Abstract
A recent review on ovarian stem cells by Horan and Williams entitled “Oocyte Stem Cells: Fact or Fantasy?” suggests that the debate on ovarian stem cells (OSCs) is still not over. They did not even discuss the presence of two distinct populations of stem cells in the ovary in their review. OSCs are located in the ovary surface epithelium and Tilly’s group reported them in the size range of 5–8 μm whereas Virant-Klun’s group has reported pluripotent, 2–4 μm OSCs. Our group reported OSCs of two distinct sizes including pluripotent very small embryonic-like stem cells (VSELs) which are smaller in size than RBCs (similar to those reported by Virant-Klun’s group) and slightly bigger (similar to those reported by Tilly’s group) tissue committed progenitors (OSCs) that presumably differentiate from VSELs. These stem/progenitor cells express receptors for follicle stimulating hormone (FSH) and are activated by FSH. Our opinion article provides explanation to several open-ended questions raised in the review on OSCs by Horan and Williams. VSELs survive chemotherapy; maintain life-long homeostasis; loss of their function due to a compromised niche results in age-related senescence and presence of overlapping pluripotent markers suggest that they may also be implicated in epithelial ovarian cancers.
Keywords: Ovary, Stem cells, VSELs, Pluripotent, Menopause, Cancer
We read the review on ovarian stem cells by Horan and Williams [1] and appreciate their efforts in highlighting the current understanding, existing lacunae, and controversies in the field. Authors raised and discussed several pertinent questions regarding various aspects of ovarian stem cells (OSCs) biology and we were indeed intrigued by the review title and the question mark suggesting that the debate on the very existence of OSCs is still not over. The present opinion article provides further clarity to certain issues raised by the authors of the review.
Do OSCs exist and are they comparable among species?
Horan and Williams [1] have very nicely discussed the historical perspective of OSCs and contributions made by Tilly’s group and others. They discussed the controversies regarding selection of markers like DDX-4 to study OSCs by flow cytometry and also doubted whether similar OSCs exist across various species. But the controversy on the use of DDX-4 to enrich OSCs is technical in nature and will subside with time and should not lead to doubting the existence of OSCs. OSCs can also be isolated by gentle scraping of ovary surface epithelium (OSE)—without the use of flow cytometry.
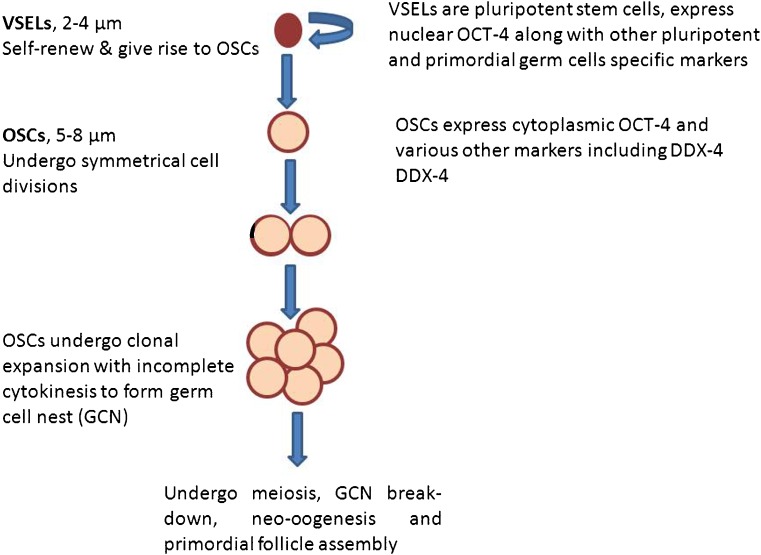
Tilly’s group [2] has focused on the bigger sized (5–8 μm) OSCs whereas Virant-Klun and her group [3] has extensively reported on the smaller sized (2–4 μm), pluripotent stem cells which are the very small embryonic-like stem cells (VSELs) located in the OSE. Our group reported the presence of two populations of stem cells including VSELs and OSCs in mouse, rabbit, marmoset, sheep, and human ovaries [4] and also published protocols for isolating the same [5]. Mouse ovary being very small in size, OSE cells are obtained by partial enzymatic digestion (not by scraping the surface) of the OSE cells [6]. Two distinct populations of stem cells exist in the OSE of various species (based on their size and marker expression) including small sized, pluripotent VSELs expressing nuclear OCT-4A and slightly bigger OSCs with cytoplasmic OCT-4B [7]. As mentioned above also, these two stem cell populations can be obtained and studied by gentle scraping of the surface of fresh as well as fixed ovarian tissue. Recently, the presence of VSELs in mouse ovary and their ability to differentiate into oocyte-like structures was confirmed by a group from Turkey [8]. Figure 1 provides available information on the two stem cell populations, specific markers to identify them and their developmental association. Smaller VSELs exist in all adult tissues [9] along with slightly bigger, tissue committed adult stem cells termed as OSCs in adult ovary, spermatogonial stem cells (SSCs) in adult testis, hematopoietic stem cells (HSCs) in the bone marrow [10, 11]. Immuno-phenotyping results further support the presence of two distinct populations of stem cells expressing OCT-4 based on their size including 2–4 μm cells (1.26 ± 0.19%) and 4–9 μm cells (6.86 ± 0.5%) within the OSE cells of sheep ovary [5]. The concern raised by Horan and Williams [1] regarding a possible mix of germ cells in the cortex while scraping the OSE is not valid. Expertise needs to be developed to apply just the right amount of pressure as the OSE cells are attached tenuously to the underlying ovarian cortex; there is no need to apply excessive pressure to collect OSE cells, rather it is a very superficial scraping and ovarian cortex (whether juvenile or adult) does not get ruptured. In fact, the possible contamination by blood cells is also avoided when stem cells are collected by gentle scraping compared to enzymatic digestion of whole ovary for flow cytometry. Thus two distinct populations of ovarian stem cells (VSELs and OSCs) exist across various species and are similar to stem cells reported in the testis (VSELs and SSCs). Two populations of “dormant” and “active” stem cells have been suggested to exist in various adult organs by other groups as well [12, 13].
Fig. 1.
Two populations of stem cells are located in the ovary surface epithelium including small, pluripotent very small embryonic-like stem cells (VSELs) and slightly bigger, ovary specific progenitors—ovary stem cells (OSCs). They are developmentally connected to each other. VSELs are equivalent to primordial germ cells (PGCs) and express both pluripotent and PGC -specific markers. VSELs self-renew and give rise to OSCs by asymmetric cell division and OSCs in turn divide rapidly, and form germ cell nest by clonal expansion
What markers can be used to study OSCs?
Reply to this question has two aspects including (i) to confirm the presence of ovarian stem cells and (ii) to sort them by flow cytometry. To confirm their presence by flow cytometry studies, either fixed and permeabilized cells are studied or live stem cells are sorted after staining for specific cell surface markers. Use of DDX-4 as a marker to sort OSCs by flow cytometry after enzymatic digestion of ovarian tissue has been debated extensively in the literature, cast a serious doubt on the existence of OSCs and led Horan and Williams to wonder whether ovarian stem cells are a fact or a fantasy? It was argued that DDX-4 is expressed in the cell cytoplasm. Zarate-Garcia et al. [14] reported FACS-sorted putative oogonial stem cells from the ovary were DDX-4 negative whereas Tilly’s group published protocols to isolate OSCs by antibody based flow sorting using antibodies specific for external epitopes of the proteins DDX-4 [15]. As discussed above, OSCs can also be enriched just by gently scraping the OSE, by avoiding flow cytometry [5]. The technical confusion due to the use of DDX-4 antibody needs to be resolved and one should not doubt existence of OSCs based on this confusion. As mentioned above, immuno-phenotyping studies on fixed sheep ovary surface epithelial cells [5] show the presence of OCT-4 positive cells in the size range of 2–10 μm. Sriraman et al. [6] have reported ovarian stem cells (VSELs) with a surface phenotype of LIN−/CD45−/SCA-1+ in adult mouse ovary.
Meiotic markers (STRA8, SCP-3, Spo1 1, Dmc 1) were reported in mouse ovaries by Tilly’s group [16]; however, SCP3 could not be detected in human ovarian cortex [17]. This discrepancy was discussed by Horan and Williams [1] who also concluded that possibly the OSCs remain quiescent and are not expected to express meiotic markers. These markers may be expressed when OSCs get activated and initiate differentiation and meiosis leading to postnatal neo-oogenesis. Parte et al. [18] have reported SCP3 expression on OSCs (isolated from adult human and sheep ovaries) after in vitro culture for 7 days. Although initial stem cells do not express SCP3, but as they differentiate in vitro, SCP3 is expressed. Similarly, c-Kit and ZP expression is expected only on developing (differentiating) oocytes from stem cells and are not specific markers for OSCs. The controversy raised by the use of DDX4 to isolate OSCs needs to be settled and additional cell surface markers like SSEA-4 in humans and SSEA-1 in mice can be used to flow sort OSCs whereas OCT-4 staining on fixed cells could be used to confirm their presence. VSELs can be flow sorted as LIN−/CD45−/CD133+/SSEA-4+ cells from human and as LIN−/CD45−/SCA-1+/SSEA-1+ cells from mouse ovaries.
Where are the OSCs localized—in OSE or bone marrow or both?
The answer to this question will be best understood once it is accepted that ovary houses two stem cell populations including VSELs and OSCs. VSELs are present in all adult organs including the bone marrow—in few numbers and serve as a backup pool of primitive stem cells that give rise to tissue specific adult stem cells (progenitors) and thus are responsible for life-long tissue homeostasis. Whenever there is damage to any adult tissue, VSELs get mobilized and differentiate en route into committed progenitors (specific to the damaged tissue) and later return to basal levels. Accumulating literature on VSELs in reproductive tissues was recently compiled [9]. Johnson et al. [19] reported bone marrow as a likely source of germ cells but their results were challenged by Eggan et al. [20] who found no evidence of germ cells in the bone marrow. The time interval of 4 days to develop parabiotic mice [including 24 h after chemotherapy and another 2–3 days after surgery for anastomosis to develop] was the time when VSELs/OSCs were possibly mobilized and could be detected in circulation. However, Eggan et al. [20] searched for germ cells in circulation after 4–5 days of surgery and this could be the underlying reason why they reported negative and contradictory results. Bhartiya et al. [9] discussed how the studies done on parabiotic mouse ovaries [20] led to confusing results. Thus to answer the question raised by Horan and Williams [1] regarding the location of ovarian stem cells, they are located in OSE and VSELs can also get mobilized from the bone marrow under conditions of stress including oncotherapy.
Do germ cell nests form in adult ovary from the stem cells?
Lei and Spradling failed to detect germ cell nests (GCN) in adult ovary using lineage tracing studies and concluding that adult ovary lacks OSCs [21]; however, Bhartiya et al. [22] had challenged their conclusions. Germ cell nests represent clonal expansion of progenitor ovarian stem cells with incomplete cytokinesis and “sphere formation” and is indeed a characteristic feature of stem cells (Fig. 1). Germ cell nests, Balbiani body formation, and cytoplasmic streaming are indeed hallmark features involved during follicular assembly in fetal ovaries [23]. When exposed to FSH, OSCs are activated and undergo proliferation and clonal expansion to form germ cell nests in vitro. Similar nest formation has also been reported when chemoablated mouse ovary is exposed to FSH [6]. Liu et al. [17] reported that besides germ cell nests formation, OSCs isolated from adult OSE in vitro also show formation of Balbiani bodies and cytoplasmic streaming. To conclude, various characteristic features of fetal OSCs like germ cell nest formation, Balbiani body and cytoplasmic streaming are recapitulated in culture of OSCs isolated from adult ovary.
Do quiescent OSCs exist?
Yes they do. VSELs are quiescent stem cells whereas OSCs undergo rapid proliferation and clonal expansion to form germ cell nests as discussed above. The general belief is that various adult tissues harbor two populations of stem cells including actively dividing and quiescent cells [12, 13]. Ovary also harbors quiescent VSELs and actively dividing OSCs of which VSELs survive chemotherapy in adult mouse ovaries [6]. Sriraman et al. [6] reported 0.02 ± 0.008% of cells as VSELs with a surface phenotype of LIN−/CD45−/SCA-1+ in normal adult mouse ovary, their numbers increase to 0.03 ± 0.017% after chemoablation and further to 0.08 ± 0.03% on treating 3-month-old chemoablated mouse with pregnant mare serum gonadotropin (PMSG) (5 IU). Thus, the concern raised by Horan and Williams [1] that PMSG effect on stem cells was studied in young 4-week-old mouse ovaries [24] and the results cannot be generalized becomes invalid. Both VSELs and OSCs express FSHR and get activated when exposed to FSH and undergo neo-oogenesis and PF assembly [25, 26]. We have also discussed that this action of FSH on ovarian stem cells is possibly mediated via alternately spliced growth factor type-1 FSHR3 [25] similar to testicular stem cells [10].
VSELs survive chemotherapy and are increased in numbers in chemoablated mouse ovary [6]. Similar results were also reported in mouse testis wherein VSELs survived chemotherapy, increased in numbers on D15 after busulphan treatment (control mouse had 3856 ± 1968.78 and busulphan-treated mouse had 23396.67 + 8830.57 VSELs in their testes along with sixfold increase in BrdU uptake), and when further stimulated by FSH treatment to chemoablated testis (0.045 ± 0.008% in untreated control and 0.1 ± 0.03% after FSH treatment). VSELs that survived chemotherapy can restore spermatogenesis when cells comprising the stem cells niche (Sertoli or bone marrow-derived mesenchymal cells) were transplanted via inter-tubular route [10, 11]. A recent meta-analysis describes positive effects of transplanting mesenchymal cells in non-functional ovaries and testis [27].
The surviving VSELs in chemoablated ovary are activated by FSH treatment and initiate neo-oogenesis and primordial follicle assembly [6]. It is the compromised somatic environment (niche) that does not allow further growth of these follicles. Also, extent of damage and recovery is dependent on the chemotherapy regimen. Anand et al. [11] recently reported that transplanting niche (Sertoli or mesenchymal) cells restored spermatogenesis from the surviving VSELs in chemoablated mouse testis. Interesting results were obtained recently by McLaughlin et al. [28] showing 4–10-fold increase in the density of non-growing follicles following non-alkylating ABVD chemotherapy regimen in patients with Hodgkin lymphoma. Authors have discussed that this could be because of stem cells ability to regenerate and undergo neo-oogenesis. Treatment with alkylating agents is relatively harsher and thus similar increase in the numbers of non-growing follicles was not seen in the group treated with OPEA-COPDAC. Quiescent stem cells exist in both adult ovary and testis, survive chemotherapy, and have the potential to regenerate the non-functional gonads.
How do OSCs function in the adult ovary? Does FSH have a role?
The role of OSCs to result in neo-oogenesis and PF assembly has been studied by several groups in vitro whereas data in vivo is yet to emerge. OSCs scraped from the OSE spontaneously differentiate into oocyte-like structures with distinct zona pellucida, exhibit polar body expulsion and also result in the formation of parthenotes [3, 4, 6]. This has been observed using human/sheep/marmoset/rabbit/mouse ovarian stem cells. It has been shown that sheep OSCs express FSHR and are activated by FSH [25]. Similar activation of human ovarian stem cells by FSH has also been reported [29].Thus the observations are not limited to only sheep but similar effects are also noted on human ovaries and it is time for reproductive biologists to take cognizance of this important observation. Tilly’s group has successfully demonstrated the ability of human OSCs to assemble as primordial follicles [2]. Human OSCs were expanded in vitro, labeled with GFP, then transplanted in ovarian cortex and later these cortical tissue pieces were transplanted in immuno-compromised mice. Primordial follicles were observed after 1–2 weeks with GFP-positive oocytes surrounded by GFP-negative granulosa cells. However, how this process of neo-oogenesis and primordial follicle assembly occurs in vivo under normal conditions in adult ovary remains to be demonstrated. In addition to the current understanding that FSH exerts an indirect effect on follicular maturation via FSHR expressed only on granulosa cells, FSH also exerts a direct action on ovarian stem cells to undergo proliferation and germ cell nest formation- ultimately leading to neo-oogenesis and follicular assembly in adult ovary.
Why menopause despite presence of OSCs in aged ovaries?
OSCs exist in adult ovaries and most likely the somatic cells comprising the niche for the stem cells get compromised with age resulting in menopause. Evidently, granulosa cells provide the ovarian microenvironment to the developing egg and their functionality is affected with age. Granulosa cells from aged women compared to younger women harbor greater degree of vacuolization and cristae formation and increased DNA mutations in the mitochondria [30]. Strong evidence in support of this concept comes from Tilly’s group who showed that OSCs from aged ovaries form functional oocytes when transplanted in a young ovary [31]. For neo-oogenesis and primordial follicle assembly in adult ovary, stem cells differentiate into oocytes and granulosa cells differentiate from the OSE cells. Thus, it is likely that OSE cells get affected with age but this remains to be demonstrated.
Therapeutic potential of OSCs
OSCs can be targeted to delay menopause, treat infertility, and restore ovarian function in cancer survivors. There may be actually no need to cryo-preserve ovarian cortical tissue and use later on to transplant or for in vitro maturation of oocytes [32, 33]. Being equivalent to primordial germ cells (natural precursors to gametes), ovarian pluripotent VSELs spontaneously differentiate into oocyte-like structures compared to ES/iPS cells which face several challenges to differentiate into gametes [34]. Regenerating non-functional ovary by transplanting mesenchymal cells is a better approach to restore ovarian function [35] similar to as discussed in chemoablated testis [36]. VSELs have escaped detection over decades because of their inability to pellet down when cells are spun at 1000–1200 rpm while processing for various experiments including flow cytometry [37].
Endogenous VSELs/OSCs or iPS cells to obtain oocytes?
Horan and Williams [1] concluded their review in an interesting manner by suggesting possible use of induced pluripotent stem cells (iPS) cells in place of OSCs to obtain oocytes in future. However, iPS cells retain the residual epigenetic memory of the somatic cells from which they are derived and also harbor genomic and mitochondrial DNA mutations which seriously restrict their clinical utility [38–40]. Thus, it may not be advisable for an aged infertile couple to use autologous adult fibroblasts to obtain iPS cells that could then be differentiated into oocytes/sperm. Even for other clinical conditions, it is being advocated to use allogeneic rather than autologous iPS cells [41]. We do not agree with this concluding remark of Horan and Williams [1] and use of iPS cells to differentiate into gametes appears to be more of a “fantasy” at this stage although success was recently achieved using mouse ES/iPS cells. Much more time is required for such a strategy to reach the clinics. On the other hand, several groups have transplanted mesenchymal cells in chemoablated mouse ovaries and testes and reported live, fertile births [27] and also a baby girl has been born on transplanting autologous mesenchymal cells directly in a POF ovary [42]. Besides being a potential source for oocytes, ovarian VSELs have also been implicated in ovarian cancer [43].
To conclude, lot of progress has been made in the field of ovarian stem cells. It is time to arrive at a consensus regarding their existence rather than getting pulled back by technical issues. More evidence including live cell imaging to demonstrate effect of FSH on ovarian stem cells leading to the formation of germ cell nests in vitro needs to emerge. Also, the scientific community needs to take cognizance of the fact that (i) the stem cells maintain ovarian homeostasis throughout life (otherwise as discussed by Tilly’s group, based on rate of follicular atresia ovary should be depleted of follicles early on in life), (ii) compromised function of the somatic cells (niche-providing cells to the OSCs) with age leads to ovarian senescence, and (iii) uncontrolled proliferation of VSELs located in the OSE possibly leads to cancer (justifying why > 90% ovarian cancers are of epithelial origin).
Acknowledgements
Authors acknowledge the earlier students and project staff in the lab who also contributed to the field. Also help from various funding agencies is also acknowledged. NIRRH accession number for the manuscript is OTH/501/07-2017.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Horan CJ, Williams SA. Oocyte stem cells: fact or fantasy? Reproduction. 2017;154(1):R23–R35. doi: 10.1530/REP-17-0008. [DOI] [PubMed] [Google Scholar]
- 2.Woods DC, Tilly JL. Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat Protoc. 2013;8(5):966–988. doi: 10.1038/nprot.2013.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virant-Klun I, Zech N, Rozman P, Vogler A, Cvjeticanin B, Klemenc P, Malicev E, Meden-Vrtovec H. Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes. Differentiation. 2008;76:843–856. doi: 10.1111/j.1432-0436.2008.00268.x. [DOI] [PubMed] [Google Scholar]
- 4.Parte S, Bhartiya D, Telang J, Daithankar V, Salvi V, Zaveri K, et al. Detection, characterization, and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovary. Stem Cells Dev. 2011; 10.1089/scd.2010.0461. [DOI] [PMC free article] [PubMed] [Retracted]
- 5.Parte S, Patel H, Sriraman K, Bhartiya D. Isolation and characterization of stem cells in the adult mammalian ovary. Methods Mol Biol. 2015;1235:203–229. doi: 10.1007/978-1-4939-1785-3_16. [DOI] [PubMed] [Google Scholar]
- 6.Sriraman K, Bhartiya D, Anand S, Bhutda S. Mouse ovarian very small embryonic-like stem cells resist chemotherapy and retain ability to initiate oocyte-specific differentiation. Reprod Sci. 2015;22(7):884–903. doi: 10.1177/1933719115576727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhartiya D. Ovarian stem cells are always accompanied by very small embryonic-like stem cells in adult mammalian ovary. J Ovarian Res. 2015; 10.1186/s13048-015-0200-0. [DOI] [PMC free article] [PubMed]
- 8.Esmaeilian Y, Atalay A, Erdemli E. Putative germline and pluripotent stem cells in adult mouse ovary and their in vitro differentiation potential into oocyte-like and somatic cells. Zygote. 2017;25(3):358–375. doi: 10.1017/S0967199417000235. [DOI] [PubMed] [Google Scholar]
- 9.Bhartiya D, Shaikh A, Anand S, Patel H, et al. Endogenous, very small embryonic-like stem cells: critical review, therapeutic potential and a look ahead. Hum Reprod Update. 2016;23(1):41–76. doi: 10.1093/humupd/dmw030. [DOI] [PubMed] [Google Scholar]
- 10.Patel H, Bhartiya D. Testicular stem cells express follicle stimulating hormone receptors and are directly modulated by FSH. Reprod Sci. 2016;23(11):1493–1508. doi: 10.1177/1933719116643593. [DOI] [PubMed] [Google Scholar]
- 11.Anand S, Bhartiya D, Sriraman K, Mallick A. Underlying mechanisms that restore spermatogenesis on transplanting healthy niche cells in busulphan treated mouse testis. Stem Cell Rev. 2016;12(6):682–697. doi: 10.1007/s12015-016-9685-1. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327(5965):542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Rosa L, De Luca M. Cell biology: dormant and restless skin stem cells. Nature. 2012;489(7415):215–217. doi: 10.1038/489215a. [DOI] [PubMed] [Google Scholar]
- 14.Zarate-Garcia L, Lane SI, Merriman JA, Jones KT. FACS-sorted putative oogonial stem cells from the ovary are neither DDX4-positive nor germ cells. Sci Rep. 2016;15(6):279–291. doi: 10.1038/srep27991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navaroli DM, Tilly JL, Woods DC. Isolation of mammalian oogonial stem cells by antibody-based fluorescence-activated cell sorting. Methods Mol Biol. 2016;1457:253–268. doi: 10.1007/978-1-4939-3795-0_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428(6979):145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Wu C, Lyu Q, Yang D, Albertini DF, Keefe DL, Liu L. Germline stem cells and neo-oogenesis in the adult human ovary. Dev Biol. 2007;306(1):112–120. doi: 10.1016/j.ydbio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Parte S, Bhartiya D, Patel H, Daithankar V, Chauhan A, Zaveri K, et al. Dynamics associated with spontaneous differentiation of ovarian stem cells in vitro. J Ovarian Res. 2014; 10.1186/1757-2215-7-25. [DOI] [PMC free article] [PubMed]
- 19.Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122(2):303–315. doi: 10.1016/j.cell.2005.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eggan K, Jurga S, Gosden R, Min IM, Wagers AJ. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature. 2006;441(7097):1109–1114. doi: 10.1038/nature04929. [DOI] [PubMed] [Google Scholar]
- 21.Lei L, Spradling AC. Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proc Natl Acad Sci U S A. 2013;110(21):8585–8590. doi: 10.1073/pnas.1306189110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhartiya D, Sriraman K, Parte S, Patel H. Ovarian stem cells: absence of evidence is not evidence of absence. J Ovarian Res. 2013; 10.1186/1757-2215-6-65. [DOI] [PMC free article] [PubMed]
- 23.Lei L, Spradling AC. Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells. Science. 2016;352(6281):95–99. doi: 10.1126/science.aad2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhartiya D, Sriraman K, Gunjal P, Modak H. Gonadotropin treatment augments postnatal oogenesis and primordial follicle assembly in adult mouse ovaries? J Ovarian Res. 2012; 10.1186/1757-2215-5-32. [DOI] [PMC free article] [PubMed]
- 25.Patel H, Bhartiya D, Parte S, Gunjal P, Yedurkar S, Bhatt M. Follicle stimulating hormone modulates ovarian stem cells through alternately spliced receptor variant FSH-R3. J Ovarian Res. 2013; 10.1186/1757-2215-6-52. [DOI] [PMC free article] [PubMed] [Retracted]
- 26.Bhartiya D, Parte S, Patel H, Sriraman K, Zaveri K, Hinduja I. Novel action of FSH on stem cells in adult mammalian ovary induces postnatal oogenesis and primordial follicle assembly. Stem Cells Int. 2016; 10.1155/2016/5096596. [DOI] [PMC free article] [PubMed]
- 27.Fazeli Z, Abedindo A, Omrani MD, Ghaderian SMH. Mesenchymal stem cells (MSCs) therapy for recovery of fertility: a systematic review. Stem Cell Rev. 2017; 10.1007/s12015-017-9765-x. [DOI] [PubMed]
- 28.McLaughlin M, Kelsey TW, Wallace WH, Anderson RA, Telfer EE. Non-growing follicle density is increased following adriamycin, bleomycin, vinblastine and dacarbazine (ABVD) chemotherapy in the adult human ovary. HumReprod. 2017;32(1):165–174. doi: 10.1093/humrep/dew260. [DOI] [PubMed] [Google Scholar]
- 29.Parte S, Bhartiya D, Manjramkar DD, Chauhan A, Joshi A. Stimulation of ovarian stem cells by follicle stimulating hormone and basic fibroblast growth factor during cortical tissue culture. J Ovarian Res. 2013; 10.1186/1757-2215-6-20. [DOI] [PMC free article] [PubMed]
- 30.May-Panloup P, Boucret L, Chao de la Barca JM, Desquiret-Dumas V, Ferré-L’Hotellier V, et al. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum Reprod Update. 2016;22(6):725–743. doi: 10.1093/humupd/dmw028. [DOI] [PubMed] [Google Scholar]
- 31.Niikura Y, Niikura T, Tilly JL. Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment. Aging (Albany NY) 2009;1:971–978. doi: 10.18632/aging.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhartiya D. Use of very small embryonic-like stem cells to avoid legal, ethical, and safety issues associated with oncofertility. JAMA Oncol. 2016;2(5):689. doi: 10.1001/jamaoncol.2016.1002. [DOI] [PubMed] [Google Scholar]
- 33.Bhartiya D, Anand S, Parte S. VSELs may obviate cryobanking of gonadal tissue in cancer patients for fertility preservation. J Ovarian Res. 2015; 10.1186/s13048-015-0199-2. [DOI] [PMC free article] [PubMed]
- 34.Bhartiya D, Anand S, Patel H, Parte S. Making gametes from alternate sources of stem cells: past, present and future. Reprod Biol Endocrinol. 2014; 10.1186/s12958-017-0308-8. [DOI] [PMC free article] [PubMed]
- 35.Bhartiya D. Letter to the editor: rejuvenate eggs or regenerate ovary? Mol Cell Endocrinol. 2017; 10.1016/j.mce.2017.03.008. [DOI] [PubMed]
- 36.Bhartiya D, Anand S. Letter to the editor: effects of oncotherapy on testicular stem cells and niche. Mol Hum Reprod. 2017;23(9):654–655. doi: 10.1093/molehr/gax042. [DOI] [PubMed] [Google Scholar]
- 37.Bhartiya D. Pluripotent stem cells in adult tissues: struggling to be acknowledged over two decades. Stem Cell Rev. 2017; 10.1007/s12015-017-9756-y. [DOI] [PubMed]
- 38.Yoshihara M, Hayashizaki Y, Murakawa Y. Genomic instability of iPSCs: challenges towards their clinical applications. Stem Cell Rev. 2017;13(1):7–16. doi: 10.1007/s12015-016-9680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reardon S. Mutated mitochondria could hold back stem-cell therapies. Nature. 2016; 10.1038/nature.2016.1975.
- 40.Kitada M, Wakao S, Dezawa M. Muse cells and induced pluripotent stem cell: implication of the elite model. Cell Mol Life Sci. 2012;69:3739–3750. doi: 10.1007/s00018-012-0994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Li W, Fu X, Xu Y. The immunogenicity and immune tolerance of pluripotent stem cell derivatives. Front Immunol. 2017;8:645. doi: 10.3389/fimmu.2017.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edessy M, Hosni HN, Shady Y, Waf Y, Bakr S, Kamel M. Autologous stem cells therapy, the first baby of idiopathic premature ovarian failure. Acta Med Int. 2016;3:19–23. doi: 10.5530/ami.2016.1.7. [DOI] [Google Scholar]
- 43.Virant-Klun I, Kenda-Suster N, Smrkolj S. Small putative NANOG, SOX2, and SSEA-4-positive stem cells resembling very small embryonic-like stem cells in sections of ovarian tissue in patients with ovarian cancer. J Ovarian Res. 2016; 10.1186/s13048-016-0221-3. [DOI] [PMC free article] [PubMed]