Abstract
Purpose
The purpose of this study is to investigate whether erythrocyte-sperm separation medium (ESSM) has effects on human sperm motility, morphology, viability, membrane maturity, acrosome integrity, and nuclear attributes before and after cryopreservation.
Methods
Semen samples from normozoospermic (n = 36) and oligozoospermic (n = 9) patients were analyzed. Samples from the same patient were divided into three aliquots: group 1 and group 2 were resuspended in sperm washing media and ESSM, respectively. Group 3 was resuspended in ESSM with blood sample to mimic the extensive number of erythrocytes in the testicular sperm extraction (TESE) material. All groups were evaluated for sperm concentration, motility, Kruger/Tygerberg strict morphology, viability by eosin-nigrosin staining, membrane maturity by hyaluronic acid-binding assay (HBA), acrosomal integrity by Pisum sativum lectin staining, chromatin maturity by aniline blue staining, and DNA integrity by TUNEL assay before and after cryopreservation.
Results
No significant difference was determined between ESSM-treated and ESSM-untreated sperm samples for the sperm parameters tested (p > 0.05). After cryopreservation, total sperm motility and viability decreased regardless of ESSM used. The percentages of sperm with Tygerberg normal morphology, intact acrosome, and HA-bound sperm were found to be lower in oligozoospermic samples before cryopreservation in each group. However, no statistically significant differences were found between oligozoospermic and normozoospermic samples when all groups were compared. Thus, ESSM treatment did not cause a significant change on sperm motility, normal morphology, viability, HA-binding capacity, chromatin maturity, and DNA fragmentation.
Conclusion
ESSM can enhance the efficiency of sperm retrieval protocol and can also decrease the time required to collect spermatozoa while not affecting sperm morphogenetic properties.
Keywords: Erythrocyte-sperm separation, TESE, Human sperm maturity, Sperm parameters, Cryopreservation
Introduction
Infertility is a common medical problem affecting more than 70 million couples around the world [1, 2] and male factors contribute to more than half of the cases [3]. Introduction of intracytoplasmic sperm injection (ICSI) is one of the most reliable methods to ensure fertilization in these cases [4]. Previously, it was assumed that men with nonobstructive azoospermia (NOA) were untreatable due to the absence of spermatozoa in the sediment of a centrifuged semen sample [5]. However, the development of surgical sperm retrieval techniques such as testicular sperm extraction (TESE) [6] and further demonstration of fertilizing ability of testicular sperm with ICSI offered a great chance for parenthood to these couples [7, 8].
The TESE process requires several surgical biopsies from the testes. In order to retrieve spermatozoa from these biopsies, mechanical [9], enzymatic [10] or a combination of both dissociation [11] techniques must be applied to the tissues in a petri dish containing medium. Even though the first successful pregnancy reported was by the use of enzymatic digestion of TESE material followed by ICSI treatment [12], it requires incubation of testicular tissues with different enzymes such as collagenase type IA or trypsin-DNAse [13] which may actually create negative effects on spermatozoa including decreased motility [10] or formation of intercellular bridges [14]. In the case of mechanical dissociation, testicular tissue pieces are minced and shredded in a petri dish containing medium to obtain spermatozoa. Although mechanical preparation of testicular tissues is quite fast in many cases, it could also cause contamination of cellular suspension by residual tissue pieces or cells such as erythrocytes. In a recent study, it has been shown that 37% of couples, who applied to infertility clinics due to NOA, have obtained live birth deliveries after TESE-ICSI treatment [15]. Although TESE-ICSI treatment is the first line of treatment for NOA [16], the success of sperm retrieval after TESE depends on the degree of testicular failure. According to the literature, spermatozoa cannot even be isolated from testicular tissues in 30–50% men with NOA even after long search [5]. Therefore, sperm extraction from testicular biopsies is crucial for the ICSI procedure. Furthermore, cryopreservation of obtained sperm is important for the infertility treatment since it provides the opportunity to perform more than one ICSI cycle [17] and reduces the necessity of repeated surgical sperm retrieval procedures which could potentially cause permanent testicular injury [18]. Thus, cryopreservation of testicular spermatozoa has significant medical, emotional, time wise, and financial benefits for patients [15].
In difficult cases with very few sperm cells, the abundant erythrocytes present after shredding the testicular biopsy makes sperm retrieval difficult by lengthening the time required to find sperm [19]. Nagy et al. (1997) showed that lysing erythrocytes in testicular biopsies that do not appear to contain visible spermatozoa facilitates the sperm recovery. The study included seven ICSI treatments out of 15 NOA cases and reported no difference in fertilization or development rates between erythrocyte lysing buffer-treated and erythrocyte lysing buffer-untreated samples [19]. Despite the use of erythrocyte-sperm separation medium (ESSM) in different clinics nowadays, the effect of this medium on sperm parameters remains uncertain. Thus, in the present study, we aimed to determine the possible effects of ESSM treatment on several sperm parameters before and after cryopreservation since in most cases, TESE-extracted sperm is cryopreserved.
Materials and methods
Selection of study samples
Since pre-epididymal testicular sperm is precious for ICSI procedure in clinics, semen samples were obtained and studied from the leftover portion of semen after routine semen analysis at the Andrology Laboratory, Department of Urology, Akdeniz University School of Medicine. All patients had a normal physical evaluation and their ages were ranging from 18 to 52. The average ages of the patients in the normozoospermic and oligozoospermic groups were 35.28 ± 1.51 and 36.67 ± 2.55, respectively. The samples were collected by masturbation into sterile wide mouth plastic jars following 2–5 days of sexual abstinence. Samples were allowed to liquefy at room temperature, then sperm concentrations were assessed according to World Health Organization (WHO) criteria 2010 [20]. Both normozoospermic (93.26 ± 16.28 × 106 sperm/Ml; n = 36) and oligozoospermic (10.26 ± 2.08 × 106 sperm/Ml; n = 9) sperm samples were included in the study (totally, n = 45 samples). In addition to sperm concentration, the sperm initial motility, morphology, viability, and sperm membrane maturity by hyaluronic acid-binding assay (HBA) were analyzed (Table 1). All studies were approved by Ethical Committee of School of Medicine in Akdeniz University (11.06.2013/114).
Table 1.
Initial characteristics of semen samples
| Initial | Concentration (×106 sperm) | Motility (%) | Morphology (%) | Viability (%) | HBA (%) |
|---|---|---|---|---|---|
| Overall | 76.66 ± 13.92 | 43.03 ± 2.74 | 15.26 ± 1.14 | 79.83 ± 1.71 | 49.52 ± 4.01 |
| Oligozoospermic | 10.26 ± 2.08 | 26.93 ± 4.42 | 15.00 ± 4.32 | 79.34 ± 2.16 | 40.63 ± 8.37 |
| Normozoospermic | 93.26 ± 16.28 | 47.05 ± 2.89 | 15.30 ± 1.16 | 79.63 ± 1.95 | 51.74 ± 4.53 |
Data are presented as (mean ± SEM); motility, morphology, viability, and HBA values indicate the percentage of total spermatozoa (%)
Experimental design and sample preparation
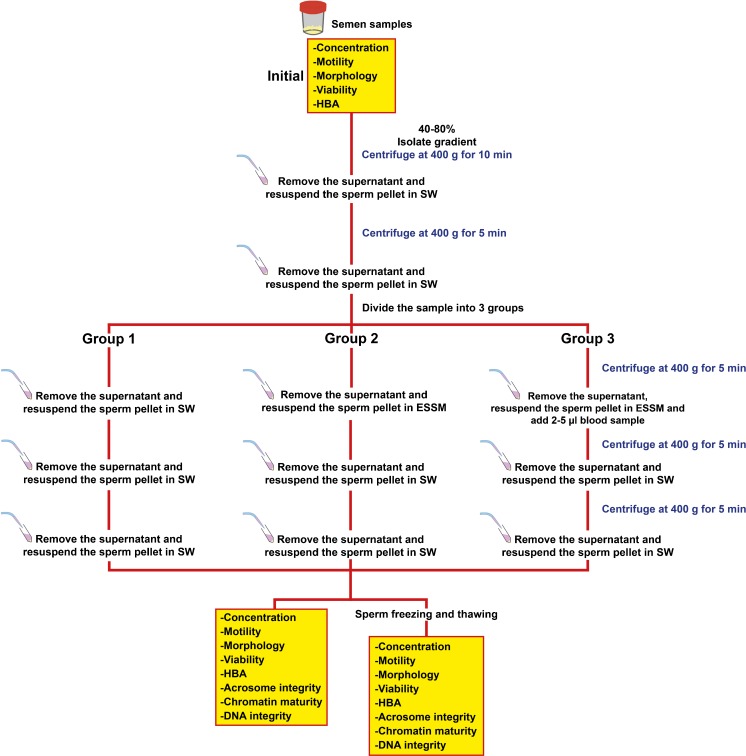
To evaluate the possible effects of ESSM on semen parameters, TESEparate™ (Korpus Biotechnology, Ankara, Turkey) ESSM was used. After assessment of initial sperm parameters, the samples were divided into three aliquots to establish experimental groups; the effect of ESSM on semen parameters was then evaluated before and after sperm cryopreservation (Fig. 1).
Fig. 1.
Experimental design of the study. HBA hyaluronic acid-binding assay, SW sperm washing medium
To remove seminal plasma, sperm samples were layered on a 40–80% isolate gradient (Irvine Scientific, Santa Ana, California) and centrifuged at 400 g for 10 min at room temperature. After resuspending the sperm pellet in sperm washing medium (Irvine Scientific, Santa Ana, California), the sperm suspension was centrifuged at 400 g for 5 min at room temperature to remove gradient solution. The sperm pellet was resuspended in sperm washing medium and total volume divided into three sterile 15 mL Falcon® centrifuge tubes. The three different tubes of sperm samples were centrifuged at 400 g for 5 min at room temperature. Group 1 (untreated) was resuspended in 3 ml of sperm washing media. Group 2 was resuspended in 3 ml of ESSM to test the effects of ESSM on sperm. Group 3 was resuspended in 3 ml of ESSM with a blood sample (2–5 μl) to mimic the extensive number of erythrocytes in TESE material. Group 3 was included to compare the potential effects of hemoglobin release on sperm in comparison to group 2 without blood present. Samples were incubated in related media for 5–10 seconds and centrifuged at 400 g for 5 min; sperm pellets were resuspended in sperm washing medium. This washing step was repeated. After the final washing step, all groups were evaluated for sperm concentration, motility, morphology, viability, membrane maturity (HBA), acrosome integrity, chromatin maturity, and DNA integrity. All parameters were evaluated before and after sperm cryopreservation.
Sperm concentration and motility determination
The semen samples were mixed before each measurement and applied to the Makler chamber. Ten squares in Makler chamber were evaluated from two drops of each sample. Sperm concentration and percent motility were determined by calculating the mean value from two drops.
Morphological assessment of sperm
5–10 μl aliquots of sperm suspension were smeared onto clean, glass slides and were allowed to air-dry. The smears were then stained with Spermac™ kit (FertiPro N.V., Beernem, Belgium) according to the manufacturer’s instructions. Sperm morphology was assessed according to Kruger/Tygerberg criteria [21, 22]. To evaluate sperm morphology, at least 200 sperm (100 sperm twice) were counted for each slide; the percentages of morphologically normal sperm were calculated [23].
Eosin-nigrosin staining for sperm viability
The underlying principle of eosin-nigrosin staining is based on cell membrane integrity [24]. Equal volumes of sperm suspension and eosin-nigrosin stain (1:1) were mixed in 1.5 ml sterile tubes, incubated 30 seconds at room temperature, and then smeared onto clean, glass slides. Spermatozoa were evaluated at ×400 magnification; at least 200 sperm (100 sperm twice) were counted for each slide; the percentages of live (unstained) and dead (stained with eosin) sperm were calculated.
Hyaluronic acid-binding assay (HBA) (membrane maturity)
Hyaluronic acid (HA) is a polysaccharide in the extracellular matrix of the cumulus oophorus [25–27]. Since sperm plasma membrane remodeling, the formation of zona pellucida and HA-binding sites are related; the hyaluronic acid-binding assay (HBA) has been suggested as a novel technique to select mature sperm with no cytoplasmic retention, persistent histones, and DNA chain breaks [28–31]. 7 μl aliquots of sperm suspension were added to the HA-coated surfaces of HA-binding slides (Origio, MidAtlantic Devices Inc., USA) [31, 32]. Gridded cover slips were placed on slides and HA-bound and HA-unbound spermatozoa were counted [30, 32]. At least 200 sperm (100 sperm twice) were counted and the final percentage of HA-bound and HA-unbound sperm was determined by averaging two counts.
Pisum sativum lectin (PSA) staining for acrosome integrity
The outer acrosomal membranes [33] and lectins are widely used to assess the acrosome reaction in vitro [34]. Pisum sativum lectin binds to α-mannosyl residues in the acrosomal matrix [35, 36] and fluorescence-labeled lectin staining was performed to assess acrosome reaction of ESSM-treated and ESSM-untreated sperm before and after cryopreservation with a previously published protocol [30]. The scoring criteria indicated as follows: (1) spermatozoa with intact acrosome were defined by bright fluorescence over the sperm head, (2) moderate fluorescence over the sperm head, (3) meridional linear fluorescence around the sperm head reflected capacitating spermatozoa, or (4) dark sperm head without fluorescence indicated acrosome-reacted dull sperm [30]. The evaluation of acrosomal status was performed using Olympus BX61 fluorescence microscope at ×600 magnification. 200 sperm (100 sperm twice) were counted and the percentage was determined by averaging two counts.
Aniline blue staining for sperm chromatin maturity
The degree of histone-protamine transition can be assessed by aniline blue staining [37]. Acidic aniline blue stains histones so that mature sperm (light) were stained with aniline blue very lightly, since they have completed histone-protamine transition. To determine whether ESSM treatment affects the histone-protamine transition, staining was performed as previously described [38–40]. 200 sperm (100 sperm twice) were counted at ×400 magnification. The percentages of mature sperm (light), moderately immature sperm (intermediate), and severely immature sperm (dark) were determined by calculating the mean value from two counts.
DNA integrity assessment by TUNEL assay
The sperm slides were prepared by adding 2 or 3 drops of PB-suc to clean poly-l-lysine-coated glass slides; 3–5-μl aliquots of the sperm suspension were added into the PB-suc drops. Glass slides were incubated overnight in a humidified chamber at 4 °C to allow the sperm to settle onto the poly-l-lysine-coated slides. The following day, spermatozoa were fixed with 3.7% formalin in PB-suc for 20 min. After removal of the formalin, the slides were allowed to air-dry. Following two PB-suc washes, the slides were exposed to permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate). After permeabilization, samples were washed with PB-suc twice and incubated with TUNEL reaction mixture in the dark at 37 °C for 1 h. The TUNEL label solution and TUNEL dilution buffer with the enzyme omitted were applied to some slides as a negative control at the performance of each experiment (Roche, Basel, Switzerland). The sperm were then examined under Olympus BX61 fluorescence microscope at ×600 magnification. On each slide studied, 200 sperm cells were randomly counted and the percentage of apoptotic cells was determined.
Sperm cryopreservation
The sperm samples (n = 30) were cryopreserved rapidly by using freezing medium containing Test yolk buffer with gentamicin sulfate (cat# 90128; Irvine Scientific, CA, USA). The sperm suspensions were transferred to cryovials and the equal volume of freezing medium was slowly added dropwise, mixing thoroughly as each drop was added. Cryovials were loaded into straws and straws were placed at a distance of 5 cm above the level of liquid nitrogen for 15 min and then immersed in liquid nitrogen.
Sperm thawing
The sperm samples were thawed in a water-bath at 37 °C for approximately 2 min. Once the sperm was thawed, the sperm was transferred immediately to sterile 15-ml centrifuge tubes, washing medium added, mixed gently, and centrifuged at 400 g for 5 min at room temperature to remove freezing medium. After centrifugation, the sperm pellet was resuspended in sperm washing medium (Sperm washing medium, Irvine Scientific, Santa Ana, CA, USA) and the same sperm parameters were evaluated as were performed before cryopreservation.
Statistical analysis
All data was exported directly to Microsoft® Office Excel® 2007 (Microsoft; Redmond, USA) and statistical analysis was performed by SigmaStat® 3.5 (Systat Software; San Jose, USA). Samples were grouped by different media conditions (groups 1, 2 and 3) or before and after cryopreservation. The differences were evaluated by one-way ANOVA or paired t test, respectively. All data were expressed as mean ± SEM and statistical significance was defined as p < 0.05.
Results
Sperm motility
In order to evaluate the potential effects of ESSM on sperm motility, the percentage of sperm motility in three groups was evaluated before and after cryopreservation (Table 2). Following the thawing of samples, there was a significant difference (p < 0.05) between raw total sperm motility (43.03 ± 2.74%) and frozen-thawed sperm total motility with either group 1 (28.67 ± 4.13%), group 2 (25.12 ± 3.64%), or group 3 (23.50 ± 3.70%). However, no significant difference was determined between group 1, group 2, and group 3 for either oligozoospermic or normozoospermic samples before cryopreservation. Similarly, after cryopreservation, sperm motility did not show significant difference between the three groups. Our data showed that total sperm motility decreased after cryopreservation regardless of the ESSM; thus, it is the cryopreservation process which resulted in a loss of motile sperm during the freezing and thawing process.
Table 2.
The percentage of sperm motility between erythrocyte-sperm separation medium-treated and erythrocyte-sperm separation medium-untreated samples before and after cryopreservation
| Overall (%) | Oligozoospermic (%) | Normozoospermic (%) | |
|---|---|---|---|
| Before cryopreservation | |||
| Group 1 | 40.93 ± 3.43 | 24.33 ± 6.07 | 45.08 ± 3.73 |
| Group 2 | 35.05 ± 3.25 | 20.44 ± 6.19 | 38.70 ± 3.53 |
| Group 3 | 34.34 ± 3.44 | 18.09 ± 5.45 | 38.40 ± 3.81 |
| After cryopreservation | |||
| Group 1 | 28.67 ± 4.13 | 13.20 ± 5.23 | 32.96 ± 4.62 |
| Group 2 | 25.12 ± 3.64 | 9.88 ± 4.35 | 29.36 ± 3.97 |
| Group 3 | 23.50 ± 3.70 | 12.20 ± 5.31 | 26.64 ± 4.25 |
Data are presented as (mean ± SEM)
p values for before and after cryopreservation were p = 0.63 and p = 0.89 (oligozoospermic samples), p = 0.37 and p = 0.58 (normozoospermic samples), respectively
Sperm morphology
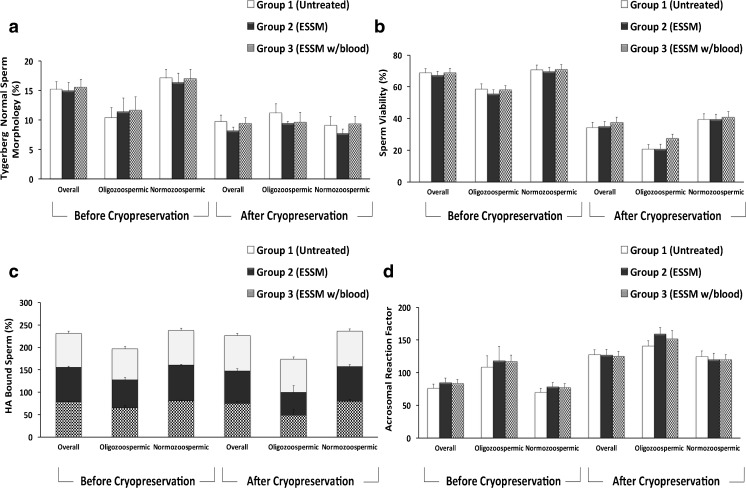
The percentage of normal sperm morphology was compared between the three groups. Data analysis was performed with both overall data and data from oligozoospermic and normozoospermic samples. As presented in Fig. 2a, the results indicated that ESSM did not have an adverse effect on sperm morphology between the three groups when compared to before and after cryopreservation for the overall data (p = 0.96 and p = 0.49, respectively). The percentage of sperm with normal morphology was found to be lower in oligozoospermic samples before cryopreservation in each group. However, the results were not any different in oligozoospermic or normozoospermic samples between groups 1, 2, and 3 either before (p = 0.92 and p = 0.69, respectively) or after cryopreservation (p = 0.60, both).
Figure 2.
a Morphologically normal sperm in ESSM-treated and ESSM-untreated sperm before and after cryopreservation. No significant difference was observed between three groups before (p = 0.96 for overall, p = 0.92 for oligozoospermic, and p = 0.69 for normozoospermic samples) and after cryopreservation (p = 0.49 for overall, p = 0.60 for both oligozoospermic and normozoospermic samples). b The percentage of alive (unstained) sperm after eosin-nigrosin staining refers to sperm viability in ESSM-treated and ESSM-untreated sperm before and after cryopreservation. No significant difference was observed between three groups before and after cryopreservation. p values for before and after cryopreservation were p = 0.65 and p = 0.05 (oligozoospermic samples), p = 0.89 and p = 0.98 (normozoospermic samples), respectively. c The percentage of HA-bound sperm showing the membrane maturity of ESSM-treated and ESSM-untreated sperm before and after cryopreservation. No significant difference was observed between three groups before and after cryopreservation. p values for before and after cryopreservation were p = 0.77 and p = 0.30 (oligozoospermic sampels), p = 0.52 and p = 0.74 (normozoospermic samples), respectively. d The percentage of the integrity of acrosome reaction. No significant difference was observed between 3 groups in overall, oligozoospermic and normozoospermic before (p = 0.56, p = 0.90, and p = 0.55, respectively) and after cryopreservation (p = 0.89, p = 0.33, and p = 0.92, respectively)
Sperm viability
According to the eosin-nigrosin staining data, sperm viability decreased significantly after cryopreservation (Fig. 2b). However, when comparing the groups to each other, we found no significant differences between either ESSM-treated (group 2 oligozoospermic 55.57 ± 2.51% and normozoospermic 69.43 ± 2.93%; group 3 oligozoospermic 58.21 ± 2.48% and normozoospermic 70.94 ± 3.03%) and ESSM-untreated sperm (group 1 oligozoospermic 58.64 ± 3.16% and normozoospermic 70.78 ± 2.97%). Even after sperm cryopreservation, the percentage of viable sperm did not show any significant difference between ESSM-treated (group 2 oligozoospermic 20.85 ± 3.11% and normozoospermic 39.57 ± 3.16%; group 3 oligozoospermic 27.48 ± 2.61% and normozoospermic 40.78 ± 3.70%) and ESSM-untreated samples (group 1 oligozoospermic 20.71 ± 2.88% and normozoospermic 39.44 ± 3.43%).
Sperm hyaluronic acid binding
To investigate whether ESSM treatment has any impact on sperm hyaluronic acid-binding capacity, a sperm HBA test was performed and the percentage of HA-bound sperm was calculated (Fig. 2c). Oligozoospermic sperm samples showed decreased sperm HBA when compared to normozoospermic samples before and after cryopreservation. However, the percentage of HA-bound sperm was not statistically significantly altered between ESSM-treated (group 2 oligozoospermic 62.75 ± 4.94% and normozoospermic 79.83 ± 2.07%; group 3 oligozoospermic 65.33 ± 4.78% and normozoospermic 80.54 ± 1.97%) and ESSM-untreated samples (group 1 oligozoospermic 68.31 ± 6.14% and normozoospermic 77.02 ± 2.45%) before and after cryopreservation (group 2 oligozoospermic 50.33 ± 15.06% and normozoospermic 77.17 ± 4.76%; group 3 oligozoospermic 49.23 ± 12.88% and normozoospermic 79.85 ± 4.33%; group 1 oligozoospermic 74.00 ± 3.06% and normozoospermic 79.35 ± 3.70%).
Acrosomal integrity of sperm
Figure 2d shows the acrosomal status of each group according to the scoring criteria indicated in materials and methods. The data demonstrated that the percentage of sperm with an intact acrosome was lower in oligozoospermic samples compared to normozoospermic samples either before or after cryopreservation. We found no statistical difference between ESSM-treated (group 2 and group 3) and ESSM-untreated sperm (group 1) before and after cryopreservation for overall data (p = 0.56 and p = 0.89, respectively). No difference was observed when oligozoospermic and normozoospermic samples from groups 1, 2, or 3 were compared either before (p = 0.90 and p = 0.55, respectively) or after cryopreservation (p = 0.33 and p = 0.92, respectively).
Sperm chromatin maturity
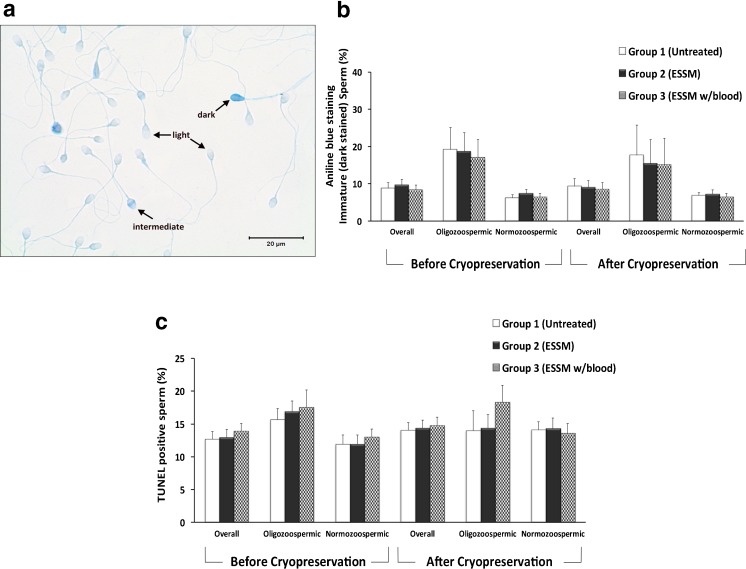
Based on the intensity of the staining, sperm cells were classified as light (mature), intermediate, or dark (arrested maturity spermatozoa) as described previously by Huszar et al. [30, 40] (Fig. 3a). The percentages of dark stained immature sperm were compared between ESSM-treated and ESSM-untreated groups (Fig. 3b). As expected, oligozoospermic samples showed the highest percentages of dark immature sperm regardless of ESSM treatment or cryopreservation. However, when the three groups were compared with each other, there was no statistically significant differences in terms of chromatin maturity for overall or oligozoospermic and normozoospermic samples (p = 0.66, p = 0.96 and 0.71, respectively). Finally, the percentages of immature sperm were not altered after cryopreservation as well (p = 0.78, p = 0.79 and 0.88).
Figure 3.
a–b Evaluation of sperm chromatin maturity in ESSM-treated and ESSM-untreated sperm before and after cryopreservation. a Mature sperm were stained with aniline blue very lightly (light), whereas immature sperm (intermediate) and severely immature sperm (dark) were stained notably due to incomplete histone-protamine transition. b The percentage of immature sperm in ESSM-treated and ESSM-untreated sperm before (p = 0.66, p = 0.96, and p = 0.71 for overall, oligozoospermic, and normozoospermic samples, respectively) and after cryopreservation (p = 0.78, p = 0.79, and p = 0.88 for overall, oligozoospermic, and normozoospermic samples, respectively). No significant difference was observed between three groups before and after cryopreservation. c DNA integrity was assessed by TUNEL assay and the percentage of apoptotic cells in ESSM-treated and ESSM-untreated sperm before and after cryopreservation. No significant difference was observed between 3 groups before (p = 0.65 for overall, p = 0.80 for oligozoospermic, and p = 0.58 for normozoospermic samples) and after cryopreservation (p = 0.93 for overall, p = 0.45 for oligozoospermic, and p = 0.94 for normozoospermic samples)
DNA integrity of sperm
In order to analyze whether ESSM treatment reveals any evidence of higher DNA fragmentation and apoptosis, we performed the TUNEL assay. The results indicated that in oligozoospermic samples particularly, group 3 had highest DNA fragmentation. However, this was not found to be statistically significant either before or after cryopreservation (p = 0.80 and p = 0.45, respectively). As reported for other parameters tested, no significant differences were determined between ESSM-treated and ESSM-untreated samples by means of TUNEL assay for overall or normozoospermic samples (p = 0.65 and p = 0.58, respectively). These results were confirmed with the data obtained after cryopreservation as well (p = 0.93 and p = 0.94, respectively) (Fig. 3c).
Conclusions
The introduction of ICSI [4] and discovery of the fertilization capacity of extracted sperm from testis [7, 41] have led to the development of different methods for testicular sperm retrieval. When the importance of extracted spermatozoa for ICSI is considered, an optimal method is needed to facilitate sperm retrieval while not creating any adverse effects on the sperm. However, potential optimal methods to obtain sperm from testicular tissue is still under debate [3].
During the process of TESE biopsy, extensive number of erythrocytes dominates the suspension and make it difficult to visualize the sperm. To improve the retrieval rate and decrease the time spent for sperm recovery, erythrocyte lysing buffer is used by some embryology laboratories. Verheyen et al. (1995) were the first to report using the erythrocyte lysing buffer to extract spermatozoa while comparing four different mechanical sperm retrieval methods. To test the toxicity of this medium, motility and vitality of capacitated donor spermatozoa were evaluated. The results indicated that there was no negative effect of the medium on sperm motility and vitality [9]. However, assessment of only sperm motility and vitality is not sufficient to determine all potential adverse effects of erythrocyte lysing buffer on sperm fertilization capacity. Apart from motility and vitality, additional sperm parameters and markers of human sperm maturity and function have been studied for many years to show sperm quality and to increase the success of infertility treatment [31, 42, 43]. Thus, in this study, we more comprehensively analyzed whether ESSM treatment has any potential side effects on several sperm parameters, including morphology, viability, HA-binding capacity, acrosome and DNA integrity, cellular maturation such as membrane, and chromatin maturity in freshly ejaculated and cryopreserved sperm.
As it is well known, sperm motility and viability are crucial features for fertilization capacity of human spermatozoon. These factors are also clinically relevant for evaluation of male factor infertility. Our data indicates that the percentage of sperm motility and viability were not statistically different between ESSM-treated and ESSM-untreated samples before and after cryopreservation. However, sperm motility and viability were decreased in each group after cryopreservation, similar to previous reports [44–46]. Thus, our data suggests a negative effect by cryopreservation rather than the effect of ESSM treatment on sperm.
Sperm morphology is another prognostic and diagnostic tool for the prediction of male fertility potential with regard to in vivo pregnancies and assisted reproductive technology (ART) outcomes [47]. Although ejaculated sperm is a heterogeneous population and many sperm in ejaculate may have abnormal morphology, previous studies have reported reduced fertility rates in ICSI with abnormal sperm morphology [48, 49]. Considering that our results clearly showed that ESSM treatment of freshly ejaculated and cryopreserved sperm do not have negative influence on sperm morphology.
In addition to sperm motility, viability, and morphology, many sperm selection techniques are being developed in several laboratories. Sperm HBA is one of the commercial kits being marketed for routine testing of sperm maturity and fertility [30, 50]. Huszar and colleagues have carried extensive studies showing that HA-bound sperm have completed membrane maturity during spermiogenetic maturation [30, 31]. For diagnostics, it is recommended for the hyaluronic acid-binding assay (HBA) to be applied before ART [43]. Thus, we analyzed the percentage of HA-bound sperm. Our results indicated that ESSM does not adversely affect hyaluronic acid-binding capacity or HA receptors or membrane maturity of human spermatozoa, while ESSM does lyse erythrocytes.
We also evaluated freshly ejaculated and cryopreserved sperm in terms of acrosome integrity between experimental groups. As it is widely known, the acrosome reaction is essential for fertilization [35]. According to our results, ESSM treatment neither affects acrosome integrity nor causes degenerative acrosome loss in all groups before and after cryopreservation. Even the acrosome-reacted sperm were increased in each group after cryopreservation; this increase is most likely the effect of sperm cryopreservation [51] rather than due to ESSM treatment.
Since nuclear immaturity of spermatozoa has been suggested to be one of the primary factors underlying male factor infertility [52], ESSM-treated sperm was also evaluated in terms of chromatin maturity and DNA strand breaks of sperm nucleus. Even though there was no statistically significant difference, in group 3, oligozoospermic samples had the highest DNA fragmentation. Previously, Salian et al. (2012) showed that frozen-thawed spermatozoa from oligozoospermic samples were more susceptible to DNA fragmentation in different ICSI media compared to normozoospermic samples [53]. Therefore, the highest DNA fragmentation in frozen-thawed oligozoospermic samples may be due to the sensitivity of oligozoospermic samples regardless of the components of different medias. It seems that ESSM treatment did not adversely affect sperm chromatin and did not result in the significant increase of DNA strand breaks in freshly ejaculated and cryopreserved sperm in experimental groups.
In this study, we evaluated not only the adverse effect of ESSM treatment by itself, but also evaluated the potential effect of hemoglobin release for sperm parameters. Considering the clinical importance of pre-epididymal TESE materials, it is of note that only post-epididymal sperm samples were included in the present study which is a limitation on our experimental design. However, it is likely that sperm suffer oxidative stress and nuclear DNA damage during transit through the male genital tract, which leads to lower ejaculated sperm quality [54, 55]. Thus, it has been suggested that testicular sperm aspiration may allow access to higher quality of sperm, leading to better IVF results. For our study, even if freshly ejaculated (post-epididymal) sperm may not truly reflect testicular samples, our data indicated that ESSM treatment and increased hemoglobin in sperm suspension do not affect freshly ejaculated and cryopreserved sperm. While previously shown data indicated that erythrocyte lysing buffer treatment of testicular spermatozoa did not have a negative effect on fertilization and development rates [19], the direct effect of erythrocyte lysing buffer on more comprehensive sperm parameters and maturation was unknown. Therefore, we studied the effects of ESSM on various semen parameters and sperm maturity markers in freshly ejaculated and cryopreserved sperm. Our data suggests that ESSM and cellular stress caused by erythrocyte lysis does not have harmful effects on the attributes of several sperm maturity markers. Usage of this medium facilitates sperm visualization by the embryologist, decreases the time spent for sperm recovery, and it also appears to be safe to use in embryology laboratories. However, further studies are required to further clarify the potential clinical usage of ESSM in clinics.
Acknowledgements
The authors would like to thank Rebecca Jaszczak from University of California San Francisco for proof reading the article.
Funding
This study was supported by Akdeniz University Research Foundation with grant number 2013.01.0103.011.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Fathalla MF. Reproductive health: a global overview. Early Hum Dev. 1992;29(1–3):35–42. doi: 10.1016/0378-3782(92)90055-L. [DOI] [PubMed] [Google Scholar]
- 2.Ombelet W, Cooke I, Dyer S, Serour G, Devroey P. Infertility and the provision of infertility medical services in developing countries. Hum Reprod Update. 2008;14(6):605–621. doi: 10.1093/humupd/dmn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–426. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 4.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–18. doi: 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- 5.Schlegel PN, Palermo GD, Goldstein M, Menendez S, Zaninovic N, Veeck LL, et al. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia. Urology. 1997;49(3):435–440. doi: 10.1016/S0090-4295(97)00032-0. [DOI] [PubMed] [Google Scholar]
- 6.Devroey P, Liu J, Nagy Z, Tournaye H, Silber SJ, Van Steirteghem AC. Normal fertilization of human oocytes after testicular sperm extraction and intracytoplasmic sperm injection. Fertil Steril. 1994;62(3):639–641. doi: 10.1016/S0015-0282(16)56958-1. [DOI] [PubMed] [Google Scholar]
- 7.Nagy Z, Liu J, Cecile J, Silber S, Devroey P, Van Steirteghem A. Using ejaculated, fresh, and frozen-thawed epididymal and testicular spermatozoa gives rise to comparable results after intracytoplasmic sperm injection. Fertil Steril. 1995;63(4):808–815. doi: 10.1016/S0015-0282(16)57486-X. [DOI] [PubMed] [Google Scholar]
- 8.Donoso P, Tournaye H, Devroey P. Which is the best sperm retrieval technique for non-obstructive azoospermia? A systematic review. Hum Reprod Update. 2007;13(6):539–549. doi: 10.1093/humupd/dmm029. [DOI] [PubMed] [Google Scholar]
- 9.Verheyen G, De Croo I, Tournaye H, Pletincx I, Devroey P, van Steirteghem AC. Comparison of four mechanical methods to retrieve spermatozoa from testicular tissue. Hum Reprod. 1995;10(11):2956–2959. doi: 10.1093/oxfordjournals.humrep.a135828. [DOI] [PubMed] [Google Scholar]
- 10.Crabbe E, Verheyen G, Tournaye H, Van Steirteghem A. The use of enzymatic procedures to recover testicular germ cells. Hum Reprod. 1997;12(8):1682–1687. doi: 10.1093/humrep/12.8.1682. [DOI] [PubMed] [Google Scholar]
- 11.Aydos K, Demirel LC, Baltaci V, Unlu C. Enzymatic digestion plus mechanical searching improves testicular sperm retrieval in non-obstructive azoospermia cases. Eur J Obstet Gynecol Reprod Biol. 2005;120(1):80–86. doi: 10.1016/j.ejogrb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Fischer R, Baukloh V, Naether OG, Schulze W, Salzbrunn A, Benson DM. Pregnancy after intracytoplasmic sperm injection of spermatozoa extracted from frozen-thawed testicular biopsy. Hum Reprod. 1996;11(10):2197–2199. doi: 10.1093/oxfordjournals.humrep.a019075. [DOI] [PubMed] [Google Scholar]
- 13.Salzbrunn A, Benson DM, Holstein AF, Schulze W. A new concept for the extraction of testicular spermatozoa as a tool for assisted fertilization (ICSI) Hum Reprod. 1996;11(4):752–755. doi: 10.1093/oxfordjournals.humrep.a019248. [DOI] [PubMed] [Google Scholar]
- 14.Dym M, Fawcett DW. Further observations on the numbers of spermatogonia, spermatocytes, and spermatids connected by intercellular bridges in the mammalian testis. Biol Reprod. 1971;4(2):195–215. doi: 10.1093/biolreprod/4.2.195. [DOI] [PubMed] [Google Scholar]
- 15.Vloeberghs V, Verheyen G, Haentjens P, Goossens A, Polyzos NP, Tournaye H. How successful is TESE-ICSI in couples with non-obstructive azoospermia? Hum Reprod. 2015;30(8):1790–1796. doi: 10.1093/humrep/dev139. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Yosef D, Yogev L, Hauser R, Yavetz H, Azem F, Yovel I, et al. Testicular sperm retrieval and cryopreservation prior to initiating ovarian stimulation as the first line approach in patients with non-obstructive azoospermia. Hum Reprod. 1999;14(7):1794–1801. doi: 10.1093/humrep/14.7.1794. [DOI] [PubMed] [Google Scholar]
- 17.Ulug U, Bener F, Karagenc L, Ciray N, Bahceci M. Outcomes in couples undergoing ICSI: comparison between fresh and frozen-thawed surgically retrieved spermatozoa. Int J Androl. 2005;28(6):343–349. doi: 10.1111/j.1365-2605.2005.00559.x. [DOI] [PubMed] [Google Scholar]
- 18.Schlegel PN, Su LM. Physiological consequences of testicular sperm extraction. Hum Reprod. 1997;12(8):1688–1692. doi: 10.1093/humrep/12.8.1688. [DOI] [PubMed] [Google Scholar]
- 19.Nagy ZP, Verheyen G, Tournaye H, Devroey P, Van Steirteghem AC. An improved treatment procedure for testicular biopsy specimens offers more efficient sperm recovery: case series. Fertil Steril. 1997;68(2):376–379. doi: 10.1016/S0015-0282(97)81534-8. [DOI] [PubMed] [Google Scholar]
- 20.Organization WH . WHO laboratory manual for the examination and processing of human semen. 5. Geneva: WHO Press; 2010. [PubMed] [Google Scholar]
- 21.Kruger TF, Menkveld R, Stander FS, Lombard CJ, Van der Merwe JP, van Zyl JA, et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986;46(6):1118–1123. doi: 10.1016/S0015-0282(16)49891-2. [DOI] [PubMed] [Google Scholar]
- 22.Menkveld R, Stander FS, Kotze TJ, Kruger TF, van Zyl JA. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod. 1990;5(5):586–592. doi: 10.1093/oxfordjournals.humrep.a137150. [DOI] [PubMed] [Google Scholar]
- 23.Prinosilova P, Kruger T, Sati L, Ozkavukcu S, Vigue L, Kovanci E, et al. Selectivity of hyaluronic acid binding for spermatozoa with normal Tygerberg strict morphology. Reprod BioMed Online. 2009;18(2):177–183. doi: 10.1016/S1472-6483(10)60253-2. [DOI] [PubMed] [Google Scholar]
- 24.Bjorndahl L, Soderlund I, Kvist U. Evaluation of the one-step eosin-nigrosin staining technique for human sperm vitality assessment. Hum Reprod. 2003;18(4):813–816. doi: 10.1093/humrep/deg199. [DOI] [PubMed] [Google Scholar]
- 25.Eppig JJ. FSH stimulates hyaluronic acid synthesis by oocyte-cumulus cell complexes from mouse preovulatory follicles. Nature. 1979;281(5731):483–484. doi: 10.1038/281483a0. [DOI] [PubMed] [Google Scholar]
- 26.Dandekar P, Aggeler J, Talbot P. Structure, distribution and composition of the extracellular matrix of human oocytes and cumulus masses. Hum Reprod. 1992;7(3):391–398. doi: 10.1093/oxfordjournals.humrep.a137656. [DOI] [PubMed] [Google Scholar]
- 27.Salustri A, Camaioni A, Di Giacomo M, Fulop C, Hascall VC. Hyaluronan and proteoglycans in ovarian follicles. Hum Reprod Update. 1999;5(4):293–301. doi: 10.1093/humupd/5.4.293. [DOI] [PubMed] [Google Scholar]
- 28.Cayli S, Jakab A, Ovari L, Delpiano E, Celik-Ozenci C, Sakkas D, et al. Biochemical markers of sperm function: male fertility and sperm selection for ICSI. Reprod BioMed Online. 2003;7(4):462–468. doi: 10.1016/S1472-6483(10)61891-3. [DOI] [PubMed] [Google Scholar]
- 29.Cayli S, Sakkas D, Vigue L, Demir R, Huszar G. Cellular maturity and apoptosis in human sperm: creatine kinase, caspase-3 and Bcl-XL levels in mature and diminished maturity sperm. Mol Hum Reprod. 2004;10(5):365–372. doi: 10.1093/molehr/gah050. [DOI] [PubMed] [Google Scholar]
- 30.Huszar G, Ozenci CC, Cayli S, Zavaczki Z, Hansch E, Vigue L. Hyaluronic acid binding by human sperm indicates cellular maturity, viability, and unreacted acrosomal status. Fertil Steril. 2003;79(Suppl 3):1616–1624. doi: 10.1016/S0015-0282(03)00402-3. [DOI] [PubMed] [Google Scholar]
- 31.Huszar G, Ozkavukcu S, Jakab A, Celik-Ozenci C, Sati GL, Cayli S. Hyaluronic acid binding ability of human sperm reflects cellular maturity and fertilizing potential: selection of sperm for intracytoplasmic sperm injection. Curr Opin Obstet Gynecol. 2006;18(3):260–267. doi: 10.1097/01.gco.0000193018.98061.2f. [DOI] [PubMed] [Google Scholar]
- 32.Sati L, Cayli S, Delpiano E, Sakkas D, Huszar G. The pattern of tyrosine phosphorylation in human sperm in response to binding to zona pellucida or hyaluronic acid. Reprod Sci. 2014;21(5):573–581. doi: 10.1177/1933719113504467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flesch FM, Voorhout WF, Colenbrander B, van Golde LM, Gadella BM. Use of lectins to characterize plasma membrane preparations from boar spermatozoa: a novel technique for monitoring membrane purity and quantity. Biol Reprod. 1998;59(6):1530–1539. doi: 10.1095/biolreprod59.6.1530. [DOI] [PubMed] [Google Scholar]
- 34.Chan JZ, Krause W, Bohring C. Computer-assisted analysis of sperm morphology with the aid of lectin staining. Andrologia. 2002;34(6):379–383. doi: 10.1046/j.1439-0272.2002.00518.x. [DOI] [PubMed] [Google Scholar]
- 35.Mendoza C, Carreras A, Moos J, Tesarik J. Distinction between true acrosome reaction and degenerative acrosome loss by a one-step staining method using Pisum sativum agglutinin. J Reprod Fertil. 1992;95(3):755–763. doi: 10.1530/jrf.0.0950755. [DOI] [PubMed] [Google Scholar]
- 36.Kohn FM, Mack SR, Schill WB, Zaneveld LJ. Detection of human sperm acrosome reaction: comparison between methods using double staining, Pisum sativum agglutinin, concanavalin A and transmission electron microscopy. Hum Reprod. 1997;12(4):714–721. doi: 10.1093/humrep/12.4.714. [DOI] [PubMed] [Google Scholar]
- 37.Auger J, Mesbah M, Huber C, Dadoune JP. Aniline blue staining as a marker of sperm chromatin defects associated with different semen characteristics discriminates between proven fertile and suspected infertile men. Int J Androl. 1990;13(6):452–462. doi: 10.1111/j.1365-2605.1990.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 38.Sati L, Huszar G. Methodology of aniline blue staining of chromatin and the assessment of the associated nuclear and cytoplasmic attributes in human sperm. Methods Mol Biol. 2013;927:425–436. doi: 10.1007/978-1-62703-038-0_36. [DOI] [PubMed] [Google Scholar]
- 39.Sati L, Ovari L, Bennett D, Simon SD, Demir R, Huszar G. Double probing of human spermatozoa for persistent histones, surplus cytoplasm, apoptosis and DNA fragmentation. Reprod BioMed Online. 2008;16(4):570–579. doi: 10.1016/S1472-6483(10)60464-6. [DOI] [PubMed] [Google Scholar]
- 40.Ovari L, Sati L, Stronk J, Borsos A, Ward DC, Huszar G. Double probing individual human spermatozoa: aniline blue staining for persistent histones and fluorescence in situ hybridization for aneuploidies. Fertil Steril. 2010;93(7):2255–2261. doi: 10.1016/j.fertnstert.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 41.Devroey P, Liu J, Nagy Z, Goossens A, Tournaye H, Camus M, et al. Pregnancies after testicular sperm extraction and intracytoplasmic sperm injection in non-obstructive azoospermia. Hum Reprod. 1995;10(6):1457–1460. doi: 10.1093/HUMREP/10.6.1457. [DOI] [PubMed] [Google Scholar]
- 42.Huszar G, Vigue L, Corrales M. Sperm creatine phosphokinase activity as a measure of sperm quality in normospermic, variablespermic, and oligospermic men. Biol Reprod. 1988;38(5):1061–1066. doi: 10.1095/biolreprod38.5.1061. [DOI] [PubMed] [Google Scholar]
- 43.Sakkas D, Ramalingam M, Garrido N, Barratt CL. Sperm selection in natural conception: what can we learn from Mother Nature to improve assisted reproduction outcomes? Hum Reprod Update. 2015;21(6):711–726. doi: 10.1093/humupd/dmv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozkavukcu S, Erdemli E, Isik A, Oztuna D, Karahuseyinoglu S. Effects of cryopreservation on sperm parameters and ultrastructural morphology of human spermatozoa. J Assist Reprod Genet. 2008;25(8):403–411. doi: 10.1007/s10815-008-9232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zribi N, Feki Chakroun N, El Euch H, Gargouri J, Bahloul A, Ammar KL. Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil Steril. 2010;93(1):159–166. doi: 10.1016/j.fertnstert.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 46.Oberoi B, Kumar S, Talwar P. Study of human sperm motility post cryopreservation. Med J Armed Forces India. 2014;70(4):349–353. doi: 10.1016/j.mjafi.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menkveld R, Holleboom CA, Rhemrev JP. Measurement and significance of sperm morphology. Asian J Androl. 2011;13(1):59–68. doi: 10.1038/aja.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kihaile P, Hirotsuru K, Kumasako Y, Misumi J, Utsunomiya T. Fertilization rates of small-head sperm in conventional IVF and ICSI. Arch Androl. 2003;49(5):327–329. doi: 10.1080/01485010390219692. [DOI] [PubMed] [Google Scholar]
- 49.Kahraman S, Akarsu C, Cengiz G, Dirican K, Sozen E, Can B, et al. Fertility of ejaculated and testicular megalohead spermatozoa with intracytoplasmic sperm injection. Hum Reprod. 1999;14(3):726–730. doi: 10.1093/humrep/14.3.726. [DOI] [PubMed] [Google Scholar]
- 50.Huszar G, Jakab A, Sakkas D, Ozenci CC, Cayli S, Delpiano E, et al. Fertility testing and ICSI sperm selection by hyaluronic acid binding: clinical and genetic aspects. Reprod BioMed Online. 2007;14(5):650–663. doi: 10.1016/S1472-6483(10)61060-7. [DOI] [PubMed] [Google Scholar]
- 51.Cross NL, Hanks SE. Effects of cryopreservation on human sperm acrosomes. Hum Reprod. 1991;6(9):1279–1283. doi: 10.1093/oxfordjournals.humrep.a137526. [DOI] [PubMed] [Google Scholar]
- 52.Katayose H, Yanagida K, Hayashi S, Kuretake S, Morozumi K, Sato A. Fertilization failure from a sperm chromatin defect in couples with unexplained infertility. J Reprod Med. 2004;49(9):727–732. [PubMed] [Google Scholar]
- 53.Salian SR, Kalthur G, Uppangala S, Kumar P, Adiga SK. Frozen-thawed spermatozoa from oligozoospermic ejaculates are susceptible to in situ DNA fragmentation in polyvinylpyrrolidone-based sperm-immobilization medium. Fertil Steril. 2012;98(2):321–325. doi: 10.1016/j.fertnstert.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 54.Suganuma R, Yanagimachi R, Meistrich ML. Decline in fertility of mouse sperm with abnormal chromatin during epididymal passage as revealed by ICSI. Hum Reprod. 2005;20(11):3101–3108. doi: 10.1093/humrep/dei169. [DOI] [PubMed] [Google Scholar]
- 55.Greco E, Scarselli F, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod. 2005;20(1):226–230. doi: 10.1093/humrep/deh590. [DOI] [PubMed] [Google Scholar]