Abstract
The Chengjiang fossil Lagerstätte (Cambrian Stage 3) from Yunnan, southern China is renowned for its soft-tissue preservation. Accordingly structures in fuxianhuiids, radiodontans and great appendage arthropods have been interpreted as the nervous and cardiovascular systems, including brains, hearts and blood vessels. That such delicate organ systems survive the fossilization process seems remarkable; given that this mode of preservation involves major taphonomic changes, such as flattening, microbial degradation, chemical alteration and replacement. Here, we document a range of taphonomic preservation states in numerous articulated individuals of Fuxianhuia protensa. We suggest that organic (partly iron mineral-replaced) bulbous structures in the head region, previously interpreted as brain tissue, along with sagittally located organic strands interpreted as part of the cardiovascular system or as nerve cords, may be better explained as microbial biofilms that developed following decomposition of the intestine, muscle and other connective tissues, forming halos surrounding the original organic remains.
Keywords: microbial biofilms, Chengjiang fossil Lagerstätte, Cambrian, nervous tissue, cardiovascular system
1. Introduction
The early Cambrian Chengjiang fossil locality represents one of the world's oldest Konservat-Lagerstätten, providing valuable insights into early metazoan evolution [1–9]. It documents a snapshot into the later stage of the Cambrian Radiation Event. Fine mudstones housing these ‘Burgess Shale-type’ fossils, in other words soft-bodied animals preserved as organic carbonaceous compressions, are dated at Chengjiang to the Cambrian Stage 3 (ca 520 Ma); equivalent to the late Atdabanian or early Botoman of the Siberian regional scale [10]. Understanding the complicated taphonomic history of this shale-hosted Lagerstätte is fundamental to the interpretation of the often exceptionally preserved metazoan remains [11,12]. This in turn impacts on reconstructions of their morphology, ecology and evolutionary significance.
Recently, several authors have interpreted structures in fuxianhuiids, radiodontans and great appendage arthropods as the nervous and cardiovascular system. Organs identified include brains, hearts and blood vessels [13–17] and these data have been employed to formulate hypotheses about evolution of the brain and eye in stem-group arthropods. In particular, the fuxianhuiid brain was described as being more like that of mandibulates rather than chelicerates [15], an observation which has to be reconciled with fuxianhuiids' basal phylogenetic position within Euarthropoda [2]; well before the chelicerate–mandibulate split. These hypotheses assume that the brain has indeed been preserved, whereby the fact that such delicate organ systems could survive the process of fossilization seems remarkable, not least in the highly labile nervous tissue. Doubts have been expressed on the fact how the different parts of brains and their innervation into antennae and eyes can be correctly identified after the compaction of an originally three-dimensional brain in sediments [18]. It has been pointed out that the interpretation of the brain parts, such as a tritocerebrum in Fuxianhuia, contradicts with current neurological hypotheses on brain evolution [18].
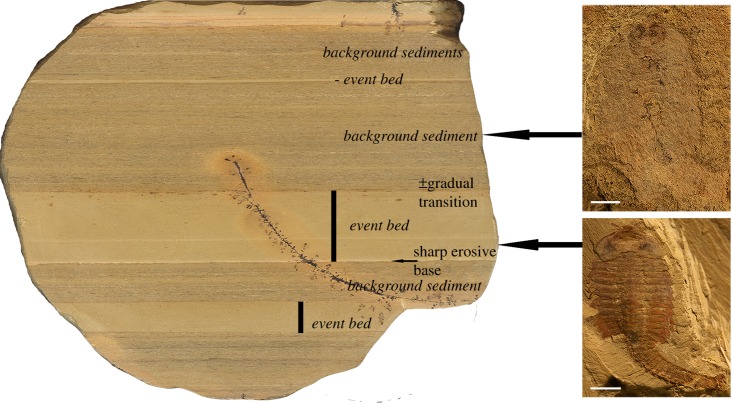
Of particular note is that claims for the identification of the cardiovascular system refer to material recovered from the thinly-laminated, so-called ‘background beds’ [11,12] (BGBs), rather than the rapidly deposited ‘event beds’ (EBs) [11,12] (figure 1) which for the most part yield better preserved material (figure 2a). In the BGBs slower sedimentation rates resulted in carcasses spending more time in the bioactive decomposition zone on the seafloor–water interface, which in turn facilitated longer periods of decay prior to burial. This time-averaging influence in the BGBs is recognizable in the fauna via, for example, a higher rate of disarticulation, preservation of less anatomical detail (figure 2b), and a shift in the composition of the taxa. Given the extended time for decay and the general lack of body appendages in Chengjiang BGBs, it seems surprising to us that these beds were able to preserve pristine details of delicate internal organ systems which: (i) lack rigid morphological structures, and (ii) are likely to be among the first parts of the body to disintegrate after death [19]. Thus, it is incumbent on us to question how such putative cardiovascular systems could be preferentially preserved under unfavourable BGB conditions without preserving other internal tissues, such as nervous tissue and how, by contrast, individuals preserved in EBs preserve putative nervous tissue without recording traces of the cardiovascular system.
Figure 1.
Event beds (EBs) and background beds (BGBs) belonging to the Chengjiang fossil Lagerstätte in a vertical section of the mid-Yu'anshan Formation mudstones. The characteristics of EBs are: 1. fine claystone probably of distant tempestites or nepheloid layer; 2. total organic carbon (TOC) (0.11–0.21%) slightly lower than in BGBs, but the preserved TOC does not allow strong early diagenetic sulphate reduction; 3. beds homogeneous or with upward grading with a sharp erosional base; 4. thanatocenosis indicating instantaneous embedding (day level), parautochthonous, >90% articulated, often with ‘soft-tissue’ preservation, partly 3D preservation and oblique embedding of carcasses; 5. diversity higher than in adjacent BGB; 6. depositional time represented in EBs is in the range of hours to days; and 7. decay rate in organic remains is lower because of oxidant depletion, but some decay due to endogenous gut bacteria. BGBs characterized by: 1. claystone with higher content of silt; 2. TOC (0.18–0.48%) slightly higher than in EBs, with abundant faecal strings and organic hash beds laminated with its base gradually from underlying EBs; 3. fossil remains slightly time-averaged, majority are disarticulated shell fragments, articulated remains very rare, often partly destroyed or with a spongy appearance and mostly without ‘soft-tissue’ preservation; 4. diversity lower than in adjacent EBs; 5. time represented in a millimetric lamina is in the range of some years; and 6. decay rate in organic remains high by aerobic respiration and microbial decomposition. Scale bar in fossils is 5 mm. (Online version in colour.)
Figure 2.
Fuxianhuia protensa from an ‘event bed’ (EB) and a ‘background bed’ (BGB) of the Chengjiang fossil Lagerstätte, showing typical differences in preservation. (a) EB fossil (ELI-JS721) showing excellent preservation of the external anatomy (e.g. the head shield, trunk tergites, antennae and stalked eyes). (b) BGB fossil (ELI-JS327A) preserving only remains of the gut and very faint segmentation in the partly decayed cuticle. Scale bar equals 5 mm. (Online version in colour.)
2. Results and discussion
(a). Taphonomic survey
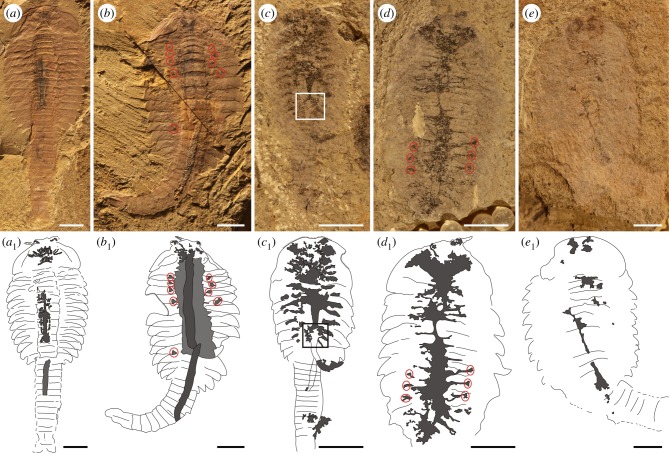
In support of our critique, we carried out a systematic taphonomic study of 801 fuxianhuiid fossils, held in the Early Life Institute, Xi'an, China (ELI). Measurements of their internal organs (head, ‘brain’, eye, antenna, gut and the surrounding halos) were obtained in order to see whether they were consistent with structures comparable to those previously interpreted either as nervous or cardiovascular systems (electronic supplementary material, figure S1 and table S1). The 801 specimens of Fuxianhuia come from nine different localities (Jianshan, Erjie, Mafang, Tanlipo, Chenggong, Yunlongsi, Ma'anshan, Haoyi village and Ercai village) within the wider Chengjiang Lagerstätte. Specifically, 635 are from the EBs and 166 from the BGBs. As expected, the rapidly-buried individuals from the EBs mostly show excellent preservation of the external anatomy (figure 2a). Sclerotized features include the head shield, trunk tergites, antennae, stalked eyes and (more rarely) the trunk appendages and specialized post-antennal head appendages. EB fossils also often preserve remains of the gut (figure 6a,b), as well as sagittally arranged organic strands (partly Fe-replaced) and in the head region organic (or Fe-replaced) bulbous structures (electronic supplementary material, figure S2).
Figure 6.
Five reconstructed decay stages of Fuxianhuia protensa from the Chengjiang fossil Lagerstätte with fossil examples and their explanatory drawings. (a) Decay stage 1 (EBs), specimen ELI-EJ0072A: external cuticle is well-preserved and small microbial biofilms occur in the head and gut (black part in a1). (b) Decay stage 2 (EBs), specimen ELI-EJ0076A: external cuticle is still well-preserved, but the gut has been ruptured and displaced (black part in b1) and the development of a microbial halo in the mid-thorax is common (grey part in b1). (c) Decay stage 3 (BGBs), specimen ELI-JS516: external cuticle is obscured and the gut mostly degraded, the microbial communities strongly radiate laterally in the muscles and connective tissues of head and thorax (black part in c1; the box marks a bulbous structure in the midgut region strongly resembling the morphology of those in the head region previously interpreted as ‘brain’ and ‘nervous tissues’). (d) Decay stage 4 (BGBs), specimen ELI-JS327B: cuticles of the trunk tergites, head shield, eyes and antennae have also begun to decompose, microbial biofilm occupies nearly the entire body cavity and head shield; labile tissues have been fully decomposed, microbes metabolize the more durable connective tissue (black part in d1). (e) Decay stage 5 (BGBs), specimen JS354B, with strongly decayed external cuticle and internal biofilms shrunken in size and fragmented into small or particulate regions (black part in e1). Red circles mark spot-like organic remains at the positions of apodemes, where stronger connective tissue may have existed.
It is these bulbous and spinose structures, and their extensions reaching into the eye stalks, that are exactly those features which have previously been interpreted as part of the nervous system [14,15,20–22]. Our data suggest that a bulbous structure in the head region of Fuxianhuia is indeed a genuine feature, albeit only occasionally present (10% of our collection, see the electronic supplementary material, table S1). When, however, a wider range of specimens are assessed there are marked inconsistencies in the way in which the bulbous structure is preserved. Specifically, its dimensions vary from about a tenth to more than half (ca 13–64%) of the width of the head region, while its appearance varies enormously, from partly rounded, quadrate, trapezoidal, rectangular, bifid, triangular, rounded, sub-rounded, oval or butterfly-shaped (figure 3), along with a variable number of spinose projections (figure 4). Interpreting this medley of shapes in the context of supposed ‘brain-like’ structures is further complicated by splitting artefacts, which owing to separation of organic films in the part and counterpart may create different appearances (figures 3a–f and 5a1,a2,b1,b2). To compensate for this effect where appropriate we restored combined images from either half of specimens (figure 5a1,2; electronic supplementary material, figures S7–S11); a procedure not previously followed in this context.
Figure 3.
Enlargement of the head of Fuxianhuia protensa from the Chengjiang fossil Lagerstätte, showing different shapes of the ‘brain’. (a) Fossil (ELI-HY030A) with quadrate-shaped ‘brain’. (b) Fossil (ELI-HY030B) with upside down triangular-shaped ‘brain’. Note the part and counterpart of one specimens with different shapes of the ‘brain’ (which is created by split artefacts). (c) Fossil (ELI-JS050A) with trapezoidal-shaped ‘brain’. (d) Fossil (ELI-JS050B) with triangular-shaped ‘brain’. (e) Fossil (ELI-EJ062A) with triangular-shaped ‘brain’. (f) Fossil (ELI-EJ062B) with irregular-shaped ‘brain’, showing some branches. (g) Fossil (ELI-EJ067B) with rectangular-shaped ‘brain’. (h) Fossil (ELI-JS0087A) with butterfly-shaped ‘brain’. (i) Fossil (ELI-EJ231A) with rounded ‘brain’. (j) Fossil (ELI-JS469B2) with sub-rounded ‘brain’. (k) Fossil (ELI-EJ129A) with oval-shaped ‘brain’. (l) Fossil (ELI-EJ264B) with bifid ‘brain’. Scale bar, 1 mm. (Online version in colour.)
Figure 4.
‘Brain-like’ structures in specimens of Fuxianhuia protensa revealing different shapes and branching morphologies. (a,a1) ELI-EJ0072A with eight branches on the left, four branches on the right. (b,b1) ELI-EJ0076A with six branches on the left, four branches on the right. (c,c1) ELI-CG752 with four branches on the left, three branches on the right. (d,d1) ELI-EJ264B with two branches on the left, four branches on the right. (e,e1) ELI-TLP0004B, round ‘brain-like’ structure with only one branch on the right. (f,f1) ELI-JS354B with one branch on the left, two branches on the right. (g,g1) ELI-JS327B, ‘brain-like’ structure almost filling the head shield without branches. (h,h1) ELI-JS359A, large ‘brain-like’ structure with four branches on the left and five branches on the right. Scale bar in (a,b,e,f,g) is 2.5 mm; (c,d,h) is 2 mm.
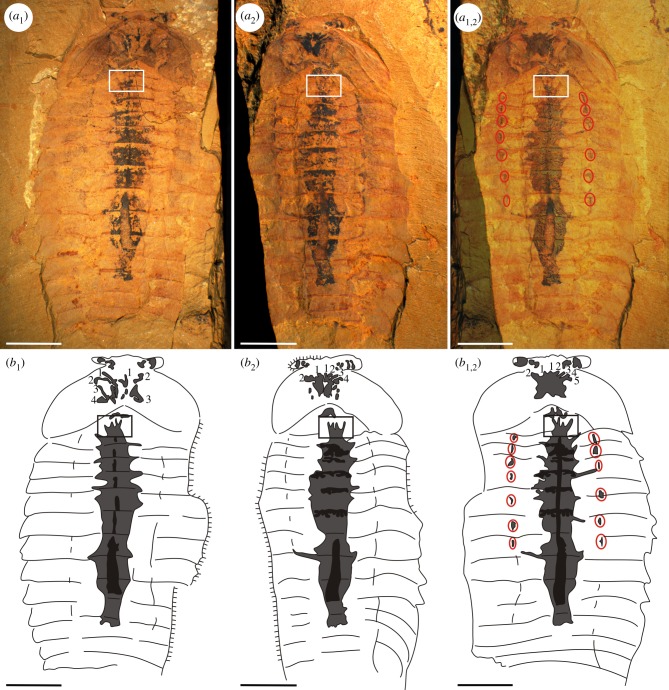
Figure 5.
Fuxianhuia protensa EJ264A, B from the Chengjiang fossil Lagerstätte. Note the different appearance of the ‘brain-like’ structures, which were created by splitting artefacts, and the gut (black) is surrounded by a rounded organic halo (dark grey) in the thoracic region. (a1) ELI-EJ264A, ‘brain-like’ structure with four branches on the left and three branches on the right. (a2) ELI-EJ264B, ‘brain-like’ structure with two branches on the left and four branches on the right. (a1,2) Combined image from both halves of the specimen, showing the complete biofilm forming the ‘brain-like’ structure which differs in appearance from either the part or counterpart. (b1,b2,b1,2) Camera lucida drawings of (a1,a2,a1,2). Box marks some spinose projections of an additional bulbous structure in the foregut-midgut transition, which are similar to more frontally positioned structures previously interpreted as ‘nerves’ emerging from a ‘brain’. Red circles mark spot-like organic remains (b1,2) at the positions of apodemes, where stronger connective tissue may have existed. Scale bar equals 5 mm.
Not only were anterior structures identified as brain tissue, but Ma et al. [15] specifically described material with three lobes on either side. In their interpretation these correspond to the optical neuro-tract, the antennal nerve and a nerve trunk for the post-antennal specialized appendage respectively. We do not dispute the observations, but rather their interpretation. Thus, our results demonstrate that the number of lobes extending from each side of this bulbous head structure is variable, ranging from none to eight (figure 4).
A further difficulty for the earlier interpretation is that the supposed ‘brain’ is often directly connected to what we interpret as structures either related to the gut (figure 6b) or some other sagittally disposed broad, organic structure (electronic supplementary material, figure S3). These gut-induced structures also show variable states of preservation, sometimes forming a tubular structure of similar width along its length, but sometimes being strongly conical (figures 5 and 6). In the thoracic region the gut is often surrounded by a rounded organic halo (sometimes replaced by Fe-minerals) and this can extend as regular elongate projections (figure 5; electronic supplementary material, figure S2). It is important to note that these strongly resemble the spinose projections of the bulbous head structure, previously interpreted as nerves emerging from a brain. In the mid gut the gut-induced structure often expands repeatedly into bulbous structures; these again are comparable to the supposed ‘brain’ of the head region (figures 6c and 7; electronic supplementary material, figure S12). It is evident, therefore, that features identified as pertaining to the ‘brain’ are, when sufficient numbers of specimens are compared, not only widely variable but occur in regions outside the cephalic region.
Figure 7.
The bulbous structure in the mid gut region of Fuxianhuia protensa, which shows a comparable morphology to the ‘brain-like’ structures in the head region. (a–e) The bulbous structures in the mid gut of specimens ELI-EJ203, EJ073A, JS403A, JS516, JS630A. (f–j) The complete specimens of ELI-EJ203, EJ073A, JS403A, JS516, JS630A. Box marks the region of the bulbous structure in the body. Scale bars: (a–e) is 1 mm; (f–j) is 5 mm. (Online version in colour.)
(b). Microbial biofilms
We suggest here an alternative interpretation for the organic halo with its lateral projections of various sizes as well as the ‘brain-like’ bulbous structures in the head region of fuxianhuiids. Instead of treating them as effectively the remains of pristine organs, we interpret them as gut-induced microbial biofilms, which metabolized the original internal tissues and were subsequently preserved along with the exoskeleton.
Decay experiments on the extant brine shrimp Artemia salina [23] demonstrated that microbial biofilms develop from endogenous gut bacteria. Following the consumption (or rupture) of the gut wall, these biofilms migrate into the body cavity. It is common that rupture of the gut occurs in the transition of the endodermal midgut to the ectodermal hindgut; the latter with chitinous re-enforcement. In general, arthropod guts may be more likely to be preserved in the fossil record by virtue of the presence of cuticle linings at the fore- and hindgut [24], plus any gut contents which help to retain the structure in the fossil even following early and strong degradation by endogenous gut bacteria. After release of the bacteria from the gut into the body cavity, they begin to consume muscle and connective tissues and other organ systems, rapidly forming a microbial halo around a gut-like tube.
The components making up the gut-tissue (and any infill) are themselves also consumed by the microbes. As a result their overall shape, including their width becomes very variable, while in later stages of decay bulbous expansions with long radiating projections may develop (figures 6 and 8; electronic supplementary material, figures S5 and S6). Since arthropods generally have an open circulatory system, decay products should eventually permeate into the extremities too (i.e. the legs, antennae and eyes). Indeed the decay experiments of Butler et al. reported microbes in the distal appendages by stage 3 of their observed decay processes ([23]: table 1). The successive stronger appearance of lateral projections (figure 6d,d1), often connecting to spot-like structures at the positions of apodemes (figure 5a1,2,b1,2) and of large symmetric bodies in the head region with ongoing decay is here explained by the later infestation of more durable cartilage-like tissues, such as the connective tissues, which form elongated ligaments, muscle tendons at the apodemes and large symmetrical bodies in the head shield [25].
Figure 8.
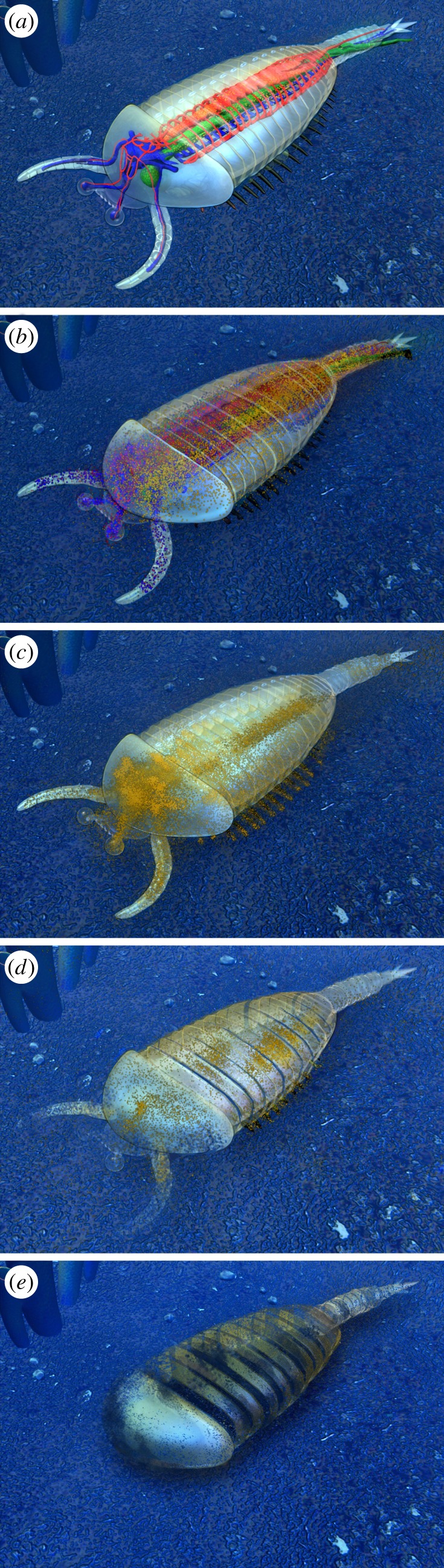
Taphonomical decay model, corresponding to the five decay stages shown in figure 6. Note: Decay stage 0 (undecayed organism) is not preserved in the Chengjiang fossil Lagerstätte.
Accordingly, the bulbous expansion in the head region of the fuxianhuiids is interpreted here as a microbial biofilm, which developed principally around the biomass concentrated in the foregut region located beneath the head shield and of connective and muscle tissues. It is not known how extensive the gut was in this region—some arthropods have an expanded area such as a ‘crop’ here [26]. We propose that following consumption of the foregut tissue, bacteria extended into the head region to consume more labile material, such as nervous, vascular and muscle tissue, eventually also metabolizing more durable connective tissues with typical structures such as inter gnathal ligaments leading to a ‘brain-like’ appearance [25]. In this way the bacteria generated variably shaped bulbous expansions, along with sharp projections; some of which also migrated into the eyes and antennae (figure 4).
(c). Taphonomic decay model
In support of our conclusions, our survey of several specimens at different stages of decay and a corresponding taphonomic decay model reveal that ‘brain-like’ organic biofilms with a variety of preserved morphologies are actually quite common in fuxianhuiids and develop early on in association with a microbial halo surrounding the midgut (figures 5 and 6). Our scheme, therefore, fits into a taphonomic model (figure 8) based on progressive stages of decay in the fuxianhuiids. Structures that could be identified as deriving from nervous tissue are indeed quite frequent, but show such a range of variability that their interpretation as the consequences of biofilm formation seems to provide a more convincing explanation.
For much the same reason we also consider that the organic strand in an otherwise decayed specimen of Fuxianhuia from a BGB, previously interpreted as part of the cardiovascular system [13], is actually a microbial halo surrounding a more centrally positioned and decayed gut-tube and ladder-like connective tissue. More specifically, the postulated heart [13] appears to be only a clay-rich area. So too may the microbial biofilm and decomposed gut indeed partly appear segmented (electronic supplementary material, figures S2, S3 and S5), but this results from the replication of the inner surface of the thoracic tergites and the bars of connective tissues associated with this. We should add here the critique of Göpel & Wirkner [27] who also doubted that these fossil structures were part of the cardiovascular system since the identification was based on: (i) the structures being interpreted as not part of the gut or nervous system, as opposed to the presence of convincing anatomical details; and (ii) the fact that the reconstruction of this structure in general [13] is unlike the vasculature of any living arthropod group.
In the arthropod Alalcomenaeus sp. from the Chengjiang Lagerstätte, a more centrally located clay infill of a gut-tube surrounded by a microbial halo was interpreted—erroneously in our opinion—as an oesophageal foramen within a nerve cord extending from a brain [16]. Note that lateral compressions of Alalcomenaeus reveal that the dark elongated strands are not located ventrally, where we would expect a nerve cord to lie [16], and are closer to a position where the arthropod gut would be predicted. In a number of other arthropod taxa similar microbial biofilms developed, but the study of their decay series remains a matter of future investigation.
Our review and re-interpretation of Fuxianhuia has implications for other studies of putative central nervous systems, for example in the Chengjiang lobopodian Paucipoda inermis, the great appendage arthropod Alalcomenaeus, the radiodontan Lyrarapax unguispinus [17,20], and the Burgess Shale arthropods Helmetia expansa, Odaraia alata [28] and Waptia fieldensis [29]. It is important to stress that we accept some organisms or specimens found in other fossil Lagerstätten may contain remnants of fossilized nervous tissues. That said, we strongly encourage future studies reporting highly labile organ systems, such as nervous tissues or cardiovascular systems, to be accompanied by a thorough taphonomic analysis to rule out biofilms. For instance, more pristine tissue preservation is recorded in fuxianhuiids from the slightly younger Xiaoshiba fossil Lagerstätte [30,31]. The reported Chengjiangocaris material preserves elongated strands that have convincingly been interpreted as ventral nerve cords [31]. In this case there were no microbial halos with lateral projections surrounding the gut and, significantly, no large bulbous, brain-like structures like those reported from in Chenjiang Fuxianhuia. Preservation of nervous tissue at Xiaoshiba thus appears plausible, although a detailed taphonomic analysis would be welcome. In summary, our challenge to the developing field of palaeoneurology is to demonstrate that specific organ systems are not merely artefacts associated with the ubiquitous natural process of decay.
(d). The value of decay experiments
In pursuit of this, decay experiments on several modern organisms have been carried out during the last decade covering most metazoan clades [19,23,32]. Muscles and nerves were consistently found to be among the first tissues to decompose under various environmental conditions affecting all investigated ecdysozoan model animals. This obviously challenges interpretations of soft-tissue preservation in fossil Lagerstätten; in particular reports of nervous and vascular tissues in fuxianhuiids [32].
Parry et al. [33] reviewed the major taphonomic factors leading to exceptional fossil preservation. They analysed several modern decay and fossilization experiments together with their experimental settings and conclusions. It should be cautioned here that they took reports of nervous and/or vascular tissue in Cambrian fuxianhuiids as granted. They also draw on other cases of excellent preservation in which labile tissues were apparently present, while more durable tissues or structures were not [34]. Because durable structures are occasionally lacking in soft-tissued organisms—which were assumed to have carried the full array of crown-group characters such as a notochord in Haikouichthys from Chengjiang—Parry et al. argued that results of modern decay experiments cannot be directly applied to the interpretation of exceptional fossil preservation. They further stressed [33] that modern decay experiments can never replicate exactly those conditions that lead to a given state of decay and diagenesis, and suggested that the fossilization process may thus be at least partly capable of selective tissue preservation.
Based on our current taphonomic study of putative soft-tissue preservation in Fuxianhuia, we question this critique of decay experiments. Such studies provide valuable model data on the resilience of different tissue types and extracellular structures. If exceptional fossils do not show tissue preservation in accordance with the expectations from decay experiments, it is imperative to challenge these results and seek a detailed explanation for why this particular tissue preservation deviates from the norm. We propose that the decay experiments of Butler et al. [23], together with our taphonomic survey, offer a more plausible explanation for why gut-induced structures are preferentially preserved, even though their tissues are consumed early on during microbial decay. Our new interpretation of organic strands within fuxianhuiids as microbial biofilms also resolves the apparent contradiction between expectations from modern decay experiments and palaeontological descriptions. Of course we cannot exclude the possibility that specific tissues can be preferentially preserved at other fossil Lagerstätten. However, if fossils are documented in which, e.g. the cardiovascular system is preserved, it becomes necessary to explain the processes which led to such an unusual situation. Simply referring to potential selectivity of tissue preservation [33] is not a substitute for convincing taphonomic models.
According to our studies of Chengjiang Fuxianhuia, we propose that reports of easily degradable structures—such as muscle, nervous, or vascular tissues—should be accompanied by a taphonomic analysis in which specific parameters are recorded. As an example of ‘best practice’, data on which decay features are recorded in the specimen with putative soft-tissue preservation, and in other accompanying individuals, should be presented. The following questions also need to be addressed. Is the array of preserved tissue types in accordance with results from decay experiments? What are the depositional circumstances and approximately how long did the carcass lay in the zone of aerobic respiration? Which diagenetic transformations occurred, and did exceptional environmental conditions exist that could promote selective tissue preservation? Finally, what is the extent of morphological variability among the putative soft-tissued organs across a range of fossil specimens?
These questions may only offer broad guidance to the analysis of the degree of decay impact and diagenetic transformation, because exceptional fossil preservation in different types of Lagerstätten is extremely complex. Despite this, our main conclusion is to encourage authors to examine whether microbial decay structures may explain complex internal features in other exceptionally preserved fossils too.
3. Material and methods
All fossils are held at the ELI, Northwest University, Xi'an, China. Photographs were taken using a Canon 5D Mark II. Energy-dispersive spectroscopy (EDS) analyses of five specimens (ELI-EJ264A, B; ELI-JS341; ELI-EJ652A, B; ELI-JS516 and ELI-JS327B) without coating were conducted on a ZEISS-Supra 40VP environmental scanning electron microscope with an Inca (EDS) system and X-max 50 mm2 detector at the FU Berlin. Drawings were made with a camera lucida on a Leica MZ125 stereomicroscope. The figures were prepared with Adobe Photoshop CS, CoralDraw X4 and Adobe Illustrator.
Supplementary Material
Acknowledgements
Special thanks for valuable comments from Simon Conway Morris. We thank Honglei Wang for the reconstruction drawing, Li Yan (Early Life Institute, Northwest University, Xi'an, China) for taking photos and preparing figures. We thank the reviewers for several helpful remarks on a previous version.
Data accessibility
This article has no additional data.
Authors' contributions
J.L. and M.S. interpreted morphological characters in the fossils and wrote the paper; J.L. and D.S. collected the fossils; M.S. developed the taphonomical model; J.A.D. contributed to the discussion; all authors discussed the results and commented on the manuscript.
Competing interests
We have no competing interests.
Funding
This work was supported by the ‘973’ Project of the Ministry of Science and Technology of China (grant 2013837100), National Program for Support of Top-notch Young Professionals and the National Natural Science Foundation of China (grants 41222014, 41172023, 41621003, 41102012), the Ministry of Education of China for Changjiang Scholars (to J.L.) and DFG grant STE814/5-1 (to M.S.). Financial supports from Ministry of Education of China, the Program of Introducing Talents of Discipline to Universities the 111 project (P201102007), Shaanxi Bureau of Science and Technology (FJ11366), the Fund from the State Key Laboratory of Continental Dynamics, Northwest University (Grants 201210128) and the support for young talents of Northwest University.
References
- 1.Chen JY, Ramsköld L, Zhou GQ. 1994. Evidence for monophyly and arthropod affinity of Cambrian giant predators. Science 264, 1304–1308. ( 10.1126/science.264.5163.1304) [DOI] [PubMed] [Google Scholar]
- 2.Chen JY, Edgecombe GD, Ramskold L, Zhou GQ. 1995. Head segmentation in early Cambrian Fuxianhuia: implications for arthropod evolution. Science 268, 1339–1342. ( 10.1126/science.268.5215.1339) [DOI] [PubMed] [Google Scholar]
- 3.Hou XG, Bergström J. 1997. Arthropods of the Lower Cambrian Chengjiang fauna southwest China. Fossils Strata 45, 1–116. [Google Scholar]
- 4.Shu DG, et al. 1999. Lower Cambrian vertebrates from south China. Nature 402, 42–46. ( 10.1038/46965) [DOI] [Google Scholar]
- 5.Shu DG, Conway Morris S, Han J, Chen L, Zhang XL, Zhang ZF, Liu HQ, Li Y, Liu JN. 2001. Primitive deuterostomes from the Chengjiang Lagerstätte (Lower Cambrian, China). Nature 414, 419–424. ( 10.1038/35106514) [DOI] [PubMed] [Google Scholar]
- 6.Hou XG, Aldridge RJ, Bergström J, Siveter DJ, Siveter DJ, Feng XH. 2004. The Cambrian fossils of Chengjiang, China: the flowering of early animal life. Oxford, UK: Blackwell. [Google Scholar]
- 7.Ramsköld L, Chen JY. 1998. An arthropod phylogeny based on fossil and recent taxa. In Arthropod fossils and phylogeny (ed. Edgecombe G.), pp. 77–93. New York, NY: Columbia University Press. [Google Scholar]
- 8.Liu JN, Steiner M, Dunlop JA, Keupp M, Shu DG, Ou Q, Han J, Zhang ZF, Zhang XL. 2011. An armoured Cambrian lobopodian from China with arthropod-like appendages. Nature 470, 526–530. ( 10.1038/nature09704) [DOI] [PubMed] [Google Scholar]
- 9.Liu JN, Dunlop JA. 2014. Cambrian lobopodians: a review of recent progress in our understanding of their morphology and evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 398, 4–15. ( 10.1016/j.palaeo.2013.06.008) [DOI] [Google Scholar]
- 10.Peng SC. 2003. Chronostratigraphic subdivision of the Cambrian of China. Geologica. Acta. 1, 135–144. [Google Scholar]
- 11.Hu SX. 2005. Taphonomy and palaeoecology of the Early Cambrian Chengjiang biota from Eastern Yunnan, China. Berl. Paläobiologische Abh. 7, 1–197. [Google Scholar]
- 12.Zhao FC, Caron JB, Hu SX, Zhu MY. 2009. Quantitative analysis of taphofacies and paleocommunities in the early Cambrian Chengjiang Lagerstätte. Palaios 24, 826–839. ( 10.2110/palo.2009.p09-004r) [DOI] [Google Scholar]
- 13.Ma XY, Cong PY, Hou XG, Edgecombe GD, Strausfeld NJ. 2014. An exceptionally preserved arthropod cardiovascular system from the early Cambrian. Nature Commu. 5, 3560 ( 10.1038/ncomms4560) [DOI] [PubMed] [Google Scholar]
- 14.Bergström J, Hou XG, Zhang XG, Clause S. 2008. A new view of the Cambrian arthropod Fuxianhuia. GFF 130, 189–201. ( 10.1080/11035890809452772) [DOI] [Google Scholar]
- 15.Ma XY, Hou XG, Edgecombe GD, Strausfeld NJ. 2012. Complex brain and optic lobes in an early Cambrian arthropod. Nature 490, 258–261. ( 10.1038/nature11495) [DOI] [PubMed] [Google Scholar]
- 16.Tanaka G, Ma XY, Hou XG, Edgecombe GD, Strausfeld NJ. 2013. Chelicerate neural ground pattern in a Cambrian great appendage arthropod. Nature 502, 364–367. ( 10.1038/nature12520) [DOI] [PubMed] [Google Scholar]
- 17.Cong PY, Ma XY, Hou XG, Edgecombe GD, Strausfeld NJ. 2014. Brain structure resolves the segmental affinity of anomalocaridid appendages. Nature 513, 538–542. ( 10.1038/nature13486) [DOI] [PubMed] [Google Scholar]
- 18.Scholtz G. 2016. Perspective—heads and brains in arthropods: 40 years after the ‘endless dispute’. In Structure and evolution of invertebrate nervous systems (eds Schmidt-Rhaesa A, Harzsch S, Purschke G), pp. 402–410. Oxford, UK: Oxford University Press. [Google Scholar]
- 19.Murdock DJE, Gabbot SE, Mayer G, Purnell MA. 2014. Decay of velvet worms (Onychophora), and bias in the fossil record of lobopodians. Evol. Biol. 14, 1–9. ( 10.1186/s12862-014-0222-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edgecombe GD, Ma XY, Strausfeld NJ. 2015. Unlocking the early fossil record of the arthropod central nervous system. Proc. R. Soc. B 370, 1684 ( 10.1098/rstb.2015.0038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma XY, Edgecombe GD, Hou XG, Goral T, Strausfeld NJ. 2015. Preservational pathways of corresponding brains of a Cambrian euarthropod. Curr. Biol. 25, 1–7. ( 10.1016/j.cub.2014.10.064) [DOI] [PubMed] [Google Scholar]
- 22.Strausfeld NJ, Ma XY, Edgecombe GD. 2016. Fossils and the evolution of the arthropod brain. Curr. Biol. 26, 989–1000. ( 10.1016/j.cub.2016.09.012) [DOI] [PubMed] [Google Scholar]
- 23.Butler AD, Cunningham JA, Budd GE, Donoghue PC. 2015. Experimental taphonomy of Artemia reveals the role of endogenous microbes in mediating decay and fossilization. Proc. R. Soc. B 282, 20150476 ( 10.1098/rspb.2015.0476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mrak P, Bogataj U, Strus J, Znidarsic N. 2017. Cuticle morphogenesis in crustacean embryonic and post embryonic stages. Arth. Strc. Develop. 46, 77–95. ( 10.1016/j.asd.2016.11.001) [DOI] [PubMed] [Google Scholar]
- 25.Bitsch C, Bitsch J. 2002. The endoskeletal structures in arthropods: cytology, morphology and evolution. Arth. Strc. Develop. 30, 159–177. ( 10.1016/S1467-8039(01)00032-9) [DOI] [PubMed] [Google Scholar]
- 26.Hopkins MJ, Chen F, Hu S, Zhang Z. 2017. The oldest known digestive system consisting of both paired digestive glands and a crop from exceptionally preserved trilobites of the Guanshan Biota (early Cambrian, China). PLoS ONE 12, e0184982 ( 10.1130/abs/2017AM-305891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Göpel T, Wirkner CS. 2015. An ‘ancient’ complexity? Evolutionary morphology of the circulatory system in Xiphosura. Zoology 118, 221–238. ( 10.1016/j.zool.2014.12.004) [DOI] [PubMed] [Google Scholar]
- 28.Ortega-Hernández J. 2015. Homology of head sclerites in Burgess Shale euarthropods. Cur. Biol. 25, 1625–1631. ( 10.1016/j.cub.2015.04.034) [DOI] [PubMed] [Google Scholar]
- 29.Strausfeld NJ. 2016. Waptia revisited: Intimations of behaviors. Arth. Strc. Develop. 45, 173–184. ( 10.1016/j.asd.2015.09.001) [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Ortega-Hernández J, Butterfield NJ, Zhang XG. 2013. Specialized appendages in fuxianhuiids and the head organization of early euarthropods. Nature 494, 468–471. ( 10.1038/nature11874) [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Ortega-Hernández J, Butterfield NJ, Liu Y, Boyan GS, Hou J, Lan T, Zhang XG. 2016. Fuxianhuiid ventral nerve cord and early nervous system evolution in Panarthropoda. Proc. Natl Acad. Sci USA 113, 2988–2993. ( 10.1073/pnas.1522434113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sansom RS. 2016. Preservation and phylogeny of Cambrian ecdysozoans tested by experimental decay of Priapulus. Sci. Rep. 6, 32817 ( 10.1038/srep32817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parry LA, et al. 2017. Soft-bodied fossils are not simply rotten carcasses: toward a holistic understanding of exceptional fossil preservation. Bioessays 40, 1700167. [DOI] [PubMed] [Google Scholar]
- 34.Parry LA, Wilson P, Sykes D, Edgecombe GE, Vinther J. 2015. A new fireworm (Amphinomidae) from the Cretaceous of Lebanon identified from three-dimensionally preserved myoanatomy. BMC Evol. Biol. 15, 256 ( 10.1186/s12862-015-0541-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.