Abstract
Although there have been advances in our understanding of carcinogenesis and development of new treatments, cancer remains a common cause of death. Many regulatory pathways are incompletely understood in cancer development and progression, with a prime example being those related to the endoplasmic reticulum (ER). The pathological sequelae that arise from disruption of ER homeostasis are not well defined. The ER is an organelle that is responsible for secretory protein biosynthesis and the quality control of protein folding. The ER triggers an unfolded protein response (UPR) when misfolded proteins accumulate, and while the UPR acts to restore protein folding and ER homeostasis, this response can work as a switch to determine the death or survival of cells. The treatment of cancer with agents that target the UPR has shown promising outcomes. The UPR has wide crosstalk with other signaling pathways. Multi-targeted cancer therapies which target the intersections within signaling networks have shown synergistic tumoricidal effects. In the present review, the basic cellular and signaling pathways of the ER and UPR are introduced; then the crosstalk between the ER and other signaling pathways is summarized; and ultimately, the evidence that the UPR is a potential target for cancer therapy is discussed. Regulation of the UPR downstream signaling is a common therapeutic target for different tumor types. Tumoricidal effects achieved from modulating the UPR downstream signaling could be enhanced by phosphodiesterase 5 (PDE5) inhibitors. Largely untapped by Western medicine for cancer therapies are Chinese herbal medicines. This review explores and discusses the value of some Chinese herbal extracts as PDE5 inhibitors.
Keywords: Endoplasmic reticulum stress, Unfolded protein response, Signaling pathways, Cancer, Phosphodiesterase 5 inhibitors
Introduction
Tumorigenesis occurs as a consequence of dysregulation of multiple signaling pathways. Some examples include mitogen activated protein kinase (MAPK), Akt and Smad4-mediated transforming growth factor-β (TGF-β) signaling (Yang and Yang 2010; Testa and Tsichlis 2005; Dhillon et al. 2007). Tolerance against death signals and the ability to survive under unfavorable environmental conditions and cellular stress are conferred on tumor cells by targets of the relevant signaling pathways, such that tumor cells grow in an uncontrolled manner and are often resistant to cancer therapies that kill dividing non-malignant cells (Klein 2000). Routine cancer therapy is generally based on arresting cancer cell growth at a specific phase in the cell cycle and enhancing cancer cell death, but it is also accompanied by primary chemoresistance and the secondary outgrowth of highly resistant clones (Otto and Sicinski 2017). Identification of a universal therapeutic target in the treatment of cancer could produce a snowball effect on activating relevant signaling pathways that can promote death, and inhibit survival, of cancer cells.
Recently, research into tumoricidal mechanisms of treatments based on endoplasmic reticulum (ER) stress has attracted attention. ER-regulated protein synthesis and folding help maintain homeostasis of the cellular microenvironment (Rutkowski and Hegde 2010). The protein folding process is prone to errors and misfolded proteins are continuously identified, unfolded, and removed from the ER for degradation. If misfolding exceeds clearance, the accumulated misfolded proteins trigger the unfolded protein response (UPR) to inhibit protein synthesis and stop the entrance of nascent peptides into the ER, while boosting ER folding mediators. Transient UPR is pro-survival; whereas, a chronic UPR triggers a series of apoptotic pathways directly or indirectly via interacting with other signaling pathways, for example, the phosphoinositol-3 kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway or the Ras/Raf/MEK/extracellular signal-regulated kinase (Ras/Raf/MEK/ERK) pathway (Verfaillie et al. 2012). Hypoxia is a feature of most tumors, because the oxygen supply cannot meet the requirement of the high proliferation rate. Therefore, cancer cells are constantly experiencing activated ER stress and activating the UPR to restore homeostasis. In this regard, the activation of the UPR is exploited by cancer cells to help them survive (Giampietri et al. 2015). Additionally, the UPR facilitates tumorigenesis by hampering the antigen-presenting function of dendritic cells, which blunts the immune elimination of cancer cells (Grootjans et al. 2016). However, activation of the UPR also facilitates cell cycle suppression and/or cell death (Brewer et al. 1999).
There is accumulating evidence that indicates that interfering with ER stress pathways may exert beneficial anticancer effects (Hazari et al. 2016). These effects are generally based on the activity of inhibitors of chaperone proteins which plays a central role in protein quality control (Sidera and Patsavoudi 2014), or the activation of pro-apoptotic pathways and inhibition of pro-survival signals that are relevant to the downstream UPR cascades (Shimodaira et al. 2014; Yang et al. 2016; Zhao et al. 2016). Multi-target therapy which targets several crucial signals in this ER stress-regulated network has shown synergistic effects compared with the use of single-specific targeted therapies (Booth et al. 2015b). Drugs that might have synergistic effects with therapies that alter ER stress are phosphodiesterase-5 (PDE5) inhibitors. PDE5 is an enzyme that degrades cyclic guanosine monophosphate (cGMP) (Shen et al. 2016). The drug combination of sildenafil, a well-known PDE5 inhibitor, and OSU-03012, a chaperone inhibitor, exerted a synergstic effect in killing glioma cell lines, concurrent with activation of the UPR cascades (Booth et al. 2015a). Although the inhibition of PDE5 has shown great potential in enhancing the tumoricidal effects of chaperone protein inhibitors, the mechanism of the interaction between cGMP signaling and the UPR remains largely unknown.
The ER had been discovered for more than half a century, but the yin-yang principle of ER action in determining the fate of tumor cells has still to be fully understood. The yin-yang principle describes the ability of the ER to promote various survival pathways that allow cells to adapt to ER stress by activating the UPR, versus the action of the UPR to destroy the cell via apoptosis if it fails to adapt to ER stress. The aim of this review is to outline the UPR-centered signaling network and its role in determining cell fate and the potential synergistic tumoricidal functions of multi-drug therapies which include chaperone protein regulators and PDE5 inhibitors.
A brief introduction of the structure and function of the ER
The microstructure of the largest but the last discovered cell organelle, ER, was described in 1945 by Porter and colleagues (Porter et al. 1945) using electron microscopy of chick fibroblasts. The morphology of its tubular network was originally described as “vesicle-like” bodies dispersed alongside the “lace-like” chains which extended in the cytosol. With advanced techniques, such as multicolour live cell imaging, details concerning the fine structure of the ER have come to light. This elaborate internal membrane system has a series of specialized functions, such as (1) secretory protein synthesis, modification, quality control and transportation; (2) lipid synthesis and distribution; (3) sterol synthesis; (4) Ca2+ storage and regulation; and (5) cell interior compartmentalization and interconnection (Westrate et al. 2015). These various functions are dependent on the delicate, dynamic, and transitional structure of the ER. The first part of this review will briefly introduce the basic form and functions of the ER. The mechanisms behind the formation of this dynamic network have been reviewed previously (Park and Blackstone 2010).
The network of the ER is a continuous lipid double-layered system comprising a nuclear envelope, peripheral ER, and cortical ER (Westrate et al. 2015). The nuclear envelope constitutes the inner and outer nuclear membranes. The peripheral ER comprises two sub-domains which are functionally and morphologically different: peripheral ER sheets (or cisternae) and peripheral ER tubules. The primary difference in structure is that ER sheets are flat and studded with ribosomes while ER tubules are highly curved and largely lack ribosomes. Hence, ER sheets and ER tubules are termed rough ER and smooth ER, respectively.
ER sheets and ER tubules are cell type-specific sub-organelles. For example, different cell types bearing different functions have different sheet or tubule ratios or ribosome densities. ER sheets are abundant in cells which exhibit a high capacity to secrete proteins, such as insulin-secreting pancreatic β cells, ER tubules are enriched in muscle cells, hepatocytes, and adrenal cortical cells which are responsible for Ca2+ storage, detoxification, and steroid hormone production, respectively (Patrizia and Afshin 2012). Having partly flat/rough and partly high curvature/smooth surfaces, the cortical ER is tethered to the plasma membrane working as a mediator of the Ca2+ concentration between the ER lumen and the extracellular environment, which regulates muscle contraction (Westrate et al. 2015). In the kidney, ultrastructural studies have demonstrated variation of the spatial organization of ER among different nephron segments. For example, in proximal tubular cells, the ER surrounds mitochondria, forming a structure like fingers in a glove. In this segment, the ER is rich in canaliculi and fenestrated saccules. In contrast, distal straight and convoluted tubular cells are only abundant in canaliculi with occasional non-fenestrated saccules. The variation of the abundance of the ER structure is likely related to the heterogeneity in function of different nephron segments. For example, in the thin limb cells that play a nonsignificant role in electrolyte transport, the ER structure is rare (Bergeron et al. 1987).
Interconnection between the ER and other organelles
As a hub for protein and lipid synthesis, translocation and secretion, the ER disperses throughout the cell and keeps an active crosstalk with other organelles. Growing polypeptides synthesized by ribosomes are imported to the rough ER lumen via the binding between the signal-recognition particle-ribosome complex and the receptor in the ER membrane (Gilmore 1991). In this way, the polypeptide chains are transferred into the ER lumen for further processing (Simon 1993). The primary quality control happens during this process when the incorrectly folded proteins are distinguished from the correctly folded ones to avoid entering the secretory pathways (Lars and Ari 2003). The correctly folded proteins are subsequently routed to the Golgi apparatus by vesicular transportation for further glycosylation and secretion (Kelley and Georgopoulos 1992; Lee et al. 2004). ER resident proteins are secreted along with other secreted proteins, but there also exist retention pathways to recall them back to the ER via COPI-coated transport vesicles (Saraste and Kuismanen 1992; Graham et al. 1993). Specifically, the KDEL receptor, a transmembrane protein of the Golgi, binds to the lysine-aspartate-glutamate-leucine sequence at the C-terminal of soluble ER protein. This is a condition for COPI-coated transport vesicles to carry soluble ER-resident protein back to the ER. However, the sequence on the ER membrane proteins is recognized directly by the COPI coats (Alberts et al. 2002). With the enzymes contained in the ER lumen, the ER also synthesizes phospholipids, cholesterol, and ceramide. Lipids can be shuttled to the Golgi apparatus via both vesicular and non-vesicular mechanisms (Meer 1993; Cockcroft 2001; Hanada et al. 2003). The ATP-dependent direct membrane contact is a prerequisite for the transport of phospholipids from the ER to the mitochondria. More specifically, various components are involved to support the contact between the ER and the mitochondria, such as mitochondria-associated membrane (MAM), ER-mitochondria encountered structures (ERMES), and acetylated microtubules. The transport for cholesterol, however, is cAMP dependent, during which process the cholesterol-binding protein “steroidogenic acute regulatory protein” plays a vital role in modulating the membrane structure of the mitochondria to facilitate the import of cholesterol into the mitochondria (Flis and Daum 2013). The mitochondria act as a buffer to restrict the accumulation of Ca2+ in the cytosol when Ca2+ leaks from the ER. The voltage-dependent anion-selective channel in the outer membranes of the mitochondria allows the diffusion of Ca2+. Further uptake of Ca2+ into the matrix of mitochondria requires specific transporters in the inner membranes of the mitochondria (Kaufman and Malhotra 2014).
ER quality control mechanisms
The protein folding process is inherently error-prone and dependent on complexity of individual proteins (Drummond and Wilke 2009). Environmental stresses, such as ultraviolet light exposure, depletion of Ca2+, osmotic stress, oxidative stress, and hypoxia or deprivation of nutrients in pathological conditions such as malignancy, increase the accumulation of improperly folded polypeptides (Walter and David 2011). If they are not removed from the ER via the process known as ER-associated degradation (ERAD), the partially folded or misfolded polypeptides are susceptible to aggregation in disordered structures (Fink 1998). The excessive accumulation of the misfolded proteins is a common characteristic of conformational disorders, such as amyloid diseases, spongiform encephalopathies, emphysema, cirrhosis, and thrombosis (Carrell and Lomas 1997).
Providentially, the ER has an orchestrated quality-control system (also known as proteostasis) to facilitate the processes of protein folding and misfolded protein degradation, in which ATP-dependent chaperones, also known as heat shock proteins (Hsps), play a crucial role (Hartl and Hayer-Hartl 2002; Saibil 2013). For example, glucose-regulated protein 94 (GRP94, gp96, Hsp90B1), a chaperone of the Hsp90 family, facilitates the effective folding of many functional proteins, such as immunoglobulins, insulin-like growth factors (IGFs), toll-like receptors, and integrins (Dersh et al. 2014). GRP78 (a 70 kDa protein which belongs to the Hsp79 family) binds to the hydrophobic surface of the non-native proteins and cooperates with Hsp110. It is capable of interfering with protein aggregation (Mattoo et al. 2013), assisting the unfolding, and refolding of misfolded proteins (Bukau and Horwich 1998; Zavilgelsky et al. 2002). GRP78 also keeps the stability of unfolded proteins until they are competent for the correct folding process under normal conditions (Sharma et al. 2010). Similarly, the glycan-dependent chaperones, calnexin and calreticulin, form a de-glycosylation and glycosylation cycle inside the ER lumen to hold the incorrectly folded proteins in the ER lumen and divert them into ERAD (Lars and Ari 2003).
Despite the support offered for primary quality control by all of the above factors, many newly synthesized proteins end up misfolded. The unwanted misfolded proteins are recognized and then retro-translocated to the cytosol for ultimate degradation by the ubiquitin-proteasome pathway, as a secondary quality control (Ruggiano et al. 2014). Misfolded proteins are flagged by glycosylation with a specific oligosaccharide structure (Man7GlcNAc2 with α 1, 6-linked mannosyl residual) (Clerc et al. 2009). The mannose-6-phosphate receptor homology domain of osteosarcoma amplified 9 (OS-9), an ER-resident lectin, recognizes α 1, 6-linked terminal mannose (Clerc et al. 2009), which targets the misfolded glycoprotein for degradation (Hirsch et al. 2009; Satoh et al. 2010). OS-9 interacts with the 3-hydroxyl-3-methylglutaryl-coenzymeA reductase degradation (HRD) ligase through the Hrd3p subunit for ubiquitination (Gauss et al. 2006). However, how the aberrant proteins are recruited to HRD ligase remains enigmatic. Finally, ubiquitinated proteins are recognized and degraded by the 26S proteasome (Voges et al. 1999).
If the ER-associated degradation capacity is insufficient, the ER will be congested with immature proteins in a process known as ER stress, initiating a series of signaling pathways collectively known as the UPR (Hendershot 2002). The UPR works on alleviating ER stress by fine-tuning the balance between (1) protein synthesis and degradation, (2) protein aggregation and disaggregation, and (3) protein influx and retro-translocation. However, if the re-establishment of homeostasis cannot be achieved, the UPR can cause cell death primarily by initiating apoptosis, which protects the organism from being harmed by toxic accumulation of misfolded proteins.
Signaling cascades elicited by the UPR
The coordinated functions regulated by the UPR are achieved by three ER stress proximal transducers: activating transcription factor-6 (ATF6), double-stranded RNA-activated protein kinase/PKR-like ER kinase (PERK), and inositol-requiring enzyme 1 (IRE-1). They constitute a complicated and dynamic signaling network which enhances the resistance of cells to ER stress. The adaptive signaling elicited by the UPR is based on a series of conformational adaptations and phosphorylation processes (Kaufman 1999; Mori 2000; Patil and Walter 2001).
All three of the stress transducers have binding sites for GRP78. In unstressed cells, the stress transducers are maintained in an inactivated state by GRP78 so as to block their activity. Under ER stress, GRP78 dissociates from these ER-residential proteins to initiate downstream cascades (Bertolotti et al. 2000).
Under the scope of the UPR, ATF6 is the only transducer that departs the ER via translocation in the membrane of the Golgi (Shen et al. 2002). ATF6 signaling functions to enhance protein folding and degradation capacity. Upon translocation from the ER to the Golgi apparatus, ATF6 undergoes proteolytic cleavage which releases a cytosolic fragment, pATF6(N) (Sasaki and Yoshida 2015). pATF6(N) then moves into the nucleus to activate the transcription of genes encoding most ER chaperones, such as GRP78, GRP94 and calreticulin, and some ERAD components, such as HRD (Adachi et al. 2008). However, knockout of the ATF6 gene did not influence the expression of proteins, which are responsible for protein translocation (Adachi et al. 2008).
PERK signaling decides the cell fate after exposure to ER stress. On ER stress, PERK phosphorylates eIF2α (eukaryotic translation initiation factor 2α) and consequently deactivates eIF2B. This results in blocking guanosine triphosphate (GTP)-dependent transportation of the initiator Met-tRNAi Met to the rough ER, which consequently downregulates global translation. Activated eIF2 also inhibits the influx of nascent polypeptides into the ER (Teske et al. 2011). The immediate result of this translation inhibition is a reduction in the rates of global protein synthesis, which saves energy consumption when oxygen and ATP levels are low. Although PERK signaling reduces the synthesis of most proteins, it preferentially increases the biosynthesis of some proteins that can help cells survive in stressed conditions, for example, inhibitor of Bruton’s tyrosine kinase (IBTKα), growth arrest and DNA-damage-inducible protein (GADD34), and Gcn4p. Alternatively, PERK signaling may increase the biosynthesis of some proteins that induce cell death (e.g., activating transcription factor 4 [ATF4]) if the stress is irreversible. IBTKα can protect cells from caspase3/7-dependent cell death (Baird et al. 2014). Activated ATF4 induces the expression of C/EBP homologous protein (CHOP), also known as growth arrest and DNA-damage-inducible protein 153 (GADD153), which can induce the apoptotic pathway (Li et al. 2014a). In contrast, activated ATF4 and CHOP also conjointly target genes, such as GADD34, that relieve translation attenuation for recovering cells from stress (Choy et al. 2015). Similarly, Gcn4p enhances biosynthesis of amino acids, which helps cells withstand starvation (Hinnebusch and Natarajan 2002). In addition, PERK inhibits the synthesis of cell cycle regulators, such as cyclin D1, resulting in cell cycle arrest in the G1 phase (Brewer et al. 1999). Consequently, if the UPR is transient, these adaptive responses promote cell survival. However, if the UPR is prolonged, overconsumption of nutrition and energy during protein synthesis causes oxidative stress which can further enhance protein misfolding by interfering with disulfide bond formation during protein folding (Han et al. 2013). This effect is enhanced by the pro-apoptotic influence of CHOP which, together, passes the molecular thresholds for induction of apoptosis.
Among all the three branches of the UPR, IRE1 signaling is the only one that is conserved in lower eukaryotes. In the case of IRE1, dissociation with GRP78 initiates the phosphorylation and dimerization of IRE1. This in turn results in removal of a 26 nucleotide intron from X-box binding protein 1 (XBP1) mRNA, leading to the synthesis of the isoform XBP1(S), which translocates into the nucleus to induce the upregulation of its target genes, the protein products of which facilitate every aspect of the secretory pathway ranging from protein folding and entry of proteins into the ER to ERAD (Walter and David 2011; Piperi et al. 2016). IRE1α is a branch of the UPR that can covert pro-survival ER stress to pro-apoptotic ER stress, depending on the activation of c-Jun amino-terminal kinase (JNK) (Brozzi et al. 2016). The prolonged activation of JNK is known to induce tissue and stimulus-specific apoptosis through mitochondria-dependent caspase activation (Dhanasekaran and Reddy 2008). The activation and function of the IRE1α/JNK pathway will be discussed in the next section. Unlike the broad expression of IRE1α, IRE1β is exclusively expressed in intestinal and bronchial epithelial cells (Nakamura et al. 2011). In contrast with the high cleavage activity of IRE1α against XBP1 mRNA, the RNase domain of IRE1β has higher cleavage potential against 28S ribosome RNA, which may cause apoptosis (Imagawa et al. 2008). Regarding the cell protective role, IRE1β is required for the production of mucins (Muc5b and Muc5ac) in the respiratory tract (Martino et al. 2013) and mucin-2 in the goblet cells in the colon (Tsuru et al. 2013). Consistent with the dual functions of the PERK branch of the UPR, the existence of IRE1 signaling further indicates that the UPR, which had been thought to be pro-survival under ER stress, could also induce cell death when ER stress is persistent and harmful.
Crosstalk between ER stress and other signaling pathways that can determine cell fate
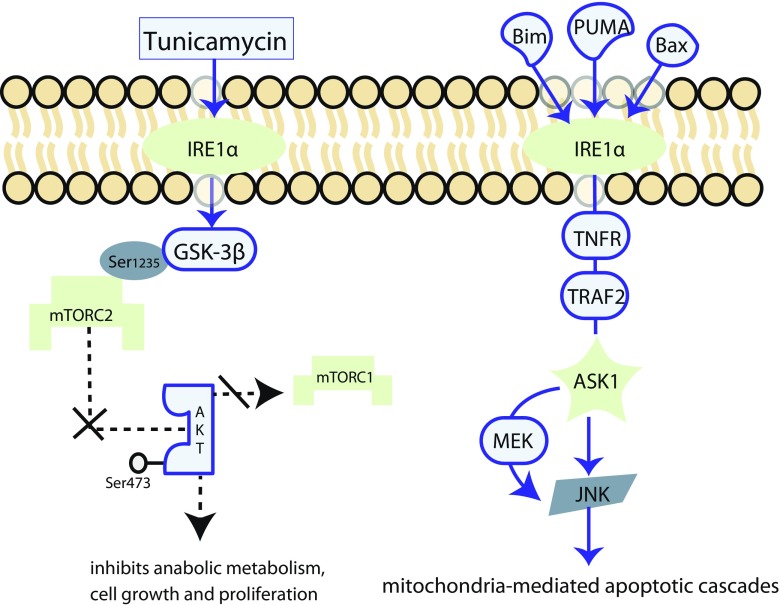
ER stress modulates crosstalk with signaling pathways that are pivotal in the control of cell fate, including PI3K/Akt/mTOR signaling, Ras/Raf/MEK/ERK signaling, and the mitochondria-mediated intrinsic pathway of apoptosis (Fig. 1). As discussed above, transient ER stress is beneficial; whereas, pathologically chronic ER stress serves to execute apoptosis. It has been consistently observed in primary cultured glial cells that the PI3K/protein kinase B (PKB, also known as Akt) signals are upregulated in exposure to short-term ER stress, but are subject to downregulation in chronic ER stress, and may facilitate cell death (Hosoi et al. 2007). PI3K/Akt/mTOR is an important signaling pathway that upregulates transcription and translation, thereby supporting cell cycling (Porta et al. 2014). The molecular mechanism of the activation of the PI3K/Akt/mTOR signaling has recently been reviewed elsewhere (Jason and Cui 2016). Briefly, sensing the environmental cues, the complex phosphatidylinositol-3,4,5-triphosphate/phosphoinositide dependent kinase 1 (PIP3/PDK1) phosphorylates Akt at Thr308, resulting in the activation of Akt (Dangelmaier et al. 2014). Activated Akt maintains the activity of the Rheb/GTP complex, which is essential for activation of mammalian target of rapamycin (mTOR) complex 1 (mTORC1) (Avruch et al. 2009). Upon activation, mTORC1 activates downstream signals which stimulate anabolic metabolism. For example, the activation of the ribosomal protein S6 kinase (S6 K) and the eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) promote protein synthesis (Magnuson et al. 2012). The activation of Akt is synergised by mTORC2 through phosphorylation of Akt on Ser473 (Yang et al. 2015). Recent evidence suggests that it is mTORC2 instead of mTORC1 that mediates adriamycin-induced apoptosis via mTORC2/Akt/nuclear factor κB (NFκB) signaling which activates transient receptor potential cation channel 6 (TRPC6) to increase intracellular Ca2+ in podocytes (Zhang et al. 2016). ER stress-mediated inhibition of the anabolic effects of the mTORC2-Akt pathway has been described (Chen et al. 2011). They found that a previously unknown phosphorylation site, Ser1235, on the rictor (rapamycin-insensitive companion of mTOR) component of mTORC2 could be phosphorylated by glycogen synthase kinase-3 β (GSK-3β), which is widely known as an Akt substrate that regulates glucose metabolism (Woodgett 2003). This occurred under hyperosmolality, tunicamycin, and thapsigargin-induced ER stress conditions, thereby hindering substrate Akt binding to mTORC2. Furthermore, using a mutagenesis technique, they found the GSK-3β-mediated inhibition of mTORC2-Akt signaling was important in inhibiting the cell proliferation and tumor growth in cultured mouse embryonic fibroblasts (MEFs) and nude mouse models which were injected with allograft MEFs bearing mutated rictor with enhanced phosphorylation at Ser1235. In a recent study, the ability of tunicamycin-induced ER stress to activate GSK-3β was completely abolished in IRE1α-deficient MEFs, but was normal in MEFs which were deficient in PERK, XBP1, or ATF6, suggesting that the activation of GSK-3β was IRE1α dependent (Kim et al. 2015).
Fig. 1.
Crosstalk between ER stress and other signaling pathways. Under ER stress, IRE1α activated GSK-3β phosphorylates mTORC2 on Ser1235, thereby hindering the binding between mTORC2 and the Ser473 site on Akt. This decreases the total amount of activated Akt, thus impairing the activation of mTORC1. Consequently, the mTORC1-initiated downstream anabolic metabolism, including protein synthesis, cell growth, and proliferation, is inhibited. The ER stress sensor, IRE1α, can be activated by the pro-apoptotic proteins, Bak, Bim, and PUMA. Upon stimulation, IRE1α induces the TRAF2/ASK1/JNK cascade, which contributes to cell death. While playing a core role in the IRE1α-initiated apoptotic signaling, ASK1 is also a member of the Raf family, which activates MEK4/MEK7-JNK signaling. This suggests that ASK1 is a coordinator in the interplay between the IRE1α-mediated apoptotic signaling and Ras/Raf/MEK/ERK signaling. GSK-3β glycogen synthase kinase-3, mTORC mTOR complex, TRAF2 tumor necrosis factor receptor (TNFR)-associated factor 2, ASK1 apoptosis signal-regulating kinase 1, JNK c-Jun amino-terminal kinase
The transmembrane ER stress sensor, IRE1, interacts with MAPK signaling (via Ras/Raf/MEK/ERK) to determine the cell fate in response to ER stress (Darling and Cook 2014). As discussed above, in addition to activation by disassociation from GRP78 complex, IRE1α can also be activated by the pro-apoptotic BH123 protein Bak and BH3-only proteins Bim and PUMA (Hetz et al. 2006; Klee et al. 2009). Upon stimulation, IRE1α induces the tumor necrosis factor receptor (TNFR)-associated factor2 (TRAF2)/apoptosis signal-regulating kinase 1 (ASK1)/JNK cascade, which contributes to cell death (Urano et al. 2000; Nishitoh et al. 2002). Knocking down of IRE1α and TRAF2 consistently inhibited cell death induced by Bim and PUMA in the presence of Bak, revealing the pro-apoptotic function of the IRE1α (Klee et al. 2009). Beyond being activated by IRE1α, JNK is an important downstream target of the multi-tier kinase module that contains Ras, RAF, MEK, and ERK (Wagner and Nebreda 2009), suggesting that Ras/Raf/MEK/ERK signaling may play a role in ER stress-induced cell death. In the interplay between the Ras/Raf/MEK/ERK signaling and the IRE1α signaling, ASK1 may function as a coordinator (Hayakawa et al. 2012). While playing a core role in IRE1α-initiated apoptotic signaling, ASK1 is also a member of the Raf family which activates MEK4/MEK7-JNK and MEK3/MEK6-p38 pathways (Ichijo et al. 1997). ASK1-deficient mice exhibited an increased resistance to ischemia-reperfusion (I/R)-induced death of cardiomyocytes. This was accompanied by a smaller increase in activating p38 and JNK compared with wild type mice, indicating that ASK1 deficiency negates the crosstalk between the IRE1α and MAPK signaling that normally promotes cell death in this stimulus scenario (Watanabe et al. 2005).
The pro-apoptotic effect induced by CHOP is relevant to the activation of the mitochondria-mediated intrinsic pathway of apoptosis whereby cytochrome C leaves the mitochondrial intermembrane space and then moves into the cytoplasm to trigger apoptosis. Prior to initiation of the intrinsic apoptotic pathway, the Bcl2 family pro-apoptotic proteins Bax or Bak aggregate to form a channel to allow the transmembrane release of cytochrome C, the process of which can be inhibited by the anti-apoptotic protein, Bcl2 (Cheng et al. 2001). Bcl2 is downregulated during CHOP-induced apoptosis in vitro (McCullough et al. 2001). The correlation between the Bcl2 protein family and CHOP-induced apoptosis has also been shown in mouse models. For example, mice bearing CHOP-deficient genes exhibited enhanced resistance to I/R-induced tubular epithelial cell death, with downregulation of pro-apoptotic Bax (Noh et al. 2015). This suggests that the mitochondria-mediated intrinsic pathway has a synergistic effect with CHOP-induced apoptosis.
As discussed above, UPR downstream cascades can induce cell apoptosis. Hence, targeting apoptotic ER-stress induced pathways might be effective in eliminating unwanted cells, such as tumor cells.
Evidence of UPR involvement in cancer
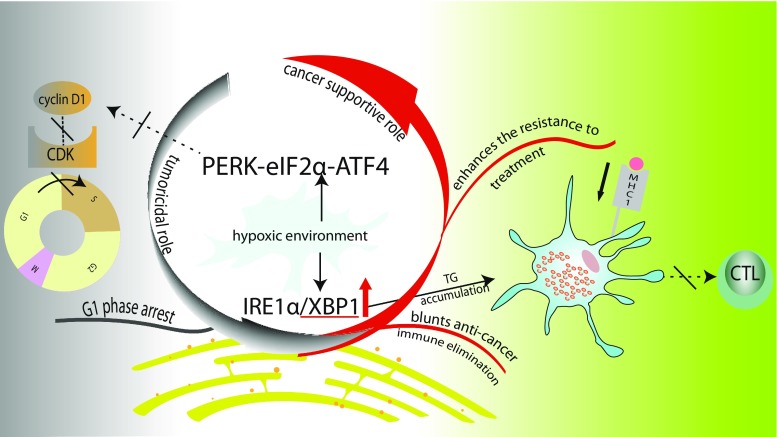
Although ER stress has been linked adversely to several diseases, including neurodegenerative diseases (Hetz and Bertrand 2014), heart diseases (Liu and Dudley 2016), diabetes (Cnop et al. 2012), and renal disease (Taniguchi and Yoshida 2015), manipulation of the ER stress can be harnessed therapeutically (Inki et al. 2008). For example, inactivating UPR cascades is an attractive target for antitumor modalities. In this section, an overview of different mechanisms that have been proposed to explain the molecular link between cancer and the induction of the ER stress is provided. The possible mechanisms of the adaptive behaviors of cancer cells based on the activation of the UPR will be described first. Then, deregulation of the immunity in the microenviroment of tumor, which is mediated by the ER stress, will be described. Finally, the cancer-killing potential of the UPR by interfering with the cell cycle will be discussed briefly (Fig. 2).
Fig. 2.
Dual roles of UPR in cancer. Cancer cells often live under hypoxic conditions which activate the IRE1 and PERK branches of the UPR to support cancer growth. On the one hand, cancer cells exploit the prosurvival UPR signaling to conquer the lethal effect of treatments. On the other hand, upregulated XBP1 induces the accumulation of triglyceride (TG) in dendritic cells, which decreases the expression of the major histocompatibility complex-1 (MHC1), thereby hindering the activation of CD8+ T cells. The immune sabotage of XBP1 contributes to a blunted immune elimination of cancer cells. In contrast, the G1 phase arrest of cancer cells is also related to the activation of the PERK branch of the UPR, suggesting that UPR has dual roles in determining the fate of cancer cells. TG triglyceride, CTL cytotoxic T lymphocyte, MCH1 major histocompatibility complex1, CDK cyclin dependent kinase
Due to uncontrolled proliferation, cancer cells often live in a condition with insufficient oxygen and nutrition. Stably expressed under conditions of hypoxia, hypoxia-inducible factor-1 (HIF-1) promotes angiogenesis via upregulating the expression of vascular endothelial growth factor (VEGF) (Liao and Johnson 2007)). Additionaly, HIF-1 decreases ATP consumption during glycolysis via stimulating the gene expression of pyruvate dehydrogenase kinase, which inhibits pyruvate dehydrogenase (Kim et al. 2006). Hence, cancer is known to inherently maintain a highly efficient proliferation rate when confronting hypoxia (Hanahan and Weinberg 2011). Apart from assisting the pro-survival signaling in tumorigenesis, the hypoxic stress also activates the IRE1 and PERK arms of the UPR, which launches downstream cascades to increase insensitivity of tumor cells toward pro-apoptotic signaling (Koumenis 2006). The activation of ER stress in response to oxygen-glucose deprivation has been reported in previous studies. For example, in primary cultures of mixed rat brain cortical cells which were deprived of oxygen and glucose, the PERK-eIF2α and IRE1-XBP1 branches of the UPR were stimulated (Badiola et al. 2011). In the context of tumors, the upregulation of GRP78 was also related to the glucose depletion (Shiu et al. 1977). It has now become clear that the activation of ER stress is a common phenomenon in tumorigenesis (Schewe et al. 2008; Hazari et al. 2016; Bi et al. 2005). The altered expression of ER stress proteins has been observed in various cancer types, including lung cancer (Tsai et al. 2013), breast cancer (Kim et al. 2016), colon cancer (Ryan et al. 2016), gastric cancer (Shen et al. 2015), pancreatic cancer (Niu et al. 2015), liver cancer (Shuda et al. 2003; Al-Rawashdeh et al. 2010), prostate cancer (Storm et al. 2016; Liu et al. 2017), kidney cancer (Fu et al. 2010), skin cancer (Shimizu et al. 2017), uterine cancer (Lin et al. 2013), ovarian cancer (Cubillos-Ruiz et al. 2015), leukemia (Buontempo et al. 2016), myeloma (Zhong et al. 2016), and gliobastoma (Epple et al. 2013).
GRP78 is the common UPR component that has upregulated expression in most of the tumor tissues which are mentioned above. GRP78 was initially identified as a protein that was upregulated in Rous sarcoma virus-transformed chick embryo fibroblasts (Shiu et al. 1977). The activation of GRP78 correlates with the severity and prognosis of cancer. For example, the activation of a rarely known splicing variant of GRP78, GRP78va, is associated with enhanced viability of leukemic cells. GRP78va is dramatically upregulated under ER stress. As a result of unleashing PERK signaling, which would be inhibited by P58IPK, the upregulation of GRP78va adapts leukemic cells to the ER stress, indicating the cancer-supporting role of the PERK branch of the UPR (Ni et al. 2009). The cancer supporting role of PERK signaling has been revalidated in other research. Meixia et al. found that xenograft tumors grown from Ki-RasV12 and Ha-RasV12-transformed MEFs with wild-type PERK- eIF2α-ATF4 were six times larger in size than tumors with a compromised PERK-eIF2α-ATF4 pathway, suggesting that inhibiting PERK-eIF2α-ATF4 activity inhibits the growth of tumors (Bi et al. 2005). The silencing of IRE1β, however, may contribute to the carcinogenesis of colorectal cancer, which is characterized by a dysregulated expression of mucins (Krishn et al. 2016), because the integrity of IRE1β is crucial for the normal characterization of mucin-2 in the colon. Knock out of IRE1β increases the ER stress level in the goblet cells of the colon mucosal layer. Increased ER stress, in this case, is characterized by an upregulation of GRP78, an increased ratio of spliced to unspliced XBP1 and the ER distention in goblet cells. Immunofluorescence microscopy indicated an overlap of the strong staining of the O-glycosylated proteins and calreticulin, which is an ER resident protein, indicating that the aberrant expression of IRE1β was responsible for the overaccumulation of mucin-2 in the ER of goblet cells. Failing to move the C-terminal peptide of mucin-2 during posttranslation may interefere with the mobility of mucin-2, which could be the reason of the overaccumulation of aberrant mucin-2 in the ER lumen (Tsuru et al. 2013). Consistently, it has been found that there was downregulated IRE1β expression in colorectal adenocarcinomas compared with normal colon (Jiang et al. 2017). Clinical studies based on archived tumor tissues demonstrate that the levels of expression of specific ER stress markers can work as predictors of cancer (summarized in Table 1).
Table 1.
Summary of the upregulation of ER stress markers in different human tumor types and the association with aggressivenss and prognosis of cancer
| Cancer types | ER stress markers | Methods | Sample size | Results | |
|---|---|---|---|---|---|
| Poor prognostic role of the upregulated ER stress markers | Endometrial adenocarcinoma (Matsuo et al. 2013) | GRP78, CHOP | IHC analysis on FFPE specimens | 246 | 1. CHOP expression paralleled the GRP78 expression in adipocytes and in the tumor. |
| 2. High visceral adipocyte GRP78 expression positively correlated with advanced-stage disease and deep myometrial invasion. | |||||
| 3. High visceral adipocyte GRP78 expression was significantly associated with decreased DFS. | |||||
| Renal cell carcinoma (RCC) (Fu et al. 2010) | GRP78 | IHC analysis on FFPE specimens and RT-PCR | 42 | 1. GRP78 expression was significantly higher in RCC tissues compared with nontumorous renal tissues. | |
| 2. The high levels of GRP78 mRNA expression and protein expression were related to the large tumor size and high clinical stage. | |||||
| Oral squamous cell carcinoma (OSCC) (Xia et al. 2014) | GRP78 | IHC analysis on FFPE specimens | 46 | 1. Patients with OSCC exhibited an upregulation of the expression of GRP78 than the health control. | |
| 2. GRP78 expression was positively correlated with tumor size, stage, grade, lymphatic invasion, and distant metastasis. | |||||
| 3. Positive GRP78 expression was inversely correlated with survival. | |||||
| Prostate cancer (Daneshmand et al. 2007) | GRP78 | IHC analysis on FFPE specimens | 153 | 1. The intensity of the GRP78 expression was markedly higher in the primary tumor compared with areas of benign epithelium. | |
| 2. Patients with strong GRP78 expression had higher risk of death and recurrence than patients with weak expression. | |||||
| Hepatitis B virus-related-hepatocellular cancer (HCC) (Lim S et al. 2005) | HSP27, HSP60, HSP70, HSP90, GRP78, and GRP94 | IHC analysis on FFPE and Western blot | 52 | 1. Expression of HSP27, HSP70, HSP90, GRP78, and GRP94 increased along with the stepwise progression of hepatocarcinogenesis. | |
| 2. There was a positive correlation between the expression of GRP78, GRP94, HSP90, and HSP70 and prognostic factors of HCC. | |||||
| 3. Strong correlation was found only in GRP78. | |||||
| Lung cancer (Wang et al. 2005) | GRP78 and GRP94 | IHC analysis on FFPE specimens and RT-PCR | 54 | 1. There was a significant overexpression of GRP94 and GRP78 in cancer tissues as compared to normal tissues. | |
| 2. The overexpression of GRP94 and GRP78 was correlated with poor differentiation and late stage. | |||||
| Melanoma (Zhuang et al. 2009) | GRP78 | IHC | 171 | 1. The IRS of GRP78 increased with the progression of melanoma. | |
| 2. The IRS of GRP78 increased with increasing tumor thickness and with increasing dermal tumor mitotic index. | |||||
| 3. Compared with patients whose IRS < 25, patients with IRS ≥ 25 had shorter DFS and OS. | |||||
| 4. The overexpression of GRP78 was not an independent predictor of DFS or OS. | |||||
| Promising prognositic role of the upregulated ER stress markers | Adenocarcinomas of the esophagus (Langer et al. 2008) | GRP78, GRP94 | IHC analysis on FFPE specimens and RT-PCR | 137 | 1. Significant higher mRNA levels of GRP78 were found in the well-differentiated tumors as compared to moderately and poorly differentiated tumors. |
| 2. A strong GRP78 ICH staining was correlated with early tumor stage. | |||||
| 3. A weak GRP94 ICH staining was correlated with early tumor stage and less lymph node involvement. | |||||
| 4. A trend toward better prognosis was found in patients with high GRP78 and GRP94 mRNA levels. | |||||
| Urothelial carcinoma of the upper urinary tract (Uematsu et al. 2010) | GRP78 | IHC analysis on FFPE | 126 | 1. There was a significantly higher incidence of GRP78 expression in low-grade invasive tumors than in high-grade invasive tumors. | |
| 2. The overexpression of GRP78 was associated with the improved DFS. | |||||
| Colorectal adenocarcinoma (Jiang et al. 2017) | IRE1β | IHC analysis on FFPE specimens, RT-PCR and Western blot | 42 | 1. IRE1β expression wassignificantly lower in cancer tissues compared with nontumorous colorectal tissues. | |
| 2. The low levels of IRE1β expression, RNA, and protein expression were related to the presence of lymph node metastasis and high clinical stage. |
ER endoplasmic reticulum, GRP glucose-regulated protein, HSP heat shock protein, CHOP C/EBP homologous protein, IHC immunohistochemical, FFPE formalin-fixed, paraffin-embedded, RCC renal cell carcinoma, RT-PCR reverse transcription polymerase chain reaction, IRS immunoreactivity score, OSCC oral squamous cell carcinoma, HCC hepatocellular carcinoma, DFS disease-free survival, OS overall survival
Blunted anti-cancer immunity is an underlying process of cancer development. By means of utilizing ER stress, tumors can successfully suppress or evade immune scavenging (Hanahan and Weinberg 2011). Over the past years, significant research efforts have elucidated the functions of the UPR in immunity. In pilot research from Giovanna et al., the link between the activation of the eIF2α/ATF4/GADD34 branch of the UPR signaling and the intact innate anti-viral immunity in response to Chikungunya virus infection was revealed (Clavarino et al. 2012). In this research, the activation of GADD34 upon sensing the double strand viral mRNA was a precondition for the translation of interleukin 6 (IL-6), interferon β (IFN-β), and PKR. IL-6 and IFN-β are cytokines that are crucial for the innate response to infection (Steinhagen et al. 2013). PKR is an eIF2α kinase, which halts viral protein synthesis by phosphorylating eIF2α (Dar et al. 2005; Dey et al. 2005). However, in a cancer setting, the pro-inflammatory molecules propel the processes of tumorigenesis (Mumm and Oft 2008). For example, secretion of IL-6 by renal tumor cells helps maintain the tumorigenic inflammatory signaling pathway (Kamińska et al. 2015). Along with the cytokines produced under the innate immune response, the antigen-presenting dendritic cell is crucial for activating T cells and subsequently elicits the adaptive immune response to eliminate cancer cells (Ni and O’Neill 1997; Chaux 1995). Although in clinical oncology, cancer immunotherapy mostly targets overcoming T cell inactivation by checkpoint inhibitors, the dentritic cell-based anticancer immunotherapy has also achieved attention. Large amounts of dendritic cells with defective functions are produced in the cancer microenvironment, thereby crippling the elimination of cancer cells (Yang and Carbone 2004). The hampered “T cell-dependent anti-tumor immunity” is partially ascribed to the activation of another UPR branch: the IRE1α/XBP1 signaling (Cubillos-Ruiz et al. 2015). In physiological conditions, tumor-irrelevant CD8+ dendritic cells constitutively activate the IRE1-α/XBP1 axis without triggering the UPR cascades, and so regulate the gene expression that maintains ER homeostasis and the phenotype of dendritic cells. Furthermore, intact IRE1-α/XBP1 signaling plays a significant role in the cross-presentation by CD8+ dendritic cells (Osorio et al. 2014). The overexpression of XBP1 has been discovered in tumor-associated dendritic cells in aggressive cancers. This overexpression has negative effects on the function of dendritic cells in the tumor microenvironment (Cubillos-Ruiz et al. 2016). For example, Cubillos-Ruiz et al. (Cubillos-Ruiz et al. 2015) found that the expression of the spliced XBP1 was positively correlated with the volume and weight of ovarian tumors in murine models, indicating the existence of an impaired antigen presenting function of the dendritic cells. They further found the lipid peroxidation product 4-hydroxy-trans-2-nonenal in ovarian cancer-associated dendritic cells stimulated the production of XBP1 and induction of ER stress. The dendritic cells which were devoid of XBP1 demonstrated significant suppression of genes, such as Agpat6, Fasn, Scd2, and Lpar1, which are involved in lipid metabolism pathways, and genes such as ATF6, Sec61α1, Pdia4, and Sec24, which are involved in the ER stress response. Large intracellular lipid bodies were only found in the XBP1-sufficient dendritic cells but not in the XBP1-deficient cells. The accumulation of triglyceride in bone-marrow-derived dendritic cells decreased the surface expression of the major histocompatibility complex-1 (MHC-1) which was loaded with ovalbumin-derived peptide epitope, thus hindering the activation of CD8+ T cells. The immune sabotage of XBP1 is revalidated in XBP1 deficient mice where the T cells exhibited enhanced capacity to hamper tumor growth. Moreover, silencing IRE1α/XBP1 signaling prolonged survival of mice bearing aggressive orthotopic ovarian tumors. These results collectively indicate that the XBP1-dependent turbulence of lipid metabolism in ovarian cancer contributes to the dysfunction of dendritic cells, which weakens the T-cell mediated anti-tumor responses.
The presence of cell cycle-mediated cancer resistance is a great challenge for antitumor therapies (Shah and Schwartz 2001). In contrast to the supportive role of the UPR in tumorigenesis by blunting the immune elimination, the UPR facilitates the tumoricidal treatment by blocking cell cycling of cancer cells. The upregulation of the G1/S phase regulator, cyclin D1, in G1 phase is found in various malignant neoplasms (Bianchi et al. 1993; Arber et al. 1996; Youssef et al. 1997; Donnellan and Chetty 1998; Drobnjak et al. 2000; Gautschi et al. 2007). As shown by Brewer et al., the tunicamycin-mediated UPR inhibits the translation of cyclin D1, thereby inhibiting cyclin D1 forming a complex with cyclin-dependent kinase. Thus, the retinoblastoma protein is not able to be phosphorylated, which results in the cell-cycle arrest in G1 phase (Brewer et al. 1999). It is worth noting that the G1 phase arrest mediated by the UPR may lead to resistance to agents that target the succeeding phases of cell cycle, indicating the importance of selecting the appropriate therapy when the intact cell cycle is interfered with by the UPR.
Evidence of the tumoricidal effect of drugs based on regulating ER stress
The importance of the UPR in tumorigenesis and malignancy has inspired great interest in modulating the response of cancer cells toward the ER stress. Accumulating evidence indicates that utilizing the pro-apoptotic pathways of the UPR has tumouricidal effects in various cancer types (Table 2). Amplifying the pro-apoptotic PERK/eIF2α/ATF4/CHOP signaling led to substantial death in breast cancer cells (Chakraborty et al. 2016). In this recently published research, the MCF-7 breast cancer cell line subjected to mephebrindole treatment exhibited a high apoptotic rate, accompanied by increased reactive oxygen species (ROS) generation, Ca2+ disequilibrium, and amplified PERK/eIF2α/ATF4/CHOP signaling. Moreover, the tumoricidal effect of mephebrindole is partially inhibited by the MAPK inhibitor SB2035880. These results were further validated in animal models, cumulatively suggesting that mephebrindole can cause death of breast cancer cells via utilizing apoptotis induced by PERK and MAPK signaling pathways.
Table 2.
Multiple pharmacological therapeutic targets for cancer based on ER stress
| Name of the agent | Origin or conventional application | Mechanism of action to exhibit the antitumor effect | Targeting tumor types |
|---|---|---|---|
| Mephebrindole (Chakraborty et al. 2016) | Derivative of 3, 3′-Diinodolylmethane, one of the active components from brassica vegetables. | To amplify the pro-apoptotic PERK/eIF2α/ATF4/CHOP signaling and the MAPK signaling. | Breast cancer |
| Temozolomide, 5-fluorouracil and CPT-11 (Pyrko et al. 2007) | Conventional antineoplastic agents | To activate the CHOP signaling and caspase 7 with the help of inhibiting the expression of GRP78, therefore enhancing the chemosensitivity. | Gliomas |
| GRP78 antibodies (Misra and Pizzo 2010; De Ridder et al. 2011) | n/a | To inhibit the PI3K/Akt pathway, to activate the pro-apoptotic pathways mediated by mitochondria and MEK4/JNK signaling | Prostate cancer and melanoma |
| OSU-03012 (Booth, Cazanave, et al. 2012) | Derivative of celecoxib with enhanced Akt inhibitory ability, but lacks the cyclooxygenase. | To enhance phosphorylation of PERK and eIF2α, resulting in reduced expression of GADD34, thus hindering the recovery of the protein synthesis under ER stress. The synergistic effect is dependent on the activation of the IRE1/JNK signaling and the inhibition on the expression of Hsp90 and GRP78. Moreover, the lethal effect is accompanied by the downregulation of the multidrug resistance protein. | Gliomas and medulloblastoma |
| Sildenafil (Booth et al. 2017; Booth et al. 2015b; Webb et al. 2015; Booth et al. 2015a) | Selective PDE5 inhibitor which is widely applied in ameliorating erectile dysfunction and pulmonary arterial hypertension | ||
| Multi-kinase inhibitors, such as sorafenib and lapatinib (Booth, Cruickshanks, et al. 2012) | Antitumor agents with multi-target actions | ||
| Bortezomib (Johnson 2015) | The first line anti-tumor agent for myeloma | To activate the pro-apoptotic PERK/eIF2α/ATF4/CHOP signaling and to inhibit 26S proteasome, which disrupts the degradation of the misfolded proteins. | Myeloma |
| Icariin (Fan et al. 2016) | The herbal medicine originated PDE5 inhibitors, which was conventionally applied to improve the reproductive functions. | To activate the pro-apoptotic PERK/eIF2α/ATF4/CHOP signaling and to upregulate the ER stress related chaperone proteins. | Esophageal squamous cell carcinoma |
ER endoplasmic reticulum, PERK [double-stranded RNA-activated protein kinase (PKR)-like ER kinase, eIF2α, eukaryotic translation initiation factor 2α, ATF4 activating transcription factor 4, CHOP, C/EBP homologous protein, MAPK mitogen-activated protein kinase, PI3K phosphoinositol-3 kinase, GADD34 growth arrest and DNA-damage-inducible protein, JNK c-Jun amino-terminal kinase, GRP78 glucose-regulated protein
The resistance toward chemotherapeutic agents is an obstacle for efficient therapy for cancer. Now it is clear that overexpression of GRP78 in multiple tumor types is positively correlated to high resistance (Roller and Maddalo 2013). Inhibiting the expression of GRP78 sensitizes cancer cells toward chemotherapy. For example, conventional antineoplastic agents, such as temozolomide, 5-fluorouracil, and CPT-11, coupled with inhibition of the expression of GRP78 launches the activation of CHOP signaling and caspase 7, thus enhancing the chemosensitivity of malignant gliomas (Pyrko et al. 2007). The NH2-terminal domain of GRP78 interacts with tissue-type plasminogen activator (t-PA) to support cell proliferation (Gonzalez-Gronow et al. 2014). In agreement with the tumorigenic role of GRP78, the presence of anti-GRP78 autoantibodies in the serum of prostate cancer patients predicts an adverse prognosis of cancer (Mintz et al. 2003). This humoral response to GRP78 is ineffective in eliminating cancer cells. In contrast, the generated anti-GRP78 autoantibody stimulated Akt phosphorylation and melanoma proliferation in the murine model (De Ridder et al. 2011). The tumoricidal effects induced by blocking the cell surface GRP78 are caused by a comprehensive alteration of the ER stress-relevant signaling pathways, including inhibition of the PI3/Akt pathway and the activation of the pro-apoptotic pathways mediated by mitochondria and MEK4/JNK signaling pathway. These data are evidenced by inhibition of tumor growth and proliferation of prostate cancer and melanoma by treatment with GRP78 antibodies (Misra and Pizzo 2010; de Ridder et al. 2012).
Currently, there are several research groups that are active and prominent in the field of ER stress and cancer therapy. Research by Dent’s group demonstrated a more than additive anti-tumor effect of multi-target therapies when the UPR and the cGMP signaling were targeted. For example, OSU-03012, which is the inhibitor of the chaperone protein GRP94 and GRP78, exhibits remarkable tumoricidal effects when combined with sildenafil. OSU-03012 is a derivate of celecoxib (Zhu et al. 2004). Although the anti-tumor potential of the non-steroidal anti-inflammatory drugs has been extensively studied (Cha and DuBois 2007), OSU-03012 lacks the cyclooxygenase, indicating the existence of additional mechanisms underlying the tumoricidal effect. Sildenafil is a well-known selective PDE5 inhibitor which is widely applied in ameliorating erectile dysfunction and pulmonary arterial hypertension through nitric oxide-mediated vasodilation (Corbin 2004). Recent research has highlighted the anti-tumor potential of sildenafil when applied together with standard chemotherapeutic agents. The mechanism is relevant to the activation of apoptosis, autophagy, and the accumulated ROS in cancer cells (Booth et al. 2014a, b; Roberts et al. 2014). Research designed by Booth and his colleagues indicated that treatment of glioma cells using sildenafil (0.5–2 μmol) and OSU-03012 (0.5–2 μmol) produced synergistic tumor toxicity (Booth et al. 2014a, b). Either knocking down the IRE1/XBP1 branch of the UPR or activating the synthesis of nitric oxide enhanced the lethal effects. Moreover, sildenafil and OSU-03012 produced a synergistic effect in activating the PERK/eIF2α signaling, as evidenced by increased phosphorylation levels of eIF2α. Additionally, inhibiting PI3K/Akt signaling enhanced the drug toxicity. Further testing in this research demonstrated that the enhancement of the toxicity depended on the activation of the IRE1/JNK-induced apoptosis and the synthesis of nitric oxide. These observations collectively indicate that there is potential crosstalk between the cGMP signaling and UPR, which regulates the proliferation and growth of tumor cells. However, the specific molecular mechanism underlying the synergistic tumoricidal effects induced by OSU-03012 and sildenafil remains largely unexplained. Although knocking down the IRE1 branch of the UPR facilitated the drug-combinatory toxicity after treatment for 24 h, it is not clear whether transient inhibition of the UPR will induce pro-survival signaling which tumor cells could potentially exploit. Moreover, it is not clear to what extent this multi-drug modality could be applied to other tumors, such as kidney cancer, which are also highly resistant to treatment. Further research based on SiRNA-mediated knockdown of the UPR and the cGMP components is required to explain the synergistic effect in more types of cancer.
The synergised anti-tumor ability of co-treatment with OSU-03012 and sildenafil could be further enhanced by multi-kinase inhibitors. Booth et al. Booth et al. 2015a) demonstrated that this drug combination therapy enhanced phosphorylation of PERK and eIF2α, resulting in reduced expression of GADD34. As mentioned previously, GADD34 helps recover the translational rate when cells are undergoing ER stress. The results here indicate that the multidrug therapy kills cancer cells by suppressing their protein synthesis. Except for activating the PERK branch of the UPR, the synergised tumor-killing ability induced by multi-drug therapy is dependent on the activation of the IRE1 signaling and the inhibition on the expression of GRP94 and GRP78. Moreover, the lethal effect is accompanied by the downregulation of the multidrug resistance protein 1 (gene name is ABCB1) and ATP-binding cassette sub-family G member 2 (gene name is ABCG2), which pump drugs out of the cell (Hegedus et al. 2009). The expression of ABC multi-drug transporters decreased with the downregulation of GRP78. Further research demonstrated that adding specific kinase inhibitors, for example, mesenchymal-epithelial translation inhibitor crizotinib and IGF1R inhibitor OSI-906, to this, drug-combination therapy could produce greater synergistic tumoricidal effects. Of note, the promising effects were cell-type specific. For example, the application of OSI-906 only enhanced the tumoricidal effects of sildenafil and OSU-03012 in glioblastoma 6 and glioblastoma 14 cells, but not in breast cancer BT474 cells. Of note, Booth et al. found that multi-drug therapy was not harmful for normal tissues in vivo, highlighting the potential of the clinical application.
In fact, some first line anti-tumor agents also help regulate chaperone proteins and the UPR signaling pathways, indicating that ER stress could be a universal therapeutic target for cancer. For example, bortezomib, which was approved by US Food and Drug Administration (FDA) for treating refractory multiple myeloma in 2003, is a 26S proteasome inhibitor (Grigoreva et al. 2015; Adams 2001). Consistent with most findings in malignancy, GRP78 is also upregulated in myeloma (Zhuang et al. 2009). Treatment with bortezomib led to the activation of the PERK branch of the UPR in myeloma cells, as evidenced by the upregulation of ATF4 and CHOP/GADD153, thereby resulting in the ER stress-induced apoptosis (Obeng et al. 2006). Additionally, 26S plays a role in relieving the protein overload. Inhibition of the 26S proteasome with bortezomib may kill myeloma via worsening the already crowded microenvironment in tumor cells (Johnson 2015).
Traditional Chinese herbal medicine shows anticancer potential via regulating ER stress
Traditional Chinese herbal medicine (TCHM) applies natural products extracted from herbs, animals, and minerals. TCHM is a rich therapy resource and has attracted tremendous attention in recent years. There is increasing clinical acceptance and marketing of monomers extracted from natural products of TCHM, such as cantharidin, arsenic trioxide, artesunate, and homoharringtonine, for their anti-neoplastic effects (Efferth et al. 2007). Herbal medicine derivatives, such as the PDE5 inhibitors icariin and icaritin, are potential anti-tumor compounds (Zhu et al. 2011; Li et al. 2014b). Icariin is the compound extracted from the genus Epimedium (also known as “Yin Yang Huo” in Chinese). Derived from icariin, icaritin exerts an 80-fold stronger PDE5 inhibitory ability than icariin (Shen et al. 2016). The most recent research has linked the anti-tumor effects of icariin to its regulatory role in ER stress (Fan et al. 2016). In this study which investigated the anti-tumor activity of icariin in human esophageal squamous cell carcinoma, treatment with icariin resulted in the death of cancer cells accompanied by upregulation of ER stress-related proteins, including PERK, GRP78, ATF4, and eIF2α. Consistent with Booth’s study (Booth et al. 2015a), the study by Fan et al. also demonstrated that suppressing the PERK branch of the UPR by silencing eIF2α blunted the tumor killing ability of icariin, as evidenced by a comprehensive downregulation of the proapoptotic signals, including caspase 9 and PUMA, and an upregulation of the anti-apoptotic Bcl2 protein. Contrary to expected findings, the result of this study demonstrated that the tumoricidal effect of icariin was related to upregulation of GRP78: downregulation of GRP78 was the mechanism underlying the synergistic effects of sildenafil and OSU-03012. As mentioned previously, activation of ER stress is dynamic, such that the drug exposure time or the time-point of measurement may influence the apparent UPR protein levels. However, the drug exposure time was 24 h prior to protein testing with Western immunoblot; this pre-test exposure time was same with Booth’s study. The disparity in results between these two teams may be related to the difference in cancer types, because the upregulation of GRP78 was also found to be a positive predictor for esophageal carcinoma (Langer et al. 2008) (Table 1). The limitation of the study was that it did not compare the baseline expression of GRP78 in normal esophageal cells and esophageal carcinoma cells. Discrepancies between the tumouricidal effect via upregulating the chaperone proteins, especially for GRP78, make the ER stress regulatory roles of PDE5 inhibitors uncertain.
Conclusion
In physiological conditions, ER stress maintains a homeostatic balance via the ER-specific UPR. Cancer exploits ER stress to overcome its cellular elimination mechanism and achieves uncontrolled proliferation. The ER stress response is now recognized as a common molecular pathway for the main pathogenic mechanisms of cancer, and there is increasing evidence that targeting ER stress pathways is promising in developing novel therapies for cancer. Due to the dual functions of ER stress in determining the fate of cancer cells and maintaining physiological homeostasis, caution must be exercised when interfering with ER stress-related signaling, because of the possibility of off-target side effects. Moreover, the duration of drug exposure should be taken into consideration due to the dynamic and time-dependent proapoptotic effects of the UPR. Finally, more cancer types that exhibit resistance to therapies should be involved in the emerging research about targeting ER stress in suppressing cancers.
Acknowledgements
This work is partly supported by the scholarship from China Scholarship Council (File No. 2016008440278).
Abbreviations
- ASK
Apoptosis signal-regulating kinase
- ATF
Activating transcription factor
- cGMP
Cyclic guanosine monophosphate
- eIF2
Eukaryotic translation initiation factor 2α
- ERAD
Endoplasmic reticulum associated degradation
- ERK
Extracellular signal-regulated kinase
- FDA
Food and Drug Administration
- GADD
Growth arrest and DNA-damage-inducible protein
- GSK
Glycogen synthase kinase
- GTP
Guanosine triphosphate
- HIF
Hypoxia-inducible factor
- HRD
3-hydroxyl-3-methylglutaryl-coenzymeA reductase degradation
- IBTKα
inhibitor of Bruton’s tyrosine kinase
- IFN
Interferon
- IGF
Insulin-like growth factor
- IL
Interleukin
- I/R
Ischemia-reperfusion
- IRE
Inositol-requiring enzyme
- MAPK
Mitogen-activated protein kinase
- MEF
Mouse embryonic fibroblasts
- MHC
Major histocompatibility complex
- mTOR
Mammalian target of rapamycin
- NFκB
Nuclear factor κB
- OSCC
Oral squamous cell carcinoma
- OS-9
Osteosarcoma amplified 9
- PDE
Phosphodiesterase
- PDK
Phosphoinositide dependent kinase
- PERK
Double-stranded RNA-activated protein kinase/PKR-like ER kinase
- PIP3
Phosphatidylinositol-3,4,5-triphosphate
- PKB
Protein kinase B
- ROS
Reactive oxygen species
- S6 K
S6 kinase
- TCHM
Traditional Chinese herbal medicine
- TFG-β
Transforming growth factor-β
- t-PA
tissue-type plasminogen activator
- TRAF
Tumor necrosis factor receptor associated factor
- TRPC
Transient receptor potential cation channel
- TNFR
Tumor necrosis factor receptor
- UPR
Unfolded protein response
- VEGF
Vascular endothelial growth factor
- XBP
X-box binding protein
- 4E–BP
4E–binding protein
References
- Adachi Y, Yamamoto K, Okada T, et al. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct Funct. 2008;33:75–89. doi: 10.1247/csf.07044. [DOI] [PubMed] [Google Scholar]
- Adams J. Proteasome inhibition in cancer: development of PS-341. Semin Oncol. 2001;28:613–619. doi: 10.1016/s0093-7754(01)90034-x. [DOI] [PubMed] [Google Scholar]
- Al-Rawashdeh FY, Scriven P, Cameron IC, et al. Unfolded protein response activation contributes to chemoresistance in hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2010;22:1099–1105. doi: 10.1097/MEG.0b013e3283378405. [DOI] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J (2002) Transport from the ER through the Golgi apparatus. In: Molecular biology of the cell, 4th edn. Garland Science, New York, pp: 726–739
- Arber N, Hibshoosh H, Moss SF, et al. Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology. 1996;110:669–674. doi: 10.1053/gast.1996.v110.pm8608874. [DOI] [PubMed] [Google Scholar]
- Avruch J, Long X, Lin Y, et al. Activation of mTORC1 in two steps: Rheb-GTP activation of catalytic function and increased binding of substrates to raptor. Biochem Soc Trans. 2009;37:223–226. doi: 10.1042/BST0370223. [DOI] [PubMed] [Google Scholar]
- Badiola N, Penas C, Miñano-Molina A, et al. Induction of ER stress in response to oxygen-glucose deprivation of cortical cultures involves the activation of the PERK and IRE-1 pathways and of caspase-12. Cell Death Dis. 2011;2:e149. doi: 10.1038/cddis.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird TD, Palam LR, Fusakio ME, et al. Selective mRNA translation during eIF2 phosphorylation induces expression of IBTKα. Mol Biol Cell. 2014;25:1686–1697. doi: 10.1091/mbc.E14-02-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron M, Gaffiero P, Thiery G. Segmental variations in the organization of the endoplasmic reticulum of the rat nephron. A stereomicroscopic study. Cell Tissue Res. 1987;247:215–225. doi: 10.1007/BF00216564. [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Zhang YH, Hendershot LM, et al. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Bi M, Naczki C, Koritzinsky M, et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24:3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi AB, Fischer SM, Robles AI, et al. Overexpression of cyclin D1 in mouse skin carcinogenesis. Oncogene. 1993;8:1127–1133. [PubMed] [Google Scholar]
- Booth L, Cazanave SC, Hamed HA et al (2012) OSU-03012 suppresses GRP78/BiP expression that causes PERK-dependant increases in tumor cell killing. Cancer Biol Ther 13:224–36 [DOI] [PMC free article] [PubMed]
- Booth L, Cruickshanks N, Ridder T et al (2012) OSU-03012 interacts with lapatinib to kill brain cancer cells. Cancer Bio Ther 13:1501–1511 [DOI] [PMC free article] [PubMed]
- Booth L, Roberts JL, Cash DR, et al. GRP78/BiP/HSPA5/Dna K is a universal therapeutic target for human disease. J Cell Physiol. 2015;230:1661–1676. doi: 10.1002/jcp.24919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth L, Roberts JL, Cruickshanks N, et al. Phosphodiesterase 5 inhibitors enhance chemotherapy killing in gastrointestinal/genitourinary cancer cells. Mol Pharmacol. 2014;85:408–419. doi: 10.1124/mol.113.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth L, Roberts JL, Cruickshanks N, et al. Regulation of OSU-03012 toxicity by ER stress proteins and ER stress-inducing drugs. Mol Cancer Ther. 2014;13:2384–2398. doi: 10.1158/1535-7163.MCT-14-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth L, Roberts JL, Tavallai M, et al. OSU-03012 and Viagra treatment inhibits the activity of multiple chaperone proteins and disrupts the blood–brain barrier: implications for anti-cancer therapies. J Cell Physiol. 2015;230:1982–1998. doi: 10.1002/jcp.24977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth L, Roberts JL, Poklepovic A at al (2017) PDE5 inhibitors enhance the lethality of pemetrexed through inhibition of multiple chaperone proteins and via the actions of cyclic GMP and nitric oxide. Oncotarget 8:1449–1468 [DOI] [PMC free article] [PubMed]
- Brewer JW, Hendershot LM, Sherr CJ, et al. Mammalian unfolded protein response inhibits cyclin D1 translation and cell-cycle progression. Proc Natl Acad Sci. 1999;96:8505–8510. doi: 10.1073/pnas.96.15.8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozzi F, Gerlo S, Grieco FA, et al. Ubiquitin D regulates IRE1alpha/c-Jun N-terminal kinase (JNK) protein-dependent apoptosis in pancreatic beta cells. J Biol Chem. 2016;291:12040–12056. doi: 10.1074/jbc.M115.704619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Buontempo F, Orsini E, Lonetti A, et al. Synergistic cytotoxic effects of bortezomib and CK2 inhibitor CX-4945 in acute lymphoblastic leukemia: turning off the prosurvival ER chaperone BIP/Grp78 and turning on the proapoptotic NF-κB. Oncotarget. 2016;7:1323–1340. doi: 10.18632/oncotarget.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell RW, Lomas DA. Conformational disease. Lancet. 1997;350:134–138. doi: 10.1016/S0140-6736(97)02073-4. [DOI] [PubMed] [Google Scholar]
- Cha YI, DuBois RN. NSAIDs and cancer prevention: targets downstream of COX-2. Annu Rev Med. 2007;58:239–252. doi: 10.1146/annurev.med.57.121304.131253. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Ghosh S, Banerjee B, et al. Mephebrindole, a synthetic indole analog coordinates the crosstalk between p38MAPK and eIF2α/ATF4/CHOP signaling pathways for induction of apoptosis in human breast carcinoma cells. Apoptosis. 2016;21:1106–1124. doi: 10.1007/s10495-016-1268-8. [DOI] [PubMed] [Google Scholar]
- Chaux P. Dendritic cells and immune function in cancer. Pathol Biol (Paris) 1995;43:897–903. [PubMed] [Google Scholar]
- Chen CH, Shaikenov T, Peterson TR, et al. ER stress inhibits mTORC2 and Akt signaling through GSK-3beta-mediated phosphorylation of rictor. Sci Signal. 2011;4:ra10. doi: 10.1126/scisignal.2001731. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Weiler S, Flavell RA, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Choy MS, Yusoff P, Lee IC, et al. Structural and functional analysis of the GADD34:PP1 eIF2α phosphatase. Cell Rep. 2015;11:1885–1891. doi: 10.1016/j.celrep.2015.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavarino G, Claudio N, Couderc T, et al. Induction of GADD34 is necessary for dsRNA-dependent interferon-beta production and participates in the control of chikungunya virus infection. PLoS Pathog. 2012;8:e1002708. doi: 10.1371/journal.ppat.1002708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc S, Hirsch C, Oggier DM, et al. Htm1 protein generates the N-glycan signal for glycoprotein degradation in the endoplasmic reticulum. J Cell Biol. 2009;184:159–172. doi: 10.1083/jcb.200809198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012;18:59–68. doi: 10.1016/j.molmed.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. Phosphatidylinositol transfer proteins couple lipid transport to phosphoinositide synthesis. Semin Cell Dev Biol. 2001;12:183–191. doi: 10.1006/scdb.2000.0235. [DOI] [PubMed] [Google Scholar]
- Corbin JD. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int J Impot Res. 2004;16(Suppl 1):S4–S7. doi: 10.1038/sj.ijir.3901205. [DOI] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Molecular pathways: immunosuppressive roles of IRE1a-XBP1 signaling in dendritic cells of the tumor microenvironment. Clin Cancer Res. 2016;22:2121–2126. doi: 10.1158/1078-0432.CCR-15-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell. 2015;161:1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshmand S, Quek ML, Lin E et al (2007) Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol 38:1547–1552 [DOI] [PubMed]
- Dangelmaier C, Manne BK, Liverani E, et al. PDK1 selectively phosphorylates Thr(308) on Akt and contributes to human platelet functional responses. Thromb Haemost. 2014;111:508–517. doi: 10.1160/TH13-06-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar AC, Dever TE, Sicheri F. Higher-order substrate recognition of eIF2alpha by the RNA-dependent protein kinase PKR. Cell. 2005;122:887–900. doi: 10.1016/j.cell.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Darling NJ, Cook SJ. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim Biophys Acta. 2014;1843:2150–2163. doi: 10.1016/j.bbamcr.2014.01.009. [DOI] [PubMed] [Google Scholar]
- De Ridder GG, Gonzalez-Gronow M, Ray R, et al. Autoantibodies against cell surface GRP78 promote tumor growth in a murine model of melanoma. Melanoma Res. 2011;21:35–43. doi: 10.1097/CMR.0b013e3283426805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ridder GG, Ray R, Pizzo SV. A murine monoclonal antibody directed against the carboxyl-terminal domain of GRP78 suppresses melanoma growth in mice. Melanoma Res. 2012;22:225–235. doi: 10.1097/CMR.0b013e32835312fd. [DOI] [PubMed] [Google Scholar]
- Dersh D, Jones SM, Eletto D, et al. OS-9 facilitates turnover of nonnative GRP94 marked by hyperglycosylation. Mol Biol Cell. 2014;25:2220–2234. doi: 10.1091/mbc.E14-03-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey M, Cao C, Dar AC, et al. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2α substrate recognition. Cell. 2005;122:901–913. doi: 10.1016/j.cell.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon AS, Hagan S, Rath O, et al. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Donnellan R, Chetty R. Cyclin D1 and human neoplasia. Mol Pathol. 1998;51:1. doi: 10.1136/mp.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobnjak M, Osman I, Scher HI, et al. Overexpression of cyclin D1 is associated with metastatic prostate cancer to bone. Clin Cancer Res. 2000;6:1891–1895. [PubMed] [Google Scholar]
- Drummond DA, Wilke CO. The evolutionary consequences of erroneous protein synthesis. Nat Rev Genet. 2009;10:715–724. doi: 10.1038/nrg2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efferth T, Li PCH, Konkimalla VSB, et al. From traditional Chinese medicine to rational cancer therapy. Trends Mol Med. 2007;13:353–361. doi: 10.1016/j.molmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Epple LM, Dodd RD, Merz AL, et al. Induction of the unfolded protein response drives enhanced metabolism and chemoresistance in glioma cells. PLoS One. 2013;8:e73267. doi: 10.1371/journal.pone.0073267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CX, Yang Y, Liu Y, et al. Icariin displays anticancer activity against human esophageal cancer cells via regulating endoplasmic reticulum stress-mediated apoptotic signaling. Sci Rep. 2016;6:21145. doi: 10.1038/srep21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink AL. Protein aggregation: folding aggregates, inclusion bodies and amyloid. Fold Des. 1998;3:R9–R23. doi: 10.1016/S1359-0278(98)00002-9. [DOI] [PubMed] [Google Scholar]
- Flis VV, Daum G. Lipid transport between the endoplasmic reticulum and mitochondria. Cold Spring Harb Perspect Biol. 2013;5:a013235. doi: 10.1101/cshperspect.a013235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu WJ, Wu XY, Li JC, et al. Upregulation of GRP78 in renal cell carcinoma and its significance. Urology. 2010;75:603–607. doi: 10.1016/j.urology.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Gauss R, Jarosch E, Sommer T, et al. A complex of Yos9p and the HRD ligase integrates endoplasmic reticulum quality control into the degradation machinery. Nat Cell Biol. 2006;8:849–854. doi: 10.1038/ncb1445. [DOI] [PubMed] [Google Scholar]
- Gautschi O, Ratschiller D, Gugger M, et al. Cyclin D1 in non-small cell lung cancer: a key driver of malignant transformation. Lung Cancer. 2007;55:1–14. doi: 10.1016/j.lungcan.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Giampietri C, Petrungaro S, Conti S, et al. Cancer microenvironment and endoplasmic reticulum stress response. Mediat Inflamm. 2015;2015:417281. doi: 10.1155/2015/417281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R. The protein translocation apparatus of the rough endoplasmic reticulum, its associated proteins, and the mechanism of translocation. Curr Opin Cell Biol. 1991;3:580–584. doi: 10.1016/0955-0674(91)90026-u. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gronow M, Gomez CF, De Ridder GG, et al. Binding of tissue-type plasminogen activator to the glucose-regulated protein 78 (GRP78) modulates plasminogen activation and promotes human. J Biol Chem. 2014;289:25166–25176. doi: 10.1074/jbc.M114.589341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TR, Scott PA, Emr SD. Brefeldin A reversibly blocks early but not late protein transport steps in the yeast secretory pathway. EMBO J. 1993;12:869. doi: 10.1002/j.1460-2075.1993.tb05727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoreva TA, Tribulovich VG, Garabadzhiu AV, et al. The 26S proteasome is a multifaceted target for anti-cancer therapies. Oncotarget. 2015;6:24733–24749. doi: 10.18632/oncotarget.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootjans J, Kaser A, Kaufman RJ, et al. The unfolded protein response in immunity and inflammation. Nat Rev Immunol. 2016;16:469–484. doi: 10.1038/nri.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Back SH, Hur J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Kumagai K, Yasuda S, et al. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Hayakawa R, Hayakawa T, Takeda K, et al. Therapeutic targets in the ASK1-dependent stress signaling pathways. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88:434–453. doi: 10.2183/pjab.88.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazari YM, Bashir A, Haq E, et al. Emerging tale of UPR and cancer: an essentiality for malignancy. Tumor Biol. 2016;37:14381–14390. doi: 10.1007/s13277-016-5343-0. [DOI] [PubMed] [Google Scholar]
- Hegedus C, Ozvegy-Laczka C, Apati A, et al. Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties. Br J Pharmacol. 2009;158:1153–1164. doi: 10.1111/j.1476-5381.2009.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot L. The mammalian endoplasmic reticulum as a sensor for cellular stress. Cell Stress Chaperones. 2002;7:222–229. doi: 10.1379/1466-1268(2002)007<0222:tmeraa>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Bernasconi P, Fisher J, et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- Hetz C, Bertrand M. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci. 2014;15:233–249. doi: 10.1038/nrn3689. [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG, Natarajan K. Gcn4p, a master regulator of gene expression, is controlled at multiple levels by diverse signals of starvation and stress. Eukaryot Cell. 2002;1:22–32. doi: 10.1128/EC.01.1.22-32.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch C, Gauss R, Horn Sabine C, et al. The ubiquitylation machinery of the endoplasmic reticulum. Nature. 2009;458:453. doi: 10.1038/nature07962. [DOI] [PubMed] [Google Scholar]
- Hosoi T, Hyoda K, Okuma Y, et al. Akt up- and downregulation in response to endoplasmic reticulum stress. Brain Res. 2007;1152:27–31. doi: 10.1016/j.brainres.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Irie KF, Takagi MF, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Imagawa Y, Hosoda A, Sasaka SI, et al. RNase domains determine the functional difference between IRE1α and IRE1β. FEBS Lett. 2008;582:656–660. doi: 10.1016/j.febslet.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Inki K, Xu W, John CR. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- Jason Y, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 2016;143:3050. doi: 10.1242/dev.137075. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zhou Y, Zheng Y, et al. Expression of inositol-requiring enzyme 1β is downregulated in colorectal cancer. Oncol Lett. 2017;13:1109–1118. doi: 10.3892/ol.2017.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE. The ubiquitin-proteasome system: opportunities for therapeutic intervention in solid tumors. Endocr Relat Cancer. 2015;22:T1–T17. doi: 10.1530/ERC-14-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamińska K, Czarnecka AM, Escudier B, et al. Interleukin-6 as an emerging regulator of renal cell cancer. Urol Oncol. 2015;33:476–485. doi: 10.1016/j.urolonc.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ, Malhotra JD. Calcium trafficking integrates endoplasmic reticulum function with mitochondrial bioenergetics. Biochim Biophys Acta. 2014;1843:2233–2239. doi: 10.1016/j.bbamcr.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- Kelley WL, Georgopoulos C. Chaperones and protein folding. Curr Opin Cell Biol. 1992;4:984–991. doi: 10.1016/0955-0674(92)90130-5. [DOI] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, et al. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kim JY, Heo SH, Song IH, et al. Activation of the PERK-eIF2 pathway is associated with tumor-infiltrating lymphocytes in HER2-positive breast cancer. Anticancer Res. 2016;36:2705–2711. [PubMed] [Google Scholar]
- Kim S, Joe Y, Kim HJ, et al. Endoplasmic reticulum stress-induced IRE1alpha activation mediates cross-talk of GSK-3beta and XBP-1 to regulate inflammatory cytokine production. J Immunol. 2015;194:4498–4506. doi: 10.4049/jimmunol.1401399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee M, Pallauf K, Alcala S, et al. Mitochondrial apoptosis induced by BH3-only molecules in the exclusive presence of endoplasmic reticular Bak. EMBO J. 2009;28:1757–1768. doi: 10.1038/emboj.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G. Better understanding of the biology of cancer cells. Ugeskr Laeger. 2000;162:5199–5204. [PubMed] [Google Scholar]
- Koumenis C. ER stress, hypoxia tolerance and tumor progression. Curr Mol Med. 2006;6:55–69. doi: 10.2174/156652406775574604. [DOI] [PubMed] [Google Scholar]
- Krishn SR, Kaur S, Smith LM, et al. Mucins and associated glycan signatures in colon adenoma-carcinoma sequence: prospective pathological implication(s) for early diagnosis of colon cancer. Cancer Lett. 2016;374:304–314. doi: 10.1016/j.canlet.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R, Feith M, Siewert JR, et al. Expression and clinical significance of glucose regulated proteins GRP78 (BiP) and GRP94 (GP96) in human adenocarcinomas of the esophagus. BMC Cancer. 2008;8:70–70. doi: 10.1186/1471-2407-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lars E, Ari H. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- Lee MCS, Miller EA, Goldberg J, et al. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- Li YM, Guo YS, Tang J, et al. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin. 2014;46:629–640. doi: 10.1093/abbs/gmu048. [DOI] [PubMed] [Google Scholar]
- Li ZJ, Yao C, Liu SF, et al. Cytotoxic effect of icaritin and its mechanisms in inducing apoptosis in human burkitt lymphoma cell line. Biomed Res Int. 2014;2014:391512. doi: 10.1155/2014/391512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- Lim S, Park SG, Yoo JH et al (2005) Expression of heat shock proteins (HSP27, HSP60, HSP70, HSP90,GRP78, GRP94) in hepatitis B virus-related hepatocellular carcinomas and dysplastic nodules. World J Gastroenterol 11:2072–2079 [DOI] [PMC free article] [PubMed]
- Lin LH, Lin ST, Chou HC. Role of asparagine synthetase in doxorubicin-induced resistance. Biomark Genomic Med. 2013;5:100–102. [Google Scholar]
- Liu J, Xiao M, Li J, et al. Activation of UPR signaling pathway is associated with the malignant progression and poor prognosis in prostate cancer. Prostate. 2017;77:274–281. doi: 10.1002/pros.23264. [DOI] [PubMed] [Google Scholar]
- Liu M, Dudley SC. Role for the unfolded protein response in heart disease and cardiac arrhythmias. Int J Mol Sci. 2016;17:52. doi: 10.3390/ijms17010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- Martino MB, Jones L, Brighton B, et al. The ER stress transducer IRE1β is required for airway epithelial mucin production. Mucosal Immunol. 2013;6:639–654. doi: 10.1038/mi.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Gray MJ, Yang DY et al (2013) The endoplasmic reticulum stress marker, glucose-regulated protein-78 (GRP78) in visceral adipocytes predicts endometrial cancer progression and patient survival. Gynecol Oncol 128:552–559 [DOI] [PMC free article] [PubMed]
- Mattoo RU, Sharma SK, Priya S, et al. Hsp110 is a bona fide chaperone using ATP to unfold stable misfolded polypeptides and reciprocally collaborate with Hsp70 to solubilize protein aggregates. J Biol Chem. 2013;288:21399–21411. doi: 10.1074/jbc.M113.479253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, et al. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meer GV. Transport and sorting of membrane lipids. Curr Opin Cell Biol. 1993;5:661–673. doi: 10.1016/0955-0674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- Mintz PJ, Kim J, Do KA, et al. Fingerprinting the circulating repertoire of antibodies from cancer patients. Nat Biotechnol. 2003;21:57–63. doi: 10.1038/nbt774. [DOI] [PubMed] [Google Scholar]
- Misra UK, Pizzo SV. Ligation of cell surface GRP78 with antibody directed against the COOH-terminal domain of GRP78 suppresses Ras/MAPK and PI 3-kinase/AKT signaling while promoting caspase activation in human prostate cancer cells. Cancer Biol Ther. 2010;9:142–152. doi: 10.4161/cbt.9.2.10422. [DOI] [PubMed] [Google Scholar]
- Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Mumm JB, Oft M. Cytokine-based transformation of immune surveillance into tumor-promoting inflammation. Oncogene. 2008;27:5913–5919. doi: 10.1038/onc.2008.275. [DOI] [PubMed] [Google Scholar]
- Nakamura D, Tsuru A, Ikegami K, et al. Mammalian ER stress sensor IRE1β specifically down-regulates the synthesis of secretory pathway proteins. FEBS Lett. 2011;585:133–138. doi: 10.1016/j.febslet.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Ni K, O’Neill HC (1997) The role of dendritic cells in T cell activation. Immunol Cell Biol 75:223–230 [DOI] [PubMed]
- Ni M, Zhou H, Wey S, et al. Regulation of PERK signaling and leukemic cell survival by a novel cytosolic isoform of the UPR regulator GRP78/BiP. PLoS One. 2009;4:e6868. doi: 10.1371/journal.pone.0006868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitoh H, Matsuzawa A, Tobiume K, et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Z, Wang M, Zhou L, et al. Elevated GRP78 expression is associated with poor prognosis in patients with pancreatic cancer. Sci Rep. 2015;5:16067. doi: 10.1038/srep16067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh MR, Kim JL, Han SJ, et al. C/EBP homologous protein (CHOP) gene deficiency attenuates renal ischemia/reperfusion injury in mice. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2015;1852:1895–1901. doi: 10.1016/j.bbadis.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Obeng EA, Carlson LM, Gutman DM, et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio F, Tavernier SJ, Hoffmann E, et al. The unfolded-protein-response sensor IRE-1[alpha] regulates the function of CD8[alpha]+ dendritic cells. Nat Immunol. 2014;15:248–257. doi: 10.1038/ni.2808. [DOI] [PubMed] [Google Scholar]
- Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17:93–115. doi: 10.1038/nrc.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Blackstone C. Further assembly required: construction and dynamics of the endoplasmic reticulum network. EMBO Rep. 2010;11:515–521. doi: 10.1038/embor.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr Opin Cell Biol. 2001;13:349–355. doi: 10.1016/s0955-0674(00)00219-2. [DOI] [PubMed] [Google Scholar]
- Patrizia A, Afshin S. Endoplasmic reticulum stress in health and disease. Netherlands: Springer; 2012. [Google Scholar]
- Piperi C, Adamopoulos C, Papavassiliou AG. XBP1: a pivotal transcriptional regulator of glucose and lipid metabolism. Trends Endocrinol Metab. 2016;27:119–122. doi: 10.1016/j.tem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 2014;4:64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter KR, Claude A, Fullam EF. A study of tissue culture cells by electronic microscopy: methods and preliminary observasions. J Exp Med. 1945;81:233. doi: 10.1084/jem.81.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrko P, Schönthal AH, Hofman FM, et al. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–9816. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- Roberts JL, Booth L, Conley A, et al. PDE5 inhibitors enhance the lethality of standard of care chemotherapy in pediatric CNS tumor cells. Cancer Biol Ther. 2014;15:758–767. doi: 10.4161/cbt.28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roller C, Maddalo D. The molecular chaperone GRP78/BiP in the development of chemoresistance: mechanism and possible treatment. Front Pharmacol. 2013;4:10. doi: 10.3389/fphar.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiano A, Foresti O, Carvalho P. ER-associated degradation: protein quality control and beyond. J Cell Biol. 2014;204:869. doi: 10.1083/jcb.201312042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski DT, Hegde RS. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J Cell Biol. 2010;189:783–794. doi: 10.1083/jcb.201003138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D, Carberry S, Murphy ÁC, et al. Calnexin, an ER-induced protein, is a prognostic marker and potential therapeutic target in colorectal cancer. J Transl Med. 2016;14:196. doi: 10.1186/s12967-016-0948-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibil H. Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol. 2013;14:630–642. doi: 10.1038/nrm3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J, Kuismanen E. Pathways of protein sorting and membrane traffic between the rough endoplasmic reticulum and the Golgi complex. Semin Cell Biol. 1992;3:343–355. doi: 10.1016/1043-4682(92)90020-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Yoshida H. Organelle autoregulation-stress responses in the ER, Golgi, mitochondria and lysosome. J Biochem. 2015;157:185–195. doi: 10.1093/jb/mvv010. [DOI] [PubMed] [Google Scholar]
- Satoh T, Chen Y, Hu D, et al. Structural basis for oligosaccharide recognition of misfolded glycoproteins by OS-9 in ER-associated degradation. Mol Cell. 2010;40:905–916. doi: 10.1016/j.molcel.2010.11.017. [DOI] [PubMed] [Google Scholar]
- Schewe DM, Aguirre-Ghiso JA. ATF6alpha-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proc Natl Acad Sci U S A. 2008;105:10519–10524. doi: 10.1073/pnas.0800939105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MA, Schwartz GK. Cell cycle-mediated drug resistance: an emerging concept in cancer therapy. Clin Cancer Res. 2001;7:2168–2181. [PubMed] [Google Scholar]
- Sharma SK, De los Rios P, Christen P, et al. The kinetic parameters and energy cost of the Hsp70 chaperone as a polypeptide unfoldase. Nat Chem Biol. 2010;6:914–920. doi: 10.1038/nchembio.455. [DOI] [PubMed] [Google Scholar]
- Shen JS, Chen X, Hendershot L, et al. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- Shen KY, Johnson DW, Gobe GC. The role of cGMP and its signaling pathways in kidney disease. Am J Physiol Renal Physiol. 2016;311:F671–Ff81. doi: 10.1152/ajprenal.00042.2016. [DOI] [PubMed] [Google Scholar]
- Shen X, Xue Y, Si Y, et al. The unfolded protein response potentiates epithelial-to-mesenchymal transition (EMT) of gastric cancer cells under severe hypoxic conditions. Med Oncol. 2015;32:1–7. doi: 10.1007/s12032-014-0447-0. [DOI] [PubMed] [Google Scholar]
- Shimizu A, Kaira K, Yasuda M, et al. Clinical and pathological significance of ER stress marker (BiP/GRP78 and PERK) expression in malignant melanoma. Pathol Oncol Res. 2017;23:111–116. doi: 10.1007/s12253-016-0099-9. [DOI] [PubMed] [Google Scholar]
- Shimodaira Y, Takahashi S, Kinouchi Y, et al. Modulation of endoplasmic reticulum (ER) stress-induced autophagy by C/EBP homologous protein (CHOP) and inositol-requiring enzyme 1α (IRE1α) in human colon cancer cells. Biochem Biophys Res Commun. 2014;445:524–533. doi: 10.1016/j.bbrc.2014.02.054. [DOI] [PubMed] [Google Scholar]
- Shiu RP, Pouyssegur J, Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977;74:3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuda M, Kondoh N, Imazeki N, et al. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J Hepatol. 2003;38:605–614. doi: 10.1016/s0168-8278(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Sidera K, Patsavoudi E. HSP90 inhibitors: current development and potential in cancer therapy. Recent Pat Anticancer Drug Discov. 2014;9:1–20. [PubMed] [Google Scholar]
- Simon S. Translocation of proteins across the endoplasmic reticulum. Curr Opin Cell Biol. 1993;5:581–588. doi: 10.1016/0955-0674(93)90126-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhagen F, McFarland AP, Rodriguez LG, et al. IRF-5 and NF-κB p50 co-regulate IFN-β and IL-6 expression in TLR9-stimulated human plasmacytoid dendritic cells: innate immunity. Eur J Immunol. 2013;43:1896–1906. doi: 10.1002/eji.201242792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm M, Sheng X, Arnoldussen YJ, et al. Prostate cancer and the unfolded protein response. Oncotarget. 2016;7:54051–54066. doi: 10.18632/oncotarget.9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Yoshida H. Endoplasmic reticulum stress in kidney function and disease. Curr Opin Nephrol Hypertens. 2015;24:345–350. doi: 10.1097/MNH.0000000000000141. [DOI] [PubMed] [Google Scholar]
- Teske BF, Baird TD, Wek RC. Methods for analyzing eIF2 kinases and translational control in the unfolded protein response. Methods Enzymol. 2011;490:333–356. doi: 10.1016/B978-0-12-385114-7.00019-2. [DOI] [PubMed] [Google Scholar]
- Testa JR, Tsichlis PN. AKT signaling in normal and malignant cells. Oncogene. 2005;24:7391–7393. doi: 10.1038/sj.onc.1209100. [DOI] [PubMed] [Google Scholar]
- Tsai HY, Yang YF, Wu AT, et al. Endoplasmic reticulum ribosome-binding protein 1 (RRBP1) overexpression is frequently found in lung cancer patients and alleviates intracellular stress-induced apoptosis through the enhancement of GRP78. Oncogene. 2013;32:4921–4931. doi: 10.1038/onc.2012.514. [DOI] [PubMed] [Google Scholar]
- Tsuru A, Fujimoto N, Takahashi S, et al. Negative feedback by IRE1beta optimizes mucin production in goblet cells. Proc Natl Acad Sci U S A. 2013;110:2864–2869. doi: 10.1073/pnas.1212484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu K, Ogata S, Nakanishi K, et al. Glucose-regulated protein 78 expression in urothelial carcinoma of the upper urinary tract. BJU Int. 2010;106:873–878. doi: 10.1111/j.1464-410X.2009.09144.x. [DOI] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- Verfaillie T, Jäger R, Samali A, et al. ER stress signaling pathways in cell survival and death. In: Patrizia A, Samali A, et al., editors. Endoplasmic reticulum stress in health and disease. Netherlands: Springer; 2012. pp. 41–73. [Google Scholar]
- Voges D, Zwickl P, Baumeister W. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem. 1999;68:1015–1068. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- Wang Q, He ZZ, Zhang JH et al (2005) Overexpression of endoplasmic reticulum molecular chaperone GRP94 and GRP78 in human lung cancer tissues and its significance. Cancer Detect Prev 29:544–551 [DOI] [PubMed]
- Walter P, David R. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Takeda T, Yamaguchi O et al (2005) Apoptosis signal-regulating kinase 1 is involved not only in apoptosis but also in non-apoptotic cardiomyocyte death. Biochem Biophys Res Commun [DOI] [PubMed]
- Webb T, Carter J, Roberts JL et al (2015) Celecoxib enhances [sorafenib + sildenafil] lethality in cancer cells and reverts platinum chemotherapy resistance. Cancer Biol Ther 16:1660–1670 [DOI] [PMC free article] [PubMed]
- Westrate LM, Lee JE, Prinz WA, et al. Form follows function: the importance of endoplasmic reticulum shape. Annu Rev Biochem. 2015;84:791–811. doi: 10.1146/annurev-biochem-072711-163501. [DOI] [PubMed] [Google Scholar]
- Woodgett JR. Physiological roles of glycogen synthase kinase-3: potential as a therapeutic target for diabetes and other disorders. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:281–290. doi: 10.2174/1568008033340153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia F, Xu JC, Zhang P et al (2014) Glucose-regulated protein 78 and heparanase expression in oral squamous cell carcinoma: correlations and prognostic significance. World J Surg Oncol 12:121 [DOI] [PMC free article] [PubMed]
- Yang F, Tang XY, Liu H, et al. Inhibition of mitogen-activated protein kinase signaling pathway sensitizes breast cancer cells to endoplasmic reticulum stress-induced apoptosis. Oncol Rep. 2016;35:2113–2120. doi: 10.3892/or.2016.4580. [DOI] [PubMed] [Google Scholar]
- Yang G, Murashige DS, Humphrey SJ, et al. A positive feedback loop between Akt and mTORC2 via SIN1 phosphorylation. Cell Rep. 2015;12:937–943. doi: 10.1016/j.celrep.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Yang G, Yang X. Smad4-mediated TGF-beta signaling in tumorigenesis. Int J Biol Sci. 2010;6:1–8. doi: 10.7150/ijbs.6.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res. 2004;92:13–27. doi: 10.1016/S0065-230X(04)92002-7. [DOI] [PubMed] [Google Scholar]
- Youssef EM, Hasuma T, Morishima Y, et al. Overexpression of cyclin D1 in rat esophageal carcinogenesis model. Jpn J Cancer Res. 1997;88:18–25. doi: 10.1111/j.1349-7006.1997.tb00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavilgelsky GB, Kotova VY, Mazhul MM, et al. Role of Hsp70 (DnaK-DnaJ-GrpE) and Hsp100 (ClpA and ClpB) chaperones in refolding and increased thermal stability of bacterial luciferases in Escherichia coli cells. Biochemistry (Mosc) 2002;67:986–992. doi: 10.1023/a:1020565701210. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Wang WW, Ren LH, et al. The mTORC2/Akt/NFκB pathway-mediated activation of TRPC6 participates in adriamycin-induced podocyte apoptosis. Cell Physiol Biochem. 2016;40:1079–1093. doi: 10.1159/000453163. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yang Y, Yao F, et al. Unfolded protein response promotes doxorubicin-induced nonsmall cell lung cancer cells apoptosis via the mTOR pathway inhibition. Cancer Biother Radiopharm. 2016;31:347–351. doi: 10.1089/cbr.2016.2079. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Zhang Y, Wang P, et al. V8 induces apoptosis and the endoplasmic reticulum stress response in human multiple myeloma RPMI 8226 cells via the PERK-eIF2alpha-ATF4 signaling pathway. Oncol Lett. 2016;12:2702–2709. doi: 10.3892/ol.2016.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Huang JW, Tseng PH, et al. From the cyclooxygenase-2 inhibitor celecoxib to a novel class of 3-phosphoinositide-dependent protein kinase-1 inhibitors. Cancer Res. 2004;64:4309–4318. doi: 10.1158/0008-5472.CAN-03-4063. [DOI] [PubMed] [Google Scholar]
- Zhu JF, Li ZJ, Zhang GS, et al. Icaritin shows potent anti-leukemia activity on chronic myeloid leukemia in vitro and in vivo by regulating MAPK/ERK/JNK and JAK2/STAT3/AKT signalings. PLoS One. 2011;6:e23720. doi: 10.1371/journal.pone.0023720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang LQ, Scolyer RA, Lee CS, et al. Expression of glucose-regulated stress protein GRP78 is related to progression of melanoma. Histopathology. 2009;54:462–470. doi: 10.1111/j.1365-2559.2009.03242.x. [DOI] [PubMed] [Google Scholar]