Abstract Abstract
The results of manual dissection of the musculature of the male genitalia in Nothybus kuznetsovorum are fully confirmed by the modern methods of Micro-CT. A comparative analysis of Neria commutata and Cothornobata sp. shows that an increase in the flexion in the genitalia of males and the displacement of syntergosternite VII to the ventral side in Cothornobata sp. caused the disappearance of the muscles ITM6–7r and ITM7–8r. In addition, this increase in flexion apparently caused the fusion of the M18 muscles into one bundle. The muscle ISM5-6c goes on to moving the second segment of the forcipate appendages of sternite V.
Keywords: Cothornobata, Morphology, musculature, Neria commutata, Nothybus kuznetsovorum, sclerites
Introduction
Micropezidae is an average-sized family of acalyptrate flies (Diptera). It comprises approximately 700 described species in 50 genera (Marshall 2010, 2012; Ferro and Carvalho 2014). The family is globally distributed, with the greatest species richness found in tropical regions (Steyskal 1968). The body of adult flies is 3.5 to 20.0 mm long (Steyskal 1987).
The family Nothybidae includes only one genus, Nothybus Rondani, 1875, distributed in the Oriental Region. These acalyptrate flies are 5.5 to 15.0 mm body long (Lonsdale and Marshall 2016).
Study of the musculature is helpful not only for specifying the functions of genital sclerites, but also for revealing the homology of some poorly traced structures (Ovtshinnikova 1989, 1994, Ovtshinnikova and Yeates 1998; Galinskaya and Ovtshinnikova 2015).
Previously specimens of Nothybus kuznetsovorum Galinskaya et Shatalkin, 2015 (Nothybidae) and Neria commutata (Czerny, 1930) (Micropezidae) were investigated by manual dissection using light microscopy (Galinskaya et al. 2016, Ovtshinnikova and Galinskaya 2017). In these studies, some muscles were thin and indistinct.
Among the Diptera the X-ray micro-computed tomography (Micro-CT) was used for revealing the morphology of the feeding apparatus of Philoliche rostrata and Ph. gulosa (Tabanidae) (Karolyi et al. 2014); for the characterization of the morphology of the venom system of Eutolmus rufibarbis (Asilidae) (Drukewitz et al. 2018); for revealing the morphology of the proboscis, the food canal and suction pump muscles of Prosoeca sp. (Nemestrinidae) (Karolyi et al. 2013); for analysis of the Drosophila larvae central nervous system (Drosophilidae) (Mizutani et al. 2007); for revealing the morphology of the late pupa of Calliphora vicina (Calliphoridae) (Metscher 2009); for performing qualitative and quantitative analyses of the morphological changes taking place during the intra-puparial period of Calliphora vicina and Lucilia sericata (Calliphoridae) (Richards et al. 2012; Hall et al. 2017; Martín-Vega et al. 2016; Martín-Vega et al. 2017a, b); and for the visualization of the three-dimensional movements of flight muscles and thoracic sclerites of Calliphora vicina (Calliphoridae) (Walker et al. 2014).
In this study X-ray micro-computed tomography (micro-CT) was utilizzed for revealing genital sclerites and muscles of Nothybus kuznetsovorum (Nothybidae), comparing it with results of manual dissection (Galinskaya et al. 2016). The sclerites and muscles of Cothornobata sp. (Micropezidae) are also revealed. Males of Micropezidae are characterized by specialized forceps-like processes of variable size, shape, and ornamentation arising from sternite V and by sternite VI sometimes with specialized processes (these processes are secondarily reduced in some spp). The examined species is characterized by elongated forcipate appendages of sternite V, and the musculature of these appendages was investigated.
Materials and methods
One specimen of Nothybus kuznetsovorum and one specimen of Cothornobata sp. were collected in Northern Vietnam, PhuTho Province, Thanh Son District, Xuan Son National Park; 21°8.333'N; 104°56.25'W; h = 300–900 m, 23 October 2014 by T.V. Galinskaya. The specimens were fixed in 70% ethanol. They were prepared for X-ray micro-computed tomography (micro-CT) analysis by contrasting with iodine as outlined in Gignac and Kley (2014) and by critical point drying. Specimens were glued with the thorax pointing downwards on the tip of a small pin of 1 mm diameter, with the tip of the male abdomen as close to the rotation axis as possible. Micro-CT scans were produced under phase contrast (40 KV, 8 W), using a 4× detector (10 s; 4.15 μm pixel size) and 10× detector (30 s; 1.89 μm pixel size).
The male genital muscles were classified into several groups (muscles of the epandrial complex, muscles of the hypandrial complex, tergosternal muscles, and pregenital muscles) and described according to the system of Ovtshinnikova and Galinskaya (Ovtshinnikova 1989, 2000; Ovtshinnikova and Galinskaya 2017). Male genital sclerites were described according to the Sinclair (2000).
Results
Nothybus kuznetsovorum Galinskaya & Shatalkin, 2015
Figure 1
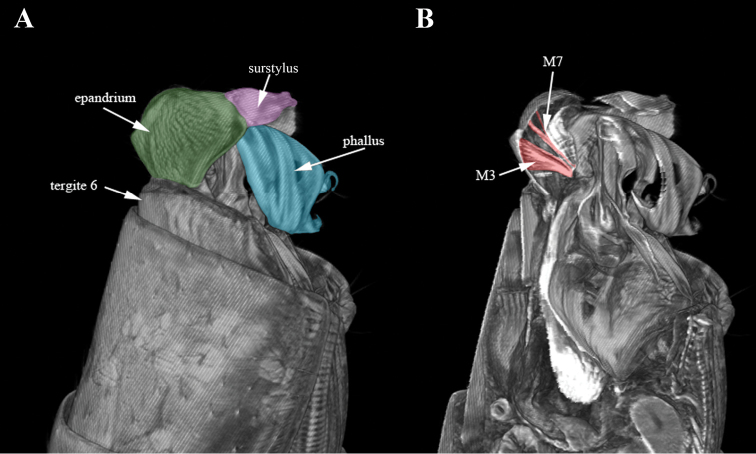
Figure 1.
Micro-CT surface rendering (A) and volume rendering of virtual sections to median, digitally stained (B) of Nothybus kuznetsovorum (Nothybidae), lateral view. Cerci shown in yellow, epandrium in dark green, phallus in light blue, surstylus in pink, syntergosternite VIII in violet, syntergosternite VII in light green, sternite VI in orange and sternite V in dark blue.
All sclerites and muscles and their places of attachments revealed using manual dissection and decribed by us previously (Galinskaya et al. 2016) have been confirmed by micro-CT. The only questionable muscle after manual dissection was M7 (Galinskaya et al. 2016). Here the presence and attachment sites of attachment of M7 are confirmed (Figure 1B).
Cothornobata sp.
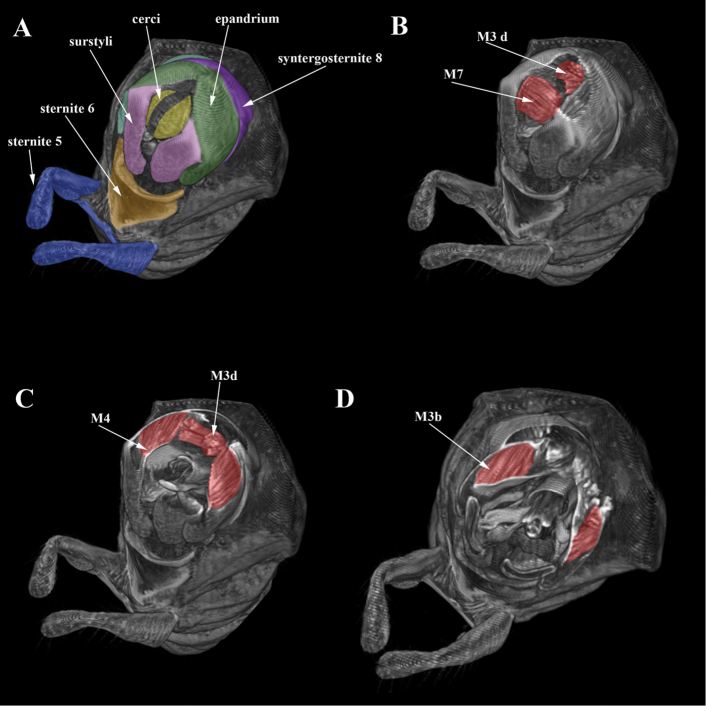
Figure 2.
Micro-CT surface rendering (A) and volume rendering of virtual sections posteriorly, digitally stained (B–D) of Cothornobata sp. (Micropezidae), posterior view.
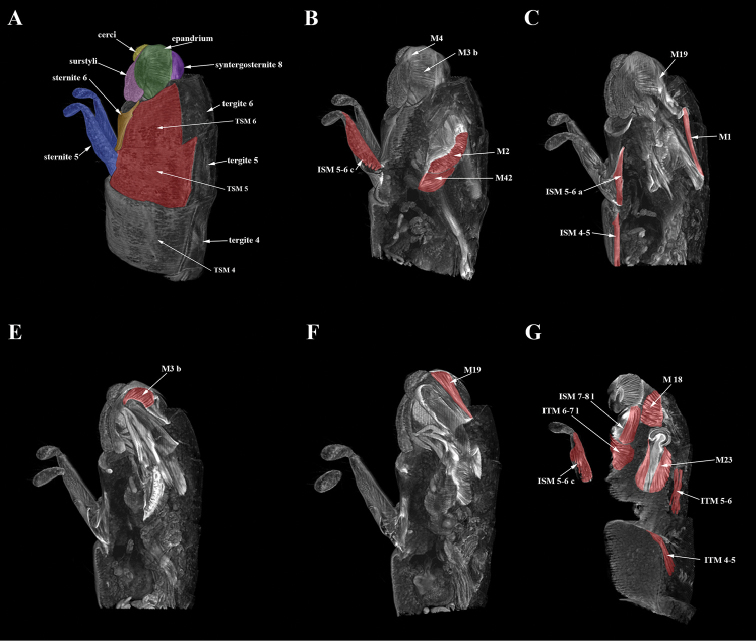
Figure 4.
Micro-CT surface rendering (A) and volume rendering of virtual sections right to median, digitally stained (B–G) of Cothornobata sp. (Micropezidae), lateral view.
Sclerites. Sternite IV elongate. Sternite V modified into elongated forcipate appendages. Sternite VI elongate. Tergite IV, V, VI not modificated. Ejaculatory apodeme placed at the level of sternites V and VI. Sternite and tergite VII fused into syntergosternite positioned on left side of body. Syntergosternite VIII spherical. Epandrium large, bearing bifurcate surstyli and small cerci.
Muscles. The muscles are grouped by the site of insertion of their proximal parts. Thin paired muscles ISM4–5 attached to basal area of sternite V (Figure 3B). Tergosternal muscle TSM4 poorly developed (Figure 4A).
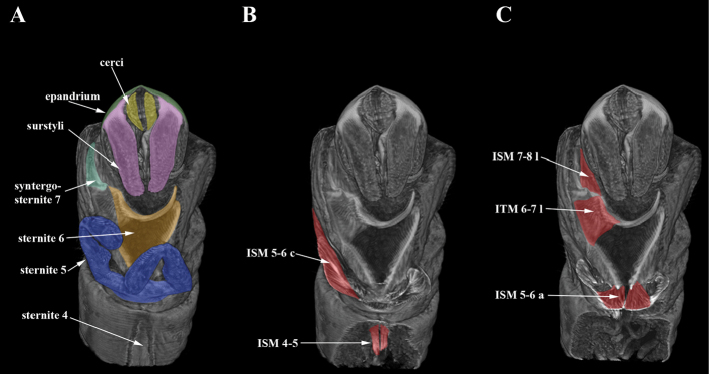
Figure 3.
Micro-CT surface rendering (A) and volume rendering of virtual sections right to median, digitally stained (B–C) of Cothornobata sp. (Micropezidae), abdominal view.
Muscles of segments V and VI. Two pairs of muscles ISM5–6 lying between sternites V and VI: proximal retractors of sternite VI ISM5–6a broadly fan-shaped, extending from basal area of sternite V to lateral surface of sternite VI (Figures 3C, 4C); median flexors of forcipate appendages ISM5–6c broadly fan-shaped, extending from lateral outgrowths of sternite V to central part of sternite VI (Figures 3B, 4B, G). Contraction of muscles ISM5–6 powers the forcipate appendages of sternite V that participate in the fixation of the female’s abdomen during copulation. Tergosternal muscles TSM5 and TSM6 in segments V–VI poorly developed (Figure 4A).
Muscles associated with segment VII are asymmetrical. Left intersegmental tergal muscle ITM6–7l wide, extending from distal area of tergite VI to left lateral margin of syntergosternite VII (Figure 4G). No muscles TSM6–7 present in sternal area between segments VI and VII. Left muscle ISM7–8l wide, extending from basal margin of syntergosternite VII to syntergosternite VIII (Figure 4G). Tergosternal muscles of segment VII absent, probably due to fusions or reductions.
Pregenital muscles. Muscle of hypandrium M18 connects syntergosternite VIII to hypandrium (Figure 4G). Retractors of epandrium M19 extending from syntergosternite VIII to middle of proximal margin of epandrium (Figure 4C, F).
Tergosternal muscles of segment VIII absent, probably due to fusions or reductions.
Long and powerful symmetrical tergosternal abductors M5 extending from latero-basal margin of epandrium to latero-distal margin of hypandrium.
Muscles of epandrial complex symmetrical. Wide and thin paired cercal muscles M7 extend from distal outgrowths of subepandrial sclerite to cerci (Figure 2B). Paired muscles of subepandrial sclerite M3 well developed, comprise two pairs of muscles: muscles M3b extending from latero-distal lobes of subepandrial sclerite to latero-distal part of epandrium (Figure 2D, 4B, E); muscles M3d extending from median bridge of subepandrial sclerite to distal part of epandrium (Figure 2B, C). Short and narrow adductors of surstylus M4 connecting laterodistal parts of epandrium to basal part of surstylus (Figure 2C, 4B).
Muscles of hypandrial complex symmetrical. Short and powerful paired phallic retractors M1 connecting distal part of phallapodeme to distal inner part of hypandrium (Figure 4C). Long and powerful paired phallic protractors M2 connecting distal part of phallapodeme to lateral and median parts of hypandrium (Figure 4B) and lying above M1. Paired retractors of pregonites M42 extending from inner distal part of hypandrium to pregonites (Figure 4B). Compressors of ejaculator M23 well developed (Figure 4G).
Discussion
Since the results of the study of the male genitalia in Nothybus kuznetsovorum using micro-CT completely coincide with the results of manual dissection, we conclude that the method of manual anatomy has not lost its significance. However, the micro-CT takes much more time than manual anatomy. The undoubted advantage of micro-CT is its higher accuracy and the fact that only one specimen is needed for the study, while manual anatomy usually needs 4–5 specimens.
We assume that the presence of the elongated appendages of sternite V in the species Cothornobata sp. can cause the presence of additional muscles ISM5–6 (due to the complication of sclerites in Cothornobata sp. comparing with Neria commutata). However, no muscle going from the basal to the distal segments of the appendages of V sternite was discovered. Additionally, we did not find the muscles ISM5–6b and ISM5–6d. Neria commutata has the lateral flexors of forcipate appendages ISM5–6d broad and short, extending from inner vanes of sternite V to those of sternite VI and occupying a considerable part of the surfaces of both sclerites; the distal retractors of sternite VI ISM5–6b are narrow, extending from the distal parts of the outgrowths of sternite V to the distal area of sternite VI. Apparently, the muscle ISM5–6c goes on to moving the distal half of the forcipate appendages of sternite V.
In Cothornobata sp., unlike Neria commutata, no muscles ITM6–7r and ITM7–8r have been detected. Neria commutata has the right muscle ITM6–7r narrow, conical, extending from the median part of tergite VI to the membrane in front of syntergosternite VII, and the right muscle ISM7–8r small and short, extending from the membrane at syntergosternite VII to syntergosternite VIII (Ovtshinnikova and Galinskaya 2017). We assume that in Cothornobata sp. these muscles are absent due to a stronger flexion of syntergosternite VII to the ventral side. Increased flexion of syntergosternite VII in Cothornobata sp. caused the disappearance of muscles ITM6–7r and ITM7–8r. Also, an increase of the flexion apparently caused the fusion of the M18 muscles into one bundle.
Acknowledgments
The authors are grateful to A.I. Shatalkin (Zoological Museum of Lomonosov Moscow State University) and V.A. Krivokhatsky (Zoological Institute of Russian Academy of Sciences) for valuable discussions and advice. The work of T.V. Galinskaya was supported by the Russian Science Foundation (project no. 14-14-00208). The work of O.G. Ovtshinnikova was supported by the Zoological Institute of Russian Academy of Sciences (State Research Program AAAA-A17-117030310205-9) and the Russian Foundation for Basic Research (projects no. 15-04-03457 and 18-04-0035). The work was carried out using a computer X-ray microtomograph Skyscan 1172, purchased from the funds of the Development Program of the Moscow University of Geological Faculty of M.V. Lomonosov Moscow State University.
Citation
Galinskaya TV, Gafurova (Gilyazetdinova) D, Ovtshinnikova OG (2018) X-ray microtomography (microCT) of male genitalia of Nothybus kuznetsovorum (Nothybidae) and Cothornobata sp. (Micropezidae). ZooKeys 744: 139–147. https://doi.org/10.3897/zookeys.744.22347
References
- Drukewitz SH, Fuhrmann N, Undheim EAB, Blanke A, Giribaldi J, Mary R, Laconde G, Dutertre S, Reumont BM. (2018) A Dipteran’s Novel Sucker Punch: Evolution of Arthropod Atypical Venom with a Neurotoxic Component in Robber Flies (Asilidae, Diptera). Toxins 10(29): 1–23. https://doi.org/10.3390/toxins10010029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro GB, Carvalho CJB. (2014) A pictorial key and diagnosis of the Brazilian genera of Micropezidae (Diptera, Nerioidea). Revista Brasileira de Entomologia 58(1): 52–62. https://doi.org/10.1590/S0085-56262014000100009 [Google Scholar]
- Galinskaya TV, Ovtshinnikova OG. (2015) Musculature of the male genitalia in the tribe Ulidiini (Diptera, Ulidiidae). Entomological Review 95(1): 31–37. https://doi.org/10.1134/S0013873815010042 [Google Scholar]
- Galinskaya TV, Shatalkin AI, Ovtshinnikova OG. (2016) Skeleton and musculature of the male genitalia in the family Nothybidae (Diptera). Entomological Review 96(9): 1182–1193. https://doi.org/10.1134/S0013873816090037 [Google Scholar]
- Gignac PM, Kley NJ. (2014) Iodine-enhanced micro-CT imaging: Methodological refinements for the study of the soft-tissue anatomy of post-embryonic vertebrates. Journal of Experimental Zoology Part B 322: 166–176. https://doi.org/10.1002/jez.b.22561 [DOI] [PubMed] [Google Scholar]
- Hall MJR, Simonsen TJ, Martín-Vega D. (2017) The ‘dance’ of life: visualizing metamorphosis during pupation in the blow fly Calliphora vicina by X-ray video imaging and micro-computed tomography. Royal Society Open Science 4: 160699. https://doi.org/10.1098/rsos.160699 [DOI] [PMC free article] [PubMed]
- Karolyi F, Morawetz L, Colville JF, Handschuh S, Metscher BD, Krenn HW. (2013) Time management and nectar flow: flower handling and suction feeding in long-proboscid flies (Nemestrinidae: Prosoeca). Naturwissenschaften 100: 1083–1093. https://doi.org/10.1007/s00114-013-1114-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolyi F, Colville JF, Handschuh S, Metscher BD, Krenn HW. (2014) One proboscis, two tasks: Adaptations to blood-feeding and nectar-extracting in long-proboscid horse flies (Tabanidae, Philoliche). Arthropod Structure and Development 43: 403–413. https://doi.org/10.1016/j.asd.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale O, Marshall SA. (2016) Revision of the family Nothybidae (Diptera: Schizophora). Zootaxa 4098(1): 1–42. https://doi.org/10.11646/zootaxa.4098.1.1 [DOI] [PubMed] [Google Scholar]
- Martín-Vega D, Hall MJR, Simonsen ThJ. (2016) Resolving Confusion in the Use of Concepts and Terminology in Intrapuparial Development Studies of Cyclorrhaphous Diptera. Journal of Medical Entomology 53(6): 1249–1251. https://doi.org/10.1093/jme/tjw081 [DOI] [PubMed] [Google Scholar]
- Martín-Vega D, Simonsen ThJ, Wicklein M, Hall MJR. (2017a) Age estimation during the blow fly intra-puparial period: a qualitative and quantitative approach using micro-computed tomography. International Journal of Legal Medicine 131(5): 1429–1448. https://doi.org/10.1007/s00414-017-1598-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Vega D, Simonsen ThJ, Hall MJR. (2017b) Looking into the puparium: Micro-CT visualization of the internal morphological changes during metamorphosis of the blow fly, Calliphora vicina, with the first quantitative analysis of organ development in cyclorrhaphous dipterans. Journal of Morphology 278: 629–651. https://doi.org/10.1002/jmor.20660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA. (2010) 55. Micropezidae (stilt-legged flies). In: Brown BV, Borkent A, Cumming JM, Wood DM, Woodley NE, Zumbado M. (Eds) Manual of Central American Diptera. vol 2. National Research Council Canada, Ottawa, 805–813.
- Marshall SA. (2012) Flies: the Natural History and Diversity of Diptera. Firefly Books, Ontario, 616 pp. [Google Scholar]
- Metscher BD. (2009) MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiology 9: 11. https://doi.org/10.1186/1472-6793-9-11 [DOI] [PMC free article] [PubMed]
- Mizutani R, Takeuchi A, Hara T, Uesugi K, Suzuki Y. (2007) Computed tomography imaging of the neuronal structure of Drosophila brain. Journal of Synchrotron Radiation 14: 282–287. https://doi.org/10.1107/S0909049507009004 [DOI] [PubMed] [Google Scholar]
- Ovtshinnikova OG. (1989) Muscles of the male genitalia of Brachycera–Orthorrhapha (Diptera). Trudy Zoologicheskogo Instituta Akademii Nauk SSSR 190: 1–166. [Google Scholar]
- Ovtshinnikova OG. (1994) On the homology of male genital sclerites of Brachycera Orthorrhapha and Cyclorrhapha (Diptera) based on musculature. Dipterological Research 5(4): 263–269. [Google Scholar]
- Ovtshinnikova OG. (2000) Muscles of the Male Genitalia of Hover Flies of the Family Syrphidae (Diptera). Kholodkovsky Memorial Lectures: a Report at the 52nd Annual Meeting, April 1, 1999, St. Petersburg, 70 pp. [In Russian] [Google Scholar]
- Ovtshinnikova OG, Yeates DK. (1998) Male genital musculature of Therevidae and Scenopinidae (Diptera: Asiloidea): structure, homology and phylogenetic implications. Australian Journal of Entomology 37(1): 27–33. https://doi.org/10.1111/j.1440-6055.1998.tb01539.x [Google Scholar]
- Ovtshinnikova OG, Galinskaya TV. (2017) The male abdominal, genital and pregenital sclerites and musculature in Neria commutata (Czerny, 1930) (Diptera, Micropezidae). Entomological Review 97(3): 282–287. https://doi.org/10.1134/S0013873817030022 [Google Scholar]
- Richards CS, Simonsen ThJ, Abel RL, Hall MJR, Schwyn DA, Wicklein M. (2012) Virtual forensic entomology: Improving estimates of minimum post-mortem interval with 3D micro-computed tomography. Forensic Science International 220: 251–264. https://doi.org/10.1016/j.forsciint.2012.03.012 [DOI] [PubMed] [Google Scholar]
- Sinclair BJ. (2000) 1.2. Morphology and terminology of Diptera male terminalia. In: Papp L, Darvas B. (Eds) Contributions to a manual of Palaearctic Diptera. vol 1. Science Herald, Budapest, 53–74.
- Steyskal GC. (1968) Family Micropezidae. In: Vanzolini PE, Papavero N. (Eds) A Catalogue of the Diptera of the Americas South of the United States. Departmento de Zoologia, Secretaria da Agricultura, Sau Paulo, 1–33.
- Steyskal GC. (1987) Micropezidae. In: McAlpine JF. (Ed.) Manual of Nearctic Diptera. vol 2. Agriculture Canada, Ottawa, 761–767.
- Walker SM, Schwyn DA, Mokso R, Wicklein M, Muller T, Doube M, Stampanoni M, Krapp HG, Taylor GK. (2014) In Vivo Time-Resolved Microtomography Reveals the Mechanics of the Blowfly Flight Motor. PLoS Biol 12(3): e1001823. https://doi.org/10.1371/journal.pbio.1001823 [DOI] [PMC free article] [PubMed]