Abstract
Antibody-mediated rejection (AMR) resulting in transplant allograft vasculopathy (TAV) is the major obstacle for long-term survival of solid organ transplants. AMR is caused by donor-specific antibodies to HLA which contribute to TAV by initiating outside-in signaling transduction pathways that elicit monocyte recruitment to activated endothelium. Mechanistic target of rapamycin (mTOR) inhibitors can attenuate TAV; therefore, we sought to understand the mechanistic underpinnings of mTOR signaling in HLA class I Ab-mediated endothelial cell activation and monocyte recruitment. We used an in vitro model to assess monocyte binding to HLA I Ab-activated endothelial cells and found mTOR inhibition reduced Ezrin/Radixin/Moesin (ERM) phosphorylation, ICAM-1 clustering and monocyte firm adhesion to HLA I Ab-activated endothelium. Further, in a mouse model of AMR, in which B6.RAG1−/− recipients of BALB/c cardiac allografts were passively transferred with donor-specific MHC I antibodies, mTOR inhibition significantly reduced vascular injury, ERM phosphorylation, and macrophage infiltration of the allograft. Taken together, these studies indicate mTOR inhibition suppresses ERM phosphorylation in endothelial cells which impedes ICAM-1 clustering in response to HLA class I Ab, and prevents macrophage infiltration into cardiac allografts. These findings indicate a novel therapeutic application for mTOR inhibitors to disrupt endothelial-monocyte interactions during AMR.
INTRODUCTION
The development of AMR persists as a major issue limiting long-term patient and allograft survival (1). AMR is mediated by the binding of HLA antibody (HLA Ab) to the mismatched donor HLA class I (HLA I) and class II (HLA II) antigens expressed on the graft endothelium, resulting in microvascular inflammation and intravascular activated mononuclear cells, with or without complement deposition (2, 3). Chronic exposure of heart, kidney and lung allografts to HLA Ab can lead to transplant allograft vasculopathy (TAV) resulting in graft dysfunction, loss and patient death (1, 4–7). Disruption of HLA Ab-mediated microvascular inflammation and leukocyte recruitment may prevent TAV. Recent experimental transplant studies depleting natural killer cells (8) or macrophages (9) support this concept.
Ligation of HLA I molecules expressed by endothelial cells (ECs) induces outside-in signaling pathways eliciting cytoskeletal remodeling, proliferation and migration (10). HLA I Ab signaling also triggers rapid mobilization of Weibel-Palade bodies (WPBs), induction of P-selectin, and recruitment of leukocytes to the activated endothelium (11–13). Mechanistic target of rapamycin (mTOR) can promote EC-leukocyte interactions, suggesting immunotherapy with rapalogs may be beneficial in preventing graft injury mediated by AMR (14–18). mTOR inhibitors are widely used for cancer and atherosclerosis (19, 20); however, they are also used as an immunosuppressive agent to prevent rejection of solid organ transplants (21). A number of studies have demonstrated efficacy of mTOR inhibitors for attenuating cardiac TAV (22, 23), yet the underlying mechanism remains unclear.
Here, we investigated the role of mTOR in monocyte recruitment to HLA I Ab-activated ECs. Our data demonstrate mTOR signaling induces ICAM-1 clustering on the EC surface which stabilizes monocyte firm adhesion, critical for transendothelial migration under conditions of physiological shear flow. Accordingly, mTOR inhibition in a murine model suppressed monocyte infiltration into allografts undergoing AMR with a concomitant decrease in capillary EC phosphorylation of downstream proteins. Taken together, these studies provide much needed mechanistic insight into the function of clinical mTOR inhibitors and their potential use in treating AMR.
MATERIALS AND METHODS
Reagents
Pan-HLA class I mAb recognizing distinct monomorphic epitopes were obtained from BioXCell (W6/32; mIgG2a; West Lebanon, NH) or Abcam (MEM-147; mIgG1; Cambridge, MA). Sheep pAb to ICAM-1/CD54, integrin αVβ3 (anti-ITGB3; mIgG1), and P-selectin were from R&D (Minneapolis, MN). Sirolimus (Rapamycin; Rapa) was purchased from LC Laboratories (Woburn, MA). Everolimus (RAD001; RAD) was from Novartis Pharma AG (Switzerland). siRNAs were designed as previously described (15) and from Dharmacon/GE Healthcare Life Sciences (Lafayette, CO). Bapta-AM, UO126, Ro-31-7549, Y27632 and rabbit pAb to mTOR, Raptor, and Rictor were from Calbiochem/EMD Millipore (Billerica, MA). Rabbit pAb to phosphorylated mTORSer2448, S6KThr389, S6RPSer235/236, AktSer473, p44/42 MAPK (ERK1/2Thr202/Tyr204), MYPT1Thr696, or EzrinThr567/RadixinThr564/MoesinThr558 (ERM) were from Cell Signaling Technology (Danvers, MA) and used as we previously described (14, 15, 24–27). Goat-anti-rabbit and anti-mouse IgG were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit IgG isotype control, horseradish peroxidase (HRP) goat-anti-rabbit IgG, and 3,3’-diaminobenzidine (DAB) HRP Substrate Kit was purchased from Vector Labs (Burlingame, CA). Vybrant™ CFDA-SE (CFSE) Cell Tracer Kit, donkey-anti-sheep IgG, Phalloidin and DAPI were from Invitrogen (Rockville, MD). Rabbit-anti-mouse C4d Ab was a gift from Dr. William Baldwin (Cleveland Clinic). Rat anti-mouse Mac2 was from Cedarlane (Hornby, Ontario, Canada). Prior to use, all antibodies were titered to determine optimal working concentrations and their specificity confirmed using appropriate positive and negative controls.
Human Cells
Use of all human cells was approved by the UCLA Institutional Review Board (IRB#10-001689; IRB#00-01-023). Primary ECs were isolated from aortic rings of deceased donor hearts or from Clonetics (EC5555; San Diego, CA) and cultured as previously described (16, 28, 29). The monocytic cell line Mono Mac 6 (MM6; a gift from Dr. Loems Ziegler-Heitbrock; Germany) was cultured as described (11). Primary human monocytes were enriched from the peripheral blood of multiple healthy volunteers using Ficoll-Paque (GE Healthcare) density centrifugation, followed by MACS pan-monocyte negative selection (Miltenyi) as we previously described (12) at an average final purity of 89.8% based on positive CD14 staining as determined by flow cytometry using an LSRFortessa (BD Biosciences). Experiments were performed with early-passage ECs from 2–3 different donors. Monocyte Fc interactions with ECs were inhibited by incubation with human IgG (12).
Monocyte static and shear-based adhesion assays
Adherence of monocytes to ECs under static conditions was measured as described (11, 12, 30). Briefly, ECs were pre-treated with 30 µg/ml 10 nM Rapa or 30 nM RAD (Figure S1) or 100nM siRNA (siRNA efficiency and specificity are shown in Figure S2), then stimulated with 1 µg/ml anti-ITGB3 or anti-HLA I for 30 min, or 1 U/ml thrombin for 4–6 h. Monocytes were fluorescently labeled with 2 mM CFSE, then added 3:1 to ECs for 20 min. Non-adherent cells were removed by washing. Images of adherent monocytes were acquired from 8–10 4× objective fields on a Nikon Eclipse Ti and quantified using Imaris 7.6.2 (Bitplane; Concord, MA).
Adherence of monocytes to ECs under laminar flow conditions was measured using a closed perfusion system (ibidi) (31). ECs (105 cells/cm2) were seeded in gelatin-coated flow chamber slides (ibidi) and cultured for 24 h. ECs were pre-treated with Rapa for 24 h or 20 µg/mL anti-ICAM-1 for 30 mins. ECs were then stimulated as above. 6.25 × 105/mL CFSE-labeled monocytes were perfused over ECs at 1 dynes/cm2. Data was acquired on a Nikon Eclipse Ti and analyzed using ImarisTrack 7.6.2 (BitPlane; Concord, MA). Exported videos show individual monocytes as objects, with each track representing an individual monocyte’s movement over the indicated time.
Flow cytometry and ELISA Assays
Cell surface expression of P-selectin was measured on adherent ECs by cell-based ELISA (11, 30, 31). Cell surface expression of ICAM-1, E-selectin or VCAM-1 was measured by flow cytometry on an LSRFortessa (BD Biosciences; San Jose, CA). ECs were detached using Accutase (Innovative Cell; San Diego, CA) to preserve epitopes (32). von Willebrand Factor was measured in culture supernatants by ELISA (Helena Laboratories; Beaumont, TX) (11, 30).
Western blots
EC lysates for Western blot analysis, with/without anti-ICAM-1 immunoprecipitation, were prepared as described (29) and quantified with Image J software (NIH; Bethesda, MD).
ICAM-1 localization and clustering
Following HLA I Ab stimulation and/or mTOR inhibition, ECs were incubated for 1 h with sheep-pAb to ICAM-1 then 30 min with donkey-anti-sheep IgG conjugated with AF594 before being fixed with 4% PFA. Fixed cells were counterstained with AF488-conjugated Phalloidin and DAPI. Cellular localization and clustering of ICAM-1 expression on ECs was quantified using three-dimensional analysis of confocal laser scanning microscopy (CLSM) images (33, 34). Images were acquired on a TCS-SP5 inverted Acousto Optical Beam Splitter (AOBS) confocal microscope (Leica) using LAS X 1.9 (Leica Biosystems, Vista, CA). Three-dimensional z-stacks were analyzed for ICAM-1 expression using Imaris 8.1 by creating a discrete quantitative “spot” objects layer from ICAM-1 labeling data in the red channel. ICAM-1 clustering was evaluated by counting the number of spots per cell, and measuring their three-dimensional sizes in voxels (volumetric pixels).
Murine Heterotopic heart transplantation
Male B6.129S7-Rag1tm1Mom (B6.RAG1−/−, H-2b) and BALB/c (H-2d) mice aged 7–10 weeks old were obtained from The Jackson Laboratory (Bar Harbor, ME) and housed under pathogen-free conditions in the Division of Laboratory Animals at UCLA. BALB/c hearts were heterotopically transplanted into B6.RAG1−/− recipients as we previously described (11). Beginning day 3 post-transplant, 30 µg/kg of donor-specific anti-MHC I (anti-H-2Kd, SF1-1.1; or anti-H-2Dd, 34-2-12) or isotype control (mIgG2a, MOPC-173) from Biolegend (San Diego, CA) were injected biweekly by tail vein. Indicated mice received intraperitoneal injection of 1 mg/kg/day Rapa. Graft function was monitored by abdominal palpation daily.
Immunohistochemistry and histopathological grading
After 30 days of treatment, mice were anesthetized by isofluorane, donor and native hearts were obtained and fixed in 10% buffered-formalin, embedded in paraffin, and sectioned at 5 µm before H&E and IHC staining was performed as we previously described for Mac-2 and C, or p-ERK, p-AKT and p-S6K (14, 15, 24–27). IHC staining for p-ERM was conducted as follows. Sections were rehydrated and antigen was recovered in a steamer with citrate buffer (pH 6.0) before endogenous peroxide activity was quenched with 3% hydrogen peroxide. Sections were blocked with normal goat serum in PBS, incubated with rabbit pAb to ERM, or isotype control overnight at 4°C then HRP goat anti-rabbit IgG for 40 min at room temperature before being developed with DAB and counterstained with hematoxylin. Staining was scored by a single blinded pathologist (35). The presence of Mac2-positive intravascular activated mononuclear cells, or positive EC staining in capillaries and intramyocardial arteries/veins for C4d or phosphorylated proteins were scored on a scale of 0–3 (0, no staining; 1, focal staining; 2, multifocal staining; 3, diffuse staining).
Statistical Analysis
Differences between groups were determined using one or two-way ANOVA using Prism 5 (GraphPad). P-values of <0.05 were considered statistically significant.
RESULTS
mTOR inhibition in ECs significantly reduces HLA I Ab-induced monocyte recruitment
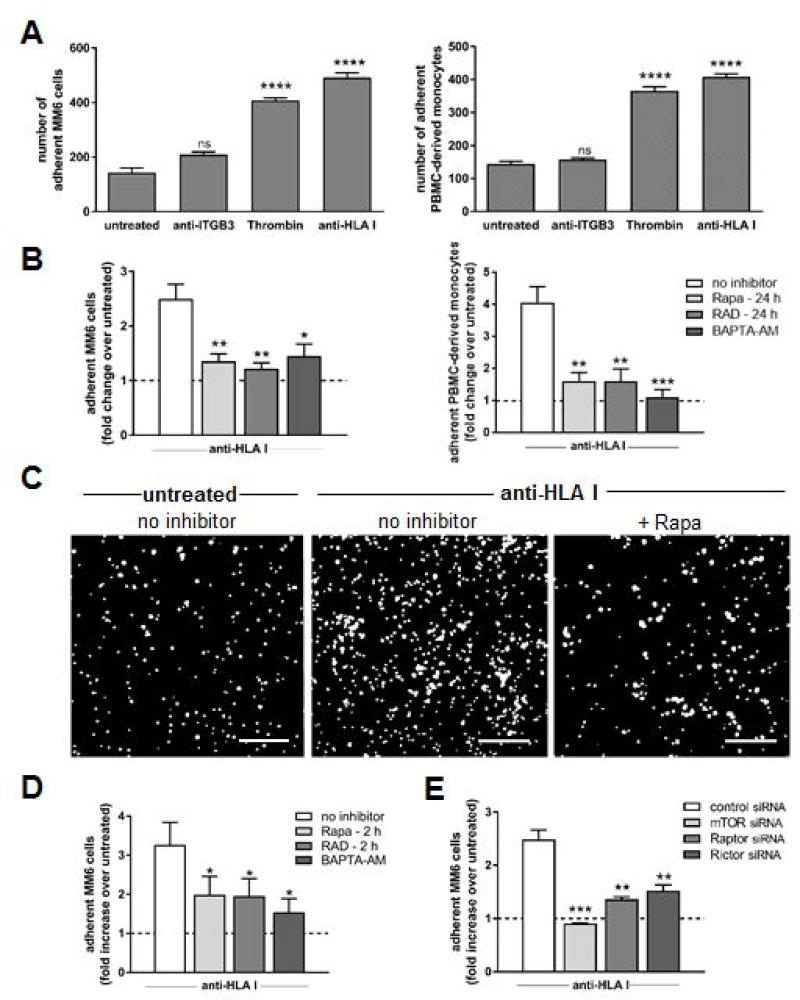
We characterized the capacity of two different FDA-approved pharmacologic mTOR inhibitors, sirolimus (Rapamycin; Rapa), and everolimus (RAD001; RAD), to halt recruitment of monocytes to HLA I Ab-activated human aortic ECs. Stimulation of ECs with HLA I Ab increased recruitment of the human monocytic cell line MonoMac6 (MM6 cells) to a level comparable to that induced by thrombin (Figure 1A, left panel). In contrast, treating ECs with control IgG (not shown) or Ab against ITGβ3 failed to evoke monocyte adhesion. Similar effects were observed using PBMC-derived monocytes freshly isolated from third-party healthy donors (Figure 1A, right panel). Pre-treatment of ECs with Rapa completely abolished enhanced monocyte recruitment to HLA I Ab-activated EC (Figure 1B, 1C), comparable to ECs pre-treated with the calcium-chelator (11) BAPTA-AM. Again, we observed similar results between propagated and PBMC-derived (Figure 1B, left vs right panel) monocytes. Collectively, these data indicate mTOR is necessary for HLA I Ab-induced monocyte binding to activated ECs.
Figure 1. Monocyte adherence to HLA class I Ab-stimulated endothelium is suppressed when the mTOR pathway is inhibited in ECs.
Primary endothelial cells (ECs) were stimulated with Ab against Integrin β3 (anti-ITGB3), HLA class I (anti-HLA I; clone W6/32) or thrombin for 5 min. CFSE-labeled MonoMac6 (MM6 cells) or human monocytes enriched from third-party peripheral blood (PBMC-derived monocytes) were pre-treated with polyclonal human IgG to block FcγR interactions, then allowed to adhere to ECs for 20 min, then un-adhered monocytes were washed off and adherent monocytes in 8–10 fields were imaged and quantified. (A) Shown are mean numbers of MM6 cells (left panel) or PBMC-derived monocytes (right panel) adherent to treated ECs over untreated ECs ± SEM from five independent experiments. ****P<0.0001, ns=not significant when comparing treated ECs to untreated ECs by two-way ANOVA with Tukey’s multiple comparisons test (B) ECs were pre-treated with Rapa, RAD or no inhibitor for 24 h, or BAPTA-AM for 30 min before monocytes were allowed to adhere. Shown are fold changes in mean number of MM6 cells (left panel) or PBMC-derived monocytes (right panel) adherent to treated ECs over untreated control ECs (dotted line) ± SEM from five independent experiments. *P<0.05, **P<0.01, ***P<0.001 for ECs given the indicated inhibitor to ECs given no inhibitor by two-way ANOVA with Tukey’s multiple comparisons test (C) Representative images showing CFSE-labeled MM6 cells adherent to the endothelial monolayer following indicated treatment. scale bars = 100 µm (D) ECs were pre-treated with no inhibitor, Rapa or RAD for 2 h (targets mTORC1 or 2 respectively), or BAPTA-AM for 30 min before MM6 cells were allowed to adhere. Shown are fold changes in mean numbers of MM6 cells adherent to treated ECs over untreated control ECs (dotted line) ± SEM from five independent experiments. *P<0.05 for ECs given the indicated inhibitor to ECs given no inhibitor by two-way ANOVA with Tukey’s multiple comparisons test (E) ECs were pre-treated with control siRNA or siRNA directed against mTOR, Raptor (mTORC1), or Rictor (mTORC2) before MM6 cells were allowed to adhere. Shown are fold changes in numbers of MM6 cells adherent to treated ECs over untreated control ECs (dotted line) ± SEM from five independent experiments. **P<0.01, ***P<0.001 for ECs given the indicated siRNA to ECs given no siRNA by two-way ANOVA with Tukey’s multiple comparisons test
mTOR can complex with regulatory-associated protein of mTOR (Raptor) to form mTOR complex 1 (mTORC1) or rapamycin-insensitive companion of mTOR (Rictor) to form mTOR complex 2 (mTORC2), with differing downstream effects. We used two complementary approaches to investigate mTOR complex involvement in HLA I Ab-mediated monocyte recruitment to ECs (Figure 1D, E). First, we treated ECs with short-term Rapa or RAD for 2 h prior to stimulation with HLA I Ab to determine the contribution of mTORC1 protein complexes to monocyte recruitment, as rapalogs require >6 h to inhibit mTORC2 formation, but inhibit mTORC1 formation rapidly (15, 16). Adherence of MM6 cells was significantly attenuated with 2 h Rapa or RAD pre-treatment of ECs (Figure 1D), although not as completely as 24 h pre-treatment to block formation of both complexes (Figure 1B). Second, we used siRNA to knockdown Raptor, Rictor or mTOR expression in ECs prior to their stimulation with HLA I Ab (Figure 1E). High efficiency and specificity of mTOR (>99%), Raptor (>85%) and Rictor (>80%) protein depletion was achieved by siRNA-mediated knockdown (Figure S2). Although silencing of either mTORC1 or mTORC2 individually suppressed adherence of MM6 cells to near baseline levels, simultaneous depletion of mTORC1 and mTORC2 via mTOR siRNA resulted in its complete suppression (Figure 1E), suggesting a synergistic interaction between mTORC1 and mTORC2 in this process. These results indicate monocyte recruitment to HLA I Ab-activated ECs is regulated via both mTORC1- and mTORC2-dependent pathways.
Additionally, results obtained using HLA I Ab directed against a different monomorphic epitope on HLA I (clone MEM-147) were comparable to those obtained with clone W6/32 (Figure S3).
In vivo blockade of mTOR ameliorates MHC class I Ab-mediated AMR
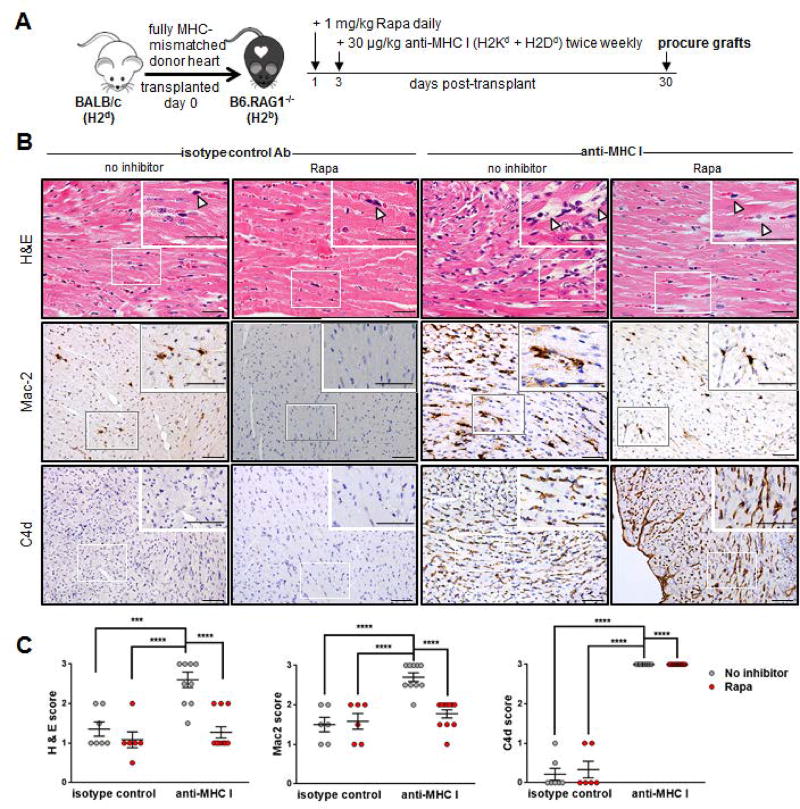
We then tested the requirement for mTOR in monocyte recruitment to HLA I Ab-activated endothelium in vivo using a clinically relevant murine model (11). B6.RAG1−/− mice (H-2b) received a complete mismatch Balb/c (H-2d) heterotopic heart transplant and were passively transferred with anti-donor MHC class I Ab biweekly for 30 days (Figure 2A). H&E and Mac2 staining confirmed MHC I Ab transfer resulted in EC swelling with associated intravascular activated mononuclear cells in the allograft that were significantly reduced in Rapa-treated animals to levels similar to isotype control-treated mice (Figure 2B, 2C). As expected, Rapa did not inhibit MHC I Ab-mediated activation of the complement cascade. These data are consistent with the in vitro experimental findings, and indicate administration of mTOR inhibitors in vivo causes a reduction in HLA I Ab-induced monocyte recruitment to the allograft.
Figure 2. Rapamycin treatment ameliorates acute injury by blocking monocyte recruitment in a murine model of cardiac antibody mediated rejection.
(A) BALB/c cardiac allografts were transplanted into B6.RAG1−/− recipients and passively transfused with 30 µg/kg anti-MHC I (anti-H2Kd + anti-H2Dd) mAb or isotype control mAb biweekly beginning 3 days post-transplant. Rapa treatment at 1mg/kg/daily was initiated 1 day post-transplant. (B) Grafts were procured at day 30 post-transplant and evaluated for microvascular abnormalities by H&E, as well as intravascular activated mononuclear cells and/or complement deposition by immunohistochemical staining for MAC2 or C4d respectively. Arrow heads in top row indicate prominent nuclei of cells in distended capillaries of anti-MHC I-treated animals that was not seen in the anti-MHC I group treated with Rapa, or either group that received control mIgG mAb where arrow heads indicate flat, thin nuclei in collapsed capillaries. scale bars = 50 µm (C) Shown are mean scores ± SEM for n≥6 per group with each dot representing one animal. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001 by two-way ANOVA with Tukey’s multiple comparisons test.
mTOR regulates ICAM-1-induced firm adhesion of monocytes to HLA I Ab stimulated ECs
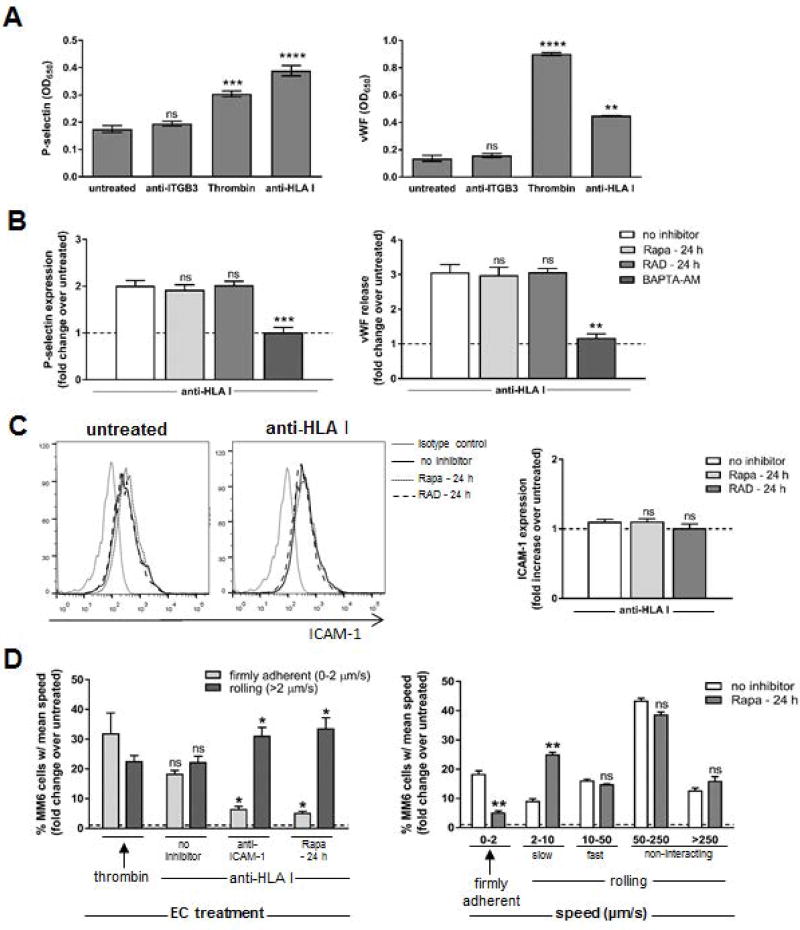
We next sought to characterize the mechanism by which mTOR regulates HLA I Ab-mediated monocyte adherence. To investigate the potential impact of mTOR inhibition on HLA I Ab-induced WPb exocytosis, we measured endothelial P-selectin surface expression and von Willebrand Factor (vWF) release (Figure 3). Stimulation of ECs with thrombin or HLA I mAb induced rapid WPb exocytosis as demonstrated by P-selectin expression and vWF release (Figure 3A), while exposure to anti-ITGβ3 did not. Pre-treatment of ECs with Rapa or RAD for 24 h (Figure 3B) or 2 h (Figure S4A) had no effect on HLA I Ab-induced WPb mobilization, as neither P-selectin expression nor vWF secretion were affected (Figures 3B, S4A) like they were upon pre-treatment with BAPTA-AM.
Figure 3. mTOR regulates ICAM-1-induced firm adhesion of monocytes to HLA class I Ab-stimulated ECs.
Primary endothelial cells (ECs) were pre-treated with Rapa, RAD or no inhibitor for 24 h, or BAPTA-AM for 30 min before they were stimulated with Ab against Integrin β3 (anti-ITGB3), HLA I (anti-HLA I; clone W6/32) or thrombin for 5 min. (A) EC P-selectin expression was detected by cell-based ELISA, and EC-release of von Willebrand Factor (vWF) was assessed by ELISA of supernatant. Shown are the average optical density values (OD650) ± SEM from 5 independent experiments. ***P<0.001, ****P<0.0001, ns=not significant when comparing treated ECs to untreated ECs by two-way ANOVA with Tukey’s multiple comparisons test (B) ECs were pre-treated with no inhibitor, Rapa or RAD for 24 h, or BAPTA-AM for 30 min. EC P-selectin expression was detected by cell-based ELISA, and EC-release of vWF was assessed by ELISA of supernatant. Shown are the mean fold change of protein expression from treated ECs over untreated ECs (dotted line) ± SEM from 5 independent experiments. ***P<0.001, ns=not significant when comparing ECs given the indicated inhibitor to ECs given no inhibitor by two-way ANOVA with Tukey’s multiple comparisons test (C) ICAM-1 expression was detected by flow cytometry. Shown is representative histograms and mean fold increase in ICAM-1 positive cells in each condition over untreated cells (dotted line on graph) from 2 independent experiments. ns=not significant when comparing ECs given the indicated inhibitor to ECs given no inhibitor by one-way ANOVA with Tukey’s multiple comparisons test (D) MM6 cells were pre-treated with polyclonal human IgG to block FcγR interactions, perfused at 1 dynes/cm2 over an endothelial monolayer pre-treated with Rapa for 24 h, then anti-ICAM-1 neutralizing Ab for 30 min then stimulated with anti-HLA I for 30 min. Three 5–10s videos (Videos S1–3) were collected in real-time for each condition, and mean velocity (mm/sec) of each MM6 cell over video duration was determined using ImarisTrack. Shown are average percentages of total MM6 cells observed with each indicated speed ± SEM from 5 independent experiments *P<0.05, ns=not significant by two-way ANOVA with Tukey’s multiple comparisons test (left panel) or **P<0.01; ***P<0.001, when comparing ECs treated with Rapa to ECs left untreated by Student’s T test (right panel).
Rapa is known to directly inhibit protein synthesis (36); however, we did not find any significant changes in the basal surface expression levels of key molecules in the leukocyte adhesion cascade (37), including ICAM-1 (Figure 3C), E-Selectin or VCAM-1 (Figure S4B) upon activating ECs with HLA I Ab, with or without Rapa pre-treatment.
Since expression levels of adhesion molecules were not altered, we chose to investigate the effects of mTOR inhibition on the adhesive function of the endothelium under physiological shear flow conditions (Figure 3D, Videos S1–3). When we perfused MM6 cells over HLA I Ab-activated endothelial monolayers, we observed increased adhesion compared to untreated control ECs (Figure 3D, left panel and Videos S1–2). However, the percent of firmly adherent MM6 cells was significantly decreased when ECs were pre-treated with Rapa compared to no inhibitor treatment (Figure 3D, left panel and Videos S2–3). The capacity of Rapa to inhibit firm adhesion of MM6 cells was comparable to treatment of ECs with ICAM-1 neutralizing Ab. Further stratification of MM6 cells by their rolling speed confirmed no difference in monocytes not directly interacting with the ECs (Figure 3D, right panel). We observed a significant decrease in the percentage of firmly adherent MM6 cells (speed 0–2 µm/s) between ECs with no inhibitor treatment (18%) and Rapa-treated ECs (5.3%) with a corresponding increase in the percentage of slow rolling MM6 cells (speed 2–10 µm/s) for these groups (9.1% vs 25.1% respectively). These data indicate the proportion of monocytes that would normally firmly adhere to HLA I Ab-stimulated ECs are no longer able to do so in the presence of mTOR inhibition, and instead continue to slowly roll along the endothelium, implicating a role for mTOR in regulating monocyte firm adhesion to ICAM-1 on ECs following HLA I Ab stimulation.
mTOR inhibition in HLA I Ab-stimulated EC impairs ICAM-1 clustering
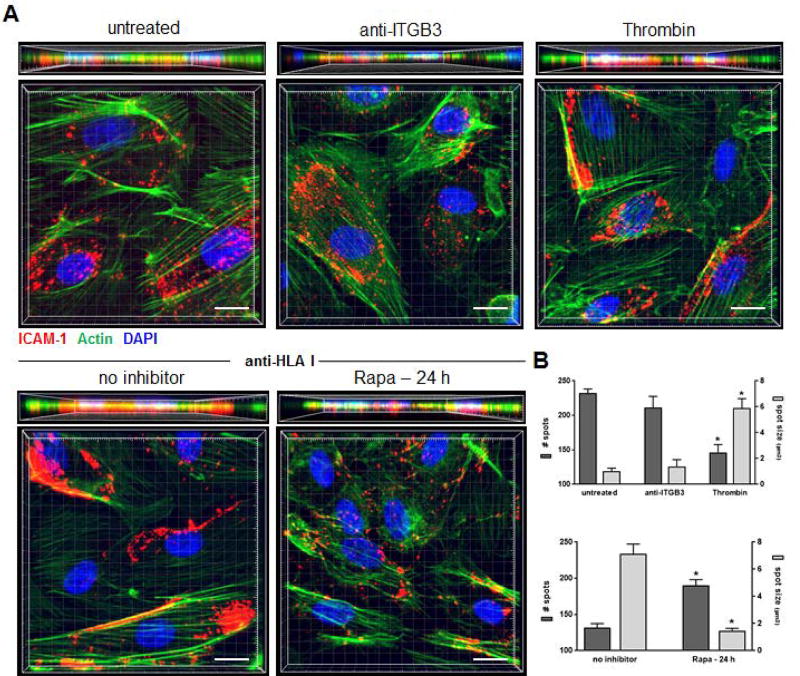
ICAM-1 clusters at the apical membrane of ECs and promotes leukocyte firm adhesion by forming a “ring” or “cup” around the adhered leukocyte (37–39). As this clustering event is essential for firm adherence, we questioned if mTOR inhibition disables ICAM-1 clustering. We first examined the capacity of HLA I Ab-stimulation to mediate ICAM-1 clustering by imaging ECs using super-resolution confocal immunofluorescence microscopy. Surface expression levels of ICAM-1 were not altered after HLA I Ab ligation (Figure 3C). Therefore, we measured ICAM-1 aggregation with the Imaris spot detection algorithm. In ECs that were untreated, or treated with negative control ITGβ3 Ab, ICAM-1 was distributed diffusely, with a large number of small sized spots (Figure 4A, 4B). Treatment with HLA I Ab triggered dramatic ICAM-1 clustering into a significantly smaller number of spots with a significantly larger size at the margins of ECs, similar to ECs treated with positive control thrombin (Figure 4A, 4B). Pre-treatment of ECs with Rapa resulted in a diffuse scattering of small-sized ICAM-1 spots distributed throughout the cell surface similar to untreated control ECs (Figure 4). These findings indicate HLA I Ab-mediated mTOR activation regulates ICAM-1 clustering, promoting monocyte firm adhesion to the endothelium.
Figure 4. mTOR is required for ICAM-1 clustering in HLA class I Ab-activated ECs.
Primary endothelial cells (ECs) were pre-treated with Rapa or no inhibitor for 24 h, before they were stimulated with Ab against Integrin β3 (anti-ITGB3), HLA I (anti-HLA I; clone W6/32) or thrombin for 5 min, then CFSE-labeled MM6 cells were allowed to adhere for 20 min. ECs were stained by immunofluorescence for ICAM-1 (red), as well as Phalloidin for actin filaments (green) and DAPI to detect cell nuclei (blue). (A) Shown are representative three-dimensional volumetric confocal images of ECs following indicated treatments. scale bars=10 µm. (B) Imaris 3D analysis software was used to quantify ICAM-1 clustering. Shown are the number of individual spots per cell detected in the red channel as well as their size, and results are expressed as the mean number of spots or mean three-dimensional size (µm3) ± SEM of each treated group over untreated ECs (n≥30 cells per group). *P<0.05 by unpaired two-tailed Student’s t test.
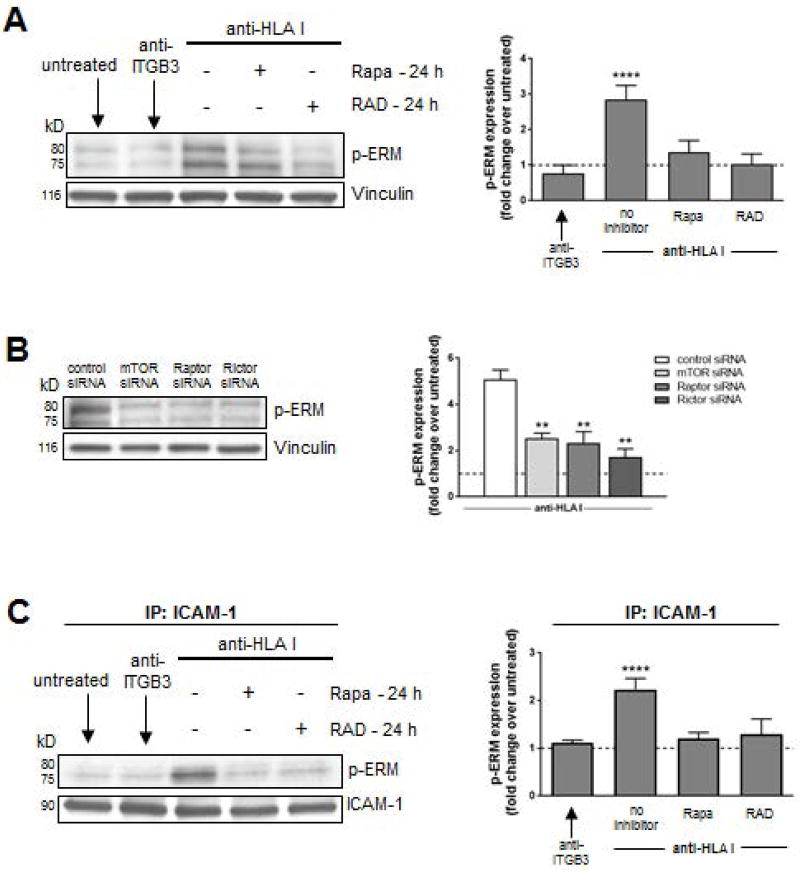
HLA I Ab-induced ERM phosphorylation is impaired by mTOR inhibition in ECs
ERM complex activation is required for ICAM-1 clustering and association with the actin cytoskeleton (40); therefore, we investigated the effect of mTOR inhibition on ERM signaling. Stimulation of ECs with HLA I Ab induced a 3-fold increase in the levels of phosphorylated ERM compared to control ECs (Figure 5A). HLA I Ab-ligation on ECs pre-treated with rapalogs for 24 h decreased phosphorylation of ERM (Figure 5A), suggesting ERM phosphorylation depends on mTOR. As a control, we verified pre-treatment of ECs with Rapa or RAD inhibited HLA I Ab-induced phosphorylation of mTOR, p70S6K, Akt, ERK, and S6RP (Figure S5A), known downstream targets of the HLA class I outside-in signaling pathway (16).
Figure 5. ERM phosphorylation is impaired by mTOR inhibition in HLA class I Ab-activated ECs.
ECs were pre-treated with Rapa or RAD for 24 h and stimulated with HLA I Ab (anti-HLA I; clone W6/32) for 10 min. (A) Cells were lysed and proteins were separated by SDS–PAGE followed by immunoblotting to detect phosphorylated EzrThr456/RadThr564/MoesThr558 (p-ERM) as well as Vinculin to confirm equal loading of proteins and were quantified by densitometry scan analysis using ImageJ. Results are expressed as the mean fold increase in p-ERM expression over untreated ECs ± SEM over 3 independent experiments. (B) ECs were transfected with either control siRNA, or siRNA directed against mTOR, Raptor (MTORC1), or Rictor (MTORC2) before treatment with anti-HLA I for 10 min. Cells were lysed after 48 h and proteins were separated by SDS–PAGE followed by immunoblotting to detect p-ERM as well as anti-Vinculin mAb to confirm equal loading of proteins and were quantified by densitometry scan analysis using ImageJ. Results are expressed as the mean fold increase in p-ERM expression over untreated ECs ± SEM over 3 independent experiments. (C) ECs were pretreated with Rapa or RAD for 24 h and stimulated with anti-HLA I for 10 min. Cells lysates were immunoprecipitated (IP) with anti-ICAM-1 followed by immunoblotting to detect p-ERM as above. Results are expressed as the mean fold increase in p-ERM expression over untreated ECs ± SEM over 3 independent experiments. **P<0.01; ****P<0.0001 by two-way ANOVA with Tukey’s multiple comparisons test.
Using siRNA to investigate the independent contribution of mTOR complexes in HLA I Ab-mediated ERM phosphorylation (Figure 5B), we found HLA I Ab-induced ERM phosphorylation in ECs was reduced 3-fold following Raptor (mTORC1) and 2.8-fold after Rictor (mTORC2) knockdown. Complete inhibition was achieved upon mTOR knockdown. We have verified that pre-treatment of ECs with Rapa or RAD inhibited HLA I-induced phosphorylation of known downstream targets of the HLA class I outside-in signaling pathway (15, 26). These experiments substantiate the synergistic role for both mTORC1 and mTORC2 in the phosphorylation of ERM.
Co-immunoprecipitation with anti-human ICAM-1 showed stimulation of ECs with HLA I Ab increased complex formation between ICAM-1 and phosphorylated ERM by 2.2-fold compared to control (Figure 5C). Pre-treatment of ECs with either mTOR inhibitor significantly reduced complex formation between ICAM-1 and phosphorylated ERM, supporting our hypothesis that mTOR signaling downstream of HLA class I engagement with Ab regulates ERM phosphorylation and physical association with ICAM-1.
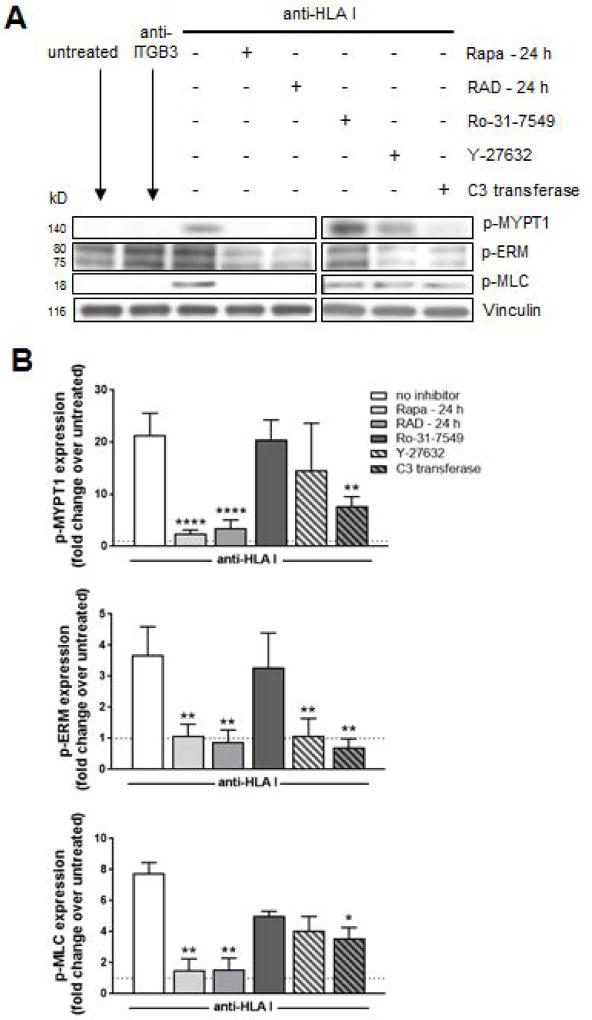
mTOR regulates HLA I Ab-induced ERM phosphorylation through the Rho pathway
EC activation leads to ERM phosphorylation through the GTP-binding protein RhoA (39, 41). Rho signals through the ROCK and PKC pathways resulting in an accumulation of phosphorylated ERM (42, 43). To examine the signaling relationships between Rho and mTOR following HLA class I ligation, we used pharmacological inhibitors targeting PKC (Ro-31-7549), ROCK (Y-27632), and Rho (C3 transferase) (Figure 6). Rho activation was determined by quantitating levels of phosphorylated myosin light chain (MLC) and myosin phosphatase target subunit 1 (MYPT1), as myosin phosphatase has been shown to regulate actin:myosin interactions (44, 45) and they are both downstream of Rho signaling. HLA I Ab-stimulation of ECs caused a significant increase in levels of phosphorylated MYPT1 and MLC compared to ECs stimulated with isotype control Ab (not shown) or untreated (Figure 6A, 6B) that was reduced to baseline levels by mTOR inhibition with Rapa or RAD, situating the mTOR pathway upstream of Rho signaling. As previously reported, rapalogs blocked HLA I Ab-mediated phosphorylation of downstream mediators of the mTOR pathway including mTOR, p70S6K, Akt, ERK and S6RP (Figure S6) (15). Inhibition of the Rho pathway via indirect ROCK inhibition or direct Rho inhibition blocked HLA I Ab-induced phosphorylation of MYPT1, MLC and ERM (Figure 6A, 6B); however, we saw no effect on inhibition of the mTOR pathway, with no changes in Akt473 or p70S6K389 (Figure S6). Pre-treatment of ECs with pharmacological inhibitors targeting Rho or ROCK, significantly reduced adherence of monocytes to HLA I Ab-activated endothelium (Figure S7). However, PKC inhibition had no effect on levels of phosphorylated target proteins or monocyte adherence (Figure 6A, 6B, S6 and S7). Collectively, these data indicate that following HLA class I engagement by Ab, mTOR signals through the Rho pathway via ROCK to mediate ERM phosphorylation and subsequent monocyte adhesion to ECs.
Figure 6. mTOR regulates HLA class I Ab-induced ERM phosphorylation through the Rho pathway.
ECs were pretreated with Rapa or RAD for 24 h, or Ro-31-7549 (PKC inhibitor), Y-27632 (ROCK inhibitor), or U0126 (C3 transferase; Rho inhibitor) for 4 h, then stimulated with HLA I Ab (anti-HLA I; clone W6/32) for 10 min. (A) Cells were lysed and proteins were separated by SDS–PAGE followed by immunoblotting to detect phosphorylated MYPT1Thr696 (p-MYPT1), p-ERM, and phosho-MLCSer19 (p-MLC), as well as Vinculin to confirm equal loading of proteins. Images from different parts of the same gel are separated by boxes. (B) Protein bands were quantified by densitometry scan analysis using ImageJ and results are expressed as the mean fold increase in phosphorylation above untreated with and without the indicated inhibitor ± SEM over 3 independent experiments. *P<0.05; **P<0.01; ****P<0.0001 by two-way ANOVA with Tukey’s multiple comparisons test.
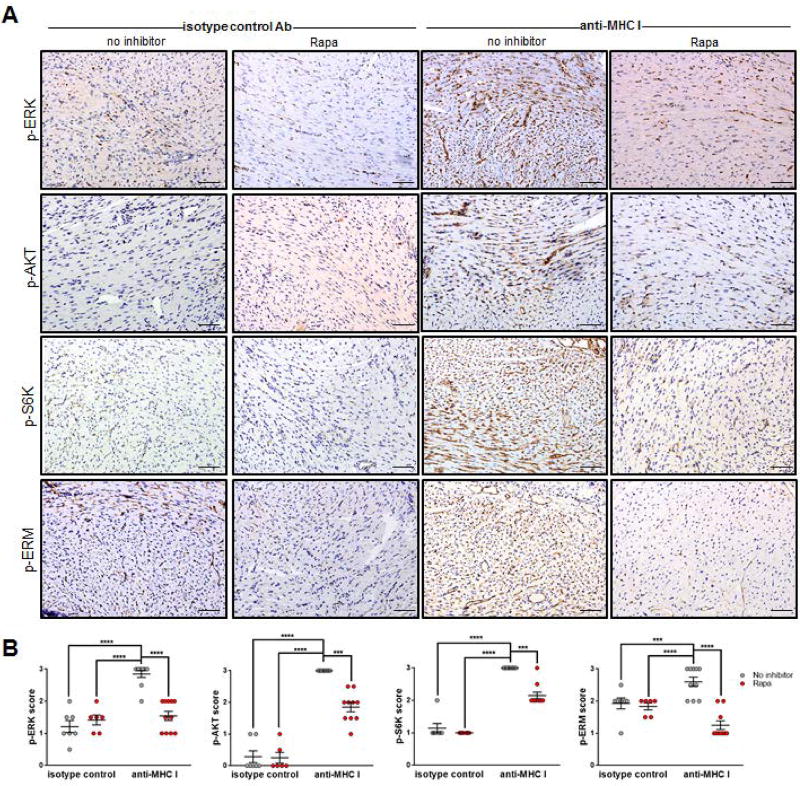
Blockade of mTOR prevents MHC class I Ab-activation of endothelial ERM phosphorylation in murine cardiac allografts during AMR
Our in vitro findings indicated that inhibiting mTOR signaling prevents HLA class I Ab-mediated ICAM-1 clustering in ECs by inhibiting ERM phosphorylation. Therefore we evaluated the effect of Rapa on MHC I Ab-induced phosphorylation of proteins in the ERM pathway in our murine model of cardiac AMR. Mice treated with anti-donor MHC class I Ab demonstrated diffuse capillary staining of phosphorylated ERK, AKT, S6K and ERM (Figure 7) compared to control mAb-treated groups. In the Rapa + MHC I Ab-treated group, expression of all four phosphorylated proteins was significantly diminished. These results provide evidence that mTOR regulates ERM protein activation in vivo similar to the regulation we observed in our in vitro studies.
Figure 7. Rapamycin treatment inhibits activation of anti-HLA class I mAb-induced mTOR signaling proteins in vivo.
BALB/c cardiac allografts were transplanted into B6.RAG1−/− recipients, passively transfused with anti-MHC I or isotype control and treated with daily Rapa before immunohistochemical analysis of proteins involved in MHC I-induced cell survival/proliferation pathways. (A) Grafts were procured at day 30 post-transplant and evaluated for p-ERK, p-AKT, p-S6K and/or p-ERM expression by immunohistochemical staining. All phosphorylated proteins are visible in cardiac vessels and endocardium (dark brown) in cardiac vessels and endocardium. scale bars = 50 µm (C) Shown are mean scores ± SEM for n≥6 per group with each dot representing one animal. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001 by two-way ANOVA with Tukey’s multiple comparisons test.
DISCUSSION
Our results provide the first evidence that ligation of HLA I Ab on ECs leads to ICAM-1 clustering and monocyte firm adhesion through an mTOR/ERM-dependent signaling pathway. Both mTORC1 and mTORC2 were involved in mTOR-mediated monocyte adherence to HLA I Ab-activated ECs. Inhibiting mTOR diminished monocyte adherence in vitro as well as monocyte recruitment and allograft injury in an in vivo model of cardiac AMR, supporting the clinical relevance of these findings.
Leukocyte adhesion to the activated endothelium is tightly regulated by the selectin family of molecules. E- and P-selectin mediate the initial steps of monocyte tethering and adhesion to ECs, whereas ICAM-1 and VCAM-1 are needed for firm adhesion (37). Since previous studies showed crosslinking HLA I on ECs by Ab induces P-selectin expression and monocyte capture (13, 30), we reasoned that mTOR inhibition might affect this process. However, while Rapa inhibits VCAM-1 expression by TNFα-activated ECs (46), neither Rapa nor RAD altered protein expression of VCAM-1, E-selectin, P-selectin, or ICAM-1 in HLA I Ab-activated ECs. Instead, mTOR inhibition abrogated ICAM-1 translocation to the site of leukocytes, which is consistent with previous studies (47).
Phosphorylation of ERM causes a conformational change allowing it to bind ICAM-1’s cytoplasmic tail at its N-terminus and the actin cytoskeleton at its C-terminus, forming a physical bridge which mediates clustering of ICAM-1 on the surface of the cell (38, 47, 48). Here, we provide several lines of evidence that HLA I Ab-mediated ICAM-1 clustering is due to upstream mTOR regulation of ERM phosphorylation. First, mTOR inhibition or protein depletion of Raptor or Rictor in HLA I Ab-stimulated ECs disrupts physical complex formation between phosphorylated ERM and ICAM-1. Second, mTOR inhibition led to a significant reduction in the expression of phosphorylated ERM. Finally, in our model of antibody-mediated cardiac transplant injury, we show a dramatic reduction in phosphorylated ERM accompanied by a significant decrease in intravascular activated mononuclear cells within the capillaries of heart allografts from mice treated with Rapa, consistent with in vitro studies.
The GTP-binding protein Rho regulates actin cytoskeletal remodeling and the formation of cell-cell and cell-substratum adhesion (49). Previous observations that ICAM-1 associates with components of the actin cytoskeleton, together with our studies showing HLA I Ab induces MLC phosphorylation to promote stress fiber formation via Rho kinase (50), suggested likely involvement of Rho family proteins in HLA I Ab-induced ERM phosphorylation. Furthermore, HLA I Ab-ligation promotes hydrolysis of phosphatidylinositol bisphosphate (PIP2) to inositol (1,4,5)-triphosphate (IP3) (51), a requirement for activation of the ERM proteins (52). Notably, RhoA plays an important role in monocyte adhesion to TNFα-activated human ECs (39) and EC activation can lead to Rho-induced ERM phosphorylation (38, 39).
Here, we show that Rho is required for HLA I Ab-mediated phosphorylation of ERM. While Rho or Rho kinase inhibition prevented HLA I-induced ERM phosphorylation, it had no effect on HLA I Ab-induced activation of the mTOR signaling, indicating mTOR is upstream of Rho. RhoA activity has been reported to be dependent on both mTORC1 and mTORC2, which mediate its expression and GTPase activity, respectively (39, 53). HLA I Ab-ligation prompted Rho activation, but also stimulated MLC/MYPT1 phosphorylation and subsequent stress fiber formation in an mTOR-dependent manner. Given its dual role in regulating ERM phosphorylation and actin stress fiber formation, it was unsurprising Rho inhibition dramatically reduced monocyte binding to HLA I Ab-activated endothelium.
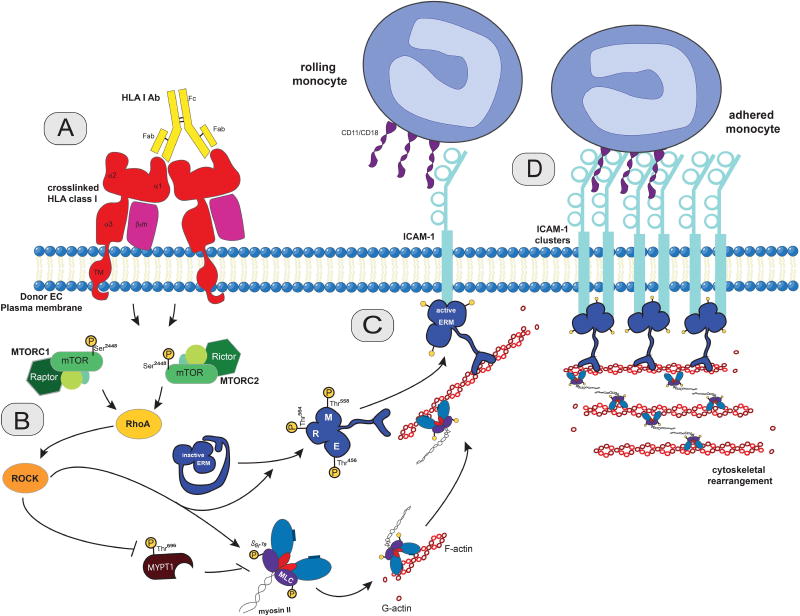
Macrophages profoundly influence various aspects of transplantation, representing important effector cells in the process of antibody-mediated rejection (4, 54). Our findings are consistent with a model in which HLA class I Ab/mTOR-dependent outside-in signaling mechanisms trigger clustering of endothelial ICAM-1 into a membrane structure that provides a platform for stable monocyte firm arrest (Figure 8). Class I mediated activation of mTOR signals through the Rho pathway to mediate ERM phosphorylation. mTOR inhibition in HLA class I antibody-stimulated ECs prevents HLA I antibody-mediated p-ERM and disrupts the association of p-ERM with ICAM-1. Although rapalogs are commonly used as a component of maintenance immunosuppressive therapy, they are primarily credited with directly suppressing T cell activation; however, our findings demonstrate a novel mechanism of suppressing monocyte recruitment to the HLA Ab-activated endothelium during AMR. mTOR inhibitors have therapeutic benefit in reducing the incidence and progression of TAV in cardiac recipients (21–23); therefore, targeting the mTOR pathway may alleviate vascular injury in the setting of AMR by reducing microvascular inflammation. We did not characterize the effect of rapamycin on the thrombin inflammatory pathway that can also mediate monocyte recruitment during AMR via PKC signaling (55). Given the observation of thrombotic microangiopathy in early kidney AMR (56), and the role of thrombin in amplifying the production of monocyte chemoattractant protein-1 (57) it would be important to determine whether or not rapamycin therapy attenuates PKC signaling. It is known that mTORC2 phosphorylates PKCs, as it phosphorylates AKT. Based on previously reported data (58–60), we would argue that long-term treatment with rapamycin is likely to affect PKCs. However, it remains to be tested in primary human endothelial cells. In addition, mTOR’s regulation of monocyte:EC adhesive interactions are likely important in other immune and inflammatory disorders including atherosclerosis and cancer. As such, the signaling pathways we identified here may be broadly applicable to other inflammatory diseases.
Figure 8. Proposed model of outside-in HLA class I-mediated regulation of ICAM-1 clustering and EC-monocyte interactions via mTOR in transplant antibody-mediated rejection.
(A) Antibody-ligation and cross-linking of HLA class I molecules on the surface of ECs initiates outside-in signaling. (B) HLA class I cross-linking triggers mTOR complex 1 (MTORC1) and complex 2 (MTORC2) signaling and downstream activation of Rho to phosphorylate ERM (p-ERM) (C) p-ERM physically associates with both the cytoskeleton (via its F-actin binding site) and ICAM-1 (via its N-terminal FERM domain). (D) This physical association results in ICAM-1 clustering at the surface of ECs and monocyte firm adhesion to ECs.
Supplementary Material
Figure S1. Determination of optimal mTOR inhibitor dosage.
Primary endothelial cells (ECs) were pre-treated with Rapa or RAD for 24 h at the indicated concentrations, then stimulated with HLA class I Ab W6/32 or MEM-147 for 5 min before CFSE-labeled monocytes (MM6 cells) were allowed to adhere for 20 min. Shown are fold changes in numbers of monocytes adherent to treated ECs over monocytes adherent to untreated control ECs (dotted line) ± SEM from five independent experiments.
Figure S2. Efficiency and specificity of mTOR, Rictor and Raptor siRNA knockdown.
ECs were transfected with mTOR, Raptor, Rictor or control siRNA for 4-6 h, then allowed to rest for 48 h before stimulation with HLA class I Ab. After 48 h, ECs were lysed and analyzed by Western blot to determine percent of maximum (% max) for mTOR, Raptor, and Rictor, as well as Vinculin to confirm equal loading of proteins. Protein bands were quantified by densitometry scan analysis using ImageJ and results are expressed as the percentage of maximal increase in protein in ECs given indicated siRNA treatment over control siRNA treated ECs ± SEM over 3 independent experiments. **P<0.01 or ***P<0.001 when comparing ECs given indicated treatment over untreated ECs by one-way ANOVA with Dunnett’s multiple comparisons test.
Figure S3. Monocyte adherence to HLA class I Ab-stimulated endothelium is suppressed when the mTOR pathway is inhibited in ECs irrespective of HLA I Ab epitope recognition.
Primary endothelial cells (ECs) were pre-treated with Rapa or RAD for 24 (mTORC1/2 inhibition) or 2 h (mTORC1 inhibition) at the indicated concentrations, then stimulated with HLA class I Ab W6/32 or MEM-147 for 5 min before CFSE-labeled monocytes (MM6 cells or third-party PBMC-derived) were allowed to adhere for 20 min. Shown are fold changes in numbers of monocytes adherent to treated ECs over monocytes adherent to untreated control ECs (dotted line) ± SEM from five independent experiments. ns=not significant when comparing ECs stimulated with MEM-147 to ECs stimulated with W6/32 by two-way ANOVA with Tukey’s multiple comparisons test.
Figure S4. mTOR does not regulate WPb exocytosis or adhesion molecule expression in HLA class I Ab-stimulated ECs irrespective of HLA I Ab epitope recognition.
Primary endothelial cells (ECs) were pre-treated with Rapa or RAD for 2 h (mTORC1 inhibition) or 24 h (mTORC1/2 inhibition) at the indicated concentrations, then (A) stimulated with HLA class I Ab W6/32 or MEM-147 for 5 min before measuring EC surface P-selectin expression by cell-based ELISA, and EC-release of von Willebrand Factor (vWF) by ELISA of supernatant, or (B) stimulated with HLA class I Ab W6/32 for 5 min before measuring E-selectin and VCAM-1 expression by flow cytometry. Shown are the mean fold increase in treated groups over untreated ECs ± SEM from 3 independent experiments. **P<0.01 by two-way ANOVA with Tukey’s multiple comparisons test.
Figure S5. Phosphorylation of known downstream targets of the mTOR pathway is inhibited by EC pre-treatment with Rapalogs
(A) ECs were pretreated with Rapa or RAD for 24 h and stimulated with anti-ITGB3 or anti-HLA I (clone W6/32) for 10 min. Cells were lysed and proteins were separated by SDS–PAGE followed by immunoblotting to detect p-mTOR, p-P70S6K, p-AKT, p-ERK, p-S6RP as well as Vinculin to confirm equal loading of proteins. Protein bands were quantified by densitometry scan analysis using ImageJ and results are expressed as the mean fold increase of phosphorylated protein in ECs given indicated treatment over untreated ECs ± SEM over 3 independent experiments. **P<0.01, ***P<0.001 or ****P<0.0001 when comparing ECs given indicated treatment over untreated ECs by two-way ANOVA with Tukey’s multiple comparisons test.
Figure S6. Phosphorylation of known downstream targets of the mTOR pathway is not inhibited by EC pre-treatment with Rho pathway inhibitors
(A) ECs were pretreated with Rapa or RAD for 24 h, or Ro-31-7549 (PKC inhibitor), Y-27632 (ROCK inhibitor), or U0126 (C3 transferase; Rho inhibitor) for 4 h, then stimulated with HLA I Ab (anti-HLA I; clone W6/32) for 10 min. Cells were lysed and proteins were separated by SDS–PAGE followed by immunoblotting to detect phosphorylated mTORSer2448 (p-mTOR), p70S6KThr389 (p-p70S6K), and AktSer473 (p-AKT), as well as Vinculin to confirm equal loading of proteins. Images from different parts of the same gel are separated by boxes. (B) Protein bands were quantified by densitometry scan analysis using ImageJ and results are expressed as the mean fold increase in phosphorylation over untreated ECs ± SEM over 3 independent experiments. *P<0.05; **P<0.01 by two-way ANOVA with Tukey’s multiple comparisons test.
Figure S7. Rho inhibitors abrogate HLA I Ab-induced monocyte adherence.
Primary endothelial cells (ECs) were pre-treated with Y-27632, Ro-31-7549 or C3 transferase for 4 h, then stimulated with HLA I Ab W6/32 or MEM-147 for 5 min before CFSE-labeled monocytes (PBMC-derived) were allowed to adhere for 20 min. Shown are numbers of adherent monocytes ± SEM from five independent experiments. *P<0.05; **P<0.01 by two-way ANOVA with Tukey’s multiple comparisons test.
Monocytes were perfused at 1 dynes/cm2 over an endothelial monolayer pre-treated with Rapa for 24 h, then anti-ICAM-1 neutralizing Ab for 30 mins then stimulated with anti-HLA I for 30 mins. Monocyte rolling and adhesion on untreated endothelial cells were acquired in 5-10s videos by the Imaris software. Exported videos show individual monocytes as objects, with each track representing a single monocyte’s movement over the indicated time. Three 5-10s videos were collected in real-time for each condition, and mean velocity (µm/sec) of each monocyte over video duration was determined using ImarisTrack.
Video S1: A representative video of monocyte activity when perfused over untreated ECs.
Video S2: A representative video of monocyte activity when perfused over anti-HLA class I Ab-treated ECs.
Video S3: A representative video of monocyte activity when perfused over Rapa + anti-HLA class I Ab-treated ECs.
Acknowledgments
The authors acknowledge Drs. Nicole M. Valenzuela, Maura Rossetti, and Michelle J. Hickey for critical review of manuscript, and LongSheng Hong and Ngan Doan for technical expertise in immunohistochemical staining. The authors thank all the organ and tissue donors and their families, for giving the gift of life and the gift of knowledge, by their generous donation.
FUNDING
This work was supported by the Ruth L. Kirschstein National Research Service Award (T32HL69766 to SS; T32CA009120 to RAS), as well as the NIH (AI042819) and Pfizer Arts grants (to EFR).
Abbreviations
- Ab
Antibody
- AMR
Antibody-mediated rejection
- ECs
Endothelial cells
- ERM
Ezrin/Radixin/Moesin
- HLA class I antigens
HLA-A, B, C antigens
- ICAM-1
Intercellular adhesion molecule 1
- MLC
Myosin light chain
- mTOR
Mechanistic Target of Rapamycin
- mTORC1
mTOR complex I
- mTORC2
mTOR complex II
- MYPT1
myosin phosphatase target subunit 1
- Rapa
Rapamycin
- TAV
Transplant allograft vasculopathy
- VCAM-1
vascular cell adhesion protein 1
- vWF
von Willebrand Factor
- WPBs
Weibel Palade Bodies
Footnotes
AUTHOR CONTRIBUTIONS
SS and RAS contributed equally to this work. SS, RAS, MF, ER, JWKW and EFR designed research studies. SS, RAS, YJ, SK, and MF conducted experiments and/or acquired data. SS, RAS, YJ, EFR and MF analyzed data. SS, RAS and EFR wrote the manuscript. MF, ER, and JWKW provided critical review of the manuscript.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. EFR received funding for this research through the Pfizer Arts grant. The other authors have no conflicts of interest to disclose.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Colvin RB. Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis. Journal of the American Society of Nephrology. 2007;18(4):1046–1056. doi: 10.1681/ASN.2007010073. [DOI] [PubMed] [Google Scholar]
- 2.Berry GJ, Burke MM, Andersen C, Bruneval P, Fedrigo M, Fishbein MC, et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. Journal of Heart and Lung Transplantation. 2013;32(12):1147–1162. doi: 10.1016/j.healun.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Abrahimi P, Liu R, Pober JS. Blood Vessels in Allotransplantation. American Journal of Transplantation. 2015;15(7):1748–1754. doi: 10.1111/ajt.13242. [DOI] [PubMed] [Google Scholar]
- 4.Fishbein GA, Fishbein MC. Morphologic and immunohistochemical findings in antibody-mediated rejection of the cardiac allograft. Human Immunology. 2012;73(12):1213–1217. doi: 10.1016/j.humimm.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Loupy A, Toquet C, Rouvier P, Beuscart T, Bories MC, Varnous S, et al. Late Failing Heart Allografts: Pathology of Cardiac Allograft Vasculopathy and Association With Antibody-Mediated Rejection. American Journal of Transplantation. 2016;16(1):111–120. doi: 10.1111/ajt.13529. [DOI] [PubMed] [Google Scholar]
- 6.Snyder LD, Wang ZW, Chen DF, Reinsmoen NL, Finlen-Copeland CA, Davis WA, et al. Implications for Human Leukocyte Antigen Antibodies After Lung Transplantation A 10-Year Experience in 441 Patients. Chest. 2013;144(1):226–233. doi: 10.1378/chest.12-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeNicola MM, Weigt SS, Belperio JA, Reed EF, Ross DJ, Wallace WD. Pathologic findings in lung allografts with anti-HLA antibodies. Journal of Heart and Lung Transplantation. 2013;32(3):326–332. doi: 10.1016/j.healun.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirohashi T, Chase CM, Della Pelle P, Sebastian D, Alessandrini A, Madsen JC, et al. A Novel Pathway of Chronic Allograft Rejection Mediated by NK Cells and Alloantibody. American Journal of Transplantation. 2012;12(2):313–321. doi: 10.1111/j.1600-6143.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitchens WH, Chase CM, Uehara S, Cornell LD, Colvin RB, Russell PS, et al. Macrophage depletion suppresses cardiac allograft vasculopathy in mice. American Journal of Transplantation. 2007;7(12):2675–2682. doi: 10.1111/j.1600-6143.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- 10.Thomas KA, Valenzuela NM, Reed EF. The perfect storm: HLA antibodies, complement, Fc gamma Rs, and endothelium in transplant rejection. Trends in Molecular Medicine. 2015;21(5):319–329. doi: 10.1016/j.molmed.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valenzuela NM, Hong L, Shen XD, Gao F, Young SH, Rozengurt E, et al. Blockade of P-Selectin Is Sufficient to Reduce MHC I Antibody-Elicited Monocyte Recruitment In Vitro and In Vivo. American Journal of Transplantation. 2013;13(2):299–311. doi: 10.1111/ajt.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valenzuela NM, Mulder A, Reed EF. HLA Class I Antibodies Trigger Increased Adherence of Monocytes to Endothelial Cells by Eliciting an Increase in Endothelial P-Selectin and, Depending on Subclass, by Engaging Fc gamma Rs. Journal of Immunology. 2013;190(12):6635–6650. doi: 10.4049/jimmunol.1201434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamakuchi M, Kirkiles-Smith NC, Ferlito M, Cameron SJ, Bao C, Fox-Talbot K, et al. Antibody to human leukocyte antigen triggers endothelial exocytosis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(4):1301–1306. doi: 10.1073/pnas.0602035104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li F, Wei J, Valenzuela NM, Lai C, Zhang QH, Gjertson D, et al. Phosphorylated S6 kinase and S6 ribosomal protein are diagnostic markers of antibody-mediated rejection in heart allografts. Journal of Heart and Lung Transplantation. 2015;34(4):580–587. doi: 10.1016/j.healun.2014.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jindra PT, Jin YP, Rozengurt E, Reed EF. HLA class I antibody-mediated endothelial cell proliferation via the mTOR pathwayle. Journal of Immunology. 2008;180(4):2357–2366. doi: 10.4049/jimmunol.180.4.2357. [DOI] [PubMed] [Google Scholar]
- 16.Jin YP, Valenzuela NM, Ziegler ME, Rozengurt E, Reed EF. Everolimus Inhibits Anti-HLA I Antibody-Mediated Endothelial Cell Signaling, Migration and Proliferation More Potently Than Sirolimus. American Journal of Transplantation. 2014;14(4):806–819. doi: 10.1111/ajt.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruneau S, Nakayama H, Woda CB, Flynn EA, Briscoe DM. DEPTOR regulates vascular endothelial cell activation and proinflammatory and angiogenic responses. Blood. 2013;122(10):1833–1842. doi: 10.1182/blood-2013-03-488486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C, Qin LF, Manes TD, Kirkiles-Smith NC, Tellides G, Pober JS. Rapamycin antagonizes TNF induction of VCAM-1 on endothelial cells by inhibiting mTORC2. Journal of Experimental Medicine. 2014;211(3):395–404. doi: 10.1084/jem.20131125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng LH, Zheng XFS. Toward rapamycin analog (rapalog)-based precision cancer therapy. Acta Pharmacologica Sinica. 2015;36(10):1163–1169. doi: 10.1038/aps.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurdi A, De Meyer GRY, Martinet W. Potential therapeutic effects of mTOR inhibition in atherosclerosis. In: Pharmacol BJC, editor. Br J Clin Pharmacol. Br J Clin Pharmacol. 2016. pp. 1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waldner M, Fantus D, Solari M. New perspectives on mTOR inhibitors (rapamycin, rapalogs, and TORKinibs) in transplantation. In: Pharmacol BJC, editor. Br J Clin Pharmacol. Br J Clin Pharmacol: Br J Clin Pharmacol. 2016. pp. 1158–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisen HJ, Kobashigawa J, Starling RC, Pauly DF, Kfoury A, Ross H, et al. Everolimus Versus Mycophenolate Mofetil in Heart Transplantation: A Randomized, Multicenter Trial. American Journal of Transplantation. 2013;13(5):1203–1216. doi: 10.1111/ajt.12181. [DOI] [PubMed] [Google Scholar]
- 23.Andreassen AK, Andersson B, Gustafsson F, Eiskjaer H, Radegran G, Gude E, et al. Everolimus Initiation With Early Calcineurin Inhibitor Withdrawal in De Novo Heart Transplant Recipients: Three-Year Results From the Randomized SCHEDULE Study. American Journal of Transplantation. 2016;16(4):1238–1247. doi: 10.1111/ajt.13588. [DOI] [PubMed] [Google Scholar]
- 24.Li F, Zhang X, Jin YP, Mulder A, Reed EF. Antibody ligation of human leukocyte antigen class I molecules stimulates migration and proliferation of smooth muscle cells in a focal adhesion kinase-dependent manner. Hum Immunol. 2011;72(12):1150–1159. doi: 10.1016/j.humimm.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jindra PT, Hsueh A, Hong L, Gjertson D, Shen XD, Gao F, et al. Anti-MHC class I antibody activation of proliferation and survival signaling in murine cardiac allografts. Journal of Immunology. 2008;180(4):2214–2224. doi: 10.4049/jimmunol.180.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jindra PT, Jin YP, Jacamo R, Rozengurt E, Reed EF. MHC class I and integrin ligation induce ERK activation via an mTORC2-dependent pathway. Biochemical and biophysical research communications. 2008;369(2):781–787. doi: 10.1016/j.bbrc.2008.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Rozengurt E, Reed EF. HLA class I molecules partner with integrin beta4 to stimulate endothelial cell proliferation and migration. Science signaling. 2010;3(149):ra85. doi: 10.1126/scisignal.2001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin YP, Korin Y, Zhang XH, Jindra PT, Rozengurt E, Reed EF. RNA interference elucidates the role of focal adhesion kinase in HLA class I-mediated focal adhesion complex formation and proliferation in human endothelial cells. Journal of Immunology. 2007;178(12):7911–7922. doi: 10.4049/jimmunol.178.12.7911. [DOI] [PubMed] [Google Scholar]
- 29.Jin YP, Singh RP, Du ZY, Rajasekaran AK, Rozengurt E, Reed EF. Ligation of HLA class I molecules on endothelial cells induces phosphorylation of Src, paxillin, and focal adhesion kinase in an actin-dependent manner. Journal of Immunology. 2002;168(11):5415–5423. doi: 10.4049/jimmunol.168.11.5415. [DOI] [PubMed] [Google Scholar]
- 30.Valenzuela NM, Hong L, Shen XD, Gao F, Young SH, Rozengurt E, et al. Blockade of p-selectin is sufficient to reduce MHC I antibody-elicited monocyte recruitment in vitro and in vivo. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(2):299–311. doi: 10.1111/ajt.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valenzuela NM, Trinh KR, Mulder A, Morrison SL, Reed EF. Monocyte Recruitment by HLA IgG-Activated Endothelium: The Relationship Between IgG Subclass and FcgammaRIIa Polymorphisms. In: Angeles UoCaL, editor. American Journal of Transplantation. 2015. p. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mutin M, George F, Lesaule G, Sampol J. Reevaluation of Trypsin-EDTA for Endothelial Cell Detachment before Flow Cytometry Analysis. Endothelium. 1996 [Google Scholar]
- 33.Sosa RA, Murphey C, Ji N, Cardona AE, Forsthuber TG. The Kinetics of Myelin Antigen Uptake by Myeloid Cells in the Central Nervous System during Experimental Autoimmune Encephalomyelitis. Journal of Immunology. 2013;191(12):5848–5857. doi: 10.4049/jimmunol.1300771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sosa RA, Murphey C, Robinson RR, Forsthuber TG. IFN-gamma ameliorates autoimmune encephalomyelitis by limiting myelin lipid peroxidation. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1505955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lones MA, Czer LSC, Trento A, Harasty D, Miller JM, Fishbein MC. CLINICAL-PATHOLOGICAL FEATURES OF HUMORAL REJECTION IN CARDIAC ALLOGRAFTS - A STUDY IN 81 CONSECUTIVE PATIENTS. Journal of Heart and Lung Transplantation. 1995;14(1):151–162. [PubMed] [Google Scholar]
- 36.Raught B, Gingras AC, Sonenberg N. The target of rapamycin (TOR) proteins. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(13):7037–7044. doi: 10.1073/pnas.121145898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Reviews Immunology. 2007;7(9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 38.van Buul JD, van Rijssel J, van Alphen FPJ, van Stalborch AM, Mul EPJ, Hordijk PL. ICAM-1 Clustering on Endothelial Cells Recruits VCAM-1. Journal of Biomedicine and Biotechnology. 2010:9. doi: 10.1155/2010/120328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wojciak-Stothard B, Williams L, Ridley AJ. Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. Journal of Cell Biology. 1999;145(6):1293–1307. doi: 10.1083/jcb.145.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heiska L, Alfthan K, Gronholm M, Vilja P, Vaheri A, Carpen O. Association of ezrin with intercellular adhesion molecule-1 and −2 (ICAM-1 and ICAM-2) - Regulation by phosphatidylinositol 4,5-bisphosphate. Journal of Biological Chemistry. 1998;273(34):21893–21900. doi: 10.1074/jbc.273.34.21893. [DOI] [PubMed] [Google Scholar]
- 41.Ishibashi T, Sakamoto T, Ohkawara H, Nagata K, Sugimoto K, Sakurada S, et al. Integral role of RhoA activation in monocyte adhesion-triggered tissue factor expression in endothelial cells. Arteriosclerosis Thrombosis and Vascular Biology. 2003;23(4):681–687. doi: 10.1161/01.ATV.0000065194.00822.C7. [DOI] [PubMed] [Google Scholar]
- 42.Zhang CY, Wu Y, Xuan ZN, Zhang SJ, Wang XD, Hao Y, et al. p38MAPK, Rho/ROCK and PKC pathways are involved in influenza-induced cytoskeletal rearrangement and hyperpermeability in PMVEC via phosphorylating ERM. Virus Research. 2014;192:6–15. doi: 10.1016/j.virusres.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 43.Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, et al. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. Journal of Cell Biology. 1998;140(3):647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ichikawa K, Ito M, Hartshorne DJ. Phosphorylation of the large subunit of myosin phosphatase and inhibition of phosphatase activity. Journal of Biological Chemistry. 1996;271(9):4733–4740. doi: 10.1074/jbc.271.9.4733. [DOI] [PubMed] [Google Scholar]
- 45.Kaneko-Kawano T, Takasu F, Naoki H, Sakumura Y, Ishii S, Ueba T, et al. Dynamic Regulation of Myosin Light Chain Phosphorylation by Rho-kinase. Plos One. 2012;7(6):10. doi: 10.1371/journal.pone.0039269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang C, Yi T, Qin LF, Maldonado RA, von Andrian UH, Kulkarni S, et al. Rapamycin-treated human endothelial cells preferentially activate allogeneic regulatory T cells. Journal of Clinical Investigation. 2013;123(4):1677–1693. doi: 10.1172/JCI66204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oh HM, Lee S, Na BR, Wee H, Kim SH, Choi SC, et al. RKIKK motif in the intracellular domain is critical for spatial and dynamic organization of ICAM-1: Functional implication for the leukocyte adhesion and transmigration. Molecular Biology of the Cell. 2007;18(6):2322–2335. doi: 10.1091/mbc.E06-08-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, et al. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. Journal of Cell Biology. 2002;157(7):1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ridley AJ. Signaling networks, Rho GTPases and cell migration. Molecular Biology of the Cell. 1999;10:117A–117A. [Google Scholar]
- 50.Ziegler ME, Souda P, Jin YP, Whitelegge JP, Reed EF. Characterization of the Endothelial Cell Cytoskeleton following HLA Class I Ligation. Plos One. 2012;7(1):14. doi: 10.1371/journal.pone.0029472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bian H, Harris PE, Mulder A, Reed EF. Anti-HLA antibody ligation to HLA class I molecules expressed by endothelial cells stimulates tyrosine phosphorylation, inositol phosphate generation, and proliferation. Human Immunology. 1997;53(1):90–97. doi: 10.1016/S0198-8859(96)00272-8. [DOI] [PubMed] [Google Scholar]
- 52.Mahon MJ. Apical membrane segregation of phosphatidylinositol-4,5-bisphosphate influences parathyroid hormone 1 receptor compartmental signaling and localization via direct regulation of ezrin in LLC-PK1 cells. Cellular Signalling. 2011;23(10):1659–1668. doi: 10.1016/j.cellsig.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barilli A, Visigalli R, Sala R, Gazzola GC, Parolari A, Tremoli E, et al. In human endothelial cells rapamycin causes mTORC2 inhibition and impairs cell viability and function. Cardiovascular Research. 2008;78(3):563–571. doi: 10.1093/cvr/cvn024. [DOI] [PubMed] [Google Scholar]
- 54.Salehi S, Reed EF. The divergent roles of macrophages in solid organ transplantation. Current Opinion in Organ Transplantation. 2015;20(4):446–453. doi: 10.1097/MOT.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahman A, Anwar KN, Uddin S, Xu N, Ye RD, Platanias LC, et al. Protein Kinase C-δ Regulates Thrombin-Induced ICAM-1 Gene Expression in Endothelial Cells via Activation of p38 Mitogen-Activated Protein Kinase. Molecular and Cellular Biology. 2001;21(16):5554–5565. doi: 10.1128/MCB.21.16.5554-5565.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meehan SM, Kremer J, Ali FN, Curley J, Marino S, Chang A, et al. Thrombotic Microangiopathy and Peritubular Capillary C4d Expression in Renal Allograft Biopsies. Clinical Journal of the American Society of Nephrology : CJASN. 2011;6(2):395–403. doi: 10.2215/CJN.05870710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen D, Carpenter A, Abrahams J, Chambers RC, Lechler RI, McVey JH, et al. Protease-activated receptor 1 activation is necessary for monocyte chemoattractant protein 1-dependent leukocyte recruitment in vivo. The Journal of experimental medicine. 2008;205(8):1739–1746. doi: 10.1084/jem.20071427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gan X, Wang J, Wang C, Sommer E, Kozasa T, Srinivasula S, et al. PRR5L degradation promotes MTORC2-mediated PKCδ phosphorylation and cell migration downstream of Gα12. Nature cell biology. 2012;14(7):686–696. doi: 10.1038/ncb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomanetz V, Angliker N, Cloëtta D, Lustenberger RM, Schweighauser M, Oliveri F, et al. Ablation of the mTORC2 component rictor in brain or Purkinje cells affects size and neuron morphology. The Journal of Cell Biology. 2013;201(2):293–308. doi: 10.1083/jcb.201205030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masui K, Tanaka K, Ikegami S, Villa GR, Yang H, Yong WH, et al. Glucose-dependent acetylation of Rictor promotes targeted cancer therapy resistance. Proc Natl Acad Sci U S A. 2015;112(30):9406–9411. doi: 10.1073/pnas.1511759112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Determination of optimal mTOR inhibitor dosage.
Primary endothelial cells (ECs) were pre-treated with Rapa or RAD for 24 h at the indicated concentrations, then stimulated with HLA class I Ab W6/32 or MEM-147 for 5 min before CFSE-labeled monocytes (MM6 cells) were allowed to adhere for 20 min. Shown are fold changes in numbers of monocytes adherent to treated ECs over monocytes adherent to untreated control ECs (dotted line) ± SEM from five independent experiments.
Figure S2. Efficiency and specificity of mTOR, Rictor and Raptor siRNA knockdown.
ECs were transfected with mTOR, Raptor, Rictor or control siRNA for 4-6 h, then allowed to rest for 48 h before stimulation with HLA class I Ab. After 48 h, ECs were lysed and analyzed by Western blot to determine percent of maximum (% max) for mTOR, Raptor, and Rictor, as well as Vinculin to confirm equal loading of proteins. Protein bands were quantified by densitometry scan analysis using ImageJ and results are expressed as the percentage of maximal increase in protein in ECs given indicated siRNA treatment over control siRNA treated ECs ± SEM over 3 independent experiments. **P<0.01 or ***P<0.001 when comparing ECs given indicated treatment over untreated ECs by one-way ANOVA with Dunnett’s multiple comparisons test.
Figure S3. Monocyte adherence to HLA class I Ab-stimulated endothelium is suppressed when the mTOR pathway is inhibited in ECs irrespective of HLA I Ab epitope recognition.
Primary endothelial cells (ECs) were pre-treated with Rapa or RAD for 24 (mTORC1/2 inhibition) or 2 h (mTORC1 inhibition) at the indicated concentrations, then stimulated with HLA class I Ab W6/32 or MEM-147 for 5 min before CFSE-labeled monocytes (MM6 cells or third-party PBMC-derived) were allowed to adhere for 20 min. Shown are fold changes in numbers of monocytes adherent to treated ECs over monocytes adherent to untreated control ECs (dotted line) ± SEM from five independent experiments. ns=not significant when comparing ECs stimulated with MEM-147 to ECs stimulated with W6/32 by two-way ANOVA with Tukey’s multiple comparisons test.
Figure S4. mTOR does not regulate WPb exocytosis or adhesion molecule expression in HLA class I Ab-stimulated ECs irrespective of HLA I Ab epitope recognition.
Primary endothelial cells (ECs) were pre-treated with Rapa or RAD for 2 h (mTORC1 inhibition) or 24 h (mTORC1/2 inhibition) at the indicated concentrations, then (A) stimulated with HLA class I Ab W6/32 or MEM-147 for 5 min before measuring EC surface P-selectin expression by cell-based ELISA, and EC-release of von Willebrand Factor (vWF) by ELISA of supernatant, or (B) stimulated with HLA class I Ab W6/32 for 5 min before measuring E-selectin and VCAM-1 expression by flow cytometry. Shown are the mean fold increase in treated groups over untreated ECs ± SEM from 3 independent experiments. **P<0.01 by two-way ANOVA with Tukey’s multiple comparisons test.
Figure S5. Phosphorylation of known downstream targets of the mTOR pathway is inhibited by EC pre-treatment with Rapalogs
(A) ECs were pretreated with Rapa or RAD for 24 h and stimulated with anti-ITGB3 or anti-HLA I (clone W6/32) for 10 min. Cells were lysed and proteins were separated by SDS–PAGE followed by immunoblotting to detect p-mTOR, p-P70S6K, p-AKT, p-ERK, p-S6RP as well as Vinculin to confirm equal loading of proteins. Protein bands were quantified by densitometry scan analysis using ImageJ and results are expressed as the mean fold increase of phosphorylated protein in ECs given indicated treatment over untreated ECs ± SEM over 3 independent experiments. **P<0.01, ***P<0.001 or ****P<0.0001 when comparing ECs given indicated treatment over untreated ECs by two-way ANOVA with Tukey’s multiple comparisons test.
Figure S6. Phosphorylation of known downstream targets of the mTOR pathway is not inhibited by EC pre-treatment with Rho pathway inhibitors
(A) ECs were pretreated with Rapa or RAD for 24 h, or Ro-31-7549 (PKC inhibitor), Y-27632 (ROCK inhibitor), or U0126 (C3 transferase; Rho inhibitor) for 4 h, then stimulated with HLA I Ab (anti-HLA I; clone W6/32) for 10 min. Cells were lysed and proteins were separated by SDS–PAGE followed by immunoblotting to detect phosphorylated mTORSer2448 (p-mTOR), p70S6KThr389 (p-p70S6K), and AktSer473 (p-AKT), as well as Vinculin to confirm equal loading of proteins. Images from different parts of the same gel are separated by boxes. (B) Protein bands were quantified by densitometry scan analysis using ImageJ and results are expressed as the mean fold increase in phosphorylation over untreated ECs ± SEM over 3 independent experiments. *P<0.05; **P<0.01 by two-way ANOVA with Tukey’s multiple comparisons test.
Figure S7. Rho inhibitors abrogate HLA I Ab-induced monocyte adherence.
Primary endothelial cells (ECs) were pre-treated with Y-27632, Ro-31-7549 or C3 transferase for 4 h, then stimulated with HLA I Ab W6/32 or MEM-147 for 5 min before CFSE-labeled monocytes (PBMC-derived) were allowed to adhere for 20 min. Shown are numbers of adherent monocytes ± SEM from five independent experiments. *P<0.05; **P<0.01 by two-way ANOVA with Tukey’s multiple comparisons test.
Monocytes were perfused at 1 dynes/cm2 over an endothelial monolayer pre-treated with Rapa for 24 h, then anti-ICAM-1 neutralizing Ab for 30 mins then stimulated with anti-HLA I for 30 mins. Monocyte rolling and adhesion on untreated endothelial cells were acquired in 5-10s videos by the Imaris software. Exported videos show individual monocytes as objects, with each track representing a single monocyte’s movement over the indicated time. Three 5-10s videos were collected in real-time for each condition, and mean velocity (µm/sec) of each monocyte over video duration was determined using ImarisTrack.
Video S1: A representative video of monocyte activity when perfused over untreated ECs.
Video S2: A representative video of monocyte activity when perfused over anti-HLA class I Ab-treated ECs.
Video S3: A representative video of monocyte activity when perfused over Rapa + anti-HLA class I Ab-treated ECs.