Abstract
Large predators are overabundant in mid-Cretaceous continental dinosaur assemblages of North Africa. Such unbalanced ecosystem structure involves, among predatory dinosaurs, typical abelisaurid or carcharodontosaurid theropods co-occurring with long-snouted spinosaurids of debated ecology. Here, we report calcium (Ca) isotope values from tooth enamel (expressed as δ44/42Ca) to investigate resource partitioning in mid-Cretaceous assemblages from Niger (Gadoufaoua) and Morocco (Kem Kem Beds). In both assemblages, spinosaurids display a distinct isotopic signature, the most negative in our dataset. This distinct taxonomic clustering in Ca isotope values observed between spinosaurids and other predators provides unambiguous evidence for niche partitioning at the top of the trophic chains: spinosaurids foraged on aquatic environments while abelisaurid and carcharodontosaurid theropods relied almost exclusively on terrestrial resources.
Keywords: ecology, Cretaceous terrestrial ecosystems, dinosaurs, calcium isotopes, spinosaurs, palaeodiet
1. Introduction
Dietary inference in extinct vertebrates allows for addressing the structure of extinct food webs and remains critical for understanding how ecosystems evolved with time and environmental changes. In modern ecosystems, the trophic cascade concept [1] illustrates the complex role of top carnivores in regulating, directly and indirectly, populations from subsequent trophic levels. By modulating the intensity of resource exploitation (top-down), along with productivity feedback loops (bottom-up), top predators can shape the structure of ecosystems. However, reconstructing long extinct ecosystems, such as those of the Mesozoic, remains challenging because taxa with unknown ecology and physiology dominated the scene.
In contrast with modern mammalian continental ecosystems where herbivores represent most of the biomass of vertebrates, large predatory theropod dinosaurs were overabundant in mid-Cretaceous continental biotas of North Africa. The origin and the significance of this overabundance is debated [2,3], but recent studies have highlighted the peculiar nature of such predator-dominated ecosystems [4,5]. These faunas include very large theropods such as abelisaurids, carcharodontosaurids and spinosaurids, which are all considered as having high food requirements owing to their elevated metabolic rates [6,7]. Their high abundance relative to herbivorous dinosaurs thus raises questions about how resources could be partitioned between them. Because of their remarkable anatomy, spinosaurids have attracted attention with previous work inferring a semi-aquatic lifestyle [7,8] associated with a diverse diet [4,5,9–18], although the respective proportions of feeding sources, whether terrestrial or aquatic, remain unknown. Indeed, if for some spinosaurids, trophic links can be assessed using direct evidence like stomach content and feeding marks [10,16], such fossils remain rare and account for a short time window. Morphology can also provide a wealth of data concerning potential trophic links, but interpretations remain hypothetical owing to the lack of modern analogues for spinosaurids and their ecosystem. The jaw morphology of spinosaurids has been repeatedly, albeit inexactly, considered analogous to that of crocodiles [14,15] and has recently been compared to that of pike conger eels [18], reflecting some uncertainty in palaeoecological inference when considering morphological evidence alone [19]. In this context, we investigate diet and resource partitioning among mid-Cretaceous Saharan vertebrates using calcium (Ca) isotopes (δ44/42Ca) of tooth enamel and scale ganoine, a method that has previously proved informative both in modern [20–22] and fossil vertebrate assemblages [22–25]. Because Ca is almost exclusively derived from food among terrestrial vertebrates [20], the systematic change between the isotopic signature of their food relative to their mineralized tissues allows inference of trophic levels and diet. Moreover, Ca is the major constituent of the mineral phase of bone and tooth enamel, i.e. hydroxylapatite (Ca10(PO4)6OH2), which gives the method two main advantages. First, the diagenetic overprinting of biogenic Ca is unlikely to occur, providing that secondary carbonates are absent or have been leached accordingly [23]. Second, minute amounts of bone or tooth enamel sample (approx. 100 µg) are sufficient for a routine measurement of the Ca isotopic ratios. The technique is thus applicable to precious fossils with minimal damage.
Our study is focused on fossils from the Aptian–Albian fluvial deposits of Gadoufaoua (Niger, approx. 113 Ma ago) [26] and of the Cenomanian Kem Kem Beds (Morocco, approx. 100 Ma ago) [27]. In these vertebrate assemblages, crocodylomorphs, spinosaurids and non-spinosaurid theropods are overabundant relative to herbivorous dinosaurs [11,26,27], making them typical examples of North African mid-Cretaceous ecosystems. Despite age differences, the two deposits share similarities in their environmental context and host a comparable diversity of vertebrates. The analysed specimens include fishes, crocodylomorphs, pterosaurs, herbivorous (sauropods, ornithopods) and predatory (theropod) dinosaurs, including spinosaurids. Fossils of the spinosaurids Suchomimus tenerensis and Cristatusaurus lapparenti [11,12] are found in Gadoufaoua, where predators such as the non-spinosaurid theropods Kryptops palaios and Eocarcharia dinops [28] occur. In the Kem Kem Beds, the spinosaurids Spinosaurus aegyptiacus and Sigilmassasaurus brevicollis [7,13,29] are associated with fossils of non-spinosaurid theropods such as Carcharodontosaurus saharicus [27].
2. Material and methods
(a). Sample collection
Samples comprising 73 tooth enamel, enameloid and fish scale ganoine from the early to middle Cretaceous of Morocco and Niger have been analysed for elemental concentration and Ca isotope composition. Niger samples, totalling seven taxa, come from the locality of Gadoufaoua belonging to the Aptian/Albian Elrhaz Formation [26]. They consist of eight spinosaurid theropod teeth (S. tenerensis or C. lapparenti), nine non-spinosaurid theropod teeth (cf. K. palaios and cf. E. dinops), two teeth of the sauropod Nigersaurus taqueti, nine teeth of the ornithopod Ouranosaurus nigeriensis, eight teeth of the pholidosaurid crocodylomorph Sarcosuchus imperator, three teeth tentatively referred to the notosuchian crocodylomorph cf. Araripesuchus sp., three teeth tentatively referred to an indeterminate large pterosaur, five teeth referred to an indeterminate pycnodont fish, two scales of an indeterminate lepisosteiform fish and one scale attributed to the lepisosteiform fish Pliodetes sp.
Moroccan samples previously analysed for their apatite oxygen and carbon isotope compositions [7] come from three localities (Bou Laâlou, Jebel Al Qabla and Takemout) from the early Cenomanian Ifezouane Formation of the Kem Kem Beds [27]. They consist of seven teeth attributed to non-spinosaurid theropods (four teeth of C. saharicus, and three teeth of an indeterminate large theropod), eight teeth referred to the spinosaurid theropods Sp. aegyptiacus or Si. brevicollis, three teeth of an indeterminate crocodylomorph, three teeth of an indeterminate large pterosaur, one scale of the amiiform fish Stromerichthys sp. and one tooth plate of the lungfish Neoceratodus africanus.
Here, we investigate dietary and ecological aspects using the Ca isotope composition of tooth enamel, enameloid and scale ganoine (δ44/42Ca) rather than bone or dentine, which are porous and incorporate secondary diagenetic minerals. These biological materials are compact and resistant to diagenetic alteration [24]. Sampling was performed using a handled micro-drill (8200 Dremel with tungsten steel solid carbide bit and diamond burr), a micro-drill station (MicroMill with tungsten steel solid carbide bit and diamond burr), and a scalpel made in stainless steel. Tooth enamel, enameloid and scale ganoine have been carefully separated from dentine and sediment using these tools.
(b). Analytical techniques
Fossil samples were placed in Teflon beakers and dissolved in 500 µl of ultrapure nitric acid (15 N) at 130°C during 1 h. Following this attack, samples were dried out, then dissolved in a dilute solution of ultrapure nitric acid (0.5 N).
A fraction of the solutions was measured for elemental concentrations of trace elements (rare earth elements (REE), strontium (Sr) and barium (Ba)) and major elements (such as Ca, magnesium (Mg) and phosphorus (P)). These measurements were performed on an inductively coupled plasma mass spectrometer (ICP-MS) (7500 Series ICP-MS, Agilent Technologies) for trace elements, and on an inductively coupled plasma atomic emission spectrometer (ICAP 6000 Series ICP spectrometer, Thermo Electron Corporation) for major elements. The reliability of measurements has been controlled through a set of blanks and standards (see measures NIST-SRM1400, USGS-MAPS-4 and USGS-MAPS-5 in the electronic supplementary material, table S1).
Ca from samples was chemically purified following previously described protocols [30]. Briefly, samples were dissolved in double distilled HCl and loaded onto columns filled with AG 50WX-12, separated from matrix including phosphates. Ca, collected together with Sr, is then further processed in HNO3 medium using Eichrom Sr-specific resin, allowing removal of Sr. The reliability of elutions have been controlled by processing and analysing standard materials (see measures of NIST-SRM1486 in the electronic supplementary material, table S1).
Ca isotope abundance ratios (44Ca/42Ca) were measured using a Neptune Plus multi-collector ICP-MS (MC-ICP-MS) following previously described methods [30,31]. After purification, Ca samples were dissolved in ultrapure 0.05 N HNO3 and Ca concentration was set at 2 mg l−1 for all samples and standards. All Ca isotope compositions are expressed using the ‘delta’ notation defined as follows:
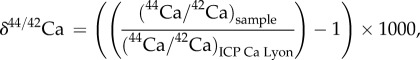 |
where (44Ca/42Ca)sample and (44Ca/42Ca)ICP Ca Lyon are the Ca isotope abundance ratios measured in sample and ICP Ca Lyon reference standard, respectively. The ICP Ca Lyon standard, used as a bracketing standard, is a Specpure Ca plasma standard solution (Alfa Aesar) previously described here: [22,23,30–32]. The SRM1486 cow bone meal standard (NIST) was used as secondary standard, in order to assess the accuracy of analytical procedure, including chemical purification.
(c). Monitoring of the isotopic measurement accuracy
Blanks realized during purification protocols have been analysed with the MC-ICP-MS. All blanks contained less than 85 ng of Ca, which is 470 times less than the Ca contained in samples. Thus, this nano-pollution does not alter the isotopic measurements of our samples, considering our measurement precision.
The mass dependency curve of the isotopic measures follows the expected relation for Ca (figure 1), with a slope value of 0.509 ± 0.016 (2 s.e., n = 74) [22,23,25,30–32], supporting the accuracy of the measures.
Figure 1.

Three isotopes plot: δ43/42Ca as a function of δ44/42Ca (‰, reference standard ICP Ca) for all samples and standards analysed for Ca isotope compositions in this study. Ca isotope compositions fall on a line with a y-axis intercept of 0.009 ± 0.015 (‰, 2 s.e.), indistinguishable from theoretical 0‰ intercept. The slope value of this line is 0.509 ± 0.016 (2 s.e.), indistinguishable from the 0.507 slope predicted by the exponential mass-dependent fractionation law. Error bars at the bottom right are average 2 s.d. for δ43/42Ca and δ44/42Ca. The two most external lines delimit the prediction interval, and the two lines accompanying the middle line correspond to the 95% confidence interval of the regression line. (Online version in colour.)
Measurements collected for the standard SRM1486 exhibit a δ44/42Ca of −1.00 ± 0.10‰ (2 s.d., n = 32), which is in the range of previously published data for this standard: −0.96 ± 0.14‰ (2 s.d., n = 17) [30], −1.04 ± 0.11‰ (2 s.d., n = 25) [22], −1.03 ± 0.13‰ (2 s.d., n = 120) [31], −1.03 ± 0.12‰ (2 s.d., n = 147) [32].
(d). Diet modelling
In order to perform diet inferences with Ca isotope data, we make use of our data to feed a simple diet mixing model. This model provides estimates of the relative contribution of Ca sources in the diet of predators thanks to δ44/42Ca. Two main parameters need to be constrained prior to using it.
First, the isotopic signature of Ca sources needs to be constrained for studied animals. Ca is more concentrated in mineralized tissues (bone, enamel, scale ganoine) than in soft tissues or in water. Thus, it has been shown that even a few per cent of mineralized tissues in diet was sufficient to constitute the major source of Ca for organisms [24]. There is fossil evidence of bone and fish scale consumption among numerous taxa of crocodylomorphs, spinosaurid and non-spinosaurid theropods [10,33]. Thus, even if this consumption can be restricted to occasional events such as feeding on juveniles for bones, the high concentration of Ca in mineralized tissues supports that these tissues were most likely the main source of Ca among these taxa. Moreover, considering the heterogeneity of modern animal data on soft tissues [20,30,34], and that blood can exhibit δ44/42Ca close to bones [30], this supports the use of δ44/42Ca values of mineralized tissues as representative of the main source of Ca for predators in our model. We have collected Ca isotope data for herbivorous dinosaurs and fishes from Gadoufaoua, which represent the most likely prey of the analysed apex predators (crocodylomorphs, spinosaurid and non-spinosaurid theropods). Thus, we have constrained the isotopic signature of the potential Ca sources to be entered in the model.
Second, our model needs an estimate of the isotopic shift between Ca from food and teeth of the predators. According to data collected on a variety of extant vertebrates (chicken, horse, seal, deer, minipigs, sheep and mouse [20,30,34]), the mean isotopic shift between Ca from diet to bones is 0.57 ± 0.10‰ (2 s.e., n = 21) and is relatively homogeneous among these vertebrates. Thus, considering that the studied predators are phylogenetically close (all are archosaurs) and that all of them share comparable Ca-cycles [20], we can reasonably assume that they exhibited a similar diet-bone isotopic offset. Only limited data have been collected on diet and enamel couples in modern faunas. Some studies show differences between these two shifts (±0.2‰; [22–24,30,35]), but these studies are often conducted on mammals, whose teeth formed in early life, and thus were not representative of the adult diet for which the comparison is made. However, in human wisdom teeth, which developed along with an adult diet, the isotopic shift between enamel and food is similar to the diet-bone shift of −0.57 ± 0.10‰ (2 s.e., n = 21) [32]. Based on these results, we assume a similar diet-enamel shift among studied taxa, and use this shift to estimate the δ44/42Ca value of the food of predators. We thus applied this model to S. imperator, spinosaurid and non-spinosaurid theropods, in order to investigate the proportion of fish and herbivorous dinosaurs in the diet of these predators.
We estimate the Ca source fractions using the estimation of food δ44/42Ca between the first and the third quartile of each population of predators, considering that it is more representative of their general diet. Moreover, to take into account the variability of δ44/42Ca values among sources, these fractions are calculated as if predators with the most depleted δ44/42Ca values fed on fishes and herbivorous dinosaurs with the most enriched δ44/42Ca values, and vice versa. In this way, we maximized the source fraction scattering, which sets the limit of diet inferences achievable with our model.
3. Results
(a). Elemental concentrations
The full dataset compiling all the elemental concentrations measured in this study is reported in the electronic supplementary material, table S1.
Among important results, the mean Ca versus P ratio is of 2.3 ± 0.1 (1 s.d.).
(b). Calcium isotope ratios
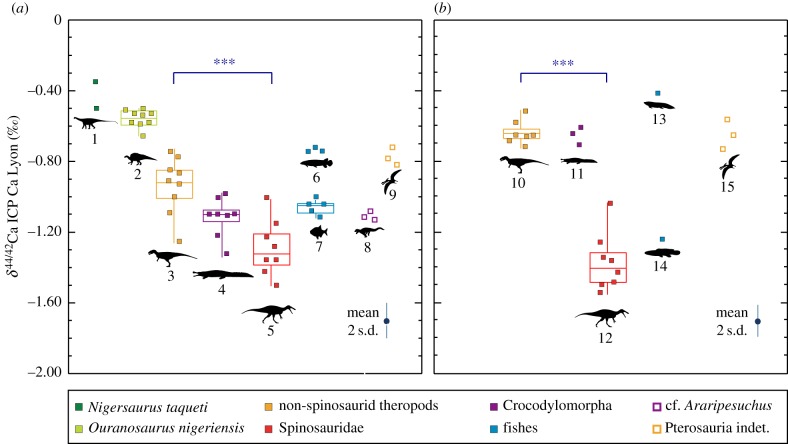
The full dataset compiling all the Ca isotopic ratios measured in this study is reported in the electronic supplementary material, table S1. The δ44/42Ca values of vertebrates from Gadoufaoua range between −0.35 and −1.51‰ with a mean 2 s.d. of 0.10‰. In this locality, the sauropod N. taqueti is the taxon which exhibits the highest δ44/42Ca with a mean value of −0.43 ± 0.11‰ (2 s.d., n = 2), whereas the spinosaurids exhibit the lowest values with −1.30 ± 0.15‰ (2 s.d., n = 8). Among the other dinosaurs, the ornithopod O. nigeriensis exhibits values at −0.56 ± 0.05‰ (2 s.d., n = 9), whereas non-spinosaurid theropods exhibit values at −0.94 ± 0.16‰ (2 s.d., n = 9). The small, supposedly terrestrial crocodylomorph, cf. Araripesuchus sp., exhibits a mean value of −1.12 ± 0.05‰ (2 s.d., n = 3), whereas for the semi-aquatic crocodylomorph S. imperator, this value is of −1.12 ± 0.13‰ (2 s.d., n = 8). For fishes, the mean values are of −0.74 ± 0.02‰ (2 s.d., n = 2) for indeterminate Lepisosteiformes, of −0.67‰ (n = 1) for Pliodetes sp., and of −1.08 ± 0.04‰ (2 s.d., n = 5) for Pycnodontiformes. Finally, pterosaurs exhibit a mean value of −0.78 ± 0.05‰ (2 s.d., n = 3). The δ44/42Ca of spinosaurid theropods is significantly lower than that of non-spinosaurid theropods (Wilcoxon's rank-sum test: p-value = 9.9 × 10−4).
The δ44/42Ca values of Kem Kem Beds vertebrates range between −0.41 and −1.56‰ with a mean 2 s.d. of 0.09‰. In Kem Kem Beds, the lungfish Ne. africanus is the taxon which exhibits the highest δ44/42Ca with a value of −0.41‰ (n = 1), whereas the other fish Stromerichthys sp. exhibits a low value of −1.26‰ (n = 1). Spinosaurids exhibit the lowest mean value of this fauna, which is −1.39 ± 0.27‰ (2 s.d., n = 8). For large non-spinosaurid theropods (C. saharicus and indeterminate theropods), this value is of −0.64 ± 0.13‰ (2 s.d., n = 7). Semi-aquatic indeterminate crocodylomorphs exhibit a mean value of −0.66 ± 0.09‰ (2 s.d., n = 3), and indeterminate pterosaurs from the family Anhangueridae exhibit a mean value of −0.67 ± 0.17‰ (2 s.d., n = 3). The δ44/42Ca of spinosaurids is significantly lower than for non-spinosaurid theropods (Wilcoxon rank-sum test: p-value = 3.1 × 10−4).
(c). Fractions of calcium sources in diet
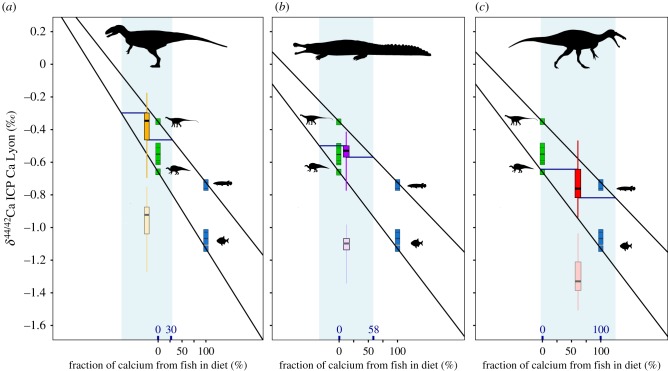
With our diet mixing model, we estimate that the maximum Ca fraction from fishes in the diet reaches 30%, 58% and 100% for non-spinosaurid theropods, S. imperator and spinosaurids, respectively. On the other hand, the maximum Ca fraction from herbivorous dinosaurs in the diet reaches 100% for all of these predators. The maximum Ca fraction derived from fishes reaches 100% for six of the eight studied spinosaurid individuals according to this model, which is possible for only two individuals of S. imperator and one non-spinosaurid theropod. However, the maximum Ca fraction derived from herbivorous dinosaurs is also 100% for four of the eight spinosaurid individuals.
4. Discussion
(a). Diagenesis
Enamel, ganoine and to a lesser extent enameloid tissues, are highly mineralized and exhibit a lower porosity than dentine or bone. They have a limited exchange surface with surrounding fluids and sediment, and for this reason, they are considered resistant to diagenesis [23,24,36]. Moreover, with almost 40% of their mass constituted of Ca, it is very unlikely that the Ca isotopic signature of these tissues can be entirely overprinted during early diagenetic processes. The Ca/P ratios measured for samples (electronic supplementary material, table S1) exhibit values of 2.3 ± 0.1 (1 s.d.), which are close to those measured in modern animals [37]. This supports that Ca isotope ratios from these fossils have not been extensively modified during diagenesis.
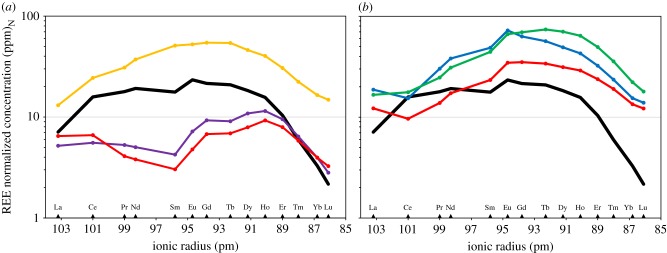
Quantifying elemental concentration of non-essential trace elements such as REE and of major elements such as Ca and P can provide information on both the autochthony and diagenetic alteration [38,39]. Among fossils from Gadoufaoua, REE enrichment profiles (normalized to the Post-Archean Australian Shale, PAAS) exhibit a similar shape with different REE enrichment intensity (figure 2a; electronic supplementary material, table S1). They can thus reasonably be considered as autochthonous [39]. Three teeth exhibit distinct REE profile shapes (figure 2a). They have thus been identified as allochthonous fossils and have not been considered in the palaeoecological discussion. The general REE profile at Gadoufaoua is ‘hat shaped’ according to the definition given by Reynard & Balter [38]. This shape, associated with measured lanthanum over ytterbium (La/Yb) ratio, and lanthanum over samarium (La/Sm) ratio (electronic supplementary material, table S1), differs from the REE profiles and ratios expected after extensive recrystallization processes during diagenesis [38,40].
Figure 2.
(a) PAAS normalized REE profiles of fossil bioapatite from the Aptian–Albian of Gadoufaoua, Niger. Concentrations are plotted on a logarithmic scale. Plain black line, typical REE profile at Gadoufaoua; yellow line, exotic profile of sample GCA5; purple line, exotic profile of sample GSA7; red line, exotic profile of sample GSP3. (b) PAAS normalized REE profiles of fossil bioapatite from the Cenomanian of Kem Kem Beds, Morocco. Concentrations are plotted on a logarithmic scale. For comparison, the plain black line represents the typical REE profile at Gadoufaoua; yellow line, typical profile of samples from the locality of Jebel Al Qabla; blue line, typical profile of samples from the locality of Bou Laâlou; red line, typical profile of samples from the locality of Takemout.
Among fossils from the Kem Kem Beds, REE enrichment profiles (normalized to the PAAS) exhibit a distinct profile between the three studied localities (figure 2b; electronic supplementary material, table S1). However, within each locality, fossils exhibit similar REE profiles. They can thus reasonably be considered as autochthonous [39]. As for Gadoufaoua, the general ‘hat shaped’ REE profile of the Kem Kem Beds [38] and the measured La/Yb and La/Sm ratios (electronic supplementary material, table S1) are different from those expected for diagenetic recrystallization processes [38,40].
Thus, the Ca/P ratios and the REE concentrations jointly support the hypothesis that the Ca isotopic composition of the fossils have not been extensively affected by diagenesis. Moreover, taxa from Gadoufaoua exhibit δ44/42Ca values consistent with their putative ecology (figure 3a). Indeed, herbivorous dinosaurs represented by N. taqueti and O. nigeriensis exhibit the highest δ44/42Ca values of this dataset, with values ranging between −0.35‰ ± 0.14 (2 s.d., n = 3) and −0.66‰ ± 0.05 (2 s.d., n = 4), respectively. Their potential predators (the pholidosaurid crocodylomorph S. imperator, spinosaurid and non-spinosaurid theropods) exhibit lower δ44/42Ca values, below −0.70‰. This isotopic distribution between herbivores and predators follows what has been observed in modern continental faunas [20], and supports, along with the observations presented above, the biogenic nature of δ44/42Ca values.
Figure 3.
δ44/42Ca data reported from Gadoufaoua (a) and Kem Kem Beds faunas (b). Groups representing at least five specimens are represented through boxplots, for which the middle line represents the median, the box limits correspond to the first and third quartiles, and the whiskers are extended between the maximum and the minimum δ44/42Ca values. The mean 2 s.d. of each measure is represented at the bottom right of each graph. The degree of significance of the difference between the theropod groups are represented with stars (*), with three stars indicating a high significance (Wilcoxon rank-sum test: p-value < 0.001). Taxonomic groups are identified by their number and represent: 1, N. taqueti (n = 2); 2, O. nigeriensis (n = 9); 3, non-spinosaurid theropods from Gadoufaoua (n = 9); 4, S. imperator (n = 8); 5, spinosaurids from Gadoufaoua (n = 8); 6, Lepisosteiformes (n = 3); 7, Pycnodontiformes indet. (n = 5); 8, cf. Araripesuchus (n = 3); 9, Pterosauria indet. (n = 3); 10, non-spinosaurid theropods from Kem Kem Beds (n = 7); 11, Crocodylomorpha indet. (n = 3); 12, spinosaurids from Kem Kem Beds (n = 8); 13, Ne. africanus (n = 1); 14, Stromerichthys sp. (n = 1); 15, Anhangueridae indet. (n = 3).
(b). Resource partitioning among predators
Compared to non-spinosaurid theropods, spinosaurids exhibit distinctly lower δ44/42Ca values (Wilcoxon rank-sum test: p-value = 9.9 × 10−4), the lowest ones of the dataset (figure 3a). This distinct pattern is also observed in the Kem Kem Beds of Morocco (figure 3b) with spinosaurids possessing significantly lower δ44/42Ca values compared to non-spinosaurid theropods (Wilcoxon rank-sum test: p-value = 3.1 × 10−4). Physiological homogeneity in Ca isotope fractionation from food to tooth enamel in these theropods is supported both by their phylogenetic proximity, all are archosaurs, and the observed homogeneity in modern vertebrates [20,30,34]. This pattern thus highlights the distinctive ecological niche of spinosaurids relative to the other theropods.
The trophic pyramid in these fluvial palaeo-ecosystems could have been supported both by herbivorous dinosaurs and fishes [4,5,26,27], which would represent two potential primary sources of Ca for predators. In Gadoufaoua, the Ca source constituted by herbivorous dinosaurs exhibits a higher δ44/42Ca value than fishes, which allows discriminating potential dietary sources among predators. Using the diet model described (see Diet modelling) for the vertebrate assemblage of Gadoufaoua, we observed that the estimated fractions of Ca originating from herbivorous dinosaurs and fishes in the diet of predators do overlap between predators. The important range of fraction between the maximum and minimum fraction estimated for each predator was expected considering the scattering of the δ44/42Ca data from fishes, herbivorous dinosaurs and predators. However, even with this important scattering in isotopic values, the difference between spinosaurid and non-spinosaurid theropods remains statistically significant, implying distinct resource partitioning between these large predators. Here, δ44/42Ca data support the idea that a majority of non-spinosaurid theropods mainly fed on herbivorous dinosaurs, with less than 30% of their Ca potentially stemming from fish sources (figure 4a). The giant crocodylomorph S. imperator exhibits an intermediate diet, possibly composed of both fishes and herbivorous dinosaurs (figure 4b). Moreover, if the diet of spinosaurid theropods potentially included a mix of fishes and herbivorous dinosaurs according to the model, these data also support that a majority of spinosaurids could be exclusively piscivorous (along with one non-spinosaurid theropod and two S. imperator individuals), and that half of them were at least partially piscivorous (figure 4c). Nevertheless, if this clearly illustrates that spinosaurids were the predators of Gadoufaoua which had the most piscivorous-oriented diet, this also leads to the conclusion that their diet was potentially a mix between fishes and herbivorous dinosaurs. Similarly, Kem Kem spinosaurids are also strongly depleted in heavy Ca, confirming a piscivorous diet in another fossil assemblage. In previous studies, several diets have been proposed for these groups of theropods. For spinosaurids, fish consumption inferred on the basis of the ecosystem structure [4,5], morphology [9,11,13–15,17,18] or direct fossil evidences [10] is thus supported by our dataset. The consumption of terrestrial prey, which is also proposed in these papers, as well as the direct evidences related to this behaviour [10,16], also remain possible according to our data. In addition, the consumption of herbivorous dinosaurs that is commonly assumed for non-spinosaurid theropods [33], such as abelisaurids and carcharodontosaurids, is also supported by our dataset. The fraction of terrestrial prey in the diet of these theropods appears to be significantly higher than that of spinosaurids. However, whether these prey have been acquired through hunting [10,14,16,17], scavenging [10,14,16] or active fishing [9,10,13–15,17,18] cannot be assessed with our dataset.
Figure 4.
Mixing model of the Ca source for non-spinosaurid theropods (a) S. imperator (b) and spinosaurids (c) from the Gadouafaoua fauna. δ44/42Ca data collected on herbivorous dinosaurs and fishes are represented, respectively, in green and blue squares. In each part of the figure, the faded lower boxplot represents the enamel δ44/42Ca values measured for each apex predator, whereas the dark upper boxplot represents the isotopic signature of their calcium source estimated to be shifted by +0.57‰ from the signature of their enamel (see Diet modelling). Boxplots are yellow for non-spinosaurid theropods (a), violet for S. imperator (b) and red for spinosaurids (c). The line in the boxplot is equal to the median, boxes are limited by their first and third quartile, and whiskers represent the maximum and the minimum values. The estimated diet fraction range of Ca derived from the fish source are indicated in bold above the x-axis and expressed in per cent.
In the model, for some non-spinosaurid theropod individuals, the inferred δ44/42Ca signature of the diet is more positive than the source with the most positive δ44/42Ca values. Four non-mutually exclusive hypotheses could be proposed to explain this inconsistency. First of all, it can be owing to the absence of a heavier source of Ca in our dataset. One of the possibilities is that this heavy source indeed corresponds to an unidentified terrestrial taxon at the bottom of the food chain. Alternatively, it can correspond to N. taqueti mineralized tissues for which only two teeth have been measured, and which exhibit the highest δ44/42Ca values of the dataset. Another possibility is that this source of heavy isotopes of Ca comes from the digestion of carbonated gastroliths. The ingestion of gastroliths among dinosaurs has been documented thanks to comparisons with modern archosaurs and fossil evidence, notably for spinosaurids [10]. Because of the heavy Ca isotope composition of carbonates [34,41,42], this consumption could buffer the δ44/42Ca towards 44Ca-enriched values among studied archosaurs. This could lead to an overestimation of the fraction of herbivorous dinosaurs in diets inferred from our mixing model, which could explain the inconsistency with non-spinosaurid theropods in our model. The third hypothesis is that this inconsistency is the result of an overestimation of the diet-enamel shift, owing to the lack of data collected on modern archosaurs, which can also lead to an overestimation of the fraction of herbivorous dinosaurs in diets (values potentially shifted towards heavy Ca). However, none of these hypotheses is susceptible to change the conclusions made on the resource partitioning among these predators or to the more piscivorous-oriented diet of spinosaurids. Indeed, a validation of these hypotheses will imply a more piscivorous diet for all these predators, even providing more support to the piscivorous-oriented diet of spinosaurids.
5. Conclusion
Our data imply that the abundance of apex predators in these peculiar African mid-Cretaceous ecosystems can be sustained simultaneously by at least two main sources: fishes and herbivorous dinosaurs, resulting in a general abundance of prey. The present Ca isotope dataset also supports the ecological partitioning of potential predatory competitors [7,8]. Indeed, the different ecological specializations of these predators resulted in different mixtures between carnivorous and piscivorous habits and are likely to have reduced the ecological competition between them. Ca isotopes support the hypothesis of spinosaurids regarded as semi-aquatic predators [7,8] and adopting a piscivorous diet, with non-spinosaurid theropods having a more terrestrial ecology and diet, feeding mainly on herbivorous dinosaurs. This ecological partitioning, combined with the abundance of prey, is consistent with the observed high abundance of predators in these faunas [4,5].
The present study underlines a complex trophic structure relying on the interaction between aquatic and terrestrial environments. One of the key points for the sustainability of these palaeoecosystems was the piscivorous-oriented diet of spinosaurids. Whether this ecological structure is a global feature among other mid-Cretaceous faunas is an open question ([7,10] and reference therein), but beyond this, Ca isotopes offer promising perspectives to study trophic structures in long extinct vertebrate assemblages.
Supplementary Material
Acknowledgements
The authors are grateful to the LABEX Lyon Institute of Origins, the TelluS INSU 2017-action INTERRVIE, and the Jurrasic Foundation for having funded this project. We thank G. Clément for allowing us to sample fossil fishes from Gadoufaoua in the collections of MNHN (Paris). For technical assistance on spectrometers, we thank E. Albalat and P. Telouk. We thank the two anonymous referees for their insightful comments on the manuscript. The animal outlines in figures 3 and 4 were retrieved from www.phylopic.org with some modifications, we thus thank their authors: Duane Raver, Todd Marshall, Nobu Tamura, Martin Kevil, Milton Tan, Scott Hartman, FunkMonk, Smokeybjb, Zimices, Heinrich Harder and T. Michael Keesey, for helping us to illustrate our study by having transferred their work to the public domain or under a creative commons licence.
Data accessibility
The dataset table compiling all elemental concentrations and isotopic ratio measurements is available as the electronic supplementary material.
Authors' contributions
The project was conceived by J.E.M., A.H., R.Am., T.T., R.Al. and V.B. R.Am. and R.Al. accessed samples for analysis. A.H. and J.E.M. sampled the specimens and conducted purification in the clean laboratory. A.H. and F.A.G. performed elemental concentration measurements. A.H., J.E.M. and T.T. performed MC-ICP-MS measurements. All authors discussed the results. The first draft version of the text was written by A.H. then subsequently, J.E.M., R.Am., T.T., R.Al., F.A.G. and V.B. contributed to it.
Competing interests
We declare we have no competing interests.
Funding
Funding for this work was provided by the LABEX Lyon Institute of Origins (ANR-10-LABX-0066) of the Université de Lyon for financial support within the programme ‘Investissements d'Avenir’ (ANR-11-IDEX-0007) of the French government operated by the National Research Agency (ANR). Consumables used in the clean laboratory for this study were partly paid for by J.E.M.'s DIUNIS project (‘Dietary Inferences Using Novel Isotope System’—TelluS INSU 2017—action INTERRVIE). Analyses and sample preparations were partly funded by the Jurassic Foundation through the Jurassic Foundation Grant Program.
References
- 1.Ripple WJ, et al. 2014. Status and ecological effects of the world's largest carnivores. Science 343, 1241484 ( 10.1126/science.1241484) [DOI] [PubMed] [Google Scholar]
- 2.McGowan AJ, Dyke GJ. 2009. A surfeit of theropods in the Moroccan Late Cretaceous? Comparing diversity estimates from field data and fossil shops. Geology 37, 843–846. ( 10.1130/G30188A.1) [DOI] [Google Scholar]
- 3.Dyke GJ. 2010. Palaeoecology: different dinosaur ecologies in deep time? Curr. Biol. 20, R983–R985. ( 10.1016/j.cub.2010.10.001) [DOI] [PubMed] [Google Scholar]
- 4.Läng E, Boudad L, Maio L, Samankassou E, Tabouelle J, Tong H, Cavin L. 2013. Unbalanced food web in a Late Cretaceous dinosaur assemblage. Palaeogeogr. Palaeoclimatol. Palaeoecol. 381–382, 26–32. ( 10.1016/j.palaeo.2013.04.011) [DOI] [Google Scholar]
- 5.Benyoucef M, Lâng E, Cavin L, Mebarki K, Adaci M, Bensalah M. 2015. Overabundance of piscivorous dinosaurs (Theropoda: Spinosauridae) in the mid-Cretaceous of North Africa: the Algerian dilemma. Cretac. Res. 55, 44–55. ( 10.1016/j.cretres.2015.02.002) [DOI] [Google Scholar]
- 6.Amiot R, Lécuyer C, Buffetaut E, Escarguel G, Fluteau F, Martineau F. 2006. Oxygen isotopes from biogenic apatites suggest widespread endothermy in Cretaceous dinosaurs. Earth Planet. Sci. Lett. 246, 41–54. ( 10.1016/j.epsl.2006.04.018) [DOI] [Google Scholar]
- 7.Amiot R, et al. 2010. Oxygen isotope evidence for semi-aquatic habits among spinosaurid theropods. Geology 38, 139–142. ( 10.1130/G30402.1) [DOI] [Google Scholar]
- 8.Ibrahim N, Sereno PC, Dal Sasso C, Maganuco S, Fabbri M, Martill DM, Zouhri S, Myhrvold N, Iurino DA. 2014. Semiaquatic adaptations in a giant predatory dinosaur. Science 345, 1613–1616. ( 10.1126/science.1258750) [DOI] [PubMed] [Google Scholar]
- 9.Taquet P. 1984. Une curieuse spécialisation du crâne de certains Dinosaures carnivores du Crétacé: le museau long et étroit des Spinosauridés. C. R. Acad. Sci. II 299, 217–222. [Google Scholar]
- 10.Charig AJ, Milner AC. 1997. Baryonyx walkeri, a fish-eating dinosaur from the Wealden of Surrey. Bull. Nat. Hist. Museum London 53, 11–70. [Google Scholar]
- 11.Sereno PC, et al. 1998. A long-snouted predatory dinosaur from Africa and the evolution of spinosaurids. Science 282, 1298–1302. ( 10.1126/science.282.5392.1298) [DOI] [PubMed] [Google Scholar]
- 12.Taquet P, Russell DA. 1998. New data on spinosaurid dinosaurs from the early Cretaceous of the Sahara. Comptes Rendus I'Académie des Sci. IIA - Earth Planet. Sci. 327, 347–353. ( 10.1016/S1251-8050(98)80054-2) [DOI] [Google Scholar]
- 13.Evers SW, Rauhut OWM, Milner AC, McFeeters B, Allain R. 2015. A reappraisal of the morphology and systematic position of the theropod dinosaur Sigilmassasaurus from the ‘middle’ Cretaceous of Morocco. PeerJ 3, e1323 ( 10.7717/peerj.1323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holtz TR. 1998. Spinosaurs as crocodiles mimics. Science 282, 1276–1277. ( 10.1126/science.282.5392.1276) [DOI] [Google Scholar]
- 15.Rayfield EJ, Milner AC, Xuan VBUI, Young PG. 2007. Functional morphology of spinosaur ‘crocodile-mimic’ dinosaurs. Soc. Vertebr. Paleontol. 27, 892–901. ( 10.1671/0272-4634(2007)27%5B892:FMOSCD%5D2.0.CO;2) [DOI] [Google Scholar]
- 16.Buffetaut E, Martill D, Escuillié F. 2004. Pterosaurs as part of a spinosaur diet. Nature 430, 33 ( 10.1038/nature02716) [DOI] [PubMed] [Google Scholar]
- 17.Hone DWE, Holtz TR. 2017. A century of spinosaurs: a review and revision of the spinosauridae with comments on their ecology. Acta Geol. Sin. 91, 1120–1132. ( 10.1111/1755-6724.13328) [DOI] [Google Scholar]
- 18.Vullo R, Allain R, Cavin L. 2016. Convergent evolution of jaws between spinosaurid dinosaurs and pike conger eels. Acta Palaeontol. Pol. 61, 825–828. ( 10.4202/app.00284.2016) [DOI] [Google Scholar]
- 19.Thomason J. 1997. Functional morphology in vertebrate paleontology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 20.Skulan J, DePaolo DJ. 1999. Calcium isotope fractionation between soft and mineralized tissues as a monitor of calcium use in vertebrates. Proc. Natl Acad. Sci. USA 96, 13 709–13 713. ( 10.1073/pnas.96.24.13709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melin AD, et al. 2014. Calcium and carbon stable isotope ratios as paleodietary indicators. Am. J. Phys. Anthropol. 154, 633–643. ( 10.1002/ajpa.22530) [DOI] [PubMed] [Google Scholar]
- 22.Martin JE, Tacail T, Adnet S, Girard C, Balter V. 2015. Calcium isotopes reveal the trophic position of extant and fossil elasmobranchs. Chem. Geol. 415, 118–125. ( 10.1016/j.chemgeo.2015.09.011) [DOI] [Google Scholar]
- 23.Martin JE, Tacail T, Balter V. 2017. Non-traditional isotope perspectives in vertebrate palaeobiology. Palaeontology 60, 485–502. ( 10.1111/pala.12300) [DOI] [Google Scholar]
- 24.Heuser A, Tütken T, Gussone N, Galer SJG. 2011. Calcium isotopes in fossil bones and teeth: diagenetic versus biogenic origin. Geochim. Cosmochim. Acta 75, 3419–3433. ( 10.1016/j.gca.2011.03.032) [DOI] [Google Scholar]
- 25.Martin JE, Vincent P, Tacail T, Khaldoune F, Jourani E, Bardet N, Balter V. 2017. Calcium isotopic evidence for vulnerable marine ecosystem structure prior to the K/Pg extinction. Curr. Biol. 27, 1641–1644. ( 10.1016/j.cub.2017.04.043) [DOI] [PubMed] [Google Scholar]
- 26.Taquet P. 1976. Géologie et paléontologie du gisement de Gadoufaoua. Cah. Paleontol. 1976, 1–247. [Google Scholar]
- 27.Cavin L, et al. 2010. Vertebrate assemblages from the early Late Cretaceous of southeastern Morocco: an overview. J. African Earth Sci. 57, 391–412. ( 10.1016/j.jafrearsci.2009.12.007) [DOI] [Google Scholar]
- 28.Sereno PC, Brusatte SL. 2008. Basal abelisaurid and carcharodontosaurid theropods from the Lower Cretaceous Elrhaz Formation of Niger. Acta Palaeontol. Pol. 53, 15–46. ( 10.4202/app.2008.0102) [DOI] [Google Scholar]
- 29.Stromer E. 1915. Das original des theropoden Spinosaurus aegyptiacus nov. gen., nov. spec. Abhandlungen der Königlich Bayer. Akad. der Wissenschaften Math. Klasse Abhandlung 28, 1–31. [Google Scholar]
- 30.Tacail T, Albalat E, Télouk P, Balter V. 2014. A simplified protocol for measurement of Ca isotopes in biological samples. J. Anal. At. Spectrom. 29, 529 ( 10.1039/c3ja50337b) [DOI] [Google Scholar]
- 31.Tacail T, Télouk P, Balter V. 2016. Precise analysis of calcium stable isotope variations in biological apatites using laser ablation MC-ICPMS. J. Anal. At. Spectrom. 31, 152–162. ( 10.1039/C5JA00239G) [DOI] [Google Scholar]
- 32.Tacail T, Thivichon-Prince B, Martin JE, Charles C, Viriot L, Balter V. 2017. Assessing human weaning practices with calcium isotopes in tooth enamel. Proc. Natl Acad. Sci. USA 114, 6268–6273. ( 10.1073/pnas.1704412114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farlow JO, Holtz TR. 2002. The fossil record of predation in dinosaurs. Paleontol. Soc. Pap. 8, 251–266. [Google Scholar]
- 34.Chu NC, Henderson GM, Belshaw NS, Hedges REM. 2006. Establishing the potential of Ca isotopes as proxy for consumption of dairy products. Appl. Geochem. 21, 1656–1667. ( 10.1016/j.apgeochem.2006.07.003) [DOI] [Google Scholar]
- 35.Clementz MT. 2012. New insight from old bones: stable isotope analysis of fossil mammals. J. Mammal. 93, 368–380. ( 10.1644/11-MAMM-S-179.1) [DOI] [Google Scholar]
- 36.Wang Y, Cerling TE. 1994. A model of fossil tooth enamel and bone diagenesis: implications for stable isotope studies and paleoenvironment reconstruction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 107, 281–289. ( 10.1016/0031-0182(94)90100-7) [DOI] [Google Scholar]
- 37.Balter V., Person A., Labourdette N., Drucker D., Renard M., Vandermeersch B. 2001. Were Neandertalians essentially carnivores? Sr and Ba preliminary results of the mammalian palaeobiocoenosis of Saint-Césaire. Comptes Rendus I'Académie des Sci. IIA - Earth Planet. Sci. 332, 59–65. [Google Scholar]
- 38.Reynard B, Balter V. 2014. Trace elements and their isotopes in bones and teeth: diet, environments, diagenesis, and dating of archeological and paleontological samples. Palaeogeogr. Palaeoclimatol. Palaeoecol. 416, 4–16. ( 10.1016/j.palaeo.2014.07.038) [DOI] [Google Scholar]
- 39.Trueman CN. 2013. Chemical taphonomy of biomineralized tissues. Palaeontology 56, 475–486. ( 10.1111/pala.12041) [DOI] [Google Scholar]
- 40.Lécuyer C, Reynard B, Grandjean P. 2004. Rare earth element evolution of Phanerozoic seawater recorded in biogenic apatites. Chem. Geol. 204, 63–102. ( 10.1016/j.chemgeo.2003.11.003) [DOI] [Google Scholar]
- 41.DePaolo DJ. 2004. Calcium isotopic variations produced by biological, kinetic, radiogenic and nucleosynthetic processes. Rev. Mineral. Geochem. 55, 255–288. ( 10.2138/gsrmg.55.1.255) [DOI] [Google Scholar]
- 42.Gussone N, Tipper E, Schmitt A, Alexander H, Frank W, Martin D, Schiller M. 2016. Calcium stable isotope geochemistry. Berlin, Germany: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset table compiling all elemental concentrations and isotopic ratio measurements is available as the electronic supplementary material.