Abstract
Purpose
The etiology of fertilization failure and polyspermy during assisted reproductive technology (ART) remains elusive. The aim of this study was to determine whether mutations in the IZUMO1 receptor (IZUMO1R) gene, which is essential for mammalian fertilization, contribute to the pathogenesis of fertilization failure or polyspermy in humans.
Methods
We recruited 215 female subjects with fertilization failure/poor fertilization, 330 females with polyspermy, and 300 matched controls. All subjects underwent IVF treatment. Peripheral blood DNA of cases was extracted and screened for mutations in IZUMO1R gene.
Results
Four rare single nucleotide polymorphisms (SNPs) of the IZUMO1R were identified among specimens from patients with fertilization failure and polyspermy but were absent in the 300 control subjects. These included a missense SNP (rs76779571 in exon 4), which was found in two fertilization failure patients, and a nonsynonymous SNP (rs61742524 in exon 1) and two synonymous SNPs (rs76781645 in exon 1 and rs377369966 in intron 2), which were found among three polyspermy cases.
Conclusions
The variations in IZUMO1R might play a role in the pathogenesis of fertilization failure and polyspermy, and the putative functions and effects of these rare variants require further studies.
Electronic supplementary material
The online version of this article (10.1007/s10815-017-1101-5) contains supplementary material, which is available to authorized users.
Keywords: Fertilization failure, Polyspermy, IZUMO1R, SNP
Background
With improvements in assisted reproductive technologies and the use of sophisticated facilities, successful in vitro fertilization (IVF) rates have approached 70–80% [1]. However, fertilization failures and polyspermy still remain a concern that leads to the overall failure of an IVF cycle.
Total fertilization failure occurs when all available oocytes fail to fertilize, and the incidence of total fertilization failure after IVF has been reported to be 3.52–20% [2]. Oocyte-related factors relevant to IVF fertilization failure include abnormal regulatory mechanisms of zona pellucida (ZP) formation, gamete recognition, and oocyte activation [1, 3–5]. Several receptors located in the oocyte membrane have been implicated in the recognition and/or fusion process, including CD9, ZP1, ZP2, ZP3, and PTGER2 [6].
Polyspermic fertilization occurs when the oocyte is fertilized by two or more spermatozoa [7–9], and the incidence of polyspermy is 3–10% in IVF cycles [9–11]. The membrane block to polyspermy is achieved through a relatively fast-acting block involving biochemical changes in the oolemma followed by a slow hardening of the ZP over the course of about an hour caused by the action of enzymes released from cortical granules after oocyte activation [12]. This slow-acting process is known as the cortical reaction [13]. A variety of oocyte-related factors contribute to the pathogenesis of polyspermy, including the degree of oocyte maturation, changes in the molecular biology of the ZP, and alterations in the cortical reaction [12–15]. It also has been shown that the phosphatidylinositol signaling pathway plays an important role in the cortical reaction, and alterations of this pathway might disrupt the membrane block leading to polyspermy [16].
JUNO (also called FOLR4), which is encoded by the IZUMO1R gene, is a glycophosphatidylinositol (GPI)-anchored receptor on the oocyte surface that is essential for fertilization [17–19]. It is one of three folate receptor paralogs in mouse whose main role is related to folate uptake. However, a previous study showed that, unlike FOLR1 and FOLR2, JUNO is unable to bind to folate [18]. In JUNO−/− female mice, the zona-free oocytes do not fuse with wild-type sperm, leading to unsuccessful fertilization [18]. Furthermore, the interaction between JUNO and IZUMO1 is a vital molecular basis of gamete recognition, and this interaction is conserved within several mammalian species, including humans. After fertilization, rapid loss of JUNO from the oolemma also suggests a mechanism for the membrane block to polyspermy in mammals [18]. We hypothesized, therefore, that mutations of IZUMO1R can explain fertilization failure or polyspermy in humans.
Human IZUMO1R is encoded by four exons and resides in a locus on chromosomal band 11q21. This study was designed to determine whether IZUMO1R variations contribute to fertilization failure or polyspermy in women undergoing IVF. In the present study, we identified one nonsynonymous SNP (rs76779571) in two fertilization failure subjects. Among polyspermy subjects, one nonsynonymous SNP (rs61742524) and two synonymous SNPs (rs76781645 and rs377369966) were found. None of these rare SNPs were found in the control group. These variations might account for the pathogenesis of fertilization failure and polyspermy.
Methods
Subjects
During 2009–2013, peripheral blood was collected from 215 women with fertilization failure or poor fertilization and from 330 women with polyspermy who were recruited from the Center for Reproductive Medicine, Shandong Provincial Hospital, Shandong University. Inclusion criteria for fertilization failure or poor fertilization were that more than 60% of the oocytes experienced fertilization failure during IVF treatment, and polyspermy cases were defined as the occurrence of three or more pronuclei in no fewer than 50% of the oocytes on the first day after IVF. Patients older than 42 years or with fewer than three retrieved oocytes were excluded. Three hundred women with regular menstrual cycles and who had normal IVF oocyte fertilization and had already given birth to at least one healthy baby and with no history of spontaneous abortion were recruited as controls. The clinical and hormone characteristics of all participants, including age, body mass index (BMI), follicle-stimulating hormone (FSH), antral follicle count (AFC), and number of retrieved oocytes, are shown in Supplementary Table S1.
DNA extraction and mutational analysis
Genomic DNA was extracted from peripheral blood samples with the use of genomic DNA extraction kits (QIAamp DNA Blood Mini kit; Qiagen). The entire coding sequence and the flanking intronic portions of IZUMO1R (Refseq DNA: NM_001199206) were amplified by polymerase chain reaction (PCR) (Gene Amp PCR System 9700; Applied Biosystems). The four primer pairs are presented in Supplementary Table S2. The PCR conditions were as follows: initial denaturation at 95 °C for 10 min; then denaturation at 95 °C for 30 s, annealing at 58 °C for 30 s, elongation at 72 °C for 45 s with 35 cycles; finally, elongation at 72 °C for 10 min. The PCR product was first analyzed by agarose gel electrophoresis and then sequenced on an automated sequencer (ABI PRISM 310; Applied Biosystems) using the ABI-Prism BigDye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems).
The sequencing results were analyzed using Sequencer 4.9 software. The rare single nucleotide polymorphism (SNP) variations identified in this study were validated by repeating the PCR amplification three times and sequencing in both directions.
Bioinformatics analysis
The deduced amino acid sequences were analyzed, and the possible impacts of the amino acid substitutions on the structure and function of JUNO were predicted with the PolyPhen-2 (Polymorphism Phenotyping v2; http://genetics.bwh.harvard.edu/pph2/) human nonsynonymous SNP prediction database.
Results
Sequencing results
All four exons and the exon–intron boundaries of IZUMO1R were screened for variations. One nonsynonymous SNP (rs76779571 in exon 4) was discovered in two fertilization failure/poor fertilization subjects (Fig. 1; Supplementary Fig. S1). Among polyspermy subjects, one nonsynonymous SNP (rs61742524 in exon 1) and two synonymous SNPs (rs76781645 in exon 1 and rs377369966 in intron 2) were identified (Fig. 1; Supplementary Fig. S2). None of these rare SNPs were found in the control group (Table 1). Clinical characteristics of the cases carrying the above variations in the IZUMO1R gene are summarized in Tables 2 and 3.
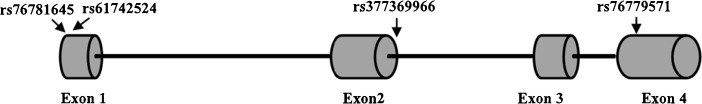
Fig. 1.
Identification of rare SNPs in the IZUMO1R gene. Schematic diagram of the IZUMO1R gene, showing two nonsynonymous variations (rs61742524 in exon 1 and rs76779571 in exon 4) and two synonymous variant (rs76781645 in exon 1 and rs377369966 in intron 2) found in the present study. Exons are a composite of human sequence NM_001199206 (GenBank) and ENST00000328458.5 (Ensembl)
Table 1.
Variants of IZUMO1R found in women with fertilization failure, polyspermy, and controls
| Phenotype | DNA sequence variation | Amino acid substitution | dbSNP ID | Location | Genotype, n (%) | Allele (%) | Probable interpretation | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | ||||||||
| Fertilization failure | c.510T>G | p.His170Gln | rs76779571 | Exon 4 | TT | 213 (99.0) | 300 (100.0) | T | 99.3 | 100 | Deleterious |
| TG | 1 (0.5) | 0 | G | 0.7 | 0 | ||||||
| GG | 1 (0.5) | 0 | |||||||||
| Polyspermy | c.9C>G | p.Cys3Trp | rs61742524 | Exon 1 | CC | 329 (99.7) | 300 (100.0) | C | 99.8 | 100 | Not deleterious |
| GC | 1 (0.3) | 0 | G | 0.2 | 0 | ||||||
| GG | 0 | 0 | |||||||||
| c.-18G>T | Synonymous | rs76781645 | Exon 1 | GG | 329 (99.7) | 300 (100.0) | C | 99.8 | 100 | Not deleterious | |
| GT | 1 (0.3) | 0 | G | 0.2 | 0 | ||||||
| TT | 0 | 0 | |||||||||
| c.329+8G>T | Synonymous | rs377369966 | Intron 2 | GG | 329 (99.7) | 300 (100.0) | G | 99.8 | 100 | Not deleterious | |
| GT | 1 (0.3) | 0 | T | 0.2 | 0 | ||||||
| TT | 0 | 0 | |||||||||
Table 2.
Baseline and laboratory characteristics of fertilization failure cases with variants in IZUMO1R
| F189 | F226 | |
|---|---|---|
| Baseline characteristics | ||
| Variant | c.510T>G | c.510T>G |
| Genotype | Homozygous | Heterozygous |
| Age at diagnosis | 30 | 31 |
| Reproductive history | G0P0A0L0 | G0P0A0L0 |
| Laboratory characteristics | ||
| No. of retrieved MII oocytes | 12 | 16 |
| No. of oocytes that failed to fertilize, n (%) | 11 (91.7) | 16 (100) |
| No. of metaphase II oocytes injected | 11 | 16 |
| No. of day 3 embryos | 9 | 13 |
| No. of fresh/frozen embryos transferred | 2/2 | 1/1 |
| Pregnancy outcome | One male infant/one male infant | None/one female infant |
Table 3.
Baseline and laboratory characteristics of polyspermy cases with variants in IZUMO1R. Note: – means that this cycle had no transfers
| P249 | P103 | P89 | |
|---|---|---|---|
| Baseline characteristics | |||
| Variant | c.9C>G | c.-18G>T | c.329+8G>T |
| Genotype | Heterozygous | Heterozygous | Heterozygous |
| Age at diagnosis | 32 | 39 | 31 |
| Reproductive history | G0P0A0L0 | G1P0A1L0 | G1P0A1L0 |
| Laboratory characteristics | |||
| No. of retrieved MII oocytes | 8 | 7 | 4 |
| No. of 0 PN oocytes, n (%) | 1 (12.5) | 1 (14.3) | 0 (0) |
| No. of 2 PN oocytes, n (%) | 3 (37.5) | 1 (14.3) | 1 (25) |
| No. of ≥ 3 PN oocytes, n (%) | 4 (50) | 5 (71.4) | 3 (75) |
| No. of day 3 embryos | 7 | 6 | 4 |
| No. of fresh/frozen embryos transferred | 2/1 | 1/– | –/1 |
| Pregnancy outcome | None/one female infant | None | One male infant |
PN pronuclei
The nonsynonymous SNP found in fertilization failure cases and clinical features
The rs76779571 SNP leading to a histidine to glutamine substitution within the functional domain of the receptor was found in fertilization failure cases F189 and F226. PolyPhen-2 predicted this variant to be possibly damaging. F189 was a homozygous carrier of the GG allele; she had 12 oocytes retrieved during her IVF treatment, one of which was degenerated, while the rest suffered from fertilization failure. Rescue intracytoplasmic sperm injection (ICSI) was performed for the 11 oocytes, and this led to nine day 3 embryos. Two embryos were transferred and resulted in the delivery of one healthy male infant. F226, a heterozygous TG allele carrier, had 16 oocytes retrieved, and all of them suffered from fertilization failure. Rescue ICSI resulted in 13 day 3 embryos. One embryo was transferred but did not result in pregnancy. Five months later, one frozen embryo from the rescue ICSI was thawed and transferred into her uterus. These two subjects’ parents were healthy, and no other family members had similar histories of infertility.
The nonsynonymous SNP found in polyspermy cases and clinical features
We detected the variation rs61742524 in the first exon, which leads to a cysteine to tryptophan substitution, in polyspermy case P249. This variation might not have an impact on the protein level as assessed by PolyPhen-2. Among eight oocytes retrieved in her IVF cycle, one was degenerated, three were fertilized with two pronuclei, and four contained three or more pronuclei. Two day 3 embryos from the two-pronuclei-fertilized oocytes were transferred but did not result in pregnancy. The third two-pronuclei-fertilized oocyte developed into a blastocyst and was cryopreserved. Two months later, the frozen embryo was thawed and transferred into her uterus, and the patient delivered a healthy female infant.
Discussion
Apart from structural, environmental, and iatrogenic causes, the etiology of fertilization failure and polyspermy remains poorly understood. Female mice lacking IZUMO1R are infertile, and JUNO-deficient oocytes cannot fuse with normal sperm [18]. Rapid shedding of JUNO from the oolemma after fertilization suggests a mechanism for the membrane polyspermic block, ensuring that oocytes normally fuse with just a single sperm [18]. Thus, JUNO has critical functions in normal single-sperm fertilization, and mutations in the IZUMO1R gene might cause dysfunction of JUNO leading to fertilization failure or polyspermy.
This is the first study to investigate IZUMO1R gene mutations or variations among human subjects with fertilization failure and polyspermy during IVF. We identified one known rare SNP (rs76779571 in exon 4) in two fertilization failure cases and three known rare SNPs (rs61742524 in exon, rs76781645 in exon 1 and rs377369966 in intron 2) in three polyspermy cases, but none of the SNPs were seen in controls. A rare functional SNP is a genetic variant that alters gene function and occurs at low frequency in a population. Rare variants can play significant roles in complex diseases, as well as in some Mendelian conditions [20], and they were more likely to be deleterious than common variants [21]. Our results suggest that IZUMO1R is implicated in the etiology of fertilization failure and polyspermy.
The rare SNP rs76779571, which results in histidine being replaced by glutamine, is predicted to have a damaging effect on the function of the IZUMO1R protein JUNO. In the homozygote carrier F189 and the heterozygote carrier F226, all oocytes suffered from fertilization failure. According to the prediction by PolyPhen-2, this variant might cause important changes in the GPI-anchor structure such that the oocyte cannot fuse with the sperm, leading to fertilization failure.
The variation rs61742524, found in the first exon and leading to a cysteine to tryptophan substitution, might not have an impact on the protein level as assessed by PolyPhen-2, but it might affect the expression of IZUMO1R at either the mRNA or the protein level, which could ultimately alter the normal function of IZUMO1R.
Of the other two synonymous variations, rs76781645 lies in the promoter region and rs377369966 lies in intron 2, and both were found in polyspermy cases. Rs76781645, located in the 5′ untranslated region, might affect gene expression by way of regulatory elements, while rs377369966 might not affect the coding sequence or alter expression but might impact the transcriptional efficiency of the gene [22]. The clinical significance of homozygosity or heterozygosity for these SNPs is unclear, and future functional studies are necessary to determine their effects.
One limitation of this study is the relatively small sample size, which might lead to random events being seen as significant. Hence, this study should be considered as providing preliminary observations and a basis for further studies on a larger scale and in other ethnic groups. Moreover, further studies are needed to elucidate the precise molecular mechanism underlying the action of these rare IZUMO1R variations in relation to the pathogenesis of fertilization failure and polyspermy.
Conclusions
In summary, we have discovered one rare IZUMO1R missense variant, rs76779571 in exon 4 (c.510T>G, p.His170Gln), in two cases with fertilization failure, and rs61742524 in exon 1 (c.9C>G, p.Cys3Trp), rs76781645 (c.-18G>T, synonymous), and rs377369966 (c.329+8G>T, synonymous) in three polyspermy cases, respectively. All SNPs were absent in control subjects. Our identification of the rare nonsynonymous SNPs in the IZUMO1R gene suggests a contribution to the etiology of fertilization failure and polyspermy.
Electronic supplementary material
(DOCX 12.7 kb)
(DOCX 14.7 kb)
(DOCX 68.6 kb)
(DOCX 44.9 kb)
Acknowledgements
We are grateful to all participants in this study.
Funding information
This study was supported by the National Science and Technology Major Project of China (2016YFC1000600), the National Natural Science Foundation of China (81622021, 31371453, 31571548, 81430029, 81490743, 81300461), the Program for New Century Excellent Talents in University (NCET-13-0355), and the Young Scholars Program of Shandong University (2015WLJH54).
Compliance with ethical standards
Ethics approval and consent to participate
Informed consents were obtained from all subjects. The study was approved by the Institutional Review Board of Reproductive Medicine of Shandong University, China.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10815-017-1101-5) contains supplementary material, which is available to authorized users.
References
- 1.Swain JE, Pool TB. ART failure: oocyte contributions to unsuccessful fertilization. Hum Reprod Update. 2008;14:431–446. doi: 10.1093/humupd/dmn025. [DOI] [PubMed] [Google Scholar]
- 2.Beck-Fruchter R, Lavee M, Weiss A, Geslevich Y, Shalev E. Rescue intracytoplasmic sperm injection: a systematic review. Fertil Steril. 2014;101:690–698. doi: 10.1016/j.fertnstert.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Neuber E, Powers RD. Is the mouse a clinically relevant model for human fertilization failures? Hum Reprod. 2000;15:171–174. doi: 10.1093/humrep/15.1.171. [DOI] [PubMed] [Google Scholar]
- 4.Rawe VY, Olmedo SB, Nodar FN, Doncel GD, Acosta AA, Vitullo AD. Cytoskeletal organization defects and abortive activation in human oocytes after IVF and ICSI failure. Mol Hum Reprod. 2000;6:510–516. doi: 10.1093/molehr/6.6.510. [DOI] [PubMed] [Google Scholar]
- 5.Kunathikom S, Makemaharn O, Suksompong S, Laokirkkiat P. Chromosomal analysis of “failed-fertilized” human oocytes resulting from in-vitro fertilization and intracytoplasmic sperm injection. J Med Assoc Thail. 2001;84:532–538. [PubMed] [Google Scholar]
- 6.Ikawa M, Inoue N, Benham AM, Okabe M. Fertilization: a sperm’s journey to and interaction with the oocyte. J Clin Invest. 2010;120:984–994. doi: 10.1172/JCI41585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahutte NG, Arici A. Failed fertilization: is it predictable? Curr Opin Obstet Gynecol. 2003;15:211–218. doi: 10.1097/00001703-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs PA, Angell RR, Buchanan IM, Hassold TJ, Matsuyama AM, Manuel B. The origin of human triploids. Ann Hum Genet. 1978;42:49–57. doi: 10.1111/j.1469-1809.1978.tb00930.x. [DOI] [PubMed] [Google Scholar]
- 9.Zaragoza MV, Surti U, Redline RW, Millie E, Chakravarti A, Hassold TJ. Parental origin and phenotype of triploidy in spontaneous abortions: predominance of diandry and association with the partial hydatidiform mole. Am J Hum Genet. 2000;66:1807–1820. doi: 10.1086/302951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trounson A, Bongso A. Fertilization and development in humans. Curr Top Dev Biol. 1996;32:59–101. doi: 10.1016/S0070-2153(08)60425-1. [DOI] [PubMed] [Google Scholar]
- 11.Ho PC, Yeung WS, Chan YF, So WW, Chan ST. Factors affecting the incidence of polyploidy in a human in vitro fertilization program. Int J Fertil Menopausal Stud. 1994;39:14–19. [PubMed] [Google Scholar]
- 12.Gardner AJ, Evans JP. Mammalian membrane block to polyspermy: new insights into how mammalian eggs prevent fertilisation by multiple sperm. Reprod Fertil Dev. 2006;18:53–61. doi: 10.1071/RD05122. [DOI] [PubMed] [Google Scholar]
- 13.Ducibella T. The cortical reaction and development of activation competence in mammalian oocytes. Hum Reprod Update. 1996;2:29–42. doi: 10.1093/humupd/2.1.29. [DOI] [PubMed] [Google Scholar]
- 14.Niwa K, Park CK, Okuda K. Penetration in vitro of bovine oocytes during maturation by frozen-thawed spermatozoa. J Reprod Fertil. 1991;91:329–336. doi: 10.1530/jrf.0.0910329. [DOI] [PubMed] [Google Scholar]
- 15.Bauskin AR, Franken DR, Eberspaecher U, Donner P. Characterization of human zona pellucida glycoproteins. Mol Hum Reprod. 1999;5:534–540. doi: 10.1093/molehr/5.6.534. [DOI] [PubMed] [Google Scholar]
- 16.Tatone C, Van Eekelen CG, Colonna R. Plasma membrane block to sperm entry occurs in mouse eggs upon parthenogenetic activation. Mol Reprod Dev. 1994;38:200–208. doi: 10.1002/mrd.1080380211. [DOI] [PubMed] [Google Scholar]
- 17.Swann K. Different triggers for calcium oscillations in mouse eggs involve a ryanodine-sensitive calcium store. Biochem J. 1992;287(Pt 1):79–84. doi: 10.1042/bj2870079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature. 2014;508:483–487. doi: 10.1038/nature13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coonrod SA, Naaby-Hansen S, Shetty J, Shibahara H, Chen M, White JM, Herr JC. Treatment of mouse oocytes with PI-PLC releases 70-kDa (pI 5) and 35- to 45-kDa (pI 5.5) protein clusters from the egg surface and inhibits sperm-oolemma binding and fusion. Dev Biol. 1999;207:334–349. doi: 10.1006/dbio.1998.9161. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein DB, Allen A, Keebler J, Margulies EH, Petrou S, Petrovski S, Sunyaev S. Sequencing studies in human genetics: design and interpretation. Nat Rev Genet. 2013;14:460–470. doi: 10.1038/nrg3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panoutsopoulou K, Tachmazidou I, Zeggini E. In search of low-frequency and rare variants affecting complex traits. Hum Mol Genet. 2013;22:R16–R21. doi: 10.1093/hmg/ddt376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plotkin JB, Kudla G. Synonymous but not the same: the causes and consequences of codon bias. Nat Rev Genet. 2011;12:32–42. doi: 10.1038/nrg2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 12.7 kb)
(DOCX 14.7 kb)
(DOCX 68.6 kb)
(DOCX 44.9 kb)