Abstract
Abiotic conditions have long been considered essential in structuring freshwater macroinvertebrate communities. Ecological drift, dispersal and biotic interactions also structure communities, and although these mechanisms are more difficult to detect, they may be of equal importance in natural communities. Here, we hypothesized that in 10 naturally replicated headwater streams in eastern Switzerland, locally dominant amphipod species would be associated with differences in environmental conditions. We conducted repeated surveys of amphipods and used a hierarchical joint species distribution model to assess the influence of different drivers on species co-occurrences. The species had unique environmental requirements, but a distinct spatial structure in their distributions was unrelated to habitat. Species co-occurred much less frequently than predicted by the model, which was surprising because laboratory and field evidence suggests they are capable of coexisting in equal densities. We suggest that niche preemption may limit their distribution and that a blocking effect related to the specific linear configuration of streams determines which species colonizes and dominates a given stream catchment, thus suggesting a new solution a long-standing conundrum in freshwater ecology.
Keywords: amphipod, aquatic ecology, community assembly, competition, metacommunity, species distributions
It is reasonable to suppose that the fundamental niches of the two species overlap, but that within the overlap [the amphipod Gammarus] pulex is successful, while [the amphipod G.] duebeni with a greater tolerance of salinity has a refuge in brackish water … This case is as clear as one could want except that Hynes is unable to explain the absence of G. duebeni from various uninhabited favorable localities in the Isle of Man and elsewhere … These disconcerting empty spaces in the distribution of Gammarus may raise doubts as to the completeness of the picture.
— G. Evelyn Hutchinson, 1957 [1]
1. Introduction
A central goal of ecology is to understand the factors determining the distribution of species, and the mechanisms of how these species are structured into communities. For instance, species distribution models based on environmental variables are commonly used to characterize species' niches—the set of abiotic and biotic conditions in which a species can survive and reproduce [1]—and then to predict where they should be found. However, other processes are also important in determining species distributions, such as dispersal [2–4], interspecific interactions like competition [5] and processes like order of arrival or ‘priority effects’ [6–8]. Particularly at the local scale, these processes may in effect prevent the coexistence of species that are otherwise similarly suited to environmental conditions [9], and which do coexist at broader spatial scales. Compared to environmental variables, factors like order of arrival and dispersal limitation are not easy to detect or quantify in observational data, and so far have been largely neglected in species distribution models despite widespread recognition of their importance [10,11].
Amphipods are one of the examples of organisms G. Evelyn Hutchinson used in his seminal 1957 remarks positing the factors shaping species coexistence (see epigraph). While the examples on plankton or plant community coexistence are much more widely referred to, the relatively species-poor family of Gammaridae amphipods (Crustacea: Amphipoda) are an enigmatic case because the individual species are highly similar to one another ecologically, for instance using the same food resources, and are speculated to fill the same niches while also predating on one another (as well as members of their own species) [12]. Furthermore, in regions such as Europe and parts of Eurasia, they are the most dominant and important decomposers in freshwater ecosystems, thus playing a key role in ecosystems and food webs. In general, dominant species in a given community can structure communities and play an essential role in determining ecosystem function [13]. This is also true for amphipods, with greater dominance by the common central European species Gammarus fossarum associated with higher decomposition rates in streams [14]. Because of such ecosystem-level effects, the distribution and potential coexistence of amphipod species are of particular interest. As noted by Hutchinson [1] and others (for instance, [15] called the amphipod species distributions a ‘problem’), mechanisms behind both these species' commonly observed coexistence, but also the equally common apparent exclusion of one by another, need clarification given that the species' niches are assumed to be so similar.
In general, when a new species arrives from the regional species pool, there are three relevant outcomes in a community, assuming that the species' abiotic requirements are met: (1) the new species cannot establish in the community; (2) it establishes and coexists with the other species; or (3) it establishes and replaces the previously dominant species. The first case can occur when the species are functionally similar and niche space is not wide enough for both to coexist (niche preemption), or even when they are dissimilar but the previously established species has modified and erased the niche of the new species; such conditions result in priority effects [16]. The second case can occur when species have different niches, or among competitors with similar niches when there are spatial storage effects due to environmental or temporal heterogeneity at a given scale [17]. The third case, meanwhile, is typical but not exclusive to invasive species. These cases illustrate that even when there is the opportunity for multiple functionally similar species to coexist, they not always do so. Furthermore, the ‘final’ outcome of species interactions after a new species' arrival depends on the temporal and spatial scale being considered. Species turnover due to competitive exclusion often occurs very slowly [18], thus coexistence in the short term may lead to species replacement (succession) over a longer time frame.
To identify the mechanisms governing the distribution and coexistence of freshwater amphipods, we surveyed 121 stream reaches distributed in 10 headwater stream catchments in eastern Switzerland, sampling throughout the network topology of main and side stems, and capturing temporal dynamics by visiting each stream seasonally for 1 year. Previous work indicated that five amphipod species were present in the downstream lake, and three of these species consistently occupy the tributary catchments [19]. These omnivorous species are comparable in size and functionally similar. They can move kilometres or tens of kilometres per year when expanding their ranges [20,21]. By comparison, the total stream length in our studied headwater catchments ranged from 2.8 to 4.8 km. No genetic differentiation has been observed in this region's native freshwater amphipods at this spatial scale [19], and patterns of population genetic differences of the same species in Germany did not indicate dispersal limitation but rather colonization history and subsequent genetic drift [22]. While there is genetic isolation by distance at larger scales [23], we thus assumed that the distribution of these different species across our comparatively small study catchments should be driven only by niche differences with respect to abiotic conditions, biotic interactions, and/or stochastic processes, rather than dispersal limitation. We also expected that we would find multiple species coexisting in at least some locations, either as a result of equalizing or stabilizing mechanisms, or as a transient state before eventual competitive exclusion. We hypothesized that:
(a) species richness would be invariable throughout the sampling region, but with different species or combinations of species in different locations comprising this diversity; or,
(b) species richness would be higher at downstream points near the lake outlet. All of the species previously found in the streams are present in the lake, so we conceptualize the lake as a regional species pool. We would expect such a pattern of species richness both because downstream points are closest to the regional species pool, and because of characteristic diversity patterns found in river networks [24]; and,
(c) species would have individual niches and habitat preferences. This has been demonstrated for amphipod species in lakes [25] and larger rivers [26], and we expected that this niche partitioning would explain why species were found in different locations. Such environmental requirements would lead to coexistence in complex habitats and/or to spatial segregation of species into non-overlapping patches within a catchment.
2. Material and methods
(a). Study location and sampling sites
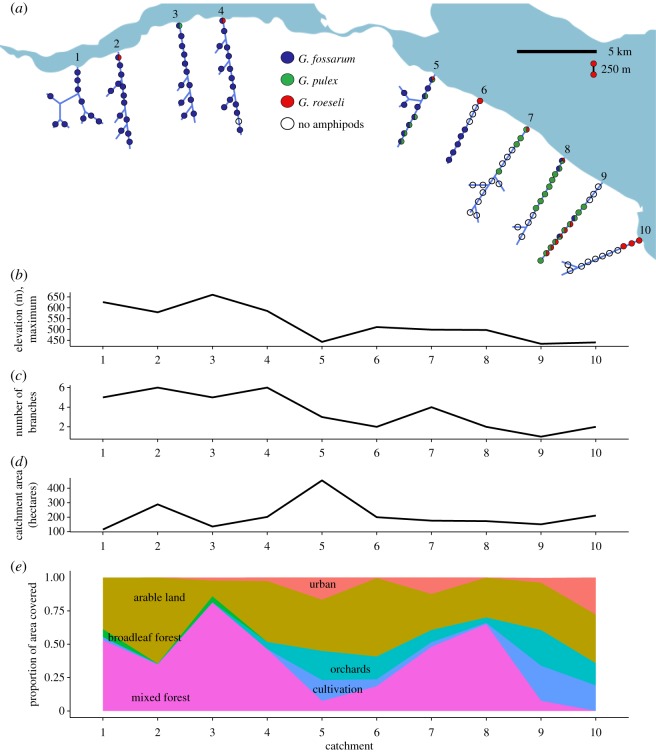
We studied 10 naturally replicated catchments in eastern Switzerland, with the headwater streams between 2.75 and 5 km in total length (including main and side stems) and running into Lake Constance (catchment sizes 115 to 453 ha). Four streams were located in the less-developed, steeper ‘Untersee’ region to the west, and six were located in the more heavily agricultural, flatter ‘Obersee’ region to the east (figure 1a). Catchments had varying land use from primarily mixed deciduous and coniferous forest to primarily agriculture, with pockets of higher density housing or industrial uses (figure 1e). In each catchment, streams were divided into 250-m segments along the main stem. Side stems less than 450 m in total length were counted as single segments, while side stems greater than this length were divided into 250-m segments beginning from the confluence with the main stem. A sampling point was established within each segment in a reach with representative habitat and stream flow, and sampling points in different segments were placed as equidistantly as possible. This resulted in a range of nine to 15 sampling points per catchment, and 121 sampling points in total.
Figure 1.
(a) Simplified diagram of 121 sampling points along the branches of 10 headwater stream catchments of Lake Constance in eastern Switzerland. Sampling points are separated by, on average, 250 m. Colours of sampling points represent whether each species was ever found at the point over a year of sampling effort; divided circles show that multiple species were found at that point. Catchments varied by (b) maximum elevation (and thus stream slope), (c) branching structure/network complexity, (d) catchment area and (e) land use.
(b). Data collection
Sampling points were visited four times at roughly three-month intervals, between April/May 2015 and January 2016. Repeat sampling points were within 10 m of points at the same location. We measured:
(1) Substrate and habitat characterization. Substrate type and complexity have previously been shown to explain local distributions of amphipods [26–28]. Thus, we measured the width of the active channel and classified habitat inside a 1 m long section using a 1 × 1 m sampling frame with 0.2 × 0.2 m gridlines. The number of grid squares comprising different substrate types was estimated visually, as impermeable surface (bedrock, solidly calcified benthic material or concrete), rocks greater than 20 cm in diameter, gravel 2.5–20 cm diameter, fine gravel less than 2.5 cm diameter, sand, mud or clay. A separate visual estimation was made of the number of grid squares covered by dead leaves, living terrestrial and large aquatic plants, roots and woody debris or moss and algae, and allowed for the layered structure of microhabitats such that the area covered by these components could sum to greater than the two-dimensional area of the stream section.
(2) Water chemistry. A water sample was collected from each sample point and, in the laboratory, measured for total phosphorus with a spectrophotometer (Varian Cary 50 Bio, Palo Alto, California, USA), and total nitrogen, dissolved organic carbon (DOC), and total organic carbon (TOC), all with a TOC analyser (Shimadzu TOC-L, Kyoto, Japan). Further variation in water chemistry was inferred to be captured indirectly through differences in land use [29], described below.
(3) Amphipod abundance and identity. After leaf collection, kicknetting was performed across the width of the stream section. Sampling effort was equal per metre of stream width, so that the total time spent kicknetting was greater in wider stream segments, and each habitat and substrate type was included in the sampling. Abundance of amphipods was estimated by order of magnitude: 0, 1–10, 10–100, 100–1000 or greater than 1000. From each sample up to approximately 40 amphipods were collected and preserved in ethanol for subsequent identification in the laboratory; individuals were chosen to represent the range of sizes present in the sample, but not including those that were too small to reliably identify to species based on morphological characters. Depending on stream temperature, the common amphipod species in this area may live 1–3 years and reach sexual maturity at six months [30]; thus we assumed that the smallest juveniles from the spring and summer sampling visits could be counted/identified as medium-sized individuals in subsequent sampling visits if they survived.
(c). Land-use data
Amphipod species distributions have previously been associated with ammonia concentration [31], which in turn is associated with agricultural run-off in our study region [32]. Thus, this and other important factors for amphipod distributions such as riparian vegetation degradation [33] and pH and dissolved oxygen [34] were assessed indirectly through land use type, integrating various unmeasured factors. All spatial analysis was done using ArcGIS 10.2.2 (ESRI, Redlands, California, USA). Spatial information about streams was extracted from the Swiss national 1 : 25 000 scale water network and digital elevation models ([35] 2003, [35] 2007). We calculated elevation of each sampling point, latitude/longitude, and its upstream distance from the outlet on Lake Constance.
Land cover within the catchments was classified using a combination of data sources. We used as the basis the CORINE land cover (2012) land-use classification [36], produced from Indian Remote Sensing (IRS) P6 LISS III and RapidEye imagery with a Minimal Mapping Unit of 25 hectares and positional accuracy of, at a minimum, 100 m. To add specificity to CORINE's agricultural classification and because orchards often have higher herbicide application, we added the area of vine and orchard fruit cultivation from a 1 : 25 000 scale vector map ([35], 2010), resulting in nine categories: discontinuous urban fabric, industrial or commercial units, non-irrigated arable land, complex cultivation patterns, fruit orchards and vine cultivation, broad-leaved forest, mixed forest, inland marshes and water bodies. The area of land falling into each land use category was calculated for each study catchment in total, as well as for a 50-m radius area at each sampling point.
(d). Statistical analysis
All statistical analyses were performed in R v. 3.3.2 [37]. The presence or absence of amphipod species was examined using the ‘HMSC’ Bayesian joint species distribution model (JDSM) package [38]. This framework incorporates aspects of traditional species distribution models by estimating the association between species and environmental and/or spatial variables, but implements the model for multiple species concurrently, which allows the residual variation from the environmental factors to be used to detect associations between species that are not driven only by shared environmental preferences ([38]; also other JDSM's, e.g. [39]). We incorporated environmental covariates and the sampling structure, with catchment and sampling point as random factors representing spatial context. We used default priors and modelled species occurrences using the Bernoulli distribution and a probit link function (additional information on model specification in electronic supplementary information, appendix SI). MCMC chains were run to 100 000 iterations, with a burn-in period of 1000 iterations and subsequently thinned to include only every 100th sample of the posterior distributions.
We primarily compared two models. The first included only the spatial random effects (S). The second included three types of factors: spatial random effects, prior amphipod occurrence and environmental covariates (SPE). The environmental covariates included those described previously: substrate and habitat information, water chemistry, latitude, elevation, distance from the stream outlet and land use at the point and catchment level. Because at the first sampling time point, there was no prior presence–absence information, these two models were made using data only from the second through fourth sampling time points. For comparison purposes, we repeated the model with random effects plus environmental factors with the complete dataset of all four time points, which necessitated excluding information about prior amphipod occurrence (SEFull). Finally, we ran two additional models using only the second through fourth time points—one with spatial random effects plus the prior presence of amphipod species (SP), and another with the random effects plus all other environmental covariates described above (SE)—the results of which are presented in electronic supplementary information, appendix SI.
Overall model fit was assessed using Tjur's R2 [40], the difference between the mean fitted value of sampling units where species are present and the mean fitted values where species are absent. Importance of environmental covariates was assessed in two ways: whether the covariate had a significant effect, and what proportion of variance it actually explained. First, parameter estimates of the association between environmental covariates and presence/absence of individual species were extracted as 95% central credible intervals. Where this interval did not overlap with zero, the covariate was deemed to have a strong directional association with the species. Secondly, the explained variance in presence/absence of each species was partitioned among all explanatory variables, which were grouped for presentation into broad categories, as well as to random effects at both sampling scales (catchment and sample point).
Finally, we assessed the potential co-occurrence of species, or ‘hypothetical species association patterns' [41], by extracting the residual correlations between species from the latent part of the model framework. A positive residual correlation indicates that species occur together more frequently than would be predicted by their calculated niches, while a negative residual correlation indicates that their niches would predict them to co-occur more frequently than they do in practice. These putative species associations are depicted by the median value of posterior samples.
3. Results
(a). Spatial and temporal patterns in distribution
Our sampling revealed a pronounced spatial pattern in species distributions, with Gammarus fossarum the only species present upstream of outlets in the western catchments and three different species (G. fossarum, G. pulex, and G. roeseli) present in eastern catchments, but rarely coexisting (figure 1). Of these three, G. fossarum and G. pulex are native species while G. roeseli is non-native but considered naturalized because it arrived in the 1800s. Across the whole study region and sampling year, mean species richness at outlet points was 1.25 species (range 0–3), and at non-outlet points was 0.69 species (range 0–2). No non-outlet point ever had three species present. We concluded that outlets were more representative of the lake's species pool of five to six species [19,42] than of stream communities, and excluded outlet points from subsequent analyses.
Site occupancy was fairly stable through time, with no change in species composition at a sampling point in 78% of the possible transitions from one time point to the next. There were few changes from single-species to multi-species occupancy (3% of possible transitions) or vice versa (2% of possible transitions). There was also one change from a point being occupied by one to a different species (0.3% of transitions; figure 2). The most common change at the sampling point level was from being occupied to being unoccupied (11% of possible transitions), in large part due to seasonal drying of some stream reaches. Few (5/17) of these dried stream reaches were reoccupied, and in all of these cases they were reoccupied by the same species which had been present before the drying event. In one of the five cases, an additional species co-established at the re-wetted stream reach. Overall, this shows that there is nearly zero turnover in species dominance amongst occupied sampling points and little chance for ‘new’ species to establish after disturbance has rendered some patches unoccupied.
Figure 2.
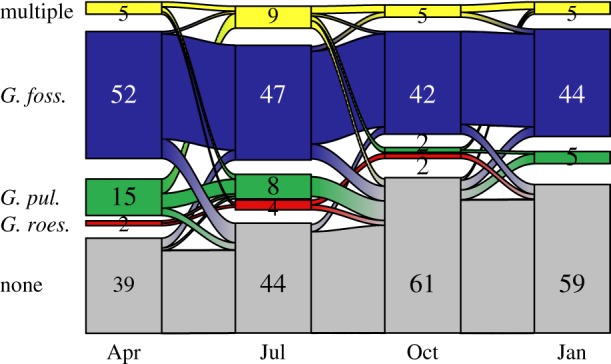
Amphipod occupancy and non-occupancy at the non-outlet sampling sites over a year of visits. Five different possible states of site occupancy are defined as follows: no amphipods (zero occupancy), occupancy by one of each one of the three amphipod species separately (G. roeseli, G. fossarum or G. pulex), or co-occupancy by multiple species. The thickness of curved line flows connecting boxes from one sampling visit to the next is scaled to the number of state transitions which occurred over the given sampling interval. At the final sampling visit, no sites were occupied solely by G. roeseli, thus this state disappears from the chart.
(b). Comparing joint species distribution models
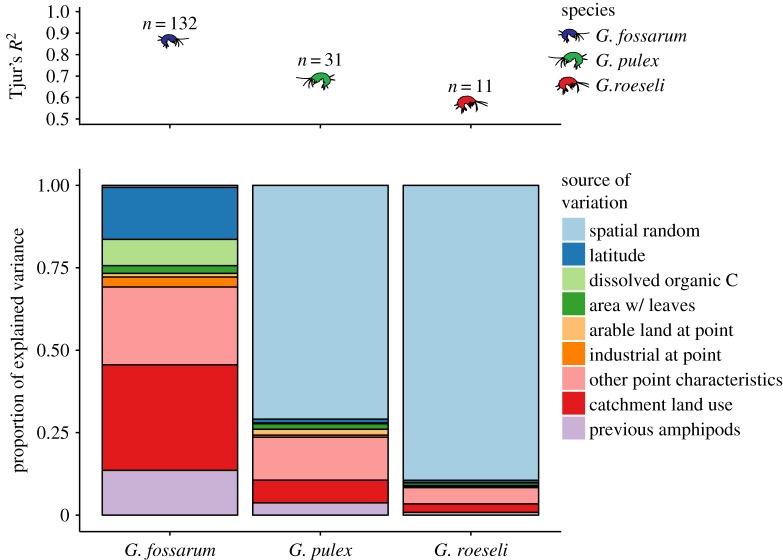
The spatial arrangement of the sampling points was important in explaining the presence and absence of different species through several different metrics. The S model using only the random effects of catchment and sampling point for the second through fourth time points (n = 390) explained 51% of the variation in species presence and absence. The full ‘SPE’ model (n = 237) explained 71% of variation. The models with either prior amphipod occurrence or environmental covariates separately had intermediate model fits (electronic supplementary information, appendix SI). By comparison, the SEFull model across all sampling time points but using only spatial arrangement and environmental covariates (n = 367) explained 64% of variation. This suggests that both environmental data and data about species distributions at prior sampling times are important and do not convey the same information.
(c). Abiotic influences on species distribution
In the SPE model, only a few variables had strong directional effects (defined using the 95% central credible interval of the posterior distribution of the association) on the presence or absence of amphipod species (electronic supplementary material, table S1). Despite their strong directional effects, these variables did not necessarily account for a large proportion of the variance in occurrence patterns (figure 3); for instance, the association between the area of substrate covered by leaves and the occurrence of G. pulex accounted for only 1.7% of the explained variation in the species' occurrence. For G. pulex, the area of the streambed covered by leaf litter, the proportion of area surrounding a point made up of arable land, and the previous presence of G. pulex were strong predictors. For G. fossarum, latitude, the proportion of catchment area covered by orchards, the DOC in the water, and the previous presence of G. fossarum were strong predictors. And for G. roeseli, the proportion of area surrounding a point used for industrial or commercial purposes and the previous presence of G. pulex were strong predictors. Other important factors in the SEFull model, such as the association between G. fossarum and previous drying or the area of moss and algae on the substrate, no longer had strong directional effects when both environment and previous species occurrence information were integrated into the same model (electronic supplementary material, table S1). Although other factors measured at the sampling point or catchment level did not have strongly directional effects, they nevertheless contributed greatly to explaining the variation in species occurrences when a variance partitioning was conducted on the SPE model (figure 3). For example, land use in the catchment accounted for 32% of the explained variation in the occurrence of G. fossarum, and 6% of the explained variation in G. pulex; while variables measured at the point level which did not have strong directional effects nevertheless combined to account for 24% of the explained variation in the occurrence of G. fossarum, 13% of explained variation in G. pulex, and 5% of the explained variation in G. roeseli. The density of posterior distributions of all associations between measured variables and species occurrences are presented in electronic supplementary material, figure S1.
Figure 3.
(a) The amount of variance in species occurrences (Tjur's R2) explained by model components for each species individually, and the number of times each species was detected in the field sampling. (b) Variance partitioning of factors used in the SPE model (spatial random effects, prior amphipod occurrence, environmental covariates) in relation to presence/absence of each species found in the study. Stacked bars show the proportion of the total explained variance, indicated in panel (a) for each species; overall, 71% of total variance in the dataset was explained by the model.
(d). Co-occurrence of amphipod species
After accounting for these factors, putative species associations between different amphipod species remained in the SPE model: weak positive correlations at the catchment level, and strong positive and negative correlations at the sampling point level (figure 4). At the sampling point level, G. fossarum rarely co-occurred with either of the other species despite somewhat similar habitat requirements (median residual correlation = −0.74 to G. pulex and −0.75 to G. roeseli). Conversely, G. pulex and G. roeseli co-occurred much more frequently (median residual correlation = 0.99) than would have been predicted either by random chance or based on their individual habitat requirements. At the catchment level, pairs of species co-occurred slightly more frequently (residual correlations of 0.10–0.25) than would have been predicted either by random chance or by the niches constructed based on our measured factors (spatial arrangement, previous species occurrence and environmental covariates).
Figure 4.
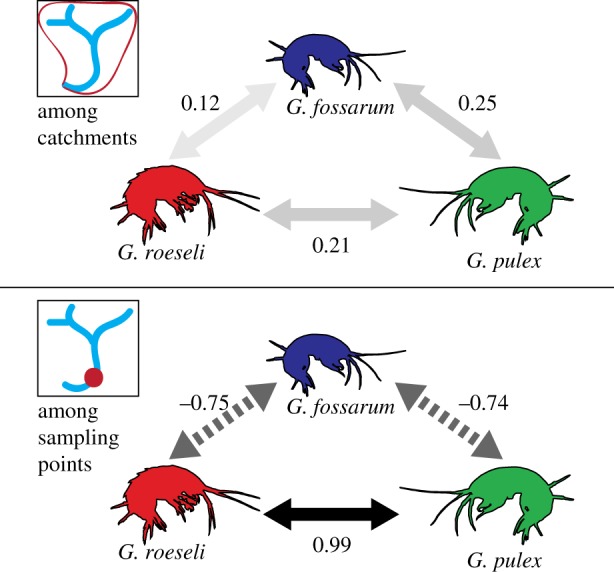
Residual associations in occurrence between different species at the catchment and sampling point level, after the influence of spatial relationships, environmental covariates and previous amphipod site occupancy have been taken into account by the SPE model. Arrows are coloured by the strength of the association; positive associations (represented by solid lines) indicate that species co-occur more frequently than would be predicted by covariates, while negative associations (represented by dashed lines) indicate that species co-occur less frequently than predicted. (Online version in colour.)
4. Discussion
It has been commonly observed that species do not always coexist where might be expected. We examined the distribution of three locally dominant and occasionally co-occurring amphipod species in order to disentangle the ecological processes behind their occupancy patterns in stream catchments. As expected, environmental factors explained part of the variation in these species’ distribution between and within catchments. Overall, however, individual environmental factors rarely had strongly positive or negative effects on species occurrences, and a large amount of variance remained unexplained by environmental variables, as is commonly found [43,44]. While we are confident that we included the most important variables in our analysis, we cannot completely exclude that an unmeasured variable that is not correlated to any of the included variables could define a niche axis along which the species segregate. We think, however, this to be unlikely, as our variable choice is based on extensive existing knowledge of relevant variables [25–28]. Importantly, these three species are not dispersal limited at the scale of our studied headwater stream catchments [19], ruling out another common mechanism shaping community composition.
Using a joint species distribution modelling approach, we show that this unexplained variance can be assigned to putative species interactions. While much experimental work on community assembly and species interactions has been done in plant communities—where individuals are immobile, order of arrival can be easily manipulated and neighbours may be removed from a community (e.g. [45])—this is more challenging when working with animals in flowing-water systems. We used an analytical approach which allowed us to infer species interactions from observational data [38,41] without performing manipulations. In past studies, competition has been assumed to be the primary species interaction shaping amphipod communities: for example, G. pulex rarely co-occurred with another sympatric species, G. duebeni, in rivers in France, and this was attributed to hypothesized strong competition between the two species [15]. However, in no amphipod distribution study that we are aware of has competition been directly measured or indirectly inferred. Our results now suggest a more nuanced role of competition.
After accounting for important environmental factors we found strong negative species interactions, but because different species are dominant in different catchments, we ruled out that one species has an absolute fitness and competitive advantage over the others; if this was true, the same species should have dominated all of our catchments. Which species dominated which catchment was also not satisfactorily explained by environmental variables alone, suggesting that the identity of the ‘winner’ is also not deterministically driven by niche differences. This we conclude because at the catchment scale species coexisted more frequently than expected based on environmental factors. In particular, in two catchments where multiple species co-occurred throughout the length of the stream, they did so at roughly equal densities over the course of the entire study period (electronic supplementary material, table S2). These two catchments were not particularly close to each other and had different land use (figure 1) and habitat characteristics (electronic supplementary material, Data), making it improbable that some particular abiotic variable promoted coexistence. The ability of the species to coexist was also found in laboratory experiments, where G. fossarum and G. roeseli each had equal (approx. 90%) survival over short-term experiments, regardless of whether maintained separately or together in mesocosms at equal densities [46]. This rules out strong, density-independent competition between the species, to the degree that would cause competitive exclusion when the species are at similar densities. And yet most strikingly, at the scale of sampling reaches we found a putative negative species association between the two most common species in the region, G. fossarum and G. pulex. Indeed, classic studies in France [15,27] and Britain [47] found that derived environmental preferences (i.e. niche differences) were insufficient in explaining the distribution of freshwater amphipods. Instead, in our data the previous occurrence of the same species at a sampling point was the only strong positive predictor of the occurrence of G. fossarum and G. pulex. Thus, it is clear that coexistence of species depends on scale [17], and we indeed saw coexistence at the catchment but only rarely at the reach scale.
Thus, what is the source of these differing patterns of coexistence, and how are strong negative association between the two most common species shaped? Neither a pure environmental filtering nor competitive exclusion perspective offers convincing explanations in our analysis. Alternatively, priority effects are thought to be common in various ecosystems [8,48,49]. They are, however, generally difficult to quantify through observational study because the history of community assembly is rarely known [16]. Several patch characteristics are associated with promoting priority effects among functionally similar species. These mechanisms typically allow early-arriving species to quickly grow to large population sizes: for instance, small patch size and a stable environment with high resource supplies and/or lack of predation [16]. In linear habitat networks such as streams, which are surrounded by an unsuitable (terrestrial) habitat matrix and where each habitat patch (stream reach) is connected to only a very small number of other patches, priority effects may play an outsized role due to spatial blocking. Notably, after a large-scale disturbance, purely aquatic animals primarily colonize stream networks from the outlet up. Thus, if a species first colonizes a stream reach near the outlets, this species encounters low resistance while dispersing further upstream and may quickly rise to high densities in these patches as well. Conversely, it may become very difficult for another newly arriving species to pass through these initial downstream habitat patches en route to suitable (potentially even empty) upstream reaches, once a prior species is present. Distributions in an overlapping set of streams, measured at a coarser scale, also showed little change over 2 years [19], however we assume that after events such as heavy pesticide application to surrounding farm fields, species turnover in a catchment could occur if the disturbance extended downstream and provided access from the regional species pool. Priority effects have been invoked to explain macroinvertebrate community composition in individual reaches [50,51], but as far as we are aware, the role of priority effects in excluding species at the catchment or network level has not yet been investigated in natural riverine systems.
There are further mechanisms supporting/consistent with the role of priority effects in structuring these amphipod communities. First, intraguild predation is thought to favour priority-effects shaping community structure (e.g. [52]). And indeed, intraguild predation is common in various Gammarus species pairs, often at a stronger intensity by one species than the other [12]. Secondly, mate limitation may also prevent new species from moving into a catchment dominated by a single other species, and is a characteristic destabilizing mechanism which can lead to priority effects [9]. Gammarus species have been shown to have varying abilities to differentiate between potential mates of different species [53,54]. Some form interspecific copulatory pairs even when mates of their own species are available, and no viable offspring can be produced [53].
5. Conclusion
We found that although part of the variation in the distribution of G. fossarum could be explained by environmental measures, multiple species rarely coexisted, even in reaches that would seem to be suitable for more than one species. This leads to a classic problem: despite knowledge of environmental conditions, it can be difficult to predict where a given species will be found if other factors are preventing it from occupying all suitable niche space [1]. Competition is often invoked as a probable cause for one species to exclude another, yet here, competing species can coexist in some circumstances, but not others. Order of arrival may be the key to understanding these different outcomes. Furthermore, river networks represent a unique spatial setting for such considerations [24], because colonization by aquatic organisms is in many cases directional (downstream to upstream or vice versa). For example, established dominant species in downstream reaches have a head start towards colonizing empty upstream patches and may prevent newly arriving species from passing through occupied habitat patches to reach empty ones. While most studies of historical contingency, community assembly and priority effects have used plant communities, we show that priority effects may be important in freshwater ecosystems as well, due in part to their specific spatial structure.
Supplementary Material
Supplementary Material
Acknowledgements
The authors thank the Kanton Thurgau Office of the Environment and all landowners whose property we crossed. We thank Pravin Ganesanandamoorthy, Elvira Mächler, Simon Flückiger and Katharina Kaelin for help with fieldwork, and the Eawag AuA laboratory for chemical analysis of water samples. Tadashi Fukami, Marcel Holyoak, Brad Taylor, and Hanna Kokko provided helpful feedback on the manuscript. We thank Victor Saito and one anonymous reviewer for their comments.
Data accessibility
The data for this study are archived in the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.75jq1) [55].
Authors' contributions
C.J.L. and F.A. jointly developed the field sampling protocol and locations. C.J.L. performed the fieldwork, analysed the data, and wrote the initial draft of the manuscript. C.J.L. and F.A. both contributed to revisions.
Competing interests
We declare we have no competing interests.
Funding
This project was funded by Swiss National Science Foundation grant no. PP00P3_150698.
References
- 1.Hutchinson GE. 1957. Concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 22, 415–427. ( 10.1101/SQB.1957.022.01.039) [DOI] [Google Scholar]
- 2.MacArthur RH, Wilson EO. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Hubbell SP. 2001. The unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Leibold MA, et al. 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett. 7, 601–613. ( 10.1111/j.1461-0248.2004.00608.x) [DOI] [Google Scholar]
- 5.Wisz MS, et al. 2013. The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biol. Rev. 88, 15–30. ( 10.1111/j.1469-185X.2012.00235.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake JA. 1991. Community-assembly mechanics and the structure of an experimental species ensemble. Am. Nat. 137, 1–26. ( 10.1086/285143) [DOI] [Google Scholar]
- 7.Chase JM. 2003. Community assembly: when should history matter? Oecologia 136, 489–498. ( 10.1007/s00442-003-1311-7) [DOI] [PubMed] [Google Scholar]
- 8.De Meester L, Vanoverbeke J, Kilsdonk LJ, & Urban MC. 2016. Evolving perspectives on monopolization and priority effects. Trends Ecol. Evol. 31, 136–146. ( 10.1016/j.tree.2015.12.009) [DOI] [PubMed] [Google Scholar]
- 9.Fukami T, Mordecai EA, Ostling A. 2016. A framework for priority effects. J. Veg. Sci. 27, 655–657. ( 10.1111/jvs.12434) [DOI] [Google Scholar]
- 10.Guisan A, Thuiller W. 2005. Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 8, 993–1009. ( 10.1111/j.1461-0248.2005.00792.x) [DOI] [PubMed] [Google Scholar]
- 11.D'Amen M, Rahbek C, Zimmermann NE, Guisan A. 2017. Spatial predictions at the community level: from current approaches to future frameworks. Biol. Rev. 92, 169–187. ( 10.1111/brv.12222) [DOI] [PubMed] [Google Scholar]
- 12.MacNeil C, Dick JTA, Elwood RW. 1997. The trophic ecology of freshwater Gammarus spp. (Crustacea: Amphipoda): problems and perspectives concerning the functional feeding group concept. Biol. Rev. 72, 349–364. ( 10.1017/S0006323196005038) [DOI] [Google Scholar]
- 13.Hillebrand H, Bennett DM, Cadotte MW. 2008. Consequences of dominance: a review of evenness effects on local and regional ecosystem processes. Ecology 89, 1510–1520. ( 10.1890/07-1053.1) [DOI] [PubMed] [Google Scholar]
- 14.Dangles O, Malmqvist B. 2004. Species richness–decomposition relationships depend on species dominance. Ecol. Lett. 7, 395–402. ( 10.1111/j.1461-0248.2004.00591.x) [DOI] [Google Scholar]
- 15.Pinkster S, Dennert DB, Stock B, Stock JH. 1970. The problem of European freshwater populations of Gammarus duebeni Liljeborg, 1852. Bijdr. tot Dierkd. 40, 116–147. [Google Scholar]
- 16.Fukami T. 2015. Historical contingency in community assembly: integrating niches, species pools, and priority effects. Annu. Rev. Ecol. Evol. Syst. 46, 1–23. ( 10.1146/annurev-ecolsys-110411-160340) [DOI] [Google Scholar]
- 17.Hart SP, Usinowicz J, Levine JM. 2017. The spatial scales of species coexistence. Nat. Ecol. Evol. 1, 1066–1073. ( 10.1038/s41559-017-0230-7) [DOI] [PubMed] [Google Scholar]
- 18.Yackulic CB. 2017. Competitive exclusion over broad spatial extents is a slow process: evidence and implications for species distribution modeling. Ecography (Cop.) 40, 305–313. ( 10.1111/ecog.02836) [DOI] [Google Scholar]
- 19.Altermatt F, Alther R, Mächler E. 2016. Spatial patterns of genetic diversity, community composition and occurrence of native and non-native amphipods in naturally replicated tributary streams. BMC Ecol. 16, 23 ( 10.1186/s12898-016-0079-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dick JTA, Elwood RW, Montgomery WI. 1994. Range expansion of the alien freshwater amphipod Gammarus pulex (L.) in the River Lagan, Co. Down. Irish Nat. J. 24, 403–404. [Google Scholar]
- 21.Bollache L, Devin S, Wattier R, Chovet M, Beisel J-N, Moreteau J-C, Rigaud T. 2004. Rapid range extension of the Ponto-Caspian amphipod Dikerogammarus villosus in France: potential consequences. Arch. für Hydrobiol. 160, 57–66. ( 10.1127/0003-9136/2004/0160-0057) [DOI] [Google Scholar]
- 22.Weiss M, Leese F. 2016. Widely distributed and regionally isolated! Drivers of genetic structure in Gammarus fossarum in a human-impacted landscape. BMC Evol. Biol. 16, 1–14. ( 10.1186/s12862-016-0723-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.AM Westram, Jokela J, Keller I. 2013. Hidden biodiversity in an ecologically important freshwater amphipod: differences in genetic structure between two cryptic species. Plos One 8:e69576 ( 10.1371/journal.pone.0069576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altermatt F. 2013. Diversity in riverine metacommunities: a network perspective. Aquat. Ecol. 47, 365–377. ( 10.1007/s10452-013-9450-3) [DOI] [Google Scholar]
- 25.Hesselschwerdt J, Necker J, Wantzen KM. 2008. Gammarids in lake constance: habitat segregation between the invasive Dikerogammarus villosus and the indigenous Gammarus roeselii. Fundam. Appl. Limnol. 173, 177–186. ( 10.1127/1863-9135/2008/0173-0177) [DOI] [Google Scholar]
- 26.Kley A, Maier G. 2005. An example of niche partitioning between Dikerogammarus villosus and other invasive and native gammarids: a field study. J. Limnol. 64, 85–88. ( 10.4081/jlimnol.2005.85) [DOI] [Google Scholar]
- 27.Piscart C, Manach A, Copp GH, Marmonier P. 2007. Distribution and microhabitats of native and non-native gammarids (Amphipoda, Crustacea) in Brittany, with particular reference to the endangered endemic sub-species Gammarus duebeni celticus. J. Biogeogr. 34, 524–533. ( 10.1111/j.1365-2699.2006.01609.x) [DOI] [Google Scholar]
- 28.Eisenring M, Altermatt F, Westram AM, Jokela J. 2016. Habitat requirements and ecological niche of two cryptic amphipod species at landscape and local scales. Ecosphere 7, 1–13. ( 10.1002/ecs2.1319) [DOI] [Google Scholar]
- 29.Johnson L, Richards C, Host G, Arthur J. 1997. Landscape influences on water chemistry in Midwestern stream ecosystems. Freshw. Biol. 37, 193–208. ( 10.1046/j.1365-2427.1997.d01-539.x) [DOI] [Google Scholar]
- 30.Pöckl M, Webb BW, Sutcliffe DW. 2003. Life history and reproductive capacity of Gammarus fossarum and G. roeseli (Crustacea: Amphipoda) under naturally fluctuating water temperatures: a simulation study. Freshw. Biol. 48, 53–66. ( 10.1046/j.1365-2427.2003.00967.x) [DOI] [Google Scholar]
- 31.Gergs R, Schlag L, Rothhaupt KO. 2013. Different ammonia tolerances may facilitate spatial coexistence of Gammarus roeselii and the strong invader Dikerogammarus villosus. Biol. Invasions 15, 1783–1793. ( 10.1007/s10530-013-0408-0) [DOI] [Google Scholar]
- 32.Abbaspour KC, Yang J, Maximov I, Siber R, Bogner K, Mieleitner J, Zobrist J, Srinivasan R. et al. 2007. Modelling hydrology and water quality in the pre-alpine/alpine Thur watershed using SWAT. J. Hydrol., 333, 413–430. ( 10.1016/j.jhydrol.2006.09.014) [DOI] [Google Scholar]
- 33.Mauchart P, Bereczki C, Ortmann-Ajkai A, Csabai Z, Szivák I. 2014. Niche segregation between two closely similar gammarids (Peracarida, Amphipoda)—native vs. naturalized non-native species. Crustaceana 87, 1296–1314. ( 10.1163/15685403-00003355) [DOI] [Google Scholar]
- 34.Meijering MPD. 1991. Lack of oxygen and low pH as limiting factors for Gammarus in Hessian brooks and rivers. Hydrobiologia 223, 159–169. ( 10.1007/BF00047637) [DOI] [Google Scholar]
- 35.Swisstopo. 2003–2010 Vector 25, Gewässernetz, DHM 25. 5704 000 000, reproduced by permission of swisstopo/JA100119, Bundesamt für Landestopographie (Art.30 Geo IV).
- 36.Bossard M, Feranec J, Otahel J. 2000. The revised and supplemented CORINE land cover nomenclature. Copenhagen, Denmark: European Environment Agency. [Google Scholar]
- 37.R Core Team. 2016. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 38.Ovaskainen O, Tikhonov G, Norberg A, Blanchet FG, Duan L, Dunson D, Roslin T, Abrego N. et al. 2017. How to make more out of community data? A conceptual framework and its implementation as models and software. Ecol. Lett. 20, 561–576. ( 10.1111/ele.12757) [DOI] [PubMed] [Google Scholar]
- 39.Pollock LJ, Tingley R, Morris WK, Golding N, O'Hara RB, Parris KM, Vesk PA, McCarthy MA. et al. 2014. Understanding co-occurrence by modelling species simultaneously with a joint species distribution model (JSDM). Methods Ecol. Evol. 5, 397–406. ( 10.1111/2041-210X.12180) [DOI] [Google Scholar]
- 40.Tjur T. 2009. Coefficients of determination in logistic regression models—a new proposal The coefficient of discrimination. Am. Statitician 63, 366–372. ( 10.1198/tast.2009.08210) [DOI] [Google Scholar]
- 41.Aivelo T, Norberg A. 2018. Parasite–microbiota interactions potentially affect intestinal communities in wild mammals. J. Anim. Ecol. 18, 438–447. ( 10.1111/1365-2656.12708) [DOI] [PubMed] [Google Scholar]
- 42.Altermatt F, Alther R, Fišer C, Jokela J, Konec M, Küry D, Mächler E, Stucki P, Westram AM. et al. 2014. Diversity and distribution of freshwater amphipod species in Switzerland (Crustacea: Amphipoda). PLoS ONE 9, e110328 ( 10.1371/journal.pone.0110328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cottenie K. 2005. Integrating environmental and spatial processes in ecological community dynamics. Ecol. Lett. 8, 1175–1182. ( 10.1111/j.1461-0248.2005.00820.x) [DOI] [PubMed] [Google Scholar]
- 44.Heino J, et al. 2015. A comparative analysis reveals weak relationships between ecological factors and beta diversity of stream insect metacommunities at two spatial levels. Ecol. Evol. 5, 1235–1248. ( 10.1002/ece3.1439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choler P, Michalet R, Callaway RM. 2001. Facilitation and competition on gradients in alpine plant communities. Ecology 82, 3295–3308. ( 10.1890/0012-9658(2001)082%5B3295:FACOGI%5D2.0.CO;2) [DOI] [Google Scholar]
- 46.Little CJ, Altermatt F. In press Species turnover and invasion of dominant freshwater invertebrates alter biodiversity–ecosystem function relationship. Ecol. Monogr. ( 10.1002/ecm.1299) [DOI] [Google Scholar]
- 47.Hynes HBN. 1954. The ecology of Gammarus duebeni Lilljeborg and its occurrence in fresh water in western Britain. J. Anim. Ecol. 23, 38–84. ( 10.2307/1660) [DOI] [Google Scholar]
- 48.Alford RA, Wilbur HM. 1985. Priority effects in experimental pond communities: competition between Bufo and Rana. Ecology 66, 1097–1105. ( 10.2307/1939161) [DOI] [Google Scholar]
- 49.Almany GR. 2003. Priority effects in coral reef fish communities. Ecology 84, 1920–1935. ( 10.1890/0012-9658(2003)084%5B1920:PEICRF%5D2.0.CO;2) [DOI] [Google Scholar]
- 50.McAuliffe JR. 1984. Competition for space, disturbance, and the structure of a benthic stream community. Ecology 65, 894–908. ( 10.2307/1938063) [DOI] [Google Scholar]
- 51.Palmer MA, Allan JD, Butman CA. 1996. Dispersal as a regional process affecting the local dynamics of marine and stream benthic invertebrates. Trends Ecol. Evol. 11, 322–326. ( 10.1016/0169-5347(96)10038-0) [DOI] [PubMed] [Google Scholar]
- 52.Blaustein L, Margalit J. 1996. Priority effects in temporary pools: nature and outcome of mosquito larva–toad tadpole interactions depend on order of entrance. J. Anim. Ecol. 65, 77 ( 10.2307/5701) [DOI] [Google Scholar]
- 53.Kolding S. 1986. Interspecific competition for mates and habitat selection in five species of Gammarus (Amphipoda: Crustacea). Mar. Biol. 91, 491–495. ( 10.1007/BF00392600) [DOI] [Google Scholar]
- 54.Dick JTA, Elwood RW. 1992. Coexistence and exclusion among Gammarus species: behavioural avoidance of interspecific precopulation by male G. pulex (Amphipoda). Oikos 64, 541–547. ( 10.2307/3545173) [DOI] [Google Scholar]
- 55.Little CJ, Altermatt F. 2018. Data from: Do priority effects outweigh environmental filtering in a guild of dominant freshwater macroinvertebrates? Dryad Digital Repository. ( 10.5061/dryad.75jq1) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Little CJ, Altermatt F. 2018. Data from: Do priority effects outweigh environmental filtering in a guild of dominant freshwater macroinvertebrates? Dryad Digital Repository. ( 10.5061/dryad.75jq1) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data for this study are archived in the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.75jq1) [55].