Abstract
This study was done in order to determine the molecular and biochemical alterations following testicular torsion (TT) and torsion-reperfusion (TR). For this purpose, 54 male Wistar rats were divided into five groups as control group (n = 6) and experimental group subjected to 1, 2, 4, and 8 h unilateral left torsion induction (n = 12 in each group). After induction of TT, testicular samples were collected from each group (n = 6), and the other six rats of each group underwent the same period of reperfusion after TT and then were sampled. Histological changes, the mRNA and protein expression of heat shock protein-70 (Hsp70), and caspase-3 were examined using reverse transcriptase-PCR (RT-PCR) and immunohistochemistry, respectively. Testicular total antioxidant capacity (TAC), glutathione peroxidase (GSH-px), and malondialdehyde (MDA) levels were evaluated. The mRNA damage and DNA fragmentation were assessed. The TT and TR significantly reduced differentiation and spermiogenesis indices (p < 0.05). The TT- and TR-induced groups exhibited a severe reduction in Hsp70 expression as well as remarkable enhancement in caspase-3 expression. The TAC and GSH-px levels were decreased and the MDA content was increased in TT- and TR-induced groups. Finally, the TT and TR enhanced mRNA damage and DNA fragmentation. The TT- and TR-induced damaging oxidative stress, diminished Hsp70 expression, and enhanced caspase-3 mRNA and protein levels result in apoptosis following 1, 2, and 4 h. Whereas, following 8 h, TT and TR initiate the necrosis by inducing energy depletion as well as severe mRNA damage.
Keywords: Torsion-reperfusion, Hsp70, Caspase-3, Apoptosis, Necrosis, Rat
Introduction
Testicular torsion (TT) is a common urologic condition in which testicular tissue, epididymis, and spermatic cord rotate around longitudinal axis. Indeed, the TT requires emergency scrotal exploration (Yurtcu et al. 2008). According to previous clinical reports, two types of TT are described; type one is occurring between prenatal and the first year of life and the second type with higher incidence during puberty (Filho et al. 2004; Lorenzini et al. 2012). Various studies have been done to show the possible mechanism(s), by which the TT adversely affects testicular tissue. It has been illustrated that the compression of spermatic veins during TT reduces blood circulation, resulting in severe hypoxia and oxidative and nitrosative stresses (Lorenzini et al. 2012; Vigueras et al. 2004). More analyses showed that the longtime (i.e., 30 days) TT is characterized by significant reduction in serum level of testosterone (Turner et al. 2005) and germinal cell damage, leading to subfertility and/or infertility (Okorie 2011; Zhang et al. 2009). On the other hand, reperfusion and recirculation of the blood flow of the affected testis, as occurs in surgical therapeutic intervention, lead to cell loss at spermatogenesis level and consequently enhance testicular lipid peroxidation ratio (Turner and Brown 1993; Yurtcu et al. 2008). It means that the histologic and molecular damages after ischemia-reperfusion mainly depend on progressive reactive oxygen species (ROS) generation especially short time after reperfusion (Filho et al. 2004; Lorenzini et al. 2012). Although TT-induced hypoxia and reperfusion-induced biochemical stresses have been identified as the main factors for testicular injury, the exact pathophysiologies of TT and/or reperfusion after TT are not completely understood.
It has been established that impaired endocrine interactions initiate the ROS- and/or reactive nitrogen species (RNS)-dependent stresses in testicular tissue (Agarwal et al. 2008). Actually, pathologic generation of ROS/RNS negatively affects the spermatogenesis, which adversely impacts the sperm DNA integrity, motility, and plasma membrane fluidity (Adibnia et al. 2016; Forlenza and Miller 2006; Gholirad et al. 2016). In line with this issue, more than 20 chaperone families have now been described, which are upregulated under the effect of various biochemical stressors including oxidative and nitrosative stresses (Dun et al. 2012; Rezazadeh-Reyhani et al. 2015). Considering the critical roles of chaperones in enhancing the cellular resistance against environmental stressors, the majority of these chaperone families are referred to cell stress responders or heat shock proteins (HSPs). The HSPs are known to be involved in regulating proper spermatogenesis (Rerole et al. 2011). Accordingly, the Hsp70-2, as main expressed chaperone during early meiosis and/or mitosis, involves in folding/refolding the proteins during different cell cycles. Moreover, the Hsp70 proteins participate in recovering the DNA and RNA damages via improving the DNA integrity as well as enhancing the RNA-binding protein stability in haploid cells (Khosravanian et al. 2014; Rezazadeh-Reyhani et al. 2015). Aside to mentioned roles, the Hsp70 is known to be responsible for regulating and maintaining the lower temperature of the testes (Sarge and Cullen 1997). Indeed, to maintain optimal sperm quality, the testicular temperature must be 2°–4° lower than the rest of the body. Therefore, slight enhancement in temperature results in protein denaturation which rapidly disrupts spermatogenesis (Comish et al. 2015; Dada et al. 2002).
Caspases, as endoproteases, are involved in cascade of events such as apoptosis and inflammation (McIlwain et al. 2013). Accordingly, the caspase family has been classified by their roles, as caspases (caspase-3, -6, -7, -8, and -9 in mammals) participating in apoptosis and those (caspase-1, -4, -5, and -12 in humans and caspase-1, -11, and -12 in mice) involving in inflammation (McIlwain et al. 2013; Oberst et al. 2011). The caspases actively cleave the proteins and are known to involve in cleaving neighboring amino acids, as well. Indeed, once caspases are activated, there seems to be an unalterable commitment leading to cell death (McIlwain et al. 2013; Sakai and Sugasawa 2014).
The present study was done to update previous findings about the time-dependent effects of TT and torsion/reperfusion (TR) on testicular tissue. In order to analyze the homeostatic status of testicles following TT and TR, the mRNA and protein levels of Hsp70 were examined. Moreover, to illustrate the cross-link between TT- and TR-induced oxidative stress and testicular hemostasis, lipid peroxidation ratio as well as testicular antioxidant status was analyzed. Moreover, the pro-necrotic and/or pro-apoptotic impact of TT and TR was investigated by evaluating the caspase-3 expression and estimating the mRNA damage, respectively. Finally, the DNA laddering test was done in order to estimate the TT- and/or TR-induced DNA fragmentation.
Methods and materials
Animals
Fifty-four 30-day-old male Wistar rats weighing between 180 ± 20 g were divided into control and experimental groups. The rats were housed in individual solid-bottom plastic cages on sawdust bedding at a constant temperature of 21–23 °C with 12-h periods of light-dark exposure. The animals were allowed access to standard food and water ad libitum. The experimental procedures and protocols performed in this study were in concordance with ethical approval for animal model research committee of the authors’ institution.
Experimental design
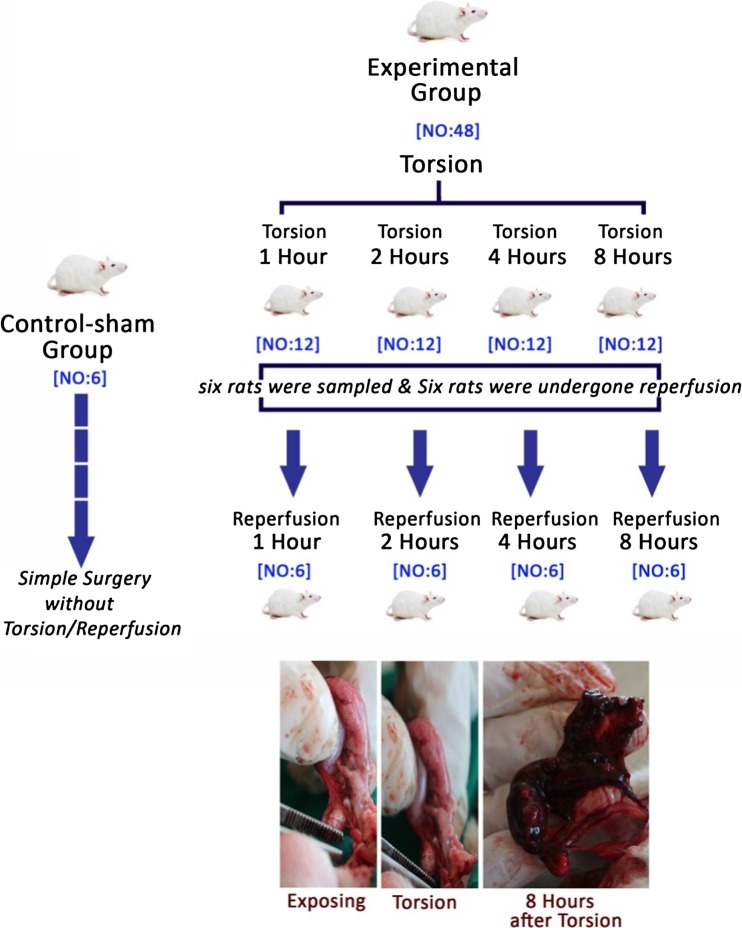
Following 1 week of acclimatization, the rats were divided into five groups as control group (N = 6) and experimental group subjected to 1, 2, 4, and 8 h unilateral TT (N = 12 in each group). Following mentioned periods, six rats from each group were euthanized using special CO2 device (URUM-ADACO, Iran) and the testicular tissues were sampled. The other six rats underwent reperfusion. Following 1, 2, 4, and 8 h post reperfusion, the animals in TR-induced groups were sampled, as well (Fig. 1).
Fig. 1.
Schematic view for grouping of the animals to euthanasia
Torsion and reperfusion
The rats were anesthetized by 5% ketamine, 40 mg/kg, and 2% xylazine (Alfasan, Woerden, The Netherlands), 5 mg/kg, intraperitoneally. Aseptically, scrotum was entered through a left inguinoscrotal incision. The tunica vaginalis was opened, and the left testicle was exteriorized. The torsion was created by rotating the left testis 720° clockwise, and then the torsion was maintained by suturing the testicle to scrotal wall with silk 3-0 (Supa, Tehran, Iran) through the tunica albuginea. Finally, the incision was closed with the silk suture. The rats were allowed to recover from anesthesia and left free in their cages. Subsequently, the reperfusion-subjected rats (n = 6) were re-anesthetized and the reperfusion process was conducted. The reperfusion was performed by counter-rotation and by re-suturing of the testicular gubernacular stump to the scrotal gubernacular stump. The testes were monitored for recirculation of blood flow. Finally, the testes were replaced into the scrotum (Filho et al. 2004). Simple incision on scrotum without TT and/or TR was conducted in control group and then the incision was closed. The animals in control group were sampled after 8 h.
Histological analyses
At the end of mentioned time points, the testicular tissues were dissected out and washed with normal saline. Then, half of the tissues were fixed in Bouin’s fixative for histological investigations and subsequently embedded in paraffin. The embedded tissues were cut by rotary microtome (5 μm) and stained with H&E technique for analyzing histomorphometric changes. For this purpose, the percentage of seminiferous tubules with less than 3–4 germinal layers (negative tubular differentiation index, TDI) and percentage of tubules with impaired spermiogenesis (negative spermiogenesis index SPI) were assessed in 20 seminiferous tubules/one cross section at two magnifications (×400 and ×1000). General histological changes were assessed and compared between groups.
Immunohistochemical staining
Immunohistochemical staining was done in order to analyze the caspase-3 and Hsp70 positive cells distribution. Before initiating the staining process, the tissue sections (5 μm) were heated at 60 °C for approximately 25 min in a hot air oven (Venticell, MMM, Einrichtungen, Germany). The sections were then de-paraffinized in xylene (two changes) and rehydrated using an alcohol gradient (96, 90, 80, 70, and 50%). The antigen retrieval process was performed in 10 mM sodium citrate buffer (pH 7.2). Immunohistochemical staining was conducted according to the manufacturer’s protocol (Biocare, USA). Briefly, endogenous peroxidase was blocked in a peroxidase blocking solution (0.03% hydrogen peroxide containing sodium acid) for 5 min. Tissue sections were then washed gently with phosphate-buffered saline (PBS, pH 7) and subsequently incubated with caspase-3 (1:500) and Hsp70 (1:600) biotinylated primary antibodies (Biocare, USA) overnight at 4 °C in humidified chamber. The sections were rinsed gently with PBS. After that, the slides were incubated with a sufficient amount of streptavidin–HRP (streptavidin conjugated to horseradish PBS containing an anti-microbial agent for 20 min). Subsequently, the tissue sections were rinsed gently in washing buffer and placed in a buffer bath. A DAB chromogen was added to the tissue sections and incubated for 10 min. In continuation, the slides were counter stained with hematoxylin for 10 s. The sections were then dipped in ammonia (0.037 ML) ten times, rinsed with distilled water, and cover slipped. Positive immunohistochemical staining was observed as brown stains under a light microscope.
Fluorescent staining for mRNA damage
In order to show mRNA damage in necrotic cells, the especial fluorescent staining was conducted. Basically, in this method, necrotic cells are characterized by gradual loss of cytoplasmic RNA and therefore represent with the flaming red orange. Meanwhile, the intact cells exhibit bright red RNA concentrated at the periphery of nuclei. For this purpose, the frozen sections were prepared by using cryostat microtome (Bright, England). The sections then were hydrated by using different (96, 90, 80, 70, and 50%) alcohol ethanol. In continuation, the slides were rinsed in acetic acid, 1% aqueous (1 ml/99 ml water) and followed by washing in distilled water for 3 min. Then, the slides were stained in acridine-orange (5 min) and de-stained in phosphate-buffer (11.876 g/1000 ml water and 9.078 g/1000 ml water, pH 6) for 1 min. After that, the sections were differentiated in calcium chloride buffer (11.0 g/1000 ml water) for 30 s. Finally, the slides were washed with phosphate-buffer and mounted with drop of buffer (Rezazadeh-Reyhani et al. 2015). The number of cells with mRNA damage per one mm2 of the tissue was counted and compared between groups.
RNA isolation and cDNA synthesis
Total RNA was extracted from 0.3 g of testicular tissue (N = 6 samples for each single group) of experimental and control animals. For this purpose, Sina-Clon RNA extraction kit (CinnaGen, Tehran, Iran) was used. To each testicular sample, 1 ml of TRIZOL Reagent was added and the tissue was then homogenized in homogenizer Precellys 24 (Bertin Technologies, Aix-en-Provence, France). Subsequently, the samples were processed according to the manufacturer’s instructions. The purity (260 nm and A260/280 = 1.8–2.0), was measured with a NanoDrop-1000 spectrophotometer (Thermo Scientific, Washington, USA). Moreover, the total RNA concentration (ng/μl) was evaluated using NanoDrop-1000 spectrophotometer and compared between groups (Molavi et al. 2014). Thereafter, the isolated RNA was stored at − 70 °C. For reverse transcription polymerase chain reaction (RT-PCR), the cDNA was synthesized in a 20-μl reaction mixture containing 1 μg RNA, 1 μl oligo (dT) primer, 4 μl 5 × reaction buffer, 1 μl RNAse inhibitor, 10 mM dNTP mix (2 μl), and M-MuLV Reverse Transcriptase (1 μl) according to the manufacturer’s protocol (Fermentas, GmbH, Germany). The cycling protocol for 20 μl reaction mix was 5 min at 65 °C, followed by 60 min at 42 °C and 5 min at 70 °C to terminate the reaction.
Reverse transcription polymerase chain reaction
The RT-PCR reaction was carried out in a total volume of 25 μl containing PCR master mix (16 μl), FWD and REV specific primers (each 1 μl, 10 pmol/μl) and cDNA as a template (2 μl), and nuclease-free water (5 μl). The PCR condition was run as follows: general denaturation at 95 °C for 3 min, 1 cycle, followed by 35–40 cycles of 95 °C for 20 s; annealing temperature (50 °C for caspase-3, 70 °C for Hsp70, and 57 °C for GAPDH) for 45 s; elongation at 72 °C for 1 min and 72 °C for 5 min. Specific primers (Okorie 2011; Pant and Srivastava 2003) were designed and manufactured by Cinna-Gen (Cinna-Gen Co.Tehran, Iran). Neuclotid sequences and products size for primers used in RT-PCR are presented in Table 1. Final PCR products were analyzed on 1.5% agarose gel electrophoresis, and densitometric analysis of the bands was done by using PCR Gel analyzing software (ATP, Tehran, Iran). The control was set at 100% and experimental samples were compared to the control.
Table 1.
Nucleotide sequences and products size for primers used in RTPCR
| Forward and reverse primers | Product size | |
|---|---|---|
| Hsp70 | F: 5′-ACCGTGGAGCCCGTGGAGAAG-3′ R: 5′-TTGGTGGGGATGGTGGAGTTG-3′ |
450 bp |
| Caspase-3 | F: 5′-TACCCTGAAATGGGCTTGTGT-3′ R: 5′-GTTAACACGAGTGAGGATGTG-3′ |
280 bp |
| GAPDH | F: 5′-GCTGAGTATGTCGTGGAGTCT-3′ R: 5′-CCAGCCCCAGCATCAAAGGTG-3′ |
320 bp |
Assessments of testicular TAC, MDA, and GSH-px
For the biochemical evaluation of oxidant-antioxidant system, the testicular tissue washed three times with 0.9% NaCl solution and 1.15% KCl was liquidified to the amount of 9 ml for each tissue. The homogenate of the tissues was prepared with the Teflon-end-on homogenizator (Elvenjempotter, Newton CT) and was centrifuged at 4000 rpm. The malondialdehyde (MDA) content was measured by using the thiobarbituric acid (TBA) reaction as described previously, and the absorbance of samples was measured at 532 nm (Pant and Srivastava 2003). We assessed the tissue total antioxidant capacity (TAC) status based on the ferric reduction antioxidant power (FRAP) assay, and the absorbance of samples was measured at 593 nm (Niehaus and Samuelsson 1968). The tissue glutathione peroxidase (GSH-px) activities were evaluated by using the measurement kit of RANSOL, and the absorbance of samples was measured at 340 nm. Finally, the protein contents of testicles were measured according to the Lowry method (Lowry et al. 1951).
Statistical analyses and imaging
All results are presented as Mean ± SD. Differences between quantitative histological and biochemical data were analyzed with one-way ANOVA, followed by Bonferroni test, using Graph Pad Prism, 4.00. A p < 0.05 was considered as statistically significant. The photomicrographs were taken by SONY onboard camera (Zeiss, Cyber-Shot, Japan).
Results
Histological findings
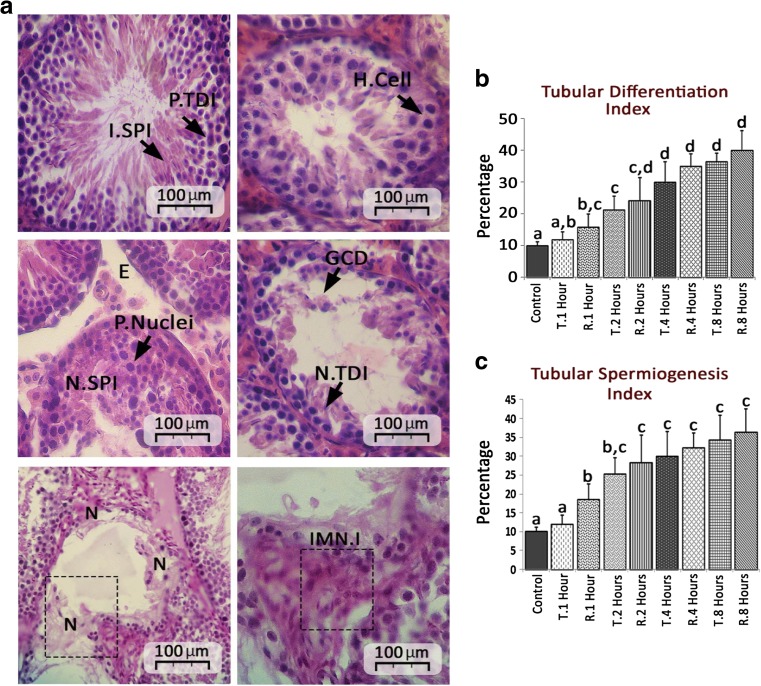
Light microscopic analyses exhibited progressive edema and cellular dissociation as well as disintegration of tubular basal membrane in both TT- and TR-induced groups (Fig. 2a). More histological analyses revealed that the TT and TR both increased the percentage of tubules with negative TDI and SPI versus control group (Fig. 2b, c). The cross sections from 8 h after TT and TR induction presented hypertrophied cells with pyknotic nuclei in 23.63 ± 7.35 and 27.17 ± 4.55% of tubules, respectively. Moreover, nuclear disappearance, eosinophilic cytoplasm, and intensive immune cells infiltration were revealed in cross sections of 8 h TT- and TR-induced group.
Fig.2.
a Note cross sections from intact and damaged seminiferous tubules; intact spermatogenesis (I.SPI), positive tubular differentiation index (P.TDI), hypertrophied cells (H.Cell), edema (E) of connective tissue, pyknotic nuclei (P.Nuclei), negative spermiogenesis (N.Spermiogenesis), germinal cells dissociation (GCD) and negative tubular differentiation (N.TDI), necrotic area (N) and immune cells infiltration (IMN.I). b Percentage of seminiferous tubules with negative tubular differentiation index (TDI). c Percentage of seminiferous tubules with negative spermiogenesis index (SPI), all data are presented in Mean percentage ± SD and different superscript letters are representing significant differences (P < 0.05, N = 6 rats in each group)
TT and TR decreased Hsp70 expression
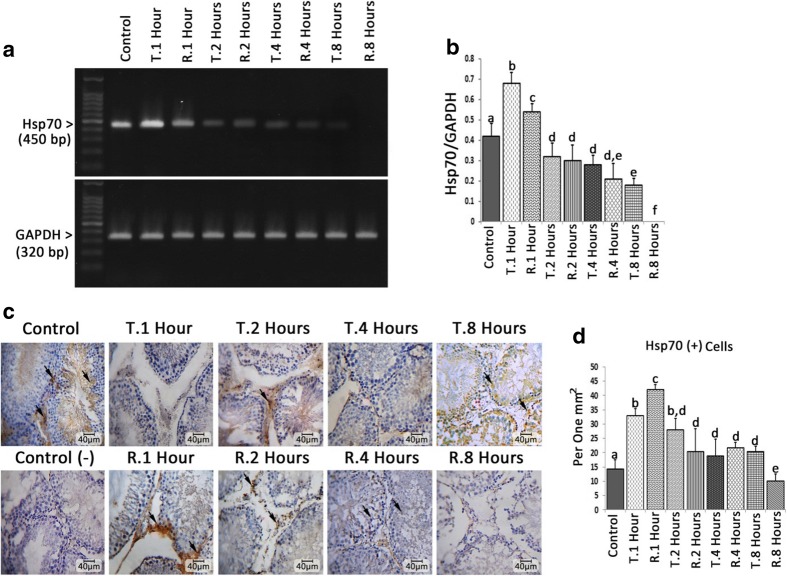
The mRNA level of Hsp70 was significantly (p < 0.05) enhanced following 1 h after TT induction, while remarkably diminished 1 h after reperfusion. Moreover, the 2, 4, and 8 h TT-induced groups represented a significant (p < 0.05) reduction in expression of Hsp70 versus control group. Generally, the RT-induced animals exhibited remarkably (p < 0.05) lower Hsp70 expression compared to TT-induced groups (Fig. 3a, b). The IHC analyses showed the same pattern represented by RT-PCR, exhibiting diminished Hsp70 expression depending on time. Accordingly, the Hsp70-positive cells distribution was decreased (p < 0.05) time dependently and the cross sections of RT-induced animals presented remarkably lower distribution of Hsp70-positive cells versus TT-induced groups. The Leydig cells were marked as precursor Hsp70 expressing cells at early stages of TT and TR (1 and 2 h after TT and/or TR), which then this situation expanded to bigger population including germinal epithelium after 4 and 8 h post TT and TR induction (Fig. 3c, d).
Fig. 3.
a The mRNA levels of Hsp70 and GAPDH were evaluated by using semi-quantitative RT-PCR. b Density of Hsp70 mRNA levels in testicular tissue that was measured by densitometry and normalized to GAPDH mRNA expression level. c Immunohistochemical staining for Hsp70. d Distribution of Hsp70-positive (arrows) cells/mm2 of testicular tissue. All data are represented in Mean ± SD. Different superscript letters are representing significant differences (P < 0.05, N = 6 rats in each group)
TT and TR enhanced the caspase-3 expression
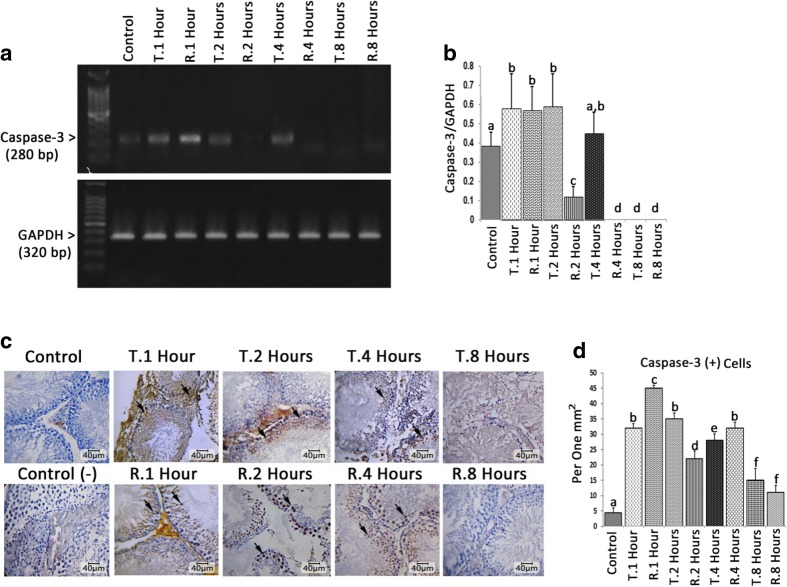
The mRNA levels of caspase-3 altered depending on condition and time. Accordingly, the animals in 1, 2, and 4 h TT-induced and those in 1 h TR-induced groups exhibited higher mRNA levels of caspase-3 compared to control group. Meanwhile, the mRNA level of caspase-3 was decreased 2 h after TR induction and was not detected 4 and 8 h after RT induction (Fig. 4a, b). Comparing the experimental and control groups with each other illustrated enhanced (p < 0.05) distribution of caspase-3-positive cells/one mm2 of TT and RT-induced groups compared to control group. The cross sections from 8 h TT- and TR-induced groups exhibited a significant reduction in caspase-3-positive cells distribution (Fig. 4c, d).
Fig. 4.
a The mRNA levels of caspase-3 and GAPDH were evaluated by using semi-quantitative RT-PCR. b Density of caspase-3 mRNA levels in testicular tissue that was measured by densitometry and normalized to GAPDH mRNA expression level. c Immunohistochemical staining for caspase-3. d Distribution of caspase-3-positive (arrows) cells/mm2 of testicular tissue. All data are represented in Mean ± SD. Different superscript letters are representing significant differences (P < 0.05, N = 6 rats in each group)
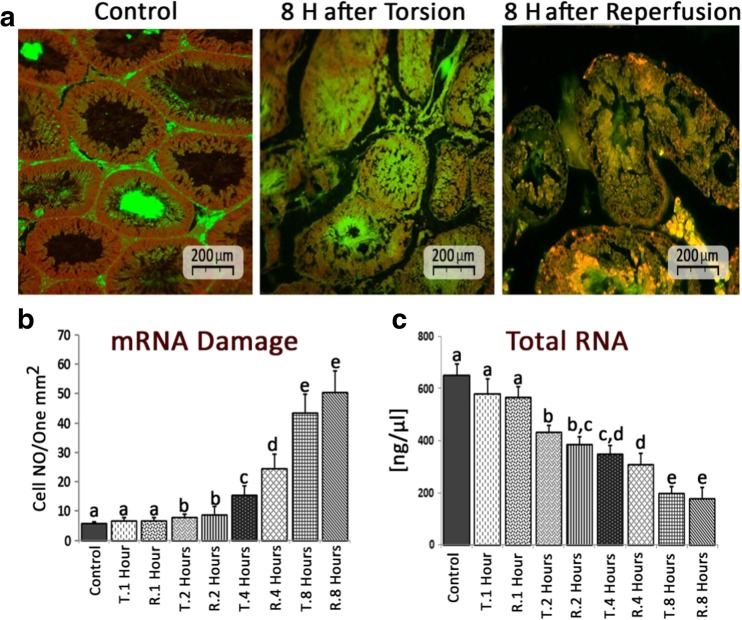
TT and TR resulted in severe mRNA damage
The special fluorescent staining for possible mRNA damage in necrotic cells showed that both TT and TR elevated the mRNA damage (representing increased necrotic cells distribution/one mm2) following 8 h (Fig. 5a, b). Moreover, the total mRNA level (ng/one μl) was evaluated in all groups. Observations showed that the TT and TR resulted in a significant (p < 0.05) reduction in total mRNA level after 2 h (Fig. 5c). Accordingly, the samples from 8 h TT- and RT-induced groups exhibited lowest mRNA in comparison to other test and control groups.
Fig. 5.
a Fluorescent staining for mRNA damage in necrotic cells; note intact mRNA with red fluorescent appearance in control group and yellowish-red (representing mRNA damage) stained necrotic cells in 8 h TT- and TR-induced groups. b Distribution of cells with mRNA damage/one mm2 of the testicular tissue. c Total mRNA volume in different groups. All data are represented in Mean ± SD. Different superscript letters are representing significant differences (P < 0.05, N = 6 rats in each group)
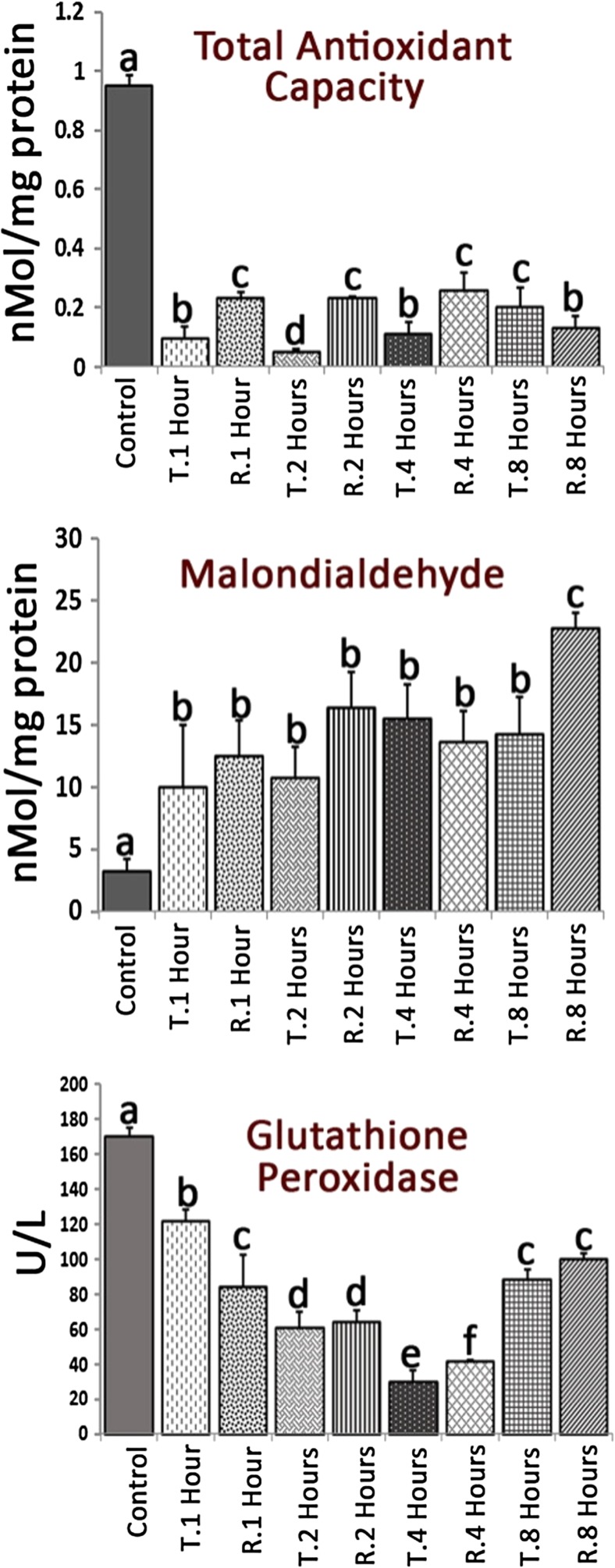
Both TT and TR diminished testicular antioxidant status
In order to estimate the testicular antioxidant status, tissue MDA content and TAC as well as GSH-px levels were analyzed. The TT- and TR-induced groups represented significantly (p < 0.05) lower TAC level compared to control animals. The testicular TAC levels were compared between TT- and RT-induced groups. The testicles belonging to 1, 2, and 4 h post TR induction represented significantly (p < 0.05) higher TAC levels compared to those from TT-induced groups at same times. In contrast, comparing to 8 h after TT, the TAC was decreased 8 h after TR induction (Fig. 6a). No statistically significant differences were observed for MDA content of the testicles during 1, 2, and 4 h after TT and TR induction (P > 0.05). However, the animals in TT- and TR-induced groups exhibited generally higher MDA content versus control group (p < 0.05, Fig. 6b). About the GSH-px, generally, the animals in TT- and TR-induced groups exhibited significantly (p < 0.05) lower GSH-px levels even 1 h after TT and/or TR (Fig. 6c).
Fig. 6.
Effect of TT and TR on testicular antioxidant status. All data are presented in Mean ± SD. Different superscript letters are representing significant differences (P < 0.05, N = 6 rats in each group)
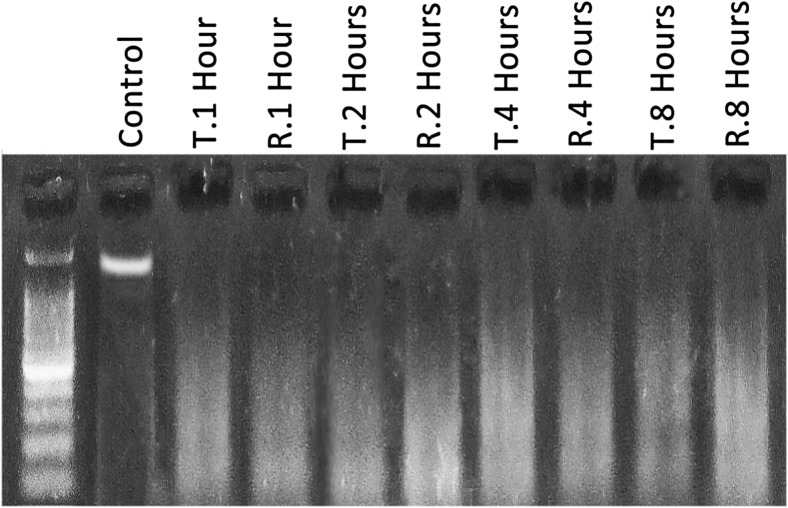
TT and TR resulted in intensive DNA fragmentation
The DNA laddering test was done in order to examine the effect of TT and TR on DNA integrity. Observations demonstrated that both TT and TR resulted in a severe DNA fragmentation. This impairment developed depending on time (Fig. 7).
Fig. 7.
DNA ladder for tissue DNA integrity; the TT and TR, in a time-dependent manner, increased DNA fragmentation. See the lanes for TT and TR-induced group, representing severe DNA fragmentation
Discussion
The present study showed that the TT and TR resulted in severe histological damages at spermatogenesis and spermiogenesis levels. Moreover, the TT and TR remarkably downregulated the Hsp70 expression and upregulated the caspase-3 expression following 1, 2, and 4 h after TT and TR induction. The animals in TT- and TR-induced groups exhibited diminished TAC level as well as enhanced MDA content versus control group. Finally, both TT and TR resulted in intensive DNA fragmentation and severe mRNA damage (especially after 4 and 8 h), as well.
It has been illustrated that severe histological damages during the TT and TR result in infertility (Adibnia et al. 2016; Ekici et al. 2012). In corroboration with this issue, here in the present study, we reveal the histological changes after TT and TR induction. Light microscopic analyses showed that both TT and TR significantly inhibited germinal cells differentiation ratio (marked with increased negative TDI) and remarkably reduced spermiogenesis potential (represented with negative SPI). It has been established that any mild downregulation of spermatogenesis remarkably affects the spermiogenesis pathway that finally reduces the sperm production (O’Donnell 2014). However, to show the exact mechanism(s), we classified the TT- and TR-induced damages into two apoptosis and/or necrosis pathways. On the other hand, there is a cross interaction between oxidative stress and apoptosis and/or necrosis. To understand the cross-link between apoptosis and oxidative stress, one should note that pathological generation of ROS following TT and/or TR adversely impacts cellular DNA, RNA, lipid, and protein backbones (Agarwal et al. 2008; Dun et al. 2012). Therefore, it would be more logical to conclude that the Hsp70 as homeostatic protein comes into play an important role in refolding the DNA and protein contents of the cells (Dun et al. 2012; Khosravanian et al. 2014; Rezazadeh-Reyhani et al. 2015). Moreover, the Hsp70s play an essential role in preserving the physiologic testicular temperature (2°–4° lower than the rest of the body), which suggests maintaining role of these proteins in successful spermatogenesis (Comish et al. 2015; Dada et al. 2002). To understand the statement, the testicular TAC, GSH-px, and MDA levels as well as Hsp70 expression were analyzed in the current study. It came clear that both TT and TR significantly diminished TAC and GSH-px levels and enhanced the MDA content versus control group. On the other hand, the TT- and TR-induced groups exhibited a remarkable reduction in mRNA and protein levels of Hsp70. Finally, the DNA laddering test was conducted to approve possible apoptosis. The DNA fragmentation was increased specifically 2 h after TT and TR induction. Taking all findings together, we can come close to this fact that progressive TT- and TR-induced oxidative stress in association with decreased Hsp70 expression results in loss of membrane and DNA, which finally initiates apoptosis. The interesting finding was that despite increased level of MDA, higher TAC levels were revealed in 1, 2, and 4 h TR-induced groups compared to the TT-induced groups at same times. In contrast to our findings, previous reports have shown that the TR results in pathologic generation of ROS (Nagakannan et al. 2012; Takhtfooladi et al. 2015). Indeed, under ischemic conditions, the ischemia-mediated proteases convert the xanthine dehydrogenase to xanthine oxidase (Ekici et al. 2012; Gladden et al. 2011), leading to high amounts of O2− and H2O2 (known oxidants) generation. Thus, this question rises up, what was the reason for upregulated TAC level? To understand the situation, one should note that despite increased TAC level, the rats in TR-induced group showed significantly higher MDA content (representing severe lipid peroxidation) and lower GSH-px (representing intensive oxidative stress) compared to TT-induced groups at same times. Therefore, we can hypothesize that, first of all, the TAC alone does not represent the real antioxidant status of the testicles, and secondly, mild enhancement in TAC level may be related to antioxidant contents of the recirculated fresh blood.
Owning the fact that the caspase-3 involves in apoptosis, the expression of caspase-3 was evaluated in the current study. Observations showed a significant enhancement in caspase-3 expression following 1, 2, and 4 h after TT and TR induction. However, the animals in 8 h TT/TR-induced groups exhibited diminished caspase-3 mRNA. Thus, it could be concluded that the TT and TR (at earlier stages) result in apoptosis via inducing caspase-3 overexpression but the question is that what happens in the later stages as 8 h post TT and TR induction? Indeed, there is a delicate cross-link between apoptosis and necrosis. The availability of intracellular ATP and caspases is known as two clearly established factors in converting an ongoing apoptotic pathway to a necrotic process (Gunther et al. 2013; Zeiss 2003). It means that remarkable reduction in caspase-3 expression and/or synthesis associated with decreased availability of ATP triggers apo-necrotic pathway. In fact, the progressive oxidative stress inevitably results in loss of mitochondrial membrane potential leading to massive ATP depletion. On the other hand, releasing of cytochrome C (as a result for mitochondrial membrane disintegrity) promotes ATP depletion, as well. All these alterations in turn initiate the cell death independent to caspases (Gunther et al. 2013; Shen et al. 2012). In addition, the pro-apoptotic activity of apoptosome mainly depends on ATP. Therefore, progressive energy depletion (ATP withdrawal) would lead to necrosis in greater proportion of cells, rather than apoptosis (Vigueras et al. 2004). Although the ATP levels of testes are not estimated in the current study, we can hypothesize that the TT/TR-induced irreversible oxidative stress (albeit 8 h after TT/TR induction) may results in uncoupled mitochondrial oxidative phosphorylation, which leads to possible energy depletion and consequently ends with necrosis. Our histological observations demonstrated eosinophilic cytoplasm of germ cells, nuclear disappearance, and massive immune cell infiltration in interstitial tissue representing local necrosis after 8 h from TT and TR induction. Moreover, fluorescent staining for mRNA damage was conducted to confirm necrosis. Observations demonstrated that despite the animals in 1, 2, and 4 h TT/TR-induced groups, the animals in 8 h TT/TR-induced group exhibited massive mRNA damage (representing necrosis) as well as diminished caspase-3 expression. Considerable point is that although the RT-PCR analyses did not exhibit the mRNA of caspase-3 4 and 8 h post TT and TR induction, the cross sections of these groups exhibited 33.68 ± 4.12, 18.28 ± 2.64, and 12.44 ± 2.87 caspase-3 positive cells per mm2 of tissue, respectively. Considering the early expression of caspase 3, even after 1 h from TT/TR induction, it can be concluded that the synthesis of caspase 3 was initiated from the early hours and this process lasted up to about 4 h. Meanwhile, after 8 h, due to reduced mRNA expression, new protein synthesis did not occur and observation of positive reactions of caspase 3 may attribute to earlier periods (for example 2 h). Thus, we believe that aside possible role of mitochondria, the TT- and TR-decreased Hsp70 expression in association with decreased caspase-3 synthesis influences the mRNA stability and consequently is able to trigger the necrosis in not all but in most of the germ cell population. In agreement with our hypothesis, previous reports have shown that the Hsp70 interacts with special RNA-binding proteins involving in stabilization of mRNA in haploid cells (Khosravanian et al. 2014; Kwon and Hecht 1991). Considering the role of mRNAs in synthesis of critical proteins (such as caspase-3 and Hsp70), we can suggest that upregulated oxidative stress accomplished with possible ATP depletion as well as decreased mRNA synthesis and/or stability makes a triangle that finally results in necrosis after 8 h.
Conclusion
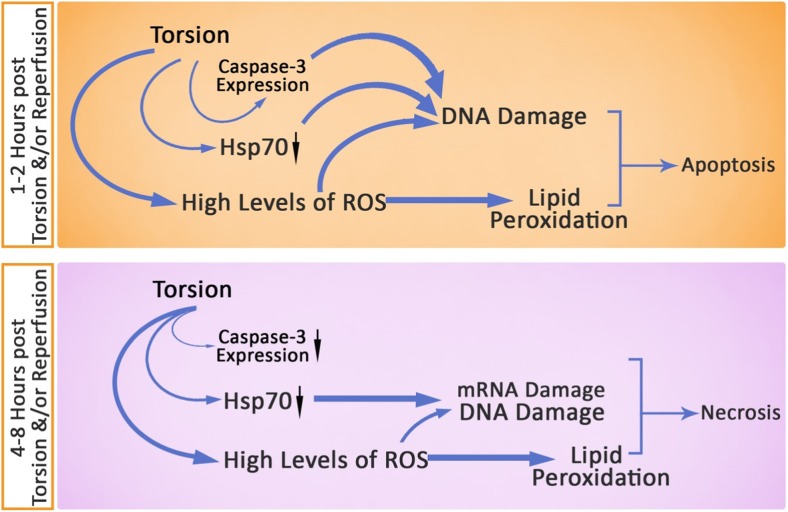
Our data showed that the TT- and TR-induced pathologic oxidative stress, diminished Hsp70 expression, and enhanced caspase-3 expression result in apoptosis following 1, 2, and 4 h. Whereas, following 8 h, the TT- and TR-induced irreversible oxidative stress in association with massive reduction in Hsp70 expression initiates the necrosis. Thus, we can conclude that performing reperfusion without complementary medications such as antioxidants does not ameliorate the TT- and/or TR-induced derangements. Moreover, considering our findings, more studies are needed to estimate the possible promoting/ameliorative effect of antioxidant injection into testicular circulation system during reperfusion (Note Fig. 8).
Fig. 8.
Schematic view for TT- and TR-induced damages. The TT- and TR-induced pathologic oxidative stress, diminished Hsp70 expression, and enhanced caspase-3 expression result in severe DNA damage and progressive lipid peroxidation and finally end with apoptosis following 1 and 2 h. Whereas, following 4 and 8 h, the TT- and TR-induced irreversible oxidative stress in association with massive reduction in Hsp70 expression as well as decreased expression of caspase-3 initiates the severe mRNA damage. All these impairments finally result in necrosis not in all but in some germ cell population
Acknowledgments
Authors wish to thank departments of Comparative Histology and Embryology and Surgery and Diagnostic Imaging, Faculty of Veterinary Medicine, Urmia University.
Funding information
The current manuscript is from thesis NO: 2D-337, which was funded by Urmia University. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Adibnia E, Razi M, Malekinejad H. Zearalenone and 17 beta-estradiol induced damages in male rats reproduction potential; evidence for ERalpha and ERbeta receptors expression and steroidogenesis. Toxicon. 2016;120:133–146. doi: 10.1016/j.toxicon.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol. 2008;59:2–11. doi: 10.1111/j.1600-0897.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- Comish PB, et al. Increasing testicular temperature by exposure to elevated ambient temperatures restores spermatogenesis in adult Utp14b (jsd) mutant (jsd) mice. Andrology. 2015;3:376–384. doi: 10.1111/andr.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dada R, Gupta N, Kucheria K. Spermatogenic alterations in men with high testiculo epididymal temperatures. Indian J Human Genetics. 2002;8:20–25. [Google Scholar]
- Dun MD, Aitken RJ, Nixon B. The role of molecular chaperones in spermatogenesis and the post-testicular maturation of mammalian spermatozoa. Hum Reprod Update. 2012;18:420–435. doi: 10.1093/humupd/dms009. [DOI] [PubMed] [Google Scholar]
- Ekici S, Dogan Ekici AI, Ozturk G, Benli Aksungar F, Sinanoglu O, Turan G, Luleci N. Comparison of melatonin and ozone in the prevention of reperfusion injury following unilateral testicular torsion in rats. Urology. 2012;80:899–906. doi: 10.1016/j.urology.2012.06.049. [DOI] [PubMed] [Google Scholar]
- Filho DW, Torres MA, Bordin AL, Crezcynski-Pasa TB, Boveris A. Spermatic cord torsion, reactive oxygen and nitrogen species and ischemia-reperfusion injury. Mol Asp Med. 2004;25:199–210. doi: 10.1016/j.mam.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Forlenza MJ, Miller GE. Increased serum levels of 8-hydroxy-2′-deoxyguanosine in clinical depression. Psychosom Med. 2006;68:1–7. doi: 10.1097/01.psy.0000195780.37277.2a. [DOI] [PubMed] [Google Scholar]
- Gholirad S, Razi M, Hassani Bafrani H (2016) Tracing of zinc and iron in experimentally induced varicocele: correlation with oxidative, nitrosative and carbonyl stress. Andrologia 49(6). 10.1111/and.12687 [DOI] [PubMed]
- Gladden JD, et al. Novel insights into interactions between mitochondria and xanthine oxidase in acute cardiac volume overload. Free Radic Biol Med. 2011;51:1975–1984. doi: 10.1016/j.freeradbiomed.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther C, Neumann H, Neurath MF, Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62:1062–1071. doi: 10.1136/gutjnl-2011-301364. [DOI] [PubMed] [Google Scholar]
- Khosravanian N, Razi M, Farokhi F, Khosravanian H. Testosterone and vitamin E administration up-regulated varicocele-reduced Hsp70-2 protein expression and ameliorated biochemical alterations. J Assist Reprod Genet. 2014;31:341–354. doi: 10.1007/s10815-013-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YK, Hecht NB. Cytoplasmic protein binding to highly conserved sequences in the 3′ untranslated region of mouse protamine 2 mRNA, a translationally regulated transcript of male germ cells. Proc Natl Acad Sci U S A. 1991;88:3584–3588. doi: 10.1073/pnas.88.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzini F, Tambara Filho R, Gomes RP, Martino-Andrade AJ, Erdmann TR, Matias JE. Long-term effects of the testicular torsion on the spermatogenesis of the contralateral testis and the preventive value of the twisted testis orchiepididymectomy. Acta Cir Bras. 2012;27:388–395. doi: 10.1590/S0102-86502012000600006. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molavi M, Razi M, Malekinejad H, Amniattalab A, Rezaie H. Vitamin E improved cypermethrin-induced damages in the ovary of rats; evidence for angiogenesis and p53 involvement. Pestic Biochem Physiol. 2014;110:27–35. doi: 10.1016/j.pestbp.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Nagakannan P, Shivasharan BD, Thippeswamy BS, Veerapur VP. Effect of tramadol on behavioral alterations and lipid peroxidation after transient forebrain ischemia in rats. Toxicol Mech Methods. 2012;22:674–678. doi: 10.3109/15376516.2012.716092. [DOI] [PubMed] [Google Scholar]
- Niehaus WG, Jr, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Oberst A, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis. 2014;4:e979623. doi: 10.4161/21565562.2014.979623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okorie CO. Unilateral testicular torsion with necrotic outcome: dilemmas of surgical timing. Urology. 2011;78:1232–1234. doi: 10.1016/j.urology.2011.08.059. [DOI] [PubMed] [Google Scholar]
- Pant N, Srivastava SP. Testicular and spermatotoxic effects of quinalphos in rats. J Appl Toxicol. 2003;23:271–274. doi: 10.1002/jat.919. [DOI] [PubMed] [Google Scholar]
- Rerole AL, Jego G, Garrido C. Hsp70: anti-apoptotic and tumorigenic protein. Methods Mol Biol. 2011;787:205–230. doi: 10.1007/978-1-61779-295-3_16. [DOI] [PubMed] [Google Scholar]
- Rezazadeh-Reyhani Z, Razi M, Malekinejad H, Sadrkhanlou R. Cytotoxic effect of nanosilver particles on testicular tissue: evidence for biochemical stress and Hsp70-2 protein expression. Environ Toxicol Pharmacol. 2015;40:626–638. doi: 10.1016/j.etap.2015.08.024. [DOI] [PubMed] [Google Scholar]
- Sakai W, Sugasawa K. FANCD2 is a target for caspase 3 during DNA damage-induced apoptosis. FEBS Lett. 2014;588:3778–3785. doi: 10.1016/j.febslet.2014.08.027. [DOI] [PubMed] [Google Scholar]
- Sarge KD, Cullen KE. Regulation of hsp expression during rodent spermatogenesis. Cell Mol Life Sci. 1997;53:191–197. doi: 10.1007/PL00000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Kepp O, Kroemer G. The end of autophagic cell death? Autophagy. 2012;8:1–3. doi: 10.4161/auto.8.1.16618. [DOI] [PubMed] [Google Scholar]
- Takhtfooladi MA, Asghari A, Takhtfooladi HA, Shabani S. The protective role of curcumin on testicular tissue after hindlimb ischemia reperfusion in rats. Int Urol Nephrol. 2015;47:1605–1610. doi: 10.1007/s11255-015-1101-2. [DOI] [PubMed] [Google Scholar]
- Turner TT, Brown KJ. Spermatic cord torsion: loss of spermatogenesis despite return of blood flow. Biol Reprod. 1993;49:401–407. doi: 10.1095/biolreprod49.2.401. [DOI] [PubMed] [Google Scholar]
- Turner TT, Bang HJ, Lysiak JJ. Experimental testicular torsion: reperfusion blood flow and subsequent testicular venous plasma testosterone concentrations. Urology. 2005;65:390–394. doi: 10.1016/j.urology.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Vigueras RM, Reyes G, Rojas-Castaneda J, Rojas P, Hernandez R. Testicular torsion and its effects on the spermatogenic cycle in the contralateral testis of the rat. Lab Anim. 2004;38:313–320. doi: 10.1258/002367704323133709. [DOI] [PubMed] [Google Scholar]
- Yurtcu M, Abasiyanik A, Avunduk MC, Muhtaroglu S. Effects of melatonin on spermatogenesis and testicular ischemia-reperfusion injury after unilateral testicular torsion-detorsion. J Pediatr Surg. 2008;43:1873–1878. doi: 10.1016/j.jpedsurg.2008.01.065. [DOI] [PubMed] [Google Scholar]
- Zeiss CJ. The apoptosis-necrosis continuum: insights from genetically altered mice. Vet Pathol. 2003;40:481–495. doi: 10.1354/vp.40-5-481. [DOI] [PubMed] [Google Scholar]
- Zhang JQ, Hu XP, Kong XZ, Huang ML, Gou JM, Liu JH, Zhang XD. Testis necrosis following repeated misdiagnosis of testicular torsion: a case report and literature review. Zhonghua Nan Ke Xue. 2009;15:445–448. [PubMed] [Google Scholar]