Abstract
Heat-related illness and injury are becoming a growing safety concern for the farmers, construction workers, miners, firefighters, manufacturing workers, and other outdoor workforces who are exposed to heat stress in their routine lives. A primary response by a cell to an acute heat shock (HS) exposure is the induction of heat-shock proteins (HSPs), which chaperone and facilitate cellular protein folding and remodeling processes. While acute HS is well studied, the effect of repeated bouts of hyperthermia and the sustained production of HSPs in the myoblast-myotube model system of C2C12 cells are poorly characterized. In C2C12 myoblasts, we found that robust HS (43 °C, dose/time) significantly decreased the proliferation by 50% as early as on day 1 and maintained at the same level on days 2 and 3 of HS. This was accompanied by an accumulation of cells at G2 phase with reduced cell number in G1 phase indicating cell cycle arrest. FACS analysis indicates that there was no apparent change in apoptosis (markers) and cell death upon repeated HS. Immunoblot analysis and qPCR demonstrated a significant increase in the baseline expression of HSP25, 70, and 90 (among others) in cells after a single HS (43 °C) for 60 min as a typical HS response. Importantly, the repeated HS for 60 min each on days 2 and 3 maintained the elevated levels of HSPs compared to the control cells. Further, the continuous HS exposure resulted in significant inhibition of the differentiation of C2C12 myocytes to myotubes and only 1/10th of the cells underwent differentiation in HS relative to control. This was associated with significantly higher levels of HSPs and reduced expression of myogenin and Myh2 (P < 0.05), the genes involved in the differentiation process. Finally, the cell migration (scratch) assay indicated that the wound closure was significantly delayed in HS cells relative to the control cells. Overall, these results suggest that a repeated HS may perturb the active process of proliferation, motility, and differentiation processes in an in vitro murine myoblast-myotube model.
Keywords: Heat shock proteins (HSP), Repeated heat shock, Myoblasts, C2C12, Proliferation, Differentiation, Myopathy
Introduction
An inevitable routine muscular activity, worsening climatic and environmental conditions, increasing chronic illnesses, and several other lifestyle preferences increase the ambient temperature shift and/or thermal stress. Thus, an adaptation to heat stress is regarded as one of the fundamental requirements of cellular life. The defense response to heat at the individual cellular level is mediated by the evolutionarily conserved and abundant group of proteins called the heat shock proteins (HSPs), which mediate a variety of functions ranging from housekeeping to stress modulation and cytoprotection (Parsell and Lindquist 1993). Under stress, the HSPs exhibit chaperone-like activity ensuring both the fidelity of protein folding and prevention of toxic aggregation of various target proteins (Bakthisaran et al. 2015). Moreover, HSPs have been found to play diverse roles in the cell including protein trafficking, cytoskeletal interactions, redox control, and apoptosis (Taylor and Benjamin 2005). A consistent theme, however, among heat shock (HS) research is that the HS response is one of the most common yet critical cytoprotective mechanisms for maintenance of the proteasome and overall cell health during conditions such as heat exposure (Bakthisaran et al. 2015; Parsell and Lindquist 1993; Richter et al. 2010; Taylor and Benjamin 2005).
Numerous studies have implicated HSPs in the pathogenesis of a broad spectrum of human diseases. Cardiomyopathies, neuropathies, myopathy, nephropathy, infectious, and several inflammatory disorders have been shown to result from either mutation and/or dysregulation of HSPs (Benndorf et al. 2014; Boncoraglio et al. 2012; Buraczynska et al. 2009; Datskevich et al. 2012; Multhoff 2006; Rajasekaran et al. 2007). Interestingly, HSP induction by acute HS has been reported to protect muscle damage (Vardiman et al. 2015) and cardiac dysfunction (Radford et al. 1996). Most of these protective roles of HSPs are linked to their ability to act as cell chaperones.
While the acute or moderate HS can be managed physiologically by the endogenous defense systems, severe and/or continuous heat stress, accompanied by over-expression of certain heat shock proteins, overburdens the physiological maneuvers leading to ineffective defense, organ damage, and lethality (Dehbi et al. 2010). For example, repeated cycles of hyperthermia were shown to dramatically increase HSP production when compared to a single, acute HS, resulting in enhanced apoptosis (Haghniaz et al. 2015). Prolonged exposure to heat stress has been shown to increase the production of reactive oxygen species (ROS) in skeletal muscle (Azad et al. 2010) that can enhance various deleterious consequences such as protein modifications, proteolysis, autophagy, and inflammation and affect skeletal muscle physiology (Montilla et al. 2014; Dodd et al. 2010; McClung et al. 2010; Whidden et al. 2010). Thus, studying the reaction of muscle cells to chronic heat exposure has far-fetching applications because (i) muscular work is an unavoidable part of life that elicits core and muscle temperature change and triggers metabolic heat production and (ii) huge population of individuals (farmers, construction workers, miners, firefighters, etc.) exposed to HS continually in their daily lives. A key ingredient to muscle movement, healing, and regeneration are myoblast cells. Myoblasts form a reservoir of cells that can undergo proliferation and differentiation and fuse to form new muscle fibers, restoring muscle health.
Very relevant to the current study is that the myoblast-myotube model of C2C12 cells that has been widely used in the past to investigate the acute or moderate effect of heat shock and HSPs on different aspects of myogenesis (Liu and Brooks 2012; Singh et al. 2010; Sun et al. 2005; Straadt et al. 2010; Ito et al. 2001). However, less investigated is the change induced by repeated and/or multiple bouts of HS and sustained activation of HSPs in the myoblast-myotube model of C2C12 cells. Therefore, in the current study, we tested how the vital cellular processes, such as proliferation, differentiation, and regeneration (migration) of C2C12 myoblasts respond to repeated HS.
Methods
Reagents
The antibodies for heat shock family proteins including HSP25 (ADA-SPA-801-F), HSP70/72 (ADA-SPA-812-F), and HSP90 (ADA-SPA-836-F) were purchased from Enzo life sciences (Farmingdale, NY). CRYAB (ab13496) was bought from Abcam (Cambridge, MA) and GAPDH (D16H11), p38 MAPK (8690), Phospho-p38 MAPK (Thr180/Tyr182; 4511), Caspase-3 (9665), and Cleaved Caspase-3 (Asp175; 9664) from Cell Signaling Technology (Danvers, MA). The immunoblot anti-rabbit (PI-1000) or mouse (PI-2000) secondary antibodies of horse-radish peroxidase conjugated with IgG were from Vector Laboratories (Burlingame, CA). Quantitative PCR primers were designed using Harvard medical school Primer Bank website and bought from Integrated DNA Technologies (IDT) (Coralville, IA). All other chemicals were purchased from Sigma-Aldrich unless otherwise stated.
Cell culture and HS exposure
C2C12 myoblast cells were cultured in proliferation medium DMEM (Gibco) supplemented with 10% FBS and differentiation medium (DMEM supplemented with 2% horse serum). Proliferation medium (PM) or differentiation medium (DM) was replaced daily. Cells were heat shocked at 43 °C for 1 h/day. The temperature 43 °C was chosen for the recurrent HS was based on the studies (Tang et al. 2005; Sonna et al. 2002). This HS treatment was specifically chosen such that we obtain and study the responses of robust heat shock rather than the mild HS at 41 °C by (Finka et al. 2015). Further, the reason for shorter (per day) treatment in contrast to (Finka et al. 2015) is to give the cells enough time to recover before the next day’s HS exposure. When not undergoing HS, the cells were kept at a constant 37 °C with 5.0% CO2 in a Heracell 150i CO2 incubator (Liu and Brooks 2012).
Experimental plan
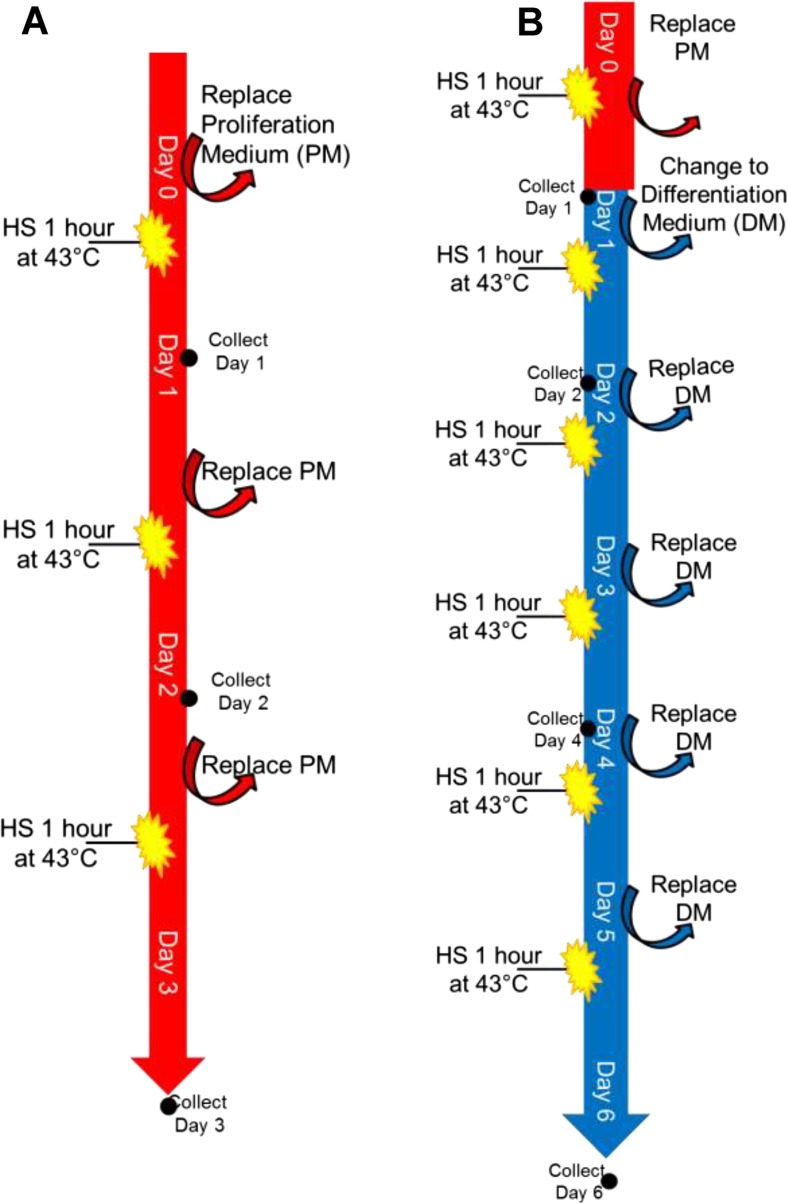
Two basic experiments were performed—one in PM and one in DM—to test the effect of HS on proliferation and differentiation. Images of the cells were recorded every day using a bright field microscopy, and experimental bias was minimized by recording images in the same marked location each day. The experimental plans for proliferation and differentiation are detailed below (Fig. 1a, b).
Fig. 1.
Experimental plan for HS in C2C12 cells. a Schematic diagram represents the experimental plan of HS in proliferation cells. The treatment includes three repeated exposures of HS before the control cells become confluent. b Describes the plan of HS in differentiation cells, wherein the treatment included six repeated bouts of HS before the control cells become completely differentiated into myotubes. This setup is followed for all the experiments and each time points or experiments carried out n = 3–4/group
Proliferation experiment
For the proliferation experiment, equal numbers of C2C12 cells were plated. On day 0, HS plates were heat shocked at 43 °C for 60 min while control plates were maintained at 37 °C. On day 1, the plates that were heat shocked on day 0 designated as HS1 (heat shock, 1 day), and control plates were harvested. On day 1, the other batch of plates were subjected to heat shock at 43 °C for 60 min, and the control plates were maintained at 37 °C. On day 2, plates designated as HS2 and control plates were harvested, while the remaining final batch of plates were heat shocked again for 60 min (43 °C). On day 3, the final HS plates (designated as HS3) and control plates were collected. This proliferation experiment was repeated at least three times. Collections occurred 24 h after each HS. Bright field microscopy images were captured on day 0 (PM-0), day 1 (PM-1), day 2 (PM-2), and day 3 (PM-3).
Differentiation experiment
The differentiation experiment followed a similar procedure as the proliferation experiment except that the cells were exposed to differentiation media on day 1 instead of day 0 to allow for adequate cell numbers before cell differentiation. To begin with, C2C12 cells were plated at equal numbers, and after day 1, the cells were completely switched to differentiation media and both the control and heat shock plates were maintained in DM thereafter throughout the experiment. The procedure of repeated heat shock continued up to 6 days of HS (5 days in DM) and was repeated at least three times and collected for protein and RNA measurements. Collections occurred 24 h after each HS. Bright field microscopy images were captured on day 0 (PM-0), day 1 (PM-1), day 2 (DM-1), day 3 (DM-2), day 4 (DM-3), day 5 (DM-4), and day 6 (DM-5).
Cytotoxicity assays
MTT assay was performed in PM to test cell viability after HS. C2C12 cells were cultured in two separate 96-well plates; one for HS cells and one for control cells. Heat shock was performed according to the proliferation experiment (60 min/day; for 3 days). Twenty four hours after each HS, n = 24 HS cells and n = 24 control cells were analyzed by adding 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (20 μl of 5 mg/ml in PBS). Formazan crystals were dissolved in 100 μl DMSO, and the absorbance was measured at 590 nm. Percentage of viability in HS cells was calculated relative to the control cells on each day.
Apoptosis assay was also conducted by PI staining with annexin-binding buffer. Cells were incubated with the PI solution for 15 min at 37 °C and then analyzed by flow cytometry. The proportion of dead cells was determined by the positive control (H2O2-induced apoptosis), and the gating established by untreated myoblast cells. Data were given as the percentage of live cells in the population.
Cell counting for quantifying cell proliferation
C2C12 cells were counted, plated equally, and collected on each day according to the proliferation HS experiment. After each collection, the number of live cells was counted using the trypan blue exclusion method. The number of live cells in each plate was then averaged and graphed over each day with error bars representing standard deviation.
Wound-healing assay
A wound-healing assay was performed in PM to test cell recovery after injury. After the C2C12 cells reached 70–80% confluence, a scratch was made in the center of the plate using a 20-μl pipet tip and continued in the general PM experimental setup. Bright field microscopy pictures were taken before and after the scratch, as well as each day up to the third day when the experiment was stopped (Ranzato et al. 2009).
Determination of HSPs by immunoblotting
Proteins were extracted from C2C12 cells using whole cell lysis buffer (10 mM HEPES, 200 mM NaCl, 0.75 mM EDTA, 0.75 mM MgCl2, and 25% glycerol) containing dithiothreitol (1 mM), phenyl methyl-sulfonyl fluoride (0.1 mM), and protease-phosphatase inhibitor cocktail (Roche and Sigma-Aldrich). Proteins were clarified by centrifugation at 6000 rpm for 5–6 min. Equal concentrations of protein lysates were mixed with 4× loading dye containing 5% β-mercaptoethanol (Bio-Rad, Hercules, CA, USA) and boiled the samples for 5–6 min followed by separated with home-made SDS-PAGE (10–12%) gels. Proteins were then electro-transferred onto a PVDF membrane (Immobilon-P IPVH00010; EMD Millipore, Billerica, MA, USA) and blocked with 10% (W/V) nonfat milk in 1× TBS-T for at least 2 h. Membranes were then incubated with respective primary antibodies (1:2000) in 1× TBS-T containing 2% bovine serum albumin for overnight at 4 °C or 2 h at room temperature (RT). Following 2× 10 min TBS-T washes, the membranes were incubated with anti-rabbit or anti-mouse (1:5000) antibody for 1 h at RT, washed 3× 10 min with 1× TBS-T, then detected by chemiluminescence using an ECL kit (ThermoFisher Scientific). Proteins were normalized to GAPDH. The immunoreactive signals were quantified by densitometry using ImageJ software (Muthusamy et al. 2012; Shanmugam et al. 2016).
Determination of mRNA levels
Quantitative PCR analysis was performed on the RNA samples collected from proliferating and differentiating myoblasts. RNA was isolated using an RNeasy extraction kit following the manufacturer’s instructions, and cDNA was synthesized with 1.25 μg of RNA using Quantitect cDNA synthesis kit (Qiagen). Messenger RNA (mRNA) levels were quantified using gene-specific primers (1 pmol) and cDNA (50 ng) in a 10-μl final reaction mixture. The genes were amplified (Table 1) and detected using a quantifast SYBR green PCR kit (Qiagen) in a Light Cycler 480 (Roche, Basel, Switzerland). The mRNA expression of Hsp25, Hsp70, Hsp90, and Cryab was determined in both proliferation and differentiation cells; additionally, the expression of Myogenin and Myh2 was performed in differentiation conditions to test the presence of differentiated myoblast cells. Fold change in the mRNA expression was calculated on the basis of cycle threshold (Ct) value, and Gapdh mRNA levels were used for normalization. Relative quantification of transcript levels was plotted as fold difference in gene expression normalized to endogenous reference gene and relative to untreated samples and was calculated using the 2−ΔΔCT method (Muthusamy et al. 2012; Shanmugam et al. 2016).
Table 1.
Complete list of real-time qPCR primer sequences
| Gene | Forward primer (5′...3′) | Reverse primer (5′...3′) |
|---|---|---|
| Hsp25 | GGTTGCCCGATGAGTGGTC | CTGAGCTGTCGGTTGAGCG |
| Hsp70 | TCTCGGCACCACCTACTCC | CCCGATCAGACGTTTGGCA |
| Hsp90 | AAACCGACTGGTGACATCCC | CCAGGTGTTTCTTTGCTGCC |
| Cryab | ACACCGGACTCTCAGAGATG | GGACCTCAATCACGTCCCC |
| Myogenin | GAGACATCCCCCTATTTCTACCA | GCTCAGTCCGCTCATAGCC |
| Myh2 | CGGAGTCCCAGGTCAACAAG | TCATTCCACAGCATCGGGAC |
| Gapdh | TGACCTCAACTACATGGTCTACA | CTTCCCATTCTCGGCCTTG |
Cell cycle analysis
C2C12 cells from both proliferation and differentiation experiments were trypsinized, and the cells were fixed in 100% ethanol at 4 °C until processing for staining. Cells were then centrifuged and washed in PBS once retrieved from storage. Cells were then stained using a solution consisting of 0.1% TritonX-100 in PBS with 0.02% RNase and 0.002% PI and incubated at room temperature in dark for 30 min. Cells were gated using untreated myoblast cells, and the relative cell population in G1, S, and G2 phase was analyzed by using control cells as a standard (Nakai and Ishikawa 2001).
Statistical analysis
Data is expressed as the mean ± SEM. To identify the statistical significance between control and their respective heat shocked groups, the data among them was analyzed using Student’s t test. In all cases, the differences were considered to be significant at p < 0.05. Statistical analysis was performed using GraphPad Prism software. Experiments involving statistical analysis were conducted with n = 3–4/group or condition.
Results
Multiple heat shock stress inhibits the proliferation of C2C12 cells
In light of the fundamental role of cell number and growth of myoblasts in determining the muscle regeneration (Yablonka-Reuveni et al. 1990), first, we determined the effects of HS on the growth of C2C12 mouse myoblasts (Figs. 1a and 2a). Our results indicate that HS significantly decreased the number of myoblasts by 50% (P < 0.05) on day 1 with a significant difference compared to the control and maintained the same level at days 2 and 3 of HS (P < 0.05). Akin to the cell counting, the cell count was decreased by 25% on day 1 HS that remained same on day 2 with a further decreased to 35% on day 3 HS when compared to the control cells (Fig. 2b). Further, the effect of HS on proliferation potential of myoblasts evaluated over the course of 3 days by bright field microscopy revealed the presence of lesser number of cells overall in any given field indicating an apparent impairment of proliferation in the HS cells when compared to the control cells (Fig. 2c).
Fig. 2.
Recurrent HS inhibits cell proliferation. a C2C12 cell viability after days 1, 2, and 3 of HS shown as a percentage. Control (n = 24) and HS (n = 24) cells were cultured in PM in 96-well plates. b MTT assay was used to measure the cell viability. The number of live cells following repeated HS cells (represented by a black line with a circle) is compared to control cells (represented by a red line with squares) at the end of each HS bout respectively. c Bright field microscopy images (× 10 magnification) showing an effect of HS on cell proliferation. All cells were maintained in PM throughout the experiment. The lower panel illustrates HS cells’ slower rate of proliferation when compared to the control cells on the upper panel. d A representative image of the immunoblot analysis for the expression of CASPASE-3 and Cleaved CASPASE-3 under proliferation condition on control and HS cells. The values are expressed as mean ± SEM
Chronic heat stress does not cause apoptosis or cell death
As the number of cells could be a resultant of induction of cell death, we performed immunoblot analysis of the cleavage of caspase-3, a central death protease of the cascade that cleaves many key cellular proteins. We found the expression of both the cleaved (active) and the uncleaved (inactive) form of caspase-3 remains unaltered under normal and repeated HS conditions in proliferating myoblasts (Fig. 2d). Further, signs of induction of programmed cell death assessed by propidium iodide (PI) staining followed by FACS remained the same between the control C2C12 myoblasts and HS (data not shown). Moreover, we did not notice a presence of large nuclei and flattened morphology, the typical characteristics of senescence following repeated HS exposure (Fig. 2c). These results indicate that neither apoptosis nor cellular senescence is a factor in reducing the cell number due to repeated HS in proliferating C2C12 myoblasts.
Recurrent heat stress disrupts the cell cycle
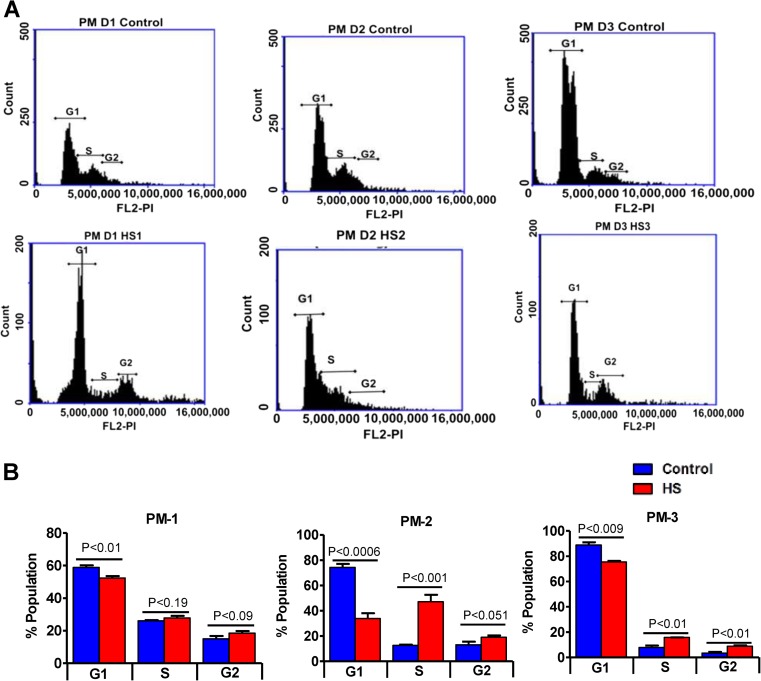
We next determined if the anti-proliferative effect of repeated HS is cell cycle related. Quantitative cell cycle FACS analysis revealed that 1–3 days HS significantly decreased the percentage of cells in G0-G1 phase with a concomitant increase in S phase cells compared to control (p < 0.01, Fig. 3a, b). Furthermore, the proportion of cells in the G2/M phase was also significantly increased upon repeated HS at the end of day 3 when compared to days 1 and 2 indicating a delay in cell cycle duration, in other words, delaying the entry of cells into mitosis (p < 0.01, Fig. 3a, b). This implies that repeated HS evokes an antiproliferative effect on C2C12 myoblasts that are grown under normal proliferating conditions.
Fig. 3.
Repeated heat stress disturbs cell cycle. a Cell cycle analysis of C2C12 myoblasts under proliferation condition with and without HS 24 h post each day of HS; the cells from control and HS group were collected and fixed with 100 % ethanol and stored at 4 °C. Cells were then stained with PI staining and analyzed using BD accuri6 for 10,000 cells from each sample. b The proportion of cells in each phase from cell cycle analysis is represented as a histogram. The values are expressed as mean ± SEM
Repeated heat stress activates transcription and translation of heat shock proteins
Following HS, cells trigger HSP synthesis to protect homeostasis. Hence, we determined whether the proliferating C2C12 myoblasts when heat stressed evokes HS response by stimulating HSPs. The transcript expression of all the three HSPs was found to be significantly increased following HS at the end of day 1 by 1.5 fold that was sustained at or above twofold after 2nd and a 3rd bout of HS (Fig. 4a). Representative protein expression patterns of HSP25, HSP70, and HSP90 are shown in Fig. 4b. Under unstressed conditions, Western blot analysis indicated that the expression of HSP25 was particularly strong followed by a faint to moderate expression of HSP90, with a barely undetectable expression of HSP70 (Fig. 4b, c). In response to HS for 60 min at 43 °C, the expression of all the three HSPs was found to be significantly elevated on each day (Fig. 4). Among the three HSPs, HSP70 showed a more pronounced induction (35-fold) than HSP25 and HSP90 (two to threefold) over the basal levels of respective HSPs (Fig. 4b, c). While the HS-induced expression of HSP25, HSP70 observed after day 1 was maintained at significantly higher levels at the end of 2nd and 3rd day of HS than the unstressed, the expression of HSP90 declined to the basal level at the end of 3rd HS exposure (Fig. 4b, c). These results indicate that the repeated bout of HS results in sustained elevation of HSP25, 70 with an exception of HSP90 protein in proliferating C2C12 myoblasts.
Fig. 4.
Recurrent heat stress-induced HSP levels in proliferating cells: a quantitative real-time qPCR showing the levels of HSP mRNA expression (Hsp25, Hsp70, Hsp90, Cryab) during cell proliferation in HS and control cells. The target gene expression was normalized to Gapdh expression and expressed as fold change from the control cells. b Immunoblot analysis of HSP 25, 70, 90, CRYAB, and GAPDH during proliferation in control and HS cells. c Densitometry analysis for protein expression of HSP 25, 70, 90 and CRYAB during proliferation, normalized to GAPDH (bottom); HS cells were compared to control cells from respective days. Data obtained from n = 3–4/group or condition, and the values are expressed as mean ± SEM
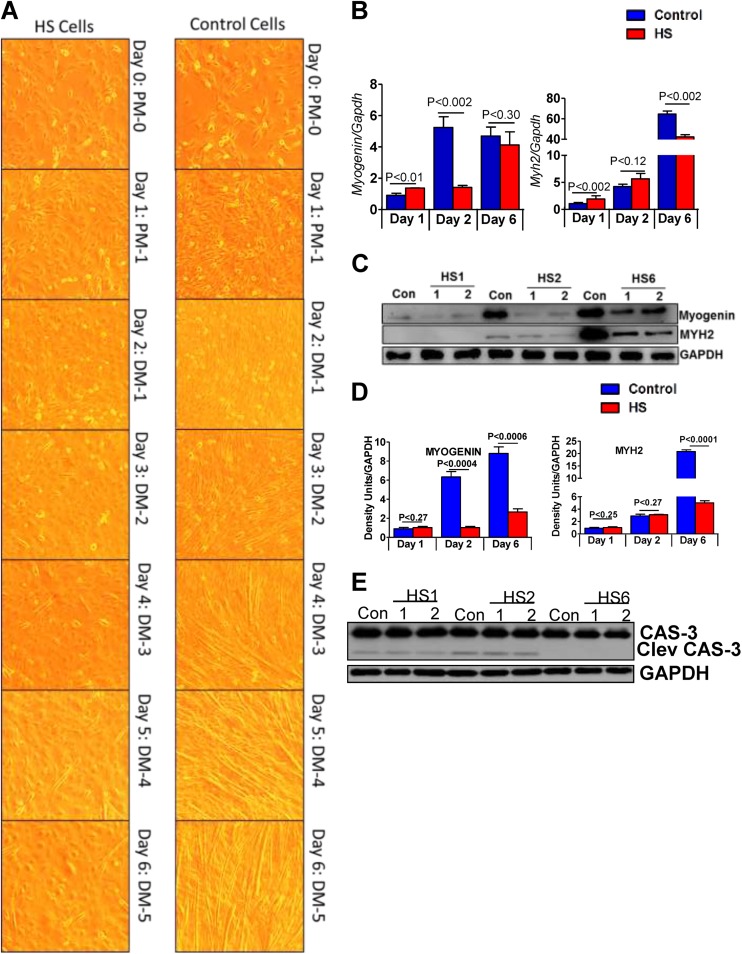
Prolonged heat–induced stress hampers C2C12 cell differentiation
As C2C12 myoblasts possess the inherent ability to undergo differentiation into mature myofibers that are critical in regenerative processes, we next investigated the effect of HS throughout the myotube development by subjecting the C2C12 myoblasts to differentiation (Fig. 1b). The microscopic data shows that the unstressed C2C12 myoblasts fused to form smaller myotubes after 4 days in differentiation media that became longer and matured into myofiber structures after days 5 and 6 of differentiation (Fig. 5a). However, the heat shocked-C2C12 cells were unable to differentiate at all the time points analyzed under conditions that were optimal for its differentiation (Fig. 5a). Notably, the sign of delayed differentiation was associated with significant reduction in the protein content of myogenin, a muscle regulatory factor involved in differentiation in conjunction with a lowered abundance of Myh2 (type IIa myosin heavy chain isoform), an actin-based motor protein required for cytoskeleton organization and muscle contraction (p < 0.05; Fig. 5b, d). Further, under differentiation condition, no significant changes were seen among control and heat shock of each group in both the uncleaved and cleaved caspase-3 (Fig. 5e). With respect to tested HSPs mRNA expression, a profile similar to that observed under proliferation conditions were observed wherein recurrent heat stress significantly induced HSP25 and 70 transcripts while the HSP90 mRNA returned to basal after a 2nd and 3rd bout of HS (Fig. 6a). The protein abundance of HSPs as measured by immunoblotting demonstrated a sustained increase in HSP25, HSP70 expression following repeated HS than the unstressed (P < 0.05; Fig. 6b, c), while the expression of HSP90 remained unchanged (Fig. 6b, c).
Fig. 5.
Multiple HS inhibits C2C12 cell differentiation. a Bright field microscopy images under × 10 magnification showing an effect of HS on cell differentiation for up to 5 days in DM. HS cells demonstrate an impaired differentiation process when compared to the control cells, most evidently on days 3–6. b qPCR showing the transcript levels of Myogenin and Myh2 in C2C12 cells grown under differentiation conditions that were either unstressed or exposed to repeated heat stress. c A representative immunoblot showing the protein expression of cell differentiation markers, MYOGENIN and MYH2, during differentiation of unstressed and heat-shocked C2C12 cells. d Scanning and densitometric analysis for the target protein(s) normalized to GAPDH. HS samples were compared to the respective day controls. e Immunoblot analysis for CASPASE-3 and Cleaved CASPASE-3 in control and HS cells
Fig. 6.
Chronic heat stress induces HSP mRNA and protein levels in differentiating cells. a qPCR showing the expression of Hsp25, Hsp70, Hsp90, and Cryab during cell differentiation in HS and control cells. b A representative immunoblot showing the protein expression changes of HSP 25, 70, and 90 in control and heat shocked C2C12 cells undergoing differentiation. GAPDH expression was used as loading control. c Densitometry quantification of protein expression of HSP 25, 70, 90 and CRYAB changes after repeated HS over control cells during differentiation. The results are expressed as density units of the target protein normalized to GAPDH. Each HS was compared with the respective day control cells d A representative immunoblot showing the protein expression changes of p-AKT (ser-473) and total AKT in control and heat-shocked C2C12 cells undergoing differentiation. GAPDH expression was used as a loading control
Sustained heat stress impairs C2C12 cell migration
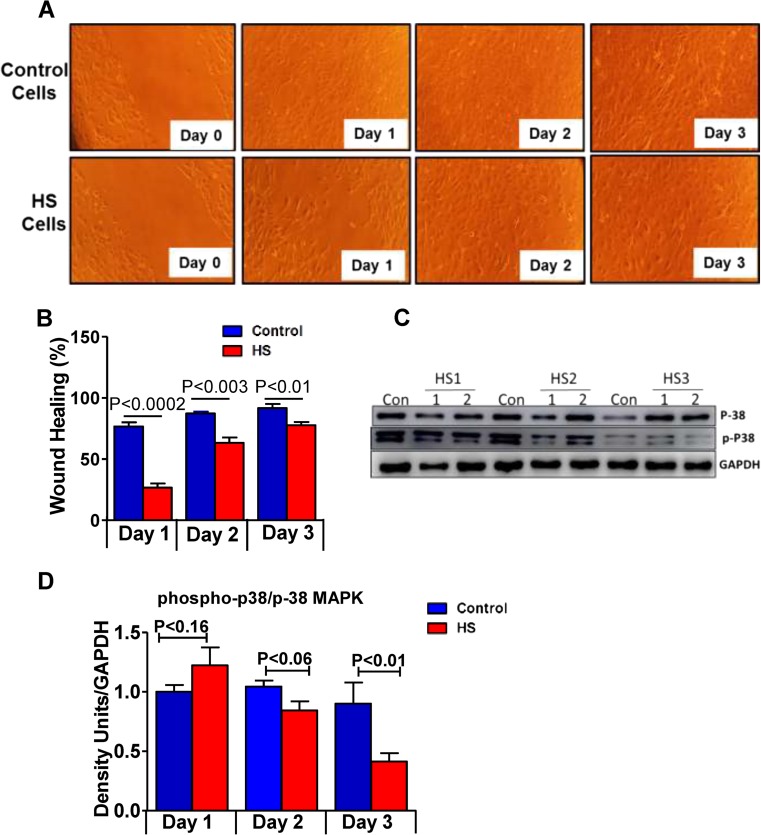
Next, to investigate the role played by concurrent HS on the migrative ability of C2C12 cells, confluent cells grown under proliferative conditions were wound scratched in the presence and absence of repeated HS and wound closure was measured by bright field imaging. In the unstressed condition, the movement of the cells appeared to progress rapidly into the wounded area, and by the end of 2nd day, the gaps were completely closed, while in the HS condition, the density of the cells in the wounded area was low and the wound closure was incomplete still after the end of 3rd day (Fig. 7a, b). We next determined p38 MAPK activation by measuring its phosphorylation status at Thr180/Tyr182 residue since p38 MAPK activity is recently shown to participate in spontaneous migration of progenitor cells (Hamanoue et al. 2016). The activity of p38 was found to be significantly decreased at the end of the 1st day of HS that was marked and sustained after repeated exposures on 2nd and 3rd day compared with unstressed C2C12 myoblasts (Fig. 7c). These results suggest that concurrent HS could reduce the migratory potential of C2C12 myoblasts, thus implying a delayed wound closure.
Fig. 7.
Consistent heat stress affects the cell migration. Cells were grown in proliferation media, and a scratch was made in all the plates before the first day of HS. Control group cells were maintained at 37 °C, and HS group was incubated at 43 °C. a Bright field microscopy images showing the migration and closure of the wound in control cells (upper panel), while the HS cells demonstrate slow wound closure when compared to control cells, (n = 3–4/group). b Histogram represents the percentage of wound healing on each day. c Immunoblot analysis of p38 and phosphorylated p38 MAPK (Thr180/Tyr182 residue) in control and heat stress cells. d Densitometry analysis for phospho and p38, normalized to GAPDH. Data obtained from n = 3–4/group or condition, and the values are expressed as mean ± SEM
Discussion
In this study, we show that repeated HS impairs proliferation, differentiation, and recovery after injury in C2C12 myoblast cells. We have also found that multiple HS cycle induces a selective regulatory pattern of sustained increase in HSP25 and 70 with either bimodal and/or unchanged regulation of HSP90 expression in these cells, which is associated with the loss of both mitogenic (proliferation) and myogenic (differentiation) events. In proliferating myoblasts, we also found that recurrent heat stress markedly elevated the expression of CryAB along with inhibition of p38 MAPK activity that could predict an unfavorable condition for growth and migration of cells (Hamanoue et al. 2016; Yamagiwa et al. 2003). While a previous HS study focused on the cytoprotective effects of acute heat shock stress (HSS) induced by mild heat shock (HS) (Liu and Brooks 2012), our study investigated the effects of recurrent hyperthermic episodes. We discovered that repeated HS impairs both proliferation and differentiation while maintaining high levels of selective HSPs throughout HS exposure and 24 h recoveries. An interesting speculation is that the C2C12 myoblast cells might be overreacting to recurrent HS exposure by producing overabundant HSPs that can tilt the homeostatic proteostatic effects to impaired proteostasis (proteotoxic). This is one of many explanations that could explain the plausible cause for myopathies in individuals exposed to chronic HS; a concept that is worth investigating further.
We present evidence that the repeated HS reduced the myoblasts proliferation, a prerequisite event for the muscle growth and regeneration (Moss and Leblond 1971). The retarded growth of C2C12 myoblasts due to repeated HS is not a reflection of cell death and could be a resultant of cell cycle arrest at S phase. Further, the increased total p38α MAPK at the end of a 3rd bout of HS supports the likelihood of restrained proliferation since a similar phenomenon of the presence of p38α MAPK has been documented to restrict the proliferation of satellite cells and their expansion (Brien et al. 2013). Our data shows, while the total p38MAPK is increased, it is interesting that a blockade of p38 MAPK activity measured in terms of its phosphorylation status is observed after repeated HS. This is in consistent with a finding that showed an inhibition of p38 MAPK activity is related to decreased protein synthesis and repression of the cellular growth in response to a mitogenic stimulation (Yamagiwa et al. 2003). This suggests a role for the p38 pathway in recurrent HS-induced C2C12 cytostasis. While the initially increased HSP25 and HSP70 in response to HS was maintained at higher levels after repeated HS, an exception in HSP90 regulation was observed that returned to basal level after repeated HS. Although the mechanism is unclear at this point, the distinct regulatory pattern of HSP90 when compared with HSP25 and HSP70 could be due to the repeated HS–induced cellular localization and distribution that can result in differential post-translational modifications, stabilization, and recycling of these HSPs. This could have an important functional significance in that when HSP90 is needed to interact with its client proteins and maintain its stability and activity; repeated HS suppressing it to basal level could affect the myogenic process of C2C12 cells (Taipale et al. 2012; Wagatsuma et al. 2011).
We observed that repeated heat stress inhibits the myogenesis process by repressing myogenin content with a concurrent decrease in myosin heavy chain expression and halting differentiation process prior to myoblast fusion. This could be attributed to the observed repression in proliferation, which results in an insufficient number of cells required for myogenesis. Consistent with our findings, an earlier study has shown that antiproliferation of satellite cells induced by angiotensin-II resulted in decreased expression of myogenin along with attenuation of skeletal muscle regeneration (Yoshida et al. 2013). Our finding related to recurrent HS-induced impairment in myogenic differentiation could be ascribed to pronounced HSP70 induction. Previous reports have demonstrated that HSP70, when released into the extracellular environment, triggers the release of cytokines such as TNF-α, IL-12, and interferon-gamma (IFN-γ) (Todryk et al. 1999), and these pro-inflammatory cytokines can, in turn, inhibit the myogenic differentiation of C2C12 cells (Langen et al. 2001). In addition, the hyper-stimulated HSP70 and its release into extracellular environment by inducing IFN-γ may also inhibit myogenesis through a direct inhibition of myogenin in C2C12 (Todryk et al. 1999; Londhe and Davie 2011).
Typically, cessation of proliferation is associated with concomitant differentiation induction and vice versa (Jones 2007; de la Serna et al. 2001) and hence, one might predict that repeated HS would favor myogenic differentiation as mitogenesis is inhibited. Conversely, our experiments confirmed that the repeated HS-induced suppression of proliferation did not trigger C2C12 myoblast differentiation processes. Although it is uncommon, the reason could be attributed to the fact that the HS-induced reduction in myoblasts proliferation presumably could have resulted in fewer myoblast number that are available to differentiate. Thus, our findings imply that repeated HS may (a) hamper the normal progression of myogenesis by perturbing both proliferation and differentiation similar to that seen in Growth and Differentiation Factor Associated Serum Protein-2 (GASP-2) knockdown (Pèrié et al. 2016), and (b) the mitogenic and myogenic processes need not always be regulated in a mutually exclusive manner, but instead could be subjected to analogous regulation.
Further, we observed a delayed resealing response after the scratch wounding seen in the repeated heat stress condition in association with increased HSP70 and HSP25 expression. While many studies have shown that an activation of HSP70 and HSP25 results in improved cell migration and wound healing (Yamasaki et al. 2010; Peterson et al. 2016), we speculate the possible explanations for the dissociation in the HSP induced-successful (acute) vs. unsuccessful (repeated) preservative attempt observed could be due to the lesions of the two cellular effects induced by recurrent heat stress, namely, (i) reduced proliferation combined with stalled cell cycle and (ii) an inhibition of basal p38 kinase that was previously demonstrated to inhibit spontaneous migration of progenitor cells (Hamanoue et al. 2016).
Conclusion
Our results show that under recurrent and severe HS, C2C12 myoblasts display a unique state of slow/reduced proliferation along with delayed differentiation without any apparent signs of apoptosis. Further, the impairment of the cellular processes such as proliferation, differentiation, and/or migration of C2C12 cells under conditions of repeated and severe HS occur despite a dramatic and/or sustained increase in HSP. This contrasts with what has been observed during acute HS exposure. Studies related to “molecular mechanisms of chronic heat-shock stress on muscle differentiation and regeneration would be relevant to our future interest”.
Acknowledgements
The authors thank Drs. Sandeep Balu Shelar and Radhakrishnan Rajesh Kumar for their assistance with cell cultures.
Funding information
This study was supported by multiple awards/research funds from the NHLBI (R01# HL118067), NIA (R03#AG042860), the AHA (BGIA#0865015F), University of Utah center for Aging (Pilot grant#2009), the Division of Cardiovascular Medicine/Department of Medicine, University of Utah, and the start-up funds (for NSR) by the Department of Pathology and School of Medicine, the University of Alabama at Birmingham, AL. This research was partially supported by the SIBS-UPS Undergraduate Research Program funded by the UAB Department of Pathology for Bolus.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Daniel J. Bolus and Gobinath Shanmugam equally contributed to this study.
Contributor Information
Madhusudhanan Narasimhan, Phone: 806.743.3793, Email: madhu.narasimhan@ttuhsc.edu.
Namakkal S. Rajasekaran, Phone: 205.996.9839, Email: rajnsr@uabmc.edu
References
- Azad MA, Kikusato M, Sudo S, Amo T, Toyomizu M. Time course of ROS production in skeletal muscle mitochondria from chronic heat-exposed broiler chicken. Comp Biochem Physiol A Mol Integr Physiol. 2010;157(3):266–271. doi: 10.1016/j.cbpa.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Bakthisaran R, Tangirala R, Rao CM. Small heat shock proteins: role in cellular functions and pathology. Biochim Biophys Acta (BBA) - Proteins and Proteomics. 2015;1854(4):291–319. doi: 10.1016/j.bbapap.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Benndorf R, Martin JL, Kosakovsky Pond SL, Wertheim JO (2014) Neuropathy- and myopathy-associated mutations in human small heat shock proteins: characteristics and evolutionary history of the mutation sites. Mutat Res Rev Mutat Res. 10.1016/j.mrrev.2014.02.004 [DOI] [PMC free article] [PubMed]
- Boncoraglio A, Minoia M, Carra S. The family of mammalian small heat shock proteins (HSPBs): implications in protein deposit diseases and motor neuropathies. Int J Biochem Cell Biol. 2012;44(10):1657–1669. doi: 10.1016/j.biocel.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Brien P, Pugazhendhi D, Woodhouse S, Oxley D, Pell JM. p38alpha MAPK regulates adult muscle stem cell fate by restricting progenitor proliferation during postnatal growth and repair. Stem cells (Dayton, Ohio) 2013;31(8):1597–1610. doi: 10.1002/stem.1399. [DOI] [PubMed] [Google Scholar]
- Buraczynska M, Swatowski A, Buraczynska K, Dragan M, Ksiazek A. Heat-shock protein gene polymorphisms and the risk of nephropathy in patients with type 2 diabetes. Clin Sci (Lond, Engl: 1979) 2009;116(1):81–86. doi: 10.1042/CS20070411. [DOI] [PubMed] [Google Scholar]
- Datskevich PN, Nefedova VV, Sudnitsyna MV, Gusev NB. Mutations of small heat shock proteins and human congenital diseases. Biochem Biokhimiia. 2012;77(13):1500–1514. doi: 10.1134/S0006297912130081. [DOI] [PubMed] [Google Scholar]
- Dehbi M, Baturcam E, Eldali A, Ahmed M, Kwaasi A, Chishti MA, Bouchama A. Hsp-72, a candidate prognostic indicator of heatstroke. Cell Stress Chaperones. 2010;15(5):593–603. doi: 10.1007/s12192-010-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd SL, Gagnon BJ, Senf SM, Hain BA, Judge AR. Ros-mediated activation of NF-kappaB and Foxo during muscle disuse. Muscle Nerve. 2010;41(1):110–113. doi: 10.1002/mus.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finka A, Sood V, Quadroni M, Rios PDL, Goloubinoff P. Quantitative proteomics of heat-treated human cells show an across-the-board mild depletion of housekeeping proteins to massively accumulate few HSPs. Cell Stress Chaperones. 2015;20(4):605–620. doi: 10.1007/s12192-015-0583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghniaz R, Umrani RD, Paknikar KM. Temperature-dependent and time-dependent effects of hyperthermia mediated by dextran-coated La0.7Sr0.3MnO3: in vitro studies. Int J Nanomedicine. 2015;10:1609–1623. doi: 10.2217/nnm.15.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanoue M, Morioka K, Ohsawa I, Ohsawa K, Kobayashi M, Tsuburaya K, Akasaka Y, Mikami T, Ogata T, Takamatsu K. Cell-permeable p38 MAP kinase promotes migration of adult neural stem/progenitor cells. Sci Rep. 2016;6:24279. doi: 10.1038/srep24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Kamei K, Iwamoto I, Inaguma Y, Kato K. Regulation of the levels of small heat-shock proteins during differentiation of C2C12 cells. Exp Cell Res. 2001;266(2):213–221. doi: 10.1006/excr.2001.5220. [DOI] [PubMed] [Google Scholar]
- Jones KA. Transcription strategies in terminally differentiated cells: shaken to the core. Genes Dev. 2007;21(17):2113–2117. doi: 10.1101/gad.1598007. [DOI] [PubMed] [Google Scholar]
- Langen RC, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kB. FASEB J: Off Publ Fed Am Soc Exp Biol. 2001;15(7):1169–1180. doi: 10.1096/fj.00-0463. [DOI] [PubMed] [Google Scholar]
- Liu CT, Brooks GA. Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes. J Appl Physiol (Bethesda, Md: 1985) 2012;112(3):354–361. doi: 10.1152/japplphysiol.00989.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londhe P, Davie JK. Gamma interferon modulates myogenesis through the major histocompatibility complex class II transactivator, CIITA. Mol Cell Biol. 2011;31(14):2854–2866. doi: 10.1128/MCB.05397-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung JM, Judge AR, Powers SK, Yan Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol. 2010;298(3):C542–C549. doi: 10.1152/ajpcell.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montilla SIR, Johnson TP, Pearce SC, Gardan-Salmon D, Gabler NK, Ross JW, Rhoads RP, Baumgard LH, Lonergan SM, Selsby JT. Heat stress causes oxidative stress but not inflammatory signaling in porcine skeletal muscle. Temperature: multidisciplinary. Biom J. 2014;1(1):42–50. doi: 10.4161/temp.28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971;170(4):421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Multhoff G. Heat shock proteins in immunity. Handb Exp Pharmacol. 2006;172:279–304. doi: 10.1007/3-540-29717-0_12. [DOI] [PubMed] [Google Scholar]
- Muthusamy VR, Kannan S, Sadhaasivam K, Gounder SS, Davidson CJ, Boeheme C, Hoidal JR, Wang L, Rajasekaran NS. Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic Biol Med. 2012;52(2):366–376. doi: 10.1016/j.freeradbiomed.2011.10.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai A, Ishikawa T. Cell cycle transition under stress conditions controlled by vertebrate heat shock factors. EMBO J. 2001;20(11):2885–2895. doi: 10.1093/emboj/20.11.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27(1):437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Pèrié L, Parenté A, Brun C, Magnol L, Pélissier P, Blanquet V. Enhancement of C2C12 myoblast proliferation and differentiation by GASP-2, a myostatin inhibitor. Biochem Biophys Rep. 2016;6:39–46. doi: 10.1016/j.bbrep.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CW, Carter RT, Bentley E, Murphy CJ, Chandler HL. Heat-shock protein expression in canine corneal wound healing. Vet Ophthalmol. 2016;19(3):262–266. doi: 10.1111/vop.12302. [DOI] [PubMed] [Google Scholar]
- Radford NB, Fina M, Benjamin IJ, Moreadith RW, Graves KH, Zhao P, Gavva S, Wiethoff A, Sherry AD, Malloy CR, Williams RS. Cardioprotective effects of 70-kDa heat shock protein in transgenic mice. Proc Natl Acad Sci U S A. 1996;93(6):2339–2342. doi: 10.1073/pnas.93.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, Zhang XQ, Stevenson TJ, Peshock RM, Leopold JA, Barry WH, Loscalzo J, Odelberg SJ, Benjamin IJ. Human αB-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130(3):427–439. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzato E, Balbo V, Boccafoschi F, Mazzucco L, Burlando B. Scratch wound closure of C2C12 mouse myoblasts is enhanced by human platelet lysate. Cell Biol Int. 2009;33(9):911–917. doi: 10.1016/j.cellbi.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;40(2):253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- de la Serna IL, Roy K, Carlson KA, Imbalzano AN. MyoD can induce cell cycle arrest but not muscle differentiation in the presence of dominant negative SWI/SNF chromatin remodeling enzymes. J Biol Chem. 2001;276(44):41486–41491. doi: 10.1074/jbc.M107281200. [DOI] [PubMed] [Google Scholar]
- Shanmugam G, Narasimhan M, Sakthivel R, Kumar RR, Davidson C, Palaniappan S, Claycomb WW, Hoidal JR, Darley-Usmar VM, Rajasekaran NS. A biphasic effect of TNF-alpha in regulation of the Keap1/Nrf2 pathway in cardiomyocytes. Redox Biol. 2016;9:77–89. doi: 10.1016/j.redox.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BN, Rao KS, Rao CM. Ubiquitin-proteasome-mediated degradation and synthesis of MyoD is modulated by αB-crystallin, a small heat shock protein, during muscle differentiation. Biochim Biophys Acta. 2010;1803(2):288–299. doi: 10.1016/j.bbamcr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Sonna LA, Gaffin SL, Pratt RE, Cullivan ML, Angel KC, Lilly CM. Selected contribution: effect of acute heat shock on gene expression by human peripheral blood mononuclear cells. J Appl Physiol. 2002;92(5):2208–2220. doi: 10.1152/japplphysiol.01002.2001. [DOI] [PubMed] [Google Scholar]
- Straadt IK, Young JF, Petersen BO, Duus JO, Gregersen N, Bross P, Oksbjerg N, Bertram HC. Metabolic profiling of heat or anoxic stress in mouse C2C12 myotubes using multinuclear magnetic resonance spectroscopy. Metabolism. 2010;59(6):814–823. doi: 10.1016/j.metabol.2009.09.029. [DOI] [PubMed] [Google Scholar]
- Sun L, Trausch-Azar JS, Ciechanover A, Schwartz AL. Ubiquitin-proteasome-mediated degradation, intracellular localization, and protein synthesis of MyoD and Id1 during muscle differentiation. J Biol Chem. 2005;280(28):26448–26456. doi: 10.1074/jbc.M500373200. [DOI] [PubMed] [Google Scholar]
- Taipale M, Krykbaeva I, Koeva M, Kayatekin C, Westover KD, Karras GI, Lindquist S. Quantitative analysis of Hsp90-client interactions reveals principles of substrate recognition. Cell. 2012;150(5):987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Khaleque MA, Jones EL, Theriault JR, Li C, Hung Wong W, Stevenson MA, Calderwood SK. Expression of heat shock proteins and heat shock protein messenger ribonucleic acid in human prostate carcinoma in vitro and in tumors in vivo. Cell Stress Chaperones. 2005;10(1):46–58. doi: 10.1379/CSC-44R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RP, Benjamin IJ. Small heat shock proteins: a new classification scheme in mammals. J Mol Cell Cardiol. 2005;38(3):433–444. doi: 10.1016/j.yjmcc.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Todryk S, Melcher AA, Hardwick N, Linardakis E, Bateman A, Colombo MP, Stoppacciaro A, Vile RG. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J Immunol (Baltimore, Md: 1950) 1999;163(3):1398–1408. [PubMed] [Google Scholar]
- Vardiman JP, Gallagher PM, Siedlik JA. Potential cytoprotective effects of heat shock proteins to skeletal muscle. In: Asea AAA, Almasoud NN, Krishnan S, Kaur P, editors. Heat shock protein-based therapies. Cham: Springer international publishing; 2015. pp. 119–127. [Google Scholar]
- Wagatsuma A, Shiozuka M, Kotake N, Takayuki K, Yusuke H, Mabuchi K, Matsuda R, Yamada S. Pharmacological inhibition of HSP90 activity negatively modulates myogenic differentiation and cell survival in C2C12 cells. Mol Cell Biochem. 2011;358(1–2):265–280. doi: 10.1007/s11010-011-0977-0. [DOI] [PubMed] [Google Scholar]
- Whidden MA, Smuder AJ, Wu M, Hudson MB, Nelson WB, Powers SK. Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J Appl Physiol. 2010;108(5):1376–1382. doi: 10.1152/japplphysiol.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Balestreri TM, Bowen-Pope DF. Regulation of proliferation and differentiation of myoblasts derived from adult mouse skeletal muscle by specific isoforms of PDGF. J Cell Biol. 1990;111(4):1623–1629. doi: 10.1083/jcb.111.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagiwa Y, Marienfeld C, Tadlock L, Patel T. Translational regulation by p38 mitogen-activated protein kinase signaling during human cholangiocarcinoma growth. Hepatology (Baltimore, Md) 2003;38(1):158–166. doi: 10.1053/jhep.2003.50257. [DOI] [PubMed] [Google Scholar]
- Yamasaki A, Ito H, Yusa J, Sakurai Y, Okuyama N, Ozawa R. Expression of heat shock proteins, Hsp70 and Hsp25, in the rat gingiva after irradiation with a CO2 laser in coagulation mode. J Periodontal Res. 2010;45(3):323–330. doi: 10.1111/j.1600-0765.2009.01239.x. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Galvez S, Tiwari S, Rezk BM, Semprun-Prieto L, Higashi Y, Sukhanov S, Yablonka-Reuveni Z, Delafontaine P. Angiotensin II inhibits satellite cell proliferation and prevents skeletal muscle regeneration. J Biol Chem. 2013;288(33):23823–23832. doi: 10.1074/jbc.M112.449074. [DOI] [PMC free article] [PubMed] [Google Scholar]