Abstract
Invertebrates represent the most plentiful component of marine biodiversity. To date, only few species have been documented for marine litter intake. Here, we report for the first time the presence of macroplastic debris in a jellyfish species. Such novel target to plastic pollution highlights an under studied vector of marine litter along marine trophic web, raising further concern over the impact on marine wildlife.
Introduction
The number of field studies showing the impacts of anthropogenic litter on marine organisms is increasing. To date, plastic ingestion has been documented for 233 marine vertebrates1. However, despite invertebrates representing the primary component of marine biodiversity, litter ingestion has been reported in only a few species2–4. Plastic ingestion is associated with both physical damages such as gut blockage, reduced energy reserves and starvation1, and potential toxicity due to persistent, bioaccumulative and toxic (PBT) substances adsorbed onto the plastic surface5–7 or those leached from the polymer matrix, such as phthalates and flame retardants, known also as endocrine disruptors8–11. Thus, plastic debris provides a pathway for these chemicals to enter marine ecosystems through plastic ingestion3,10,12.
Here we present the first evidence of marine litter internalised by the mauve stinger Pelagia noctiluca (Forsskål, 1775), the most abundant jellyfish species in the Mediterranean Sea13.
As with the distribution of plastic debris, the dispersal of this species mainly depends on the combined effect of local winds and currents14–16, concentrating this organism in regions with a high concentration of floating litter. P. noctiluca is considered an opportunistic predator, feeding on zooplankton of a broad size and taxonomic range, as a result of an adaptive strategy in the open ocean where potential prey are highly diverse17. The mauve stinger also represents a key food source for pelagic top predators. The jellyvorous guild in the Mediterranean includes two specialists (ocean sunfish and loggerhead sea turtle) and several opportunist feeders (Bluefin tuna, little tunny, spearfish, swordfish and blue butterfish), most of which are of commercial interest18,19.
Furthermore, medusae are known to supply an important contribution of carbon into pelagic and deep seafloor communities after blooms senescence20.
Hence, any impact of marine litter on this species might have consequences on its predators as well as on the marine primary productivity at an ecosystem scale.
Results
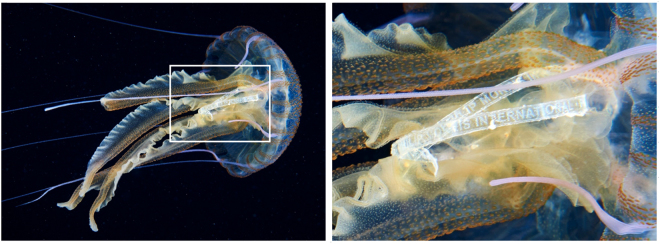
During the Mediterranean campaign of the “AQUATILIS EXPEDITION”21 on Ponza Island (Tyrrhenian Sea), specimens of P. noctiluca were found along with floating anthropogenic litter of different size, colour, shape and type. Some of these materials were found trapped among the oral lobes of the jellyfish (Fig. 1) or retained inside their hood (Fig. S1). To the best of our knowledge, no similar observations have been reported so far for jellyfish, although their blooms have been observed in regions of plastic accumulation22.
Figure 1.
Interaction between plastic debris and jellyfish. High resolution images showing a swimming mauve stinger P. noctiluca observed in the field (left) with a plastic lace of a famous cigarette brand among the oral lobes (white square, enlarged on the right).
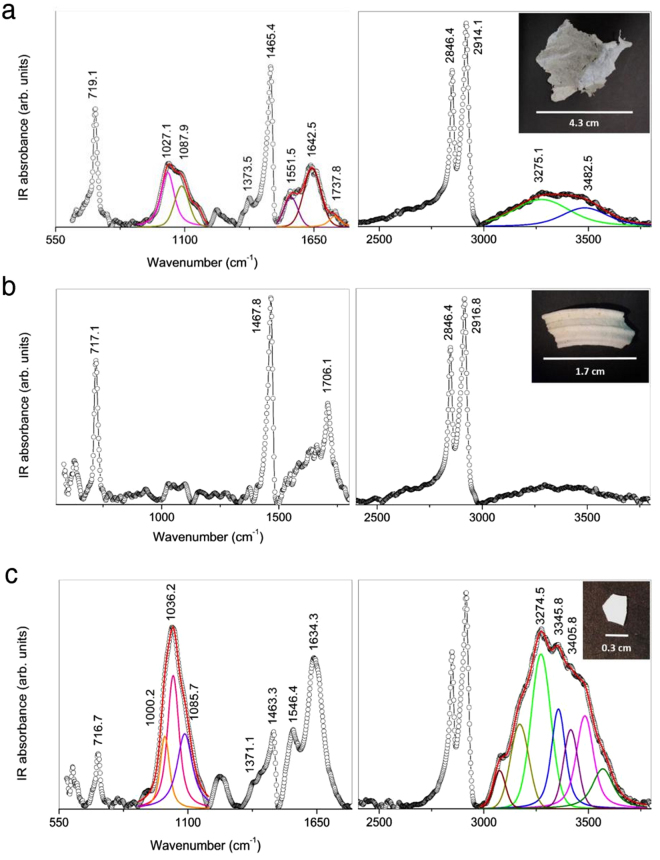
Although only a small sample size was investigated, field observations showed a strong interaction between P. noctiluca and drifting litter. In order to determine the effective ingestion of anthropogenic litter, a total of 20 specimens of P. noctiluca were collected using hand nets. Four individuals presented plastic debris inside the gastrovascular cavity. The characterization of such litter through ATR-FTIR and Raman spectroscopy revealed two macro-sized plastics (>1 cm) constituted by high-density polyethylene (HD-PE) (Figs 2a, S2A), a flame retardant polyethylene (PE) (Figs 2b, S2B) and a zinc-rich paint fragment (Fig. 2c) (see also Supporting Information).
Figure 2.
Characterization of marine litter internalised by jellyfish. ATR-FTIR spectra of the marine debris (shown in the square on the right) extracted from specimens of the mauve stinger P. noctiluca from Tyrrhenian Sea, classified as HD-PE sheet (a), PE fragment (b) and metal-based paint fragment (c).
Discussion
We hypothesize that the presence of marine litter in the gastrovascular cavity of P. noctiluca specimens (Fig. 2) was due to active ingestion of such fragments wrongly recognised as food. Marine organisms have been reported to ingest plastics as a result of visual23–26 or tactile27 misidentification, or attracted by flavouring organic compounds on the plastic surface23,28.
In cnidarians, the discharge mechanism of cnidocytes is evolutionarily conserved among different classes29. Prey capture and feeding consist in a sequence of chemically-mediated behaviours: (a) discharge of cnidocytes by compounds usually associated with cell membranes, mucin and chitin of the prey, such as N-acetylated sugars; (b) retraction of tentacles triggered by endogenous compounds to move captured prey to the mouth; (c) ingestion of the prey, promoted by a reversed ciliary beating on the mouth and pharynx30,31.
Due to its specific chemical and physical properties, drifting weathered litter may be mistaken by nematocysts as food. PE (considering both low- and high-density) is one of the main polymers produced worldwide32 and accounts for the majority (52%) of the marine litter found in Mediterranean surface waters33. Given its specific gravity, (ranging from 0.91 to 0.9434), it usually floats near the sea surface, closely behaving as neuston organisms, thus potentially inducing a meccano- and chemoreceptors reaction of cnidarians, which mistake it as prey. Laboratory experiments conducted on hard corals35 showed nematocyst discharge and ingestion of different microplastics (including PE), suggesting that the presence of phagostimulants potentially related to toxic compounds found in the polymer matrix are promoting chemoreception. Other marine pollutants also induce feeding behaviour in Cnidaria36,37. For instance, heavy metals, which can be found adsorbed to plastic debris in the marine environment6,7, have been shown to affect the chemoreceptors in P. noctiluca38.
The mauve stinger can consume prey equivalent to >50% of its body weight (wet weight) per day17, with the ability to ingest gelatinous prey of considerable size compared to its bell dimension39. This aspect further supports our hypothesis of an active ingestion of large anthropogenic fragments in P. noctiluca.
Studies on corals showed a retention rate of 5.7% of microplastics in mesenterial tissue within the gut cavity after 24 h of recovery35,40]. This is surprising, since anthozoans can digest food rapidly retaining non-food particles for an average of 50 minutes41–43. The residence time of plastics could be related to the polymer, size and shape, and further studies are needed to elucidate the fate of the ingested plastics in Cnidaria.
The biomass of gelatinous zooplankton in the epipelagic region of the Mediterranean usually ranges between 1–10 kg 100 m−3, with the biomass of P. noctiluca reaching values up to 24 kg 100 m−3 44, making it a valuable food source for several pelagic organisms18,19,45. Studies using stable isotopes suggest that gelatinous zooplankton, such as the mauve stinger represent between 30 and 60% of the diet of the Bluefin tuna19. Therefore, an important amount of marine litter may be transferred through jellyfish to pelagic predators19 suggest that fish, such as tuna, are unable to consume the required biomass of jellyplankton in a single meal, thus continuous consumption of gelatinous plankton and associated plastic debris could be possible.
Our findings highlight the vulnerability of medusae to plastic pollution found suspended in the water column and transported through currents. The ability of jellyfish, and specifically P. noctiluca, to internalise low-density macroplastic and other anthropogenic debris leads to a reinterpretation of the impact of marine litter on their common predators, previously thought only to mistake floating plastics as prey.
Here, we hypothesise that jellyfish could act as vector of plastics along marine trophic webs, raising further concern on the impact of plastics on marine organisms. Further studies are urgently needed to understand any potential effect for this species and the real extent of trophic transfer of plastic debris.
Methods
This study was carried on within the “AQUATILIS EXPEDITION”21 on the Mediterranean Sea around Ponza Island (Tyrrhenian Sea, Italy) in September 2016.
Due to its bathymetric profile, the site is particularly exposed to prominent surface and upwelling streams. This complex hydrodynamism likely concentrates planktonic species and floating litter in the same geographic area.
Mauve stinger specimens observed during the night diving campaign clearly contained anthropogenic material trapped among oral lobes and retained in the gastrovascular cavity (Figs 1, S1). Of the specimens collected on board (N = 20; average bell size = 4.7 cm), three large plastic debris were extracted from the gastrovascular cavity and one piece of polystyrene foam colonized by barnacles was detached from the oral lobes. All fragments were further analysed through Attenuated total reflection Fourier-transform infrared (ATR-FTIR) and UV Resonant Raman spectroscopies. ATR-FTIR spectra were collected at room temperature by a Bomem DA8 FTIR spectrometer using a thermoelectrically cooled deuterated triglycene sulphate (DTGS) detector in combination with a KBr beam splitter and a Globar source. Fragments without any a priori treatment were placed in a Golden Gate ATR system based on ATR technique. Spectra were recorded in the 500 ÷ 4000 cm−1 region. Each spectrum was collected in a dry atmosphere in order to avoid unnecessary contributions from air humidity, with a resolution of 4 cm−1 and an average of 100 repetitive scans to ensure high reproducibility and good signal-to-noise ratio. All spectra were normalized for taking into account the effective number of absorbers, no mathematical corrections were applied (e.g. smoothing), and all spectroscopic manipulations such as subtraction of a baseline were performed using Spectra Calc GRAMS software (Galactic Industries, Salem, NH, USA). When necessary, the experimental spectrum bandwidth was performed by curve fitting into Voigt profiles, using the routine provided by the PeakFit 4.0 software package. The statistical parameters defined in the software manual were used as a guide for the “best-fit” and were released during the iterative procedure until convergence was achieved. “Best-fit” has been defined as r2 ∼ 0.99999 for all investigated samples. For the recognition of polymer, the KnowItAll IR Spectral Library (Bio-Rad Laboratories Inc., USA) was used, resulting in a score of 93.2% (Fig. 2a), 94.10% (Fig. 2b) and 87.56% (Fig. 2c).
UV Resonant Raman measurements were carried out at the IUVS beamline at Elettra synchrotron Radiation facility (Trieste, Italy). An excitation source of 266 nm with beam-power of 1 mW was employed. The scattered light was collected by using a backscattering geometry configuration. A single Czery-turner spectrometer (focal distance of 750 mm, equipped with an 1800 g/mm holographic grating) coupled with a Peltier-cooled back-thinned CCD was used to acquire the final Raman spectra,with a spectral resolution of 5 cm−1.
Data availability
No datasets were generated or analysed during the current study.
Electronic supplementary material
Acknowledgements
The authors gratefully acknowledge Kirstie Jones-Williams (British Antarctic Survey, Cambridge, UK) for the revision of the manuscript.
Author Contributions
A.M. and E.B. wrote the manuscript, with the contribution of I.C. A.S. and A.M. provided pictures and samples from the field. V.V. and C.V. performed the analysis using ATR-FTIR spectroscopy. D.F. and R.B. performed the analysis using UV Raman spectroscopy. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
A. Macali and E. Bergami contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-24427-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gall SC, Thompson RC. The impact of debris on marine life. Mar. Pollut. Bull. 2015;92:170–179. doi: 10.1016/j.marpolbul.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 2.Law KL. Plastics in the Marine Environment. Ann. Rev. Mar. Sci. 2017;9:205–229. doi: 10.1146/annurev-marine-010816-060409. [DOI] [PubMed] [Google Scholar]
- 3.GESAMP. Sources, fate and effects of microplastics in the marine environment: part two of a global assessment Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection. Rep. Stud. No. 93 (2016).
- 4.Sun X, et al. Ingestion of microplastics by natural zooplankton groups in the northern South China Sea. Mar. Pollut. Bull. 2017;115(1):217–224. doi: 10.1016/j.marpolbul.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Van A, et al. Persistent organic pollutants in plastic marine debris found on beaches in San Diego, California. Chemosphere. 2012;86:258–263. doi: 10.1016/j.chemosphere.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 6.Rochman CM, Hentschel BT, The SJ. Long-Term Sorption of Metals Is Similar among Plastic Types: Implications for Plastic Debris in Aquatic Environments. PLoS ONE. 2014;9(1):e85433. doi: 10.1371/journal.pone.0085433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner A. Heavy metals, metalloids and other hazardous elements in marine plastic litter. Mar. Pollut. Bull. 2016;111:136–142. doi: 10.1016/j.marpolbul.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Lithner D, Larsson Å, Dave G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total. Environ. 2011;409:3309–3324. doi: 10.1016/j.scitotenv.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 9.Bejgarn S, MacLeod M, Bogdal C, Breitholtz M. Toxicity of leachate from weathering plastics: An exploratory screening study with Nitocra spinipes. Chemosphere. 2015;132:114–119. doi: 10.1016/j.chemosphere.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka K, et al. Facilitated Leaching of Additive-Derived PBDEs from Plastic by Seabirds’ Stomach Oil and Accumulation in Tissues. Environ. Sci. Technol. 2015;49:11799–11807. doi: 10.1021/acs.est.5b01376. [DOI] [PubMed] [Google Scholar]
- 11.Jang M, et al. Widespread detection of a brominated flame retardant, hexabromocyclododecane, in expanded polystyrene marine debris and microplastics from South Korea and the Asia-Pacific coastal region. Environ. Pollut. 2017;231:785–794. doi: 10.1016/j.envpol.2017.08.066. [DOI] [PubMed] [Google Scholar]
- 12.Setälä O, Fleming-Lehtinen V, Lehtiniemi M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014;185:77–83. doi: 10.1016/j.envpol.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Canepa A. et al. Jellyfish Blooms. Springer Netherlands (2014).
- 14.Yahia MD, Goy J, Yahia-Kéfi OD. Distribution et écologie des Méduses (Cnidaria) du golfe de Tunis (Méditerranée sud occidentale) Oceanol. Acta. 2003;26(5):645–655. doi: 10.1016/j.oceact.2003.05.002. [DOI] [Google Scholar]
- 15.Cózar A, et al. Plastic Accumulation in the Mediterranean Sea. PLoS ONE. 2015;10(4):e0121762. doi: 10.1371/journal.pone.0121762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedrotti M, et al. Changes in the floating plastic pollution of the Mediterranean Sea in relation to the distance to land. PloS ONE. 2016;11(8):e0161581. doi: 10.1371/journal.pone.0161581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larson RJ. A Note on the Feeding, Growth, and Reproduction of the Epipelagic Scyphomedusa Pelagia noctiluca (Forskål) Biol. Ocean. 1987;4(4):447–454. [Google Scholar]
- 18.Milisenda G, et al. Jellyfish as prey: frequency of predation and selective foraging of Boops boops (Vertebrata, Actinopterygii) on the mauve stinger Pelagia noctiluca (Cnidaria, Scyphozoa) PLoS ONE. 2014;9(4):e94600. doi: 10.1371/journal.pone.0094600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardona L, Álvarez de Quevedo I, Borrell A, Aguilar A. Massive Consumption of Gelatinous Plankton by Mediterranean Apex Predators. PLoS ONE. 2012;7(3):e31329. doi: 10.1371/journal.pone.0031329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sweetman AK, Smith CR, Dale T, Jones DO. Rapid scavenging of jellyfish carcasses reveals the importance of gelatinous material to deep-sea food webs. Proc. R. Soc. Lond. B Biol. Sci. 2014;281:1796. doi: 10.1098/rspb.2014.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semenov, A. AQUATILIS EXPEDITION: a world of oceans to explore. http://aquatilis.tv/aquatilis-expedition/ (2013).
- 22.Ziveri P. Plastic debris and jellyfish swarms spotted in Mediterranean. UAB MedSeA (MEDiterranean Sea Acidification in a changing climate) Project note. http://www.uab.cat/web/latest-news/news-detail/plastic-debris-and-jellyfish-%20warms-spotted-in-mediterranean-1096476786473.html?noticiaid=1345656667649 (2013).
- 23.Kastelein RA, Lavaleije MSS. Foreign bodies in the stomach of a female harbour porpoise (Phocoena phocoena) from the North Sea. Aquat. Mamm. 1992;18(2):40–46. [Google Scholar]
- 24.De Pierrepont JF, et al. Stomach contents of English Channel cetaceans stranded on the coast of Normandy. J. Mar. Biol. Assoc. UK. 2005;85:1539–1546. doi: 10.1017/S0025315405012762. [DOI] [Google Scholar]
- 25.Schuyler QA, et al. Mistaken identity? Visual similarities of marine debris to natural prey items of sea turtles. BMC Ecol. 2014;14:14. doi: 10.1186/1472-6785-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mrosovsky N, Ryan GD, James MC. Leatherback turtles: the menace of plastic. Mar. Pollut. Bull. 2009;58:287–289. doi: 10.1016/j.marpolbul.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Moore CJ. Synthetic polymers in the marine environment: a rapidly increasing, long-term threat. Environ. Res. 2008;108:131–139. doi: 10.1016/j.envres.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 28.Brillant M, MacDonald B. Postingestive selection in the sea scallop (Placopecten magellanicus) on the basis of chemical properties of particles. Mar. Biol. 2002;141:457–465. doi: 10.1007/s00227-002-0845-2. [DOI] [PubMed] [Google Scholar]
- 29.Morabito R, Marino A, La Spada G. Nematocytes’ activation in Pelagia noctiluca (Cnidaria, Scyphozoa) oral arms. J. Comp. Physiol. A. 2012;198(6):419–426. doi: 10.1007/s00359-012-0720-7. [DOI] [PubMed] [Google Scholar]
- 30.Thorington GU, Hessinger DA. Efferent mechanisms of discharging cnidae: a nematocyst release response in the sea anemone tentacle. Biol. Bull. 1998;195:145–155. doi: 10.2307/1542822. [DOI] [PubMed] [Google Scholar]
- 31.Lindstedt KJ. Biphasic feeding response in a sea anemone: control by asparagine and glutathione. Science. 1971;173:333–334. doi: 10.1126/science.173.3994.333. [DOI] [PubMed] [Google Scholar]
- 32.Plastics – the Facts 2016: an analysis of European latest plastics production, demand and waste data; http://www.plasticseurope.org/Document/plastics–the-facts-2016-15787.aspx?Page=DOCUMENT&FolID=2.
- 33.Suaria G, et al. The Mediterranean Plastic Soup: synthetic polymers in Mediterranean surface waters. Sci. Rep. 2016;6:37551. doi: 10.1038/srep37551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrady AL. Microplastics in the marine environment. Mar. Pollut. Bull. 2011;62:1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 35.Allen AS, Seymour AC, Rittschof D. Chemoreception drives plastic consumption in hard coral. Mar. Poll. Bull. 2017;124(1):198–205. doi: 10.1016/j.marpolbul.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 36.Morabito R, Marino A, Dossena S, La Spada G. Nematocyst discharge in Pelagia noctiluca (Cnidaria, Scyphozoa) oral arms can be affected by lidocaine, ethanol, ammonia and acetic acid. Toxicon. 2014;83:52–58. doi: 10.1016/j.toxicon.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Ormond RF, Caldwell S. The effect of oil pollution on the reproduction and feeding behaviour of the sea anemone Actinia equina. Mar. Pollut. Bull. 1982;13:118–122. doi: 10.1016/0025-326X(82)90367-8. [DOI] [Google Scholar]
- 38.Morabito R, Dossena S, La Spada G, Marino A. Heavy metals affect nematocysts discharge response and biological activity of crude venom in the jellyfish Pelagia noctiluca (Cnidaria, Scyphozoa) Cell. Physiol. Biochem. 2014;34(2):244–254. doi: 10.1159/000362979. [DOI] [PubMed] [Google Scholar]
- 39.Tilves U, et al. Predation by the scyphozoan Pelagia noctiluca on Mnemiopsis leidyi ctenophores in the NW Mediterranean Sea. J. Plankton Res. 2013;35(1):218–224. doi: 10.1093/plankt/fbs082. [DOI] [Google Scholar]
- 40.Hall NM, Berry KLE, Rintoul L, Hoogenboom MO. Microplastic ingestion by scleractinian corals. Mar. Biol. 2015;162:725–732. doi: 10.1007/s00227-015-2619-7. [DOI] [Google Scholar]
- 41.Boschma H. On the feeding reactions and digestion in the coral polyp Astrangia danae, with notes on its symbiosis with zoöxanthellae. Biol. Bull. 1925;49:407–439. doi: 10.2307/1536652. [DOI] [Google Scholar]
- 42.Nicol JAC. Digestion in sea anemones. J. Mar. Biol. Assoc. UK. 1959;38:469–477. doi: 10.1017/S0025315400006895. [DOI] [Google Scholar]
- 43.Yonge CM. Digestive processes in marine invertebrates and fishes. J. Cons. Int. Explor. Mer. 1931;6:175–212. doi: 10.1093/icesjms/6.2.175. [DOI] [Google Scholar]
- 44.Lilley et al. Global patterns of epipelagic gelatinous zooplankton biomass. Mar. Biol. 158, 2429–2436.
- 45.Revelles M, Cadorna L, Aguilar A, Fernández G. The diet of pelagic loggerhead sea turtles (Caretta caretta) off the Balearic archipelago (western Mediterranean): relevance of long-line baits. J. Mar. Biol. Ass. U.K. 2007;87:805–813. doi: 10.1017/S0025315407054707. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.