Abstract
Characterization of the effects of long-term alcohol consumption on the brain would be aided by the development of behavioral assays that are relatively easy to implement in animal models of alcohol use disorders. Assessing unconditioned behaviors such as drug-elicited yawning in models that permit long-term alcohol ingestion may be a valuable complement to more invasive and costly procedures. The present studies investigated previous unexpected findings of ethanol-induced yawning in nonhuman primates. Subjects were adult male rhesus monkeys (n=8), all of which had experience self-administering intravenous cocaine for several years. One group also had experience consuming 2.0 g/kg ethanol over one hour per day, 5 days per week, for 6.8–12.0 months. All monkeys received saline or ethanol (0.25–1.0 g/kg) infused intravenously over 10 minutes and the number of yawns elicited during the infusion was counted. A second experiment in the ethanol-experienced monkeys examined whether ethanol-induced yawning could be blocked by PG01037 (1.0, 3.0 mg/kg, i.v.), a selective antagonist at dopamine D3 receptors (D3R). Ethanol significantly and dose-dependently increased yawns in the ethanol-experienced, but not ethanol-naïve animals. In the ethanol-experienced monkeys, this effect of ethanol was blocked by the D3R antagonist. The pharmacology of yawning is complex and a good deal of model development remains to be performed to characterize the potential involvement of other neurotransmitter systems. Nonetheless, drug-elicited yawning may be a useful unconditioned behavioral assay to assess the effects of long-term alcohol consumption in established nonhuman primate models.
Keywords: alcohol, animal model, ethanol, nonhuman primates, yawning
Introduction
Excessive alcohol drinking persists as a major public health problem, resulting in ~80,000 deaths and costing >$223 billion per year in the US alone (Kanny et al. 2013). Considering the complex nature of alcoholism and many neurotransmitter systems involved, it is unsurprising that existing pharmacotherapies are not effective in all treatment seekers (Miller et al. 2011). Development of new medications is hindered by an incomplete understanding of brain changes produced by chronic alcohol consumption. To overcome this obstacle, characterizing the effects of long-term alcohol consumption is critical, as the development of addiction is not an acute process. Rather, in vulnerable individuals, initial experimentation sets into motion a cascade of brain changes, eventually leading to neuroadaptative and neurodegenerative processes that facilitate the behavioral changes that lead to addiction (e.g., Koob & Le Moal, 1997; Crews 1999). Moreover, it is only after long-term drug use that behavioral, legal and health problems are at their worst; individuals presenting for treatment have typically accumulated years of alcohol exposure. The importance of identifying basic mechanisms of alcohol’s action and alcohol-related pathology is emphasized as Goal 1 in NIAAA’s Draft Strategic Plan (https://www.niaaa.nih.gov/about-niaaa/our-work/strategic-plan).
There are several obstacles to addressing these questions in alcoholics, including uncertainty about drinking history (duration, pattern and total drug exposure), polysubstance abuse, comorbid psychiatric disorders and other difficulties inherent in research with human subjects. Studies in laboratory animal models can provide experimental control to obviate these confounds. To generate the most clinically meaningful information, animal models should closely recapitulate clinical phenomena including durations of drug exposure. Although much information can be gained about the neuropharmacology of ethanol in rodents, the ability to generate clinically relevant phenotypes related to long-term drinking is limited. Nonhuman primates (NHP) are the animal model most similar to humans in terms of genetics, neuroanatomy, neurochemistry and pharmacokinetics (Weerts et al. 2007; Phillips et al. 2014). In the present context, a critical advantage of NHP is that studies can be conducted in the same subjects over many years. Thus, NHP models are highly translational with respect to the neurobiological consequences of long-term alcohol use (e.g. Grant et al., 2008; Chen et al., 2011; Barr, 2013).
Spontaneous yawning is an unconditioned behavior that occurs in many species including rats, monkeys and humans. During recent studies of ethanol/cocaine interactions in rhesus monkeys (Czoty, 2015, 2016), yawning produced by the dopamine D3 receptor (D3R) agonist quinpirole was enhanced after monkeys consumed ethanol for 8 weeks under binge-like conditions. Moreover, we observed that monkeys yawned during intravenous ethanol infusions. Although ethanol-induced yawning has been reported previously in rodents in the context of ethanol withdrawal, particularly in rats exposed to ethanol in utero (e.g. Brus et al., 1995; Sobrian et al., 2005), these observations in adult monkeys were unexpected. The present studies systematically assessed the ability of ethanol to increase yawning and further characterized the involvement of D3R, a substrate in which there is interest as a potential target for pharmacotherapies for alcohol use disorder (Heidbreder and Newman, 2010). The effect of ethanol (0.25–1.0 g/kg administered as an i.v. infusion over 10 minutes) on yawning was assessed in two groups of monkeys. One group, composed of monkeys that was serving as subjects in a cocaine/ethanol polysubstance abuse research program (Czoty, 2015, 2016), had a history of both ethanol drinking and i.v. cocaine self-administration, while a second group only had experience self-administering cocaine. In the former group, we also assessed whether ethanol-induced yawning could be blocked PG01037, a D3R antagonist with high affinity (Ki=0.7 nM) and high selectivity for D3R (>130-fold selective vs. D2 and >530-fold selective vs. D4 receptors and >65-fold selective at serotonin receptors; Grundt et al., 2005, 2007).
Materials and Methods
Subjects
Eight adult male rhesus monkeys (Macaca mulatta) with chronically indwelling intravenous catheters and subcutaneous vascular access ports served as subjects. All had between three and six years experience self-administering cocaine, typically 5 days per week, which continued for the duration of these studies (Table 1). Four monkeys (R-1758, R-1605, R-1606, R-1607) also had experience drinking ethanol in the home cage as described previously (Czoty, 2015). Briefly, these animals drank 2.0 g/kg ethanol (in a solution of 4% ethanol and 6% Tang in water) which was provided in a 500-ml bottle attached to each monkey’s cage for one hour per day, five days per week. The duration of exposure to this drinking regimen ranged from 146 to 252 sessions, with lifetime ethanol intake ranging from 244.9 to 383.4 g/kg (Table 1). Time since the end of this regimen ranged from two weeks to 7.6 months; no ethanol was consumed since that time, including the duration of this study. The other four subjects (R-1661, R-1688, R-1689, R-1691) were ethanol-naïve. Monkeys were individually housed in stainless steel cages and fed enough food (Purina LabDiet 5045, fresh fruit and vegetables) to maintain healthy weights as determined by daily observation and periodic veterinary examinations. Water was available ad libitum in the home cage. All procedures were approved by the Wake Forest University Animal Care and Use Committee and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Table 1.
Pharmacological history of subjects
| Monkeys with cocaine and ethanol experience | |||||
|---|---|---|---|---|---|
| Cocaine duration (months) |
Cocaine intake (mg/kg) |
Ethanol sessions (#, months) |
Ethanol intake (g/kg) |
Time since last ethanol (months) |
|
| R-1605 | 68.3 | 441.4 | 146, 6.8 | 244.9 | 6.2 |
| R-1606 | 69.0 | 397.0 | 161, 7.2 | 298.3 | 7.6 |
| R-1607 | 69.0 | 528.6 | 252, 12.0 | 352.7 | 3.0 |
| R-1758 | 36.8 | 259.4 | 205, 10.0 | 383.4 | 0.5 |
| Monkeys with only cocaine experience | ||
|---|---|---|
| Cocaine duration (months) |
Cocaine intake (mg/kg) |
|
| R-1692 | 56.5 | 1092.7 |
| R-1689 | 57.9 | 1128.5 |
| R-1688 | 57.9 | 1381.9 |
| R-1661 | 63.9 | 669.15 |
Yawning experiments
On the day of an experiment, monkeys were seated in a primate chair (Primate Products, Redwood City, CA), and transferred to a familiar procedure room that contained a camera for video recording. White noise was used to mask extraneous sounds. A 30- or 60-ml syringe was filled with ethanol (4% in water) in a volume calculated to deliver a specific dose (0.25, 0.5 or 1.0 g/kg) and placed in an infusion pump (Cole-Parmer Instrument Co., Vernon Hills, IL). The pump was connected to the monkey’s catheter via tubing and a 20-gauge Huber Point Needle (Access Technologies, Skokie, IL). Ethanol solution was infused over 10 minutes, during which time the monkey was recorded. Videos were subsequently viewed and yawns were counted by two observers; typically one observer was blinded to the treatment conditions.. A yawn is readily identifiable as a full extension of the jaws, withdrawal of lips, and exposure of teeth (Code and Tang, 1991); thus, minimal training of observers is necessary. Each observer recorded the time each yawn was noted, so that discrepancies be readily identified. In most cases, the two observers were in agreement. When a discrepancy occurred (i.e., the total yawn count differed by 1 between observers), the time of the discrepancy was identified from written records, the video was advanced to the specific time point and a third observer, blinded to the treatment the monkey received, made a final determination as to whether a yawn occurred.
In a second experiment conducted in the four ethanol-experienced monkeys, an intravenous administration of saline or the D3R antagonist PG01037 (1.0 and 3.0 mg/kg) was administered, followed 10 minutes later by an infusion of 1.0 g/kg ethanol. Yawns occurring in the 10 minutes after the saline or PG01037 injection and in the 10 minutes following the subsequent ethanol injection were counted. Higher doses of PG01037 were not tested because higher doses have been shown to disrupt food-maintained responding in macaques (Czoty and Nader, 2015; John et al., 2015b). For both groups of monkeys, the most recent cocaine self-administration session occurred within one week prior to any yawning experiment, but no cocaine was self-administered on the day of a yawning experiment.
Blood ethanol concentration determination
For each monkey, one blood sample (50 microliters) was collected from the saphenous vein 30 minutes after the end of an infusion of 0.5 or 1.0 g/kg ethanol. Blood samples were sealed in airtight vials containing 500 microliters of distilled water and 20 microliters of 10% isopropanol (internal standard) and stored at −4°C until assayed using gas chromatography (Agilent 7890A GC system with G1888 Network Headspace Autosampler Santa Clara, CA) supplied with a flame ionization detector and Agilent ChemStation integrator.
Drugs and data analysis
Ethanol (95% ethyl alcohol) was obtained from The Warner-Graham Company (Cockeysville, MD) and diluted each morning with water. PG01037 was synthesized in the Medicinal Chemistry Section, NIDA-IRP according to a previously reported procedure (Grundt et al., 2005). For both experiments, the primary dependent variable was the number of yawns observed. In the first experiment, yawning data were analyzed with a two-way ANOVA with repeated measures using the ethanol doses that both groups received (0.0, 0.5 and 1.0 g/kg) and ethanol experience (+/−) as factors, followed by post-hoc multiple comparisons testing using the Holm-Sidak method. In the second experiment, averaged data were similarly analyzed using infusion (saline, 1.0 g/kg ethanol) and PG01037 dose (0.0, 1.0, 3.0 mg/kg) as factors in the ANOVA. Differences were considered significant at the 95% level of confidence (p < 0.05).
Results
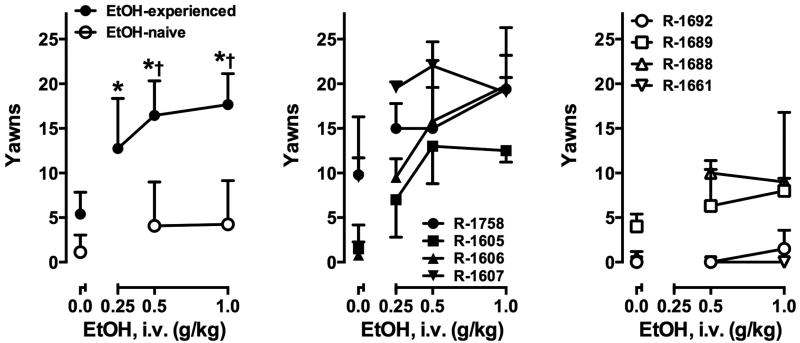
Intravenous ethanol administration significantly and dose-dependently increased the number of yawns in ethanol-experienced monkeys, but not in ethanol-naive monkeys (Fig. 1). A two-way ANOVA revealed a significant main effect of group (F1,6=19.99, p<0.01) and ethanol dose (F2,12=26.19, p<0.001) and a significant interaction (F2,12=15.08, p<0.001). Post-hoc testing indicated that the groups did not differ after saline administration, but differed significantly after administration of 0.5 and 1.0 g/kg ethanol (both p<0.001). Note that the 0.25 mg/kg Ethanol dose was not administered to the ethanol-naive group and therefore not included in the 2-way ANOVA. A separate one-way ANOVA on data from the ethanol-experienced group indicated that yawns after 0.25 g/kg ethanol differed significantly from baseline (p<0.05). The number of yawns elicited by ethanol was not related to lifetime cocaine intake in either group, nor was it related to lifetime ethanol intake or time since last ethanol drinking in the ethanol-exposed group (Table 1). Following i.v. ethanol administration, blood ethanol concentrations were not significantly different between groups (Table 2), nor did yawns vary according to BEC in either group.
Figure 1.
Left: Mean ± SEM yawns elicited by intravenous saline (0.0) and ethanol infusions in monkeys with (filled symbols) and without (open symbols) a history of ethanol consumption (n=4 per group). *, p<0.05 versus yawns produced by saline infusion. †, p<0.05 versus ethanol-naïve monkeys at the same ethanol dose. Center, right: Individual subject data for monkeys exposed to both cocaine and ethanol and those exposed only to cocaine, respectively.
Table 2.
Blood ethanol concentrations (mg/dl) in each experimental group. Blood samples were collected 30 minutes after a 10-minute infusion of ethanol (0.5 or 1.0 g/kg).
| Monkeys with cocaine and ethanol experience | ||
|---|---|---|
| 0.5 g/kg Ethanol | 1.0 g/kg Ethanol | |
| R-1605 | 63.26 | 143.79 |
| R-1606 | 57.27 | 156.15 |
| R-1758 | 57.38 | 128.70 |
| R-1607 | 54.86 | 142.26 |
|
| ||
| Mean ± SD | 58.44 ± 3.52 | 142.73 ± 11.23 |
| Monkeys with cocaine experience only | ||
|---|---|---|
| 0.5 g/kg Ethanol | 1.0 g/kg Ethanol | |
| R-1692 | 60.04 | 126.53 |
| R-1689 | 48.94 | 126.11 |
| R-1688 | 66.76 | 145.17 |
| R-1661 | 31.49 | 143.02 |
|
| ||
| Mean ± SD | 51.81±15.41 | 135.21 ± 10.30 |
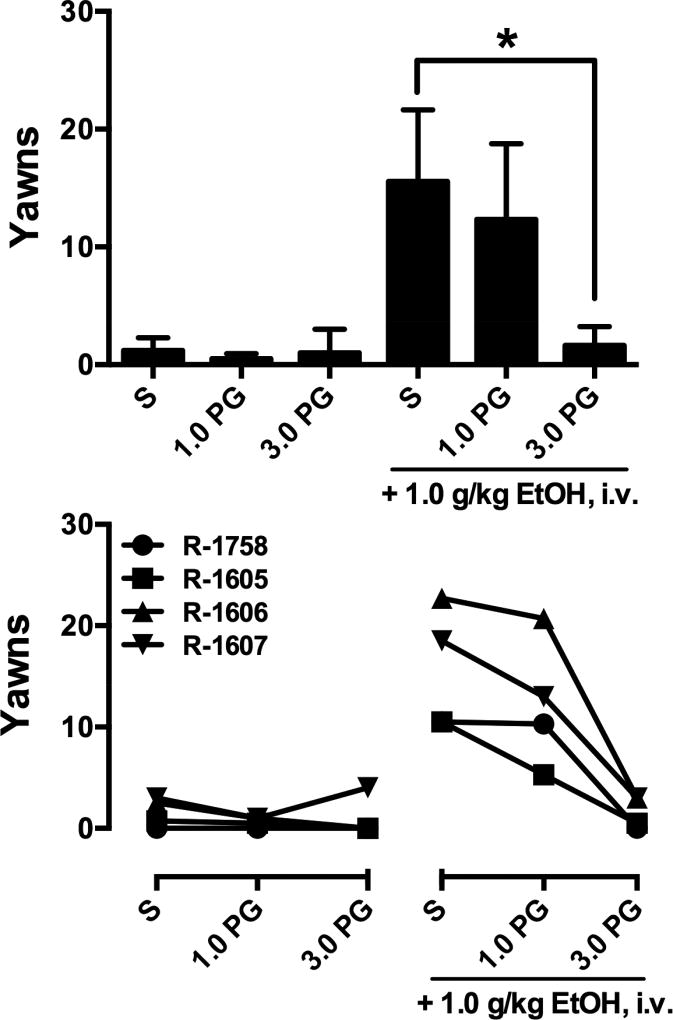
To evaluate the role of D3R in ethanol-induced yawning, the second experiment utilized the D3R-selective antagonist PG01037. A one-way ANOVA revealed a main effect of treatment (F2,6=22.89, p<0.01; Fig. 2, top). Post-hoc analysis indicated that yawns were not significantly increased in the 10 minutes after infusion of PG01037. The number of yawns elicited by a subsequent infusion of 1.0 g/kg ethanol was attenuated by 3.0 mg/kg PG01037. Inspection of individual subject data (Fig. 2, bottom) demonstrated that effects of PG01037 appeared to be dose-dependent in all four monkeys.
Figure 2.
Yawns elicited by saline (S) and PG01037 (PG) alone and in combination with saline (S) or 1.0 g/kg Ethanol in four ethanol-experienced monkeys. Top: mean ±SEM data, *, p<0.05. Bottom: Individual subjects’ data.
Discussion
In a previous study of cocaine-ethanol polysubstance abuse, in adult male rhesus monkeys who had experience self-administering cocaine, yawning elicited by the D3R agonist quinpirole was increased after several weeks of ethanol consumption (Czoty, 2015). In addition, we observed that monkeys yawned during intravenous ethanol infusions (Czoty, 2015, 2016). The present studies were designed to systematically assess the ability of ethanol to elicit yawns, to determine the contribution of a history of ethanol versus cocaine consumption on these effects and to further assess the role of D3R in ethanol-induced yawning.
In the first experiment, intravenous ethanol infusions (0.25–1.0 g/kg over 10 minutes) significantly and dose-dependently increased yawning in monkeys who had experience drinking ethanol, replicating and extending results reported earlier in the same monkeys (Czoty, 2015). These subjects had experience self-administering both cocaine and ethanol. To more clearly define the importance of chronic ethanol exposure, effects of intravenous ethanol infusions were also examined in a group of monkeys who also had experience self-administering cocaine but had never been exposed to ethanol. In that group, ethanol did not reliably elicit yawns. Blood ethanol concentrations were similar in the two groups, suggesting that differences in yawning were not the result of differences in ethanol metabolism. The results suggest that the ability of ethanol to produce yawns was not the result of a history of cocaine exposure, but rather the result of chronic ethanol drinking. It remains possible, however, that combined cocaine and ethanol exposure is necessary. Future studies conducted in monkeys exposed only to ethanol will better clarify the influence of duration and amount of ethanol exposure on the ability of acute ethanol administration to produce yawning. Sex should be considered an important variable in such studies; sex differences in spontaneous and drug-induced yawning have been reported in rats and monkeys (e.g., Serra et al., 1984; Urba-Holmgren et al., 1990; Martelle et al., 2014).
Our previous study (Czoty, 2015) indicated that long-term ethanol exposure increased quinpirole-induced yawning, an effect that has been thoroughly characterized in rats as D3R-mediated (Collins et al., 2007; Baladi et al., 2011). Consistent with a D3R mechanism, in the present studies, yawning produced by infusion of 1.0 g/kg ethanol was blocked by the D3R antagonist PG01037. Taken together the data suggest that chronic ethanol exposure may increase D3R sensitivity, and this may play an indirect role in the ability of ethanol to produce yawning. Indeed, increased D3R binding in the hypothalamus has been reported using the D3R-preferential PET ligand [11C]PHNO, in alcohol-dependent patients (Erritzoe et al 2014).
In addition to D3R, many other neurotransmitters may be involved in the observed effects. Yawning can be elicited by stimulation of muscarinic acetylcholine receptors, serotonin (5-HT)2C receptors and NMDA-type glutamate receptors (Mogilnicka and Klimek, 1977; Argiolas and Melis, 1998; Collins and Eguibar, 2010). In addition, spontaneous and drug-induced yawning can be modulated by drugs acting at several other receptors including dopamine D2, mu opioid, 5-HT1A, GABAA, GABAB, alpha2- and beta-adrenergic and cannabinoid receptors (Argiolas and Melis, 1998; Nakamura-Palacios et al., 2002; Collins and Eguibar, 2010). Importantly, these neurotransmitter systems have also been shown to be affected by chronic ethanol consumption in humans and animal models (e.g. Koob et al., 1998). Future studies of the influence of chronic ethanol drinking on yawning must examine these neurotransmitter systems. It is likely that such studies will reveal specific receptors affected by ethanol. The ability to implement the model may be diminished if multiple receptor systems are found to influence changes in ethanol-induced yawning.
Some primatologists have suggested that yawning in primates is a displacement activity elicited by stress whereas others have described it as an affiliative or aggressive behavior (e.g., Maestripieri et al., 1992; Leone et al. 2014). Thus it is possible that monkeys may yawn due to some stress experienced during the experiment. However yawning as a displacement behavior is typically observed as the result of socially derived stress (e.g., Gould et al., 2017). In the present setting, monkeys are individually tested in a familiar room. It also seems unlikely that the ethanol infusion itself produced discomfort or agitation, because these effects would have occurred in all animals. Moreover, no other physical reactions to an acute painful stimulus, such as vocalizations, were observed. It could be hypothesized that infusion of ethanol in ethanol-experienced monkeys induces some form of craving, and the lack of available ethanol creates a stressful situation. Although this cannot be ruled out, it seems unlikely that monkeys were in a state of withdrawal; the time since last ethanol consumption varied widely across monkeys (see Table 1), and the amount of ethanol-elicited yawning did not vary as a function of abstinence duration (Fig. 1). For example, the monkey with the highest lifetime ethanol intake and shortest duration of abstinence (R-1758) displayed yawns intermediate to other monkeys.
Development of efficient, reliable models that could be incorporated into ongoing nonhuman primate studies would aid in understanding the biological consequences of chronic alcohol drinking. In such studies, much information has been gained from ex vivo study of the brain and in vivo brain imaging techniques. However, the application of these techniques is limited by the need to euthanize animals and by cost, respectively. Moreover, most such studies in NHPs require time- and labor-intensive training of subjects. Taken together, a growing body of data supports the potential of ethanol-induced yawning as a model that may be of utility for future nonhuman primate studies designed to characterize the brain changes that occur during chronic alcohol use. For example, longitudinal brain scans designed to assess the effects of a ethanol exposure might provide more clinically relevant information if they were scheduled based on functional endpoints (i.e., the status of yawning elicited by ethanol or other drugs), rather than time-based endpoints (e.g., scanning at 6 and 12 months of drinking). Future studies are needed to better characterize the time course of development of ethanol-elicited yawning, and to characterize the extent of involvement of other neurotransmitter systems.
Highlights.
Ethanol elicited yawns in monkeys with extensive histories of cocaine and alcohol self-administration.
This effect was not observed in monkeys having only cocaine experience.
Ethanol-elicited yawns were blocked by prior administration of an antagonist selective for dopamine D3 receptors.
Ethanol-elicited yawning may be a useful tool to characterize the effects of long-term ethanol drinking.
Acknowledgments
The authors thank Phillip Epperly (Wake Forest School of Medicine) for technical assistance and Jianjing Cao (NIDA-IRP) for the synthesis of PG01037. This work was supported by the National Institutes of Health [grants R01 DA 021658, R01 DA012460, ZIA DA 000424 and T32 AA 007565]. The funding sources had no involvement in the study design; data collection, analysis or interpretation; writing of the report or decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argiolas A, Melis MR. The neuropharmacology of yawning. European Journal of Pharmacology. 1998;343:1–16. doi: 10.1016/s0014-2999(97)01538-0. [DOI] [PubMed] [Google Scholar]
- Baladi MG, Newman AH, France CP. Influence of body weight and type of chow on the sensitivity of rats to the behavioral effects of the direct-acting dopamine receptor agonist quinpirole. Psychopharmacology. 2011;217:573–585. doi: 10.1007/s00213-011-2320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS. Non-human primate models of alcohol-related phenotypes: the influence of genetic and environmental factors. Current Topics in Behavioral Neuroscience. 2013;13:223–249. doi: 10.1007/7854_2011_142. [DOI] [PubMed] [Google Scholar]
- Brus R, Felinska W, Rykaczewska M, Kostrzewa RM, Szkilnik R, Plech A. Prenatal ethanol diminishes reactivity of presumed dopamine D3 receptors in rats. Polish Journal of Pharmacology. 1995;47:109–114. [PubMed] [Google Scholar]
- Chen G, Cuzon Carlson VC, Wang J, Beck A, Heinz A, Ron D, Lovinger DM, Buck KJ. Striatal involvement in human alcoholism and alcohol consumption, and withdrawal in animal models. Alcohol Clinical and Experimental Research. 2011;34:1739–1748. doi: 10.1111/j.1530-0277.2011.01520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Code RA, Tang AH. Yawning produced by dopamine agonists in rhesus monkeys. European Journal of Pharmacology. 1991;201:235–238. doi: 10.1016/0014-2999(91)90351-p. [DOI] [PubMed] [Google Scholar]
- Collins GT, Eguibar JR. Neuropharmacology of yawning. In: Walusinski O, editor. Frontiers of Neurology and Neuroscience, vol. 28, The Mystery of Yawning in Physiology and Disease. Basel: S Karger Publishers; 2010. pp. 90–106. [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen J, Wang S, Woods JH. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology. 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT. Alcohol and Neurodegeneration. CNS Drug Reviews. 1999;5:379–394. [Google Scholar]
- Czoty PW. Effects of chronic binge-like ethanol consumption on cocaine self-administration in rhesus monkeys. Drug and Alcohol Dependence. 2015;153:278–285. doi: 10.1016/j.drugalcdep.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW. Lack of effect of ethanol on cocaine prime-induced reinstatement of extinguished cocaine self-administration in rhesus monkeys. Behav Pharmacol. 2016;27:633–636. doi: 10.1097/FBP.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Nader MA. Effects of oral and intravenous administration of buspirone on food-cocaine choice in socially housed male cynomolgus monkeys. Neuropsychopharmacology. 2015;40:1072–1083. doi: 10.1038/npp.2014.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erritzoe D, Tziortzi A, Bargiela D, Colasanti A, Searle GE, Gunn RN, Beaver JD, Waldman A, Nutt DJ, Bani M, Merlo-Pich E, Rabiner EA, Lingford-Hughes A. In vivo imaging of cerebral dopamine D3 receptors in alcoholism. Neuropsychopharmacology. 2014;39:1703–1712. doi: 10.1038/npp.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RW, Czoty PW, Porrino LJ, NAder MA. Social status in monkeys: effects of social confrontation on brain function and cocaine self-administration. Neuropsychopharmacology. 2017;42:1093–1102. doi: 10.1038/npp.2016.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Stafford J, Thiede A, Kiley C, Odagiri M, Ferguson B. Who is at risk? Population characterization of alcohol self-administration in nonhuman primates helps identify pathways to dependence. Alcohol Research & Health. 2008;31:289–297. [PMC free article] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. Journal of Medicinal Chemistry. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi JK, Jenkins BG, Luedtke RR, Newman AH. Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: potential substance abuse therapeutic agents. Journal of Medicinal Chemistry. 2007;50:4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Annals of the New York Academy of Sciences. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanny D, Liu Y, Brewer RD, Lu H Centers for Disease Control and Prevention (CDC) Binge drinking - United States, 2011. MMWR Surveillance Summary. 2013;62(Supplement 3):77–80. [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clinical and Experimental Research. 1998;22:998–1040. [PubMed] [Google Scholar]
- Leone A, Ferrari PF, Palagi E. Different yawns, different functions? Testing social hypotheses on spontaneous yawning in Theropithicus gelada. Scientific Reports. 2014;4:4010. doi: 10.1038/srep04010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9:147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Schino G, Aureli F, Triosi A. A modest proposal: displacement activities as an indicator of emotions in primates. Animal Behaviour. 1992;44:967–979. [Google Scholar]
- Martelle SE, Nader SH, Czoty PW, John WS, Duke AN, Garg PK, Garg S, Newman AH, Nader MA. Further characterization of quinpirole-elicited yawning as a model of dopamine D3 receptor activation in male and female monkeys. Journal of Pharmacology and Experimental Therapeutics. 2014;350:205–211. doi: 10.1124/jpet.114.214833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PM, Book SW, Stewart SH. Medical treatment of alcohol dependence: a systematic review. The International Journal of Psychiatry in Medicine. 2011;42:227–266. doi: 10.2190/PM.42.3.b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilnicka E, Klimek V. Drugs affecting dopamine neurons and yawning behavior. Pharmacology, Biochemistry and Behavior. 1977;7:303–305. doi: 10.1016/0091-3057(77)90224-6. [DOI] [PubMed] [Google Scholar]
- Nakamura-Palacios EM, Amodeo Bueno OF, Takahashi RN, Tufik S. Acute or chronic effects of cannabinoids on spontaneous or pharmacologically induced yawning in rats. Pharmacology, Biochemistry and Behavior. 2002;74:205–212. doi: 10.1016/s0091-3057(02)00991-7. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, ‘t Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA, Voytko ML. Why primate models matter. American Journal of Primatology. 2014;76:801–827. doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra G, Collu M, Serra A, Gessa GL. Estrogens antagonize apomorphine-induced yawning in rats. European Journal of Pharmacology. 1984;104:383–386. doi: 10.1016/0014-2999(84)90418-7. [DOI] [PubMed] [Google Scholar]
- Sobrian SK, Jones BL, James H, Kamara FN, Holson RR. Prenatal ethanol preferentially enhances reactivity of dopamine D1 but not D2 or D3 receptors in offspring. Neurotoxicology and Teratology. 2005;27:73–93. doi: 10.1016/j.ntt.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Urba-Holmgren R, Trucios N, Holmgren B, Eguibar JR, Gavito A, Cruz G, Santos A. Genotypic dependency of spontaneous yawning frequency in the rat. Behavioural Brain Research. 1990;40:29–35. doi: 10.1016/0166-4328(90)90039-h. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE, Goodwin AK. The value of nonhuman primates in drug abuse research. Experimental and Clinical Psychopharmacology. 2007;15:309–327. doi: 10.1037/1064-1297.15.4.309. [DOI] [PubMed] [Google Scholar]