Abstract
Extracellular Hsp70 (eHsp70) can act as damage-associated molecular pattern (DAMP) via Toll-like receptors TLR2 and TLR4, and stimulate immune and inflammatory responses leading to sterile inflammation and propagation of already existing inflammation. It was found elevated in the blood of patients with chronic obstructive pulmonary disease (COPD), who might suffer occasional bacterial colonizations and infections. We used a monocytic THP-1 cell line as a cellular model of systemic compartment of COPD to assess inflammatory effects of eHsp70 when present alone or together with bacterial products lypopolysaccharide (LPS) and lypoteichoic acid (LTA). THP-1 cells were differentiated into macrophage-like cells and treated with various concentrations of recombinant human Hsp70 protein (rhHsp70), LPS (TLR4 agonist), LTA (TLR2 agonist), and their combinations for 4, 12, 24, and 48 h. Concentrations of IL-1α, IL-6, IL-8, and TNF-α were determined by ELISA. Cell viability was assessed by MTS assay, and mode of cell death by luminometric measurements of caspases-3/7, -8, and -9 activities. rhHsp70 showed cell protecting effect by suppressing caspases-3/7 activation, while LPS provoked cytotoxicity through caspases-8 and -3/7 pathway. Regarding inflammatory processes, rhHsp70 alone induced secretion of IL-1α and IL-8, but had modulatory effects on release of all four cytokines when applied together with LPS or LTA. Combined effect with LPS was mainly synergistic, and with LTA mainly antagonistic, although it was cytokine- and time-dependent. Our results confirmed pro-inflammatory function of extracellular Hsp70, and suggest its possible implication in COPD exacerbations caused by bacterial infection through desensitization or inappropriate activation of TLR2 and TLR4 receptors.
Keywords: THP-1 cells, Extracellular Hsp70, LPS, LTA, COPD
Introduction
Heat shock protein 70 (Hsp70) is a stress-inducible 72-kDa intracellular protein with functions in prevention of protein aggregation, facilitation of protein refolding, and chaperoning of proteins. Its expression is up-regulated in various states of physiological and environmental stresses, such as infections, inflammation, cellular injury, or heat stress (Pockley et al. 2008). In the case of elevated cellular stress, Hsp70 can also be released from cells. Different mechanisms have been proposed, including translocation across the plasma membrane and release associated with lipid vesicles, an endolysosomal pathway, and the passive release after cell death by necrosis (Johnson and Fleshner 2006). Extracellular Hsp70 (eHsp70) then acts as damage-associated molecular pattern (DAMP), “danger signal” for the immune system. eHsp70 has chaperone and cytokine regulating activity (chaperokine) and can stimulate immune and inflammatory responses leading to sterile inflammation and propagation of already existing inflammation (Asea et al. 2002; Vabulas et al. 2002; Pouwels et al. 2014). eHsp70 has been shown to activate the host innate immune and inflammatory responses by engaging TLR2 and TLR4 receptors, via signaling pathways dependent on myeloid differentiation factor 88 (MyD88), which in turn activate nuclear factor κB (NF-κB) and mitogen-activated protein kinases (MAPK), causing release of pro-inflammatory cytokines (Midwood and Piccinini 2010; Giuliano et al. 2011). People suffering from a variety of inflammatory and infective diseases have chronically elevated basal levels of eHsp70 relative to healthy, aged-matched controls (Pockley 2003). In addition, an increase of eHsp70 in the blood was reported in healthy organisms after exposure to acute stressors (Johnson and Fleshner 2006). It was suggested that the release of Hsp70 could aggravate an already active host immune response, thereby leading to poor outcome. Alternatively, eHsp70 could serve a cytoprotective function at lower levels as a normal response to infection or stress, and once a certain critical threshold is surpassed, it could potentiate the dysregulated inflammatory response (Giuliano et al. 2011). Thus, it is possible that in vivo function of eHsp70 is context-dependent, such that in a normal physiological state eHsp70 facilitates immunity, whereas in a pathophysiological state eHsp70 exacerbates inflammatory diseases (Johnson and Fleshner 2006; Pockley et al. 2008; Giuliano et al. 2011). eHsp70 was found elevated in the blood of patients with chronic obstructive pulmonary disease (COPD) and some other inflammatory diseases (Hacker et al. 2009).
COPD is one of the leading causes of morbidity and mortality in the world, with the prevalence of 12% in individuals over the age of 40 years (Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017). It is characterized by progressive airflow limitation associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases, most common being cigarette smoke. Exacerbations and comorbidities contribute to the overall severity in individual patients. Patients with COPD develop abnormal inflammatory reaction, and its chronic presence can lead to destruction of lung parenchyma (emphysema) and chronic bronchitis (Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017). Besides its pulmonary manifestations, COPD has also a systemic component characterized by chronic inflammation, oxidative stress, activation of immune cells, and elevated concentration of pro-inflammatory cytokines in the blood (Agusti et al. 2003). Patients with COPD are susceptible to occasional bacterial colonizations and infections which can lead to exacerbations and worsening of already active inflammation.
In this study, we used a monocytic THP-1 cell line as a cellular model of systemic compartment of COPD to assess inflammatory effects of eHsp70 in COPD and its interactions with bacterial products lypopolysaccharide (LPS), a TLR4 agonist, and lypoteichoic acid (LTA), a TLR2 agonist, that could potentially lead to the worsening of existing inflammation. The development of inflammation was assessed by measuring concentrations of pro-inflammatory cytokines interleukin (IL)-1α, IL-6, IL-8, and tumor necrosis factor (TNF)-α secreted into the cell culture media.
Materials and methods
Cell culture
THP-1 monocytic cell line (American Type Culture Collection, USA) was cultured in an RPMI 1640 medium containing 10% fetal bovine serum, 2 mM L-glutamine, 1 mM pyruvate, and 1% penicillin and streptomycin, at 37 °C in a humidified 5% CO2 atmosphere. Cells were grown in 75-cm2 culture flasks, and subcultured every 4 or 5 days by three to five times dilution. Before treatments, cells were seeded on 12- or 96-well plates and induced into macrophage-like cells with 20 nM phorbol 12-myristate 13-acetate (PMA) for 48 h.
Treatments of THP-1 cells
Cells were treated with low endotoxin recombinant human Hsp70 protein (rhHsp70) (Enzo Life Sciences, USA). Endotoxin concentration in rhHsp70 was 2.7 EU/mg, determined by Limulus amebocyte lysate (LAL) test by manufacturer. Equivalent amount of LPS was used as negative control in all experiments. Cells were also treated with LPS (positive control for TLR4 activation) and LTA (positive control for TLR2 activation). LPS was isolated from Escherichia coli O111:B4 (Sigma-Aldrich, USA). LTA was isolated from Staphylococcus aureus (Invivogen, France), and had endotoxin concentration of 10 EU/mg. Concentrations of rhHsp70 (0.3–3 μg/ml), LPS (0.1 μg/ml), and LTA (1 μg/ml) were used in this study based on our preliminary results. Three independent experiments (n = 3) were performed for all analysis (cytokines and viability measurements).
Measurement of cytokine concentration
THP-1 cells were seeded on 12-well cell culture plates (1 × 106 cells per well) and differentiated for 48 h with 20 nM PMA. Cells were treated with rhHsp70, LPS, LTA, and their combinations for 4, 12, 24, and 48 h. Cell medium was harvested and frozen at − 80 °C until analysis.
Concentrations of IL-1α, IL-6, IL-8, and TNF-α were determined in cell supernatants by DuoSet ELISA kits (R&D Systems, USA).
A 96-well plate was coated with 100 μl per well of cytokine specific capture antibody diluted in PBS and incubated overnight at room temperature. Each well was washed three times with 400 μl of washing buffer. The plate was then blocked with 300 μl of reagent diluent and incubated at room temperature for a minimum of 1 h followed by washing. Then, 100 μl of samples or standards was added and incubated at room temperature for 2 h, followed by washing. After that, 100 μl of the specific detection antibody was added and the plate was incubated for 2 h at room temperature. After washing, 100 μl of streptavidin-HRP was added, and the plate was incubated for 20 min at room temperature in the dark, followed by washing. 100 μl of substrate solution (equal volume of hydrogen peroxide and tetramethylbenzidine) was added and the plate was once again incubated for 20 min at room temperature in the dark. Reaction was stopped by adding 50 μl of stop solution (2 N sulfuric acid). The optical density was determined at 450 nm, using a microplate reader (VICTOR3 1420 Multilabel counter, Perkin Elmer).
MTS assay
THP-1 cells were seeded on 96-well cell culture plates (5 × 104 cells per well) and differentiated for 48 h with 20 nM PMA. Cells were treated with rhHsp70, LPS, LTA, and their combinations for 24 h. Afterwards, 20 μl of MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (Promega, USA) reagent was added, and cells were incubated for 1 h in a humidified 5% CO2 atmosphere. Absorbance was measured at 490 nm using a microplate reader (VICTOR3 1420 Multilabel counter, Perkin Elmer, USA).
Caspases activity (caspase-9, -8, -3/7)
THP-1 cells were seeded on white 96-well cell culture plates (2 × 104 cells per well for caspase-3/7, 2.5 × 104 cells per well for caspases-8 and -9) and differentiated for 48 h with 20 nM PMA. Cells were then treated for 2, 4, 6, and 8 h. After incubation, activities of caspases-9, -8, and -3/7 were measured according to the manufactures protocol using the Caspase-Glo® luminescent assay kits (Promega, USA). Briefly, 100 μl of reagent for specific caspase was added to each well and the plate was gently mixed at 300–500 rpm for 30 s. the plate was then incubated at room temperature for 3 h. Luminescence of samples was measured using a microplate reader (VICTOR3 1420 Multilabel counter, Perkin Elmer).
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). Unpaired t test or analysis of variance (ANOVA) followed by post hoc testing by Sidak method were used for statistical analysis. The level of P < 0.05 was considered statistically significant. Data were analyzed using GraphPad Prism 6.01 software (GraphPad Software Inc., USA).
When assessing combined effects of rhHsp70 with LPS and LTA, we compared our measured values with the calculated (expected) values. The values were calculated as the mean value obtained after exposure to one substance alone (rhHsp70) plus the mean value obtained after exposure to the second substance (LPS or LTA) (Šegvić Klarić et al. 2014):
Significant difference between expected (calculated) and measured values was determined using t test. The level of P < 0.05 was considered statistically significant.
The results were as follows: a synergistic effect was implied if the measured values were significantly above the expected values; an antagonistic effect was implied if the measured values were significantly below the expected values.
Results
Inflammatory effects of eHsp70
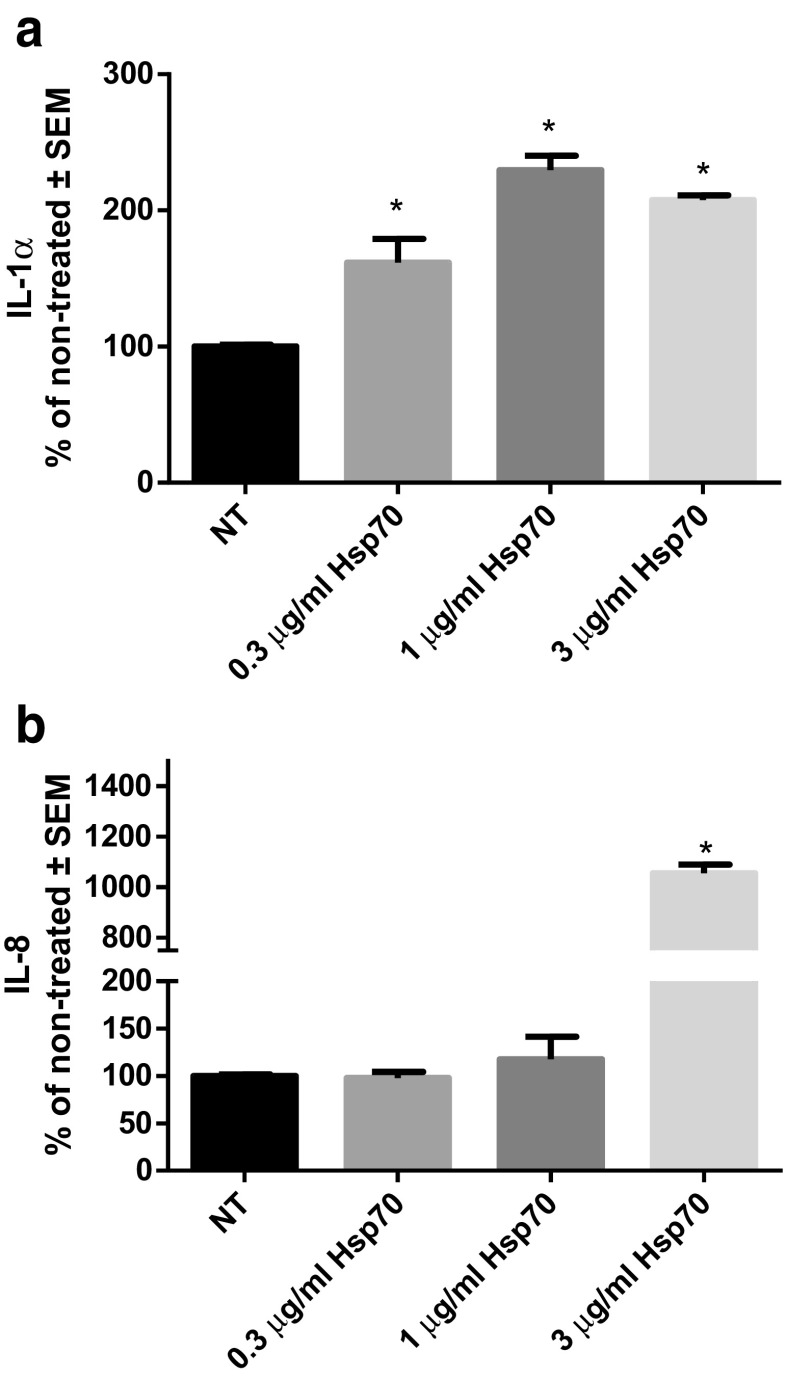
Production of pro-inflammatory cytokines IL-1α, IL-6, IL-8, and TNF-α was measured in differentiated THP-1 cells treated for 24 h with different concentrations of rhHsp70 (0.3, 1, and 3 μg/ml). Cytokine concentrations in cell culture media of non-treated (control) cells are expressed as 100%, and those of treated cells as percentages of concentration of non-treated cells. All tested concentrations of rhHsp70 significantly induced IL-1α secretion (Fig. 1a). The level of IL-8 was increased only after treatment with 3 μg/ml rhHsp70 (Fig. 1b). However, rhHsp70 failed to induce IL-6 and TNF-α (data not shown).
Fig. 1.
Concentration-dependent effects of rhHsp70 on production of IL-1α (a) and IL-8 (b). Data are presented as mean ± SEM and tested by ANOVA followed by multiple comparison Sidak method. NT, non-treated cells. a *P = 0.0097, P = 0.0034, and P = 0.0012, for 0.3, 1, and 3 μg/ml rhHsp70, respectively vs. non-treated cells. b *P < 0.0001 vs. non-treated cells
Modulation of inflammatory effects of LPS and LTA with different eHsp70 concentrations
LPS and LTA are known agonists of TLR4 and TLR2 receptors, respectively. In order to mimic simultaneous bacterial infection and inflammation propagated by eHsp70, we treated cells with 0.1 μg/ml LPS or 1 μg/ml LTA together with various rhHsp70 concentrations (0.3, 1, and 3 μg/ml) for 24 h. Cytokines IL-1α, IL-6, IL-8, and TNF-α were measured in cell supernatants.
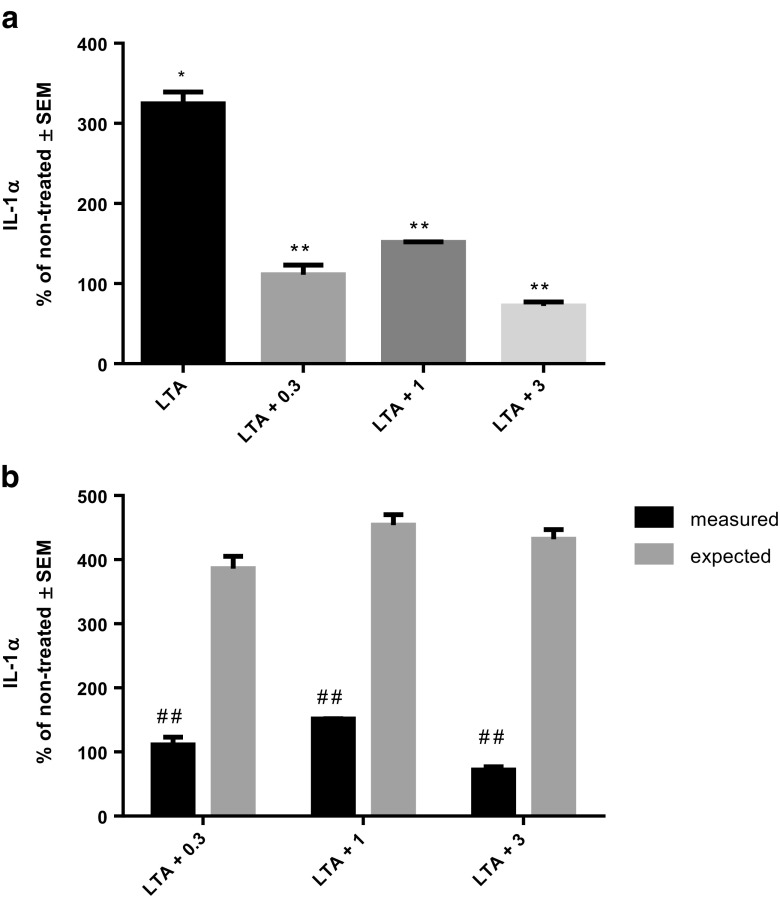
Treatment of THP-1 cells with LTA provoked strong IL-1α secretion. However, when cells were treated with combination of LTA and various concentrations of rhHsp70, release of IL-1α was similar to non-treated cells (Fig. 2a). Interaction between LTA and all applied concentrations of rhHsp70 was antagonistic, since combined treatment stimulated lower IL-1α secretion than LTA alone (Fig. 2b). Compared to the cells treated with LPS alone, IL-1α secretion was not significantly different in combinations with any concentration of rhHsp70 (P = 0.0868), and it had no significant interactions (data not shown).
Fig. 2.
Effect of rhHsp70 and LTA combination on production of IL-1α after 24 h treatment. a Production of IL-1α after 24 h treatment with 1 μg/ml LTA alone and in combination with 0.3 (LTA + 0.3), 1 (LTA + 1), and 3 (LTA + 3) μg/ml rhHsp70. Data are presented as mean ± SEM and tested by ANOVA followed by multiple comparison Sidak method. *P = 0.0247 vs. non-treated cells. **P = 0.0002, P = 0.0005, and P = 0.0001, for combination of LTA with 0.3, 1, and 3 μg/ml rhHsp70, respectively vs. LTA alone. b rhHsp70 and LTA interactions on IL-1α secretion. Dark bars represent the measured values and gray bars the expected values. Data are expressed as mean ± SEM. Tested using unpaired t test. Double number signs indicate statistically significant antagonistic effect; P = 0.0067, P = 0.00029, and P = 0.0019 for combination of LTA with 0.3, 1, and 3 μg/ml rhHsp70, respectively
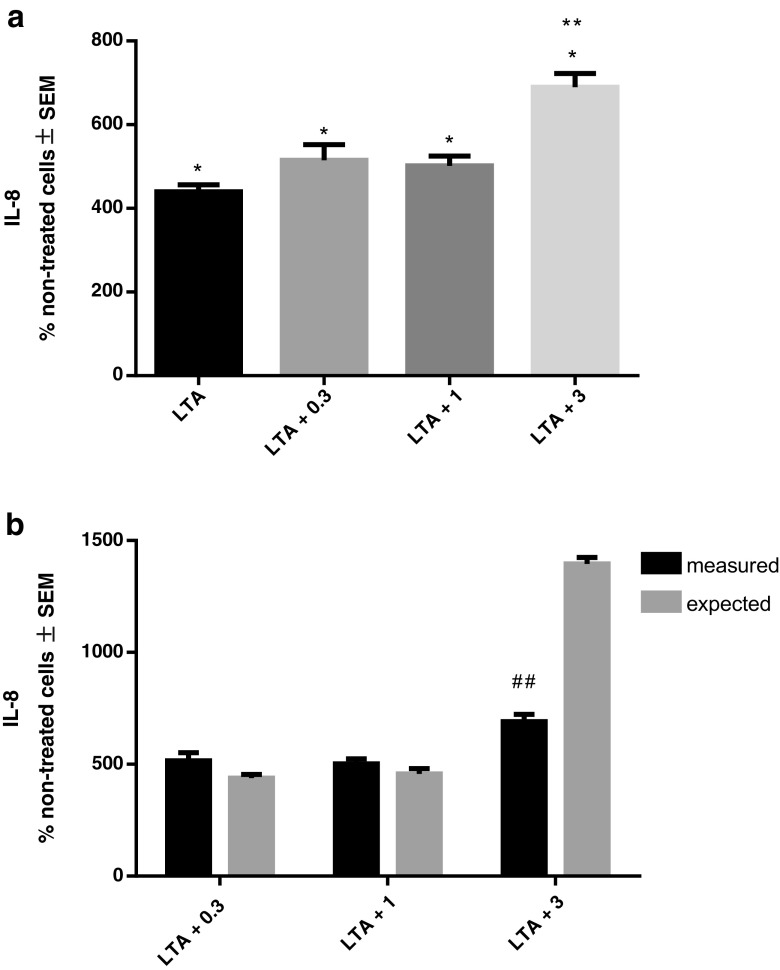
When measuring IL-8, LTA induced significant release of this cytokine as well. We observed that only 3 μg/ml rhHsp70 in combination with LTA stimulated a significantly higher concentration of IL-8 then LTA alone (Fig. 3a). Still, the type of interaction between rhHsp70 and LTA was antagonistic (Fig. 3b) On the other hand, secretion of IL-8 did not differ significantly when combined treatment with LPS was applied as compared to LPS alone (data not shown). However, the calculated interactions between 3 μg/ml rhHsp70 and LPS were significantly antagonistic (data not shown).
Fig. 3.
Effect of rhHsp70 and LTA combination on production of IL-8 after 24 h treatment. a Production of IL-8 after 24 h treatment with 1 μg/ml LTA alone and in combination with 0.3 (LTA + 0.3), 1 (LTA + 1), and 3 (LTA + 3) μg/ml rhHsp70. Data are presented as mean ± SEM and tested by ANOVA followed by multiple comparison Sidak method. *P = 0.0024 for LTA alone vs. non-treated cells. *P = 0.0002, P = 0.0005, and P = 0.0001, for combination of LTA with 0.3, 1, and 3 μg/ml rhHsp70, respectively vs. non-treated cells. **P = 0.0196 vs. LTA alone. b rhHsp70 and LTA interactions on IL-8 secretion. Dark bars represent the measured values and gray bars the expected values. Data are expressed as mean ± SEM. Tested using unpaired t test. Double number signs indicate statistically significant antagonistic effect; P = 0.0038 for combination of LTA with 3 μg/ml rhHsp70
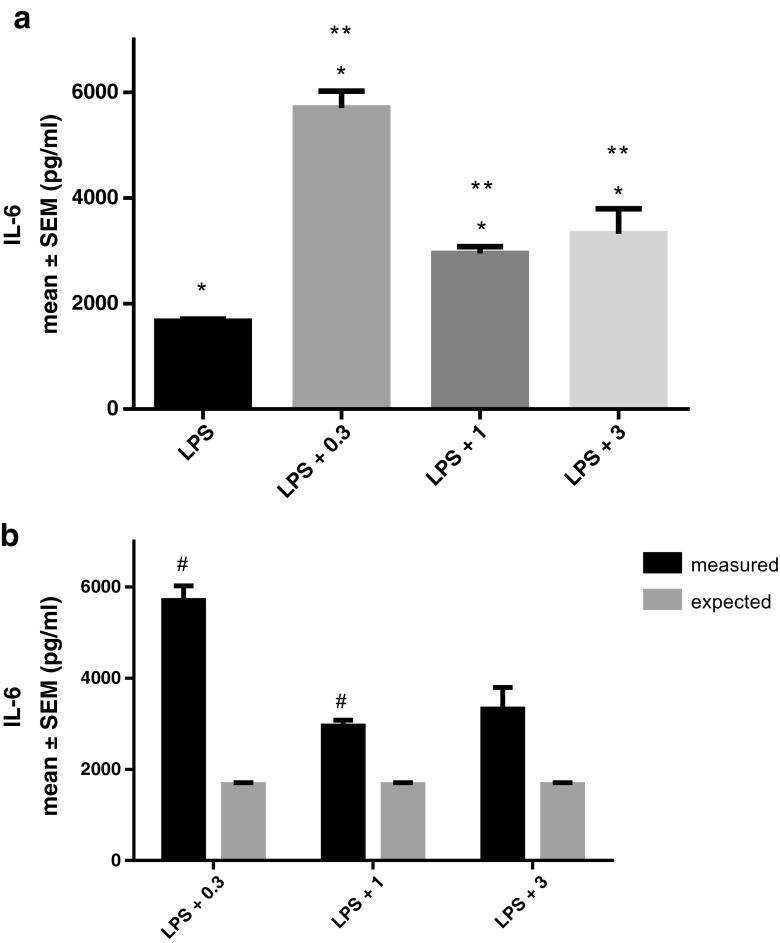
IL-6 and TNF-α concentrations were below detection limit in non-treated THP-1 cells. Therefore, we expressed the levels of those cytokines in treated cells in absolute values as pg/ml. Treatment with LPS alone significantly stimulated IL-6 secretion. A concentration of 0.3, 1, or 3 μg/ml rhHsp70 applied together with LPS showed significantly increased IL-6 levels compared to LPS alone (Fig. 4a). Those effects were synergistic for 0.3 and 1 μg/ml rhHsp70 combination (Fig. 4b). LTA alone or in combination with different concentrations of rhHsp70 did not induce significant changes in IL-6 secretion (data not shown).
Fig. 4.
Effect of rhHsp70 and LPS combination on production of IL-6 after 24 h treatment. a Production of IL-6 after 24 h treatment with 0.1 μg/ml LPS alone and in combination with 0.3 (LPS + 0.3), 1 (LPS + 1), and 3 (LPS + 3) μg/ml rhHsp70. Data are presented as mean ± SEM and tested by ANOVA followed by multiple comparison Sidak method. *P < 0.001 vs. non-treated cells. **P = 0.0018, P = 0.0104, and P = 0.0400, for combination of LPS with 0.3, 1, and 3 μg/ml rhHsp70, respectively vs. LPS alone. b rhHsp70 and LPS interactions on IL-6 secretion. Dark bars represent the measured values and gray bars the expected values. Data are expressed as mean ± SEM. Tested using unpaired t test. A number sign indicates statistically significant synergistic effect, P = 0.0063 and P = 0.0103 for combination of LPS with 0.3 and 1 μg/ml rhHsp70, respectively
Secretion of TNF-α was stimulated after treatment with LPS and LTA. Still, combined treatment of LPS or LTA and 0.3, 1, or 3 μg/ml rhHsp70 did not differ significantly when compared to LPS or LTA alone after 24 h treatment (data not shown), although calculated interactions showed synergism between LPS or LTA and 3 μg/ml rhHsp70 (data not shown).
Time-dependent interactions of LPS and LTA with eHsp70
Next, we wanted to explore if inflammatory effects of combined treatments were time-dependent. We have selected 1 μg/ml rhHsp70 concentration, and we treated THP-1 cells for 4, 12, 24, and 48 h. IL-1α was detected only at a 24-h time-point. However, other cytokines showed time-dependent secretion.
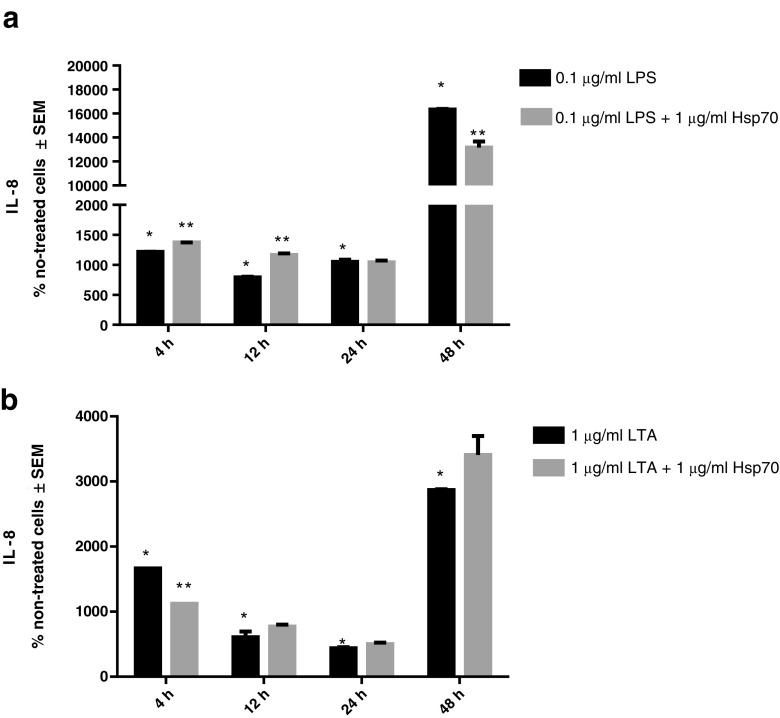
Secretion of IL-8 increased after treatment with LPS or LTA alone at all time-points. Compared to the cells treated with LPS alone, IL-8 secretion was significantly higher in the cells treated with rhHsp70 and LPS combination for 4 h and for 12 h while it was lower in cells treated together with LPS and rhHsp70 for 48 h (Fig. 5a). Cells treated with 1 μg/ml rhHsp70 and LTA produced significantly less IL-8 after 4 h than the cells treated with LTA alone (Fig. 5b).
Fig. 5.
Production of IL-8 after 4, 12, 24, and 48 h treatment with LPS (a) and LTA (b) alone and in combination with rhHsp70. Data are presented as mean ± SEM and tested by unpaired t test. a *P < 0.0001 after 4 h, P = 0.0047 after 12 h, P = 0.0020 after 24 h, and P < 0.0001 after 48 h of treatment with LPS alone vs. non-treated cells. **P = 0.0004 after 4 h, P = 0.0051 after 12 h, and P = 0.0247 after 48 h of treatment with combination of rhHsp70 and LPS vs. LPS alone. b *P < 0.0001 after 4 h, P = 0.0113 after 12 h, P = 0.0315 after 24 h, and P < 0.0001 after 48 h of treatment with LTA alone vs. non-treated cells. **P < 0.0001 after 4 h of treatment with combination of rhHsp70 and LTA vs. LTA alone
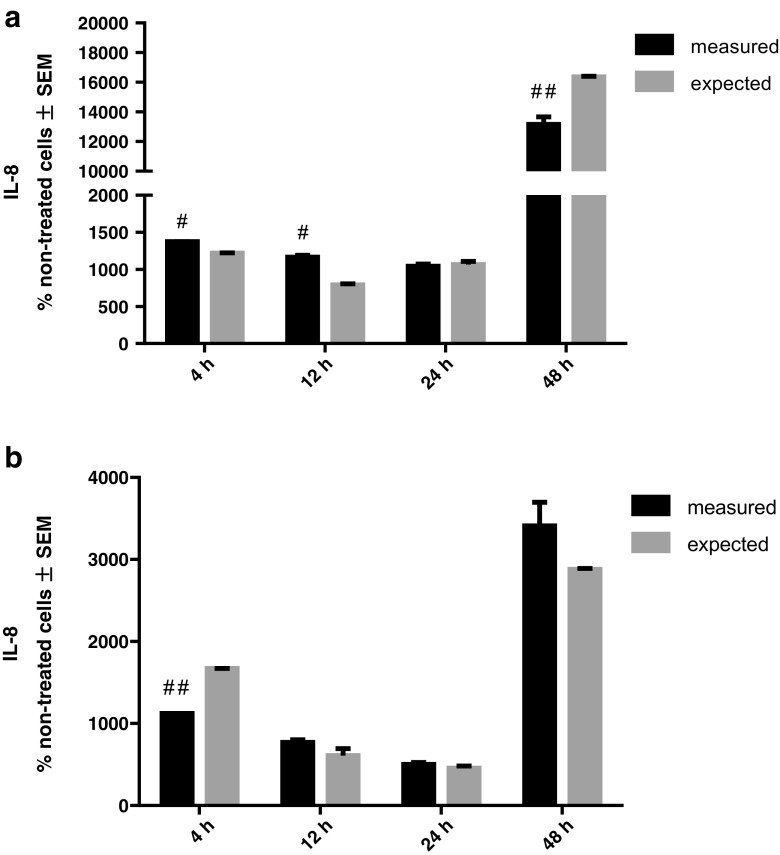
We calculated rhHsp70 and LPS or LTA interactions on IL-8 secretion (Fig. 6). Combination of rhHsp70 and LPS showed significant synergistic effect after 4 and 12 h and antagonistic effect after 48 h. On the other hand, combination of 1 μg/ml rhHsp70 and LTA showed significant antagonistic effect after 4 h.
Fig. 6.
rhHsp70 and LPS (a) or LTA (b) interactions on IL-8 secretion. Dark bars represent the measured values and gray bars the expected values. Data are expressed as mean ± SEM. Tested using unpaired t test. A number sign indicates statistically significant synergistic effect; P = 0.0006 after 4 h and P = 0.0054 after 12 h for combination of LPS and rhHsp70. Double number signs indicate statistically significant antagonistic effect; P = 0.0254 after 48 h for combination of LPS and rhHsp70; P < 0.0001 after 4 h for combination of LTA and rhHsp70
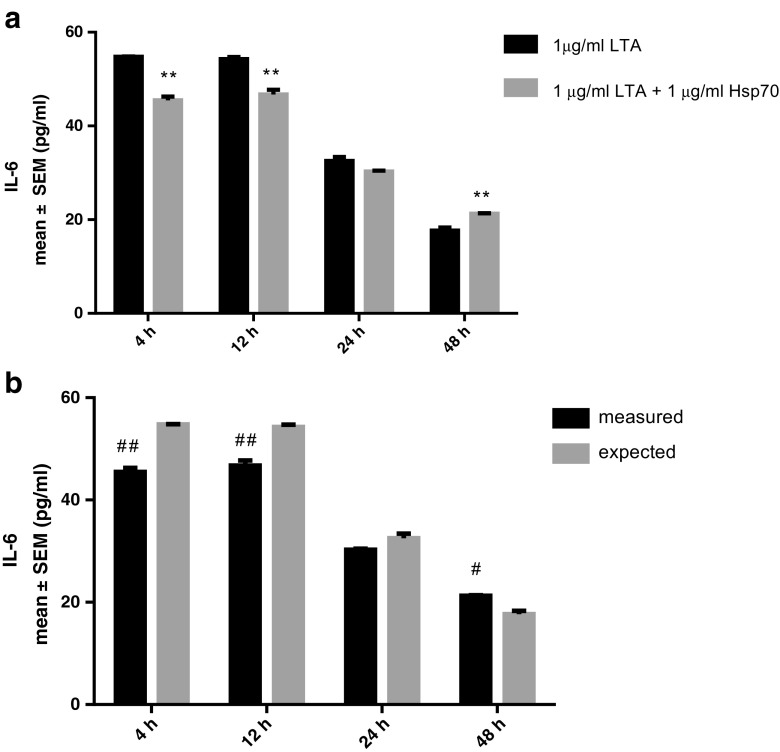
As for IL-6, cells treated with combination of LTA and rhHsp70 had lower IL-6 secretion compared to those treated with LTA alone after 4 and 12 h with an antagonistic type of interaction, while after 48 h significantly more IL-6 was secreted, and that effect was synergistic (Fig. 7). Combined treatment with LPS showed a synergistic type of interaction only after 24 h treatment (data not shown).
Fig. 7.
Effect of rhHsp70 and LTA combination on production of IL-6 after 4, 12, 24, and 48 h treatment. a Production of IL-6 after 4, 12, 24, and 48 h treatment with LTA alone and in combination with rhHsp70. Data are presented as mean ± SEM and tested by unpaired t test. **P = 0.0072 after 4 h, P = 0.0198 after 12 h, and P = 0.0337 after 48 h for combination of rhHsp70 with LTA vs. LTA alone. b rhHsp70 and LTA interactions on IL-6 secretion. Dark bars represent the measured values and gray bars the expected values. Data are expressed as mean ± SEM. Tested using unpaired t test. A number sign indicates statistically significant synergistic effect; P = 0.0337 after 48 h. Double number signs indicate statistically significant antagonistic effect P = 0.0072 after 4 h and P = 0.0198 after 12 h
Finally, secretion of TNF-α was higher after treatment with LPS and LTA at all time-points. Compared to the cells treated with LPS alone, combination of LPS and 1 μg/ml rhHsp70 had antagonistic type of effect on TNF-α production after 12 h. Treatment with LTA together with rhHsp70 exhibited antagonistic effects after 4, 12, and 48 h (Fig. 8).
Fig. 8.
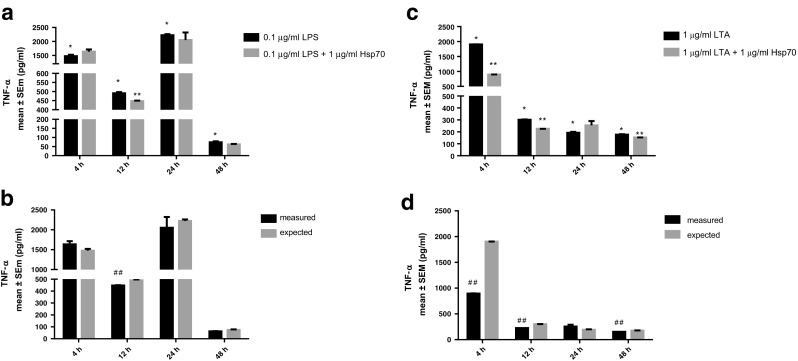
Effect of rhHsp70 and LPS or LTA combination on production of TNF-α after 4, 12, 24, and 48 h treatment. Upper panel: production of TNF-α after 4, 12, 24, and 48 h treatment with LPS (a) or LTA (c) alone and in combination with rhHsp70. Data are presented as mean ± SEM and tested by unpaired t test. a *P < 0.0001 after 4, 12, and 24 h, P = 0.0020 after 48 h of treatment with LPS vs. non-treated cells. **P = 0.0292 after 12 h for combination of rhHsp70 and LPS vs. LPS alone. c *P < 0.0001 after 4 and 12 h, P = 0.0179 after 24 h, and P = 0.0001 after 48 h of treatment with LTA vs. non-treated cells. **P < 0.0001 after 4 h, P = 0.0012 after 12 h, and P = 0.0283 after 48 h for combination of rhHsp70 and LTA vs. LTA alone. Lower panel: rhHsp70 and LPS (b) or LTA (d) interactions on TNF-α secretion. Dark bars represent the measured values and gray bars the expected values. Data are expressed as mean ± SEM. Tested using unpaired t test. Double number signs indicate statistically significant antagonistic effect P = 0.0292 after 12 h for combination of rhHsp70 and LPS; P < 0.0001 after 4 h, P = 0.0012 after 12 h, and P = 0.0283 after 48 h for combination of rhHsp70 and LTA
Cytotoxicity of eHsp70, LPS, and LTA
To explore cytotoxicity of eHsp70, LPS, and LTA, cells metabolic activity and possible apoptotic cell death were assessed by MTS assay and by caspase activity measurements, respectively.
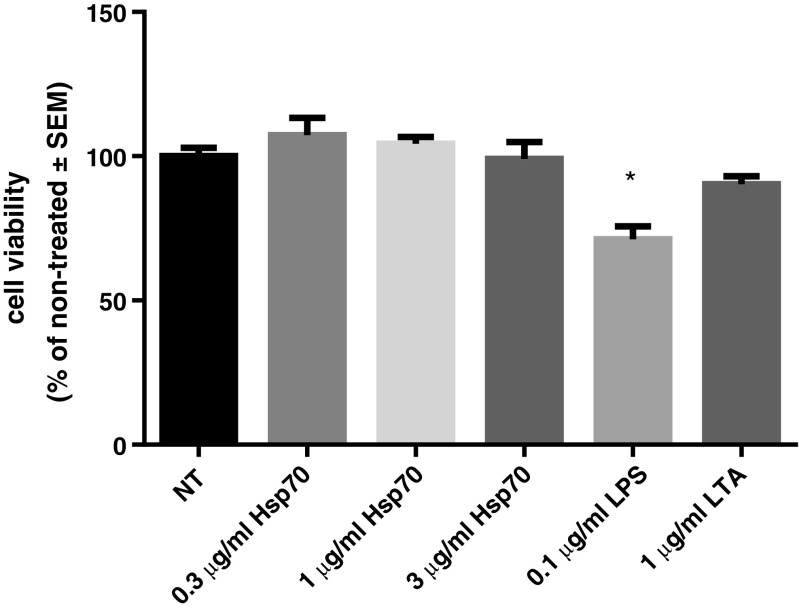
Results of the MTS assay showed that rhHsp70 did not influence cell viability, nor did the treatment with LTA alone, when compared to the control cells (expressed as 100%). However, 0.1 μg/ml LPS caused significant cytotoxic effect (Fig. 9). On the other hand, when we compared combined treatments with LTA or LPS and different rhHsp70 concentrations with individual LPS or LTA treatments, we did not observe any significant differences in viability. To confirm those results, we calculated possible rhHsp70 and LPS or LTA cytotoxicity interactions. Combination of 0.3, 1, or 3 μg/ml rhHsp70 and LPS or LTA showed no significant interaction (data not shown).
Fig. 9.
Effect of treatment with rhHsp70, LPS, and LTA on cell viability. Data are presented as mean ± SEM and tested by ANOVA followed by multiple comparison Sidak method. *P = 0.0007 vs. non-treated cells
In addition, activities of caspases-9, -8, and -3/7 in THP-1 cells were measured after 2, 4, 6, and 8 h treatment with rhHsp70, LPS, or LTA. Compared to control cells, activity of caspase-9 did not differ with aforementioned treatments (data not shown). LPS provoked significant caspase-8 activation after 6 and 8 h, while LTA and rhHsp70 did not activate caspase-8 (data not shown). Furthermore, we did not observe significant difference in caspase-3/7 activity between non-treated cells and cells treated with rhHsp70 after 2, 4, or 6 h, whereas the activity of caspase-3/7 was decreased after 8 h treatment for all rhHsp70 concentrations examined. LTA failed to activate caspase-3/7 at any time-point, while LPS caused significant activation of caspase-3/7 after 4 and 6 h compared to non-treated cells (Fig. 10).
Fig. 10.
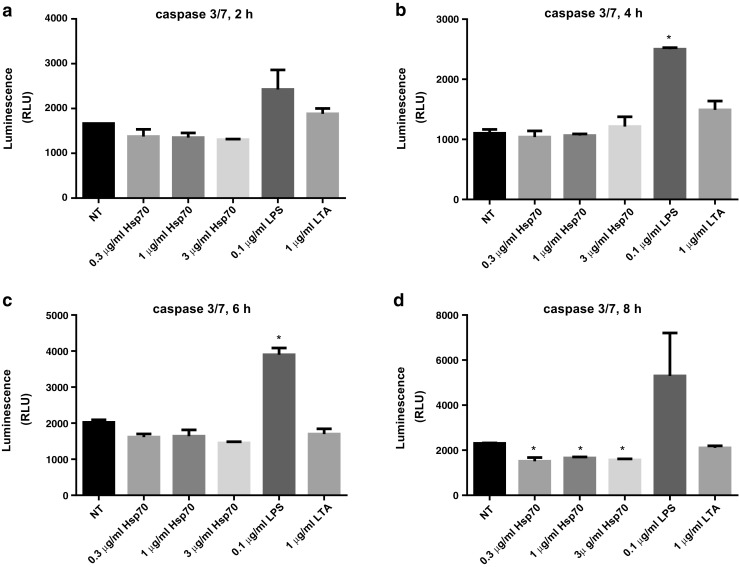
Apoptotic effects of rhHsp70, LPS, and LTA on THP-1 cells after 2 (a), 4 (b), 6 (c), and 8 (d) h of treatment, as assessed by the caspase 3/7 activity. Data are represented as mean ± SEM and tested by ANOVA followed by multiple comparison by Sidak method. P < 0.05 is considered statistically significant. b *P = 0.0036 vs. non-treated. c *P = 0.0052 vs. non-treated. d *P = 0.0103, P = 0.0221, P = 0.0140 for 0.3, 1, and 3 μg/ml rhHsp70, respectively vs. non-treated cells
Discussion
In this study, we explored the effects of extracellular Hsp70 when presented alone or together with well-known pathogen-associated molecular pattern (PAMP) molecules, LPS, and LTA, on inflammatory response determined by the levels of secreted pro-inflammatory cytokines (IL-1α, IL-6, IL-8, and TNF-α). We also assessed cell viability and mode of cell death by MTS assay and caspases-3/7, -8, and -9 activity measurements, respectively. We used a human monocytic cell line THP-1 as a cellular model of systemic compartment in COPD.
COPD is one of the most prevalent human diseases in the world, affecting about 12% of the population older than 40 years (Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017). It is characterized by chronic inflammation which in turn induces destruction of parenchyma and small airway fibrosis leading to airflow limitation (Dong et al. 2013; Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017). Innate and adaptive immunity cells, as well as structural cells, such as epithelial cells and fibroblasts, are involved in the process of inflammation, and they secrete various mediators of inflammation (Barnes and Celli 2009; Dong et al. 2013; Rovina et al. 2013). Inflammation in COPD can be sterile (mediated by DAMPs), mediated by pathogens (PAMPs), or could be a consequence of their combined actions, as bacterial colonization can be present both in stabile COPD and during its exacerbations (Wouters et al. 2007; Sethi et al. 2012).
It was shown that extracellular Hsp70 is a DAMP molecule that activates monocytes and macrophages via binding to TLR2 and TLR4 receptors, causing a pro-inflammatory cytokine production through MyD88/IRAK/NF-κB signal transduction pathway activation in a CD14-dependent fashion (Thériault et al. 2005; Ferat-Osorio et al. 2014).
In our study, rhHsp70 provoked IL-1α and IL-8 secretion. However, IL-6 and TNF-α were not induced, which is in accordance with Ferat-Osorio et al. who even found down-regulated expression of TNF-α gene after treatment with Hsp70, albeit in primary monocytes, not THP-1 cells (Ferat-Osorio et al. 2014). In addition, eHsp70 was previously found to stimulate pro-inflammatory effect in other types of cells different from monocytic-macrophagic lineage (Chase et al. 2007; Wheeler et al. 2009; Giuliano et al. 2011; Mathur et al. 2011). Despite aforementioned evidence of eHsp70 pro-inflammatory effects, some researchers challenged these assumptions, and considered those effects to be false positive, i.e., due to endotoxin (LPS) contamination of recombinant Hsp70 protein (Tsan and Gao 2004; De Maio and Vazquez 2013). To rule out this possibility, we used low endotoxin Hsp70, with its level being 2.7 EU/mg, as determined by LAL test. Equivalent dose of LPS was included in all our experiments, and did not induce a pro-inflammatory effect in the THP-1 cell line (data not shown), and neither did so in our previous research on NCI-H292 bronchial epithelial cells (non-published results). Also, in our preliminary experiments with polymyxin B, boiling of rhHsp70, and application of specific antibodies against Hsp70, TLR2, and TLR4, we have excluded that the effects on cytokine secretion and cell viability might be from endotoxin or some other contaminants, rather than from rhHsp70 itself.
LPS is a component of gram-negative bacteria and is able to induce inflammatory response via TLR4 receptor, while LTA is a constituent of the cell wall of gram-positive bacteria and TLR2 agonist. TLR2 and TLR4 activation can induce NF-κB and MAPK kinase pathways, leading to the production of TNF-α, IL-1, IL-6, IL-8, and IL-10 (Hutchison et al. 2000; Dokladny et al. 2010; Vinokurov et al. 2012). Therefore, expectedly, when we treated THP-1 cells with LPS or LTA, production of cytokines was induced, with LPS having a stronger effect. In addition, effects of LPS and LTA seem to be time-dependent.
As bacterial colonization and infections are occasionally present in COPD patients, we explored a combined effect of eHsp70 and LPS or LTA. Combination of rhHsp70 and LPS had a synergistic effect on IL-8 secretion at early time-points, but it becomes antagonistic after 48 h. Effect of combination of rhHsp70 and LPS on IL-6 secretion is synergistic only after 24 h, while its effect on TNF-α secretion is antagonistic after 12 h. Dual treatment with rhHsp70 and LTA mostly has antagonistic effects: IL-1α secretion is significantly lower after 24 h, IL-8 after 4 and 24 h, IL-6 after 4 and 12 h, and TNF-α after 4, 12, and 48 h. However, the effect is synergistic for IL-6 secretion after 48 h. It could be concluded that effects of LPS together with rhHsp70 are mainly synergistic, and those for LTA and rhHsp70 are mainly antagonistic, although those effects are time- and cytokine-dependent. Some previous publications reported that extracellular Hsp70 preferentially interacts with TLR2, rather than TLR4 (Jean-Pierre et al. 2006; Joly et al. 2010; Mathur et al. 2011), and therefore the antagonistic effect might be a consequence of modulation of the receptor sensitivity by eHsp70 and interference with LTA binding, while eHsp70 does not interfere with LPS binding to TLR4 (Bangen et al. 2007). Explanation of different synergistic/antagonistic effects on cytokines production at different time-points might also be an alteration of TLR2 and TLR4 receptor expression by eHsp70 (Jean-Pierre et al. 2006; Tulapurkar et al. 2015). Another possible option is secretion or elevated expression of other molecules that could affect the combined effects by activating positive or negative feedback mechanisms that can in the end render receptors less responsive or unresponsive (Gupta et al. 2013; Quintana and Cohen 2005; Aneja et al. 2006). It was shown in several studies that if low eHsp70 concentrations are added before LPS, eHsp70 was able to induce endotoxin tolerance and can even act protective against LPS in sepsis in rats (Aneja et al. 2006; Abboud et al. 2008; Garcia et al. 2013; Hsu et al. 2014). Also, besides TLR2 and TLR4, other receptors such as CD40, CD91, scavenger receptors, and c-type lectin receptor DECTIN might be implicated in eHsp70 inflammatory activity (Thériault et al. 2005). Further investigations are needed to elucidate underlying molecular pattern involved in inflammatory processes provoked by extracellular Hsp70.
Besides inflammation, another important aspect of understanding mechanisms of diseases is cell death, as activation of TLR2 and TLR4 receptors could lead to inflammation and/or programmed cell death. Inflammation could also cause necrotic cell death, leading to the leakage of intracellular content and further propagation of inflammation. Normally, necrotic cells are immunostimulatory, in contrast to apoptotic cells which are immunologically silent (Zhang et al. 2010; Ofengeim and Yuan 2013; Vanden Berghe et al. 2014). Apoptosis, or programmed cell death, is a vital part of normal function of the immune system and inappropriate apoptosis is associated with various diseases (Elmore 2007). Cysteinyl aspartate-specific proteases, caspases, play a central role in the apoptotic process. They can be activated by two pathways, intrinsic or mitochondrial via initiator caspase-9 activation, and extrinsic or death receptors pathway via initiator caspase-2, -8, and/or -10 activation. Both pathways converge on downstream executioner caspases-3, -6, and/or -7 (Duprez et al. 2009).
We assessed cell viability using MTS assay, and explored whether apoptotic pathways were activated by determining activities of caspases-9, -8, and -3/7. rhHsp70 did not cause a decrease in THP-1 cell viability, and neither did LTA. However, LPS caused a significant reduction in metabolic activity of the cells. It was reported that Hsp70 did not provoke cytotoxic effects when applied on HL-60 promyelocytic leukemia cells (Wheeler et al. 2009) or neutrophiles (Vinokurov et al. 2012), and LTA was found to decrease viability of dermal fibroblasts only at very high doses (Choi 2010), while it did not affect viability of A549 alveolar epithelial cells (Liu et al. 2012). Contrary to our results, Huang et al. found that only doses larger than ≥ 10 μg/ml LPS affect cell viability in THP-1 cell line (Huang et al. 2012). Combinations of rhHsp70 with LPS or LTA did not show any significant interactions on cell viability. It was shown before that pre-treatment with Hsp70 can have a protective effect against cytotoxicity of various agents, including LPS and LTA (Aneja et al. 2006; Vinokurov et al. 2012; Franco et al. 2016), but we added rhHsp70 and LPS or LTA simultaneously and this could be the reason for a discrepancies of results.
In our study, treatment of THP-1 cells with rhHsp70, LTA, or LPS did not induce mitochondrial apoptotic pathway (caspase-9), nor did rhHsp70 or LTA induce death receptor apoptotic pathway (caspase-8). In contrast, LPS induced apoptosis by the way of activation of caspases-8 and -3/7, which is in accordance with some previous studies (Xaus et al. 2000; Munshi et al. 2002). In addition, we observed a significantly lower activity of executioner caspases-3/7 in THP-1 cells after 8 h treatment with rhHsp70. It is a well-established fact that intracellular Hsp70 has anti-apoptotic function (Joly et al. 2010), but anti-apoptotic functions of eHsp70 have also been demonstrated. Luo et al. reported that eHsp70 prevents activation of caspases-3 and -9 dependent on cytochrome c, and Franco et al. reported that extracellular exposure to Hsp70 is able to lower caspase-3 activation induced by hydrogen peroxide (Luo et al. 2012; Franco et al. 2016). Exact mechanisms of eHsp70 effect on cell death are not thoroughly explored and further investigation is needed.
In conclusion, we demonstrated that extracellular Hsp70 can induce inflammation (secretion of IL-1α and IL-8) in THP-1 cells. Furthermore, when added in combination with LPS or LTA, antagonistic or synergistic interactions were observed even in situations when rhHsp70 alone did not exhibit a significant pro-inflammatory effect. Also, it seems that eHsp70 can suppress apoptosis execution. Hsp70 was found elevated in sera of COPD patients (Hacker et al. 2009), and those patients are proven to be susceptible to bacterial colonization and infections (Wouters et al. 2007). Therefore, we suggest that extracellular Hsp70 might be implicated in inflammatory processes in a stable phase of COPD as well as during its exacerbations, as TLR2 and TLR4 desensitization or inappropriate activation might lead to the worsening of the patient’s condition.
Acknowledgements
This work has been fully supported by the Croatian Science Foundation under the project number IP-2014-09-1247.
References
- Abboud PA, Lahni PM, Page K, et al. The role of endogenously produced extracellular HSP72 in mononuclear cell reprogramming. Shock. 2008;30:285–292. doi: 10.1097/SHK.0b013e318164e2c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti AGN, Noguera A, Sauleda J, et al. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:347–360. doi: 10.1183/09031936.03.00405703. [DOI] [PubMed] [Google Scholar]
- Aneja R, Odoms K, Dunsmore K, et al. Extracellular heat shock protein-70 induces endotoxin tolerance in THP-1 cells. J Immunol. 2006;177:7184–7192. doi: 10.4049/jimmunol.177.10.7184. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, et al. Novel signal transduction pathway utilized by extracellular HSP70. Role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Bangen JM, Schade FU, Flohé SB. Diverse regulatory activity of human heat shock proteins 60 and 70 on endotoxin-induced inflammation. Biochem Biophys Res Commun. 2007;359:709–715. doi: 10.1016/j.bbrc.2007.05.167. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- Chase MA, Wheeler DS, Lierl KM, et al. Hsp72 induces inflammation and regulates cytokine production in airway epithelium through a TLR4- and NF-kappaB-dependent mechanism. J Immunol. 2007;179:6318–6324. doi: 10.4049/jimmunol.179.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY. Anti-inflammatory activity of Houttuynia cordata against lipoteichoic acid-induced inflammation in human dermal fibroblasts. Chonnam Med J. 2010;46:140–147. doi: 10.4068/cmj.2010.46.3.140. [DOI] [Google Scholar]
- De Maio A, Vazquez D. Extracellular heat shock proteins: a new location, a new function. Shock. 2013;40:239–246. doi: 10.1097/SHK.0b013e3182a185ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokladny K, Lobb R, Wharton W, et al. LPS-induced cytokine levels are repressed by elevated expression of HSP70 in rats: possible role of NF-κB. Cell Stress Chaperones. 2010;15:153–163. doi: 10.1007/s12192-009-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Guo L, Liao Z, et al. Increased expression of heat shock protein 70 in chronic obstructive pulmonary disease. Int Immunopharmacol. 2013;17:885–893. doi: 10.1016/j.intimp.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Duprez L, Wirawan E, Vanden BT, Vandenabeele P. Major cell death pathways at a glance. Microbes Infect. 2009;11:1050–1062. doi: 10.1016/j.micinf.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferat-Osorio E, Sánchez-Anaya A, Gutiérrez-Mendoza M, et al. Heat shock protein 70 down-regulates the production of toll-like receptor-induced pro-inflammatory cytokines by a heat shock factor-1/constitutive heat shock element-binding factor-dependent mechanism. J Inflamm (Lond) 2014;11:19. doi: 10.1186/1476-9255-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco L, Terrinca J, Rodríguez AB, et al. Extracellular heat shock proteins protect U937 cells from H2O2-induced apoptotic cell death. Mol Cell Biochem. 2016;412:19–26. doi: 10.1007/s11010-015-2604-y. [DOI] [PubMed] [Google Scholar]
- Garcia JJ, Martin-Cordero L, Hinchado MD, et al. Effects of habitual exercise on the eHsp72-induced release of inflammatory cytokines by macrophages from obese Zucker rats. Int J Sports Med. 2013;34:559–564. doi: 10.1055/s-0032-1327650. [DOI] [PubMed] [Google Scholar]
- Giuliano JS, Lahni PM, Wong HR, Wheeler DS. Pediatric sepsis—part V: extracellular heat shock proteins: alarmins for the host immune system. Open Inflamm J. 2011;4:49–60. doi: 10.2174/1875041901104010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) (2017) Global strategy for the diagnosis, management and prevention of COPD. In: www.goldcopd.org. www.goldcopd.org
- Gupta A, Cooper ZA, Tulapurkar ME, et al. Toll-like receptor agonists and febrile range hyperthermia synergize to induce heat shock protein 70 expression and extracellular release. J Biol Chem. 2013;288:2756–2766. doi: 10.1074/jbc.M112.427336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker S, Lambers C, Hoetzenecker K, et al. Elevated HSP27, HSP70 and HSP90α in chronic obstructive pulmonary disease: markers for immune activation and tissue destruction. Clin Lab. 2009;55:31–40. [PubMed] [Google Scholar]
- Hsu J-H, Yang R-C, Lin S-J, et al. Exogenous heat shock cognate protein 70 pretreatment attenuates cardiac and hepatic dysfunction with associated anti-inflammatory responses in experimental septic shock. Shock. 2014;42:540–547. doi: 10.1097/SHK.0000000000000254. [DOI] [PubMed] [Google Scholar]
- Huang M-Y, Liao M-H, Wang Y-K, et al. Effect of lavender essential oil on LPS-stimulated inflammation. Am J Chin Med. 2012;40:845–859. doi: 10.1142/S0192415X12500632. [DOI] [PubMed] [Google Scholar]
- Hutchison ML, Bonell EC, Poxton IR, Govan JRW. Endotoxic activity of lipopolysaccharides isolated from emergent potential cystic fibrosis pathogens. FEMS Immunol Med Microbiol. 2000;27:73–77. doi: 10.1111/j.1574-695X.2000.tb01414.x. [DOI] [PubMed] [Google Scholar]
- Jean-Pierre C, Perni SC, Bongiovanni AM, et al (2006) Extracellular 70-kd heat shock protein in mid-trimester amniotic fluid and its effect on cytokine production by ex vivo-cultured amniotic fluid cells. In: American Journal of Obstetrics and Gynecology. pp 694–698 [DOI] [PubMed]
- Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol. 2006;79:425–434. doi: 10.1189/jlb.0905523. [DOI] [PubMed] [Google Scholar]
- Joly A-L, Wettstein G, Mignot G, et al. Dual role of heat shock proteins as regulators of apoptosis and innate immunity. J Innate Immun. 2010;2:238–247. doi: 10.1159/000296508. [DOI] [PubMed] [Google Scholar]
- Liu F-L, Chuang C-Y, Tai Y-T, et al. Lipoteichoic acid induces surfactant protein-A biosynthesis in human alveolar type II epithelial cells through activating the MEK1/2-ERK1/2-NF-κB pathway. Respir Res. 2012;13:88. doi: 10.1186/1465-9921-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Tao L, Lin P, et al. Extracellular heat shock protein 72 protects Schwann cells from hydrogen peroxide-induced apoptosis. J Neurosci Res. 2012;90:1261–1269. doi: 10.1002/jnr.22810. [DOI] [PubMed] [Google Scholar]
- Mathur S, Walley KR, Wang Y, et al. Extracellular heat shock protein 70 induces cardiomyocyte inflammation and contractile dysfunction via TLR2. Circ J. 2011;75:2445–2452. doi: 10.1253/circj.CJ-11-0194. [DOI] [PubMed] [Google Scholar]
- Midwood KS, Piccinini AM (2010) DAMPening inflammation by modulating TLR signalling. Mediat Inflamm. 2010 [DOI] [PMC free article] [PubMed]
- Munshi N, Fernandis AZ, Cherla RP, et al. Lipopolysaccharide-induced apoptosis of endothelial cells and its inhibition by vascular endothelial growth factor. J Immunol. 2002;168:5860–5866. doi: 10.4049/jimmunol.168.11.5860. [DOI] [PubMed] [Google Scholar]
- Ofengeim D, Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol. 2013;14:727–736. doi: 10.1038/nrm3683. [DOI] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Muthana M, Calderwood SK. The dual immunoregulatory roles of stress proteins. Trends Biochem Sci. 2008;33:71–79. doi: 10.1016/j.tibs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Pouwels SD, Heijink IH, ten Hacken NHT, et al. DAMPs activating innate and adaptive immune responses in COPD. Mucosal Immunol. 2014;7:215–226. doi: 10.1038/mi.2013.77. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Cohen IR. Heat shock proteins as endogenous adjuvants in sterile and septic inflammation. J Immunol. 2005;175:2777–2782. doi: 10.4049/jimmunol.175.5.2777. [DOI] [PubMed] [Google Scholar]
- Rovina N, Koutsoukou A, Koulouris NG (2013) Inflammation and immune response in COPD: where do we stand? Mediat Inflamm. 2013 [DOI] [PMC free article] [PubMed]
- Šegvić Klarić M, Medić N, Hulina A, et al. Disturbed Hsp70 and Hsp27 expression and thiol redox status in porcine kidney PK15 cells provoked by individual and combined ochratoxin A and citrinin treatments. Food Chem Toxicol. 2014;71:97–105. doi: 10.1016/j.fct.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Sethi S, Mahler DA, Marcus P, et al. Inflammation in COPD: implications for management. Am J Med. 2012;125:1162–1170. doi: 10.1016/j.amjmed.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Thériault JR, Mambula SS, Sawamura T, et al. Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS Lett. 2005;579:1951–1960. doi: 10.1016/j.febslet.2005.02.046. [DOI] [PubMed] [Google Scholar]
- Tsan M-F, Gao B. Endogenous ligands of toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- Tulapurkar ME, Ramarathnam A, Hasday JD, Singh IS (2015) Bacterial lipopolysaccharide augments febrile-range hyperthermia-induced heat shock protein 70 expression and extracellular release in human THP1 cells. PLoS One. 10.1371/journal.pone.0118010 [DOI] [PMC free article] [PubMed]
- Vabulas RM, Wagner H, Schild H. Heat shock proteins as ligands of toll-like receptors. Curr Top Microbiol Immunol. 2002;270:169–184. doi: 10.1007/978-3-642-59430-4_11. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, et al. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- Vinokurov M, Ostrov V, Yurinskaya M, et al. Recombinant human Hsp70 protects against lipoteichoic acid-induced inflammation manifestations at the cellular and organismal levels. Cell Stress Chaperones. 2012;17:89–101. doi: 10.1007/s12192-011-0288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DS, Chase MA, Senft AP, et al. Extracellular Hsp72, an endogenous DAMP, is released by virally infected airway epithelial cells and activates neutrophils via Toll-like receptor (TLR)-4. Respir Res. 2009;10:31. doi: 10.1186/1465-9921-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters EFM, Celis MPM, Breyer MK, et al. Co-morbid manifestations in COPD. Respir Med COPD Update. 2007;3:135–151. doi: 10.1016/j.rmedu.2007.08.003. [DOI] [Google Scholar]
- Xaus J, Comalada M, Valledor AF, et al. LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-alpha. Blood. 2000;95:3823–3831. [PubMed] [Google Scholar]
- Zhang D, Lin J, Han J. Receptor-interacting protein (RIP) kinase family. Cell Mol Immunol. 2010;7:243–249. doi: 10.1038/cmi.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]