Abstract
Neutrophil cytosolic factor 4 (NCF4) is a member of the nicotinamide adenine dinucleotide phosphate oxidase subunit. This protein functions as an essential factor in the host defense against the progression of bacterial infection. To explore the variability of the NCF4 gene and the susceptibility of cows to mastitis, NCF4 functional single nucleotide polymorphism (SNP) of the 3′ untranslated region (3′UTR) and its targeted microRNA (miRNA) were identified. One SNP g.18475 A>G in the 3′UTR of NCF4 was found within the binding seed region of bta-miR-2426. We constructed two recombinant pMIR-REPORT™ vectors with the A or G allele in the g.18475 locus and transiently co-transfected the vectors in human embryo kidney 293T (HEK 293T) cells, along with bta-miR-2426 mimics. A luciferase assay indicated that this SNP affects the binding of NCF4 and bta-miR-2426. In addition, the association analysis results showed that cows with the GG genotype in SNP g.18475 A>G had a relatively lower SCS value than cows with the AA genotype. Finally, quantitative real-time PCR (RT-qPCR) results showed that the cows with genotype GG had a relatively higher expression of NCF4 mRNA compared to the cows with genotype AA. NCF4 expression was regulated by the miRNA–mRNA interaction mechanism, and an important role for NCF4 in mastitis susceptibility in dairy cow was suggested.
Keywords: Bovine, NCF4, Mastitis, SCS, Bta-miR-2426, 3′UTR SNP
Introduction
Mastitis is among the most prevalent and costly diseases in the dairy industry, and losses are incurred due to reduced milk production, discarded milk, early culling, veterinary services, and labor costs. Despite the implementation of management practices and genetic selection approaches, bovine mastitis control continues to be inadequate (Thompson-Crispi et al. 2014). Novel genetic strategies have recently been demonstrated to reduce mastitis incidence by taking advantage of a cow’s natural ability to mount appropriate immune responses against invading pathogens. The feasibility of breeding for resistance based on one single nucleotide polymorphism (SNP) or a combination of SNPs depends on the degree of variation that each SNP contributes to mastitis resistance. Furthermore, microRNAs (miRNAs) were differentially expressed in bovine mammary epithelial cells upon challenge with mastitis-causing pathogens, thereby suggesting a role for miRNA in regulating host responses to mastitis (Lawless et al. 2013; Jin et al. 2014). Indeed, many studies have demonstrated that the bovine immune response is under both genetic and epigenetic control, and using this information in breeding strategies would help improve udder health (Hou et al. 2012; Li et al. 2013).
The innate immune system serves as the first line of defense against invading pathogens and functions as the primary initiator of the inflammatory response (Oviedo-Boyso et al. 2007). Reactive oxygen species (ROS) are important in regulating the host immune response (Wada et al. 2013). Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is a membrane-bound enzyme complex that generates reactive oxygen species critical for pathogen elimination upon phagocytosis and for the regulation of proinflammatory signaling in phagocytic cells (Conway et al. 2012). NCF4 is a cytosolic oxidase-associated protein that is a subunit of the NADPH oxidase complex (Gustafson et al. 2014). NCF4 mediates downregulation of NADPH oxidase through interactions with its SH3 domain. Genetic polymorphisms of NADPH oxidase, including those of NCF4, regulate subunit expression and enzyme activity (Schirmer et al. 2008). Previous studies indicated that variation in human NCF4 is associated with inflammatory bowel disease, Crohn’s disease, and rheumatoid arthritis risk (Olsson et al. 2007; Rioux et al. 2007; Wu et al. 2015). Neutrophils collected from NCF4−/− mice exhibited severe defects in NADPH oxidase regulation and oxidant-dependent bacterial elimination (Ellson et al. 2006). NCF4 might be essential in innate immune responses and in the progression of bacterial infections. However, characterization of functional genetic polymorphisms of bovine NCF4 and their roles in cattle mastitis susceptibility have not been reported.
MiRNAs are single-stranded, endogenous, noncoding small RNAs (18–26 nucleotides in size). MiRNAs have emerged as novel critical gene expression regulators that affect many biological processes, including mammalian development, differentiation, and function (Zhu et al. 2013). In particular, several miRNAs have been demonstrated to have important roles in bovine infection and immunity (Li et al. 2012; Lawless et al. 2014; Wang et al. 2014). MiRNAs bind to complementary sequences in the 3′ untranslated region (3′UTR) of their target messenger RNAs (mRNAs), inhibiting translation of the target mRNA into protein or accelerating mRNA degradation (Bartel 2004; Ha and Kim 2014). Increasing evidence suggests that mutations, such as SNPs, in miRNA-binding sites may result in altered protein levels and may cause diseases, including bovine mastitis, a disease of the bovine mammary gland with significant economic effects on the dairy industry. For example, Li et al. (2012) identified an SNP (g.+2776 A>G) in the bovine HMGB1 3′UTR region that affected the binding affinity of the HMGB1 gene and its target bta-miR-223. Moreover, the SNP was also associated with bovine mastitis resistance traits in Chinese Holstein cattle. One A2M gene 3′UTR mutation (c.4659_4661delC) altered the binding affinity of bta-miR-2898 and A2M, participated in the regulation of the A2M expression, and was involved in the immune function of mastitis susceptibility in dairy cows (Wang et al. 2014).
However, whether any 3′UTR SNPs of NCF4 interfere with target miRNA gene regulation and affect mastitis susceptibility remains largely unknown. The aims of this study were as follows: (1) to identify the 3′UTR SNP of the NCF4 gene, (2) to explore whether SNP was present in the target miRNA seed-binding region, (3) to investigate whether the 3′UTR SNP affects the binding activity of target miRNAs and NCF4 by transfection in HEK 293T cells, and (4) to determine whether the functional SNP is related to mastitis resistance in dairy cows.
Materials and methods
Experiment samples
Four hundred fifty-four Chinese Holstein cattle were randomly selected from five dairy cattle farms in Jinan and Qingdao Agriculture Development Area, PR China. These individuals included 17 sire families aged 4–7 years. Bovine blood samples were collected from the jugular veins and mixed with anticoagulant acid–citrate–dextrose (ACD) solution (0.48% citric acid, 1.32% sodium citrate, and 1.47% dextrose) at a ratio of 6:1 (blood/ACD). The blood sample collections were permitted by the cattle owners. Genomic DNA was extracted from the blood samples using the TIANamp Genomic Extraction Kit (Tiangen, Beijing, China). The quantity and quality of DNA were analyzed spectrophotometrically. DNA samples were stored at − 20 °C for subsequent analysis. Milk somatic cell count was measured using a milk composition analyzer (Foss MilkoScan FT 6000). The corresponding milk somatic cell scores (SCSs) were provided by the Dairy Herd Improvement Laboratory, Dairy Cattle Research Center, Shandong Academy of Agricultural Sciences.
A total of 13 mammary tissue samples were aseptically collected from a commercial bovine slaughter farm in Jinan, Shandong Province, China. One of the mammary tissue samples was collected and stored in liquid nitrogen for RNA isolation, whereas the others were used for pathogen identification. Total RNA was extracted and reverse-transcribed to cDNA using PrimeScript® 1st Strand cDNA Synthesis Kit (Takara) and miScript Reverse Transcriptase Mix (Qiagen) according to the manufacturer’s instructions.
Screening of NCF4 gene 3′UTR-SNP
One primer pair NCF4–3′UTR (forward: ACATCGCCCTCAACTACCGT, reverse: GGGAACAAGGTGAGGAAACA) was designed to amplify the 3′UTR region of bovine NCF4 gene (GenBank Accession No. NC_007303.5). The PCR products of 20 samples were evaluated by electrophoresis on 1% agarose gels after staining with ethidium bromide, and sent to BGI Ltd. for sequencing. The sequenced results were analyzed using the DNASTAR Lasergene 7.1 software to search for SNPs.
Genotyping of g.18475 A>G and association analysis
Direct sequencing of PCR products containing the 3′UTR fragment was performed to genotype the SNP g.18475 A>G. The association between the genotypes and SCS was analyzed using a general least squares model using SAS statistical analysis software. The linear model was expressed as follows:
Yijkl = µ + Gi + Pj + Ek + Fl + eijklwhere Yijkl is the observed value, μ is the overall mean, Gi is the fixed effect of genotype, Pj is the fixed effect of age, Hk is the fixed effect of farm, El is the fixed effect of season, and eijkl is the random residual error. Values with P < 0.05 were considered significant.
Prediction of target miRNA
To identify the putative miRNA-binding sites of bovine NCF4 3′UTRs harboring the SNP g.18475 A>G, we predicted the output results using RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/submission.html) (Rehmsmeier et al. 2004) and TargetScan 5.2 (http://www.targetscan.org/).
Plasmid constructs for 3′UTR of NCF4
The NCF4 3′UTR plasmid constructs were established according to our previous method (Gao et al. 2014). The brief process is as follows. To construct the wild-type pMIR-3′UTR-A and mutant pMIR-3′UTR-G plasmids, the 3′UTR fragment of NCF4 containing g.18475 A>G SNP and the putative binding sequences with bta-miR-2426 were amplified, digested with MluI and HindШ enzymes, purified using the gel/PCR extraction kit (BioTek, China), and cloned into the pMIR-REPORT™ vector (Ambion) with T4 DNA ligase. The plasmids were isolated using a Plasmid Mini Kit (Omega) according to the manufacturer’s instructions. Finally, the plasmids containing genotypes AA and GG were obtained and then confirmed by sequencing.
Cell culture, transfection, and dual-luciferase reporter assays
Human epithelial kidney 293T (HEK 293T) cells were cultured in Dulbecco’s modified Eagle’s medium, with 10% fetal bovine serum, penicillin (100 U/L), and streptomycin (100 mg/L) at 37 °C in 5% CO2 atmosphere. For the luciferase reporter assays, HEK 293T cells were inoculated in 48-well plates and transfected with 400 ng luciferase expression constructs (pMIR-3′UTR-A or pMIR-3′UTR-G plasmids), 400 ng miR-2426 mimics, or 400 ng mimics control (RiboBio) using Lipofectamine™ 2000 (Invitrogen). The mimics control was used as a negative control. The luciferase activity was measured 48 h after transfection with the Dual-Luciferase® Reporter Assay System (Promega). The results were normalized to the activity of Renilla luciferase.
RT-qPCR analysis of NCF4 mRNA
The relative expression of NCF4 mRNA in the mammary tissues of cows with different genotypes in the SNP (g.18475 A>G) locus was determined via qPCR using the primer (F: 5′-TACCGCCAGTTCTACGCCTTAC-3′; R: 5′-CATCTCGGCAATCTCCTGTTTC-3′; product size = 138 bp). The primers of the housekeeping internal control β-actin gene were designed according to the method of Li et al. (2012). qPCR was performed with SYBR Green (TaKaRa, Dalian, China) on a Roche LightCycler 480 machine (Roche Applied Science, Mannheim, Germany). The following qPCR profiles were used: 50 °C for 2 min and 94 °C for 3 min, followed by 40 cycles of 94 °C for 30 s, 55 °C for 40 s, and 68 °C for 15 s. The last stage for the dissociation curve analysis was 95 °C for 15 s, 60 °C for 15 s, and 95 °C for 15 s. All qPCR reactions were performed in triplicate. The relative expression levels of NCF4 mRNA were normalized by β-actin, the housekeeping internal control gene. Data were analyzed using the 2−ΔΔCt method.
Relative expression of bta-miR-2426
A miScript SYBR Green PCR Kit (Qiagen) with a primer specific to bta-miR-2426 (AAGGAAGTGGCTTGGGGAAAG) was used to detect mature miRNA using RT-qPCR, with bta-let-7g as the internal control. The miRNA expression from each sample was analyzed in triplicate. Relative gene expression was determined using a previously described method (Huang et al. 2011). The relative quantity of miRNA was shown as a fold change. Student’s t test was used to determine the significance of the fold changes.
Results
Identification of 3′UTR SNP g.18475 A>G in NCF4 gene
We amplified and sequenced the 3′UTR region of bovine NCF4 using a specific primer pair NCF4–3′UTR. Based on the sequence alignment results, only one SNP g.18475 A>G was identified in the 3′UTR of the bovine NCF4.
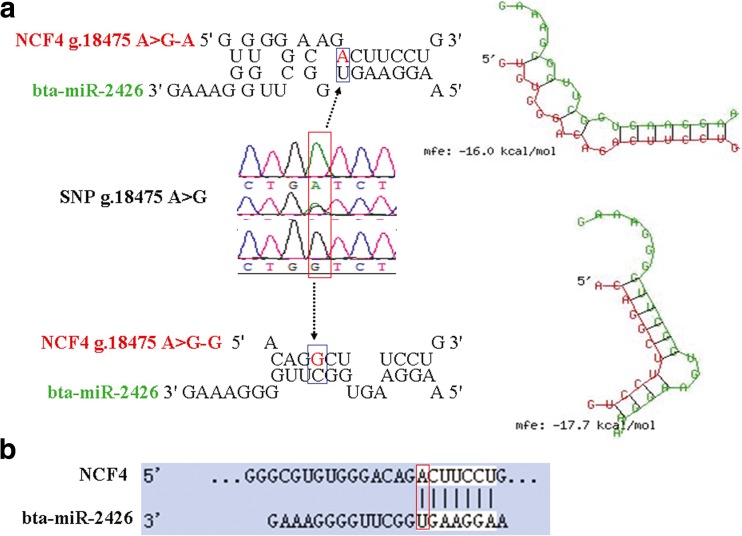
Software prediction determines SNP g.18475 A>G location in the seed region of bta-miR-2426
To identify whether the SNP g.18475 A>G is present in the seed sequence of miRNA, we computationally predicted nominated miRNAs and found that only bta-miR-2426 had a high likelihood of targeting NCF4 gene SNP g.18475 A>G. By RNAhybrid software, we found that SNP g.18475 A>G was located within the bta-miR-2426 seed region of the binding site (Fig. 1). bta-miR-2426 is a novel miRNA, as determined by performing the Illumina ultra-high-throughput sequencing approach on a cell line derived from the adult bovine kidney upon viral infection (Glazov et al. 2009). Through RNAhybrid software analysis, the binding energy of bta-miR-2426–g.18475 A>G-A was calculated to be − 16 kcal/mol, whereas that of bta-miR-2426–g.18475 A>G-G was calculated to be − 17.7 kcal/mol. According to RNAhybrid prediction, the A allele was the active allele that increases the binding energy of bta-miR-2426 compared with the G allele. The TargetScan software prediction result showed that the A allele at g.18475 A>G created a binding site with a bta-miR-2426 seeding sequence, whereas the G allele eliminated that binding site. Therefore, we speculated that SNP g.18475 A>G may influence the expression of NCF4 by altering bta-miR-2426–NCF4 regulatory interactions. We further performed several experiments to validate whether NCF4 is one of the targets of bta-miR-2426.
Fig. 1.
SNP (g.18475 A>G) located in the seed region of bta-miR-2426 binding to the 3′UTR of the bovine NCF4 gene. a RNAhybrid prediction results of bta-miR-2426/target NCF4 3′UTR. b Targets predicted in NCF4 for bta-miR-2426 by TargetScan
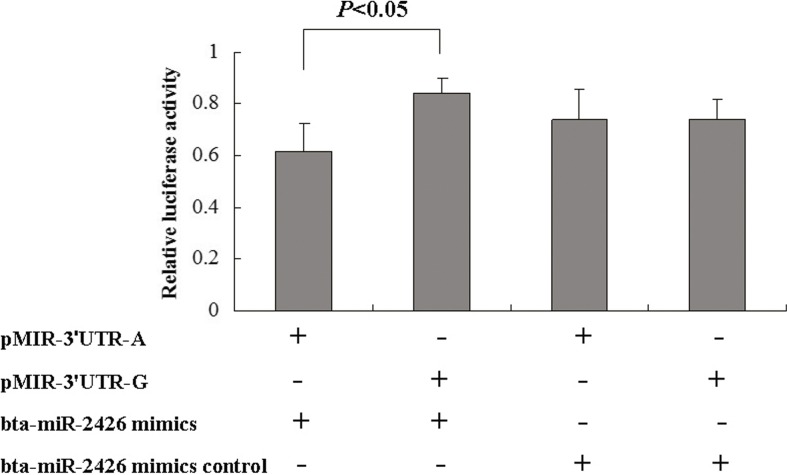
Effect of SNP g.18475 A>G on the luciferase assay containing NCF4–3′UTR fragments
In the present study, we found SNP g.18475 A>G in the 3′UTR of the NCF4 gene. We tested whether the 3′UTR SNP g.18475 A>G affected the luciferase reporter gene activity containing NCF4–3′UTR fragments. We cloned different NCF4 3′UTR fragments with A or G allele in locus 18475, constructed two recombinant pMIR-REPORT™ vector plasmids, and transiently co-transfected them into HEK 293T cells with 50-nM bta-miR-2426 mimics. The dual-luciferase assay results showed that the luciferase reporter gene activity in the presence of allele 18475G plasmid (0.8426 ± 0.0524) had a significantly higher value compared with that of allele 18475A construct (0.6179 ± 0.1041) (P < 0.05) (Fig. 2). Recalling that allele G eliminates the binding site, this result shows that bta-miR-2426 reduced the luciferase reporter gene activity, and the g.18475 A>G-AA genotype exhibited higher luciferase reporter gene activity compared with the g.18475 A>G-GG genotype. This result is consistent with the computational prediction result of miRNA–gene interaction. The SNP g.18475 A>G may affect the luciferase assay of NCF4–3′UTR fragments containing G allele and A allele plasmids.
Fig. 2.
Differential expression ratios of Luc reporters carrying the AA or GG genotype in SNP g.18475 A>G of NCF4 gene 3′UTR. HEK 293T cells were transiently co-transfected with constructs (pMIR-3′UTR-A or pMIR-3′UTR-G plasmids) and miR-2426 mimics scrambled with the negative control (mimics-control) for 24 h. Firefly luciferase activity was normalized to Renilla luciferase activity. Data are from the three transfection experiments with assays performed in six replications
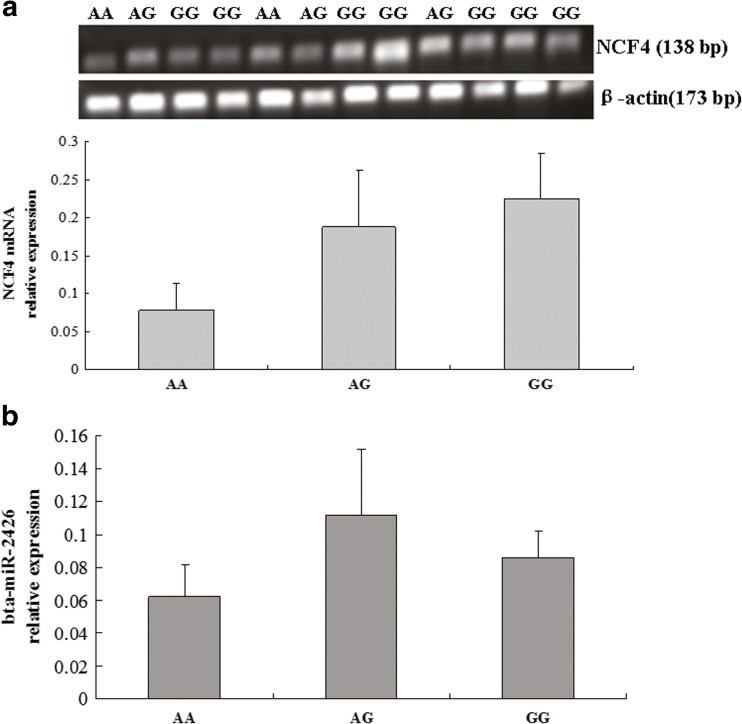
Expression of bovine NCF4 mRNA and bta-miR-2426 in the mammary tissues of cows with different genotypes
The relative quantification in the mammary tissues was performed using RT-qPCR to illustrate the relationship between different genotypes in the SNP g.18475 A>G locus and NCF4 gene expression. We obtained the genotype of the 13 mammary tissue samples of cows. The numbers of genotypes AA, AG, and GG were 2, 3, and 7, respectively. The cows with genotype GG had a relatively higher expression (0.2256 ± 0.0596) of NCF4 mRNA compared to the cows with genotype AA (0.0785 ± 0.0352) (P > 0.05) (Fig. 3a). The results showed that SNP g.18475 A>G in the NCF4 3′UTR may affect NCF4 mRNA expression. It is consistent with the results of the luciferase assay. Moreover, the relative expression levels of bta-miR-2426 for cows with genotypes AA, AG, and GG were 0.0623 ± 0.0190, 0.1116 ± 0.0396, and 0.0856 ± 0.0163, respectively (Fig. 3b). The statistical data demonstrated that the bta-miR-2426 expression had no difference between the two genotypes AA and GG (P > 0.05).
Fig. 3.
Relative expression of NCF4 mRNA (a) and bta-miR-2426 (b) in the mammary tissues of cows with genotypes AA, AG, and GG in the SNP g.18475 A>G locus. The NCF4 mRNA and bta-miR-2426 relative expression is presented as mean ± SEM. Vertical bars denote the SEM
Association analysis between 3′UTR SNP genotypes and mastitis
The association analysis between SNP and SCS was performed on 454 Chinese Holstein cows. We first amplified the PCR product containing the mutation fragment and then genotyped each individual by direct sequencing. The frequencies of AA, AG, and GG genotypes were 16.08, 36.34, and 47.58%, respectively. Subsequently, results of statistical analysis showed that cows with the GG genotype in SNP g.18475 A>G had a relatively lower SCS value than that of cows (4.09 ± 0.17) with the AA genotype (4.52 ± 0.30) (Table 1).
Table 1.
Least squares mean and standard error of SCS in different genotypes of NCF4 gene SNP g.18475 A>G in Chinese Holstein
| Genotype | Sample number | Genotypic frequencies (%) | Allelic frequencies (allele) (%) | SCS |
|---|---|---|---|---|
| AA | 73 | 16.08 | 34.25 (A) 65.75 (G) |
4.52 ± 0.30 |
| AG | 165 | 36.34 | 4.15 ± 0.20 | |
| GG | 216 | 47.58 | 4.09 ± 0.17 |
MiRNAs regulate gene expression post-transcriptionally by binding to their target mRNAs, usually at the 3′UTR, to mediate translational repression or direct degradation of the target mRNAs (Goda et al. 2015). SNPs in the 3′UTRs of several immune-related genes are associated with mastitis resistance or susceptibility by affecting miRNA-regulated gene or protein expression (Li et al. 2012; Wang et al. 2014). NCF4 (also called p40phox), a key component of NADPH oxidase, plays an important role in host defense against microbial pathogens (Conway et al. 2012). In the NCF4 gene 3′UTR region, one SNP g.18475 A>G was identified by DNA sequencing and alignment. Bioinformatics analysis found that SNP g.18475 A>G was located in the binding site of bta-miR-2426. We wanted to know whether 3′UTR SNP g.18475 A>G interferes with NCF4 gene expression by altering miRNA–mRNA regulatory interactions, which ultimately lead to differences in mastitis susceptibility in Chinese Holstein cattle. We first postulated that SNP g.18475 A>G may influence NCF4 gene expression by modulating the binding between bta-miR-2426 and NCF4. Then, we designed a series of experiments to verify this hypothesis. The luciferase reporter assay showed that NCF4 expression was significantly upregulated in the GG genotype compared with that in the AA genotype. In addition, RT-qPCR analysis was performed to examine the effect of this SNP on NCF4 mRNA expression between the two genotypes of cow mammary tissues. As expected, the cows with GG genotype mammary tissues revealed a relatively higher NCF4 mRNA expression compared with those with AA genotype tissues.
When dairy cattle suffer from mastitis, a large number of somatic cells are influxed into the mammary glands. Thus, SCS is used as an alternative trait to breed for mastitis susceptibility and low levels of SCS have been associated with increased risk of clinical mastitis (Suriyasathaporn et al. 2000; Jamrozik and Schaeffer 2012; Koeck et al. 2012). In this work, the association analysis between SNP and SCS was performed on 454 Chinese Holstein cows. The association analysis results showed that the cows with the GG genotype in SNP g.18475 A>G had a relatively lower SCS value than that of cows with the AA genotype. These findings further supported the notion that SNP g.18475 A>G may be a functional marker that can modulate the binding affinities of bta-miR-2426 and target NCF4. However, the genotype effect of one SNP may be influenced by other SNPs, and the effect is a reflection of interactions of multiple SNPs (Fallin et al. 2001). This requires us to increase the number of samples for further study.
In summary, we identified an SNP g.18475 A>G in the 3′UTR by modulating the binding activity of bovine NCF4 and bta-miR-2426, which may ultimately lead to differences in mastitis susceptibility in Chinese Holstein cattle.
Funding information
This study was supported by grants from the National Natural Science Foundation of China (31371255, 31401050, 31672397, and 31671286), the Major Project of National Transgenics in China (2014ZX08007-001), the National Cow Industrial Technology System Program (CARS-37), the Cow Innovation Team of the Shandong Province Modern Agricultural Industry Technology System (SDAIT-12-011-02), and the Natural Science Foundation of Shandong Province (ZR2013CQ035).
Compliance with ethical standards
All experiments were carried out according to the Regulations for the Administration of Affairs Concerning Experimental Animals published by the Ministry of Science and Technology, China, in 2004 and approved by the Animal Care and Use Committee from the Dairy Cattle Research Center, Shandong Academy of Agricultural Sciences, Shandong, PR China.
Contributor Information
Jifeng Zhong, Phone: +86-531-88604132, Email: zhongjifeng@sdox.cn.
Jinming Huang, Phone: +86-531-88604132, Email: huangjinm@sina.com.
References
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Conway KL, Goel G, Sokol H, Manocha M, Mizoguchi E, Terhorst C, Bhan AK, Gardet A, Xavier RJ. p40phox expression regulates neutrophil recruitment and function during the resolution phase of intestinal inflammation. J Immunol. 2012;189:3631–3640. doi: 10.4049/jimmunol.1103746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellson CD, Davidson K, Ferguson GJ, O’Connor R, Stephens LR, Hawkins PT. Neutrophils from p40phox−/− mice exhibit severe defects in NADPH oxidase regulation and oxidant-dependent bacterial killing. J Exp Med. 2006;203:1927–1937. doi: 10.1084/jem.20052069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallin D, Cohen A, Essioux L, Chumakov I, Blumenfeld M, Cohen D, Schork NJ. Genetic analysis of case/control data using estimated haplotype frequencies: application to APOE locus variation and Alzheimer’s disease. Genome Res. 2001;11(1):143–151. doi: 10.1101/gr.148401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Ju ZH, Zhang Y, Huang J, Zhang X, Qi C, Li J, Zhong J, Li G, Wang C. Association of TNP2 gene polymorphisms of the bta-miR-154 target site with the semen quality traits of Chinese Holstein bulls. PLoS One. 2014;9:e84355. doi: 10.1371/journal.pone.0084355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazov EA, Kongsuwan K, Assavalapsakul W, Horwood PF, Mitter N, Mahony TJ. Repertoire of bovine miRNA and miRNA-like small regulatory RNAs expressed upon viral infection. PLoS One. 2009;4:e6349. doi: 10.1371/journal.pone.0006349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda N, Murase H, Kasezawa N, Goda T, Yamakawa-Kobayashi K. Polymorphism in microRNA-binding site in HNF1B influences the susceptibility of type 2 diabetes mellitus: a population based case-control study. BMC Med Genet. 2015;16:75. doi: 10.1186/s12881-015-0219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson HL, Yao S, Goldman BH, et al. Genetic polymorphisms in oxidative stress-related genes are associated with outcomes following treatment for aggressive B-cell non-Hodgkin lymphoma. Am J Hematol. 2014;89:639–645. doi: 10.1002/ajh.23709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- Hou Q, Huang J, Ju Z, et al. Identification of splice variants, targeted microRNAs and functional single nucleotide polymorphisms of the BOLA-DQA2 gene in dairy cattle. DNA Cell Biol. 2012;31:739–744. doi: 10.1089/dna.2011.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JM, Ju ZH, Li QL, et al. Solexa sequencing of novel and differentially expressed microRNAs in testicular and ovarian tissues in Holstein cattle. Int J Biol Sci. 2011;7:1016–1026. doi: 10.7150/ijbs.7.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamrozik J, Schaeffer LR. Test-day somatic cell score, fat-to-protein ratio and milk yield as indicator traits for sub-clinical mastitis in dairy cattle. J Anim Breed Genet. 2012;129:11–19. doi: 10.1111/j.1439-0388.2011.00929.x. [DOI] [PubMed] [Google Scholar]
- Jin W, Ibeagha-Awemu EM, Liang G, Beaudoin F, Zhao X, Guan LL. Transcriptome microRNA profiling of bovine mammary epithelial cells challenged with Escherichia coli or Staphylococcus aureus bacteria reveals pathogen directed microRNA expression profiles. BMC Genomics. 2014;15:181. doi: 10.1186/1471-2164-15-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeck A, Miglior F, Kelton DF, Schenkel FS. Alternative somatic cell count traits to improve mastitis resistance in Canadian Holsteins. J Dairy Sci. 2012;95:432–439. doi: 10.3168/jds.2011-4731. [DOI] [PubMed] [Google Scholar]
- Lawless N, Foroushani AB, McCabe MS, O’Farrelly C, Lynn DJ. Next generation sequencing reveals the expression of a unique miRNA profile in response to a gram-positive bacterial infection. PLoS One. 2013;8:e57543. doi: 10.1371/journal.pone.0057543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless N, Vegh P, O’Farrelly C, Lynn DJ. The role of microRNAs in bovine infection and immunity. Front Immunol. 2014;5:611. doi: 10.3389/fimmu.2014.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LM, Huang J, Zhang X, et al. One SNP in the 3′-UTR of HMGB1 gene affects the binding of target bta-miR-223 and is involved in mastitis in dairy cattle. Immunogenetics. 2012;64:817–824. doi: 10.1007/s00251-012-0641-1. [DOI] [PubMed] [Google Scholar]
- Li L, Huang J, Ju Z, et al. Multiple promoters and targeted microRNAs direct the expressions of HMGB3 gene transcripts in dairy cattle. Anim Genet. 2013;44:241–250. doi: 10.1111/age.12007. [DOI] [PubMed] [Google Scholar]
- Olsson LM, Lindqvist AK, Kallberg H, Padyukov L, Burkhardt H, Alfredsson L, Klareskog L, Holmdahl R. A case–control study of rheumatoid arthritis identifies an associated single nucleotide polymorphism in the NCF4 gene, supporting a role for the NADPH-oxidase complex in autoimmunity. Arthritis Res Ther. 2007;9:R98. doi: 10.1186/ar2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo-Boyso J, Valdez-Alarcón JJ, Cajero-Juárez M, Ochoa-Zarzosa A, López-Meza JE, Bravo-Patiño A, Baizabal-Aguirre VM. Innate immune response of bovine mammary gland to pathogenic bacteria responsible for mastitis. J Inf Secur. 2007;54:399–409. doi: 10.1016/j.jinf.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Rehmsmeier M, Steffen P, Hochsmann M, Giegerich R. Fast and effective prediction of microRNA/target duplexes. RNA. 2004;10:1507–1517. doi: 10.1261/rna.5248604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux JD, Xavier RJ, Taylor KD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer M, Hoffmann M, Kaya E, Tzvetkov M, Brockmoller J. Genetic polymorphisms of NAD(P)H oxidase: variation in subunit expression and enzyme activity. Pharmacogenomics. 2008;8:297–304. doi: 10.1038/sj.tpj.6500467. [DOI] [PubMed] [Google Scholar]
- Suriyasathaporn W, Schukken YH, Nielen M, Brand A. Low somatic cell count: a risk factor for subsequent clinical mastitis in a dairy herd. J Dairy Sci. 2000;83:1248–1255. doi: 10.3168/jds.S0022-0302(00)74991-5. [DOI] [PubMed] [Google Scholar]
- Thompson-Crispi K, Atalla H, Miglior F, Mallard BA. Bovine mastitis: frontiers in immunogenetics. Front Immunol. 2014;5:493. doi: 10.3389/fimmu.2014.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Sunaga H, Ohkawara R, Shimba S. Aryl hydrocarbon receptor modulates NADPH oxidase activity via direct transcriptional regulation of p40phox expression. Mol Pharmacol. 2013;83:1133–1140. doi: 10.1124/mol.112.083303. [DOI] [PubMed] [Google Scholar]
- Wang XG, Huang JM, Feng MY, Ju ZH, Wang CF, Yang GW, Yuan JD, Zhong JF. Regulatory mutations in the A2M gene are involved in the mastitis susceptibility in dairy cows. Anim Genet. 2014;45:28–37. doi: 10.1111/age.12099. [DOI] [PubMed] [Google Scholar]
- Wu PB, Dai JF, Wang Q, Zhang G, Tan SY, Li M, Ye HL. Association between NCF4 rs4821544T/C polymorphism and inflammatory bowel disease risk in Caucasian: a meta-analysis. Inflamm Res. 2015;64:825–831. doi: 10.1007/s00011-015-0866-1. [DOI] [PubMed] [Google Scholar]
- Zhu S, Pan W, Qian YC. MicroRNA in immunity and autoimmunity. J Mol Med. 2013;91:1039–1050. doi: 10.1007/s00109-013-1043-z. [DOI] [PubMed] [Google Scholar]