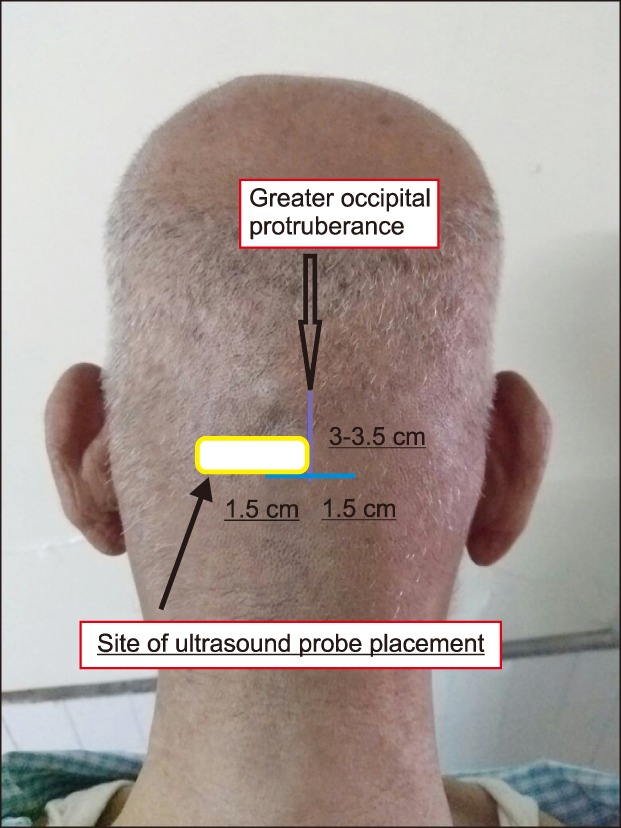
Abstract
The Epidural blood patch is considered the gold standard for managing postdural puncture headache when supportive measures fail. However, it is a procedure which can lead to another inadvertent dural puncture. Other potential adverse events that could occur during a blood patch are meningitis, neurological deficits, and unconsciousness. The bilateral greater occipital nerve block has been used for treating chronic headaches in patients with PDPH with a single injection. This minimally invasive, simple procedure can be considered for patients early, along with other supportive treatment, and an epidural blood patch can be avoided.
Keywords: Epidural blood patch, Greater occipital nerve, Nerve block, Pain management, Postdural puncture headache, Ultrasound
INTRODUCTION
Around 10–40% of patients who have a lumbar puncture for any indication (spinal anaesthesia, inadvertent dural puncture, diagnostic procedures, or intrathecal injections) experience headache [1]. The headache is referred to as a postdural puncture headache (PDPH) if it is persistent, is orthostatic (is aggravated by a standing position), is relieved by rest, and is fronto-occipital in location. Some patients experience diplopia, dizziness, and tinnitus [2].
PDPH not only increases the suffering of the patient, it also prolongs the duration of the hospital stay and adds to the overall cost of treatment. PDPH is initially managed conservatively with adequate hydration, analgesics (such as weak opioids), acetaminophen, non-steroidal anti-inflammatory agents, caffeine, laxatives, and bed rest [3].
When conservative measures fail to provide symptomatic relief, patients are offered an autologous epidural blood patch (EBP). EBP involves injection of 15–20 ml of autologous blood into the epidural space around the area of the dural puncture (DP). The clot formed by this injection seals off the dural tear, thereby preventing further cerebrospinal fluid (CSF) leak. An EBP has shown a success rate of around 70%–90% [4]. The patients who still suffers from headache after EBP are usually counselled to have another EBP.
MAIN BODY
1. Problems of an EBP
An EBP involves again identifying the epidural space with a Tuohy's needle. Theoretically, there are chances of another DP during this intervention. In such a situation, the magnitude of the problem doubles because now there is another tear which will also drain out CSF.
Undesirable complications like meningitis, arachnoiditis, seizures, loss of hearing or vision, radicular pain, and neural deficits have also been reported after an EBP [5].
Minimally invasive interventions to treat PDPH: A bilateral trans-nasal sphenopalatine ganglion block (SPGB) is a minimally invasive, bed-side intervention which can be offered to patients with PDPH. The SPG is a parasympathetic ganglion located superficially in the pterygopalatine fossa. A swab stick dipped local anesthetic is introduced trans-nasally, bilaterally, with the neck in extension. It can be left at the site where resistance is encountered for 15–20 minutes [6]. Pain physicians perform this block using a fluoroscope and radio-opaque contrast medium using different approaches, such as the trans-oral, sub-zygomatic and lateral infratemporal [7]. But the trans-nasal SPGB does not need all this. Although the evidence of its efficacy is scarce, patients have benefitted from this intervention and an EBP was eventually deferred.
Another minimally invasive peripheral nerve block which has been used quite successfully is a bilateral greater occipital nerve block (GONB). The GONB has been in use for more than a decade to treat complex headache syndromes of varying etiologies with encouraging results.
In this narrative review, we shall be discussing the anatomy of the GON, the approaches for the GONB, a review of existing literature, and the importance of using real time ultrasound while performing this block.
2. Anatomy
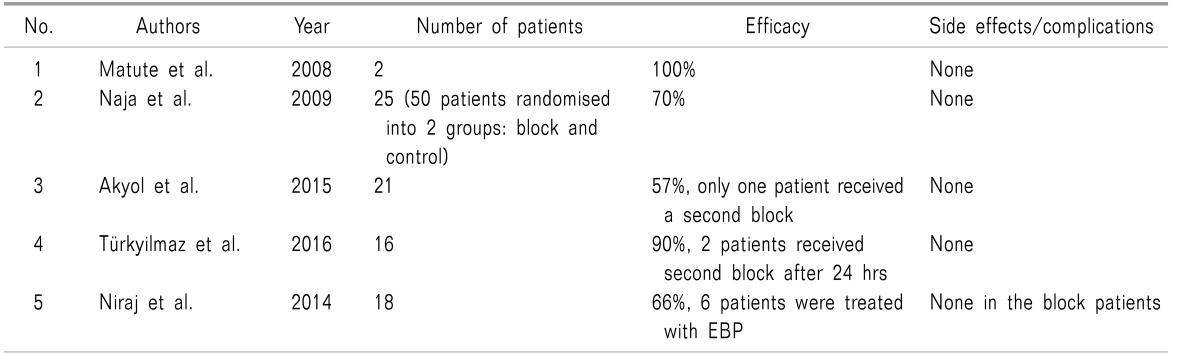
The greater occipital nerve (GON) is formed by the medial branch of the dorsal ramus of C2 that runs between the posterior arch of the atlas and the lamina of the axis. It originates, along with the lesser occipital nerve, between the first and second cervical vertebrae (Fig. 1). The GON ascends after emerging from below the suboccipital triangle beneath the obliquus capitis inferior (OCI) muscle and divides into a larger medial branch and smaller lateral branches.
Fig. 1. The origin of greater occipital nerve and its course along the occipital region (Modified from http://www.kidport.com/RefLib/Science/HumanBody/SkeletalSystem/Skull.htm). The figure has been edited and labelled at appropriate places.
The medial branch of the 2nd cervical dorsal ramus provides the motor branches to the muscles it traverses and terminates as the GON. The nerve becomes superficial at about 2.5 to 5 cm infero-lateral to the occipital protuberance. Along with the C2 ramus, the C1 ramus of the spinal nerve innervates the OCI muscle. There are several branches of the GON that supply the skin of the back of the scalp as far anterior as the vertex of the skull [8,9].
3. Techniques
1) Using a nerve stimulator
A bilateral GONB can be performed with variable degrees of success using the landmark technique, with nerve stimulator guidance. However, the variable course of the nerve on either sides could lead to an unsuccessful block either on one side or bilaterally. Use of ultrasonography has increased the success rate of the GONB.
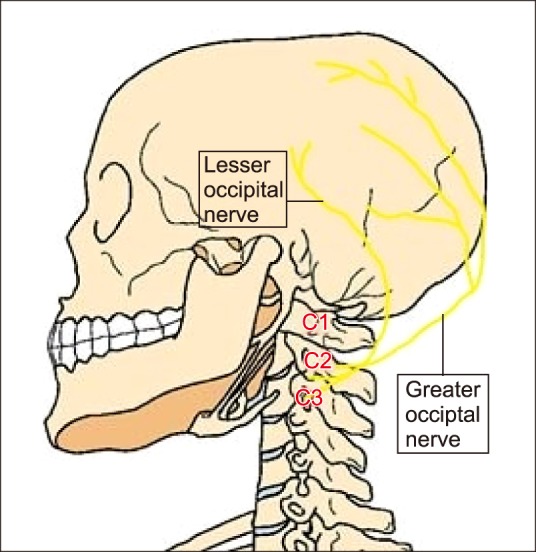
The GON block using landmark technique: Before the use of ultrasound in regional anesthesia, the GON block was performed using the landmark technique with a reasonable success rate. In landmark technique, the external occipital protuberance is identified with the patient either in prone position and the neck flexed, or in a sitting position with flexion of the neck. A point is marked 3–3.5 cm in the midline by drawing an imaginary line down from the external occipital protuberance. Another point is marked at 1.5–2 cm from this point on both sides laterally (Fig. 2). This is the point of entry for the needle [10,11].
Fig. 2. Relevant landmark for locating the needle entry using a landmark technique. The same point could be marked prior to performing an ultrasound scan also (Modified from http://resizeandsave.online/openphoto.php? img=http://images.fineartamerica.com/images-medium-large-5/anatomy-of-human-skull-rear-view-leonello-calvetti.jpg). The figure has been labelled at appropriate places.
The GON is usually located medial to the arterial pulsation. Ducic et al. [12] collected data involving 125 subjects (112 patients and 13 cadavers). They reported that the nerve in 98.5 percent subjects pierced the body of the semispinalis capitis muscle, and in 6.1 percent of individuals it is split by fibers of this muscle or in the trapezial tunnel. Not only this, they found that in 43.9 percent of situations, the course of the nerve was asymmetric on the two sides. This piece of information is very important because if landmark technique is relied upon, chances of an unsuccessful block are greater.
The success rate of the landmark technique could be increased by using a nerve stimulator while performing the block. After identifying the greater occipital artery pulsations and after skin infiltration with lidocaine, an initial nerve stimulator setting of 2–2.5 mA could be used with a 1 Hz frequency, 9 volts of voltage and a voltage current duration of 1 ms.
The needle is introduced medial to the arterial pulsation and a point is identified where the patient experiences a tingling sensation. The correct point of injection is where the tingling sensation persists at a current of 0.3 mA [13,14]. Eom and Kim [15] used an 8 MHz, portable TCD machine and identified the pulsations of the greater occipital artery with the patient in prone position in a patient with occipital neuralgia. The authors highlighted the importance of imaging while performing GONB so as to avoid failure.
2) USG for the GON block
The use of USG has improved the success rate of almost all the peripheral nerve block injections in the practice of acute and chronic pain management. Walker et al. [16] had mentioned in the Cochrane database systematic review, that in experienced hands, use of USG for performing a peripheral nerve block increases the success rate, reduces complications and side effects, and results in a better onset time.
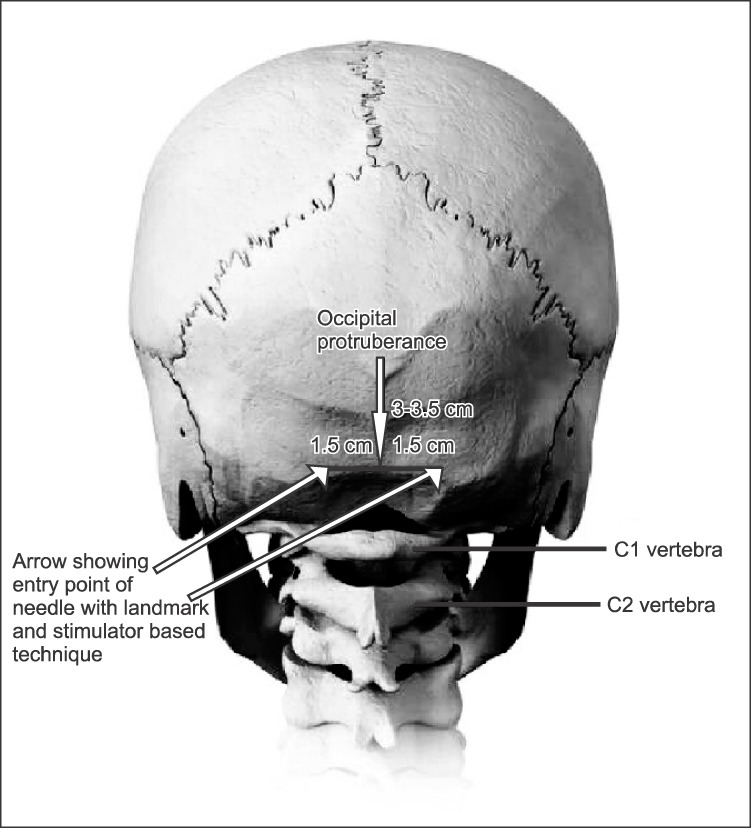
For the GON block, a high frequency linear array probe with a frequency of 8–13 MHz is required as the location of structures of importance are quite superficial. One can begin scanning by putting the probe at the midline with the patient in either in sitting or prone position with the neck flexed to facilitate imaging (Fig. 3)
Fig. 3. The probe placement while performing a greater occipital nerve block. The entry of needle in plane, with the needle entering from lateral to medial direction (Consent have been obtained from the patient to display the shaved skull with appropriate labelling for academic purpose).
The scanning can be started from 3–4 cm below the external occipital protuberance at the midline and then moving the probe laterally. The muscles that needs to be identified are the trapezius, semispinalis capitis (SSC), splenius capitis, and the obliquus capitis inferior (OCI) muscle (Fig. 4).
Fig. 4. Ultrasonographic view of relevant anatomy i.e. greater occipital artery, semispinalis muscle, obliquus capitis inferior muscle and greater occipital nerve medial to arterial pulsations.
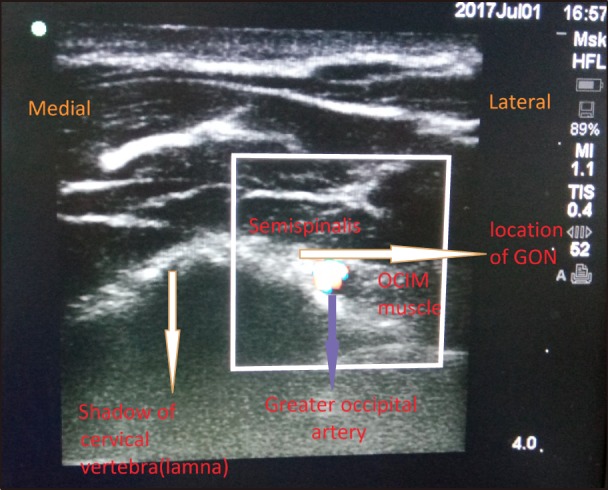
The lamina of the C2 vertebra could be identified here as a bony shadow. The GON usually lies in the myofascial plane between the OCI and SSC muscle, medial to the greater occipital artery. The artery could be easily located by using a color Doppler in this position.
Greher et al. [17] performed 20 bilateral GONBs using an ultrasound in 10 embalmed cadavers and found that by using USG, a bilateral GONB could be successfully administered either at the level of the superior nuchal line or at the level of C2. Zipfel et al. [18] had almost 96% success when the GON block for refractory occipital neuralgia or craniofacial pain syndromes was administered under ultrasound guidance.
Binici et al. [19] performed a successful bilateral ultrasound-guided GON block in a 72-year-old high-risk patient with multi comorbidities for excision of lipoma from the posterior region of neck. Shim et al. [20] also used ultrasound-guidance for performing the GON block for patients with headaches of varying etiologies and found it to be effective and reliable.
4. The mechanism of action of the GON block in PDPH
It must be understood that the idea of the GON block is to relieve the distressing headache experienced by a patient who has a DP. The injection does not address the ongoing CSF leak. Other supportive measures like hydration, analgesics, bed rest, laxatives should be continued. The sensory neurons in the upper cervical spinal cord are quite close to trigeminal nucleus caudalis (TNC) neurons. Therefore, the sensory input from both the cervical and trigeminal fibers finally gets transmitted to the TNC neurons.
When a bilateral GONB is performed, there is a ‘winding down’ of central sensitisation due to interruption of afferent input to the dorsal horn and TNC neurons, temporarily. This relieves the headache due to PDPH as well as those due to other chronic pain syndromes [21,22].
5. Review of existing literature
When we searched PubMed, Scopus, Medline and Google scholar databases with the keywords: greater occipital nerve block and headache, a lot of case series, techniques, and review articles can be found. Blumenfeld et al. [23] published a narrative review describing the expert consensus recommendations for the performance of peripheral nerve blocks for headaches.
The indications for the nerve blocks, that they suggested were primary headache disorders, secondary headache disorders, and cranial neuralgias. They reviewed greater occipital, lesser occipital, supratrochlear, supraorbital, and auriculotemporal injections in detail. Levin [24] also described the various nerve blocks which can be useful in the management of refractory headaches.
Along with the blocks described by Blumenfeld et al. [23], they also discussed the supratrochlear and supraorbital nerves, sphenopalatine ganglion, and cervical spinal roots along with the facet joints of the upper cervical spine for managing headache. However, when we used the following keywords: greater occipital nerve, postdural puncture, and headache; we found few recent case series and USG guided techniques. The published articles which have described the efficacy of bilateral GONB in PDPH have been presented in a tabular form in Table 1.
Table 1. Efficacy of Bilateral Greater Occipital Nerve Block in Patients Who Had Postdural Puncture Headache.
One of the earliest descriptions of the GON block for treating PDPH was by Matute et al. in 2008 [25]. The authors described their experience with 2 patients with PDPH who were offered a bilateral GONB with 4 ml of 0.25% bupivacaine and 20 mg triamcinolone, using the landmark technique. Both patients were effectively treated with bilateral GONBs.
Uyar Türkyilmaz et al. [26] presented their retrospective data on 16 patients with PDPH due to spinal anaesthesia for caesarean section, who were treated with bilateral GONBs (landmark technique) using levo-bupivacaine and dexamethasone. The block was repeated the next day if the VAS score was more than 3, without dexamethasone.
Niraj et al. [27] published their data of 18 patients who agreed to bilateral GONB for relieving PDPH. The patients treated were the ones who had inadvertent DP while locating an epidural space (obstetric-17, non-obstetric-1). Out of 18, 12 patients (i.e. 66% of patients) were relieved by bilateral, landmark based GONB, using steroid and local anaesthetic. The remaining 34% (i.e. 6 patients) were treated with an EBP. Out of this group, one patient had headache and photophobia for which she was readmitted. The patient was offered a repeat EBP or GONB. Patient opted for a GONB after which the symptoms resolved completely after 3 h.
Akin Takmaz et al. [28] performed bilateral GONB with landmark technique using a nerve stimulator in a 29-year-old male who had PDPH not responding to conservative measures after a spinal anaesthetic for an inguinal hernia repair. The block, which was given using only local anaesthetic, relieved the pain in 2 m.
Naja et al. [29] randomised 50 patients with PDPH due to spinal anaesthesia for caesarean sections and lower limb surgeries into 2 groups of 25 each. In one group, bilateral greater and lesser occipital nerve blocks were administered using a nerve stimulator. Patients in second group were managed conservatively with hydration, bed rest, and analgesics. In the block group, complete pain relief was observed in 68.4% of patients after 1–2 blocks, with the remaining 31.6% patients who receiving up to 4 blocks. Visual analogue scales were significantly lower in the block group (P < 0.01), with less analgesic consumption during follow-up (P < 0.05) compared with the control group. Overall hospital stays of patients who were in the block group were significantly shorter compared to the control group (P < 0.001).
Akyol et al. [30] administered bilateral, ultrasound-guided GONB in 21 patients who developed PDPH after receiving spinal anesthetic. All patients had significant pain relief which lasted for more than 24 hours after a single injection. Only one patient received a second block. One reason for the successful single shot blocks in these patients could be use of real time USG for locating the nerve. As described earlier, the nerves do not always have an identical course on both sides and not necessarily always present in the myofascial plane, medial to the arterial pulsations.
6. Complications/adverse effects of GONB
GONB is a very safe regional anaesthesia technique. Few problems that have been encountered are vasovagal syncope, transient dizziness, tenderness after injection, hypersensitivity, and worsening of migraine headaches after injection. Alopecia has been noticed at the site of injection which could be due to the addition of a steroid in the local Anesthetic [31]. Transient facial nerve palsy has been reported after GONB [32]. Local anaesthesia systemic toxicity has not been reported with GONB which could be because of the small volume of local anesthetic which is used for injection. Secondly, use of a ultrasound and meticulous aspiration before injection will make LAST a rare possibility. However, cardiac arrhythmias, seizures, respiratory and depression are possible complications due to the proximity of the site of injection to the greater occipital vessels and vertebral artery.
CONCLUSIONS
Bilateral GONB is a minimally invasive, low-volume, safe peripheral nerve block which can be offered to patients who are suffering from PDPH when conservative management is ineffective. Using real-time USG guidance can increase the success rate of the block and a second injection can be avoided.
Even after a successful block, conservative measures like hydration, bed rest, analgesics, and laxatives should be continued because the block addresses only the headache. Even after a partially successful block, it can be repeated if the patient is willing to undergo the procedure. The primary insult, i.e. a tear in the dural membrane due to the anesthetic technique (spinal or epidural catheter placement) needs time to heal.
If the patient continues to suffer from PDPH even after bilateral GONB on more than one occasion, an EBP should be considered. Bilateral GONB can also be offered to a patient who did not receive complete pain relief after an EBP and is not willing to undergo a repeat EBP.
References
- 1.Evans RW, Armon C, Frohman EM, Goodin DS. Assessment: prevention of post-lumbar puncture headaches: report of the therapeutics and technology assessment subcommittee of the american academy of neurology. Neurology. 2000;55:909–914. doi: 10.1212/wnl.55.7.909. [DOI] [PubMed] [Google Scholar]
- 2.Oedit R, van Kooten F, Bakker SL, Dippel DW. Efficacy of the epidural blood patch for the treatment of post lumbar puncture headache BLOPP: a randomised, observer-blind, controlled clinical trial [ISRCTN 71598245] BMC Neurol. 2005;5:12. doi: 10.1186/1471-2377-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arevalo-Rodriguez I, Ciapponi A, Roqué i Figuls M, Muñoz L, Bonfill Cosp X. Posture and fluids for preventing post-dural puncture headache. Cochrane Database Syst Rev. 2016;3:CD009199. doi: 10.1002/14651858.CD009199.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boonmak P, Boonmak S. Epidural blood patching for preventing and treating post-dural puncture headache. Cochrane Database Syst Rev. 2010:CD001791. doi: 10.1002/14651858.CD001791.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Desai MJ, Dave AP, Martin MB. Delayed radicular pain following two large volume epidural blood patches for postlumbar puncture headache: a case report. Pain Physician. 2010;13:257–262. [PubMed] [Google Scholar]
- 6.Nair AS, Rayani BK. Sphenopalatine ganglion block for relieving postdural puncture headache: technique and mechanism of action of block with a narrative review of efficacy. Korean J Pain. 2017;30:93–97. doi: 10.3344/kjp.2017.30.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mojica J, Mo B, Ng A. Sphenopalatine ganglion block in the management of chronic headaches. Curr Pain Headache Rep. 2017;21:27. doi: 10.1007/s11916-017-0626-8. [DOI] [PubMed] [Google Scholar]
- 8.Cho JC, Haun DW, Kettner NW, Scali F, Clark TB. Sonography of the normal greater occipital nerve and obliquus capitis inferior muscle. J Clin Ultrasound. 2010;38:299–304. doi: 10.1002/jcu.20693. [DOI] [PubMed] [Google Scholar]
- 9.Natsis K, Baraliakos X, Appell HJ, Tsikaras P, Gigis I, Koebke J. The course of the greater occipital nerve in the suboccipital region: a proposal for setting landmarks for local anesthesia in patients with occipital neuralgia. Clin Anat. 2006;19:332–336. doi: 10.1002/ca.20190. [DOI] [PubMed] [Google Scholar]
- 10.Loukas M, El-Sedfy A, Tubbs RS, Louis RG, Jr, Wartmann CH, Curry B, et al. Identification of greater occipital nerve landmarks for the treatment of occipital neuralgia. Folia Morphol (Warsz) 2006;65:337–342. [PubMed] [Google Scholar]
- 11.Güvençer M, Akyer P, Sayhan S, Tetik S. The importance of the greater occipital nerve in the occipital and the suboccipital region for nerve blockade and surgical approaches: an anatomic study on cadavers. Clin Neurol Neurosurg. 2011;113:289–294. doi: 10.1016/j.clineuro.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Ducic I, Moriarty M, Al-Attar A. Anatomical variations of the occipital nerves: implications for the treatment of chronic headaches. Plast Reconstr Surg. 2009;123:859–863. doi: 10.1097/PRS.0b013e318199f080. [DOI] [PubMed] [Google Scholar]
- 13.Naja ZM, El-Rajab M, Al-Tannir MA, Ziade FM, Tawfik OM. Repetitive occipital nerve blockade for cervicogenic headache: expanded case report of 47 adults. Pain Pract. 2006;6:278–284. doi: 10.1111/j.1533-2500.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 14.Naja Z, Al-Tannir M, El-Rajab M, Ziade F, Baraka A. Nerve stimulator-guided occipital nerve blockade for postdural puncture headache. Pain Pract. 2009;9:51–58. doi: 10.1111/j.1533-2500.2008.00238.x. [DOI] [PubMed] [Google Scholar]
- 15.Eom KS, Kim TY. Greater occipital nerve block by using transcranial Doppler ultrasonography. Pain Physician. 2010;13:395–396. [PubMed] [Google Scholar]
- 16.Walker KJ, McGrattan K, Aas-Eng K, Smith AF. Ultrasound guidance for peripheral nerve blockade. Cochrane Database Syst Rev. 2009:CD006459. doi: 10.1002/14651858.CD006459.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Greher M, Moriggl B, Curatolo M, Kirchmair L, Eichenberger U. Sonographic visualization and ultrasound-guided blockade of the greater occipital nerve: a comparison of two selective techniques confirmed by anatomical dissection. Br J Anaesth. 2010;104:637–642. doi: 10.1093/bja/aeq052. [DOI] [PubMed] [Google Scholar]
- 18.Zipfel J, Kastler A, Tatu L, Behr J, Kechidi R, Kastler B. Ultrasound-guided intermediate site greater occipital nerve infiltration: a technical feasibility study. Pain Physician. 2016;19:E1027–E1034. [PubMed] [Google Scholar]
- 19.Binici O, Kuyrukluyıldız U, Şahin M, Alagöl A, Yılmaz İ. Ultrasound-guided bilateral greater occipital nerve block for mass excision. Turk J Anaesthesiol Reanim. 2015;43:437–439. doi: 10.5152/TJAR.2015.15975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shim JH, Ko SY, Bang MR, Jeon WJ, Cho SY, Yeom JH, et al. Ultrasound-guided greater occipital nerve block for patients with occipital headache and short term follow up. Korean J Anesthesiol. 2011;61:50–54. doi: 10.4097/kjae.2011.61.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashkenazi A, Levin M. Greater occipital nerve block for migraine and other headaches: is it useful? Curr Pain Headache Rep. 2007;11:231–235. doi: 10.1007/s11916-007-0195-3. [DOI] [PubMed] [Google Scholar]
- 22.Selekler MH. Greater occipital nerve blockade: trigeminicervical system and clinical applications in primary headaches. Agri. 2008;20:6–13. [PubMed] [Google Scholar]
- 23.Blumenfeld A, Ashkenazi A, Napchan U, Bender SD, Klein BC, Berliner R, et al. Expert consensus recommendations for the performance of peripheral nerve blocks for headaches: a narrative review. Headache. 2013;53:437–446. doi: 10.1111/head.12053. [DOI] [PubMed] [Google Scholar]
- 24.Levin M. Nerve blocks in the treatment of headache. Neurotherapeutics. 2010;7:197–203. doi: 10.1016/j.nurt.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matute E, Bonilla S, Gironés A, Planas A. Bilateral greater occipital nerve block for post-dural puncture headache. Anaesthesia. 2008;63:557–558. doi: 10.1111/j.1365-2044.2008.05531.x. [DOI] [PubMed] [Google Scholar]
- 26.Uyar Türkyilmaz E, Camgöz Eryilmaz N, Aydin Güzey N, Moraloğlu Ö. Bilateral greater occipital nerve block for treatment of post-dural puncture headache after caesarean operations. Braz J Anesthesiol. 2016;66:445–450. doi: 10.1016/j.bjane.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Niraj G, Kelkar A, Girotra V. Greater occipital nerve block for postdural puncture headache (PDPH): a prospective audit of a modified guideline for the management of PDPH and review of the literature. J Clin Anesth. 2014;26:539–544. doi: 10.1016/j.jclinane.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Akin Takmaz S, Unal Kantekin C, Kaymak C, Başar H. Treatment of post-dural puncture headache with bilateral greater occipital nerve block. Headache. 2010;50:869–872. doi: 10.1111/j.1526-4610.2010.01656.x. [DOI] [PubMed] [Google Scholar]
- 29.Naja ZM, El-Rajab M, Al-Tannir MA, Ziade FM, Tawfik OM. Occipital nerve blockade for cervicogenic headache: a double-blind randomized controlled clinical trial. Pain Pract. 2006;6:89–95. doi: 10.1111/j.1533-2500.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 30.Akyol F, Binici O, Kuyrukluyildiz U, Karabakan G. Ultrasound-guided bilateral greater occipital nerve block for the treatment of post-dural puncture headache. Pak J Med Sci. 2015;31:111–115. doi: 10.12669/pjms.311.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Afridi SK, Shields KG, Bhola R, Goadsby PJ. Greater occipital nerve injection in primary headache syndromes--prolonged effects from a single injection. Pain. 2006;122:126–129. doi: 10.1016/j.pain.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Strauss L, Loder E, Rizzoli P. Transient facial nerve palsy after occipital nerve block: a case report. Headache. 2014;54:1651–1655. doi: 10.1111/head.12403. [DOI] [PubMed] [Google Scholar]