Abstract
During the 20th century parenteral vaccination of dogs at central-point locations was the foundation of successful canine rabies elimination programs in numerous countries. However, countries that remain enzootic for canine rabies have lower infrastructural development compared to countries that have achieved elimination, which may make traditional vaccination methods less successful. Alternative vaccination methods for dogs must be considered, such as oral rabies vaccine (ORV). In 2016, a traditional mass dog vaccination campaign in Haiti was supplemented with ORV to improve vaccination coverage and to evaluate the use of ORV in dogs. Blisters containing live-attenuated, vaccine strain SPBNGAS-GAS were placed in intestine bait and distributed to dogs by hand. Serum was collected from 107 dogs, aged 3 – 12 months with no reported prior rabies vaccination, pre-vaccination and from 78/107 dogs (72.9%) 17 days post-vaccination. The rapid florescent focus inhibition test (RFFIT) was used to detect neutralizing antibodies and an ELISA to detect rabies binding antibodies. Post-vaccination, 38/41 (92.7%) dogs that received parenteral vaccine had detectable antibody (RFFIT >0.05 IU/mL), compared to 16/27 (59.3%, p < 0.01) dogs that received ORV or 21/27 (77.8%) as measured by ELISA (>40% blocking, p < 0.05). The fate of 291 oral vaccines was recorded; 283 dogs (97.2%) consumed the bait; 272 dogs (93.4%) were observed to puncture the blister, and only 14 blisters (4.8%) could not be retrieved by vaccinators and were potentially left in the environment. Pre-vaccination antibodies (RFFIT >0.05 IU/mL) were detected in 10/107 reportedly vaccine-naïve dogs (9.3%). Parenteral vaccination remains the most reliable method for ensuring adequate immune response in dogs, however ORV represents a viable strategy to supplement existing parental vaccination campaigns in hard-to-reach dog populations. The hand-out model reduces the risk of unintended contact with ORV through minimizing vaccine blisters left in the community.
Keywords: Rabies Virus, Canine Rabies Vaccine, Oral Rabies Vaccine, Immune Response, Serology
Introduction
Globally, canine rabies is responsible for more human deaths than any other zoonotic disease[1], and can still be found in 122 primarily low-resource countries[2]. Provision of post-exposure prophylaxis to persons with bites from suspected rabid animals is integral to reducing human burden; however, vaccination of the reservoir species (most often dogs) is recognized as the most cost-effective and permanent solution to rabies prevention[3–5]. To interrupt transmission of canine rabies the World Health Organization (WHO) recommends ≥70% of dogs need to be vaccinated, and this level of herd immunity must be maintained for 3 – 7 years for elimination[6]. Elimination has been achieved in numerous high-income countries through parenteral vaccination at central point locations. However, there have been few low and middle income countries that have achieved this goal nationally.
Many multifaceted barriers prohibit effective vaccination through parenteral routes in low-income countries including lack of funding, infrastructure and political will, poorly organized campaigns, owner’s inability to control their dogs, vaccinator’s inability to reach dogs without extraordinary effort, and large proportions of free-roaming dogs[3, 7–10]. Oral rabies vaccine (ORV) for free roaming dogs has been proposed to reach dog populations inaccessible via parenteral vaccination routes[6]. While ORV for dogs has been utilized in numerous settings[11–15], it is not yet widely integrated into existing vaccination campaigns. For this study, a highly attenuated rabies virus, SPBNGAS-GAS, was selected for ORV. SPBNGAS-GAS is derived from SAD L16, a cDNA clone of the ORV strain SAD B19, two mutations at amino acid positions 194 and 333 of the glycoprotein and an additional identically modified glycoprotein gene are incorporated to enhance its safety profile compared to conventional attenuated oral rabies virus vaccines[16, 17].
In Haiti, canine rabies remains enzootic with current estimates of 130 annual human rabies deaths due to bites from dogs[2, 18]. Barriers to canine rabies elimination persist in Haiti including economic, educational, and cultural factors that make vaccine delivery and rabies surveillance difficult[19]. Furthermore, an estimated 80% of Haiti’s dog population is allowed to roam freely, which negatively impacts the success of central point, parenteral vaccination efforts[19]. The Haiti Ministry of Agriculture, Natural Resources, and Rural Development (MARNDR) has steadily increased the canine rabies vaccination coverage through parenteral vaccination campaigns conducted at central point locations, yet national vaccination coverage has not surpassed 50% of dogs. In 2016, the US Centers for Disease Control and Prevention (CDC) evaluated a dog vaccination campaign organized by MARNDR in partnership with Christian Veterinary Mission, Humane Society International, and IDT-Biologics in Croix-des-Bouquets, Haiti to understand how new methods for vaccination realize national rabies elimination. Methods evaluated included central point, door-to-door, ORV, and capture-vaccinate-release. Here we report the serologic response of previously unvaccinated dogs that received ORV or parenteral rabies vaccine to determine if ORV is a viable strategy for improving vaccination coverage in Haiti.
Materials and Methods
Study Design
This study was conducted during a two-week, dog vaccination campaign in Croix-des-Bouquets, Haiti in 2016, in which 10,000 dogs received parenteral vaccine and 590 dogs ORV. Dogs fitting inclusion criteria (aged 3 – 12 months, owner-reported rabies vaccine-naïve, and owner consent) were enrolled in a sero-survey to compare primary immune response to parenteral and oral vaccination. Veterinary care is not routinely available in this community, access to rabies vaccine is typically only available through annual government sponsored campaigns, making previous history of rabies vaccination unlikely for dogs fitting the inclusion criteria.
Dogs were restrained within 5 minutes of primary vaccination and blood was collected (1.5 – 3 mL). Owners were contacted 17–20 days post-vaccination, and post-vaccination serum was obtained. Sample size was determined by the Fleiss method[20], with alpha = 0.05, beta = 80%, failure in parenteral = 5%, and failure in oral = 25%. The sample size was calculated at 49 dogs for both parenteral and oral. This study was conducted in compliance with approved CDC animal care and use protocol 2757DOTMULX.
Vaccines
7,500 doses of Rabvac 1 (lot: 4130242A, expiration: 08 Sep 2017, Boehringer Ingelheim Vetmedica, Saint Joseph, MO, USA) were procured by CDC. Rabvac 1 was depleted by day 11 of the 14 day campaign, so MARNDR opted to use 3,000 doses of vaccine that was five-months expired, Rabisin R (lot: L399308, expiration: 20 Mar 2016, Merial, Leon, France).
590 doses of ORV was provided by IDT-Biologika, Dessau-Rosslau, Germany (Vaccine strain: SPBNGAS-GAS[21], lot: 0010716, expiration: Oct 2016, experimental batch). A PVC-blister sealed with aluminum foil containing ORV was placed inside 7 – 10 cm of boiled beef or swine intestine. Swine was superior due to the size of the blister.
Oral Vaccination
Prior to distribution of the oral vaccine a thorough safety review was conducted by CDC, MARNDR, and IDT Biologika as per WHO recommendations[6]. During the campaign, consent was obtained from the dog owner before ORV use, and flyers with a phone number to report adverse events or exposures to persons through vaccine contact or the dog’s saliva within 48 hours post-vaccination were provided to the owner (Fig. S1). A hand-out and retrieval model was conducted[6], to ensure that very few vaccine blisters were left in the environment. An enhanced bite and bait contact surveillance system was operated during the campaign and for 1 week post-campaign to improve detection of bites from orally vaccinated dogs or bait contact events[6, 22]. Data were collected on the dog’s response to the ORV bait, puncture of the blister, and ability of the vaccinator to retrieve the blister when not ingested by the dog. Owners of dogs orally vaccinated were not given vaccination certificates; while, owners of dogs vaccinated parenterally, regardless of product, received a rabies vaccination certificate.
Vaccine Conditions
Rabvac 1 arrived in two shipments (3,750 vaccines each); one shipment maintained cold-chain (4–8°C), the second shipment arrived at ambient temperature (presumed duration of 2 – 4 days) and was then properly-stored (4–8°C) during the 1-week prior to the campaign. Before use, the antigen content in improperly-stored vaccine was measured using an antigen capture assay as previously described[23] and was indistinguishable from properly-stored vaccine. Expired vaccine was evaluated post-hoc, but aluminum adjuvant in its formulation interfered with accurate antigen counts.
Serology
Dogs selected for parenteral vaccination were split between three groups: properly-stored (n = 27), improperly-stored (n = 27), and expired (n = 18). Rabies virus neutralizing antibody (rVNA) was measured in serum samples by the rapid fluorescent focus inhibition test (RFFIT) according to a standard protocol[24]. For RFFIT >0.05 IU/mL was considered positive. Serum samples from dogs which received ORV or had pre-vaccination rVNA were also tested using a blocking ELISA (O.K. Servis BioPro, Prague, Czech Republic) according to the manufacturer’s instructions. For ELISA >40% blocking was considered positive. Seroconversion is used to indicate the number of samples that meet the specified cut-off.
Case-Control Follow-up Survey
A semi-structured interview was conducted with owners of dogs that had rVNA pre-vaccination. Dogs without pre-vaccination rVNA and matched based on location of residence, were selected randomly as controls from the pool of dogs for which blood had been collected (i.e. fit inclusion criteria). Information regarding vaccination history, known fights with other dogs, unexplainable wounds, and time spent roaming freely were captured (Fig. S2).
Data Analysis
Results were compared between oral and parenteral at 0.05 IU/mL and within the parenteral group for improperly-stored, properly-stored, and expired vaccines. Geometric mean titers were calculated for each group. Mid-p exact 1-tailed p-values, odds ratios, and 95% confidence intervals were calculated using OpenEpi.
Results
Of 590 oral baits distributed using a hand-out method, the final disposition of 291 were tracked as part of this study (Table 1). 235 baits (80.8%) were distributed to dogs on private property, 50 (17.2%) were distributed to dogs in the street, and for six (2%) the location was not recorded; all had discrete owners. Overall, 283 dogs (97.2%) accepted the bait, and vaccinators reported 272 dogs (93.4%) perforated the blister, suggesting vaccine exposure. Of 291 baits, 277 blisters (95.2%) were ingested or recovered, only 14 baits (4.8%) were not recovered by the vaccinators (Table 2).
TABLE 1.
Dog’s Reaction to the Bait and ORV Blister
| Bait Acceptance | Bait not Accepted | Bait Accepted | TOTAL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Blister Exposure | None | Blister perforated, not ingested | Blister perforated and ingested | Blister not perforated | Blister fate unknown | |||||||
| Location of Bait Offering | n | % | n | % | n | % | n | % | n | % | n | % |
|
|
|
|
||||||||||
| On private propertya | 8 | 3.4% | 120 | 51.1% | 100 | 42.6% | 7 | 3.0% | 0 | 0.0% | 235 | 80.8% |
| On the Street | 0 | 0.0% | 29 | 58.0% | 17 | 34.0% | 0 | 0.0% | 4 | 8.0% | 50 | 17.2% |
| Not Recorded | 0 | 0.0% | 3 | 50.0% | 3 | 50.0% | 0 | 0.0% | 0 | 0.0% | 6 | 2.0% |
|
|
|
|
||||||||||
| TOTAL | 8 | 2.7% | 152 | 52.2% | 120 | 41.2% | 7 | 2.4% | 4 | 1.4% | 291 | |
|
|
|
|
||||||||||
does not imply confined or accessible dogs
TABLE 2.
Recovery of Vaccine Blister Using a Hand-out Method for Oral Rabies Vaccination of Dogs
| ORV Team (total ORV baits) | Blisters Left with the Dog | Blisters Taken by Dog, Fate Unknown | Total Unrecovered Blistersa | Total Blister Recovered or Ingested by Dog |
|---|---|---|---|---|
| A (84 ORV baits) | 2 (2.4%) | 0 (0.0%) | 2 (2.4%) | 82 (97.6%) |
| B (85 ORV baits) | 4 (4.7%) | 0 (0.0%) | 4 (4.7%) | 81 (95.3%) |
| C (80 ORV baits) | 3 (3.8%) | 0 (0.0%) | 3 (3.8%) | 77 (96.2%) |
| D (42 ORV baits) | 1 (2.4%) | 4 (9.5%) | 5 (11.9%) | 37 (88.1%) |
|
| ||||
| TOTAL (291 ORV baits) | 10 (3.4%) | 4 (1.4%) | 14 (4.8%) | 277 (95.2%) |
Refers to ORV blisters that were not ingested and could not be recovered by the vaccination team.
Vaccinators collected pre-vaccination serum from 107 dogs which fit the inclusion criteria: 72 parenterally and 35 orally vaccinated dogs. Parenterally vaccinated dogs were further sub-divided by properly-stored (n = 27), improperly-stored (n = 27), and expired vaccines (n = 18). Of the 107 enrolled dogs, 78 (72.9%) were located 17 days post-vaccination for serum collection: 49/72 dogs (68.0%) parenterally and 29/35 dogs (82.8%) orally vaccinated. Due to poor ability to follow-up on many dogs, the power is 47.9% (goal was 80%).
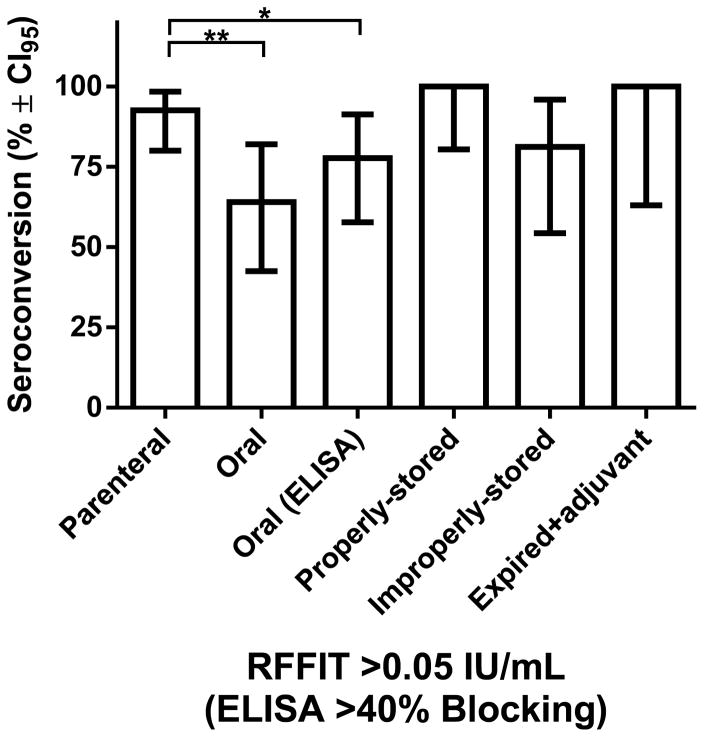
Ten dogs had rVNA pre-vaccination and were not included in aggregate analysis: eight parenterally and two orally vaccinated dogs. Thus, 38/41 (92.7%) parenterally vaccinated dogs in aggregate had detectable rVNA (>0.05 IU/mL) with a GMT of 1.3 IU/mL, and 16/27 (59.3%) orally vaccinated dogs had detectable rVNA (>0.05 IU/mL, p < 0.01) with a GMT of 0.5 IU/mL (Fig. 1). When binding antibodies for the orally vaccinated dogs were measured using a blocking ELISA, 21 dogs (77.8%) had detectable antibodies (>40% blocking, p < 0.05). Seroconversion was not statistically different for dogs that punctured and ingested the blister compared to the group that punctured then expectorated the blister (data not shown).
Figure 1. Comparison of Rabies Antibodies in Rabies Vaccine-naïve Dogs by Vaccine Type and Assay.
Serum collected from 78 dogs, aged 3 – 12 months with no reported prior rabies vaccination, 17 days after rabies vaccination was tested by RFFIT or ELISA. Dogs received either parenteral or oral vaccine. The group that received parenteral vaccine was further sub-divided into dogs that received properly-stored, improperly-stored or expired (with adjuvant) vaccines. Only dogs without detectable rVNA (<0.05 IU/mL) pre-vaccination by RFFIT were included in the analysis. The percentage of dogs with >0.05 IU/mL rVNA by RFFIT or >40% blocking by ELISA and the 95% confidence interval is shown. Using the mid-p exact (1-tailed) test the difference between parenteral and oral vaccination was significant by RFFIT (** p <0.01) and ELISA (* p <0.05). No significant differences were found based on the storage conditions of parenteral vaccine. See Table S1 for complete results.
Dogs receiving properly-stored and improperly-stored parenteral vaccine did not have significantly different antibody responses; 100% and 81.3% respectively (>0.05 IU/mL, p > 0.05, Fig. 1). The GMT for dogs that received properly-stored, improperly-stored, and expired vaccine (with adjuvant) was 1.2 IU/mL, 0.8 IU/mL, and 5.5 IU/mL respectively. Similar to Lankester, et. al. this finding supports that high-quality veterinary vaccines can break cold-chain and maintain immunogenicity[25]. However, each vaccine product and cold-chain disruption event should be critically evaluated. Vaccines that undergo >37°C or >6 month excursions outside of cold-chain were not assessed by Lankester, et. al. or this study.
Ten dogs (9.3%) enrolled in the study had >0.05 IU/mL rVNA pre-vaccination (Table 3). Seven of these samples were also positive for binding antibodies using the ELISA (Table 3). The GMT for these sero-positive dogs was 0.25 IU/mL pre-vaccination and 5.34 IU/mL post-vaccination (21.6-fold increase) compared to 1.29 IU/mL (4.1-fold lower than cases) for matched controls post-vaccination.
Table 3.
Rabies antibody titers for vaccine-naïve dogs with pre-vaccination RFFIT titer >0.05 IU/mL and matched controls (pre-vaccination RFFIT <0.05 IU/mL), before and after primary rabies vaccination
| Pre-Rabies Vaccination | Post-Rabies Vaccination | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Groupa | RFFIT Titer | IU/mL | RFFIT Interpretation | ELISAb | ELISA Interpretation | RFFIT Titer | IU/mL | Interpretation | |
|
|
|
||||||||
| Pre-Vaccination rVNA | PI | 1:25 | 0.18 | Positive | 38% | Negative | 1:1400 | 10 | Positive |
| PE | 1:56 | 0.45 | Positive | 99% | Positive | 1:1300 | 10 | Positive | |
| PP | 1:33 | 0.24 | Positive | 88% | Positive | 1:200 | 1.5 | Positive | |
| PP | 1:7 | 0.052 | Positive | 78% | Positive | 1:250 | 1.8 | Positive | |
| PI | 1:180 | 1.3 | Positive | 75% | Positive | 1:22652 | 170 | Positive | |
| O | 1:29 | 0.21 | Positive | 13% | Negative | 1:1100 | 8.1 | Positive | |
| PI | 1:7 | 0.052 | Positive | 10% | Negative | 1:1400 | 10 | Positive | |
| O | 1:250 | 1.8 | Positive | 89% | Positive | 1:65 | 0.48 | Positive | |
| PE | 1:11 | 0.081 | Positive | 80% | Positive | 1:270 | 2 | Positive | |
| PI | 1:54 | 0.43 | Positive | 77% | Positive | Not available for follow-up | |||
|
|
|
||||||||
| Controls | O | <1:5 | <0.04 | Negative | 11% | Negative | 1:900 | 6.7 | Positive |
| O | <1:5 | <0.04 | Negative | 1% | Negative | <1:5 | <0.04 | Negative | |
| O | <1:5 | <0.04 | Negative | 12% | Negative | <1:5 | <0.04 | Negative | |
| PE | <1:5 | <0.04 | Negative | −8% | Negative | 1:85 | 0.63 | Positive | |
| PE | <1:5 | <0.04 | Negative | 20% | Negative | 1:3125 | 23 | Positive | |
| PP | <1:5 | <0.04 | Negative | 3% | Negative | 1:50 | 0.36 | Positive | |
| PP | <1:5 | <0.04 | Negative | −7% | Negative | 1:625 | 4.6 | Positive | |
| PP | <1:5 | <0.04 | Negative | −17% | Negative | 1:1000 | 7.4 | Positive | |
| PP | <1:5 | <0.04 | Negative | −2% | Negative | 1:1300 | 9.6 | Positive | |
| PI | <1:5 | <0.04 | Negative | 8% | Negative | 1:95 | 0.7 | Positive | |
Vaccine received: PI, parenteral improperly-stored; PE, parenteral expired; PP, parenteral properly-stored; O, oral.
ELISA values represent percent blocking (positive >40% blocking).
Six dog owners for dogs with pre-vaccination rVNA and 6 dog owners for dogs without pre-vaccination rVNA (matched controls) participated in a follow-up survey to evaluate risk factors for rabies exposure in their dogs (Table 4). Lack of previous vaccination was confirmed in all 12 dogs. Dogs with pre-vaccination rVNA were more likely to always be free roaming, have unexplained wounds, have a history of fighting with other dogs, or have both wounds and history of fighting; and less likely to receive veterinary care (Table 4). One owner of a dog with pre-vaccination rVNA reported that the dog had eaten the remains of a dead dog. In a previous study, 10% of found-dead dogs in Haiti were confirmed rabies-positive[22].
Table 4.
Comparison of Rabies Exposure Factors for Dogs with Pre-vaccination Rabies Antibodies (Cases) Compared to Dogs without Pre-vaccination Rabies Antibodies (Controls)
| Exposure Variable | Response | Cases | Controls | Odds Ratio (95% CI)a | P-valueb |
|---|---|---|---|---|---|
| History of Rabies Vaccination | Yes | 0 (0%) | 0 (0%) | NA | NA |
| No | 6 (100%) | 6 (100%) | |||
|
| |||||
| Free Roaming | Always | 5 (83%) | 3 (50%) | 4.0 (0.2 – 88.7) | 0.25 |
| Sometimes | 1 (17%) | 3 (50%) | |||
|
| |||||
| History of Wounds | Yes | 4 (67%) | 2 (33%) | 3.0 (0.3 – 28.8) | 0.38 |
| No | 2 (33%) | 4 (67%) | |||
|
| |||||
| History of Dog Fighting | Yes | 4 (67%) | 3 (50%) | 2.0 (0.2 – 22.1) | 0.63 |
| No | 2 (33%) | 3 (50%) | |||
|
| |||||
| History of Wounds and Fighting | Yes | 5 (83%) | 3 (50%) | 3.0 (0.3 – 28.8) | 0.38 |
| No | 1 (17%) | 3 (50%) | |||
|
| |||||
| Veterinary Care | Yes | 1 (17%) | 2 (33%) | 0.5 (0.02 – 14.9) | 0.5 |
| No | 5 (83%) | 4 (67%) | |||
Matched odds-ratio and 95% confidence interval.
Although sample size is too small (follow-up with owners is difficult in Haiti) to determine significant associations, the direction of the odds-ratio measures are consistently supportive of higher-risk rabies exposure factors for dogs that had pre-vaccination rVNA.
Discussion
Recently numerous programs have achieved rabies control in low-resource settings, yet these programs have not yet achieved sufficient, sustainable coverage to eliminate canine rabies nationally[4, 26, 27]. Expansion of rabies elimination programs in low-resource countries has been constrained by logistics of vaccine procurement, distribution, and trained personnel[3]. Many enzootic countries have high proportions of free-roaming dogs which can further hamper vaccination efforts[9, 19]. Reliance upon central-point, parenteral vaccination may be inadequate in settings with logistical constraints and inaccessible dog populations [8, 28, 29]. Oral vaccination of dogs may overcome some of these limitations[30].
Post-Vaccination Safety Evaluation
No deaths were reported among the 590 baited dogs, and no calls were received regarding human exposure to the baits or vaccine. Furthermore, of 32 dog bites reported in the vaccination zone during the campaign and 2-weeks post-campaign, none were from dogs that had been orally baited. The hand-out model was crucial to reducing ORV exposure among community members, as only 14 (4.8%) vaccine blisters were left in the community. Even this residual rate may be inflated since some baits were likely ingested after the vaccinators left the dog’s territory. These data support that in Haiti, using a hand-out method with SPBNGAS-GAS vaccine in an intestine bait, the likelihood of ORV exposure to people and non-target animals is low.
Performance of ORV
Successful oral vaccination is complex and requires a series of critical steps, including that the animal is attracted to the bait, the blister is punctured, and the vaccine has adequate contact time with the oral mucosa. Any breakdown in these steps can result in vaccination failure. The SPBNGAS-GAS vaccine, packed inside a boiled intestine bait, was highly effective at attracting dogs (97.2% acceptance). Recent ORV studies in dogs and wolves, focusing on this critical step, found acceptance ranging from 47% to 93%[15, 31, 32]. Bait acceptance for dogs in the Philippines using the identical bait and blister was similar (96.1%)[30]. Furthermore, vaccinators noted that 93.4% of dogs punctured the blister, indicating vaccine exposure. Food scarcity for dogs in Haiti likely contributed to bait uptake[19], and in Haiti and other countries with similar dog populations, oral vaccines in an intestine bait are likely to be well-accepted and vaccine exposure in dogs is likely to occur at a high rate.
Overall, 77.8% of orally-vaccinated dogs had evidence of rabies antibodies post-vaccination, which is consistent with reported conversion rates in orally vaccinated wildlife[33–36]. The study design may have unintentionally influenced antibody production. As all dogs selected for this study were free-roaming, vaccinators had to judiciously plan the post-prandial capture of the dog. Although not quantified, supervisors noted that vaccinators captured some dogs before the bait was fully consumed, which could have led to decreased oral mucosal contact and negatively impacted seroconversion. The high rate of antibody production among orally vaccinated dogs in Haiti suggests SPBNGAS-GAS vaccine could be used to improve vaccination coverage among inaccessible dog populations where parenteral vaccination has failed to reach desired coverage levels.
Serial serum sampling was not feasible in this study due to logistical and political constraints: the presidential election occurred 35 days post-vaccination and a low follow-up rate made identification of a meaningful sample unlikely after the election. Sampling 17 days post-vaccination is well supported from previous literature, in which dogs orally vaccinated had peak rVNA detection between 14 and 30 days post-vaccination[30, 31, 37] and 15 days post-vaccination for parenterally vaccinated dogs[38]. While serial sampling was not feasible for this study, another evaluation of SAD B19 ORV in dogs in the Philippines, found 43% of dogs seroconverted (RFFIT ≥ 0.5 IU/mL) by day 15 and that the rate increased to 71% by 29 days post-bait consumption[30]. Thus, our results likely represent a minimum expected seroconversion rate for SPBNGAS-GAS ORV in Haiti.
This study only evaluated the primary immune responses in vaccine-naïve dogs < 1 year old, which results in lower antibody production compared to previously vaccinated or older dogs[39, 40]. Numerous studies have shown higher rates of seroconversion after primary vaccination than we detected[25, 41]. The health status of dogs in this study may have led to reduced responses to both oral and parenteral vaccination. Schildecker et.al. described a 30% annual death rate for dogs in Haiti, and an average body condition score of 3 out of 10, indicating that dogs in Haiti are overall malnourished and potentially immunosuppressed[19]. While seroconversion rates as measured at the >0.5 IU/mL level were lower than expected (Table S1), the most critical measure of successful vaccination is evidence of antibody production[42], and at >0.05 IU/mL, the vast majority of dogs in this study succeeded (Fig. 1). Even animals orally vaccinated with rVNA titers <0.05 IU/mL may be protected from rabies if an anamnestic response is induced[37]. For this reason, we choose to use a blocking ELISA which may provide a better correlate of protection for ORV than RFFIT [43].
Evidence of Rabies Exposures
Rabies is referred to as universally fatal, and once signs appear in animals this is typically true[1]. However, not every rabies exposure leads to infection and disease, with some studies showing that as few as 18% of rabies exposures result in development of disease and death[44, 45]. Wildlife surveys have detected that 1% – 40% of reservoir species may have rVNA in the absence of any vaccination programs[46–49]. This likely reflects prior rabies exposure and aborted infection, rather than recovery from acute disease. In Tanzania and Sri Lanka, 1.3% and 1.6% of dogs, respectively, had >0.5 IU/mL rVNA pre-vaccination[25, 41]. Similarly, we found 2/107 dogs (1.8%) had >0.5 IU/mL pre-vaccination (Table 3); while, 10/107 dogs (9.3%) had >0.05 IU/mL pre-vaccination. A follow-up survey conducted with owners confirmed that these dogs were still alive, and had never received rabies vaccine. The survey also identified elevated risk factors for rabies exposure, including fighting with other dogs and presence of wounds. While the sample size was too small to determine if any of the factors were significant, the direction of the associations indicates higher risk for rabies exposure in dogs with pre-vaccination rVNA compared to dogs without, and leads to speculation that these dogs had past rabies exposure resulting in rVNA. This finding that 9.3% of dogs surveyed may have been exposed to rabies virus suggests a high rabies exposure rate in Haiti. A conclusion supported by previous studies in Haiti[22]. The majority of dogs with pre-vaccination rVNA had robust anamnestic response to vaccination, possibly representing a mode of non-vaccine acquired immunity in Haiti’s dog population.
In many settings, inaccessible dogs cannot be reached through parenteral methods. Therefore, the finding that using a hand-out model, an intestine bait, and SPBNGAS-GAS ORV in Haiti was safe and highly effective for vaccinating dogs could improve vaccination coverage in communities with large populations of inaccessible dogs, and thereby decrease canine-mediated human rabies deaths.
Supplementary Material
Highlights.
Dogs in Croix-des-Bouquets, Haiti received oral rabies vaccine by a hand-out method
78 dogs 3–12 months old were tested pre- and post-vaccination for rabies antibodies
77.8% of orally vaccinated dogs had evidence of rabies antibodies after 17 days
95.2% of oral vaccine blisters were ingested by the dog or retrieved by vaccinators
9.3% of dogs with no reported rabies vaccination had pre-existing rabies antibodies
Acknowledgments
We thank David Guerrier and Emanuel Maciel for assistance in the field; Humane Society International, O.K. Servis BioPro, Cuc Tran, Richard Franka, Michael Niezgoda, Emily Pieracci, Mary Reynolds, Jennifer McQuiston, and Inger Damon for support of this study. Supported in part by a contract between Solution One Industries, Inc. and CDC. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
- CDC
US Centers for Disease Control and Prevention
- GMT
geometric mean titer
- IU
international unit
- MARNDR
Haiti Ministry of Agriculture, Natural Resources, and Rural Development
- ORV
oral rabies vaccine
- rVNA
rabies virus neutralizing antibodies
- RFFIT
rapid fluorescent focus inhibition test
- WHO
World Health Organization
Footnotes
Disclaimer
Use of trade names and commercial sources are for identification only and do not imply endorsement by the U.S. Department of Health and Human Services or Department of Agriculture. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the authors’ institutions.
Conflicts of Interest
Ad Vos is full-time employee of a company that manufactures oral rabies vaccine bait.
References
- 1.Fooks AR, Banyard AC, Horton DL, Johnson N, McElhinney LM, Jackson AC. Current status of rabies and prospects for elimination. Lancet. 2014;384:1389–99. doi: 10.1016/S0140-6736(13)62707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, et al. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis. 2015;9:e0003709. doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace RM, Undurraga EA, Blanton JD, Cleaton J, Franka R. Elimination of Dog-Mediated Human Rabies Deaths by 2030: Needs Assessment and Alternatives for Progress Based on Dog Vaccination. Front Vet Sci. 2017;4:9. doi: 10.3389/fvets.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elser JL, Hatch BG, Taylor LH, Nel LH, Shwiff SA. Towards canine rabies elimination: Economic comparisons of three project sites. Transbound Emerg Dis. 2017;00:1–11. doi: 10.1111/tbed.12637. [DOI] [PubMed] [Google Scholar]
- 5.Anderson A, Shwiff SA. The Cost of Canine Rabies on Four Continents. Transbound Emerg Dis. 2015;62:446–52. doi: 10.1111/tbed.12168. [DOI] [PubMed] [Google Scholar]
- 6.Meslin FX, editor. World Health Organization. Oral vaccination of dogs against rabies. Geneva: World Health Organization; 2007. [Google Scholar]
- 7.Castillo-Neyra R, Brown J, Borrini K, Arevalo C, Levy MZ, Buttenheim A, et al. Barriers to dog rabies vaccination during an urban rabies outbreak: Qualitative findings from Arequipa, Peru. PLoS Negl Trop Dis. 2017;11:e0005460. doi: 10.1371/journal.pntd.0005460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muthiani Y, Traore A, Mauti S, Zinsstag J, Hattendorf J. Low coverage of central point vaccination against dog rabies in Bamako, Mali. Prev Vet Med. 2015;120:203–9. doi: 10.1016/j.prevetmed.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Jackman J, Rowan AN. Free-roaming dogs in developing countries: The benefits of capture, neuter, and return programs. In: Salem D, Rowan AN, editors. The State of Animals IV. Washington, DC: Humane Society Press; 2007. pp. 55–78. [Google Scholar]
- 10.World Health Organization. Report of WHO Consultation on Dog Ecology Studies Related to rabies Control. Geneva: World Health Organization; 1988. [Google Scholar]
- 11.Frontini MG, Fishbein DB, Garza Ramos J, Flores Collins E, Balderas Torres JM, Quiroz Huerta G, et al. A field evaluation in Mexico of four baits for oral rabies vaccination of dogs. Am J Trop Med Hyg. 1992;47:310–6. doi: 10.4269/ajtmh.1992.47.310. [DOI] [PubMed] [Google Scholar]
- 12.Cliquet F, Gurbuxani JP, Pradhan HK, Pattnaik B, Patil SS, Regnault A, et al. The safety and efficacy of the oral rabies vaccine SAG2 in Indian stray dogs. Vaccine. 2007;25:3409–18. doi: 10.1016/j.vaccine.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 13.Hammami S, Schumacher CL, Cliquet F, Barrat J, Tlatli A, Ben Osman R, et al. Safety evaluation of the SAG2 rabies virus mutant in Tunisian dogs and several non-target species. Vet Res. 1999;30:353–62. [PubMed] [Google Scholar]
- 14.Ben Youssef S, Matter HC, Schumacher CL, Kharmachi H, JemLi J, Mrabet L, et al. Field evaluation of a dog owner, participation-based, bait delivery system for the oral immunization of dogs against rabies in Tunisia. Am J Trop Med Hyg. 1998;58:835–45. doi: 10.4269/ajtmh.1998.58.835. [DOI] [PubMed] [Google Scholar]
- 15.Darkaoui S, Boue F, Demerson JM, Fassi Fihri O, Yahia KI, Cliquet F. First trials of oral vaccination with rabies SAG2 dog baits in Morocco. Clin Exp Vaccine Res. 2014;3:220–6. doi: 10.7774/cevr.2014.3.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faber M, Faber ML, Papaneri A, Bette M, Weihe E, Dietzschold B, et al. A single amino acid change in rabies virus glycoprotein increases virus spread and enhances virus pathogenicity. J Virol. 2005;79:14141–8. doi: 10.1128/JVI.79.22.14141-14148.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faber M, Pulmanausahakul R, Hodawadekar SS, Spitsin S, McGettigan JP, Schnell MJ, et al. Overexpression of the rabies virus glycoprotein results in enhancement of apoptosis and antiviral immune response. J Virol. 2002;76:3374–81. doi: 10.1128/JVI.76.7.3374-3381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace RM, Etheart MD, Doty J, Monroe B, Crowdis K, Augustin PD, et al. Dog-Mediated Human Rabies Death, Haiti, 2016. Emerg Infect Dis. 2016;22:1963–5. doi: 10.3201/eid2211.160826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schildecker S, Millien M, Blanton JD, Boone J, Emery A, Ludder F, et al. Dog Ecology and Barriers to Canine Rabies Control in the Republic of Haiti, 2014–2015. Transbound Emerg Dis. 2016;64:1433–1442. doi: 10.1111/tbed.12531. [DOI] [PubMed] [Google Scholar]
- 20.Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. Hoboken, NJ: John Wiley & Sons; 2013. [Google Scholar]
- 21.Blanton JD, Self J, Niezgoda M, Faber ML, Dietzschold B, Rupprecht C. Oral vaccination of raccoons (Procyon lotor) with genetically modified rabies virus vaccines. Vaccine. 2007;25:7296–300. doi: 10.1016/j.vaccine.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace RM, Reses H, Franka R, Dilius P, Fenelon N, Orciari L, et al. Establishment of a High Canine Rabies Burden in Haiti through the Implementation of a Novel Surveillance Program [corrected] PLoS Negl Trop Dis. 2015;9:e0004245. doi: 10.1371/journal.pntd.0004245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith TG, Ellison JA, Ma X, Kuzmina N, Carson WC, Rupprecht CE. An electrochemiluminescence assay for analysis of rabies virus glycoprotein content in rabies vaccines. Vaccine. 2013;31:3333–8. doi: 10.1016/j.vaccine.2013.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yager ML, Moore SM. The Rapid Fluorescent Focus Inhibition Test. In: Rupprecht C, Nagarajan T, editors. Current Laboratory Techniques in Rabies Diagnosis, Research and Prevention. San Diego, CA: Academic Press; 2015. pp. 199–214. [Google Scholar]
- 25.Lankester FJ, Wouters PA, Czupryna A, Palmer GH, Mzimbiri I, Cleaveland S, et al. Thermotolerance of an inactivated rabies vaccine for dogs. Vaccine. 2016;34:5504–11. doi: 10.1016/j.vaccine.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Kayali U, Mindekem R, Yemadji N, Vounatsou P, Kaninga Y, Ndoutamia AG, et al. Coverage of pilot parenteral vaccination campaign against canine rabies in N’Djamena, Chad. Bull World Health Organ. 2003;81:739–44. [PMC free article] [PubMed] [Google Scholar]
- 27.Anyiam F, Lechenne M, Mindekem R, Oussigere A, Naissengar S, Alfaroukh IO, et al. Cost-estimate and proposal for a development impact bond for canine rabies elimination by mass vaccination in Chad. Acta Trop. 2016 doi: 10.1016/j.actatropica.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Lechenne M, Oussiguere A, Naissengar K, Mindekem R, Mosimann L, Rives G, et al. Operational performance and analysis of two rabies vaccination campaigns in N’Djamena, Chad. Vaccine. 2016;34:571–7. doi: 10.1016/j.vaccine.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 29.Gibson AD, Handel IG, Shervell K, Roux T, Mayer D, Muyila S, et al. The Vaccination of 35,000 Dogs in 20 Working Days Using Combined Static Point and Door-to-Door Methods in Blantyre, Malawi. PLoS Negl Trop Dis. 2016;10:e0004824. doi: 10.1371/journal.pntd.0004824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Estrada R, Vos A, De Leon R, Mueller T. Field trial with oral vaccination of dogs against rabies in the Philippines. BMC Infect Dis. 2001;1:23. doi: 10.1186/1471-2334-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berentsen AR, Bender S, Bender P, Bergman D, Gilbert AT, Rowland HM, et al. Bait flavor preference and immunogenicity of ONRAB baits in domestic dogs on the Navajo Nation, Arizona. J Vet Behav. 2016;15:20–4. [Google Scholar]
- 32.Sillero-Zubiri C, Marino J, Gordon CH, Bedin E, Hussein A, Regassa F, et al. Feasibility and efficacy of oral rabies vaccine SAG2 in endangered Ethiopian wolves. Vaccine. 2016;34:4792–8. doi: 10.1016/j.vaccine.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 33.Cliquet F, Robardet E, Must K, Laine M, Peik K, Picard-Meyer E, et al. Eliminating rabies in Estonia. PLoS Negl Trop Dis. 2012;6:e1535. doi: 10.1371/journal.pntd.0001535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins AH, Borden MD, Windmiller BS, Niezgoda M, Marcus LC, O’Brien SM, et al. Prevention of the spread of rabies to wildlife by oral vaccination of raccoons in Massachusetts. J Am Vet Med Assoc. 1998;213:1407–12. [PubMed] [Google Scholar]
- 35.Sidwa TJ, Wilson PJ, Moore GM, Oertli EH, Hicks BN, Rohde RE, et al. Evaluation of oral rabies vaccination programs for control of rabies epizootics in coyotes and gray foxes: 1995–2003. J Am Vet Med Assoc. 2005;227:785–92. doi: 10.2460/javma.2005.227.785. [DOI] [PubMed] [Google Scholar]
- 36.Zienius D, Pridotkas G, Lelesius R, Sereika V. Raccoon dog rabies surveillance and post-vaccination monitoring in Lithuania 2006 to 2010. Acta Vet Scand. 2011;53:58. doi: 10.1186/1751-0147-53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orciari LA, Niezgoda M, Hanlon CA, Shaddock JH, Sanderlin DW, Yager PA, et al. Rapid clearance of SAG-2 rabies virus from dogs after oral vaccination. Vaccine. 2001;19:4511–8. doi: 10.1016/s0264-410x(01)00186-4. [DOI] [PubMed] [Google Scholar]
- 38.Wallace RM, Pees A, Blanton JB, Moore SM. Risk factors for inadequate antibody response to primary rabies vaccination in dogs under one year of age. PLoS Negl Trop Dis. 2017;11:e0005761. doi: 10.1371/journal.pntd.0005761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cliquet F, Verdier Y, Sagne L, Aubert M, Schereffer JL, Selve M, et al. Neutralising antibody titration in 25,000 sera of dogs and cats vaccinated against rabies in France, in the framework of the new regulations that offer an alternative to quarantine. Rev Sci Tech. 2003;22:857–66. doi: 10.20506/rst.22.3.1437. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy LJ, Lunt M, Barnes A, McElhinney L, Fooks AR, Baxter DN, et al. Factors influencing the antibody response of dogs vaccinated against rabies. Vaccine. 2007;25:8500–7. doi: 10.1016/j.vaccine.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Pimburage RMS, Gunatilake M, Wimalaratne O, Balasuriya A, Perera K. Sero-prevalence of virus neutralizing antibodies for rabies in different groups of dogs following vaccination. BMC Vet Res. 2017;13:133. doi: 10.1186/s12917-017-1038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore SM, Pralle S, Engelman L, Hartschuh H, Smith M. Rabies vaccine response measurement is assay dependent. Biologicals. 2016;44:481–6. doi: 10.1016/j.biologicals.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Moore SM, Gilbert A, Vos A, Freuling CM, Ellis C, Kliemt J, et al. Rabies Virus Antibodies from Oral Vaccination as a Correlate of Protection against Lethal Infection in Wildlife. Trop Med Infect Dis. 2017;2:31. doi: 10.3390/tropicalmed2030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Babes V. Traite de la rage. 1912. [Google Scholar]
- 45.Baltazard M, Ghodssi M. Prevention of human rabies; treatment of persons bitten by rabid wolves in Iran. Bull World Health Organ. 1954;10:797–803. [PMC free article] [PubMed] [Google Scholar]
- 46.Berentsen AR, Johnson SR, Gilbert AT, VerCauteren KC. Exposure to Rabies in Small Indian Mongooses (Herpestes auropunctatus) from Two Regions in Puerto Rico. J Wildl Dis. 2015;51:896–900. doi: 10.7589/2015-01-016. [DOI] [PubMed] [Google Scholar]
- 47.Chang JC, Tsai KJ, Hsu WC, Tu YC, Chuang WC, Chang CY, et al. Rabies Virus Infection in Ferret Badgers (Melogale moschata subaurantiaca) in Taiwan: A Retrospective Study. J Wildl Dis. 2015;51:923–8. doi: 10.7589/2015-04-090. [DOI] [PubMed] [Google Scholar]
- 48.Ellison JA, Gilbert AT, Recuenco S, Moran D, Alvarez DA, Kuzmina N, et al. Bat rabies in Guatemala. PLoS Negl Trop Dis. 2014;8:e3070. doi: 10.1371/journal.pntd.0003070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bigler WJ, McLean RG, Trevino HA. Epizootiologic aspects of raccoon rabies in Florida. Am J Epidemiol. 1973;98:326–35. doi: 10.1093/oxfordjournals.aje.a121562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.