Abstract
Small heat shock proteins (sHsps) belong to the family of heat shock proteins (Hsps): some are induced in response to multiple stressful events to protect the cells while others are constitutively expressed. Until now, it was believed that Hsps, including sHsps, are present inside the cells and perform intracellular functions. Interestingly, several groups recently reported the extracellular presence of Hsps, and sHsps have also been detected in sera/cerebrospinal fluids in various pathological conditions. Secretion into the extracellular milieu during many pathological conditions suggests additional or novel functions of sHsps in addition to their intracellular properties. Extracellular sHsps are implicated in cell-cell communication, activation of immune cells, and promoting anti-inflammatory and anti-platelet responses. Interestingly, exogenous administration of sHsps showed therapeutic effects in multiple disease models implying that extracellular sHsps are beneficial in pathological conditions. sHsps do not possess signal sequence and, hence, are not exported through the classical Endoplasmic reticulum-Golgi complex (ER-Golgi) secretory pathway. Further, export of sHsps is not inhibited by ER-Golgi secretory pathway inhibitors implying the involvement of a nonclassical secretory pathway in sHsp export. In lieu, lysoendosomal and exosomal pathways have been proposed for the export of sHsps. Heat shock protein 27 (Hsp27), αB-crystallin (αBC), and Hsp20 are shown to be exported by exosomes. Exosomes packaged with sHsps have beneficial effects in in vivo disease models. However, secretion mechanisms and therapeutic use of sHsps have not been elucidated in detail. Therefore, this review aimed at highlighting the current understanding of sHsps (Hsp27, αBC, and Hsp20) in the extracellular medium.
Keywords: Small heat shock proteins, Exosomes, Plasma, Export
Introduction
Small heat shock proteins (sHsps) constitute a structurally divergent group of heat shock proteins (Hsps) characterized by a conserved sequence of 80–100 amino acid residues termed the α-crystallin domain (Basha et al. 2012; Garrido et al. 2012; Gusev et al. 2002; Sun and MacRae 2005a). The α-crystallin domain is located towards a highly flexible and variable C-terminal extension and is usually preceded by a poorly conserved N-terminal region. The molecular mass of sHsp subunits ranges from 13 to 43 kDa. The sHsps occur as homo- or heteromeric complexes, comprising 2–40 subunits. The subunits assemble into large globular complexes up to 1 mDa and are often polydispersed and dynamic with readily exchangeable subunits. The functional significance of sHsps is attributed to their ability to prevent in vitro aggregation of unfolded proteins through hydrophobic interactions (Horwitz 1992; Horwitz et al. 1998; Reddy et al. 2006). Subsequently, sHsps transfer the bound proteins to ATP-dependent chaperones such as Hsp70. Failure in appropriate protein folding will eventually result in proteasomal degradation of the unfolded proteins (Haslbeck et al. 2005; Vos et al. 2008). Thus, molecular chaperone function of sHsps may be limited to storing (holding of aggregation-prone proteins as folding-competent intermediates and conferring enhanced stress resistance to cells by preventing aggregation of proteins). There are ten members in the sHsp family: Hsp27/Hsp25 of rodents (HSPB1), MKBP (myotonic dystrophy protein kinase; HSPB2), HSPB3, αA-crystallin (αAC; HSPB4), αB-crystallin (αBC; HSPB5), Hsp20 (HSPB6), cvHsp (HSPB7), Hsp22/H11/H2IG1 (HSPB8), HSPB9, and ODF (sperm outer dense fiber protein; HSPB10). While some sHsps are tissue-specific (MKBP, HSPB3, αAC, cvHsp, HSPB9, and ODF), the rest are ubiquitous (Hsp27, αBC, Hsp20, and Hsp22) (Garrido et al. 2012) Small Hsps can also modulate intracellular redox status suggesting that sHsps are not only involved in the cellular defense mechanisms against protein aggregation but also participate in essential physiological processes including cytoskeletal reorganization and apoptosis. Traditionally, Hsps including sHsps are considered to possess intracellular chaperone activity and confer protection from stress-induced cell death (Arrigo 2005; Sun and MacRae 2005b). However, the emerging evidence envisioned that there is the existence of extracellular Hsps that might contribute toward the alleviation of pathological conditions. Extracellular Hsps are first reported by Tytell et al. (1986) in giant squid axon functioning as a glia-axon transfer protein. Similarly, Hsp70 has been reported to be present in the extracellular milieu independent of ER-Golgi-mediated protein trafficking (Hightower and Guidon 1989). At the molecular level, Hsps lack classical N-terminus signal sequence required for conventional protein secretory pathway (Mambula et al. 2007) similar to that of immune-modulators such as IL-1α, IL-1β, IL-16, and IL-18 (Prudovsky et al. 2003). Extracellular release of Hsps has been reported during necrotic cell death (Basu et al. 2000). However, Hightower and Guidon (1989) noticed that the presence of lysine amino acid analogues/aminoethyl cysteine prevented the release of Hsp70 into extracellular milieu, suggesting that only a structurally intact protein is secreted out from the cells. In support of these results, subsequent studies demonstrated the extracellular presence of large (higher molecular weight) Hsps including Hsp60 and Hsp70 (Luo et al. 2008; Mambula and Calderwood 2006; Merendino et al. 2010; Vega et al. 2008).
Existing literature shed light on the unconventional release of Hsps and their possible extracellular functions in disease conditions including cancer (Calderwood et al. 2007; De Maio 2011; De Maio and Vazquez 2013) (Santos et al. 2017). Adding to this, the presence of sHsps (Hsp27, αBC, and Hsp20) is reported to be present in an extracellular milieu with undefined functions. Intriguingly, these studies also opened up new exploratory research avenues with the possibility of identifying novel functions performed by these secreted sHsps. Therefore, the current review tries to summarize the possible export mechanisms, functions, and therapeutic effects of extracellular sHsps with special emphasis on Hsp27, αBC, and Hsp20, which are known to be well characterized to date.
Hsp27
Hsp27, a member of the sHsp family, responds to stress and acts as a chaperone to facilitate appropriate folding of proteins (Rogalla et al. 1999). Hsp27 acts as an antioxidant and reduces reactive oxygen species (Mehlen et al. 1997) and also protects cells from programmed cell death by interacting with mediators of mitochondria (Havasi et al. 2008). Contrasting observations on the functional role of Hsp27 suggests that Hsp27 can positively and negatively regulate actin polymerization during cellular stress conditions (Benndorf et al. 1994; Huot et al. 1996; Miron et al. 1991).
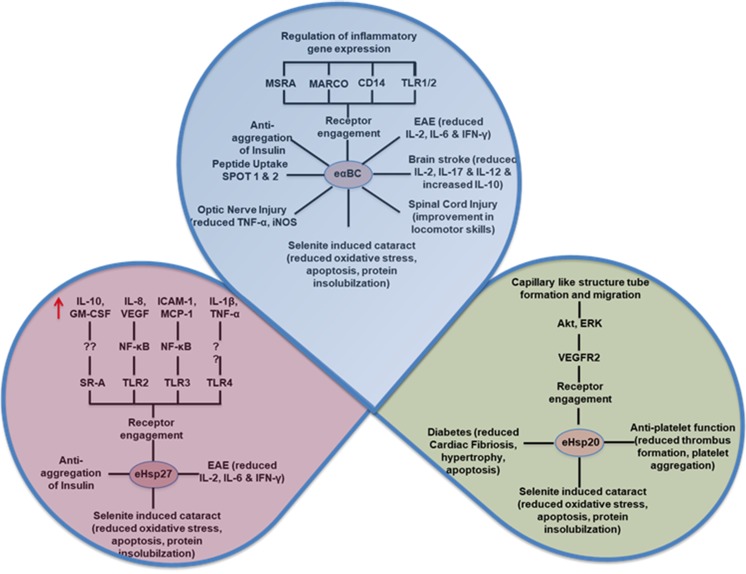
Extracellular release of Hsp27 from tumor cells with unknown functions has been reported in plasma (4-fold increase) and interstitial fluid (2500-fold increase) in breast cancer patients (Banerjee et al. 2011; Ciocca et al. 1984; Fanelli et al. 1998; Feng et al. 2005; Thuringer et al. 2015). It is reported that secretion of Hsp27 is involved in differentiation of monocytes to macrophages. The secreted protein might be involved in immunological signaling by interacting with plasma membrane proteins thereby promoting tumor growth. Therefore, knock-down or knock-out approach or inhibition of secretion of Hsp27 in tumor cell lines or animal models might be an effective strategy in reducing the tumor growth. The secretion of Hsp27 in the extracellular medium is increased under stressful/pathological insults with different fold changes (Table 1 ). Elevated levels of Hsp27 are found in the plasma of type 1 diabetic patients in the European population. Interestingly, high levels of Hsp27 are correlated with distal symmetrical polyneuropathy in diabetes and have been reported to be a marker for diabetic neuropathy (Gruden et al. 2008). Further, studies from our laboratory reported altered expression and phosphorylation of intracellular sHsps in target tissues of diabetic rat model (Reddy et al. 2015; Reddy et al. 2014; Reddy et al. 2013; Reddy and Reddy 2015; Reddy and Reddy 2016). In addition, we recently reported elevated levels of Hsp27 in the plasma of nephropathy patients in type 2 diabetes, and its levels were positively correlated with serum creatinine thereby suggesting that Hsp27 might serve as a marker for diabetic nephropathy (Jakhotia et al. 2017). Also, Hsp27 protein and its antibodies are elevated in insulin resistance in glucose-intolerant patients with a history of cardiovascular diseases (Burut et al. 2010). Further, the release of Hsp27 from platelets is accompanied by phosphorylation in type 2 diabetic patients and is correlated with accelerated aggregation of platelets (Tokuda et al. 2015). Taken together, these studies strongly suggest that Hsp27 is released into the serum in diabetes as perhaps a response to stressful events in hyperglycemia. Hsp27 and Hsp70 are also elevated in the serum of patients who smoke and have chronic obstructive pulmonary disease (COPD), and these Hsps were found to be diagnostic markers for COPD (Hacker et al. 2009). Extensive tissue damage in COPD patients with chronic disease symptoms correlates with the extracellular release of Hsp27 into circulation (1.8-fold vs healthy). Hsp27 is released not only into plasma but also into cerebrospinal fluid in the central nervous system during ischemia (Hecker and McGarvey 2011). Moreover, elevated serum levels of Hsp27 have been detected in multiple sclerosis (Ce et al. 2011), gastric adenocarcinoma (Huang et al. 2010), pancreatitis (Liao et al. 2009), thymic epithelial tumors, and myasthenia gravis (Janik et al. 2016). Very recently, secretion of Hsp27 into circulation in ovarian cancer (OC) patients has been noticed and considered to be a potential biomarker for prognosis and diagnosis for OC (Stope et al. 2017). The functions of extracellular Hsp27 are unknown, although a few studies have reported that it is involved in signaling by binding to the receptors of immune and endothelial cells (Schmitt et al. 2007; Binder et al. 2004). Additionally, a few studies reported its role in cardiovascular diseases as it was found in the serum of patients with acute coronary syndromes (Park et al. 2006), atherosclerosis (Martin-Ventura et al. 2004), and reperfusion after ischemic clamping during heart bypass surgery (Jin et al. 2014). Additionally, Hsp27 has been shown to be an atheroprotective agent by decreasing the uptake of atherogenic lipids and thereby attenuating inflammation (Rayner et al. 2008). The addition of exogenous Hsp27 to in vitro macrophage culture reduced the acetylated LDL uptake by 41% with a concomitant increase in the expression of anti-inflammatory (IL-10 and GM-CSF) and pro-inflammatory mediators (IL-1β and TNF-α) (Rayner et al. 2008; Salari et al. 2013). Extracellular Hsp27 reduced the LDL uptake by binding to the scavenger receptor-A (SR-A). It reduced the ability of SR-A to engulf acetylated LDL on the surface of macrophages and acquired the foam cell phenotype (Rayner et al. 2008). Hsp27 also activated the NF-kB in mouse coronary endothelial cells by interacting with TLR2 and TLR4 and resulted in upregulation of monocyte chemoattractant protein-1 (MCP-1) and intercellular adhesion molecule-1 (ICAM-1) (Jin et al. 2014). Similarly, Hsp27 engagement on TLR3 was implicated in the activation of NF-κB and secretion of IL-8 and vascular endothelial growth factor (VEGF) in human microvascular endothelial cells (HMECs) (Thuringer et al. 2013). Recombinant Hsp27 rapidly internalized into the endosomal compartment (within 15–30 min) with the pool of TLR3, which is required for NF-κB activation in HMECs. Treatment of bone marrow-derived dendritic cells with recombinant Hsp27 also activated IL-6, TNF-α, IL-1β, IL-12p35, and IL-12p40 by interacting with TLR4 (Yusuf et al. 2009). Estrogen receptor-β is also known to interact with Hsp27 (Al-Madhoun et al. 2007; Miller et al. 2005). In addition to inducing inflammation, extracellular Hsp27 shows chaperone and therapeutic activity in in vitro and experimental disease models. Recombinant Hsp27 inhibited the DTT-induced aggregation of insulin in vitro, indicating that exogenous Hsp27 has effective chaperone activity (Kurnellas et al. 2012). Likewise, intraperitoneal injection of recombinant Hsp27 reduced the paralytic symptoms by modulating inflammatory cytokines IL-2, IL-6, and IFN-λ in the experimental auto-immune encephalomyelitis (EAE) mouse model at the peak of the disease (Kurnellas et al. 2012). However, ceasing of Hsp27 supplementation resulted in complete return of the paralytic symptoms, indicating that Hsp27 acts as a biological inhibitor, and the therapeutic effects seem to depend on consistent circulatory levels of Hsp27, rather than inducing a long-acting immunological state of reduced inflammation or tolerance (Kurnellas et al. 2012). A short sequence of Hsp27 (73 to 92 amino acids; 1 μg/animal) ameliorated the paralytic symptoms in EAE mouse model, suggesting that peptides of Hsp27 also have effective therapeutic activity similar to that of the full-length protein (Kurnellas et al. 2012). In another study, the sequence of 93 to 113 amino acids of Hsp27 (10 μg/ml) protected the HeLa cells from STS- or H2O2-induced apoptosis by inhibiting the release of cytochrome c from mitochondria and activating caspase-3 (Nahomi et al. 2015). Intraperitoneal (i.p.) administration of the same peptide (93 to 113 amino acids; 50 μg/animal) for 6 days prevented selenite-induced cataract development in mice by inhibiting the oxidative stress, protein insolubilization, and apoptosis indicating that peptides of Hsp27 are effective in preventing disease phenotype (Nahomi et al. 2015). Detection of injected peptide (i.p.) in the lens confirmed that the peptide is able to traffic through barriers to execute its function. Finally, these observations culminate to suggest that Hsp27 is mainly involved in modulating the inflammatory response by interacting with certain receptors (secretion of cytokines), chaperone activity (prevention or suppression of aggregation of proteins), cell migration (upregulation of MCP-1 and ICAM-1), cell death (preventing apoptosis), and cell proliferation (interacting with ER-β) (Fig. 1).
Table 1.
Secretion of sHsps into the extracellular medium in pathological conditions
| sHsp | Pathology | Fold change | Effect | References |
|---|---|---|---|---|
| Hsp27 | Breast cancer | 4-fold in serum and 2500-fold increase in tumor interstitial fluid. | Induced differentiation of monocytes to macrophages, anergy in T-cell, and neovascularization | Banerjee et al. (2011) |
| Hepatocellular carcinoma (HCC) | Increased levels of Hsp27 found in HCC patients analyzed by 2-DE based serum proteome | Developed as a biomarker for HCC | Feng et al. (2005) | |
| Colon cancer | 4-fold increase (secreted into culture medium from primary tumor derived SW480 cells) | Increased the endothelial gap junction coupling by promoting the phosphorylation of connexin 43 | Thuringer et al. (2015) | |
| Diabetic neuropathy | 2-fold increase | Novel biomarker of diabetic neuropathy | Gruden et al. (2008) | |
| Insulin resistance | Increased antigen and antibodies | Related to glucose intolerance | Burut et al. (2010) | |
| Chronic obstructive pulmonary disease (COPD) | 1.8-fold increase (COPD with severe disease) | Diagnostic marker for immune activation and tissue destruction in COPD | Hacker et al. (2009) | |
| Multiple sclerosis (MS) | 4.8-fold increase in the attack phase | Guiding the accurate detection of an attack in MS | Ce et al. (2011) | |
| Gastric adenocarcinoma (GA) | 1.7-fold increase | Marker for detection of GA | Huang et al. (2010) | |
| Thymic epithelial tumors (TET) | 1.5-fold increase | Can be developed as diagnostic marker for TET | Janik et al. (2016) | |
| Myasthenia gravis | 2.3-fold increase | Can be developed as diagnostic marker for TET | Janik et al. (2016) | |
| Acute coronary syndromes | 2.3-fold increase | Represent the systemic inflammation and oxidative stress | Park et al. (2006) | |
| Atherosclerosis | 20-fold decrease | Could be developed as potential index of atherosclerosis | Martin-Ventura et al. (2004) | |
| Ovarian cancer (OC) | The secretion of Hsp27 increased after overexpression (2.38-fold) and heat shock | Non-invasive biomarker for OC | Stope et al. (2017) | |
| αBC | Obesity | Increased in Conditioned media of cultured primary human adipocytes derived from obese patients | Could be developed as a novel adipokine | Lehr et al. (2012) |
| Renal cell carcinoma (RCC) | Increased | Biomarker of RCC | Holcakova et al. (2008) | |
| Multiple sclerosis | Increased auto-antibodies | Inflammatory response | Celet et al. (2000); van Noort et al. (2006) | |
| Multiple sclerosis | 5-fold increase | Anti-inflammatory response | Rothbard et al. (2012) | |
| Experimental auto-immune myelitis | 3-fold increase | Anti-inflammatory response | Rothbard et al. (2012) | |
| Neuro-Behçet’s disease | Increased auto-antibodies | Inflammatory response | Celet et al. (2000) | |
| Guillain–Barré syndrome | Increased auto-antibodies | Inflammatory response | Celet et al. (2000); Hegen et al. (2010) | |
| Chronic inflammatory demyelinating polyneuropathy | Hegen et al. (2010) | |||
| Brain stroke in mice | 2.7-fold at 12 h, 1.7-fold at 2 days, 0.5-fold compared to naïve mice | Anti-inflammatory response | Arac et al. (2011) | |
| Ischemic brain stroke in humans | 1.7-fold at < 4 h, 1.2-fold at 24 h, 1.4 at 48 h in younger patients compared to 0.3-fold in healthy ones | Anti-inflammatory response | Arac et al. (2011) | |
| Hsp20 | Cardiomyopathy | 126-fold increase | Regulator of platelet function | Kozawa et al. (2002) |
| Acute dissecting aneurysm | 11-fold increase | Acts as anti-platelet regulator | Niwa et al. (2000) | |
| Myocardial ischemia/reperfusion (I/R) | 3.4-fold increase | Acts as a cardiokine which mediates angiogenesis by directly interacting with VEGFR2 | Zhang et al. (2012) |
Fig. 1.
Functions of extracellular heat shock protein 27 (eHsp27), αB-crystallin (eαBC), and Hsp20 (eHsp20). TLR: toll-like receptor; SR-A: scavenger receptor-A; VEGF: vascular endothelial growth factor; GM-CSF: granulocyte macrophage colony stimulating factor; ICAM-1: intercellular adhesion molecule1; MCP-1: monocyte chemoattractant protein-1; TNF-α: tumor necrosis factor; IL-6: interleukin-6; MSRA: macrophage scavenger receptor A; MARCO: macrophage scavenger receptor with collagenous structure; TNF-α: tumor necrosis factor; IL-2: interleukin-2; SOPT1: sodium oligopeptide transporter 1; VEGFR2: vascular endothelial growth factor receptor 2
Export of Hsp27
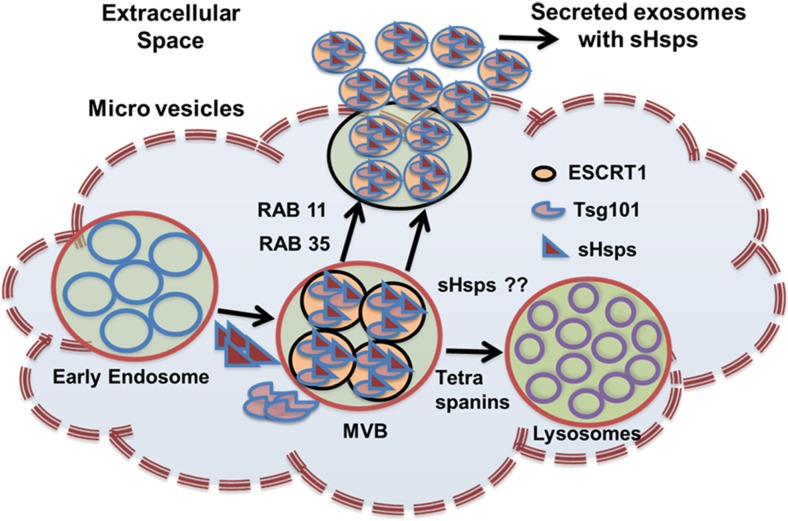
As discussed earlier, sHsps are not secreted through the classical ER-Golgi secretory pathway. Until now, lysoendosomal or exosomal/extracellular vesicular pathway, direct protein translocation, and release after cell death (necrosis) have been proposed toward the release of Hsps (De Maio 2011; De Maio and Vazquez 2013). Among these, it is unclear whether the release of Hsps after cell death is a valid mechanism for export of Hsps. Some recent studies have provided evidence for the release of sHsps via the lysoendosomal or exosomal pathway (Batulan et al. 2016; Rayner et al. 2009; Sreekumar et al. 2010; Zhang et al. 2012). Rayner et al. (2008) reported that treating macrophages with acetylated LDL or estradiol-induced secretion of Hsp27 and co-localization with lysotracker or lysosomal marker LAMP. Another group also reported the co-localization of non-phosphorylatable mutants (S15A/S82A) of Hsp27 fused to GFP with lysosomal marker, LAMP-1, indicating that Hsp27 also exits into the extracellular space through secretory lysosomal-like vesicles (Lee et al., 2012). Utilization of phosphomimetic forms of Hsp27 (S15D/S28D) further confirmed that phosphorylation of Hsp27 at specific serine residues is crucial for its secretion exclusively through the endosomal pathway. Another exit of Hsp27 is through the exosomal pathway, and numerous studies are supporting the release of Hsp27 through the exosomes. Rayner et al. (2009) showed that extracellular levels of Hsp27 are reduced upon treatment of human macrophages with exosomal inhibitor dimethyl amiloride. Nafar et al. (2016) detected Hsp27 in membrane fractions of exosomes originating from rat astrocytes upon treatment with amyloid-β. Under physiological conditions, exosomes are positive for Hsp27, and exposure of lymphoblastoid cells to heat stress (42 °C for 3 h) enhanced the quantity of exosomes with a ratio of 1.2:1 compared to that of exosomes from the control cells. Heat stress not only increased the quantity of exosomes but also selectively enriched Hsp27 along with Hsp70 and Hsp90 (Clayton et al. 2005). Exosomes are small-membrane microvesicles ranging from 4 to 100 nm in diameter and are found in all biological fluids. Lipids (lipid rafts) and proteins are major components of exosome membranes. Exosomes are released into the extracellular space after fusion with the plasma membrane and contain membranous and lumen portions. The inward budding of the plasma membrane forms early endosomes, and the membrane of early endosomes buds inwardly into various late endosomes. Late endosomes contain intraluminal bodies that are also called multivesicular bodies (MVBs). These MVBs fuse with the plasma membrane to release intraluminal bodies, i.e., exosomes, while the microvesicles, ranging from 100 to 1000 nm directly bud off from the plasma membrane. In the recent past, the exosomes have become crucial in understanding the cell-to-cell communication and as delivery agents of miRNA. Large Hsps including Hsp70 are localized to the membrane (surface) of exosomes (Gastpar et al. 2005) as well as to the luminal compartment (Clayton et al. 2005; Jia et al. 2017). It is also subject to debate whether sHsps are localized in the luminal or membrane portion in exosomes. While the presence of Hsp27 in lumen or membrane of exosomes is still under debate, studies proposed that Hsps are localized in the lumen and as well as on the surface. Localization of Hsps in the lumen but not on the exosome surface suggests that Hsps in exosomes may not interact with target cells through cell surface Hsp receptors. However, the presence of Hsps on the surface can also be accepted, as it is important for secretion of Hsps into circulation and interaction with other cell surface receptors. Furthermore, the presence of sHsps on the cell surface is corroborated by the fact that Hsp27 interacts with cell surface receptors such as SR-A and TLRs (Yusuf et al. 2009). Hsp27 was seen to be released into the extracellular medium through exosomes when OVCAR-3 and SK-OV-3 cells were cultured in vitro (Stope et al. 2017). Blocking of the ER-Golgi pathway with brefeldin-A has not affected the secretion of Hsp27 suggesting secretion of Hsp27 may depend on intracellular levels rather than the phosphorylation status of Hsp27. In support to this, Stope et al. (2017) demonstrated that phosphorylation did not affect the secretion of sHsps. This study further provided significant insights into the understanding of the factors that influence the secretion of Hsp27. Interestingly, heat shock treatment not only increased the expression of intracellular Hsp27 but also enhanced the incorporation of Hsp27 by 10-fold into extracellular exosomes imparting a crucial role of the exosomal pathway in Hsp27 secretion.
αBC
Recently, many studies have demonstrated the extracellular presence and therapeutic functions of exogenous αBC in multiple in vitro cell culture as well as in in vivo disease models. The increased secretion of αBC into the culture medium has been shown during primary human adipocyte culture from obese patients and corroborates with its increased level in plasma and adipose tissue from the patients (Lehr et al. 2012). Therefore, it is concluded that αBC also acts as an adipokine. The secretion of αBC into plasma under several disease conditions with altered fold change is listed in Table 1. One study identified that αBC can act as a biomarker for renal cell carcinoma with its increased levels in serum samples of patients (Holcakova et al. 2008). An increasing number of studies also have been reported that the export of αBC into the extracellular milieu is through the exosomes. Sreekumar et al. (2010) reported the presence of αBC in secreted exosomes when retinal pigment epithelial cells (RPE) were cultured in exosome-free medium (to avoid contamination from endogenous exosomes present in the serum). The presence of αBC in exosomes has been further confirmed by performing immunogold labeling and immunoblotting for CD63, as a marker for exosomes (Sreekumar et al. 2010). Multiple factors including stress stimuli, cell polarity, and inflammation can induce the release of αBC. For example, αBC secreted within exosomes by highly polarized human RPE cells toward the apical photoreceptor facing neural retina confers neuroprotection. Extracellular αBC can also act as an auto-antigen by eliciting auto-antibodies in multiple sclerosis (van Noort et al. 2006). Subsequently, many studies reported the presence of auto-antibodies in CSF and serum of Neuro-Behçet’s disease, Guillain–Barré syndrome, and inflammatory neurologic disorders (Celet et al. 2000; Hegen et al. 2010). However, the perception of αBC as an auto-antigen in these disorders is equivocal as recent studies reported that αBC through its chaperone activity can bind immunoglobulin with a high affinity, which contradicts the αBC as an auto-antigen (Rothbard et al. 2011). Of note, αBC promotes anti-inflammatory rather than proinflammatory response. Exogenous administration of 50 μg/100 g of body weight of α-crystallin (combination of αAC and αBC) reduced the expression of cytokines including IL-1α, TNF-α, and NO in the silver nitrate-induced rat inflammation model. Also, α-crystallin treatment in the same experimental model reduced the levels of neurotransmitters including dopamine, 5-hydroxytryptamine, and norepinephrine and increased their metabolites such as 3,4-dihydroxyphenylacetic acid, homovanillic acid, and 5-hydroxyindoleacetic acid in the neocortex and hippocampus regions of the brain (Masilamoni et al. 2005). Additionally, the same group reported alleviation of inflammation-induced expression of NF-κB and GFAP and decreased activation of acetylcholine esterase activity in neurons upon α-crystallin treatment (Masilamoni et al. 2006). However, the mechanism responsible for extracellular αBC in promoting an anti-inflammatory response upon administration remains unclear. The receptors including CD14, TLR1, and TLR2 are a prerequisite for αBC-mediated activation of HEK-293 cells secreting alkaline phosphatase under the control of transfected NF-kB (van Noort et al. 2013). Exogenous αBC activated the cells expressing CD14 and TLR1/2 through initial binding with macrophage scavenger receptor A (MSRA) and macrophage scavenger receptor with collagenous structure (MARCO) (van Noort et al. 2013). Further, it also inhibited the lymphocyte and neutrophil infiltration in the cigarette smoke-induced lung inflammation mouse model (van Noort et al. 2013). It has been observed that there is an increased level of αBC in plasma of individuals with the disease in advanced stages than that of individuals with later stages of the disease in stroke patients (Arac et al. 2011). Intriguingly, increased levels of αBC have been noticed in patients with stroke at the time of presentation in the hospital (< 4 h from the point of stroke) and subsided within the next 48 h similar to that of mice suffering from a stroke. Supplementation of αBC i.p. to the αBC knock-out mice suffering from brain stroke reduced the lesion size. Administration of αBC 1 h before and 12 h after the onset of stroke in mice did not reduce the lesion size compared to the PBS-treated group. However, administration of αBC 1 h before and 12 and 24 h after followed by daily administration for 7 days after the stroke onset in mice led to a reduction in the lesion size compared to that of PBS-treated group, implicating the neuroprotective function to αBC. Furthermore, systemic augmentation of αBC reduced both the stroke volume and inflammatory cytokines (decreased proinflammatory cytokines and increased anti-inflammatory cytokines) in the stroke-induced mouse model (Arac et al. 2011). Intraperitoneal administration of αBC rapidly and efficiently reduced the paralytic symptoms in EAE mouse model. It is also noted that extracellular αBC does not directly affect T- or B-lymphocyte proliferation; rather, it limits the inflammation induced by TLRs and innate immunity. In support to this, Rothbard et al. (2012) reported that systemic administration of αBC in the EAE (induced by adoptive transfer of myelin-specific Th17 lymphocytes) mice did not ameliorate the clinical symptoms. Another study reported that intravenous administration of human recombinant αBC attenuated the spinal cord injury (SCI) in mice by ameliorating secondary tissue damage, improving locomotor skills, and reducing the recruitment of inflammatory macrophages (Klopstein et al. 2012). The extracellularly administered αBC is able to enter into some of the tissues including the spinal cord. The intravenous administration of αBC is able to enter injured spinal cord when injected into a SCI mouse model and showed 2-fold increase in protein levels at the site of injury 12 h post-injection as compared to a saline-treated group. Altogether, these results indicate that αBC can enter into inflammatory site in neuronal tissues and can show an anti-inflammatory response. However, endogenous αBC protein levels were reduced by 20% in recombinant human αBC-treated SCI mice compared with that of the physiological levels in normal spinal cord (Klopstein et al. 2012). The injected αBC protein has been shown to enter into the white matter at the injured epicenter and into white and gray matter in the adjacent areas (45% taken up by neurons, 38.8% by astrocytes, and 30.7% by oligodendrocytes) and not found in microglial cells, as well as granulocytes. αBC enhanced the survival of retinal ganglion cells, inhibited the retinal microglia cells, and reduced the expression of TNFα and iNOS in the rat model of optic nerve crush (Wu et al. 2014). Wu et al. (2014) administered αBC in a dose-dependent manner (0.05, 0.5, and 5 g/kg of body weight) to optic nerve crush rat model, but 5 g/kg of b.w. showed agitated behavior indicating that higher concentration of αBC is toxic to rats. Therefore, one should be cautious about using higher concentrations of αBC for retinal optic neuropathy, and one has to study the range of concentrations that are deleterious or beneficial to other diseases. However, the mechanism by which extracellular αBC exhibits beneficial functions remains largely unknown. One study revealed that αBC was able to bind to > 70 proteins in plasma of multiple sclerosis, amyloidosis, and rheumatoid arthritis patients, mouse model of EAE, and interestingly, these proteins have been identified to be part of proinflammatory signature including acute phase proteins, members of complement, and coagulation cascade (Rothbard et al. 2012). A study reported that αBC or its peptide blocks the function of platelets by preventing their aggregation in in vitro, ex vivo, and in vivo mouse models induced by thrombin or botrocetin (Kozawa et al. 2001). Inhibition of platelet aggregation is mediated by suppression of Ca2+ mobilization and activation of protein kinase C (Kozawa et al. 2001). Furthermore, αBC attenuates the ADP-induced granule secretion via p38 MAPK and p42 MAPK (Enomoto et al. 2009) upon incubation with platelets. Not only full-length αBC but also the intravenous administration of αBC peptides also inhibited thrombus formation in vivo indicating that both extracellular full-length and peptide αBC are important in the regulation of platelet function with an application in anti-platelet therapy (Kanno and Matsuno 2006). αBC is also found to be instrumental in performing extracellular chaperone activity in addition to anti-inflammatory and anti-platelet functions. In support to this, Mannini et al. (2012) showed that extracellular addition of αBC reduced the misfolded oligomer-induced toxicity even at a low level rather than overexpressing in the cytosol implicating the potential role for extracellular αBC to that of cytosolic counterpart. Exogenous recombinant αBC (12.5 μg/ml) protected the human lens’ epithelial cells from heat- and oxidative stress-induced cell death indicating that exogenous αBC displays anti-apoptotic activity (Christopher et al. 2014). It is also shown that uptake of αBC is enhanced by fusion with cell penetration peptides (CPP) including TAT protein from human immunodeficiency virus and glycoprotein-C from hepatitis C virus. Cell penetration peptides fused to αBC including TAT-αBC and gC-αBC tagged with fluorophore showed enhanced uptake in HLE-B3 cells within 60 min of exposure to cell. The exogenously added gC-αBC was able to co-localize with endogenous αBC in cytoplasm as revealed by confocal microscopy indicating the efficiency of CPP in increasing the cellular uptake. It is also established that administration of αBC (i.p.) peptides inhibits protein insolubilization, epithelial cell apoptosis, and finally opacification in selenite-induced cataract in mice (Nahomi et al. 2013). This group administered the increasing dose of 2.5, 5, and 10 μg/animal of both αBC peptide and acetyl-αBC peptide at 6 h prior to selenite injection in rat pups as a single injection separately. For multiple doses, the first dose was given 6 h prior to sodium selenite injection, followed by subsequent injections each day for 4 days. Both the peptides significantly inhibited cataract progression at 10 μg (single dose), as well as multiple doses. Treatment with 5 μg of both peptides also significantly inhibited cataract progression with multiple injections, while acetyl-αBC alone inhibited the cataract with single injection, indicating a dose-dependent therapeutic effect of native as well as modified αBC peptides. This study suggests that peptides of αBC are capable of crossing the blood/aqueous barrier to inhibit the opacification of the lens and shows the efficiency of exogenous αBC peptides in ameliorating the disease phenotype. The above findings suggest that the beneficial effects of exogenous administration of αBC are not only limited to a single disease or mechanism but also to multiple diseases or mechanisms where inflammation, cell death, and protein aggregation are involved in disease initiation or progression. The functions of extracellular αBC were depicted in Fig. 1.
Export of αBC
There is also compelling evidence suggesting the presence of αBC in exosomes. Like Hsp27, αBC secretion occurs independently of the classical secretory pathway wherein inhibiting the classical secretory pathway by using brefeldin and tunicamycin did not alter the secretion of αBC. It has been shown that αBC is secreted from the apical side of polarized human RPE cells (Sreekumar et al. 2010). In exosomes, αBC resides in detergent-resistant membrane microdomains, which are essential sites for exosome biogenesis. Treatment of human RPE cells with β-methyl cyclodextrin (lipid raft and cholesterol depletor) as well as dimethyl amiloride (exosome inhibitor and inhibitor of H+/Na+ and Na+/Ca2+ exchanger) significantly inhibited αBC secretion, indicating the need of lipid rafts and exosomes for αBC secretion. Further, the secretory levels of αBC from human RPE cells are only a small fraction to that of the steady-state levels found in mitochondria and cytosolic compartments (Sreekumar et al. 2010). The polarization of cells is also equally important for secretion of αBC, which is further corroborated by the fact that a 5-fold increase in αBC secretion has been found in polarized RPE cells, compared to that of the non-polarized cells. Stress also affects the polarization and secretion of αBC. In support to this, oxidative stress induced by H2O2 in RPE cells induced the increased secretion of exosomes on the basolateral side compared to the apical side, and the secretion of αBC is found to be more from the exosomal fraction of the basolateral side when compared to non-stressed cells. Moreover, αBC has been shown to co-localize with tetraspanin CD63 in some of the MVBs/exosomes and perinuclear Golgi in ARPE cells. Interestingly, αBC did not co-localize to some of the CD63 positive exosomes/MVBs in the peripheral regions of the cell (Bhat and Gangulam 2011). Sreekumar et al. (2013) first reported the uptake of the αBC-derived peptide by two novel sodium-dependent oligopeptide transporters SOPT1 and SOPT2 in fetal RPE (fRPE) cells. These transporters are involved in transporting of endogenous and other synthetic opioid peptides and the iron regulatory peptide hormone hepcidin. αBC-derived peptides are transported into fRPE cells by competing with DADLE and deltorphin II that are substrates for SOPT1 and SOPT2. αBC-derived peptides have a high affinity for SOPT1 and SOPT2. On the contrary to Hsp27, exosomal secretion of αBC is influenced by post-translational modification including phosphorylation and O-GlcNAcylation (Kore and Abraham 2016). Phosphorylation and prevention of O-GlcNAcylation deterred the incorporation and exosomal secretion of αBC. αBC plays an crucial role in exosome biogenesis. Using shRNA, inhibition of αBC expression resulted in aggregated staining patterns of CD63 (LAMP3) and inhibited exosome secretion from ARPE19 cells (Gangalum et al. 2016). Further, inhibition of αBC expression increased the number of vacuoles and enlarged (fused) vesicles with increased expression of CD63 (LAMP3) and LAMP1 (a marker of lysosomes) indicating the shift toward the endolysosomal pathway which is further supported by increased levels of Rab7 (a marker for late endosome). This study demonstrates that αBC is crucial in the biogenesis of exosomes and that their secretion and inhibition of αBC cause shift into the endolysosome pathway. Further studies are required to address other mechanistic details of sHsps in the biogenesis of exosomes. It is possible that αBC might be involved in passaging the MVBs to the plasma membrane for the release of exosomes because in the absence of αBC, markers including Rab7 and LAMP1 increased, indicating that MVBs progress toward lysosomes instead of progressing toward the plasma membrane. However, it is also noted that exosome biogenesis normally occurs in many cell types without the involvement of αBC. Therefore, one should address whether αBC acts in a specific manner according to cell type.
Hsp20
Hsp20 is an important member of the sHsp family and is also referred to as HSPB6. It is expressed in multiple tissues with abundant expression in skeletal, cardiac, and smooth muscle cells (Fan et al. 2005; Salinthone et al. 2008). A few studies have reported the secretion of Hsp20 into the extracellular medium in pathological conditions. Whether secretion of Hsp20 in pathological conditions is advantageous or deleterious remains to be elucidated. Kozawa et al. (2002) reported that there is an elevated level of Hsp20 in plasma of cardiomyopathic hamsters. These researchers detected both dissociated and aggregated forms of Hsp20 with elevated levels of the dissociated form in plasma of cardiomyopathic hamsters. Furthermore, circulating levels of Hsp20 increased 3.4-fold in cardiac-specific Hsp20-overexpressed transgenic mice cardiomyocytes in vitro (Zhang et al. 2012). These researchers also reported the secretion of Hsp20 through exosomes rather than through the classical ER-Golgi secretory pathway. Extracellular Hsp20 promoted the capillary-like structure tube formation and migration in HUVEC cells by interacting with VEGFR2, which is associated with activation of Akt and ERK (Zhang et al. 2012). Hsp20 levels were also elevated up to 11-fold in plasma of acute dissecting aneurysm patients. Extracellular Hsp20 shows anti-platelet activity by inhibiting thrombin-induced platelet aggregation in a dose-dependent manner by inhibiting receptor-mediated calcium influx in vitro and ex vivo (Matsuno et al. 1998; Niwa et al. 2000). Nevertheless, Hsp20 levels were markedly lower in a carotid artery endothelial injury model indicating that Hsp20 is secreted into circulation in response to endothelial injury to act as a regulator of platelets extracellularly (Kozawa et al. 2002). The intravenous administration of Hsp20 into hamsters prevented thrombus formation after endothelial injury, and its peptide also reduced the platelet aggregation implicating the anti-platelet function of exogenous Hsp20 (Kanno and Matsuno 2006). Intraperitoneal administration of recombinant Hsp20 (10 μg/day for 20 days) has shown similar therapeutic activity along with other sHsps by reducing the paralytic symptoms in the EAE mouse model, suggesting that exogenous Hsp20 acts as a therapeutic protein by decreasing the inflammation (Rothbard et al. 2012). In vitro treatment of HeLa cells with Hsp20-peptide (G71HFSVLLDVKHFSPEEIAVK91) caused it to translocate to the nucleus from the cytosol during thermal stress. Hsp20 peptide also prevented apoptosis by blocking the release of cytochrome c from mitochondria and caspase-3 activation in HeLa cells (Nahomi et al. 2015). Intraperitoneal administration of 50 μg of Hsp20 peptide/animal/day for six consecutive days inhibited the protein aggregation and oxidative stress thereby preventing the cataract formation in selenite-treated rats suggesting that exogenous Hsp20 peptide shows therapeutic activity by anti-aggregation and anti-oxidant activities (Nahomi et al. 2015). Previous studies have reported the secretion of Hsp20 through exosomes rather than a classical ER-Golgi secretory pathway. Accumulating evidence suggests that exosomes secreted from cardiomyocytes contain Hsp20 and play crucial role in cardiovascular pathology. Exosomes secreted from diabetic rat cardiomyocytes contain lower levels of Hsp20 compared to that of healthy controls. Exosomes secreted from diabetic cardiomyocytes from cardiac-specific overexpressing-Hsp20 mice (conditional transgenic mice overexpressing Hsp20 in the heart) showed increased levels of Hsp20 compared to those in control rats (Wang et al. 2016). Cardiac-specific overexpression of Hsp20 increased the biogenesis of exosomes upon interaction with tumor susceptibility gene 101 (Tsg101). Tsg101 is the component of Endosomal Sorting Complex Required for Transport-1 (ESCRT-1) which recognizes the ubiquitin tagged proteins and required for the sorting of endocytic ubiquitinated cargos into multivesicular bodies (MVBs). Further, cardiac-specific overexpression of Hsp20 increased the exosomes packed with Hsp20 and protected the cells from hyperglycemia-induced cell death in in vitro and in streptozotocin-induced diabetic mouse model in vivo, altogether suggesting that Hsp20 is instrumental in cardioprotection by increasing the biogenesis of exosomes (Wang et al. 2016). The functions of extracellular Hsp20 are depicted in Fig. 1.
Export of Hsp20
Zhang et al. (2012), for the first time, reported the secretion of Hsp20 from rat cardiomyocytes through exosomes. Hsp20 is released into the extracellular medium in physiological and myocardial infarction conditions, but its secretion is increased in myocardial infarction. It implies that secretion of Hsp20 via exosomes is increased under stress conditions such as ischemia/reperfusion. Interestingly, increased secretion of Hsp20 from cardiomyocytes of cardiac-specific overexpressing Hsp20 mice correlated with increased expression of Rab11a, Rab11b, Rab35, and Tsg101 that are involved in exosome generation/biogenesis. It has also been proven that recombinant Hsp20 is known to interact with Tsg101 but not with Rab11 a/b and Rab35 indicating that Hsp20 increases the generation of exosomes by interacting with Tsg101 (Wang et al. 2016). These studies strongly support that Hsp20 is strongly associated with the biogenesis of exosomes. It is also shown that extracellular Hsp20 promotes angiogenesis by acting as a VEGF-receptor agonist. Furthermore, the same research group has demonstrated that exosomes derived from cardiomyocytes of conditional transgenic-Hsp20 mice are enriched with SOD1, survivin, p-Akt, and Hsp20 and are capable of protecting the mouse cardiac endothelial cells from high glucose-induced stress (Wang et al. 2016). Moreover, exosomes derived from cardiomyocytes of transgenic-Hsp20 mice attenuated the cardiac fibrosis, hypertrophy, and apoptosis when injected into diabetic mice. All these studies indicate that Hsp20 promotes exosome biogenesis and exosomes encased Hsp20 protect the cells from multiple stressful conditions.
Hsp22
Hsp22 is also called as HSPB8 or H11 kinase or E2IG1. Abundant expression of Hsp22 has been noticed in skeletal muscle, heart, retina, brain, and spinal cord. Hsp22 plays an important role in neuronal cell survival by regulating the mitochondrial pathway. However, the extracellular presence of Hsp22 is least studied. Hsp22 is also known to be involved in inflammation of several pathologies. Immunohistochemistry and Western blot analysis in synovial tissues of rheumatoid arthritis patients showed increased expression of Hsp22 (Roelofs et al. 2006). It is also shown that Hsp22 activates dendritic cells upon its engagement with TLR4. Wilhelmus et al. (2009) reported the association of Hsp22 with cerebral amyloid angiopathy, hereditary cerebral hemorrhage, amyloidosis (Dutch type), and senile plaques in Alzheimer’s disease. Hsp22 also induced the secretion of IL-6 in human brain pericytes, astrocytes, and microglia. However, the secretory pathway associated with Hsp22 extracellular levels is yet to be elucidated.
Conclusion and future prospects
Heat shock proteins are chaperones that prevent the aggregation of proteins and safeguard the cells from vicious insults. Previous studies reported the intracellular presence of Hsps. However, recently, this perception has changed owing to the extracellular presence of Hsps. Hsp27, αBC, and Hsp20 are secreted into the extracellular medium by a non-classical pathway. Secreted sHsps play an instrumental role in cell-to-cell communication, signaling, immunity, and inflammation. These proteins may act as part of the larger protein complex, for example by interacting with auto-antibodies in plasma or acting by binding to the cell surface receptors on immune/endothelial cells. sHsps also play a pivotal role in the biogenesis of exosomes. These proteins have also been shown to have therapeutic activity when administered extracellularly in in vitro and in vivo disease models. They attenuated the disease phenotype by chaperone, anti-apoptotic, anti-inflammatory, and anti-platelet activities. Many theories have been proposed to explain the secretion of sHsps into the extracellular medium including endolysosomal and exosomal pathways, direct translocation, and release after cell death. Among these, endolysosomal or exosomal pathways are shown to be predominant pathways utilized for the export of sHsps. Hsp27 shown to be secreted via exosomes and endolysosomes while αBC and Hsp20 via exosomes. The crucial role of sHsps in exosome biogenesis/generation should be studied in greater detail as αBC and Hsp20 are involved in exosome biogenesis and their interacting partners like Tsg 101 in the case of Hsp20. The contributory role of all the available sHsps in exosome biogenesis remains to be unanswered till date. Additionally, switching of secretory pathways from exosomes to endolysosomes and vice versa by sHsps and cell type-specific pathways is yet to be elucidated in greater detail. It is also possible that sHsps are involved in exosome biogenesis by participating in the ESCRT machinery in the generation of exosomes. The ESCRT multiprotein complex contains a set of cytosolic proteins that are incorporated into the endosomes by limiting membrane proteins. Proteins recruited to endosomes are usually tagged with ubiquitin on their cytosolic domains. Various types of ESCRT machinery are used in the biogenesis of exosomes including ESCRT-0, -I, -II, and -III. ESCRT-0 is involved in the trafficking of the ubiquitin-tagged proteins to ESCRT-I that are recognized by Tsg-101. It has been established that Hsp20 mediates biogenesis of exosomes by directly interacting with Tsg101. Hsp20 has a high affinity for Tsg101 and less affinity for Rab11 a/b and Rab35 indicating that Hsp20 might be part of the ESCRT-I complex helping Tsg101 to recognize ubiquitin proteins. These studies also support the idea that Hsp20 is localized in the lumen of exosomes as Tsg101 is used as an internal marker of exosomes. Another hypothesis is that, owing to the anti-aggregation effect of sHsps, they might participate in ESCRT complexes to sort out the proteins into vesicles. sHsps might also decide the fate of MVBs in fusion with lysosomes or plasma membrane as suggested in the case of αBC. The role of sHsps in exosome biogenesis was depicted in Fig. 2.
Fig. 2.
Hypothetical model for export of small heat shock proteins (sHsps) into the extracellular medium. sHsps may be part of the ESCRT-I complex and involved in the biogenesis of exosomes by directly interacting with Tsg101, an important component of the ESCRT-I machinery, and they might also act as a branch point to decide the fusion of MVBs with plasma membrane or lysosome
After all these observations, a lot to be resolved regarding the mechanisms by which sHsps find their way to the extracellular milieu with varied functions: in-depth molecular mechanistic details and interactions involved in secretion, role of sHsp auto-antibodies in blood, elucidating the presence of sHsps on the membrane/luminal portion of extracellular vesicles, determining how they bind to cell surface receptors and communicate with distant cells, determining the half-life of extracellular sHsps, and explicating several activities of extracellularly administered sHsp and how they cross the various barriers to exert their effect. Furthermore, studies should be conducted to exploit the benefits of exosomes packed with sHsps in various disease models. The therapeutic effect of exosomes encased with Hsp27, αBC, and Hsp20 should be studied by injecting them in disease models including neurodegeneration, cancer, diabetes, and ischemia/reperfusion. All these studies are likely to help develop extracellular sHsps as therapeutic molecules and as native proteins/peptides/exosomes encased with sHsps, encapsulation of sHsps with nano- and microparticles or cell penetration peptides.
Acknowledgments
This work is supported by the Science and Engineering Research Board-Early Career Research (SERB-ECR) grant (ECR 2017/000277/LS), Government of India to VSR. MSK is supported by a National Institutes of Health grant (NHLBI 1R01 HL134801) and a USA Department of Defense grant (W81XWH-17-1-0666). JT is supported by Research Initiation Grant (BITS/GAU/RIG/2017/77) and OPERA award (FR/SCM/160317/BIO) from BITS-Pilani, Hyderabad Campus, India. GBR is supported by grants from SERB (SB/S0/HS-192/2013), Department of Biotechnology (BT/PR10658/PFN/20/806/201) and Department of Health Research (DHR/HRD/Fellowship/SUG-04/2015-16), Government of India.
Contributor Information
V. Sudhakar Reddy, Phone: 91-40-27197293, Email: sudhakarnin@gmail.com.
G. Bhanuprakash Reddy, Phone: 91-40-27197252, Email: bhanu@ninindia.org, Email: geereddy@yahoo.com.
References
- Al-Madhoun AS, Chen YX, Haidari L, Rayner K, Gerthoffer W, McBride H, O’Brien ER. The interaction and cellular localization of HSP27 and ERbeta are modulated by 17beta-estradiol and HSP27 phosphorylation. Mol Cell Endocrinol. 2007;270:33–42. doi: 10.1016/j.mce.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Arac A, Brownell SE, Rothbard JB, Chen C, Ko RM, Pereira MP, Albers GW, Steinman L, Steinberg GK. Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proc Natl Acad Sci U S A. 2011;108:13287–13292. doi: 10.1073/pnas.1107368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo AP. Heat shock proteins as molecular chaperones. Med Sci (Paris) 2005;21:619–625. doi: 10.1051/medsci/2005216-7619. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Lin CF, Skinner KA, Schiffhauer LM, Peacock J, Hicks DG, Redmond EM, Morrow D, Huston A, Shayne M, Langstein HN, Miller-Graziano CL, Strickland J, O’Donoghue L, De AK. Heat shock protein 27 differentiates tolerogenic macrophages that may support human breast cancer progression. Cancer Res. 2011;71:318–327. doi: 10.1158/0008-5472.CAN-10-1778. [DOI] [PubMed] [Google Scholar]
- Basha E, O’Neill H, Vierling E. Small heat shock proteins and alpha-crystallins: dynamic proteins with flexible functions. Trends Biochem Sci. 2012;37:106–117. doi: 10.1016/j.tibs.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Batulan Z, Pulakazhi Venu VK, Li Y, Koumbadinga G, Alvarez Olmedo DG, Shi C, O’Brien ER. Extracellular release and signaling by heat shock protein 27: role in modifying vascular inflammation. Front Immunol. 2016;7:285. doi: 10.3389/fimmu.2016.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- Bhat S, Gangulam RK. Secretion of αB-crystallin via exosomes. New clues to the function of human retinal pigment epithelium. Commun Integr Biol. 2011;4:739–741. doi: 10.4161/cib.17610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder RJ, Vatner R, Srivastava P. The heat-shock protein receptors: some answers and more questions. Tissue Antigens. 2004;64(4):442–451. doi: 10.1111/j.1399-0039.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- Burut DF, Borai A, Livingstone C, Ferns G. Serum heat shock protein 27 antigen and antibody levels appear to be related to the macrovascular complications associated with insulin resistance: a pilot study. Cell Stress Chaperones. 2010;15:379–386. doi: 10.1007/s12192-009-0152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK, Mambula SS, Gray PJ, Jr, Theriault JR. Extracellular heat shock proteins in cell signaling. FEBS Lett. 2007;581:3689–3694. doi: 10.1016/j.febslet.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Ce P, Erkizan O, Gedizlioglu M. Elevated HSP27 levels during attacks in patients with multiple sclerosis. Acta Neurol Scand. 2011;124:317–320. doi: 10.1111/j.1600-0404.2010.01475.x. [DOI] [PubMed] [Google Scholar]
- Celet B, Akman-Demir G, Serdaroglu P, Yentur SP, Tasci B, van Noort JM, Eraksoy M, Saruhan-Direskeneli G. Anti-alpha B-crystallin immunoreactivity in inflammatory nervous system diseases. J Neurol. 2000;247:935–939. doi: 10.1007/s004150070049. [DOI] [PubMed] [Google Scholar]
- Christopher KL, Pedler MG, Shieh B, Ammar DA, Petrash JM, Mueller NH. Alpha-crystallin-mediated protection of lens cells against heat and oxidative stress-induced cell death. Biochim Biophys Acta. 2014;1843:309–315. doi: 10.1016/j.bbamcr.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocca DR, Adams DJ, Edwards DP, Bjercke RJ, McGuire WL. Estrogen-induced 24K protein in MCF-7 breast cancer cells is localized in granules. Breast Cancer Res Treat. 1984;4:261–268. doi: 10.1007/BF01806037. [DOI] [PubMed] [Google Scholar]
- Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci. 2005;118:3631–3638. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- De Maio A. Extracellular heat shock proteins, cellular export vesicles, and the stress observation system: a form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones. 2011;16:235–249. doi: 10.1007/s12192-010-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maio A, Vazquez D. Extracellular heat shock proteins: a new location, a new function. Shock. 2013;40:239–246. doi: 10.1097/SHK.0b013e3182a185ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto Y, Adachi S, Matsushima-Nishiwaki R, Niwa M, Tokuda H, Akamatsu S, Doi T, Kato H, Yoshimura S, Ogura S, Iwama T, Kozawa O. alphaB-crystallin extracellularly suppresses ADP-induced granule secretion from human platelets. FEBS Lett. 2009;583:2464–2468. doi: 10.1016/j.febslet.2009.06.036. [DOI] [PubMed] [Google Scholar]
- Fan GC, Chu G, Kranias EG. Hsp20 and its cardioprotection. Trends in cardiovascular medicine. 2005;15:138–141. doi: 10.1016/j.tcm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Fanelli MA, Cuello Carrion FD, Dekker J, Schoemaker J, Ciocca DR. Serological detection of heat shock protein hsp27 in normal and breast cancer patients. Cancer Epidemiol Biomark Prev. 1998;7:791–795. [PubMed] [Google Scholar]
- Feng JT, Liu YK, Song HY, Dai Z, Qin LX, Almofti MR, Fang CY, Lu HJ, Yang PY, Tang ZY. Heat-shock protein 27: a potential biomarker for hepatocellular carcinoma identified by serum proteome analysis. Proteomics. 2005;5:4581–4588. doi: 10.1002/pmic.200401309. [DOI] [PubMed] [Google Scholar]
- Gangalum RK, Bhat AM, Kohan SA, Bhat SP. Inhibition of the expression of the small heat shock protein alphaB-crystallin inhibits exosome secretion in human retinal pigment epithelial cells in culture. J Biol Chem. 2016;291:12930–12942. doi: 10.1074/jbc.M115.698530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido C, Paul C, Seigneuric R, Kampinga HH. The small heat shock proteins family: the long forgotten chaperones. Int J Biochem Cell Biol. 2012;44:1588–1592. doi: 10.1016/j.biocel.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65:5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruden G, Bruno G, Chaturvedi N, Burt D, Schalkwijk C, Pinach S, Stehouwer CD, Witte DR, Fuller JH, Perin PC. Serum heat shock protein 27 and diabetes complications in the EURODIAB prospective complications study: a novel circulating marker for diabetic neuropathy. Diabetes. 2008;57:1966–1970. doi: 10.2337/db08-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusev NB, Bogatcheva NV, Marston SB. Structure and properties of small heat shock proteins (sHsp) and their interaction with cytoskeleton proteins. Biochemistry (Mosc) 2002;67:511–519. doi: 10.1023/a:1015549725819. [DOI] [PubMed] [Google Scholar]
- Hacker S, Lambers C, Hoetzenecker K, Pollreisz A, Aigner C, Lichtenauer M, Mangold A, Niederpold T, Zimmermann M, Taghavi S, Klepetko W, Ankersmit HJ. Elevated HSP27, HSP70 and HSP90 alpha in chronic obstructive pulmonary disease: markers for immune activation and tissue destruction. Clin Lab. 2009;55:31–40. [PubMed] [Google Scholar]
- Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- Havasi A, Li Z, Wang Z, Martin JL, Botla V, Ruchalski K, Schwartz JH, Borkan SC. Hsp27 inhibits Bax activation and apoptosis via a phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem. 2008;283:12305–12313. doi: 10.1074/jbc.M801291200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker JG, McGarvey M. Heat shock proteins as biomarkers for the rapid detection of brain and spinal cord ischemia: a review and comparison to other methods of detection in thoracic aneurysm repair. Cell Stress Chaperones. 2011;16:119–131. doi: 10.1007/s12192-010-0224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegen H, Wanschitz J, Ehling R, Deisenhammer F, Loscher WN, Reindl M, Berger T. Anti-alpha B-crystallin immunoreactivity in Guillain-Barre syndrome and chronic inflammatory demyelinating polyneuropathy. J Peripher Nerv Syst: JPNS. 2010;15:150–152. doi: 10.1111/j.1529-8027.2010.00264.x. [DOI] [PubMed] [Google Scholar]
- Hightower LE, Guidon PT., Jr Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–266. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- Holcakova J, Hernychova L, Bouchal P, Brozkova K, Zaloudik J, Valik D, Nenutil R, Vojtesek B. Identification of alphaB-crystallin, a biomarker of renal cell carcinoma by SELDI-TOF MS. Int J Biol Markers. 2008;23:48–53. doi: 10.1177/172460080802300108. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J, Huang QL, Ding L, Bova MP. Lens alpha-crystallin: chaperone-like properties. Methods Enzymol. 1998;290:365–383. doi: 10.1016/s0076-6879(98)90032-5. [DOI] [PubMed] [Google Scholar]
- Huang Q, Ye J, Chen W, Wang L, Lin W, Lin J, Lin X. Heat shock protein 27 is over-expressed in tumor tissues and increased in sera of patients with gastric adenocarcinoma. Clin Chem Lab Med. 2010;48:263–269. doi: 10.1515/CCLM.2010.043. [DOI] [PubMed] [Google Scholar]
- Huot J, Houle F, Spitz DR, Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56:273–279. [PubMed] [Google Scholar]
- Jakhotia S, Sivaprasad M, Shalini T, Reddy PY, Viswanath K, Jakhotia K, Sahay R, Sahay M, Reddy GB (2017) Circulating levels of Hsp27 in microvascular complications of diabetes: prospects as a biomarker of diabetic nephropathy. J Diabetes Complications. 10.1016/j.jdiacomp.2017.10.004 [DOI] [PubMed]
- Janik S, Schiefer AI, Bekos C, Hacker P, Haider T, Moser J, Klepetko W, Mullauer L, Ankersmit HJ, Moser B. HSP27 and 70 expression in thymic epithelial tumors and benign thymic alterations: diagnostic, prognostic and physiologic implications. Sci Rep. 2016;6:24267. doi: 10.1038/srep24267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Chen Y, Wang Q, Jayasinghe U, Luo X, Wei Q, Wang J, Xiong H, Chen C, Xu B, Hu W, Wang L, Zhao W, Zhou J. Exosome: emerging biomarker in breast cancer. Oncotarget. 2017;8:41717–41733. doi: 10.18632/oncotarget.16684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Cleveland JC, Ao L, Li J, Zeng Q, Fullerton DA, Meng X. Human myocardium releases heat shock protein 27 (HSP27) after global ischemia: the proinflammatory effect of extracellular HSP27 through toll-like receptor (TLR)-2 and TLR4. Mol Med. 2014;20:280–289. doi: 10.2119/molmed.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno Y, Matsuno H. The possibility of novel antiplatelet peptides: the physiological effects of low molecular weight HSPs on platelets. Curr Pharm Des. 2006;12:887–892. doi: 10.2174/138161206776056047. [DOI] [PubMed] [Google Scholar]
- Klopstein A, Santos-Nogueira E, Francos-Quijorna I, Redensek A, David S, Navarro X, Lopez-Vales R. Beneficial effects of alphaB-crystallin in spinal cord contusion injury. J Neurosci: Off J Soc Neurosci. 2012;32:14478–14488. doi: 10.1523/JNEUROSCI.0923-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kore RA, Abraham EC. Phosphorylation negatively regulates exosome mediated secretion of cryAB in glioma cells. Biochim Biophys Acta. 2016;1863:368–377. doi: 10.1016/j.bbamcr.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozawa O, Matsuno H, Niwa M, Hatakeyama D, Kato K, Uematsu T. AlphaB-crystallin, a low-molecular-weight heat shock protein, acts as a regulator of platelet function. Cell Stress Chaperones. 2001;6:21–28. doi: 10.1379/1466-1268(2001)006<0021:bcalmw>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozawa O, Matsuno H, Niwa M, Hatakeyama D, Oiso Y, Kato K, Uematsu T. HSP20, low-molecular-weight heat shock-related protein, acts extracellularly as a regulator of platelet functions: a novel defense mechanism. Life Sci. 2002;72:113–124. doi: 10.1016/s0024-3205(02)02144-6. [DOI] [PubMed] [Google Scholar]
- Kurnellas MP, Brownell SE, Su L, Malkovskiy AV, Rajadas J, Dolganov G, Chopra S, Schoolnik GK, Sobel RA, Webster J, Ousman SS, Becker RA, Steinman L, Rothbard JB. Chaperone activity of small heat shock proteins underlies therapeutic efficacy in experimental autoimmune encephalomyelitis. J Biol Chem. 2012;287:36423–36434. doi: 10.1074/jbc.M112.371229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Lee HJ, Choi SH, Jin YB, An HJ, Kang JH, Yoon SS, Lee YS. Soluble HSPB1 regulates VEGF-mediated angiogenesis through their direct interaction. Angiogenesis. 2012;15:229–242. doi: 10.1007/s10456-012-9255-3. [DOI] [PubMed] [Google Scholar]
- Lehr S, Hartwig S, Lamers D, Famulla S, Muller S, Hanisch FG, Cuvelier C, Ruige J, Eckardt K, Ouwens DM, Sell H, Eckel J. Identification and validation of novel adipokines released from primary human adipocytes. Mol Cell Proteomics: MCP. 2012;11:M111 010504. doi: 10.1074/mcp.M111.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao WC, Wu MS, Wang HP, Tien YW, Lin JT. Serum heat shock protein 27 is increased in chronic pancreatitis and pancreatic carcinoma. Pancreas. 2009;38:422–426. doi: 10.1097/MPA.0b013e318198281d. [DOI] [PubMed] [Google Scholar]
- Luo X, Zuo X, Zhang B, Song L, Wei X, Zhou Y, Xiao X. Release of heat shock protein 70 and the effects of extracellular heat shock protein 70 on the production of IL-10 in fibroblast-like synoviocytes. Cell Stress Chaperones. 2008;13:365–373. doi: 10.1007/s12192-008-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849–7857. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- Mambula SS, Stevenson MA, Ogawa K, Calderwood SK. Mechanisms for Hsp70 secretion: crossing membranes without a leader. Methods. 2007;43:168–175. doi: 10.1016/j.ymeth.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannini B, Cascella R, Zampagni M, van Waarde-Verhagen M, Meehan S, Roodveldt C, Campioni S, Boninsegna M, Penco A, Relini A, Kampinga HH, Dobson CM, Wilson MR, Cecchi C, Chiti F. Molecular mechanisms used by chaperones to reduce the toxicity of aberrant protein oligomers. Proc Natl Acad Sci U S A. 2012;109:12479–12484. doi: 10.1073/pnas.1117799109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Ventura JL, Duran MC, Blanco-Colio LM, Meilhac O, Leclercq A, Michel JB, Jensen ON, Hernandez-Merida S, Tunon J, Vivanco F, Egido J. Identification by a differential proteomic approach of heat shock protein 27 as a potential marker of atherosclerosis. Circulation. 2004;110:2216–2219. doi: 10.1161/01.CIR.0000136814.87170.B1. [DOI] [PubMed] [Google Scholar]
- Masilamoni JG, Vignesh S, Kirubagaran R, Jesudason EP, Jayakumar R. The neuroprotective efficacy of alpha-crystallin against acute inflammation in mice. Brain Res Bull. 2005;67:235–241. doi: 10.1016/j.brainresbull.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Masilamoni JG, Jesudason EP, Baben B, Jebaraj CE, Dhandayuthapani S, Jayakumar R. Molecular chaperone alpha-crystallin prevents detrimental effects of neuroinflammation. Biochim Biophys Acta. 2006;1762:284–293. doi: 10.1016/j.bbadis.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Matsuno H, Kozawa O, Niwa M, Usui A, Ito H, Uematsu T, Kato K. A heat shock-related protein, p20, plays an inhibitory role in platelet activation. FEBS Lett. 1998;429:327–329. doi: 10.1016/s0014-5793(98)00626-7. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Hickey E, Weber LA, Arrigo AP. Large unphosphorylated aggregates as the active form of hsp27 which controls intracellular reactive oxygen species and glutathione levels and generates a protection against TNFalpha in NIH-3T3-ras cells. Biochem Biophys Res Commun. 1997;241:187–192. doi: 10.1006/bbrc.1997.7635. [DOI] [PubMed] [Google Scholar]
- Merendino AM, Bucchieri F, Campanella C, Marciano V, Ribbene A, David S, Zummo G, Burgio G, Corona DF, Conway de Macario E, Macario AJ, Cappello F. Hsp60 is actively secreted by human tumor cells. PLoS One. 2010;5:e9247. doi: 10.1371/journal.pone.0009247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H, Poon S, Hibbert B, Rayner K, Chen YX, O'Brien ER. Modulation of estrogen signaling by the novel interaction of heat shock protein 27, a biomarker for atherosclerosis, and estrogen receptor beta: mechanistic insight into the vascular effects of estrogens. Arterioscler Thromb Vasc Biol. 2005;25:e10–e14. doi: 10.1161/01.ATV.0000156536.89752.8e. [DOI] [PubMed] [Google Scholar]
- Miron T, Vancompernolle K, Vandekerckhove J, Wilchek M, Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991;114:255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafar F, Williams JB, Mearow KM. Astrocytes release HspB1 in response to amyloid-beta exposure in vitro. J Alzheimer’s Dis: JAD. 2016;49:251–263. doi: 10.3233/JAD-150317. [DOI] [PubMed] [Google Scholar]
- Nahomi RB, Wang B, Raghavan CT, Voss O, Doseff AI, Santhoshkumar P, Nagaraj RH. Chaperone peptides of alpha-crystallin inhibit epithelial cell apoptosis, protein insolubilization, and opacification in experimental cataracts. J Biol Chem. 2013;288:13022–13035. doi: 10.1074/jbc.M112.440214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahomi RB, DiMauro MA, Wang B, Nagaraj RH. Identification of peptides in human Hsp20 and Hsp27 that possess molecular chaperone and anti-apoptotic activities. Biochem J. 2015;465:115–125. doi: 10.1042/BJ20140837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Kozawa O, Matsuno H, Kato K, Uematsu T. Small molecular weight heat shock-related protein, HSP20, exhibits an anti-platelet activity by inhibiting receptor-mediated calcium influx. Life Sci. 2000;66:PL7–P12. doi: 10.1016/s0024-3205(99)00566-4. [DOI] [PubMed] [Google Scholar]
- van Noort JM, Verbeek R, Meilof JF, Polman CH, Amor S. Autoantibodies against alpha B-crystallin, a candidate autoantigen in multiple sclerosis, are part of a normal human immune repertoire. Mult Scler. 2006;12:287–293. doi: 10.1191/135248506ms1271oa. [DOI] [PubMed] [Google Scholar]
- van Noort JM, Bsibsi M, Nacken PJ, Gerritsen WH, Amor S, Holtman IR, Boddeke E, van Ark I, Leusink-Muis T, Folkerts G, Hennink WE, Amidi M. Activation of an immune-regulatory macrophage response and inhibition of lung inflammation in a mouse model of COPD using heat-shock protein alpha B-crystallin-loaded PLGA microparticles. Biomaterials. 2013;34:831–840. doi: 10.1016/j.biomaterials.2012.10.028. [DOI] [PubMed] [Google Scholar]
- Park HK, Park EC, Bae SW, Park MY, Kim SW, Yoo HS, Tudev M, Ko YH, Choi YH, Kim S, Kim DI, Kim YW, Lee BB, Yoon JB, Park JE. Expression of heat shock protein 27 in human atherosclerotic plaques and increased plasma level of heat shock protein 27 in patients with acute coronary syndrome. Circulation. 2006;114:886–893. doi: 10.1161/CIRCULATIONAHA.105.541219. [DOI] [PubMed] [Google Scholar]
- Prudovsky I, Mandinova A, Soldi R, Bagala C, Graziani I, Landriscina M, Tarantini F, Duarte M, Bellum S, Doherty H, Maciag T. The non-classical export routes: FGF1 and IL-1alpha point the way. J Cell Sci. 2003;116:4871–4881. doi: 10.1242/jcs.00872. [DOI] [PubMed] [Google Scholar]
- Rayner K, Chen YX, McNulty M, Simard T, Zhao X, Wells DJ, de Belleroche J, O’Brien ER. Extracellular release of the atheroprotective heat shock protein 27 is mediated by estrogen and competitively inhibits acLDL binding to scavenger receptor-A. Circ Res. 2008;103:133–141. doi: 10.1161/CIRCRESAHA.108.172155. [DOI] [PubMed] [Google Scholar]
- Rayner K, Sun J, Chen YX, McNulty M, Simard T, Zhao X, Wells DJ, de Belleroche J, O’Brien ER. Heat shock protein 27 protects against atherogenesis via an estrogen-dependent mechanism: role of selective estrogen receptor beta modulation. Arterioscler Thromb Vasc Biol. 2009;29:1751–1756. doi: 10.1161/ATVBAHA.109.193656. [DOI] [PubMed] [Google Scholar]
- Reddy VS, Reddy GB. Emerging role for alphaB-crystallin as a therapeutic agent: pros and cons. Curr Mol Med. 2015;15:47–61. doi: 10.2174/1566524015666150114112853. [DOI] [PubMed] [Google Scholar]
- Reddy VS, Reddy GB. Role of crystallins in diabetic complications. Biochim Biophys Acta. 2016;1860:269–277. doi: 10.1016/j.bbagen.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Reddy GB, Kumar PA, Kumar MS. Chaperone-like activity and hydrophobicity of alpha-crystallin. IUBMB Life. 2006;58:632–641. doi: 10.1080/15216540601010096. [DOI] [PubMed] [Google Scholar]
- Reddy VS, Raghu G, Reddy SS, Pasupulati AK, Suryanarayana P, Reddy GB. Response of small heat shock proteins in diabetic rat retina. Invest Ophthalmol Vis Sci. 2013;54:7674–7682. doi: 10.1167/iovs.13-12715. [DOI] [PubMed] [Google Scholar]
- Reddy VS, Kumar CU, Raghu G, Reddy GB. Expression and induction of small heat shock proteins in rat heart under chronic hyperglycemic conditions. Arch Biochem Biophys. 2014;558C:1–9. doi: 10.1016/j.abb.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Reddy VS, Jakhotia S, Reddy PY, Reddy GB. Hyperglycemia induced expression, phosphorylation, and translocation of alphaB-crystallin in rat skeletal muscle. IUBMB Life. 2015;67:291–299. doi: 10.1002/iub.1370. [DOI] [PubMed] [Google Scholar]
- Roelofs MF, Boelens WC, Joosten LA, Abdollahi-Roodsaz S, Geurts J, Wunderink LU, Schreurs BW, van den Berg WB, Radstake TR. Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J Immunol. 2006;176:7021–7027. doi: 10.4049/jimmunol.176.11.7021. [DOI] [PubMed] [Google Scholar]
- Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo AP, Buchner J, Gaestel M. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 1999;274:18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- Rothbard JB, Zhao X, Sharpe O, Strohman MJ, Kurnellas M, Mellins ED, Robinson WH, Steinman L. Chaperone activity of alpha B-crystallin is responsible for its incorrect assignment as an autoantigen in multiple sclerosis. J Immunol. 2011;186:4263–4268. doi: 10.4049/jimmunol.1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard JB, Kurnellas MP, Brownell S, Adams CM, Su L, Axtell RC, Chen R, Fathman CG, Robinson WH, Steinman L. Therapeutic effects of systemic administration of chaperone alphaB-crystallin associated with binding proinflammatory plasma proteins. J Biol Chem. 2012;287:9708–9721. doi: 10.1074/jbc.M111.337691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salari S, Seibert T, Chen YX, Hu T, Shi C, Zhao X, Cuerrier CM, Raizman JE, O'Brien ER. Extracellular HSP27 acts as a signaling molecule to activate NF-kappaB in macrophages. Cell Stress Chaperones. 2013;18:53–63. doi: 10.1007/s12192-012-0356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinthone S, Tyagi M, Gerthoffer WT. Small heat shock proteins in smooth muscle. Pharmacol Ther. 2008;119:44–54. doi: 10.1016/j.pharmthera.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos TG, Martins VR, Hajj GNM (2017) Unconventional secretion of heat shock proteins in cancer. Int J Mol Sci 18(5):946 [DOI] [PMC free article] [PubMed]
- Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol. 2007;81(1):15–27. doi: 10.1189/jlb.0306167. [DOI] [PubMed] [Google Scholar]
- Sreekumar PG, Kannan R, Kitamura M, Spee C, Barron E, Ryan SJ, Hinton DR. AlphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS One. 2010;5:e12578. doi: 10.1371/journal.pone.0012578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar PG, Chothe P, Sharma KK, Baid R, Kompella U, Spee C, Kannan N, Manh C, Ryan SJ, Ganapathy V, Kannan R, Hinton DR. Antiapoptotic properties of alpha-crystallin-derived peptide chaperones and characterization of their uptake transporters in human RPE cells. Invest Ophthalmol Vis Sci. 2013;54:2787–2798. doi: 10.1167/iovs.12-11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stope MB, Klinkmann G, Diesing K, Koensgen D, Burchardt M, Mustea A. Heat shock protein HSP27 secretion by ovarian cancer cells is linked to intracellular expression levels, occurs independently of the endoplasmic reticulum pathway and HSP27’s phosphorylation status, and is mediated by exosome liberation. Dis Markers. 2017;2017:1575374. doi: 10.1155/2017/1575374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, MacRae TH. Small heat shock proteins: molecular structure and chaperone function. Cell Mol Life Sci. 2005;62:2460–2476. doi: 10.1007/s00018-005-5190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, MacRae TH. The small heat shock proteins and their role in human disease. FEBS J. 2005;272:2613–2627. doi: 10.1111/j.1742-4658.2005.04708.x. [DOI] [PubMed] [Google Scholar]
- Thuringer D, Jego G, Wettstein G, Terrier O, Cronier L, Yousfi N, Hebrard S, Bouchot A, Hazoume A, Joly AL, Gleave M, Rosa-Calatrava M, Solary E, Garrido C. Extracellular HSP27 mediates angiogenesis through Toll-like receptor 3. FASEB J. 2013;27:4169–4183. doi: 10.1096/fj.12-226977. [DOI] [PubMed] [Google Scholar]
- Thuringer D, Berthenet K, Cronier L, Solary E, Garrido C. Primary tumor- and metastasis-derived colon cancer cells differently modulate connexin expression and function in human capillary endothelial cells. Oncotarget. 2015;6:28800–28815. doi: 10.18632/oncotarget.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H, Kuroyanagi G, Tsujimoto M, Enomoto Y, Matsushima-Nishiwaki R, Onuma T, Kojima A, Doi T, Tanabe K, Akamatsu S, Iida H, Ogura S, Otsuka T, Iwama T, Tanikawa T, Ishikawa K, Kojima K, Kozawa O. Release of phosphorylated HSP27 (HSPB1) from platelets is accompanied with the acceleration of aggregation in diabetic patients. PLoS One. 2015;10:e0128977. doi: 10.1371/journal.pone.0128977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytell M, Greenberg SG, Lasek RJ. Heat shock-like protein is transferred from glia to axon. Brain Res. 1986;363:161–164. doi: 10.1016/0006-8993(86)90671-2. [DOI] [PubMed] [Google Scholar]
- Vega VL, Rodriguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De Maio A. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol. 2008;180:4299–4307. doi: 10.4049/jimmunol.180.6.4299. [DOI] [PubMed] [Google Scholar]
- Vos MJ, Hageman J, Carra S, Kampinga HH. Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry. 2008;47:7001–7011. doi: 10.1021/bi800639z. [DOI] [PubMed] [Google Scholar]
- Wang X, Gu H, Huang W, Peng J, Li Y, Yang L, Qin D, Essandoh K, Wang Y, Peng T, Fan GC. Hsp20-mediated activation of exosome biogenesis in cardiomyocytes improves cardiac function and angiogenesis in diabetic mice. Diabetes. 2016;65:3111–3128. doi: 10.2337/db15-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmus MM, Boelens WC, Kox M, Maat-Schieman ML, Veerhuis R, de Waal RM, Verbeek MM. Small heat shock proteins associated with cerebral amyloid angiopathy of hereditary cerebral hemorrhage with amyloidosis (Dutch type) induce interleukin-6 secretion. Neurobiol Aging. 2009;30:229–240. doi: 10.1016/j.neurobiolaging.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Wu N, Yu J, Chen S, Xu J, Ying X, Ye M, Li Y, Wang Y. Alpha-crystallin protects RGC survival and inhibits microglial activation after optic nerve crush. Life Sci. 2014;94:17–23. doi: 10.1016/j.lfs.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Yusuf N, Nasti TH, Huang CM, Huber BS, Jaleel T, Lin HY, Xu H, Elmets CA. Heat shock proteins HSP27 and HSP70 are present in the skin and are important mediators of allergic contact hypersensitivity. J Immunol. 2009;182:675–683. doi: 10.4049/jimmunol.182.1.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang X, Zhu H, Kranias EG, Tang Y, Peng T, Chang J, Fan GC. Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS One. 2012;7:e32765. doi: 10.1371/journal.pone.0032765. [DOI] [PMC free article] [PubMed] [Google Scholar]