Abstract
Heat shock cognate 70 (HSC70) is a class of highly conserved proteins which functions as a molecular chaperon, participates in tolerance processes, and is involved in protein folding, degradation, targeting, translocation, and protein complex remodeling. In this study, the mRNA expression level of the Haliotis diversicolor HSC70 (HdHSC70) gene was detected by quantitative real-time PCR in different tissues and under different stresses. The results showed that the HdHSC70 gene was ubiquitously expressed in seven selected tissues. The highest expression level was detected in gills (P < 0.05). The expression level of the HdHSC70 gene was significantly upregulated by thermal stress, hypoxia stress, Vibrio parahaemolyticus infection, and combined thermal and hypoxia stress. The upregulation occurred at the early stage of stress. These results indicated that the HdHSC70 is an important component in the immune system of H. diversicolor and is involved in the early stress response. Meanwhile, 5′-flanking region sequence (2013 bp) of the HdHSC70 gene was cloned; it contains a putative core promoter region, heat shock element, CpG, and transcription elements including NF-1, Sp1, Oct-1, interferon consensus sequence binding protein (ICSBP), etc. In HEK 293T cells, the 5′-flanking region sequence is able to drive expression of the enhanced green fluorescent protein (EGFP), proving its promoter function. The promoter activity increased after high-temperature treatment, which may be the immediate reason why the expression of the HdHSC70 gene was significantly upregulated by thermal stress. After the ICSBP-binding site was mutated, we found the luciferase activity significantly reduced, which suggested that the ICSBP-binding site has a certain enhancement effect on the activity of the HdHSC70 promoter.
Keywords: HSC70, Pathogen, Hypoxia, Thermal, Transcriptional regulation, Haliotis diversicolor
Introduction
As a species of marine gastropod mollusks, the small abalone Haliotis diversicolor is one of the most important cultured abalones in the coastal provinces of southern China (Ke and You 2011). However, due to the deterioration of its living environment, the natural resources of the abalone are greatly reduced (Fujino et al. 1992). Although this species has a higher tolerance to high temperatures compared to other species of abalone (Ke et al. 2000; Liu et al. 2008), water temperature is still one of the important factors that affects the growth of H. diversicolor. High temperature in seawater has resulted in changes in the respiration and metabolic rates in marine benthic organisms (Wang et al. 2012). In addition, Vibrio parahaemolyticus has become a main pathogen of cultured H. diversicolor in summer, which causes the outbreak of mass mortality of cultured abalone (Cheng et al. 2004; Liu et al. 2000).
Heat shock proteins (HSPs) are a group of highly conserved proteins that act as molecular chaperones in almost all living organisms by preventing protein aggregation and promoting orthometric refolding of denatured proteins during environmental stress (Cheng et al. 2015; Shen et al. 2016). These proteins represent a predominant self-protecting mechanism adapting to extreme changes in temperature and can also be induced by various stress factors, such as starvation (Jiang et al. 2012), chemical exposure (Shu et al. 2011), and heavy metal pollution (Shi et al. 2016). Based on sequence similarity and molecular size, five major HSP families have been identified (HSP100, HSP90, HSP70, HSP60, and the small HSPs) (Gu et al. 2012; Shen et al. 2016). Among them, the HSP70 family is one of the most highly conserved proteins and participates in various cellular processes, including protein translocation, cell cycle, apoptosis, and degradation of denatured proteins (Daugaard et al. 2007; Thanaphum and Haymer 1998). This family is expressed in two different patterns (Boorstein et al. 1994; Karlin and Brocchieri 1998). The first group is heat shock protein 70 (HSP70), which expresses itself with a very low level under non-pressure conditions (Bahar et al. 2013), but its transcription and translation are quickly induced by a hundred-fold under many kinds of stress (Choi et al. 2014; Mahroof et al. 2005). In contrast, another group, heat shock cognate70 (HSC70), is constitutively expressed at high levels in normal conditions. It has been reported that the HSC70 gene does not change under exposure to stress such as hot or cold shocks, but it has also been reported that the HSC70 gene expression patterns vary in response to different kinds of stress conditions, such as thermal, starvation, and heavy metal exposure (Chuang et al. 2007; Sun et al. 2016a, b). However, to date, it has not been reported whether the expression of the HSC70 gene in abalone is induced by various types of stress or not.
As the most important link in the process of gene expression, the transcriptional regulation of eukaryotes mainly regulates the initiation of gene transcription and the intensity of gene expression through the interaction of the promoter with the corresponding transcription factors (Bansal et al. 2014; Grünberg and Hahn 2013). At present, the transcriptional regulation of HSPs in mammals (Deb et al. 2013), plants (Gong et al. 2009), fish (Li et al. 2012), and many other species has been studied. However, the transcriptional regulation of HSC70 has not been well characterized, especially in animal cells. In aquatic animals, Chuang et al. (2007) studied the HSC70 promoter of tiger shrimp and confirmed the essential role of the core promoter region, the perfect heat shock elements (HSE) and the near CAAT box in the transcription. However, that article has not reported any transcription factors related to the promoter. Zhao et al. (2013) studied the Litopenaeus vannamei HSC70 promoter and found that deletion of the NF-kappaB binding site resulted in a significant increase in the expression of the reporter gene. However, to date, no studies have been done on the transcriptional activation or regulation of HdHSC70. In general, the presence of transcription factor recognition sequences in the 5′ untranslated region (5′-UTR) of the target messenger RNA (mRNA) is responsible for regulation of transcription of the target gene. Defining the cis- and/or reactive components of 5′-UTR is a necessary first step in understanding the mechanism of molecular transcriptional control (Li et al. 2012).
In our preliminary experiments, we observed that the expression level of HdHSC70 was upregulated by heat shock. Based on current knowledge and our observations, we hypothesized that (1) the expression of the HdHSC70 gene may be induced not only by high temperature but also by hypoxia and infection of V. parahaemolyticus, (2) single stress and two stresses may stimulate different expression of HdHSC70, and (3) the change of HdHSC70 expression levels is related to the transcriptional regulation. Testing these hypotheses will lead to a better understanding of the HdHSC70 gene expression pattern and its regulatory mechanisms in abalone.
Materials and methods
Animals and preparation of samples
Adult small abalones (body length 5.18 ± 0.50 cm, weight 15.7 ± 2.50 g) were purchased from the Peiyang abalone farm (Xiamen, China) in August 2015. These abalones were fed with sea tangle once a day and maintained in recycling systems at a temperature of 25 °C and dissolved oxygen (DO) of 6.2 mg/L. They were held for 8 days to be ready for the experiment.
At least six healthy small abalones were dissected for different tissues. Hemocytes (He) were collected by cutting off the foot quickly and were isolated by centrifugation at 2000×g at 4 °C for 10 min from hemolymph. Soon afterwards, hemocytes were immediately stored in liquid nitrogen until being used for RNA isolation and quantitative real-time PCR (qRT-PCR). Hepatopancreas (Hp) and gills (Gi), mucous gland (Mg), digestive tract (Di), mantle (Ma), and muscle (Mu) were immediately stored in liquid nitrogen until being used for RNA isolation and qRT-PCR.
Animals were randomly divided into four groups: (1) thermal stress group: according to the method of Huang et al. (2013a, b), the stress condition was set to temperature (Tm) = 31 °C. The temperature of the control group was set at 25 °C. The temperature of the experimental group was set at 31 °C which was achieved by increasing from 25 °C by 1 °C per hour. When the temperature reached 28 °C, it was set as the first phase and 31 °C as the second phase. Four, 24, 96, and 192 h at 31 °C were set as the 3,4,5,6 phase, respectively. (2) Hypoxia stress group: according to the method of Cai et al. (2014), the stress condition was set to dissolved oxygen (DO) = 2 mg/L. Samples were taken at 4, 24, 96, and 192 h, respectively. (3) Bacterial challenge group: the injection concentration was determined according to the method of Sun et al. (2016a, b). All individuals were injected with 50 mL live V. parahaemolyticus in 0.9% NaCl (1.1 × 108 cfu/mL) into the foot muscle, with the control injected with 50 mL of 0.9% NaCl. Samples were taken at 0, 4, 12, and 24 h. (4) Thermal and hypoxia combined stress group: according to the method of Zhang et al. (2014), the stress conditions were set to Tm = 30 °C and DO = 4 mg/L. At the beginning of the joint stress experiment, DO content of water was 4 mg/L, and 30 °C was achieved by increasing from 25 °C with 1 °C per hour. The temperature of 30 °C was maintained and gills were sampled at 0, 4, 24, 96, and 192 h. At least six abalone gills were sampled at different time phases in all four group experiments.
Determination of HdHSC70 expression after stresses
Total RNA was extracted using total RNA Kit II (Omega, USA) according to the manufacturer’ s protocol from different tissues of all samples. The complementary DNA (cDNA) was synthesized in a system including 1 μg total RNA and 2 μL 10 mM random primers by M-MLV reverse transcriptase (Promega, USA). The synthesized cDNA was stored at − 20 °C until use.
qRT-PCR was carried out in LightCycler 480 Roche real-time thermal cycler in accordance with the manual with a 20-μL reaction volume containing 9 μL of 1:100 diluted original cDNA, 1 μL of 10× SYBR Green Master Mix (Promega, USA), and 0.5 μL of each primer (10 mM). The comparative CT method (ΔCT = CT of the target gene minus CT of the β-actin gene and ΔΔCT = ΔCT of any sample minus calibrator sample) for the relative quantification of gene expression was used to calculate the relative expression level of all these genes. Six biological replicates were tested, and each sample was assayed in triplication. The t test was used to determine the difference in the mean values among the treatments. The difference was considered as significant when P < 0.05.
Cloning of the 5′-flanking regions of the HdHSC70 gene
The 5′-flanking regions of the HdHSC70 gene (GenBank accession number FJ812176.1) were isolated using the Tail-PCR and Universal Genome Walker Kit (TaKaRa, Japan). The primer sequences used in this study are listed in Table 1. PCR products were then purified and cloned into the pMD19-T simple vector (TaKaRa, Japan) and sent for sequencing at Sangon (Shanghai, China). The sequences were analyzed using the online transcription element search software Alibaba2 (http://www.gene-regulation.com/pub/programs/alibaba2/index.html).
Table 1.
Primers used in this study
| Primer name | Primer sequence (5′ → 3′) | Used for |
|---|---|---|
| hsc70-r1 | TCCATCGGCTGAGAAGACTG | Genome Walking and Tail-PCR |
| hsc70-r2 | CAGGAAGGTCAAGGCGGTTC | |
| hsc70-r3 | CGATAACGAGATTTGCTAAACTGG | |
| hsc70-r4 | ACATCATCGGCTACACACGG | |
| hsc70-r5 | GCAAGTCAAAAGTCATTCCG | |
| c-70-1 | CGGGGTACCATAGTGATAAAGCCGTTGTTGC | Hsc70 promoter activity |
| c-70-2 | CGGGGTACCGTCTGACCCATTGTTGATGCGA | |
| c-70-3 | CGGGGTACCTCACGAGATGTGTACGGGAGT | |
| c-70-4 | CGGGGTACCTTATTGCTGTGAGAGCGGCGTTTG | |
| c-70-5 | CGGGGTACCTGACTCCAAACAAGTGACTACCT | |
| c-70-6 | CGGGGTACCCACAACCTGAAGAGAAACTGAA | |
| c-70-7 | CGGGGTACCCCATCGGCTGAGAAGACTGT | |
| c-70-r | CCGCTCGAGTCTTCTCTGCCCTCCGCTTC | |
| c-70-r1 | CCGCTCGAGCAGTCTTCTCAGCCGATGGA | |
| c-rt-f | TTGATCTTGGCGGTGGTACA | Real-time quantitative PCR |
| c-rt-r | CTGGGTGCTGGAGGAAAGTG | |
| b-actin F | CCGTGACCTTACAGACTACCT | |
| b-actin R | TACCAGCGGATTCCATAC |
Generation of reporter plasmid constructs
To analyze the HdHSC70 basal promoter activities, multiple promoter fragments of the HdHSC70 gene were generated by PCR and cloned into the pGL3-Basic luciferase reporter vector. First, the universal reverse primers were used in combination with different forward specific primers to generate DNA fragment and cloned into pMD19-T simple vector (TaKaRa, Japan). Second, the promoter fragment constructs were digested with Kpn I and Xho I and subcloned into Kpn I/Xho I-cut pGL3-Basic reporter vector. Finally, all plasmid constructs were verified by sequencing and purified with an E.Z.N.A.™ Endo-free Plasmid Mini Kit (OMEGA, USA) for transfection.
After determining the transcription factor that may play an important role, the binding site of this transcription factor is mutated and the plasmid is constructed. The method is consistent with the previous method.
Transfection and luciferase assays
HEK 293T cells were routinely cultured in DMEM high glucose medium. Transfection experiments were performed in 48-well culture plates. Briefly, 24 h before transfection, recipient cells were seeded into wells at a density of 1–3 × 105 cells/well. The cells were transfected with 1 μg of reporter construct DNA and internal reference plasmid in 50 μL medium without FBS per well using 1 μL Lipofectin 2000 (Invitrogen) according to the manufacturer’s recommendations. At 24 h post transfection, EGFP (enhanced green fluorescent protein) expression was observed using an inverted fluorescence microscope.
After transfection, each cell sample was suspended in 60 μL of 1× Passive Lysis Buffer (PLB). After centrifugation, the supernatant of each sample was taken as 15 μL. The firefly and Renilla luciferase activities were measured by the Dual-Luciferase® Reporter Assay System (Promega, Shanghai, China) with a tube luminescence meter (Berthold Technologies, Germany) reader. The ratio of luciferase activity and the luciferase relative activity were calculated. All the data were obtained from three independent transfection experiments performed in triplicate.
Results
Expression of the HdHSC70 gene
The expression of HdHSC70 was assessed by qRT-PCR in different tissues from healthy small abalone. HdHSC70 was expressed in all examined tissues with high levels in Gi, He, and Hp (P < 0.05) (Fig. 1). Because the gill is a very important site of pathogen entry and the highest expression level of HdHSC70 was detected in Gi, the Gi was set as the experimental tissue in a separate stress group.
Fig. 1.
Expression level of HdHSC70 in different tissues of H. diversicolor. He hemocytes, Ma mantle, Gi gills, Mu muscle, Hp hepatopancreas, Mg mucous gland, Di digestive tract (The different letters on the error bars represent significant differences, P < 0.05)
For the thermal stress group, the expression level of HdHSC70 in the Gi was significantly higher (~ 15-fold) than the control group at the temperature of 31 °C (0 h) (P < 0.05), and then, the expression decreased rapidly (Fig. 2).
Fig. 2.
Expression level of the HdHSC70 gene after thermal stress in Gi. 1 (28 °C), 2 (31 °C, 0 h), 3 (31 °C, 4 h), 4 (31 °C, 24 h), 5 (31 °C, 96 h), 6 (31 °C, 192 h). The significant difference between the challenged and the control group is indicated by a (*) at P < 0.05
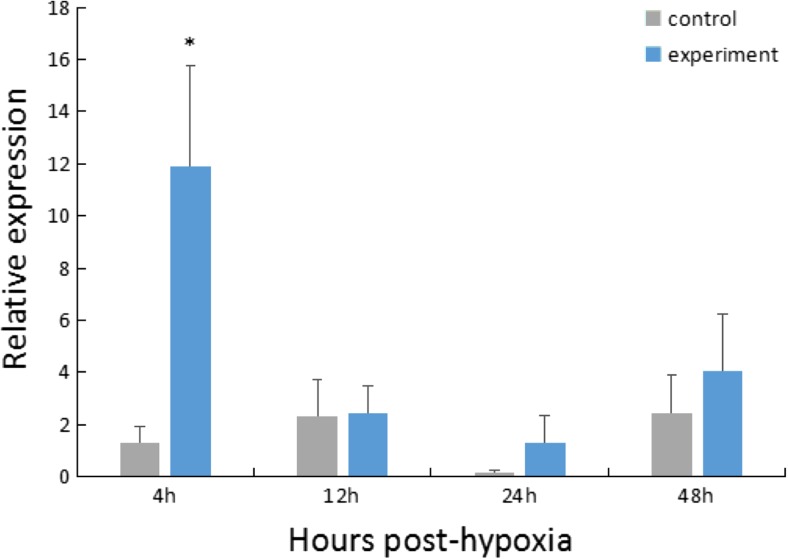
For the hypoxia stress group, the expression of HdHSC70 in the Gi was significantly higher (~ 10-fold) than the control group at 4 h after stress (P < 0.05), and then, the expression level of HdHSC70 decreased rapidly (Fig. 3).
Fig. 3.
Expression level of the HdHSC70 gene after hypoxia stress in Gi. The significant difference between the challenged and the control group is indicated by a (*) at P < 0.05
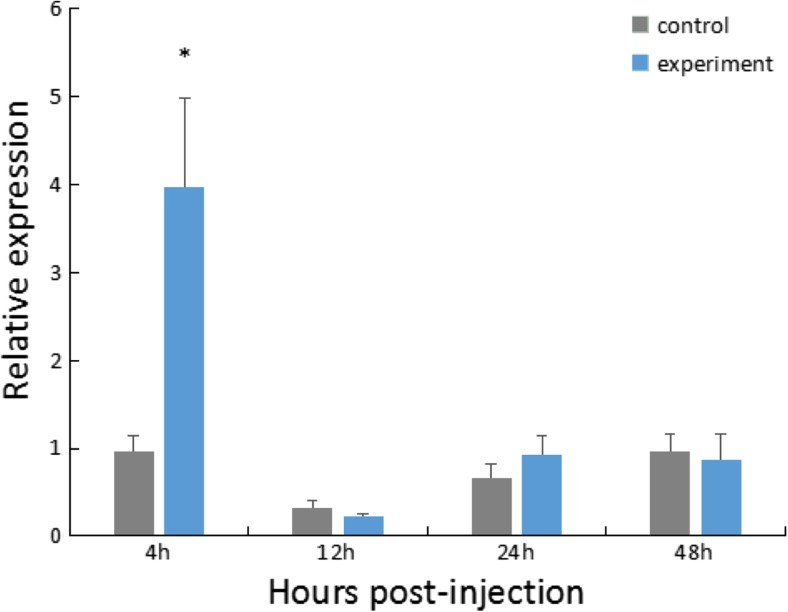
For the V. parahaemolyticus infection group, the expression of HdHSC70 in the Gi was significantly higher (~ 4-fold) than the control group at 4 h after injection (P < 0.05), and then, the expression level of HdHSC70 decreased rapidly (Fig. 4).
Fig. 4.
Expression level of HdHSC70 after injection of V. parahaemolyticus in Gi. The significant difference between the challenged and the control group is indicated by a (*) at P < 0.05
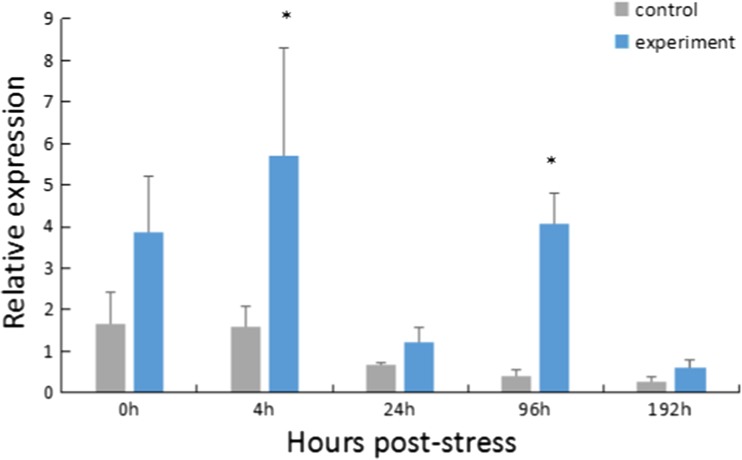
For the thermal and hypoxia combined stress group, the expression of HdHSC70 in the Gi was significantly higher than the control group at 4 and 96 h after stress (P < 0.05) (Fig. 5).
Fig. 5.
Expression level of HdHSC70 after both thermal and hypoxia stress in Gi. The significant difference between the challenged and the control group is indicated by a (*) at P < 0.05
5′ upstream sequences of the HdHSC70 gene
The 5′ flanking sequence (2013 bp) of the HdHSC70 gene was obtained (Fig. 6) by Tail-PCR and Genome Walker methods. The core promoter, transcription initiation site, CpG island, and potential transcription factor binding sites were predicted using the software NNPP, TFSEARCH, and MethPrimer. The position of the transcription initiation site (TSS) was defined as one, in the region of − 40 ± 10 bp containing the putative core promoter region. The promoter of the HdHSC70 gene has a TATA box between − 30 and − 32 bp upstream of the transcription initiation site (A) and has a CpG island with a length of 108 bp and has many transcription factor binding sites, such as ATF, NF-1, TBP, Sp1, Oct-1, C/EBPalp, and many HSE.
Fig. 6.
Nucleotide sequence of the 5′ flanking region of the HdHSC70 gene. Intron regions are shown in italic. Positions of potential binding sited for transcription factors are underlined. The overlapping binding sites are shaded. The transcriptional site and start codon are blacked
Activity of the HdHSC70 promoter in vitro
To identify the promoter activity of the HdHSC70 gene, the complete 2013 bp 5′-upstream region was inserted into the pEGFP-1 vector (pEGFP-Hsc70) and used to drive expression of the EGFP gene in HEK293T cells. Under a fluorescence microscope, EGFP fluorescent signals could be observed (Fig. 7).
Fig. 7.
EGFP expression of the HdHSC70 promoter in HEK293T cells at 24 h post transfection with pEGFP-Hsc70 which used the HdHSC70 full-length promoter (A and a), pEGFP-N1 as a positive control (B and b), and the promoter-less pEGFP-1 as a negative control (C, c). Fluorescent fields are shown in (A, B, and C), and bright fields are observed in (a, b, and c) separately
To identify the core promoter region of the HdHSC70 gene, two constructed reporter plasmids were prepared and transfected into HEK293T cells; the one containing the complete 2013 bp 5′-upstream region was named pGL-c70-1r, and the one removing the core promoter region was named pGL-c70-1r1. The activity of pGL-c70-1r was significantly higher than pGL-c70-1r1 and negative control (pGL3-Basic, plasmid without inserts) (P < 0.05). The pEGFP-N1 promoter was used as a positive control; a high fluorescence activity as expected was detected. No green fluorescence protein expression was detected in pEGFP-1 which was the negative control (Fig. 8).
Fig. 8.
Analysis of the relative activity of the HdHSC70 gene containing and removing the predicted core promoter region of the plasmid. The significant difference is indicated by an asterisk at P < 0.05 as compared with the control (basic)
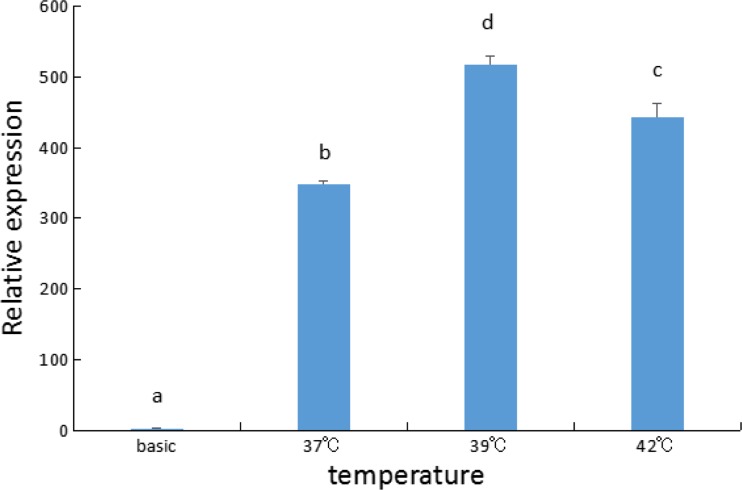
To determine if expression of the luciferase reporter driven by the HdHSC70 promoter was also induced by heat shock, HEK293T cells were exposed under high temperature stress. Thirty-seven, 39, and 42 °C were selected as the induction temperature; the treatment time was 40 min. We found that under the induction of 39 and 42 °C, luciferase activity was significantly increased (P < 0.05), and the activity in 39 °C was the highest, and significantly higher than in 37 and in 42 °C (P < 0.05) (Fig. 9).
Fig. 9.
Changes of HdHSC70 promoter activity in HEK293T cells (The different letters on the error bars represent significant differences, P < 0.05)
In order to identify important transcription factor binding sites in the HdHSC70 promoter region, we amplified a continuous truncated promoter fragment of the HdHSC70 gene by PCR. The truncated 5′ flank regions of HdHSC70 were cloned into luciferase reporter vectors (named as pGL-c70-1r, pGL-c70-2r, pGL-c70-3r, pGL-c70-4r, pGL-c70-5r, and pGL-c70-6r). The constructs were used to transfect into HEK293T cells. All truncated promoters had detectable activities compared with the control (pGL3-Basic, plasmid without inserts). There was a significant difference between pGL-c70-5r and pGL-c70-6r (P < 0.05) (Fig. 10).
Fig. 10.
Promoter activity analysis of the serial deletion constructs from the 5′-flanking region of the HdHSC70 gene. The relative luciferase activity is presented as the average firefly luciferase activity normalized to the Renilla luciferase activity. Six plasmids showed positive but variable promoter activities (P < 0.05). The significant difference between pGL-c70-6r and pGL-c70-5r is indicated by a (**) at P < 0.01
The difference between pGL-c70-5r and pGL-c70-6r is the part of − 252 to − 103 bp. There are many predicted transcription factor binding sites in this region, such as interferon consensus sequence binding protein (ICSBP), Oct-1, Cos, and Sp1. Since there is only one ICSPB binding site in the promoter fragment of HdHSC70, we hypothesized that ICSBP was an important transcriptional binding factor for HdHSC70 and decided to mutate it. The binding site of the transcription factor ICSBP is AAGAGAAACT, and the recombinant plasmid was named as pGL-wt. When we mutated the AG into TC, the sequence becomes AAGTCAAACT, and the mutant recombinant plasmid was named as pGL-mut. The results showed that after the ICSBP-binding site was mutated, the luciferase activity was significantly reduced (P < 0.05) (Fig. 11).
Fig. 11.
Activity analysis of the site-directed mutation plasmid pGL-mut-ic. Plasmid pGL-mut-ic was constructed by mutating the putative ICSBP-binding site from pGL-c70-5r. The pGL3-Basic plasmid was served as a negative control (The different letters on the error bars represent significant differences, P < 0.05)
Discussion
HdHSC70 mRNA expression in tissues
Studies of the heat shock protein 70 family have confirmed its importance in protein metabolism under normal physiological conditions and in cellular protection under stress conditions (Wang et al. 2013a, b), indicating that the study of the distribution of HdHSC70 mRNA in different tissues would help us understand the mechanism of its response to stress. qRT-PCR results showed that the HdHSC70 gene was expressed in all examined tissues with highest expression in Gi, He, Hp, especially in Gi (Fig. 1). Gi is the main structure of the suspension filter, and as a respiratory organ, it is directly stimulated by a variety of environmental stresses. It has been reported in mollusks that He and Hp play an important role in the innate immune system (Gueguen et al. 2003; Song et al. 2011). Therefore, compared with other tissues, Gi, He and Hp may be more sensitive to environmental stresses. Many of the immune-related genes such as AbNF-kB and SaAkirin2 reported in the H. diversicolor also have higher expression levels in Gi, He, and Hp (Zhang et al. 2014).
HdHSC70 mRNA expression after stresses
The general pattern of the HSC70 gene expression is actively expressed in normal cells, but is not influenced by stress. Zhang et al. (2010) and Yoshimi et al. (2009) have reported that expression of HSC70 in corn earworm and Chironomidae did not increase under cold, heat stress, or exposure to heavy metals. However, some researchers have reported that it can also be induced. Sun et al. (2016) and Chuang et al. (2007) found that in Apolygus lucorum and Penaeus monodon, the HSC70 expression was significantly higher under high temperature stress. Interestingly, Shim et al. found the expression of HSC70 in Tetranychus urticae was not increased under cold or heat stress, but significantly increased in response to starvation (Shim et al. 2006). These different results indicate that the expression of HSC70 is plastic, which may depend on the type, intensity, and duration of environmental stresses. The HdHSC70 also appears to possess a stress-associated role. Our experimental results showed that whether after hypoxia stress, V. parahaemolyticus injection, or combined thermal and hypoxia exposure, the HdHSC70 was expressed at a significantly higher level compared to the control group at some time phases. Based on the function of the HdHSC70 gene, the transcriptional upregulation related to stresses suggests that HdHSC70 is an important factor in cell protection and survival in H. diversicolor.
How organisms cope with rapid changes in their environment is a major question in biology. We found that elevated expression of HdHSC70 was detected at the early stage of stresses exposure, so we assume that the HdHSC70 was involved in the early stress response. Li et al. obtained a similar result that HSC70 mRNA expression level in most tissues was rapidly upregulated after bacterial infection in Schizothorax prenanti (Li et al. 2015). During stress, large amounts of HSC70 mRNA might be synthesized instantly to protect against damage, resulting in a big increase in HSC70 mRNA level compared to the basal expression. Additionally, HSC70 is commonly accepted as a biomarker for different classes of environmental stresses assessments in various animal species (Mukhopadhyay et al. 2010). Based on this fast and sensitive response, HdHSC70 is appropriate to be a biomarker for environmental stresses in H. diversicolor.
In the high temperature and hypoxia combined stress group, the expression level of HdHSC70 gene was significantly increased at 4 h, then decreased rapidly at 24 h, and then increased significantly again at 96 h. The expression of HSP in the organism after stress enables the cells, tissues, and even the whole organism to obtain a higher resistance to stress. However, as the organism is unable to continue to cope with the stress, it will once again start the expression of heat shock-related genes (And and Robertson 2003; Wang et al. 2006). We speculate that the abalone cannot adapt to double stresses for a long time, since HdHSC70 has a significant increase at 96 h.
The HdHSC70 5′ upstream sequence
The heat shock element (HSE) is necessary for transcriptional activation of eukaryotic HSP genes, and it has some characteristics of enhancers (Karn et al. 2011). The sequence of HSE is highly conserved, which is based on the core sequence containing five nucleotides (-nGAAn-), where n represents nucleotides that are conservatively low but very important in evolution. Usually, HSE contains at least two (-nGAAn-), and the monomer is attached in the form of -nGAAn nGAAn- or -nTTCnnGAAn- (Zhao et al. 2011). The 5′ regulatory region of the P. monodon HSC70 gene contains a long HSE with three -nGAAn-, and the HSC70 mRNA expression was induced after the heat shock by about 8-fold (Chuang et al. 2007). The 5′ regulatory region of the Crassostrea hongkongensis HSC70 gene contains six HSEs, and the HSC70 mRNA expression can be induced about 20-fold after heat shock (Zhang 2010). There is no canonical correlative rule between the number of HSE and the degree of induced expression by a heat shock. However, increasing the number of HSE more or less may enhance the expression of the heat shock protein (Amin et al. 1987; Brade et al. 2000). The 5′ regulatory region of the HdHSC70 gene contains five HSE elements; this may be related to the HdHSC70 mRNA expression that was induced after the heat shock by about 15-fold. On the other hand, the results from Amin et al. (1987) showed that the intensity of HSP70 initiation depends on the spatial arrangement of HSE and TATA boxes, the number of HSEs, and the location of HSE in Drosophila melanogaster. Therefore, although the number of HSEs can influence the activity of the promoter, it is not the sole factor that determines the activity of the promoter. More experiments are needed to explore the relationship between HSE and HSC70 gene expression.
The TATA box is one of the most important binding sites in the eukaryotic polymerase II promoter and is one of the most common motifs in the promoter (Wang et al. 2004). In transcription initiation, transcription factors TF2 and TATA frames form stable complexes and then bind to DNA in a certain order by other transcription factors to form a transcription initiation complex and then start transcription (Zhang et al. 2010). The TATA box is an important component in the core promoter region. Branchiostoma belcheri HSP70a (Li et al. 2012) and the human HSP70 gene (Hunt and Morimoto 1985) core promoter region contained a TATA box. We found that the HdHSC70 core promoter region also contained a TATA box. When we deleted the core promoter region, the promoter activity was significantly reduced, inextricably linked to the absence of the TATA box.
In vitro expression assay of the HdHSC70 promoter
The HEK293T cell line has been widely used in vertebrate promoter functional analysis. For example, Kuang et al. (2015) and Wang et al. (2013) used the cell lines to study the human miR-1908 promoter and goldfish two kiss promoters. In invertebrates, Huang et al. used 293 cell lines to study the Pinctada fucata IRF-2 promoter (Huang et al. 2013). Because there is no stable H. diversicolor cell line available now, the HEK293T cell line was used for the promoter assay in this study.
In general, in order to further characterize the function of the gene promoter, the detection of the promoter activity and the determination of the transcription initiation site are carried out. The activity of the complete 2013 bp promoter in this study was verified using the fluorescent expression on the transfected cells with the promoter-EGFP vector (Fig. 7). The luciferase activity with the promoter-luciferase vector was used to identify the core promoter region of the HdHSC70 gene (Fig. 8). In previous studies, many researchers used the same method to study gene promoters, for example, the research of the P. monodon and L. vannamei HSC70 promoter (Chuang et al. 2007; Zhao et al. 2013).
The HdHSC70 promoter was regulated by high temperature stress
The heat shock protein gene promoter is an inducible promoter. Under high temperature or other stresses, the activity of the promoter will increase and can significantly increase the transcription level of the gene (Pei et al. 2007; Yi et al. 2006). Chuang et al. used Sf21 cells to study the HSC70 promoter of tiger shrimp (P. monodon) and found that the activity of luciferase increased significantly after virus infection (Chuang et al. 2007). Zhuang et al. (2011) used the BmN cell line to study the Bombyx mori HSP70 promoter and found that the activity of luciferase was significantly increased after heat treatment. Our result was consistent with their reports: a significant increase in luciferase activity of HdHSC70 after treatment of HEK 293T cells at 39 and 42 °C (Fig. 9). Our findings indicated that the activity of HdHSC70 promoter can be increased by high temperature stress, which is consistent with the increase of HdHSC70 mRNA expression under stress.
However, our result showed that the HdHSC70 promoter activity at 42 °C was lower than at 39 °C (Fig. 9). In the study of human HSP70B promoter, Li et al. (2014) explored the thermal induction conditions in human breast cancer MCF-7 cells and found that cell viability decreased significantly when the cells were induced at 42 °C for 40 min. This may explain why the temperature is higher but the activity is reduced.
Serial deletions and mutation on the activity of the HdHSC70 promoter
During gene transcriptional regulation, the promoter controls downstream gene expression under the synergistic effect of transcription factors, which contain positive and negative regulation. Shao et al. studied the proximal promoter activities of the ajarginase gene in Apostichopus japonicus and found that the transcription factor NF-kB/Rel and STAT5 could significantly inhibit the ajarginase promoter activity (Shao et al. 2016). Zhao et al. have studied the HSC70 gene of L. vannamei and found that NF-kappaB could significantly inhibit the expression of HSC70 (Zhao et al. 2013). By studying these transcription factors that play a key role in regulation, we can provide a theoretical basis for further understanding of the promoter regulatory mechanism. In our study, the pGL-c70-6r plasmid decreased the promoter activity to a significantly low level (P < 0.05) (Fig. 10). It indicated that the deleted site (− 252 to − 103 bp) has an important role in the basic transcription of HdHSC70 promoter and this location may contain an important transcription factor. After the ICSBP-binding site was mutated, we found the ICSBP-binding site in − 252 to − 103 bp has a certain enhancement effect on the activity of the HdHSC70 promoter (Fig. 11).
Interferon consensus sequence binding protein (ICSBP) is a member of the interferon regulatory factor (IRF) family of proteins that is expressed exclusively in cells of the immune system and plays an important role in cellular immunity (Wang et al. 2000). Some reports have provided evidence that ICSBP plays a critical role in modulating the immune response by influencing the differentiation and maturation of immune cells and by affecting the cytokine expression (Scheller et al. 1999; Tamura and Ozato 2002). HSC70 can participate in immune function and help the body to stimulate the immune response to the environment. Therefore, we speculate that ICSBP is also involved in the body’s immune response by enhancing the expression of HdHSC70. Interleukin-18 (IL-18) is an important cytokine in the regulation of immune responses. Studies have shown that there is an ICSBP-binding site located on the murine IL-18 promoter, and it is involved in the upregulation of the IL-18 gene transcription in murine macrophages (Kim et al. 2000). The result is similar to the effect of ICSBP on HdHSC70. However, the specific regulatory mechanism of ICSBP on HdHSC70 remains to be studied in depth.
Funding information
The work was supported by the Natural Science Foundation of China (No. 41176152, No. 41006105).
Contributor Information
Yilei Wang, Phone: 86-592-618 6780, Email: ylwang@jmu.edu.cn.
Ziping Zhang, Phone: 86-591-8375 8852, Email: zhangziping@hotmail.com.
References
- Amin J, Mestril R, Schiller P, Dreano M, Voellmy R. Organization of the Drosophila melanogaster hsp70 heat shock regulation unit. Mol Cell Biol. 1987;7(3):1055–1062. doi: 10.1128/MCB.7.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar MH, Hegedus D, Soroka J, Coutu C, Bekkaoui D, Dosdall L. Survival and Hsp70 gene expression in Plutella xylostella and its larval Parasitoid diadegma insulare varied between slowly ramping and abrupt extreme temperature regimes. PLoS One. 2013;8:e73901. doi: 10.1371/journal.pone.0073901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal M, Kumar A, Yella VR. Role of DNA sequence based structural features of promoters in transcription initiation and gene expression. Curr Opin Struct Biol. 2014;25:77–85. doi: 10.1016/j.sbi.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Boorstein WR, Ziegelhoffer T, Craig EA. Molecular evolution of the HSP70 multigene family. J Mol Evol. 1994;38:1–17. doi: 10.1007/BF00175490. [DOI] [PubMed] [Google Scholar]
- Brade AM, Ngo D, Szmitko P, Li PX, Liu FF, Klamut HJ. Heat-directed gene targeting of adenoviral vectors to tumor cells. Cancer Gene Ther. 2000;7(12):1566–1574. doi: 10.1038/sj.cgt.7700267. [DOI] [PubMed] [Google Scholar]
- Cai XH, Huang YT, Zhang X, Wang SH, Zhou ZH, et al. Cloning, characterization, hypoxia and heat shock response of hypoxia inducible factor-1 (HIF-1) from the small abalone Haliotis diversicolor. Gene. 2014;534:256–264. doi: 10.1016/j.gene.2013.10.048. [DOI] [PubMed] [Google Scholar]
- Cheng W, Hsiao IS, Hsu CH, Chen JC. Change in water temperature on the immune response of Taiwan abalone Haliotis diversicolor supertexta and its susceptibility to Vibrio parahaemolyticus. Dis Aquat Org. 2004;17:235–243. doi: 10.1016/j.fsi.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Cheng W, Lei J, Fox CW, Johnston JS, Zhu-Salzman K. Comparison of life history and genetic properties of cowpea bruchid strains and their response to hypoxia. J Insect Physiol. 2015;75:5–11. doi: 10.1016/j.jinsphys.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Choi BG, Hepat R, Kim Y. RNA interference of a heat shock protein, Hsp70, loses its protection role in indirect chilling injury to the beet armyworm, Spodoptera exigua. Comp Biochem Physiol A Mol Integr Physiol. 2014;168:90. doi: 10.1016/j.cbpa.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Chuang KH, Ho SH, Song YL. Cloning and expression analysis of heat shock cognate 70 gene promoter in tiger shrimp (Penaeus monodon) Gene. 2007;405:10–18. doi: 10.1016/j.gene.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Daugaard M, Rohde M, Jäättelä M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Deb R, Sajjanar B, Singh U, Kumar S, Brahmane MP, Singh R, et al. Promoter variants at AP2 box region of Hsp70.1 affect thermal stress response and milk production traits in Frieswal cross bred cattle. Gene. 2013;532:230–235. doi: 10.1016/j.gene.2013.09.037. [DOI] [PubMed] [Google Scholar]
- Fujino K, Arai K, Kudo M, Takahashi K, Uematsu T, Okamura S. Estimating rate of gene-centromere recombination at some isozyme loci in Haliotis diversicolor and H. discus hannai. Nippon Suisan Gakkaishi. 1992;58(3):445–459. doi: 10.2331/suisan.58.445. [DOI] [Google Scholar]
- Gong WN, Wan FH, Xie BY, Guo JY, Zhou Y. Cloning and sequence analysis of promoters in hsp90 and hsp17.66 genes from Ageratina adenophora. J Trop Subtrop Bot. 2009;5:006. [Google Scholar]
- Grünberg S, Hahn S. Structural insights into transcription initiation by RNA polymerase II. Trends Biochem Sci. 2013;38:603–611. doi: 10.1016/j.tibs.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Huang LX, Shen Y, Huang LH, Feng QL. Hsp70 and small Hsps are the major heat shock protein members involved in midgut metamorphosis in the common cutworm, Spodoptera litura. Insect Mol Biol. 2012;21:535–543. doi: 10.1111/j.1365-2583.2012.01158.x. [DOI] [PubMed] [Google Scholar]
- Gueguen Y, Cadoret JP, Flament D, Barreauroumiguière C, Girardot AL, Garnier J, et al. Immune gene discovery by expressed sequence tags generated from hemocytes of the bacteria-challenged oyster, Crassostrea gigas. Gene. 2003;303:139–145. doi: 10.1016/S0378-1119(02)01149-6. [DOI] [PubMed] [Google Scholar]
- Huang Y, Cai X, Zou Z, Wang S, Wang G, Wang Y, Zhang Z. Molecular cloning, characterization and expression analysis of three heat shock responsive genes from Haliotis diversicolor. Fish Shellfish Immunol. 2013;36:590–599. doi: 10.1016/j.fsi.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Huang XD, Liu WG, Wang Q, Zhao M, Wu SZ, Guan YY, et al. Molecular characterization of interferon regulatory factor 2 (IRF-2) homolog in pearl oyster Pinctada fucata. Fish Shellfish Immunol. 2013;34(5):1279–1286. doi: 10.1016/j.fsi.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Hunt C, Morimoto RI. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985;82:6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Zhai H, Wang L, Luo L, Sappington TW, Zhang L. Cloning of the heat shock protein 90 and 70 genes from the beet armyworm, Spodoptera exigua, and expression characteristics in relation to thermal stress and development. Cell Stress Chaperones. 2012;17:67–80. doi: 10.1007/s12192-011-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin S, Brocchieri L. Heat shock protein 70 family: multiple sequence comparisons, function, and evolution. J Mol Evol. 1998;47:565–577. doi: 10.1007/PL00006413. [DOI] [PubMed] [Google Scholar]
- Karn H, Ovsenek N, Heikkila JJ. Examination of the DNA sequence-specific binding properties of heat shock transcription factor in Xenopus laevis embryos. Biochem Cell Biol. 2011;70:1006–1013. doi: 10.1139/o92-144. [DOI] [PubMed] [Google Scholar]
- Ke CH, You WW. Advances in genetics and breeding of Haliotis diversicolor. J Xiamen Univ. 2011;50:425–430. [Google Scholar]
- Ke CH, Tian Y, Zhou SQ, Li FX. Preliminary studies on hybridization of three species of abalone. Mar Sci. 2000;24:39–40. [Google Scholar]
- Kim YM, Im JY, Han SH, Kang HS, Choi I. IFN-gamma up-regulates IL-18 gene expression via IFN consensus sequence-binding protein and activator protein-1 elements in macrophages. J Immunol. 2000;165:3198–3205. doi: 10.4049/jimmunol.165.6.3198. [DOI] [PubMed] [Google Scholar]
- Kuang Q, Li J, You L, Shi C, Ji C, Guo X, et al. Identification and characterization of NF-kappaB binding sites in human miR-1908 promoter. Biomed Pharmacother. 2015;74:158–163. doi: 10.1016/j.biopha.2015.08.018. [DOI] [PubMed] [Google Scholar]
- Li D, Li G, Wang K, Liu X, Li W, Chen X, et al. Isolation and functional analysis of the promoter of the amphioxus Hsp70a gene. Gene. 2012;510:39. doi: 10.1016/j.gene.2012.08.031. [DOI] [PubMed] [Google Scholar]
- Li Y, Jing L, Li H, Wang X, Yan Z, Fan Y, et al. Construction of an HSP 70B′ promoter-driven heat-inducible vectors pHSPshTERT and its anti-proliferative effect in breast cancer MCF-7 cells. Chin J Cancer Biother. 2014;21:130–135. [Google Scholar]
- Li J, Zhang H, Zhang X, Yang S, Yan T, Song Z. Molecular cloning and expression of two heat-shock protein genes (HSC70/HSP70) from Prenant’s schizothoracin (Schizothorax prenanti) Fish Physiol Biochem. 2015;41:573–585. doi: 10.1007/s10695-015-0030-4. [DOI] [PubMed] [Google Scholar]
- Liu PC, Chen YC, Huang CY, Lee KK. Virulence of Vibrio parahaemolyticus isolated from cultured small abalone, Haliotis diversicolor supertexta, with withering syndrome. Lett Appl Microbiol. 2000;31:433. doi: 10.1046/j.1365-2672.2000.00843.x. [DOI] [PubMed] [Google Scholar]
- Liu XD, Yan YH, Wang ZY, Cai MY, Ke CH. A preliminary study on tolerance to high temperature and low salinity of Haliotis diversicolor reeve. J Jimei Univ. 2008;13:301–303. [Google Scholar]
- Mahroof R, Zhu KY, Subramanyam B. Changes in expression of heat shock proteins in Tribolium castaneum (Coleoptera: Tenebrionidae) in relation to developmental stages, exposure time, and temperature. Ann Entomol Soc Am. 2005;98:100–1077. doi: 10.1603/0013-8746(2005)098[0100:CIEOHS]2.0.CO;2. [DOI] [Google Scholar]
- Mukhopadhyay I, Nazir A, Saxena DK, Chowdhuri DK. Heat shock response: hsp70 in environmental monitoring. J Biochem Mol Toxicol. 2010;17:249–254. doi: 10.1002/jbt.10086. [DOI] [PubMed] [Google Scholar]
- Pei H, Hu H, Zhang X, Su C, Song X. Cloning and functional analysis of the heat-inducible promoter at HSP70b. Chin Agric Sci Bull. 2007;23:82–86. [Google Scholar]
- Scheller M, Foerster J, Heyworth CM, Waring JF, Löhler J, Gilmore GL, et al. Altered development and cytokine responses of myeloid progenitors in the absence of transcription factor, interferon consensus sequence binding protein. Blood. 1999;94:3764–7371. [PubMed] [Google Scholar]
- Shao Y, Li C, Zhang W, Xu W, Duan X, Li Y, et al. Cloning and comparative analysis the proximal promoter activities of arginase and agmatinase genes in Apostichopus japonicus. Dev Comp Immunol. 2016;65:299–308. doi: 10.1016/j.dci.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Shen Q, Zhao L, Xie G, Wei P, Yang M, Wang S, et al. Cloning three Harmonia axyridis (Coleoptera: Coccinellidae) heat shock protein 70 family genes: regulatory function related to heat and starvation stress. J Entomol Sci. 2016;50:168–185. doi: 10.18474/JES14-30.1. [DOI] [Google Scholar]
- Shi J, Fu M, Zhao C, Zhou F, Yang Q, Qiu L. Characterization and function analysis of hsp60 and hsp10 under different acute stresses in black tiger shrimp, penaeus monodon. Cell Stress Chaperones. 2016;21(2):1–18. doi: 10.1007/s12192-015-0660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JK, Jung DO, Park JW, Kim DW, Ha DM, Lee KY. Molecular cloning of the heat-shock cognate 70 (Hsc70) gene from the two-spotted spider mite, Tetranychus urticae, and its expression in response to heat shock and starvation. Comp Biochem Physiol B Biochem Mol Biol. 2006;145:288–295. doi: 10.1016/j.cbpb.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Shu Y, Du Y, Wang J. Molecular characterization and expression patterns of Spodoptera litura heat shock protein 70/90, and their response to zinc stress. Comp Biochem Physiol A Mol Integr Physiol. 2011;158:102–110. doi: 10.1016/j.cbpa.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Song X, Zhang H, Wang L, Zhao J, Mu C, Song L, et al. A galectin with quadruple-domain from bay scallop Argopecten irradians is involved in innate immune response. Dev Comp Immunol. 2011;35:592–602. doi: 10.1016/j.dci.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhao J, Sheng Y, Xiao YF, Zhang YJ, Bai LX, et al. Identification of heat shock cognate protein 70 gene (Alhsc70) of Apolygus lucorum and its expression in response to different temperature and pesticide stresses. Insect Sci. 2016;23:37–49. doi: 10.1111/1744-7917.12193. [DOI] [PubMed] [Google Scholar]
- Sun Y, Xin Z, Wang G, Shi L, Zeng X, Wang Y, Zhang Z. PI3K-AKT signaling pathway is involved in hypoxia/thermal-induced immunosuppression of small abalone Haliotis diversicolor. Fish Shellfish Immunol. 2016;59:492–508. doi: 10.1016/j.fsi.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Tamura T, Ozato K. ICSBP/IRF-8: its regulatory roles in the development of myeloid cells. J Interf Cytokine Res Off J Int Soc Interf Cytokine Res. 2002;22:145–152. doi: 10.1089/107999002753452755. [DOI] [PubMed] [Google Scholar]
- Thanaphum S, Haymer D. A member of the hsp70 gene familyfrom the Mediterranean fruit fly, Ceratitis capitata. Insect Mol Biol. 1998;7:63–72. doi: 10.1046/j.1365-2583.1998.71051.x. [DOI] [PubMed] [Google Scholar]
- Wang IM, Contursi C, Masumi A, Ma X, Trinchieri G, Ozato K. An IFN-gamma-inducible transcription factor, IFN consensus sequence binding protein (ICSBP), stimulates IL-12 p40 expression in macrophages. J Immunol (Baltimore, Md: 1950) 2000;165:271–279. doi: 10.4049/jimmunol.165.1.271. [DOI] [PubMed] [Google Scholar]
- Wang H, Li X, Bajić VB. Neural-statistical model of tata-box motifs in eukaryotes. In: Bajiśc VB, editor. Bioinformatics and biocomputing. New York: PhysicaMVerlag; 2004. pp. 123–156. [Google Scholar]
- Wang X, Xu C, Wang X, Wang D, Wang Q, Zhang B. Heat shock response and mammal adaptation to high elevation (hypoxia) Sci China Ser C Life Sci. 2006;49:500–512. doi: 10.1007/s11427-006-2027-9. [DOI] [PubMed] [Google Scholar]
- Wang H, Li K, Zhu JY, Fang Q, Ye GY, Wang H, et al (2012) Cloning and expression pattern of heat shock protein genes from the endoparasitoid wasp, pteromalus puparum in response to environmental stresses. Arch Insect Biochem Physiol 79(4-5):247–263 [DOI] [PubMed]
- Wang Q, Sham KW, Ogawa S, Li S, Parhar IS, Cheng CH, et al. Regulation of the two kiss promoters in goldfish (Carassius auratus) by estrogen via different ERα pathways. Mol Cell Endocrinol. 2013;375:130–139. doi: 10.1016/j.mce.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Wang TT, Wang N, Liao XL, Meng L, Liu Y, Chen SL. Cloning, molecular characterization and expression analysis of heat shock cognate 70 (Hsc70) cDNA from turbot (Scophthalmus maximus) Fish Physiol Biochem. 2013;39:1377–1386. doi: 10.1007/s10695-013-9792-8. [DOI] [PubMed] [Google Scholar]
- Yi SY, Sun AQ, Sun Y, Yang JY, Zhao CM, Liu J. Differential regulation of Lehsp23.8 in tomato plants: analysis of a multiple stress-inducible promoter. Plant Sci. 2006;171:398. doi: 10.1016/j.plantsci.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Yoshimi T, Odagiri K, Hiroshige Y, Yokobori S, Takahashi Y, Sugaya Y, Miura T. Induction profile of HSP70-cognate genes by environmental pollutants in Chironomidae. Environ Toxicol Pharmacol. 2009;28:294–301. doi: 10.1016/j.etap.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Zhang ZH (2010) Clone of heat shock protein 70 (HSP70) genes in Crassostrea hongkongensis and response of the HSC70 gene to pollutants. Dissertation. Jinan University (In Chinese)
- Zhang XH, Qi YX, Wang YQ, Wang ZB. Prediction and analysis of CpG island and TATA-box in fruit fly promotor region. China J Bioinforma. 2010;8:82–90. [Google Scholar]
- Zhang X, Huang Y, Cai X, Zou Z, Wang G, Wang S, et al. Identification and expression analysis of immune-related genes linked to Rel/NF-κB signaling pathway under stresses and bacterial challenge from the small abalone Haliotis diversicolor. Fish Shellfish Immunol. 2014;41:200–208. doi: 10.1016/j.fsi.2014.08.022. [DOI] [PubMed] [Google Scholar]
- Zhao KK, Cui YG, Liu JY. Research progress of heat shock protein 10. Med Recapitulate. 2011;1:1–3. [Google Scholar]
- Zhao C, Zhang X, Li F, Huan P, Xiang J. Functional analysis of the promoter of the heat shock cognate 70 gene of the Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2013;34:397–401. doi: 10.1016/j.fsi.2012.09.022. [DOI] [PubMed] [Google Scholar]
- Zhuang LF, Wei H, Lin JR, Zhong BX. Identification of Bombyx mori hsp70 promoter and its function. Chin J Cell Biol. 2011;33:503–509. [Google Scholar]