Conidiobolus infection and prevent dissemination, the patient underwent a total of eight sinus endoscopy procedures over the next several weeks to surgically debride mucosal tissue due to persistently abnormal endoscopic findings and the presence of hyphae with necrosis on pathology. During this time, sinus CT scan showed worsening mucosal thickening of the left maxillary and ethmoid sinuses (Fig. 3b). Despite fungal elements on biopsy specimens, all cultures after initial biopsy were negative for fungal growth. When it was determined that further surgery would breach his cranial vault and lead to CSF leak, debridements were discontinued.
Ongoing disease despite aggressive surgeries combined with poorly effective anti-fungal agents against this MDR pathogen led to exploration of adjuvant therapies. Augmentation of neutrophil support was initiated on induction day 15 to overcome the patient’s underlying immunocompromised state with daily sargramostim (recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF)) at 250μg/m2, granulocyte transfusions, and hyperbaric oxygen therapy (HBOT). After 30 days of neutrophil supportive therapy, production of endogenous neutrophils occurred and his chest CT scan showed improvements of the right upper lobe consolidation and bilateral lower lobe ground glass opacities.
The patient, in total, received 20 granulocyte transfusions from 16 different unrelated volunteer donors along with 53 sessions of HBOT. The granulocytes were collected and prepared at the Blood Center of Wisconsin where donors can give 2 granulocyte donations separated by 1 week time in a 3 month period. Donors were ABO matched while HLA typing was not required. The average number of granulocytes given per transfusion was 3.59 × 1010 granulocytes (range 2.4 × 1010 – 6.6 × 1010). Each donor received filgrastim (G-CSF) for mobilization of granulocytes. The post granulocyte transfusion absolute neutrophil count (ANC) mean was 282/μL (range 50–990/μL) where mean time to blood draw for ANC post-transfusion was 10.4 hours (range 3.7–16 hours).
Each HBOT session lasted 90 minutes and occurred at a depth of 2 atmospheres using 100% oxygen. After holding chemotherapy for 35 days, he received modified-maintenance treatment with 50% daily dosage of mercaptopurine (37.5mg/m2) due to intermediate thiopurine methyltransferase (TPMT) enzyme activity (TPMT*1/TPMT*3A), weekly oral methotrexate (20mg/m2/dose) and single dose of vincristine (2mg). After 3 weeks of modified-maintenance treatment, bone marrow evaluation was negative for residual leukemia by both morphology and flow cytometry and consolidation chemotherapy was then initiated.
In order to minimize the risk of recurrent fungal disease while delivering immunosuppressive leukemia therapy, he remained on LAmB and GM-CSF until beginning maintenance chemotherapy. HBOT and granulocyte transfusions were discontinued post-consolidation. The sinus CT scan without contrast prior to delayed intensification demonstrated clearance of the sinuses. GM-CSF maintenance dose was 100μg/m2 three times weekly, which was increased to 250μg/m2 given daily during neutropenic periods. During therapy with LAmB, he suffered toxicities of electrolyte abnormalities and nephrotoxicity with elevated creatinine. He required potassium and magnesium supplementation, and in attempt to ameliorate nephrotoxicity, pre- and post-LAmB volume expansion with 0.9% NaCl was given. Toxicities, improving clinical stability, patient’s desire for improved quality of life, and entering maintenance therapy where severe neutropenia is less likely were factors that contributed to the discontinuation of LAmB. The patient is now 10 months into maintenance chemotherapy without evidence of active Conidiobolus infection and remains off anti-fungal agents and neutrophil support. With this regimen he has had no recrudescence or dissemination of fungal disease and his leukemia remains in remission.
Discussion
Invasive fungal infections are a major cause of morbidity and mortality in hematologic malignancies. MDR fungal infections present additional challenges in pediatric oncology patients. This report highlights our regimen used to treat an invasive MDR fungal infection.
Our patient suffered from a potentially life-threatening, invasive, MDR Conidiobolus infection, the management of which was complicated by the need for concomitant myelosuppressive chemotherapy. Early diagnosis of sinusitis as being caused by Conidiobolus was essential in recognizing the limitations of conventional antifungal therapy. This infection progressed despite aggressive surgical debridement of infected tissue and antifungal therapy, but was ultimately controlled by addition of granulocyte transfusions, administration of GM-CSF, and use of HBOT.[3]
Conidiobolus spp. are historically highly resistant to antifungal agents, which was confirmed in this case with elevated minimum inhibitory concentrations (MIC) to nearly all agents tested.[9] Terbinafine is an ineffective systemic antifungal agent as the medication largely accumulates in skin, nails, and skin appendages without significant systemic effect. However, terbinafine was used in our patient as it could possibly act synergistically with other antifungal agents to increase their efficacy. Nonetheless, we believe that with the relative in vitro inadequacy of systemic antifungal agents, surgical debridement and augmentation of his innate host defense with granulocyte transfusions and GM-CSF was important for his survival and optimal outcome in eradicating Conidiobolus from his tissues. Although we cannot state definitively within the context of a case report the exact relative contributions of the multidisciplinary therapeutic interventions, we can state that clinical improvement was temporally related to the addition of granulocyte transfusions and GM-CSF. We hypothesize that they had a significant contribution since there was no highly effective antifungal agent available and that the serial debridements, while aggressive, stopped short of clean margins to prevent breaching the skull base.
Neutrophils, macrophages and immunoregulatory cytokines contribute to innate antifungal immunity. Neutrophils and macrophages detect fungal pathogens through surface pattern recognition receptors.[10] Macrophages phagocytose and kill inhaled conidia. If germination of conidia occurs and evades host defence, then proinflammatory cytokines, chemokines and anaphylatoxins generated during complement activation attract neutrophils to destroy hyphae. Neutrophils can also phagocytose conidia and prevent germination.[11] When germinating conidia and hyphal elements become too large for neutrophils to phagocytose, extracellular killing occurs through degranulation and resultant hyphal respiratory reduction.
Our patient suffered from both quantitative and qualitative defects in innate host defences. Correcting his persistent neutropenia by granulocyte transfusions was essential for control of his fungal sinusitis and preventing dissemination of the MDR Conidiobolus. GM-CSF was administered for upregulation of neutrophil and monocyte function during granulocyte transfusion and myelosuppressive chemotherapy.[12] Building upon earlier reports in successful treatment of sino-orbital mucormycosis, we also administered HBOT for further control of the Conidiobolus sinusitis.[13]
Granulocyte transfusions are sometimes utilized to treat patients with profound neutropenia and invasive fungal disease. In vitro findings suggest that harvested granulocytes have preserved function and quickly hone to the infection site despite their short half-life.[14] Successful outcomes have been reported for other invasive mycoses when patients with profound neutropenia were supported with granulocyte transfusions.[15,16] However, controversy still exists as to whether potential clinical benefit outweighs side effects including febrile reactions, pulmonary inflammation, and HLA alloimmunization.[17] Our patient tolerated his granulocyte transfusions well.
GM-CSF is a cytokine that stimulates production of neutrophils and increases migratory capacity and superoxide production.[18] It also aids in monocyte-directed cellular immunity by enhancing phagocytic function and increasing superoxide production in response to medically important fungi, including Aspergillus spp., Scedosporium spp., and the Mucorales.[12,19] Currently, GM-CSF is approved for neutropenia associated with stem cell transplantation and is being assessed in clinical trials as an immunostimulatory adjuvant (NCT01495637, NCT02451488).
HBOT uses a chamber to provide increased atmospheric pressure while delivering 100% oxygen. In vitro analysis of HBOT at 2–3 atmospheres show fungal growth inhibition, potentiation of antifungal drugs and an increase in oxidative radicals that contribute to fungal damage.[20] A retrospective review shows that HBOT may aid in the treatment of zygomycosis.[13]
In summary, a multidisciplinary team approach is necessary for managing patients with invasive fungal infections. The successful management strategy in this patient with MDR Conidiobolus infection combined aggressive support of neutrophil function, serial surgical debridement and anti-fungal therapy was effective in treating invasive MDR Conidiobolus sinusitis, preventing disseminated disease, and allowing delivery of myelosuppressive chemotherapy. These described management strategies could be applied to other MDR fungal infections in immunocompromised patients.
Acknowledgments
Disclosures and Acknowledgements: Dr. Walsh was supported in part for this work as a Scholar in Mucormycosis of the Henry Schueler Foundation.
References
- 1.Ozsevik SN, Sensoy G, Karli A, et al. Invasive fungal infections in children with hematologic and malignant diseases. J Pediatr Hematol Oncol. 2015;37:e69–72. doi: 10.1097/MPH.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 2.Groll AH, Castagnola E, Cesaro S, et al. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or allogeneic haemopoietic stem-cell transplantation. The Lancet Oncology. 2014;15:e327–e340. doi: 10.1016/S1470-2045(14)70017-8. [DOI] [PubMed] [Google Scholar]
- 3.Mor M, Gilad G, Kornreich L, et al. Invasive fungal infections in pediatric oncology. Pediatric blood & cancer. 2011;56:1092–1097. doi: 10.1002/pbc.23005. [DOI] [PubMed] [Google Scholar]
- 4.Shaikh N, Hussain KA, Petraitiene R, et al. Entomophthoramycosis: a neglected tropical mycosis. Clinical Microbiology and Infection. 2016 doi: 10.1016/j.cmi.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Walsh TJ, Renshaw G, Andrews J, et al. Invasive zygomycosis due to Conidiobolus incongruus. Clin Infect Dis. 1994;19:423–430. doi: 10.1093/clinids/19.3.423. [DOI] [PubMed] [Google Scholar]
- 6.Wuppenhorst N, Lee MK, Rappold E, et al. Rhino-orbitocerebral zygomycosis caused by Conidiobolus incongruus in an immunocompromised patient in Germany. J Clin Microbiol. 2010;48:4322–4325. doi: 10.1128/JCM.01188-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radhakrishnan N, Sachdeva A, Oberoi J, et al. Conidiobolomycosis in relapsed acute lymphoblastic leukemia. Pediatric blood & cancer. 2009;53:1321–1323. doi: 10.1002/pbc.22259. [DOI] [PubMed] [Google Scholar]
- 8.Payne SJ, Mitzner R, Kunchala S, et al. Acute Invasive Fungal Rhinosinusitis: A 15-Year Experience with 41 Patients. Otolaryngol Head Neck Surg. 2016;154:759–764. doi: 10.1177/0194599815627786. [DOI] [PubMed] [Google Scholar]
- 9.Guarro J, Aguilar C, Pujol I. In-vitro antifungal susceptibilities of Basidiobolus and Conidiobolus spp. strains. J Antimicrob Chemother. 1999;44:557–560. doi: 10.1093/jac/44.4.557. [DOI] [PubMed] [Google Scholar]
- 10.Brakhage AA, Bruns S, Thywissen A, et al. Interaction of phagocytes with filamentous fungi. Curr Opin Microbiol. 2010;13:409–415. doi: 10.1016/j.mib.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Mircescu MM, Lipuma L, van Rooijen N, et al. Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J Infect Dis. 2009;200:647–656. doi: 10.1086/600380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gil-Lamaignere C, Simitsopoulou M, Roilides E, et al. Interferon- gamma and granulocyte-macrophage colony-stimulating factor augment the activity of polymorphonuclear leukocytes against medically important zygomycetes. J Infect Dis. 2005;191:1180–1187. doi: 10.1086/428503. [DOI] [PubMed] [Google Scholar]
- 13.John B, Chamilos G, Kontoyiannis D. Hyperbaric oxygen as an adjunctive treatment for zygomycosis. Clinical Microbiology and Infection. 2005;11:515–517. doi: 10.1111/j.1469-0691.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 14.Bashir S, Stanworth S, Massey E, et al. Neutrophil function is preserved in a pooled granulocyte component prepared from whole blood donations. Br J Haematol. 2008;140:701–711. doi: 10.1111/j.1365-2141.2008.06996.x. [DOI] [PubMed] [Google Scholar]
- 15.Quillen K, Wong E, Scheinberg P, et al. Granulocyte transfusions in severe aplastic anemia: an eleven-year experience. Haematologica. 2009;94:1661–1668. doi: 10.3324/haematol.2009.010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dignani MC, Anaissie EJ, Hester JP, et al. Treatment of neutropenia-related fungal infections with granulocyte colony-stimulating factor-elicited white blood cell transfusions: a pilot study. Leukemia. 1997;11:1621–1630. doi: 10.1038/sj.leu.2400811. [DOI] [PubMed] [Google Scholar]
- 17.Estcourt LJ, Stanworth SJ, Hopewell S, et al. Granulocyte transfusions for treating infections in people with neutropenia or neutrophil dysfunction. The Cochrane Library. 2016 doi: 10.1002/14651858.CD005339.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bober LA, Grace MJ, Pugliese-Sivo C, et al. The effect of GM-CSF and G-CSF on human neutrophil function. Immunopharmacology. 1995;29:111–119. doi: 10.1016/0162-3109(94)00050-p. [DOI] [PubMed] [Google Scholar]
- 19.Gil-Lamaignere C, Winn RM, Simitsopoulou M, et al. Inteferon gamma and granulocyte-macrophage colony-stimulating factor augment the antifungal activity of human polymorphonuclear leukocytes against Scedosporium spp.: comparison with Aspergillus spp. Med Mycol. 2005;43:253–260. doi: 10.1080/13693780412331271072. [DOI] [PubMed] [Google Scholar]
- 20.Farina C, Marchesi G, Passera M, et al. In vitro activity of Amphotericin B against zygomycetes isolated from deep mycoses: a comparative study between incubation in aerobic and hyperbaric atmosphere. Medical mycology. 2012;50:427–432. doi: 10.3109/13693786.2011.614964. [DOI] [PubMed] [Google Scholar]


