Abstract
Background
Adenosine or regadenoson are often used with pharmacologic stress testing. Adenosine may trigger atrioventricular block (AVB). Despite its higher selectivity, regadenoson has also been associated with AVB. We studied the incidence of de novo AVB with these agents.
Methods
A comprehensive search of SCOPUS was performed from inception to March 2016. Studies of at least 10 patients, using adenosine and/or regadenoson with SPECT-MPI, reporting rates of AVB were selected for further review.
Results
Thirty four studies were pooled including 22,957 patients. Adenosine was used in 21 studies and regadenoson in 15. Both were administered in two studies. The estimated incidence of overall and high-grade AVB was 3.81% (95% CI 1.99%–6.19%) and 1.93% (95% CI 0.77%–3.59%), respectively. The incidence of AVB (8.58%; 95% CI 5.55%–12.21% vs 0.30%; 95% CI 0.04%–0.82%, respectively, P < .001) and high-grade AVB (5.21%; 95% CI 2.81%–8.30% vs 0.05%; 95% CI < .001%–0.19% respectively, P < .001) were higher with adenosine compared to regadenoson.
Conclusion
AVB is seen in about 4% of patients undergoing vasodilator stress test. Both overall and high-grade AVB are more frequent with adenosine compared to regadenoson. (J Nucl Cardiol 2017)
Keywords: Vasodilator, adenosine, regadenoson, atrioventricular block, single photon emission computed tomography
BACKGROUND
Non-invasive pharmacologic stress testing has a central role in the evaluation of patients with suspected or known coronary artery disease (CAD), not only for diagnostic but also for prognostic purposes. It is estimated that over 9 million single photon emission computed tomography (SPECT) studies are performed annually in the US, making it the stress testing method of choice.1 Exercise is generally the preferred stressor, however, in certain cases, pharmacologic induction of hyperemia is preferred, namely when patients cannot exercise adequately due to physical limitations and in patients with baseline ECG abnormalities (left bundle branch block, pre-excitation pattern, permanent ventricular pacing).2 Pharmacologic stress is also used for risk stratification purposes in clinically stable patients soon after acute myocardial infarction and in cases with presumptive acute coronary syndrome, for both diagnosis and risk stratification, when maximal exercise is not felt to be safe.2
The two most widely used coronary vasodilators in the current era are adenosine and regadenoson.3–6 Adenosine acts by non-selectively activating all four of its receptors, including A1, A2a, A2b and A3.2,7 Activation of A1 receptors is responsible for suppression of the sinus (SA) and atrioventricular (AV) nodes leading to varying degrees of bradycardia and AV block (AVB). These are the same receptors that also mediate its anti-adrenergic properties, which could account for its cardio-protective effects in the cascade of ischemia– reperfusion and mediate preconditioning. Activation of A2a receptors dilates the coronary and systemic vasculature, whereas A2b results in myocardial preconditioning. Binding to the A3 receptors induces activation of the mast cells and constriction of the smooth muscles of the airways.2,7
In contrast to adenosine, regadenoson is a highly selective A2a receptor agonist (almost 13 times higher affinity for A2a receptors) with minimal if any binding to A2b and A3 receptors.2 Compared to adenosine, regadenoson is easily administered as weight-independent bolus dose and since its approval by the Food and Drug Administration (FDA) in 2008, it is now the pharmacologic stressor of choice in the US, comprising 76% of the vasodilator market share and 59% of overall stress SPECT use.6
The safety profile for both adenosine and regadenoson has been extensively studied.3–5 With respect to occurrence of bradyarrhythmias associated with these two agents, all three degrees of AVB have been reported with adenosine. Regarding regadenoson, it should be noted that despite its high selectivity for the A2a receptors, it may still activate the A1 receptors, which could lead to AVB. Several cases of complete heart block and/or sinus arrest and asystole have been recently reported in the literature8–15 and as of June 2016, there were 72 cases of regadenoson-related complete heart block reported to the FDA.16 Given the above, the current guidelines endorsed by the American Society of Nuclear Cardiology (ASNC), the American College of Cardiology and the American Heart Association clearly state that use of either one of these agents (adenosine and regadenoson) in patients with known high-degree AVB and SA node dysfunction is contraindicated, unless the patient has a functioning pacemaker device.2 Nevertheless, the incidence of de novo AVB and SA with adenosine and regadenoson has not been comprehensively studied. In this meta-analysis, we report on the comparison of the incidence of de novo AVB and SA node dysfunction as a result of administration of adenosine or regadenoson during SPECT-MPI.
METHODS
Inclusion and Exclusion Criteria
This meta-analysis followed the guidelines for Meta-analysis of Observational Studies in Epidemiology (MOOSE).17 Studies published between 1952 and March 2016 that reported electrocardiographic changes and/or abnormalities in patients undergoing myocardial testing using adenosine and/or regadenoson were identified and underwent further analysis using pre-specified inclusion criteria as follows: (1) SPECT-MPI was the imaging method utilized, (2) adenosine and/or regadenoson were the vasodilators used for stress testing at doses equal to or less than the ones routinely used for stress SPECT-MPI (adenosine at 140 mcg/kg/min and regadenoson as a 400 mcg intravenous bolus injection), (3) the study included more than 10 patients receiving either one of the agents, (4) rates of AVB were reported. The primary outcome of interest was the incidence of AVB. Secondary outcomes were the incidence of bradycardia, sinus pause, sinus arrest, and asystole. Case reports or case series of fewer than 10 patients were excluded, as were editorials, comments, letters, review articles, guidelines, studies using other imaging modalities (vasodilator echocardiography, vasodilator cardiac magnetic resonance imaging, positron emission tomography), and invasive testing using adenosine or regadenoson (invasive fractional flow reserve). Studies on heart transplant recipients were excluded. In addition, one study18 used a cross-over design, where patients first received adenosine and were later randomized to receive either regadenoson or placebo. In order to avoid introducing non-independence by including patients in the analysis more than once, we included results from the adenosine arm only in our meta-analysis. In contrast, two-arm studies, such as the integrated ADVANCE MPI trial5 and the study by Zahid et al19 were each treated as two separate single-arm studies for the purpose of this meta-analysis.
Search Strategy and Quality Assessment
An electronic literature search in SCOPUS20 (which includes Biobase, Compendex, Embase, Fluidex, Geobase, Medline and Word Textile Index) was performed independently by two authors (EA and LB). A predefined set of keywords was used which was verified by a third researcher (NSB) (Supplement Section 1). All studies on human subjects, in English language, in either full text or abstract form were eligible for inclusion. Abstracts and oral presentations from the American College of Cardiology, American Heart Association, American College of Chest Physicians and American Society of Nuclear Cardiology were screened and corroborated by searching SCOPUS. The resulting titles and abstracts were then uploaded into a reference management software database. Following initial review of all abstracts, relevant studies were selected for retrieval of their full text for more in-depth assessment. In cases where there were two or more publications of the same patient population, the one describing the most complete dataset was selected for inclusion and further analysis. Additional relevant studies were retrieved from review of the reference lists of the initially extracted studies. Extraction of data and assessment of study quality followed regarding authorship, type (abstract vs full text) and year of publication, study population, study design (retrospective, prospective, historically matched case-control, randomized trials) and electrocardiographic changes of interest (presence and type of AVB, bradycardia, sinoatrial block, sinus arrest, sinus pause, asystole). Methodological quality of the studies selected for inclusion was done by investigator EA and confirmed by NSB using the Newcastle–Ottawa score (NOS) (Supp Table 1).21 The NOS system is a well-validated tool used to assess the quality of non-randomized studies used in meta-analyses. Each study is scored based on three aspects: (a) the selection of the study groups, (b) the degree of comparability among the different groups, and (c) the ascertainment of either the endpoint or the exposure of interest in cohort or case–control studies respectively. Any discrepancy in extraction and/or analysis of the data was resolved by consensus among the investigators. The consensus process involved weekly or biweekly meetings where discussions among the investigators were undertaken, with mandatory recognition of errors by one of the reviewers.
Study Endpoints
Primary endpoint was assessment of the incidence rates of overall and high-grade AVB. Overall AVB included first, second and third degree AVB. High-grade AVB included second and/or third degree AVB.22 Secondary endpoints were assessment of the incidence rates of SA node dysfunction including bradycardia, sinoatrial block, sinus arrest, and asystole.
Statistical Analysis
Statistical analyses were performed using R statistical software, version 3.0.23,24 The number of subjects experiencing AVB and high-grade AVB was extracted from each study.
Due to the very low incidence of AVB, the arcsine transformation was used to obtain a pooled estimate of the incidence. For meta-analyses of very rare events, this transformation is more appropriate than the commonly used logit transformation, as it can accommodate studies with no observed events, without requiring a continuity correction.25,26 Both fixed-effects and random-effects models were considered; the random-effects models were estimated using restricted maximum likelihood. Statistical tests for heterogeneity were performed using the Cochrane’s Q statistic, with the assumption of homogeneity being considered invalid for P values < .1. When homogeneity could not be assumed, we reported summary estimates from the random-effects models.
Meta-regression analyses were used to assess whether the incidence of AVB significantly varied by vasodilator (i.e., adenosine vs regadenoson). We also used meta-regression methods to investigate whether patient age and prior history of diabetes were significantly associated with incidence of AVB. Advanced age is inherently linked with higher degree of conduction system degeneration and fibrosis and has been shown to correlate with the incidence of adenosine-related conduction disturbance.3 Patients with diabetes and underlying cardiac autonomic neuropathy may be at higher risk for developing conduction derangement following adenosine or regadenoson. Other covariates that might also be related with a higher risk for SA and AV node dysfunction or cardiac autonomic neuropathy, such as use of beta blockers, calcium channel blockers, and chronic kidney disease lacked sufficient data to allow for analysis.
Finally, potential publication bias was assessed using funnel plots with the Egger test.27 Two-tailed p values less than 0.05 were considered to be statistically significant.
RESULTS
Selection and Characteristics of Studies
A total of 2,125 studies were identified, of which 1646 used adenosine and 479 used regadenoson as the pharmacological stress agent. A total of 34 studies were included in the final analysis (Figure 1).3,5,18,19,28–57 Adenosine was the vasodilator used in 21 studies,3,5,18,19,28–44 regadenoson was administered in 155,19,45–57 and both agents were used in two studies.5,19 For the integrated ADVANCE MPI trial, which included the results from both ADVANCE MPI 1 and 2 trials, we included the data for the randomized adenosine vs regadenoson scans.5 In the study by Hendel et al, regadenoson-stress SPECT-MPI was performed in patients who had previously demonstrated ischemia in adenosine-stress scans.50 No data were provided regarding the adenosine scans, therefore, this was treated as a regadenoson study. Furthermore, regadenoson was given either at the usually administered dose of 400 mcg in half the patients and at a higher non-traditional dose of 500 mcg.50 Based on our inclusion criteria, only the subset of patients who received regadenoson at 400mcg were considered for further analysis.50
Figure 1.
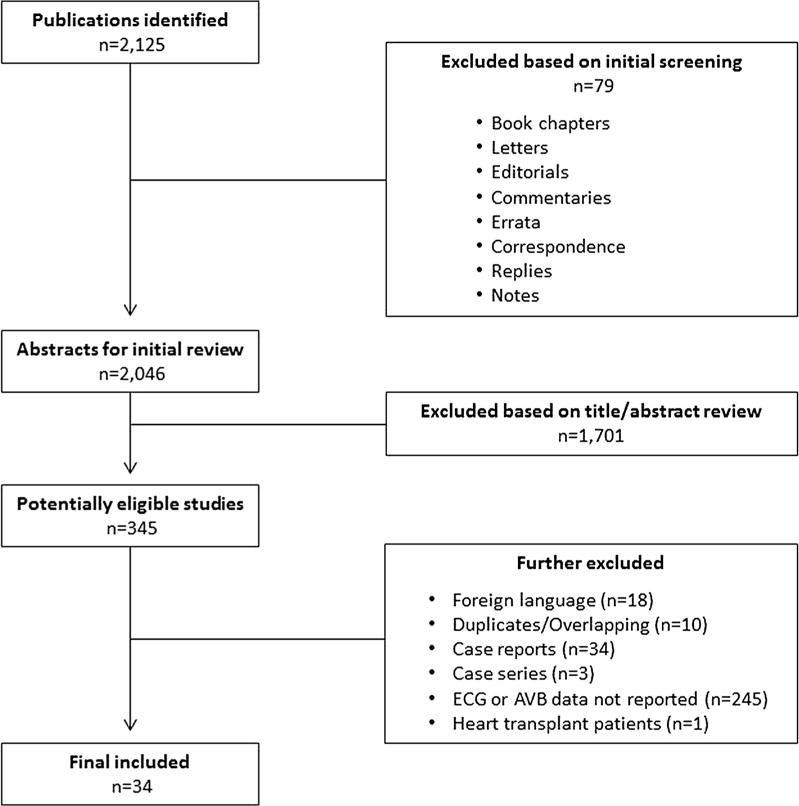
Flow diagram of included studies.
In the final analysis, the 34 studies included data on a total of 22,957 patients. These included 8,285 patients who received regadenoson and 14,672 patients who received adenosine. Percentage of male patients was not available for two studies in the adenosine group.35,36 For the remaining studies, 56.4% of patients receiving regadenoson and 54% of the patients receiving adenosine were male. The mean age of participants was not available for one study in the regadenoson group5 and eight studies in the adenosine group.5,30–32,34–36,41 For the remaining studies, the mean age was 54 years for patients receiving regadenoson and 57 years for patients receiving adenosine. The characteristics of the studies using adenosine are listed in Supp. Table 1 and those using regadenoson in Supp. Table 2. We note that: 1) in the study by Lakireddy et al patients were administered adenosine infusion for 6 min at a rate of 56 mcg/kg as opposed to the routinely administered dose of 140 mcg/kg/min,37 2) in the study by Alkoutami et al all the patients (n = 11) receiving adenosine were nonagenarians (i.e., 90–99 years old).31
Incidence of Overall AVB
AVB was observed in 1,314 of 22,957 (5.72%) patients. There was evidence of significant heterogeneity in the incidence of overall AVB (Q = 1,677.92, P < .001). Notably, when the adenosine and regadenoson studies were considered separately, we observed significant heterogeneity in the incidence of overall AVB for both the adenosine (Q = 343.61, P < .001) and the regadenoson (Q = 139.24, P < .001) studies.Tables 1 and 2 show the distribution of the different types of AVB among the adenosine and regadenoson studies, respectively. The random-effects estimate of the incidence of overall AVB was 3.81% (95% CI 1.99%–6.19%). The incidence rate of AVB in the adenosine group was significantly higher than that of the regadenoson group (8.58%; 95% CI 5.55%–12.21% vs 0.30%; 95% CI 0.04%–0.82%, respectively P < .001, OR 30.6; 95% CI 11.0–85.3) (Figure 2).
Table 1.
Distribution of first, second, and third degree AVB among studies with administration of adenosine
| Author | Year | Total number of patients | Number of patients with AVB | 1st degree AVB | Mobitz type I 2nd degree AVB | Mobitz type II 2nd degree AVB | Complete heart block |
|---|---|---|---|---|---|---|---|
| Abreu28 | 1991 | 607 | 527 | 43 | 33 | 4 | 0 |
| Al-Mallah29 | 2011 | 306 | 253 | 28 | 22 | 3 | |
| Alkoutami30 | 1998 | 97 | 83 | 8 | 5 | 1 | |
| Alkoutami31 | 1998 | 11 | 6 | 0 | 0 | 4 | 1 |
| Alkoutami32 | 1999 | 600 | 448 | 79 | 61 | 12 | |
| Bouvier33 | 1998 | 84 | 78 | 6 | 0 | ||
| Cerqueira3 | 2008 | 9,256 | 8,550 | 256 | 378 | 72 | |
| Cerqueira5 | 1994 | 631 | 579 | 43 | 9 | 0 | |
| Chun34 | 2006 | 36 | 33 | 3 | 0 | 0 | |
| Geloo35 | 1998 | 101 | 82 | 0 | 19 | ||
| Geloo36 | 1998 | 135 | 19 | ||||
| Lakkireddy37 | 2008 | 158 | 135 | 16 | 7 | 0 | 0 |
| Mahmood38 | 1994 | 90 | 88 | 2 | 0 | 0 | 0 |
| O’keefe39 | 1993 | 117 | 110 | 0 | 2 | 3 | 2 |
| Patel40 | 1995 | 69 | 68 | 0 | 0 | 0 | 1 |
| Shaw41 | 1992 | 58 | 58 | 0 | 0 | 0 | 0 |
| Sun42 | 2015 | 1,168 | 1,092 | 16 | 58 | 2 | |
| Sundram43 | 2009 | 108 | 104 | 0 | 4 | 0 | |
| Thomas44 | 2000 | 793 | 792 | 0 | 1 | 0 | |
| Thomas18 | 2009 | 60 | 59 | 0 | 1 | 0 | |
| Zahid19 | 2013 | 187 | 166 | 0 | 20 | 1 |
AVB, atrioventricular block
Table 2.
Distribution of first, second, and third degree AVB among studies with administration of regadenoson
| Author | Year | Total number of patients | Number of patients with AVB | 1st degree AVB | Mobitz type I 2nd degree AVB | Mobitz type II 2nd degree AVB | Complete heart block |
|---|---|---|---|---|---|---|---|
| Aljaroudi45 | 2010 | 411 | 410 | 0 | 0 | 1 | |
| Aljaroudi46 | 2013 | 514 | 514 | 0 | 0 | 0 | 0 |
| Aljaroudi47 | 2011 | 336 | 336 | 0 | 0 | 0 | 0 |
| Cerqueira5 | 2008 | 1,240 | 1,205 | 34 | 1 | 0 | 0 |
| Douky48 | 2013 | 300 | 293 | 0 | 7 | 0 | 0 |
| Douky49 | 2012 | 248 | 241 | 7 | 0 | 0 | 0 |
| Hendel50 | 2005 | 18 | 17 | 1 | 0 | 0 | 0 |
| Husain51 | 2012 | 1,370 | 1,370 | 0 | 0 | 0 | 0 |
| Kwon52 | 2010 | 1,263 | 1,260 | 3 | |||
| Palani53 | 2011 | 1,049 | 1,049 | 0 | 0 | 0 | 0 |
| Prenner54 | 2012 | 672 | 671 | 0 | 0 | 0 | 1 |
| Ross55 | 2013 | 75 | 75 | 0 | 0 | 0 | 0 |
| Thomas56 | 2008 | 49 | 49 | 0 | 0 | 0 | 0 |
| Thompson57 | 2013 | 531 | 531 | 0 | 0 | 0 | 0 |
| Zahid58 | 2013 | 209 | 208 | 1 | 0 | 0 | 0 |
AVB, atrioventricular block
Figure 2.
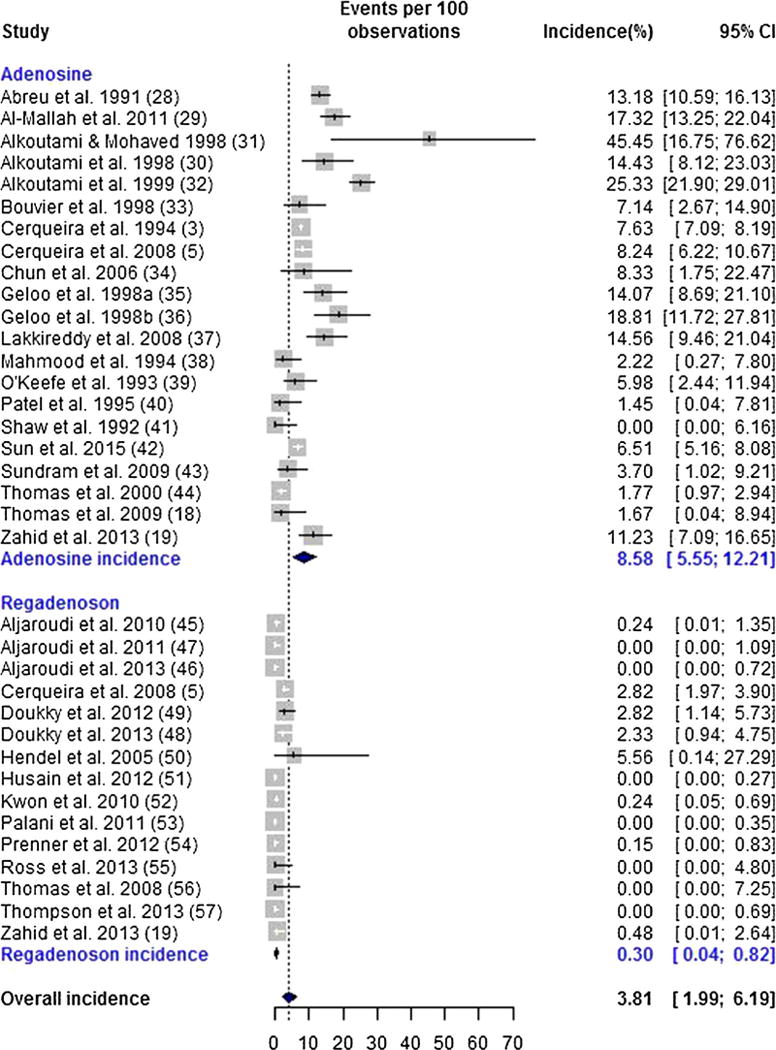
Forrest plot for overall atrioventricular block.
Incidence of High-Grade AVB
A total of 33 studies (19 adenosine, 14 regadenoson) reported sufficient information to calculate the incidence of high-grade AVB.3,5,18,19,28–32,34–43,45–51,53–57 High-grade AVB was observed in 754 of 20,817 (3.62%) patients. As with the incidence of overall AVB, there was evidence of significant heterogeneity in the incidences of high-grade AVB (Q = 1026.89, P < .001). The pooled random-effects estimate of the incidence of high-grade AVB was 1.93% (95% CI 0.77%–3.59%). The incidence rate of high-grade AVB in the adenosine group was significantly higher compared to the regadenoson group (5.21%; 95% CI 2.81%–8.30% vs 0.05%; 95% CI < 0.001%–0.19%, respectively, P < .001, OR 77.2; 95% CI 20.3–293.0). (Figure 3).
Figure 3.
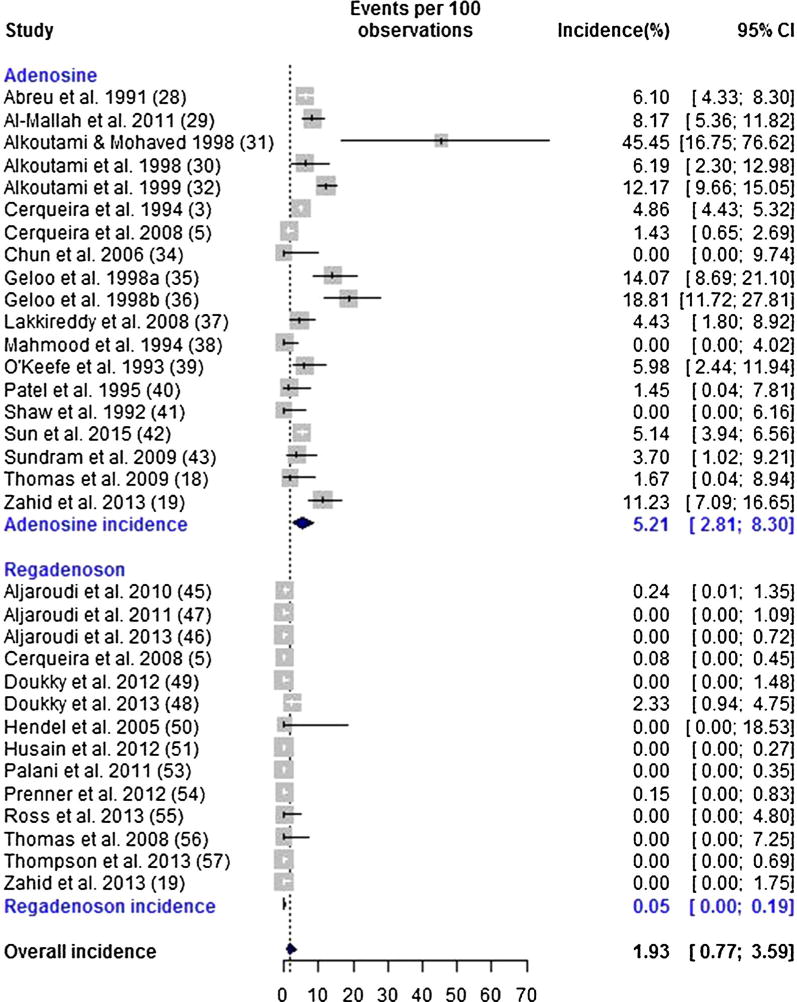
Forrest plot for high-grade atrioventricular block.
Association between Study-level Covariates and Incidence of Overall AVB
Meta-regression was used to assess whether patient age or prior history of diabetes was significantly associated with incidence of AVB. The age and diabetes history of individual patients were not available to us, so mean (or median) patient age and percentage of patients with diabetes were used as covariates in the meta-regression.
The mean patient age was available for 26 studies3,18,19,28,29,33,37–40,42–57 and median age was available for three studies.5,30,32 There was no significant association between mean (median) patient age and incidence of overall AVB (P > .2). The percentage of patients with diabetes was available for 20 studies.5,18,19,29,32,38,41,42,45–49,51–55,57 There was no significant association between percentage of patients with diabetes and incidence of AVB (P = .11).
Incidence of SA Node Dysfunction
None of the included studies using regadenoson reported the incidence of bradycardia, sinus pause or sinus arrest. In the adenosine group, there were 28 reported cases of bradycardia,3,29,38,43 one of which was specified as sinus bradycardia38 and 6 reported cases of sinus pause.29,39 Among patients receiving adenosine, there were 8 cases of transient sinoatrial block reported in the same study42 and one case of asystole reported by Alkoutami et al which was the study assessing the incidence of AVB in nonagenarian subjects.31 The above are summarized in Table 3.
Table 3.
Occurrence of SA node dysfunction among studies with administration of adenosine
| Side effect | Total number of patients | Number of patients affected |
|---|---|---|
| Bradycardia3,29,38,43 | 9,760 | 28 |
| Sinoatrial block42 | 1,168 | 8 |
| Sinus pause29,39 | 423 | 6 |
| Asystole31 | 11 | 1 |
SA, sinoatrial
Publication Bias
No evidence of publication bias was detected for the incidence of AVB using the Egger test (P > .2) (Figure 4).
Figure 4.
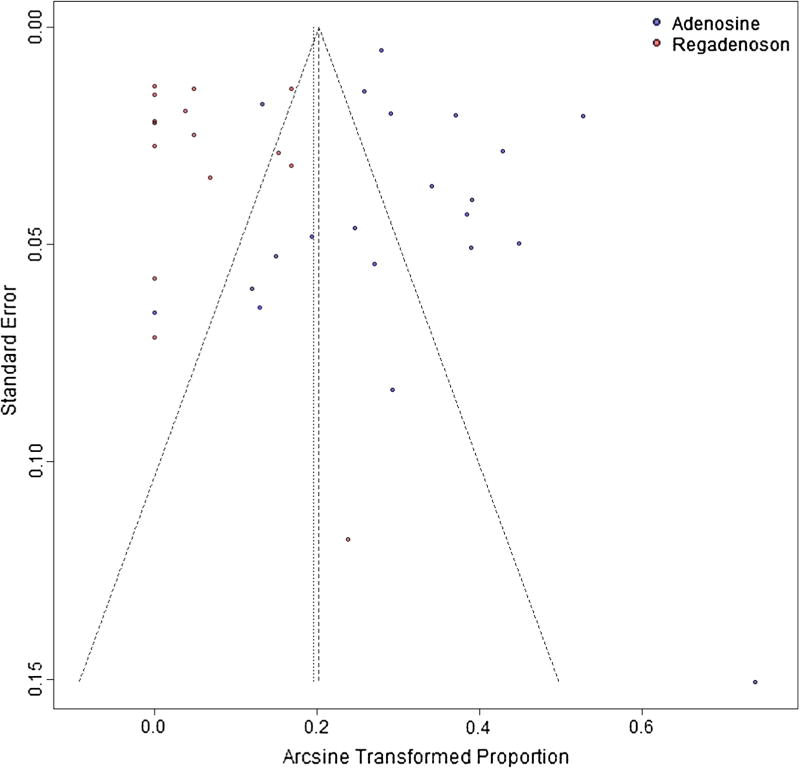
Funnel plot for publication bias.
DISCUSSION
Our meta-analysis is the first to systematically review the available literature and directly compare the rates of de novo AVB in patients undergoing pharmacologic SPECT-MPI with either adenosine or regadenoson. The rates of SA node dysfunction were also analyzed as a secondary endpoint. Based on our analysis, overall AVB is observed in approximately 4% (95% CI 2%–6%) of patients undergoing vasodilator stress SPECT-MPI using either adenosine or regadenoson. The incidence of overall AVB (first degree, second degree type I, second degree type II and third degree) was significantly higher in studies using adenosine compared to regadenoson (8.58%; 95% CI 5.55%–12.21% vs 0.30%; 95% CI 0.04%–0.82%, adenosine vs regadenoson, P < .001, OR 30.6 95% CI 11.0–85.3). The incidence of high-grade AVB—defined as second or third degree AVB—was seen in around 2% (95% CI 1–4%) of patients. Similarly to what was seen for overall AVB, the incidence of high-grade AVB was significantly higher in studies using adenosine compared to regadenoson (5.21%; 95% CI 2.81%–8.30% vs 0.05%; 95% CI < 0.001%–0.19% respectively, P < .001, OR 77.2; 95% CI 20.3–293.0). No cases of SA node dysfunction were reported in any of the studies using regadenoson, as opposed to the ones using adenosine, with the majority being cases of bradycardia3,29,38,43 and very few cases of transient sinoatrial block,42 sinus pause29,39 and only one case of asystole seen in the small study of nonagenarians.31 Neither mean (or median) patient age nor prior history of diabetes, examined as covariates, exhibited significant association with rates of de novo AVB.
AVB with Adenosine
Adenosine binds to all four adenosine receptor subtypes (A1, A2-alpha, A2-beta and A3) and by activating the A1 receptors, it mediates negative dromotropic, chronotropic, and inotropic as well as anti-adrenergic effects.58 The largest scale trial to examine the safety profile of adenosine was the ADENOSCAN trial which reported data on over 9000 patients.3 The overall rate of AVB in this study was 7.6% (N = 706 patients out of a total of 9256) which fell within the 95% CI of our meta-analysis for adenosine (5.6–12.2%). First degree AVB was seen in 36% of patients experiencing AVB, second degree AVB in 54% and complete heart block in 10% (or 0.78% of all patients included). The vast majority of episodes of AVB (72%) resolved spontaneously with no intervention needed.3 In 19% of cases, the dose of adenosine had to be decreased and in 8% of patients, the adenosine infusion had to be stopped due to AVB. Aminophylline was administered in 6 cases of AVB, however, even in these patients, the episode of AVB had already resolved spontaneously.3 As opposed to our meta-analysis, age was shown to be the only factor associated with an increased risk of AVB, with a cut-off of 70 years old correlating with a significant difference in the incidence of AVB (7.05% vs 9.44%, P < .001, age < 70 years and ≥ 70 years old respectively).3 No other clinical or ECG characteristics (including conduction abnormalities) or medications including beta blockers and calcium channel blockers, were found to correlate with increased incidence of AVB. Furthermore, the occurrence of AVB was not thought to be a manifestation of ischemia, since there was no correlation between AVB and ECG changes or perfusion defects on imaging. The authors underlined the transient nature of the effects of adenosine on AV node and also stressed the importance of avoiding fast bolus intravenous injections of adenosine, as such handling could lead to high blood levels and increase the risk for AVB.3
Since the ADENOSCAN trial, other smaller trials reported similar rates of AVB associated with administration of adenosine infusion for a stress SPECT-MPI study.5,28,32,42,59 The only study in our meta-analysis deviating from the afore-mentioned rates for AVB was the one by Alkoutami et al in 1998, which was a very small retrospective study of eleven nonagenarian patients.31 This study reported 4 cases of type II second degree AVB and one case of complete heart block. This high incidence (45%) of AVB could have been related to the advanced age of the patients and their predisposition and sensitivity for conduction disorders. Similarly to the ADENOSCAN trial, none of the cases of AVB were persistent.31
We observed significant heterogeneity in incidences of both overall and high-grade AVB. We used meta-regression analyses to explore two potential sources of this heterogeneity: age and diabetes history. However, these analyses were limited by the absence of patient-level data; as a result, we were only able to examine the relationship between mean participant age and proportion of subjects with diabetes in each trial and the estimated incidence of AVB. Furthermore these study-level covariates were only available for a subset of studies, reducing statistical power to detect any associations and possibly introducing selection bias.60
AVB with Regadenoson
Regadenoson, despite having a much higher selectivity for the A2a adenosine receptors, may still activate A1 receptors (13 times weaker affinity to A1 compared to A2a).2,61,62 The A1 receptors are located on the SA and AV nodes and such an activity mediated by regadenoson may result in sinus node dysfunction and AVB.61,62 This is the basis for the contraindication in use of regadenoson in patients with known underlying sinus node dysfunction and/or AVB undergoing stress SPECT-MPI without a functioning pacemaker.1 The ADVANCE MPI multi-center trial established the non-inferiority and safety of regadenoson compared to adenosine.4,5 Overall AVB was seen in 2.8% of the patients in the ADVANCE MPI trial which is higher compared to the incidence in our meta-analysis (0.04%–0.82%) but the incidence of high-grade AV block in that study was 0.08% which was consistent with the pooled estimate from our analysis (< 0.001%–0.19%).5 Interestingly, even the one patient that developed high-grade AVB with regadenoson in ADVANCE MPI had Wenckebach block. The absence of type 2s degree or third degree AVB in this large trial may be attributed to selection of patients, since those with known history of high-grade AVB or who developed high-grade AVB following baseline assessment with adenosine were excluded from the study and did not subsequently receive regadenoson.4,5
In contrast to the results of ADVANCE MPI, there have been isolated case reports and small case series of patients undergoing regadenoson-stress SPECT-MPI, who went on to develop high-grade AVB or sinus arrest sometimes even leading to asystole.8–13 As of 2016, there were 47 cases of complete heart block and 25 cases of sinus arrest related to regadenoson use that were reported to the FDA via its adverse event reporting system (FAERS).16 Of the total of eight so far reported cases, two were cases of third degree AVB8,11 one of which led to transient loss of consciousness11 and the rest were cases of transient asystole sometimes preceded by sinus arrest12,13 or AVB.9,10 In five patients, cardiopulmonary resuscitation needed to be performed.10,12,13 None of the patients had any associated neurologic and/or cardiovascular sequelae. All patients had normal LV systolic function on their SPECT studies and only one case was associated with subtle perfusion defect.10 These case reports did not meet the inclusion criteria of our meta-analysis, and thus their findings are not reflected in our results of regadenoson-related AVB. There is, however, a very valid point to be made: there is a real (albeit small) risk of high-grade AVB and SA node dysfunction with regadenoson in everyday clinical practice. Clinicians should be aware of this risk and develop plans for appropriate interventions when it occurs.
The mechanism underlying SA and AV node suppression which might be induced following administration of regadenoson is not clear. In some cases, episodes were preceded by nausea and vomiting and this has led to the hypothesis that an exaggerated vasovagal reaction (Bezold-Jarisch reflex) along with A2a receptor-mediated vagal stimulation in the area postraema (found on the floor of the fourth ventricle and linked with nausea and vomiting) might be the underlying mechanism.63–65 This can be particularly risky in certain susceptible individuals, namely those unlikely to be able to tolerate hypotension, such as the elderly, significant left ventricular outflow obstruction, severe ischemic left ventricular dysfunction or prior history of stroke. Another plausible explanation could be the exaggerated autonomic response mediated by activation of A2a receptors in the sympathetic afferent nerves which may in turn result in an augmented sympathetic activity and reflex vagal stimulation.64,65 Finally, the production of endogenous adenosine following regadenoson administration might also account for some of the cases of regadenoson-related high-grade AVB and asystole. Patients with severe coronary disease or significant collateral circulation are at risk for experiencing myocardial ischemia with regadenoson which might be due to either coronary steal or a sudden decrease in systemic blood pressure. Ischemia may lead to increased production of adenosine, which may activate the A1 receptors in the SA and AV nodes and eventually suppress conduction.15
LIMITATIONS
Our study is the first to systematically review the incidence of de novo AVB associated with use of adenosine and regadenoson at dosages typically administered during SPECT-MPI studies. There are, however, certain limitations. First, there was a significant heterogeneity observed when assessing the occurrence of both overall as well as high-grade AVB. This is stemming from the diverse clinical and methodological characteristics of the studies included, with a typical example of the retrospective study by Alkoutami et al in 1998, assessing the rate of AVB in a small cohort of nonagenarians.18 In this small study, the incidence of high-grade AVB was significantly higher compared to the rest (45% vs 3.48%). Another example is the fact that Lakkireddy et al in their study used adenosine at a dose of 56 mcg/kg instead of the traditionally used 140 mcg/kg/min.24
Significant heterogeneity was also noted with respect to the way studies reported on second and third degree AVB. The majority did not specify the type of second degree AVB3,5,16,17,19,29–33 while some reported collectively on second and third degree AVB,22,23 first and second degree AVB,20 or all AVB41 (see Table 3). This hindered our ability to reliably distinguish between type I and II second degree AVB. As a result, our definition of high-grade AVB encompassed third degree and any case of second degree AVB.
Another limitation is related to the design of two the studies that were included in both the adenosine as well as the regadenoson arms of our meta-analysis.5,33 The Integrated ADVANCE MPI trial5 and the study by Zahid et al33 included patients who underwent pharmacologic stress SPECT-MPI using both vasodilators, however, since these were administered to different groups of patients, they were treated as two single-arm studies. It should be stressed though that this methodological approach does not take into account any non-independence between the two single-arm studies, which may exist due to the fact that they were collected under the same protocol by the same investigative team at the same time. Nevertheless, we do not expect this non-independence to significantly impact our results and therefore elected to include these studies.
The incidence of de novo high-grade AVB observed with administration of regadenoson was trivial and there were no manifestations of SA node dysfunction. In contradistinction, as afore-mentioned, there has been a notable number of cases of complete heart block as well as sinus arrest and asystole reported in the literature (as isolated case reports or small case series) and in the FAERS.51–57 This highlights the difference in risk observed between real life practice and research reports and stresses the importance of thorough pre-test patient assessment and careful selection. From this perspective, our results may not reflect everyday clinical practice and may underestimate true risk of high-grade AVB and SA node dysfunction.
CONCLUSION
In conclusion, the incidence of overall AVB reported in the literature of patients undergoing vasodilator stress SPECT-MPI was estimated from our meta-analysis at 3.81% (95% CI 1.99%–6.19%) and the incidence of high-grade AVB at 1.93% (95% CI 0.77%–3.59%). Both overall and high-grade AVB were significantly more frequent with administration of adenosine compared to regadenoson. Based on the results of this meta-analysis and taking into account the case reports and case series reporting episodes of SA and AV node suppression—sometimes so severe as to require cardiopulmonary resuscitation—we recommend adopting an approach of individualized assessment of patients prior to administration of either adenosine or regadenoson. This will minimize risk of development of adverse conduction system effects.
NEW KNOWLEDGE GAINED
The risk of AVB with vasodilator stress SPECT-MPI, albeit infrequent, does exist and is higher with adenosine compared to the selective A2a agonist, regadenoson. Nevertheless, high-grade AVB does occur with regadenoson. Each patient’s history and risk factors should be closely examined prior to administration of pharmacologic stress agents to reduce risk of SA and AV node suppression.
Supplementary Material
Acknowledgments
Dr. Hage reports grant support from Astellas Pharma.
Abbreviations
- SPECT
Single photon emission computed tomography myocardial perfusion imaging
- MPI
Myocardial perfusion imaging/images
- AVB
Atrioventricular block
- SA
Sinoatrial
- CAD
Coronary artery disease
- HTN
Hypertension
- HLD
Hyperlipidemia
- CKD
Chronic kidney disease
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12350-017-1081-y) contains supplementary material, which is available to authorized users.
All editorial decisions for this article, including selection of reviewers and the final decision, were made by guest editor Nagara Tamaki, MD.
Disclosures
No other disclosures from the rest of the authors.
References
- 1.Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63:380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol. 2016;23:606–39. doi: 10.1007/s12350-015-0387-x. [DOI] [PubMed] [Google Scholar]
- 3.Cerqueira MD, Verani MS, Schwaiger M, Heo J, Iskandrian AS. Safety profile of adenosine stress perfusion imaging: Results from the adenoscan multicentertrial registry. J Am Coll Cardiol. 1994;23:384–9. doi: 10.1016/0735-1097(94)90424-3. [DOI] [PubMed] [Google Scholar]
- 4.Iskandrian AE, Bateman TM, Belardinelli L, Blackburn B, Cerqueira MD, Hendel RC, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: Results of the ADVANCE phase 3 multicenter international trials. J Nucl Cardiol. 2007;14:645–58. doi: 10.1016/j.nuclcard.2007.06.114. [DOI] [PubMed] [Google Scholar]
- 5.Cerqueira MD, Nguyen P, Staehr P, Underwood SR, Iskandrian AE, On behalf of the ADVANCE-MPI Trial Investigators Effects of age, gender, obesity, and diabetes on the efficacy and safety of the selective A2A agonist regadenoson versus adenosine in myocardial perfusion imaging: Integrated ADVANCE-MPI trial results. J Am Coll Cardiol Imaging. 2008;1:307–16. doi: 10.1016/j.jcmg.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Ghimire G, Hage FG, Heo J, Iskandrian AE. Regadenoson: A focused update. J Nuc Cardiol. 2013;20:284–8. doi: 10.1007/s12350-012-9661-3. [DOI] [PubMed] [Google Scholar]
- 7.Zoghbi GJ, Iskandrian AE. Selective adenosine agonists and myocardial perfusion imaging. J Nuc Cardiol. 2012;19:126–41. doi: 10.1007/s12350-011-9474-9. [DOI] [PubMed] [Google Scholar]
- 8.Pandit A, Unzek Freiman S. Complete heart block associated with regadenoson: A real side effect. J Nucl Cardiol. 2012;19:1236–9. doi: 10.1007/s12350-012-9610-1. [DOI] [PubMed] [Google Scholar]
- 9.Kureshi F, Abdallah MS, Bateman TM. Regadenoson-induced complete heart block and asystole: A real possibility nuclear laboratories should be aware of. J Nucl Cardiol. 2016 doi: 10.1007/s12350-016-0755-1. [DOI] [PubMed] [Google Scholar]
- 10.Rosenblatt J, Mooney D, Dunn T, Cohen M. Asystole following regadenoson infusion in stable outpatients. J Nuclear Cardiol. 2014;21:862–8. doi: 10.1007/s12350-014-9898-0. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal V, DePuey EG. Advanced heart block and unresponsiveness after regadenoson administration during myocardial SPECT study. Int J Cardiol. 2014;176:e49–51. doi: 10.1016/j.ijcard.2014.07.058. [DOI] [PubMed] [Google Scholar]
- 12.Grady EC, Barron JT, Wagner RH. Development of asystole requiring cardiac resuscitation after the administration of regadenoson in a patient with pulmonary fibrosis receiving n-acetylcysteine. J Nuclear Cardiol. 2011;18:521–5. doi: 10.1007/s12350-011-9373-0. [DOI] [PubMed] [Google Scholar]
- 13.Brinkert M, Reyes E, Walker S, Latus K, Maenhout A, Mizumoto R, et al. Regadenoson in Europe: First-year experience of regadenoson stress combined with submaximal exercise in patients undergoing myocardial perfusion scintigraphy. Eur J Nuclear Med Mol Imaging. 2014;41:511–21. doi: 10.1007/s00259-013-2619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Underwood SR, Latus KA, Reyes E, Standbridge K, Walker S, Wechalekar K. Regadenoson-induced bradycardia and hypotension: Possible mechanism and antidote. J Nucl Cardiol. 2014;21:1040. doi: 10.1007/s12350-014-9968-3. [DOI] [PubMed] [Google Scholar]
- 15.Hage FG. Regadenoson for myocardial perfusion imaging: Is it safe? J Nucl Cardiol. 2014;21:871–6. doi: 10.1007/s12350-014-9922-4. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Food and Drug Administration. Open FDA. 2016 https://openfda.shinyapps.io/dash/
- 17.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 18.Thomas GS, Thompson RC, Miyamoto MI, Ip TK, Rice DL, Milikien D, et al. The RegEx trial: A randomized, double-blind, placebo- and active-controlled pilot study combining regadenoson, a selective A(2A) adenosine agonist, with low-level exercise, in patients undergoing myocardial perfusion imaging. J Nucl Cardiol. 2009;16:63–72. doi: 10.1007/s12350-008-9001-9. [DOI] [PubMed] [Google Scholar]
- 19.Zahid M, Kapila A, Eagan CE, Yusko DA, Miller ED, Missenda CD. Prevalence and significance of electrocardiographic changes and side effect profile of regadenoson compared with adenosine during myocardial perfusion imaging. J Cardiovasc Dis Res. 2013;4:7–10. doi: 10.1016/j.jcdr.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: Strengths and weaknesses. FASEB J. 2008;22:338–42. doi: 10.1096/fj.07-9492LSF. [DOI] [PubMed] [Google Scholar]
- 21.Ottawa Hospital Research Institute. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (last accessed November 20th 2016)
- 22.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, et al. ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2012;61:e6–75. doi: 10.1016/j.jacc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Schwarzer G. Meta-Analysis with R. R package version 2.3.0. 2013 http://CRAN.R-project.org/package=meta.
- 24.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft. 2010;36:1–48. [Google Scholar]
- 25.Andreano A, Rebora P, Valsecchi MG. Measures of single arm outcome in meta-analyses of rare events in the presence of competing risks. Biom J. 2015;57:649–60. doi: 10.1002/bimj.201400119. [DOI] [PubMed] [Google Scholar]
- 26.Rücker G, Schwarzer G, Carpenter J, Olkin I. Why add anything to nothing? The arcsine difference as a measure of treatment effect in meta-analysis with zero cells. Stat Med. 2009;28:721–38. doi: 10.1002/sim.3511. [DOI] [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abreu A, Mahmarian JJ, Nishimura S, Boyce TM, Verani MS. Tolerance and safety of pharmacologic coronary vasodilation with adenosine in association with thallium-201 scintigraphy in patients with suspected coronary artery disease. J Am Coll Cardiol. 1991;18:730–5. doi: 10.1016/0735-1097(91)90796-c. [DOI] [PubMed] [Google Scholar]
- 29.Al-Mallah MH, Arida M, Garcia-Sayan E, Assal C, Zegarra GT, Czerska B, et al. Safety of adenosine pharmacologic stress myocardial perfusion imaging in orthotopic cardiac transplant recipients: A single center experience of 102 transplant patients. Int J Cardiovasc Imaging. 2011;27:1105–11. doi: 10.1007/s10554-010-9749-2. [DOI] [PubMed] [Google Scholar]
- 30.Alkoutami G, Reddy J, Movahed A. The incidence of atrioventricular block during adenosine pharmacologic stress testing in patients with left bundle branch block. Chest. 1998;114:S311. [Google Scholar]
- 31.Alkoutami G, Mohaved A. The safety of dipyridamole and adenosine pharmacologic stress testing in nonagenarian population. Chest. 1998;114:338S. [Google Scholar]
- 32.Alkoutami GS, Reeves WC, Movahed A. The safety of adenosine pharmacologic stress testing in patients with first-degree atrioventricular block in the presence and absence of atrioventricular blocking medications. J Nucl Cardiol. 1999;6:495–7. doi: 10.1016/s1071-3581(99)90021-1. [DOI] [PubMed] [Google Scholar]
- 33.Bouvier F, Höjer J, Hulting J, Ruiz H, Samad B, Jensen-Urstad M. Myocardial perfusion scintigraphy (SPECT) during adenosine stress can be performed safely early on after thrombolytic therapy in acute myocardial infarction. Clin Physiol. 1998;18:97–101. doi: 10.1046/j.1365-2281.1998.00079.x. [DOI] [PubMed] [Google Scholar]
- 34.Chun KA, Lee J, Lee SW, Ahn BC, Ha JH, Cho IH, et al. Direct comparison of adenosine and adenosine 5′-triphosphate as pharmacologic stress agents in conjunction with Tl-201 SPECT: Hemodynamic response, myocardial tracer uptake, and size of perfusion defects in the same subjects. J Nucl Cardiol. 2006;13:621–8. doi: 10.1016/j.nuclcard.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Geloo N, Alkoutami G, Mohaved A. The frequency of atrioventricular block during adenosine pharmacologic stress testing in patients with resting sinus tachycardia. Chest. 1998;114:317S. [Google Scholar]
- 36.Geloo N, Alkoutami G, Mohaved A. The frequency of atrioventricular block during adenosine pharmacologic stress testing in patients with resting sinus bradycardia. Chest. 1998;114:317S. [Google Scholar]
- 37.Lakkireddy D, Aronow WS, Bateman T, McGhee I, Nair C, Khan IA. Does beta blocker therapy affect the diagnostic accuracy of adenosine single-photon-emissioncomputed tomographic myocardial perfusion imaging? Am J Ther. 2008;15:19–23. doi: 10.1097/MJT.0b013e31804c71a7. [DOI] [PubMed] [Google Scholar]
- 38.Mahmood S, Gupta NK, Gunning M, Bomanji JB, Jarritt PH, Ell PJ. 201Tl myocardial perfusion SPET: Adenosine alone or combined with dynamic exercise. Nucl Med Commun. 1994;15:586–92. doi: 10.1097/00006231-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 39.O’Keefe JH, Jr, Bateman TM, Barnhart CS. Adenosine thallium 201 is superior to exercise thallium-201 for detecting coronary artery disease in patients with left bundle branch block. J Am Coll Cardiol. 1993;21:1332–8. doi: 10.1016/0735-1097(93)90305-k. [DOI] [PubMed] [Google Scholar]
- 40.Patel R, Bushnell DL, Wagner R, Stumbris R. Frequency of false-positive septal defects on adenosine/201T1 images in patients with left bundle branch block. Nucl Med Commun. 1995;16:137–9. [PubMed] [Google Scholar]
- 41.Shaw L, Miller DD, Kong BA, Hilton T, Stelken A, Stocke K, et al. Determination of perioperative cardiac risk by adenosine thallium-201 myocardial imaging. Am Heart J. 1992;124:861–9. doi: 10.1016/0002-8703(92)90965-x. [DOI] [PubMed] [Google Scholar]
- 42.Sun H, Tian Y, Zheng L, Pan Q, Xie B. Electrocardiographic profile of adenosine pharmacological stress testing. Exp Ther Med. 2015;9:1178–84. doi: 10.3892/etm.2015.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sundram F, Notghi A, Smith NB. Pharmacological stress myocardial perfusion scintigraphy: Use of a modified adenosine protocol in patients with asthma. Nucl Med Commun. 2009;30:217–25. doi: 10.1097/MNM.0b013e32831ec568. [DOI] [PubMed] [Google Scholar]
- 44.Thomas GS, Prill NV, Majmundar H, Fabrizi RR, Thomas JJ, Hayashida C, et al. Treadmill exercise during adenosine infusion is safe, results in fewer adverse reactions, and improve myocardial perfusion image quality. J Nucl Cardiol. 2000;7:439–46. doi: 10.1067/mnc.2000.108030. [DOI] [PubMed] [Google Scholar]
- 45.Aljaroudi W, Hermann D, Hage F, Heo J, Iskandrian AE. Safety of regadenoson in patients with end-stage renal disease. Am J Cardiol. 2010;105:133–5. doi: 10.1016/j.amjcard.2009.08.663. [DOI] [PubMed] [Google Scholar]
- 46.AlJaroudi WA, Alraies MC, Cerquiera MD, Jaber WA. Safety and tolerability of regadenoson in 514 SPECT MPI patients with and without coronary artery disease and submaximal exercise heart rate response. Eur J Nucl Med Mol Imaging. 2013;40:341–8. doi: 10.1007/s00259-012-2296-4. [DOI] [PubMed] [Google Scholar]
- 47.Aljaroudi W, Iqbal F, Koneru J, Bhambhvani P, Heo J, Iskandrian AE. Safety of regadenoson in patients with end-stage liver disease. J Nucl Cardiol. 2011;18:90–5. doi: 10.1007/s12350-010-9288-1. [DOI] [PubMed] [Google Scholar]
- 48.Doukky R, Rangel MO, Dick R, Wassouf M, Alqaid A, Margeta B. Attenuation of the side effect profile of regadenoson: A randomized double-blind placebo-controlled study with aminophylline in patients undergoing myocardial perfusion imaging and have severe chronic kidney disease—the ASSUAGE-CKD trial. Int J Cardiovasc Imaging. 2013;29:1029–37. doi: 10.1007/s10554-012-0166-6. [DOI] [PubMed] [Google Scholar]
- 49.Doukky R, Morales Demori R, Jain S, Kiriakos R, Mwansa V, Calvin JE. Attenuation of the side effect profile of regadenoson: A randomized double-blinded placebo-controlled study with aminophylline in patients undergoing myocardial perfusion imaging. “The ASSUAGE trial”. J Nucl Cardiol. 2012;19:448–57. doi: 10.1007/s12350-012-9533-x. [DOI] [PubMed] [Google Scholar]
- 50.Hendel RC, Bateman TM, Cerqueira MD, Iskandrian AE, Leppo JA, Blackburn B, et al. Initial clinical experience with regadenoson, a novel selective A2A agonist for pharmacologic stress single-photon emission computed tomography myocardial perfusion imaging. J Am Coll Cardiol. 2005;46:2069–75. doi: 10.1016/j.jacc.2005.05.097. [DOI] [PubMed] [Google Scholar]
- 51.Husain Z, Palani G, Cabrera R, Karthikeyan AS, Dhanalakota S, Pathmanathan S, et al. Hemodynamic response, arrhythmic risk, and overall safety of regadenoson as a pharmacologic stress agent for myocardial perfusion imaging in chronic obstructive pulmonary disease and bronchial asthma patients. Int J Cardiovasc Imaging. 2012;28:1841–9. doi: 10.1007/s10554-011-0003-3. [DOI] [PubMed] [Google Scholar]
- 52.Kwon DH, Cerqueira MD, Young R, Houghtaling P, Lieber E, Menon V, et al. Lessons from regadenoson and low level treadmill/regadenoson myocardial perfusion imaging initial clinical experience in 1263 patients. J Nucl Cardiol. 2010;17:853–7. doi: 10.1007/s12350-010-9229-z. [DOI] [PubMed] [Google Scholar]
- 53.Palani G, Husain Z, Salinas RC, Karthikeyan V, Karthikeyan AS, Ananthasubramaniam K. Safety of regadenoson as a pharmacologic stress agent for myocardial perfusion imaging in chronic kidney disease patients not on hemodialysis. J Nucl Cardiol. 2011;18:605–11. doi: 10.1007/s12350-011-9378-8. [DOI] [PubMed] [Google Scholar]
- 54.Prenner BM, Bukofzer S, Behm S, Feaheny K, McNutt BE. A randomized, double-blind, placebo controlled study assessing the safety and tolerability regadenoson in subjects with asthma chronic obstructive pulmonary disease. J Nucl Cardiol. 2012;19:681–92. doi: 10.1007/s12350-012-9547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ross MI, Wu E, Wilkins JT, Gupta D, Shen S, Aulwes D, et al. Safety and feasibility of adjunctive regadenoson injection at peak exercise during exercise myocardial perfusion imaging: The Both Exercise and Regadenoson Stress Test (BERST) trial. J Nucl Cardiol. 2013;20:197–204. doi: 10.1007/s12350-013-9679-1. [DOI] [PubMed] [Google Scholar]
- 56.Thomas GS, Tammelin BR, Schiffman GL, Marquez R, Rice DL, Milikien D, et al. Safety of regadenoson, a selective adenosine A2A agonist, in patients with chronic obstructive pulmonary disease: A randomized, double-blind, placebo-controlled trial (RegCOPD trial) J Nucl Cardiol. 2008;15:319–28. doi: 10.1016/j.nuclcard.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Thompson RC, Patil H, Thompson EC, Thomas GS, Al-Amoodi M, Kennedy KF, et al. Regadenoson pharmacologic stress for myocardial perfusion imaging: A three-way comparison between regadenoson administered at peak exercise, during walk recovery, or no-exercise. J Nucl Cardiol. 2013;20:214–21. doi: 10.1007/s12350-012-9660-4. [DOI] [PubMed] [Google Scholar]
- 58.Dhalla AK, Shryock JC, Shreeniwas R, Belardinelli L. Pharmacology and therapeutic applications of A1 adenosine receptor ligands. Curr Top Med Chem. 2003;3:369–85. doi: 10.2174/1568026033392246. [DOI] [PubMed] [Google Scholar]
- 59.Alkoutami GS, Reeves WC, Movahed A. The frequency of atrioventricular block during adenosine stress testing in young, middle-aged, young-old, and old-old adults. Am J Geriatr Cardiol. 2001;10:159–61. doi: 10.1111/j.1076-7460.2001.00004.x. [DOI] [PubMed] [Google Scholar]
- 60.Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 61.Garnock-Jones KP, Curran MP. Regadenoson. Am J Cardiovasc Drugs. 2012;10:65–71. doi: 10.2165/10489040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 62.Dhalla AK, Wong MY, Wang WQ, Biaggioni I, Belardinelli L. Tachycardia caused by A2A adenosine receptor agonists is mediated by direct sympathoexcitation in awake rats. J Pharmacol Exp Ther. 2006;316:695–702. doi: 10.1124/jpet.105.095323. [DOI] [PubMed] [Google Scholar]
- 63.Minic Z, O’Leary DS, Scislo TJ. Nucleus tractus solitarii A(2a) adenosine receptors inhibit cardiopulmonary chemoreflex control of sympathetic outputs. Auton Neurosci. 2014;180:32–42. doi: 10.1016/j.autneu.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang MJ, Park MS, Shin IC, Koh HC. Modification of cardiovascular response of posterior hypothalamic adenosine A(2) receptor stimulation by adenylate cylase, guanylate cyclase and by K(ATP) channel blockade in anesthetized rats. Neurosci Lett. 2003;344:57–61. doi: 10.1016/s0304-3940(03)00402-6. [DOI] [PubMed] [Google Scholar]
- 65.Palmer ED. The abnormal upper gastrointestinal vasovagal reflexes that affect the heart. Am J Gastroenterol. 1976;66:29. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


