Abstract
Purpose
The objective of this study was to evaluate the effects of multiple growth factors on the development of individually cultured murine embryos.
Methods
Embryos produced by in vitro fertilization using in vitro (IVM) or in vivo (IVO) matured oocytes from three strains of mice (CF1, Swiss Webster, B6D2F1) were cultured individually (10 μl) in the absence (control) or presence of growth factors (paf, epidermal growth factor [EGF], insulin-like growth factor 1 [IGF-1], and granulocyte-macrophage colony-stimulating factor [GM-CSF]). Blastocyst formation, hatching, and blastocyst cell numbers (trophectoderm, inner cell mass, and total) were evaluated on days 4 and 5 of culture. Post-hatching development of CF1 IVO embryos was also evaluated in vitro and in vivo.
Results
The presence of growth factors did not improve the proportion of embryos forming blastocysts or initiating hatching for any of the types of embryos tested. The only significant (P < 0.05) effect of growth factors was a decrease in the proportion of embryos that formed blastocysts by day 5 in CF1 IVM embryos. The presence of growth factors also did not affect blastocyst cell numbers. For CF1 IVO embryos, the presence of growth factors during culture did not affect the proportion of embryos that attached to fibronectin-coated dishes, the size of the resulting outgrowths, or in vivo development following transfer.
Conclusion
Combinations of paf, EGF, GM-CSF, and IGF-1 did not improve development of murine embryos cultured individually in a sequential medium containing a defined protein source.
Keywords: Culture medium, Growth factors, Individual/single culture, Mouse strain, Blastocyst
Introduction
Recent advances in analytical technologies have made it possible to detect subtle changes in the composition of the culture medium made by individual preimplantation embryos. The appearance or disappearance of nutrients from the medium reflects the metabolic activity of the embryo [1, 2]. The embryo also secretes a variety of proteins into the surrounding medium, which can be analyzed to gain insight on the signaling between the embryo and the maternal endometrium [3, 4]. New imaging technologies also allow the developmental kinetics of individual embryos to be monitored by time-lapse microscopy [5]. In addition to important information on the biology of early embryos, the knowledge gained from these technologies will facilitate the development of clinical assays for embryo viability that could be used to select embryos for transfer [6, 7]. As technology continues to improve, it will be possible to evaluate multiple aspects of viability on individual embryos prior to transfer. However, the utility of these assays for both basic research and clinical application will be limited by our ability to successfully culture single embryos.
Numerous studies have indicated that embryo development is improved when embryos are cultured in groups [8, 9]. Results are consistent across multiple species [10–12], and the benefits of group culture can even be achieved when using embryos from different species [13]. These benefits are dependent on the volume of the medium, as well as the distance between neighboring embryos, suggesting that the effects of group culture are due to soluble, diffusible growth factors secreted by the embryo(s) [8, 14–16]. In vivo, these growth factors likely mediate communication between the embryo and maternal tissues [17–19], since embryos express receptors for the growth factors secreted by maternal tissues and the maternal tissues contain receptors for growth factors secreted by the embryo [20]. In vitro, group culture results in the accumulation of embryo-derived growth factors that enhance development [20, 21]. Therefore, if the culture medium was supplemented with a combination of growth factors that mimics those produced by the maternal tissues and/or other embryos, it should be possible to better support the in vitro development of individual embryos.
The objective of this study was to evaluate the effects of multiple growth factors on the development of individually cultured murine embryos. Embryonic development (blastocyst formation and hatching), blastocyst cell number (total, trophectoderm, and inner cell mass), post-hatching attachment and outgrowth, and in vivo development post-embryo transfer were analyzed to determine the effects of growth factor supplementation on embryo viability.
Materials and methods
Animals
All protocols were approved by the Colorado Center for Reproductive Medicine’s Ethics in Research Committee and followed animal care and use guidelines, as described by the Guide for the Care and Use of Laboratory Animals [22]. Mice were maintained on a 12:12-h light/dark cycle in single (male) or group (female) housing with ad libitum access to food and water.
General culture conditions
All cultures were performed in standard, tissue culture incubators with a humidified atmosphere maintained at 37 °C. Due to the elevation of our laboratory (Lone Tree, CO, ~ 1830 m above sea level), gas concentrations were increased to 7.5% CO2 and 6.5% O2 (equivalent to 6% CO2 and 5% O2 at sea level). All media were prepared in our laboratory and equilibrated for at least 4 h before use to achieve a final, equilibrated pH between 7.2 and 7.3. Unless stated otherwise, all cultures were performed in media covered with oil (OvOil, Vitrolife, Englewood, CO, USA) in tissue culture-treated dishes (Primaria™, Corning, Corning, NY, USA).
Oocyte collection: in vitro maturation
Female mice (B6D2F1/Hsd hybrid [BDF1], CF1 non-Swiss albino outbred [Hsd:NSA], and Swiss Webster outbred [SW; Hsd:ND4]; Envigo Laboratories, Indianapolis, IN) were treated with 5 IU pregnant mare’s serum gonadotropin (PMSG; Calbiochem, Billerica, MA, USA). Cumulus-oocyte complexes (COC) were collected 46 to 48 h post-PMSG into a MOPS-buffered medium [2] containing 5% fetal calf serum (Hyclone, Thermo Fisher Scientific, Waltham, MA, USA). COC with multiple layers of cumulus cells were selected and matured for 17 to 18 h in 50 μl drops (10 COC/drop) of medium. The maturation medium [23, 24] contained 0.5 mM glucose, 0.2 mM pyruvate, 6.0 mM lactate, 0.5 mg/ml insulin, 0.275 mg/ml transferrin, 0.25 ng/ml selenium, 10 ng/ml epidermal growth factor (EGF), 2 mg/ml fetuin, and 2.5 mg/ml recombinant human albumin (AlbIX; Novozymes, Bagsvaerd, Denmark).
Oocyte collection: in vivo maturation
Female mice were treated with 5 IU human chorionic gonadotropin (hCG; Calbiochem, Billerica, MA, USA) 48 h after receiving 5 IU PMSG. Ovulated COCs were collected from the oviducts 16 to 18 h after hCG injection into a MOPS-buffered medium [2] containing 5% fetal calf serum.
In vitro fertilization
Spermatozoa were collected from the cauda epdidymides and vas deferentia of BDF1 males (≥ 10 weeks). Spermatozoa were capacitated for 1 h in fertilization medium (25.0 mM NaHCO3, 3 mM glucose, 4.0 mg/ml BSA, 2.0 mM Ca2+, and 0.2 mM Mg2+) [23] and then co-cultured with COC (10 COC/50 μl drop fertilization medium, 1 × 106 spermatozoa/ml) for 6 h.
Embryo culture
Individual embryo culture was performed in 60-well culture dishes (one embryo in 10 μl/well, Nunc Mini Tray 163118, Thermo Fisher Scientific). Although our laboratory typically cultures groups of 10 embryos in 20 μl drops of medium (1 embryo per 2 μl of medium), 10 μl was used to minimize the accumulation of autocrine growth factors that might occur in 2 μl of medium and avoid the negative effects of culturing a single embryo in 20 μl of medium [16]. Embryos were cultured in a defined (2.5 mg/ml recombinant human albumin), sequential system for 48 in the first step medium (0.5 mM glucose, 0.3 mM pyruvate, 10.0 mM L-lactate, 0.5 mM alanyl-glutamine, 0.01 mM EDTA, and both nonessential and essential amino acids), followed by 64 h in the second step medium (3.0 mM glucose, 0.1 mM pyruvate, 6.0 mM L-lactate, 1.0 mM alanyl-glutamine, vitamins, and both nonessential and essential amino acids) [2]. Cleavage was evaluated 24 h post-insemination, and blastocyst formation and hatching were assessed on days 4 (D4) and 5 (D5; 96 and 114 h from placement into the first step culture medium, respectively).
Immunofluorescent staining of blastocysts
On D5, hatching and hatched blastocysts were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) for 15 to 20 min and stored in PBS with 0.5% BSA (MP Biomedicals, Solon, OH, USA) until staining. Blastocysts were washed in PBS with 0.1% Triton X100 (TX100), permeabilized in PBS with 1.0% TX100 (30 min), and blocked in PBS with 0.1% TX100 and 0.5% BSA (2 h), before incubation with primary antibodies. Anti-human SOX2 (Biogenex, Fremont, CA, USA, rabbit monoclonal) and CDX2 (Biogenex, mouse monoclonal) were used to detect cells of the inner cell mass (ICM) and trophectoderm (TE), respectively [24, 25]. Secondary antibodies (Alexa Fluor 488 donkey anti-rabbit IgG and Alexa Fluor 555 goat anti-mouse IgG; Invitrogen, Thermo Fisher Scientific) were then used for SOX2 and CDX2, respectively. Blastocysts were mounted on a glass slide with ProLong Gold Antifade Reagent (Life Technologies, Thermo Fisher Scientific) and evaluated (400×) using a fluorescent microscope and MetaMorph software (Molecular Devices, Sunnyvale, CA).
Trophectoderm attachment and outgrowth
On D5, hatching and hatched blastocysts were transferred to a 24-well plate with fibronectin-coated wells (Corning 356241; Corning, NY, USA). Each well contained one embryo and 0.5 ml of the control (no growth factors) second step embryo culture medium [2] supplemented with insulin (2.5 μg/ml), transferrin (1.38 μg/ml), and selenium (1.68 ng/ml; Cellgro, Corning). Attachment to the surface of the dish was evaluated 48 and 96 h after the initiation of culture. After 96 h of culture, a digital image of the entire embryo was taken and used to determine the total area (arbitrary units) of the outgrowth using Metamorph software.
Non-surgical embryo transfers
On day 3.5 (~ 84 h) of culture, expanded and minimally hatching blastocysts were selected and incubated in 20 μl drops of EmbryoGlue (Vitrolife) under oil for 1 h prior to transfer. Recipient female SW mice were housed overnight with vasectomized CF1 males, and those with a visible vaginal plug were used as recipients 72 h later (day 3). Embryos (15 to 17 per female) from a single treatment were placed into the uterus using the NSET™ device (Paratechs, Lexington, KY) [26, 27]. Implantation (resorptions and viable fetuses) and fetal development were assessed on day 15.5 of development (12 days post-transfer).
Experimental design
A group of four growth factors were selected for evaluation using previously published concentrations that had elicited positive effects on murine embryos (Table 1). Since the beneficial effects of paf (P) and EGF (E) have been shown to be stage specific [28–30], these growth factors were only present in the first (P) or second (E) stages of culture (P-E). In contrast, insulin-like growth factor 1 (IGF-1, I) [31, 32] and granulocyte-macrophage colony-stimulating factor (GM-CSF, G) [33, 34] were evaluated in both stages of culture in the presence of paf and EGF (PGI-EGI).
Table 1.
Concentrations of growth factors previously shown to improve the development of murine embryos
| Growth factor | Test concentration | Reference |
|---|---|---|
| β-Acetyl-γ-O-hexadecyl-L-α-phosphatidylcholine (paf)a | 100 ng/ml | 28, 31 |
| Epidermal growth factor (EGF)b | 5 ng/ml | 29, 57 |
| Granulocyte-macrophage colony-stimulating factor (GM-CSF)c | 2 ng/ml | 33, 35 |
| Insulin-like growth factor 1 (IGF-1)d | 50 ng/ml | 31, 57 |
aSigma P4904
bSigma SRP3196, recombinant mouse
cSigma SRP3201, recombinant mouse
dSigma I8779, recombinant mouse
Three strains of mice (BDF1, CF1, and SW) and two sources of oocytes (in vitro or in vivo matured) were used to produce embryos with varying degrees of developmental competence [24]. Outgrowth and embryo transfer experiments were only conducted with CF1 embryos derived from in vivo matured oocytes, since these embryos exhibit an intermediate level of developmental competence compared to embryos produced from IVM oocytes from CF1 females (low quality) or embryos produced from IVO oocytes from BDF1 or SW female (high quality). Based on blastocyst formation, hatching, and cell numbers, P-E and PGI-EGI produced similar results. Therefore, outgrowth and embryo transfer experiments only compared embryos that had been cultured in the absence of growth factors (control) or in the presence of PGI-EGI, to minimize the number of evaluated treatment groups.
Statistical analysis
Embryonic development was analyzed using the generalized linear mixed model (GLIMMIX) procedure in SAS. The proportions of embryos developing to the blastocyst or hatching blastocyst stage were based on the number of cleaved (≥ 2-cell) embryos 24 h post-insemination. Each embryo was scored as a 1 or 0 depending on whether or not it achieved the desired stage of development (e.g., blastocyst, hatching blastocyst, or attached blastocyst) and analyzed using a binomial error distribution and a probit link function. The fixed factor in the model was treatment (control, P-E, or PGI-EGI), and replicate (day of oocyte collection) was included as a random factor.
Blastocyst cell numbers (control, P-E, or PGI-EGI) and trophectoderm outgrowths (control or PGI-EGI) were analyzed using the mixed model procedures in SAS with treatment as the only fixed factor. Results from embryo transfers (control or PGI-EGI) were summarized as the proportions of females or embryos and analyzed using 2 × 2 contingency tables (graphpad.com/quickcals/contingency2/).
Results
Blastocyst formation and hatching
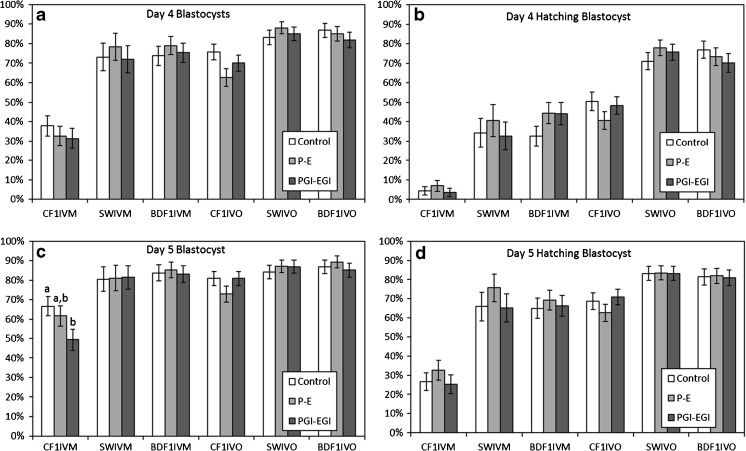
For each combination of mouse strain (CF1, SW, or BDF1) and type of oocyte (IVM or IVO), three to eight replicates were performed involving a total of 50 to 137 oocytes per treatment. The proportion of oocytes that cleaved ranged from 63.3 to 98.9% and averaged 86.5% across treatments, resulting in 37 to 120 embryos per treatment. Embryo development (per two-cell embryo) varied widely across treatments, with the proportion of embryos forming blastocysts on D4 ranging from 37.8 ± 5.1 to 86.8 ± 3.6% and the proportion of embryos hatching on D5 ranging from 26.7 ± 4.7 to 83.2 ± 3.6% for embryos cultured in the absence of growth factors (Fig. 1). For all parameters, development of embryos derived from in vitro matured oocytes from CF1 females had the lowest development and embryos derived from in vivo matured oocytes from SW or BDF1 females had the highest development.
Fig. 1.
Blastocyst formation (a, c) and hatching (b, d) on days 4 and 5 for embryos from in vitro (IVM) or in vivo (IVO) matured oocytes collected from CF1, SW, or BDF1 females and cultured in the absence (control) or presence of growth factors (P-E or PGI-EGI). Different letters indicate significant (P < 0.05) differences between treatments within a type of embryo (strain and oocyte source)
The presence of growth factors (P-E or PGI-EGI) did not improve (P > 0.05) the proportion of embryos forming blastocysts or initiating hatching by D4 or D5 for any of the types of embryos tested (Fig. 1). The only significant (P < 0.05) effect of growth factors on embryo development was a decrease in the proportion of embryos that formed blastocyst by D5 when in vitro matured oocytes from CF1 females were cultured with PGI-EGI (Fig. 1).
Blastocyst cell numbers
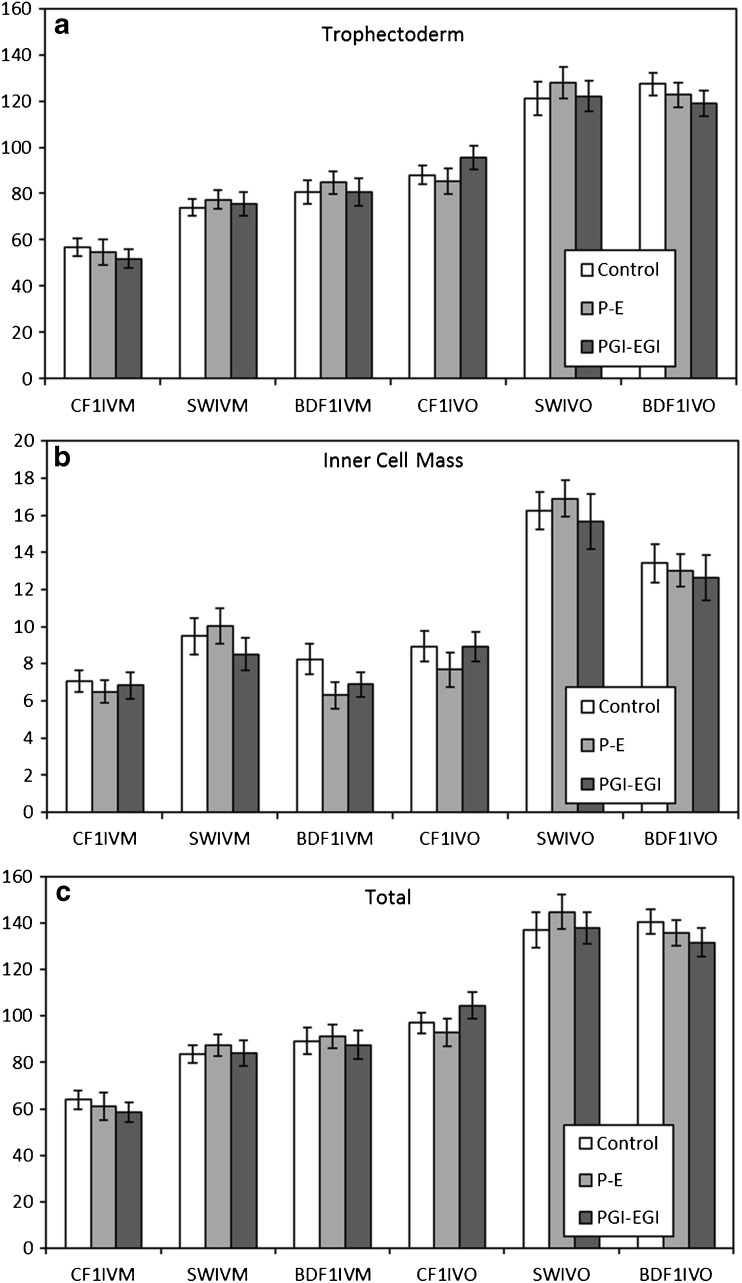
For each combination of mouse strain and type of oocytes, 20 to 46 blastocysts per treatment were evaluated for the number of TE and ICM cells, as well as the total number of cells per blastocyst (TE + ICM). The presence of growth factors did not affect (P > 0.05) the allocation of cells or the total number of cells within the blastocysts (Fig. 2).
Fig. 2.
Cell numbers (trophectoderm (a), inner cell mass (b), and total (c)) of hatching and hatched blastocysts on day 5 from in vitro (IVM) or in vivo (IVO) matured oocytes collected from CF1, SW, or BDF1 females and cultured in the absence (control) or presence of growth factors (P-E or PGI-EGI). There were no significant effects (P < 0.05) of growth factors for any of the types of embryos
Trophectoderm attachment and outgrowth
When D5 hatching or hatched embryos from in vivo matured oocytes from CF1 females were placed into fibronectin-coated wells, the proportions of embryos that had attached at 48 h (55.2 and 66.7%) and 96 h (96.6 and 83.3%) were not different (P > 0.05) between control (n = 29) and growth factor-exposed (PGI-EGI, n = 30) embryos, respectively (Table 2). The area of the outgrowths at 96 h was also not affected (P > 0.05) by the presence of growth factors (Table 2).
Table 2.
Attachment and outgrowth of hatching and hatched blastocysts cultured on fibronection-coated wells
| Treatment | Number | Attached at 48 h | Attached at 96 h | Area at 96 h (arbitrary units) |
|---|---|---|---|---|
| Control | 29 | 55.2% | 96.6% | 109,094 ± 9687 |
| Growth factors (PGI-EGI) | 30 | 66.7% | 83.3% | 121,690 ± 10,553 |
There were no significant differences (P > 0.05) between treatments
Embryo transfers
Following non-surgical embryo transfers, 55.6 and 62.5% of recipients receiving control or growth factor-treated (PGI-EGI) embryos, respectively, became pregnant (i.e., had at least one implantation site when evaluated at day 15.5, Table 3). The proportion of all recipients (44.4%) and the proportion of pregnant recipients (80.0%) with at least one fetus were numerically higher for embryos cultured with growth factors compared to control embryos (25.0 and 40.0%, respectively), but these differences were not significant (P > 0.05; Table 3). Overall fetal development per transferred embryo was < 10% and was not affected by growth factor exposure (P > 0.05; Table 3).
Table 3.
Summary of implantation and fetal development on day 15.5 following non-surgical transfer of blastocysts that had been cultured in the absence (control) or presence of growth factors (PGI-EGI)
| Control | Growth factor (PGI-EGI) | |
|---|---|---|
| %All recipients | n = 8 | n = 9 |
| With ≥ 1 implantation site (pregnant) | 62.5% | 55.6% |
| With ≥ 1 fetus | 25.0% | 44.4% |
| %Embryos transferred to all recipients | n = 122 | n = 137 |
| Implanted | 18.9% | 21.2% |
| Formed fetus | 2.5% | 4.4% |
| %Pregnant recipients | n = 5 | n = 5 |
| With ≥ 1 fetus | 40.0% | 80.0% |
| %Embryos transferred to pregnant recipients | n = 77 | n = 75 |
| Implanted | 29.9% | 38.7% |
| Formed fetus | 3.9% | 8.0% |
| %Implanted embryos | n = 23 | n = 29 |
| Formed fetus | 13.0% | 20.7% |
There were no significant differences (P > 0.05) between treatments
Discussion
It is now possible to analyze the transcriptome, proteome, metabolome, and secretome of individual embryos, as well as their developmental kinetics. When combined, these analyses will greatly expand our understanding of early embryo development and may be useful for selecting embryos for transfer. However, the utility of these assays for both the clinician and the researcher is dependent on our ability to successfully culture individual embryos. In vivo, embryos are exposed to a variety of growth factors that are secreted by the oviductal and uterine epithelia, as well as the embryo itself. Although most embryo culture media do not contain exogenous growth factors, it is thought that culturing embryos in groups concentrates embryo-secreted growth factors in the medium, which then supports development. In contrast, when embryos are cultured individually, there may be insufficient growth factors in the medium to support normal development. We hypothesized that the addition of growth factors to the culture medium used for individual mouse embryos would improve in vitro and in vivo development, but our results did not support this hypothesis. Although the tested growth factors have been reported to stimulate embryo development in other studies, the literature regarding the effects of growth factors is inconsistent [28, 30, 32, 34–40]. There are a number of possible reasons for these discrepancies, including the concentration of the growth factor, the quality of the embryo being tested, the culture conditions being used, and the developmental endpoints being evaluated.
The effects of growth factors can be dose dependent, and, in some cases, excessive concentrations can negate beneficial effects and sometimes even be inhibitory [31, 35, 39, 41, 42]. We did not perform dose-response experiments for each of the tested growth factors, but we did use concentrations that had previously been shown to be effective for murine embryos (Table 1). It is possible that the specific, recombinant preparations of the growth factors tested here had different potencies than those used in other studies. Alternatively, the use of multiple growth factors (one to three present in each stage of culture) could alter the effective dose of each growth factor. If multiple growth factors stimulate the same pathways [43–47], the combined effect of low concentrations of multiple growth factors may be equivalent to a higher, ineffective, or inhibitory dose of a single growth factor.
The strain of mouse used for oocyte or embryo collections, as well as the developmental stage of the oocyte or embryo at collection, can affect the sensitivity of the resulting embryo to culture conditions [42, 48–50]. We have recently demonstrated that embryos resulting from IVM and IVF provide a sensitive assay for the detection of contaminants in the culture environment [24]. Similar correlations have been reported between embryo quality and responsiveness to growth factors [51–53]. Based on these previous findings, we evaluated embryos from three strains of mice derived from in vitro or in vivo matured oocytes and in vitro fertilization in order to assess the effects of growth factors on embryos with a range of developmental potentials. For example, the proportion of control embryos that had initiated hatching on D4 ranged from 4.4% for in vitro matured oocytes from CF1 females to 76.9% for in vivo matured oocytes from BDF1 females. However, we were unable to find a strain of mouse and/or source of oocyte that demonstrated improved in vitro development (blastocyst formation, hatching, or blastocyst cell number) in the presence of growth factors.
The effects of growth factors on embryo development can also be dependent on the culture conditions. Many of the studies demonstrating positive effects of growth factors have used sub-optimal culture conditions or embryos that have been stressed in some way. Culturing with low concentrations of protein or in atmospheric oxygen can increase the beneficial effects of growth factors [53–56]. Similarly, the response to growth factors can be more pronounced for embryos exposed to heat or oxidative stress or embryos that have previously been cryopreserved [30, 40, 57]. Although embryos in the present study were presumably stressed by culturing them individually, we still did not observe beneficial effects of the growth factors. It is possible that the growth factors secreted by a single embryo in just 10 μl of medium accumulate in sufficient amounts to support development in the absence of additional, exogenous growth factors. Alternatively, the presence of protein, the use of reduced O2, and the use of a medium containing amino acids may have allowed the embryos to better cope with the stress of individual culture, even in the absence of growth factors [58, 59].
Trophectoderm outgrowth, implantation, and fetal development were also unaffected by growth factors, but this portion of our study was limited and should be interpreted cautiously. Only one strain of mouse (CF1) and one source of oocytes (IVO) were evaluated for these endpoints. A large proportion (> 70%) of embryos from these oocytes were capable of reaching the blastocyst stage by day 5; > 60% of those were hatching, and the embryos displayed cells of both the TE (CDX2+) and ICM (SOX2+) lineages, so we had assumed that these embryos would provide a suitable model to evaluate the effects of growth factor exposure on post-hatching development. It is difficult to speculate why so few developed into fetuses following transfer. There is limited data concerning the outcomes of embryo transfers using CF1 × BDF1 IVF embryos, but it is possible for ~ 40% of transferred embryos to develop into pups [60, 61]. However, these studies used different culture conditions (group culture with BSA) and transfer protocols (surgical) than we used in the present study (individual culture with recombinant albumin and non-surgical transfers). While the low fetal development may have prevented us from detecting significant effects of the growth factors on this parameter, comparisons of the proportions of females becoming pregnant or the proportions of embryos implanting remain useful measures of the effects of growth factors that are often used with embryos from sensitive strains of mice [50, 62].
In conclusion, two different sources of oocytes (in vitro and in vitro matured) from three strains of mice were used to produce six different types of embryos with varying degrees of developmental competence. When these embryos were individually cultured with combinations of paf, EGF, GM-CSF, and IGF-1 in a sequential medium system containing a defined protein source, blastocyst formation, hatching, and blastocyst cell number and allocation were not affected. While our culture conditions may have been able to minimize some of the stress associated with individual culture and support blastocyst development in the absence of growth factors, the low fetal development of embryos from CF1 IVO oocytes suggests deficiencies in the environment for individual embryo culture that were not overcome by the tested growth factors.
Acknowledgments
The authors would like to thank Erik Strait, Caitlyn Graham, and Brittany Winters for excellent care of the mice used for this study.
References
- 1.Cortezzi SS, Cabral EC, Trevisan MG, Ferreira CR, Setti AS, DPdAF B, RdCS F, Iaconelli A, Eberlin MN, Borges E. Prediction of embryo implantation potential by mass spectrometry fingerprinting of the culture medium. Reproduction. 2013;145:453–462. doi: 10.1530/REP-12-0168. [DOI] [PubMed] [Google Scholar]
- 2.Krisher RL, Heuberger A, Paczkowski M, Stevens J, Popsil C, Prather RS, Sturmey RG, Herrick JR, Schoolcraft WB. Applying metabolomic analyses to the practice of embryology: physiology, development and ART. Reprod Fertil Dev. 2015;27:602–620. doi: 10.1071/RD14359. [DOI] [PubMed] [Google Scholar]
- 3.Katz-Jaffe MG, McReynolds S, Gardner DK, Schoolcraft WB. The role of proteomics in defining the human embryonic secretome. Mol Hum Reprod. 2009;15:271–277. doi: 10.1093/molehr/gap012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominguez F, Gadea B, Mercader A, Esteban FJ, Pellicer A, Simón C. Embryologic outcome and secretome profile of implanted blastocysts obtained after coculture in human endometrial epithelial cells verus the sequential system. Fertil Steril. 2010;93:774–782. doi: 10.1016/j.fertnstert.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Rubio I, Galán A, Larreategui Z, Ayerdi F, Bellver J, Herrero J, Meseguer M. Clinical validation of embryo culture and selection by morphokinetic analysis: a randomized, controlled trial of the EmbryoScope. Fertil Steril. 2014;102:1287–1294. doi: 10.1016/j.fertnstert.2014.07.738. [DOI] [PubMed] [Google Scholar]
- 6.Krisher RL, Schoolcraft WB, Katz-Jaffe MG. Omics as a window to view embryo viability. Fertil Steril. 2015;103:333–341. doi: 10.1016/j.fertnstert.2014.12.116. [DOI] [PubMed] [Google Scholar]
- 7.Gardner DK, Meseguer M, Rubio C, Treff NR. Diagnosis of human preimplantation embryo viability. Hum Reprod Update. 2015;21:727–747. doi: 10.1093/humupd/dmu064. [DOI] [PubMed] [Google Scholar]
- 8.Lane M, Gardner DK. Effect of incubation volume and embryo density on the development and viability of mouse embryos in vitro. Hum Reprod. 1992;7:558–562. doi: 10.1093/oxfordjournals.humrep.a137690. [DOI] [PubMed] [Google Scholar]
- 9.Ebner T, Shebl O, Moser M, Mayer RB, Arzt W, Tews G. Group culture of human zygotes is superior to individual culture in terms of blastulation, implantation and life birth. Reprod BioMed Online. 2010;21:762–768. doi: 10.1016/j.rbmo.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 10.Gardner DK, Lane M, Spitzer A, Batt PA. Enhanced raates of cleavage and development for sheep zygotes cultured to the blastocyst stage in vitro in the absence of serum and somatic cells: amino acids, vitamins, and culturing embryos in groups stimulate development. Biol Reprod. 1994;50:390–400. doi: 10.1095/biolreprod50.2.390. [DOI] [PubMed] [Google Scholar]
- 11.Wydooghe E, Heras S, Dewulf J, Piepers S, Van den Abbeel E, De Sutter P, Vandaele L, Van Soom A. Reprod Fertil Dev. 2014;26:717–724. doi: 10.1071/RD13043. [DOI] [PubMed] [Google Scholar]
- 12.Spindler RE, Wildt DE. Quality and age of companion felid embryos modulate enhanced development by group culture. Biol Reprod. 2002;66:167–173. doi: 10.1095/biolreprod66.1.167. [DOI] [PubMed] [Google Scholar]
- 13.Spindler RE, Crichton EG, Agca Y, Loskutoff N, Critser J, Gardner DK, Wildt DE. Improved felid embryo development by group culture is maintained with heterospecific companions. Theriogenology. 2006;66:82–92. doi: 10.1016/j.theriogenology.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Stokes PJ, Abeydeera LA, Leese HJ. Development of porcine embryos in vivo and in vitro; evidence for embryo ‘cross talk’ in vitro. Dev Biol. 2005;284:62–71. doi: 10.1016/j.ydbio.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Gopichandran N, Leese HJ. The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos. Reproduction. 2006;131:269–277. doi: 10.1530/rep.1.00677. [DOI] [PubMed] [Google Scholar]
- 16.Kelley RL, Gardner DK. In vitro culture of individual mouse preimplantation embryos: the role of embryo density, microwells, oxygen, timing and conditioned media. Reprod BioMed Online. 2017; In Press; 10.1016/j.rbmo.2017.02.001. [DOI] [PubMed]
- 17.Armant DR. Blastocysts don’t go it alone. Extrinsic signals fine-tune the intrinsic developmental program of trophoblast cells. Dev Biol. 2005;280:260–80. [DOI] [PMC free article] [PubMed]
- 18.Singh M, Chaudhry P, Asselin E. Bridging endometrial receptivity and implantation: network of hormones, cytokines, and growth factors. J Endocrinol. 2011;210:5–14. doi: 10.1530/JOE-10-0461. [DOI] [PubMed] [Google Scholar]
- 19.Maillo V, Sánchez-Calabuig MJ, Loperaa-Vasquez R, Hamdi M, Guiterrez-Adaan A, Lonergan P, Rizos D. Oviductal response to gametes and early embryos in mammals. Reproduction. 2016;152:R127–R141. doi: 10.1530/REP-16-0120. [DOI] [PubMed] [Google Scholar]
- 20.Thouas GA, Dominguez F, Green MP, Viella F, Simon C, Gardner DK. Soluble ligands and their receptors in human embryo development and implantation. Endocr Rev. 2015;36:92–130. doi: 10.1210/er.2014-1046. [DOI] [PubMed] [Google Scholar]
- 21.O’Neill C. The potential roles for embryotrophic ligands in preimplantation embryo development. Hum Reprod Update. 2008;14:275–88. [DOI] [PubMed]
- 22.National Research Council (U.S.A.) Committee for the update of the guide for the care and use of laboratory animals. Guide for the care and use of laboratory animals. 8. Washington D.C.: National Academies Press; 2011. [PubMed] [Google Scholar]
- 23.Herrick JR, Strauss KJ, Schneiderman A, Rawlins M, Stevens J, Schoolcraft WB, Krisher RL. The beneficial effects of reduced magnesium during the oocyte-to-embryo transition are conserved in mice, domestic cats, and humans. Reprod Fertil Dev. 2015;27:323–331. doi: 10.1071/RD13268. [DOI] [PubMed] [Google Scholar]
- 24.Herrick JR, Paik T, Strauss KJ, Schoolcraft WB, Krisher RL. Building a better mouse embryo assay: effects of mouse strain and in vitro maturation on sensitivity to contaminants of the culture environment. J Assist Reprod Genet. 2016;33:237–245. doi: 10.1007/s10815-015-0623-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakhtari A, Ross PJ. DPPA3 prevents cytosine hydroxymethylation of the maternal pronucleus and is required for normal development in bovine embryos. Epigenetics. 2014;9:1271–1279. doi: 10.4161/epi.32087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steele KH, Hester JM, Stone BJ, Carrico KM, Spear BT, Fath-Goodin A. Nonsurgical embryo transfer device compared with wurgery for embryo transfer in mice. J Am Assoc Lab Anim Sci. 2013;52:17–21. [PMC free article] [PubMed] [Google Scholar]
- 27.Stone BJ, Steele KH, Fath-Goodin A. A rapid and effective nonsurgical artificial insemination protocol using the NSET™ device for sperm transfer in mice without anesthesia. Transgenic Res. 2015;24:775–781. doi: 10.1007/s11248-015-9887-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Neill Autocrine mediators are required to act on the embryo by the 2-cell stage to promote normal development and survival of mouse preimplantation embryos in vitro. Biol Reprod. 1998;58:1303–1309. doi: 10.1095/biolreprod58.5.1303. [DOI] [PubMed] [Google Scholar]
- 29.Dardik A, Schultz RM. Blastocoel expansion in the preimplantation mouse embryo: stimulatory effect of TGF-α and EGF. Development. 1991;113:919–930. doi: 10.1242/dev.113.3.919. [DOI] [PubMed] [Google Scholar]
- 30.Desai N, Lawson J, Goldfarb J. Assessment of growth factor effects on post-thaw development of cryopreserved mouse morulae to the blastocyst stage. Hum Reprod. 2000;15:410–418. doi: 10.1093/humrep/15.2.410. [DOI] [PubMed] [Google Scholar]
- 31.O’Neill C. Evidence for the requirement of autocrine growth factors for development of mouse preimplantation embryos in vitro. Biol Reprod. 1997;56:229–37. [DOI] [PubMed]
- 32.Spanos S, Becker DL, Winston RML, Hardy K. Anti-apoptotic action of insulin-like growth factor-I during human preimplantation embryo development. Biol Reprod. 2000;63:1413–1420. doi: 10.1095/biolreprod63.5.1413. [DOI] [PubMed] [Google Scholar]
- 33.Chin PY, Macpherson AM, Thompson JG, Lane M, Robertson SA. Stress response genes are suppressed in mouse preimplantation embryos by granulocyte-macrophage colony-stimulating factor (GM-CSF) Hum Reprod. 2009;24:2997–3009. doi: 10.1093/humrep/dep307. [DOI] [PubMed] [Google Scholar]
- 34.Sjöblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor promotes human blastocyst development in vitro. Hum Reprod. 1999;14:3069–3076. doi: 10.1093/humrep/14.12.3069. [DOI] [PubMed] [Google Scholar]
- 35.Robertson SA, Sjöblom C, Jasper MJ, Norman RJ, Seamark RF. Granulocyte-macrophage colony-stimulating factor promotes glucose transport and blastomere viability in murine preimplantation embryos. Biol Reprod. 2001;64:1206–1215. doi: 10.1095/biolreprod64.4.1206. [DOI] [PubMed] [Google Scholar]
- 36.Wood SA, Kaye PL. Effects of epidermal growth factor on preimplantation mouse embryos. J Reprod Fertil. 1989;85:575–582. doi: 10.1530/jrf.0.0850575. [DOI] [PubMed] [Google Scholar]
- 37.Radonjic-Lazovic G, Roudebush WE. The effect of short- vs. long-term platelet-activating factor exposure on mouse preimplantation embryo development. Early Pregnancy. 1995;1:196–200. [PubMed] [Google Scholar]
- 38.de Moraes AAS, Hansen PJ. Granulocyte-macrophage colony-stimulating factor promotes development of in vitro produced bovine embryos. Biol Reprod. 1997;57:1060–1065. doi: 10.1095/biolreprod57.5.1060. [DOI] [PubMed] [Google Scholar]
- 39.Chi MMY, Schlein AL, Moley KH. High insulin-like growth factor 1 (IGF-1) and insulin concentrations trigger apotosis in the mouse blastocyst via down-regulation of the IGF-1 receptor. Endocrinology. 2000;141:4784–4792. doi: 10.1210/endo.141.12.7816. [DOI] [PubMed] [Google Scholar]
- 40.Jousan FD, Hansen PJ. Insulin-like growth factor-I as a survival factor for the bovine preimplantation embryo exposed to heat shock. Biol Reprod. 2004;71:1665–1670. doi: 10.1095/biolreprod.104.032102. [DOI] [PubMed] [Google Scholar]
- 41.Campbell JM, Nottle MB, Vassiliev I, Mithcell M, Lane M. Insulin increases epiblast cell number of in vitro cultured mouse embryos via the PI3K/GSK3/p53 pathway. Stem Cells Dev. 2012;21:2430–2441. doi: 10.1089/scd.2011.0598. [DOI] [PubMed] [Google Scholar]
- 42.Jin XL, O’Neil C. Systematic analysis of the factors that adversely affect the rate of cell accumulation in mouse embryos during their culture in vitro. Reprod Biol Endocrinol. 2014;12:35. doi: 10.1186/1477-7827-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heo JS, Han HJ. PKC and MAPKs pathway mediate EGF-induced stimulation of 2-deoxyglucose uptake in mouse embryonic stem cells. Cell Physiol Biochem. 2006;17:145–158. doi: 10.1159/000092076. [DOI] [PubMed] [Google Scholar]
- 44.Navarrete Santos A, Ramin N, Tonack S, Fischer B. Cell lineage-specific signaling of insulin and insulin-like growth factor I in rabbit blastocysts. Endocrinology. 2008;149:515–524. doi: 10.1210/en.2007-0821. [DOI] [PubMed] [Google Scholar]
- 45.Jousan FD, Oliveira LJ, Hansen PJ. Short-term culture of in vitro produced bovine preimplantation embryos with insulin-like growth factor-I prevents heat shock induced apoptosis through activation of the phosphatidyl 3-kinase/Akt pathway. Mol Reprod Dev. 2008;75:681–688. doi: 10.1002/mrd.20830. [DOI] [PubMed] [Google Scholar]
- 46.Bonilla AQS, Ozawa M, Hansen PJ. Timing and dependence upon mitogen-activated protein kinase signaling for pro-development actions of insulin-like growth factor 1 on the preimplantation bovine embryo. Growth Hormon IGF Res. 2011;21:107–111. doi: 10.1016/j.ghir.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Jeong W, Kim J, Bazer FW, Song G. Proliferation-stimulating effect of colony-stimulating factor 2 on porcine trophectoderm cells is mediated by activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase. PLoS One. 2014;9:e88731. doi: 10.1371/journal.pone.0088731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chatot CL, Lewis JL, Torres I, Ziomek CA. Development of 1-cell embryos from different strains of moce in CZB medium. Biol Reprod. 1990;42:432–440. doi: 10.1095/biolreprod42.3.432. [DOI] [PubMed] [Google Scholar]
- 49.Hadi T, Hammer MA, Algire C, Richards T, Baltz JM. Similar effects of osmolarity, glucose, and phosphate on cleavage past the 2-cell stage in mouse embryos from outbred and F1 hybrid females. Biol Reprod. 2005;72:179–187. doi: 10.1095/biolreprod.104.033324. [DOI] [PubMed] [Google Scholar]
- 50.Highet AR, Bianco-Miotto T, Pringle KG, Peura A, Bent S, Zhang J, Nottle MB, Thompson JG, Roberts CT. A novel embryo culture media supplement that improves pregnancy rates in mice. Reproduction. 2017;153:327–340. doi: 10.1530/REP-16-0517. [DOI] [PubMed] [Google Scholar]
- 51.Kawamura K, Chen Y, Shu Y, Cheng Y, Qiao J, Behr B, Reijo Pera RA, Hsueh AJW. Promotion of human early embryonic development and blastocyst outgrowth in vitro using autocrine/paracrine growth factors. PLoS One. 2012;7:e49328. doi: 10.1371/journal.pone.0049328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dobbs KB, Khan FA, Sakatani M, Moss JI, Ozawa M, Ealy AD, Hansen PJ. Regulation of pluripotency of inner cell mass and growth and differentiation of trophectoderm of the bovine embryo by colony stimulating factor 2. Biol Reprod 2013;89:141,1–10. [DOI] [PubMed]
- 53.Ziebe S, Loft A, Povlsen BB, Erb K, Agerholm I, Aasted M, Gabrielsen A, Hnida C, Zobel DP, Munding B, Bendz SH, Robertson SA. A randomized clinical trial to evaluate the effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) in embryo culture medium for in vitro fertilization. Fertil Steril. 2013;99:1600–1609. doi: 10.1016/j.fertnstert.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 54.Wei Z, Park KW, Day BN, Prather RS. Effect of epidermal growth factor on preimplantation development and its receptor expression in porcine embryoss. Mol Reprod Dev. 2001;60:457–462. doi: 10.1002/mrd.1110. [DOI] [PubMed] [Google Scholar]
- 55.Karagenc L, Lane M, Gardner DK. Granulocyte-macrophage colony-stimulating factor stimulates mouse blastocyst inner cell mass development only when media lack human serum albumin. Reprod BioMed Online. 2005;10:511–518. doi: 10.1016/S1472-6483(10)60829-2. [DOI] [PubMed] [Google Scholar]
- 56.Yoon J, Juhn KM, Ko JK, Yoon SH, Ko Y, Lee CY, Lim JH. Effects of oxygen tension and IGF-I on HIF-1α protein expression in mouse blastocysts. J Assist Reprod Genet. 2013;30:99–105. doi: 10.1007/s10815-012-9902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurzawa R, Glabowski W, Baczkowski T, Wiszniewska B, Marchlewicz M. Growth factors protect in vitro cultured embryos from the consequences of oxidative stress. Zygote. 12:231–40. [DOI] [PubMed]
- 58.Gardner DK. Dissection of culture media for embryos: the most important and less important components and characteristics. Reprod Fertil Dev. 2008;20:9–18. doi: 10.1071/RD07160. [DOI] [PubMed] [Google Scholar]
- 59.Biggers JD, Summer MC. Choosing a culture medium: making informed choices. Fertil Steril. 2008;90:473–483. doi: 10.1016/j.fertnstert.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 60.Donjacour A, Liu X, Lin W, Simbulan R, Rinaudo PF. In vitro fertilization affects growth and glucosemetabolism in a sex-specific manner in an outbred mouse model. Biol Reprod 2014;90:80,1-10. [DOI] [PMC free article] [PubMed]
- 61.Summers MC, Bhatnagar PR, Lawitts JA, Biggers JD. Fertilization in vitro of mouse ova from inbred and outbred strains: complete preimplantation embryo development in glucose-supplemented KSOM. Biol Reprod. 1995;53:431–437. doi: 10.1095/biolreprod53.2.431. [DOI] [PubMed] [Google Scholar]
- 62.Takeuchi M, Seki M, Furukawa E, Takahashi A, Saito K, Kobayashi M, Ezoe K, Fukui E, Yoshizawa M, Matsumoto H. Improvement of implantation potential in mouse blastocysts derived from IVF by combined treatment with prolactin, epidermal growth factor and 4-hydroxyestradiol. Mol Hum Reprod. 2017;23:557–570. doi: 10.1093/molehr/gax035. [DOI] [PubMed] [Google Scholar]